Abstract
OBJECTIVE
Obesity is associated with microvascular insulin resistance, which is characterized by impaired insulin-mediated microvascular recruitment. Glucagon-like peptide 1 (GLP-1) recruits skeletal and cardiac muscle microvasculature, and this action is preserved in insulin-resistant rodents. We aimed to examine whether GLP-1 recruits microvasculature and improves the action of insulin in obese humans.
RESEARCH DESIGN AND METHODS
Fifteen obese adults received intravenous infusion of either saline or GLP-1 (1.2 pmol/kg/min) for 150 min with or without a euglycemic insulin clamp (1 mU/kg/min) superimposed over the last 120 min. Skeletal and cardiac muscle microvascular blood volume (MBV), flow velocity and blood flow, brachial artery diameter and blood flow, and pulse wave velocity (PWV) were determined.
RESULTS
Insulin failed to change MBV or flow in either skeletal or cardiac muscle, confirming the presence of microvascular insulin resistance. GLP-1 infusion alone increased MBV by ∼30% and ∼40% in skeletal and cardiac muscle, respectively, with no change in flow velocity, leading to a significant increase in microvascular blood flow in both skeletal and cardiac muscle. Superimposition of insulin to GLP-1 infusion did not further increase MBV or flow in either skeletal or cardiac muscle but raised the steady-state glucose infusion rate by ∼20%. Insulin, GLP-1, and GLP-1 + insulin infusion did not alter brachial artery diameter and blood flow or PWV. The vasodilatory actions of GLP-1 are preserved in both skeletal and cardiac muscle microvasculature, which may contribute to improving metabolic insulin responses and cardiovascular outcomes.
CONCLUSIONS
In obese humans with microvascular insulin resistance, GLP-1’s vasodilatory actions are preserved in both skeletal and cardiac muscle microvasculature, which may contribute to improving metabolic insulin responses and cardiovascular outcomes.
Introduction
Conduit arteries regulate total tissue blood flow and tissue delivery of nutrients, oxygen, and hormones. Microvasculature provides endothelial surface area for the extraction of nutrients, oxygen, and hormones into the tissue interstitium. Thus, both conduit arteries and microvasculature are vital in the maintenance of tissue health and as such subject to fine regulation.
Vascular endothelia express abundant insulin receptors (1) as well as glucagon-like peptide 1 (GLP-1) receptors (2). Both insulin and GLP-1 cause vasodilatation. Insulin through a receptor-mediated and nitric oxide (NO)-dependent process dilates the conduit artery to increase tissue total blood flow and relaxes the precapillary arterioles to increase microvascular perfusion in both laboratory animals (3) and humans (4,5). Similarly, GLP-1, a hormone produced by intestinal L cells in response to nutrient ingestion, exerts a potent vasodilatory effect on the conduit and resistance arteries as well as muscle microvasculature in rats and healthy humans (6–9).
The vascular effects of insulin are clearly attenuated in insulin-resistant laboratory animals and humans. Insulin-mediated muscle microvascular recruitment is reduced or abolished in rats that receive lipid infusion (10), are obese (11), or are fed a high-fat diet (HFD) (6,12). Similarly, insulin fails to increase conduit artery blood flow and recruit muscle microvasculature in humans with evidence of insulin resistance such as obesity (5), diabetes (13), or receipt of systemic lipid infusion to raise plasma free fatty acid (FFA) concentrations (14,15). We recently demonstrated that GLP-1 infusion acutely recruits muscle microvasculature, likely via a protein kinase A (PKA)-NO–mediated pathway, in laboratory rodents (7,8) and healthy humans (16,17), along with increased brachial artery diameter and total blood flow (16,17). In rats fed an HFD or receiving systemic lipid infusion, acute GLP-1 infusion was able to recruit microvasculature and improve insulin sensitivity in muscle (6), and treatment of HFD-fed rats with GLP-1 receptor agonist liraglutide for 4 weeks restored vascular insulin responses and endothelial function (18). It is unknown whether the vasodilatory actions of GLP-1 are preserved in humans with obesity and vascular insulin resistance.
In the current study, we evaluated the effects of insulin and GLP-1 at physiologically relevant concentrations on skeletal and cardiac muscle microvasculature and conduit arteries as well as their relations to insulin-mediated whole-body glucose disposal in obese humans. As insulin and GLP-1 act via different receptors and signaling pathways (PI 3-kinase vs. cAMP-PKA pathway) to elicit endothelium-mediated NO production, we hypothesize that humans with class I obesity exhibit vascular insulin resistance but have preserved responses to the vasodilatory action of GLP-1 in both skeletal and cardiac muscle microvasculature.
Research Design and Methods
Study Subjects
All volunteers were screened for study eligibility at the University of Virginia Clinical Research Unit. At the screening visit, vital signs, weight, and height were measured and BMI was calculated. Comprehensive metabolic panels, complete blood counts, lipid profiles, and pregnancy tests (if female) were acquired. Individuals with normal screening parameters and no exclusion criteria were invited to participate in the study. Persons with chronic medical illness such as diabetes, hyperlipidemia, hypertension or hypotension, anemia, or intracardiac or intrapulmonary shunt were excluded from the study. Other exclusion criteria included a first-degree relative with type 2 diabetes mellitus (T2DM), current smoking or having quit smoking within 6 months, use of supplements that could potentially affect vascular function (e.g., fish oil, vitamins E and C), pregnancy or lactation, or known hypersensitivity to perflutren.
A total of 15 obese adults (4 males and 11 females), mean ± SEM age 26.6 ± 2.2 years and BMI 33.7 ± 1.2 kg/m2, participated in the study. All women were studied in the follicular phase in their menstrual cycles. See Supplementary Table 1 for detailed participant characteristics.
Study Protocols
After study enrolment, participants returned for a treadmill Vo2max test using the Bruce protocol and body composition measurement using air displacement plethysmography (Bod-Pod; Life Management, Concorde, CA). They were then studied in random order under three separate study protocols, with at least a 2-week interval between the admissions.
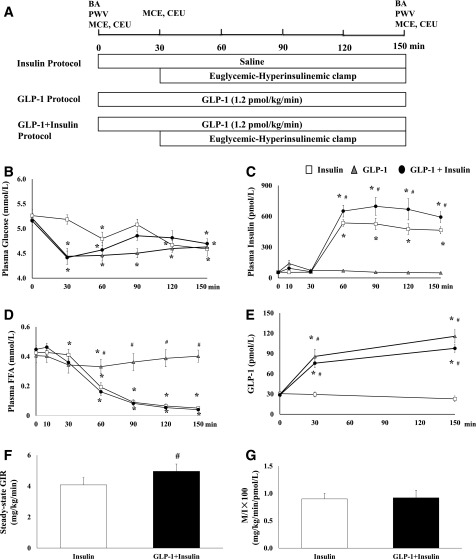
For each study, participant was admitted to the Clinical Research Unit at 0700 h after refraining from exercise and caffeine for 24 h and undergoing a fast from 2000 h the night before. A catheter was placed in the antecubital vein for infusions of normal saline, dextrose, microbubbles, GLP-1, and insulin. A second catheter was placed distal to the antecubital vein for blood sampling. Baseline vascular parameters were then determined, including brachial artery diameter, flow velocity and blood flow, pulse wave velocity (PWV), and cardiac and skeletal muscle microvascular blood volume (MBV), microvascular flow velocity (MFV), and microvascular blood flow (MBF), using myocardial contrast echocardiography (MCE) and contrast-enhanced ultrasound (CEU). Subject was then studied under one of the following protocols (Fig. 1A).
Figure 1.
Study protocol, plasma biochemical parameters, and GIRs. A: Study protocol: each subject received an intravenous infusion of either normal saline or GLP-1 (1.2 pmol/kg/min) for 150 min with or without a euglycemic insulin clamp (1 mU/kg/min) superimposed over the last 120 min. BA, brachial artery measurements. B: Plasma glucose concentrations. C: Plasma insulin concentrations. D: Plasma FFA concentrations. E: Plasma GLP-1 concentrations. F: Steady-state GIRs during insulin infusion and GLP-1 + insulin infusion. G: Steady-state GIRs corrected by plasma insulin concentrations. Compared with 0 min, *P < 0.05. Compared with insulin protocol, #P < 0.05.
Insulin Protocol
Each participant received a systemic infusion of normal saline for a total of 150 min. After MCE and CEU measurements were obtained at 30 min, a 2-h hyperinsulinemic-euglycemic clamp (1 mU/kg/min) was begun. Plasma glucose concentrations were monitored every 5 min and maintained at ∼10 mg/dL below the baseline levels to avoid arterial hyperglycemia using 20% dextrose infused at variable rates. MCE and CEU measurements, brachial artery parameters, and PWV were determined again at the end of insulin clamp.
GLP-1 Protocol
Each subject received a systemic infusion of GLP-1 at a rate of 1.2 pmol/kg/min for a total of 150 min. MCE and CEU measurements were repeated at 30 and 150 min. Brachial artery parameters and PWV were measured again at 150 min. GLP-1 infusion at the dose selected raised plasma GLP-1 concentrations to the postprandial levels and increased brachial arterial blood flow by ∼30% (P < 0.005), skeletal muscle MBV by ∼40% (P < 0.001), and cardiac muscle MBV by nearly 60% (P < 0.001) in healthy young adults (16).
GLP-1 + Insulin Protocol
Each participant received a systemic infusion of GLP-1 at a rate of 1.2 pmol/kg/min for a total of 150 min. After MCE and CEU measurements were obtained at 30 min, subject received a 2-h hyperinsulinemic-euglycemic clamp as detailed in the insulin protocol. MCE and CEU measurements, brachial artery parameters, and PWV were determined again at the end of insulin clamp.
During each study, subjects remained supine and vital signs were measured every 30 min. Blood samples were collected at baseline and then every 30 min for glucose, insulin, and FFA measurements. Additional samples were collected at 0, 30, and 150 min for GLP-1 measurements.
The study protocols were carried out in accordance with the 2013 Declaration of Helsinki of the World Medical Association and were approved by the University of Virginia Institutional Review Board. Each participant gave written informed consent before study enrolment.
Measurement of Microvascular Parameters in Cardiac and Skeletal Muscle
MCE and CEU were performed with the subject in the left decubitus position using a SONOS 7500 ultrasound system and an S3 phased array transducer (Philips Medical Systems, Andover, MA) as previously described (14,19–21). Definity microbubbles (Lantheus Medical Imaging, North Billerica, MA) were infused systemically to trace the microvasculature. Once the blood microbubble concentrations reached steady state (within 2–3 min), intermittent ultraharmonic imaging was performed with the pulsing intervals of 1, 2, 3, 4, 5, and 8 cardiac cycles in the cardiac muscle (mechanic index 0.8) and 1, 2, 3, 4, 5, 8, 12, 16, and 20 cardiac cycles in the skeletal muscle (mechanic index 1.5). At each pulsing interval, three images were captured digitally. Cardiac imaging included the apical two-, three-, and four-chamber views. The skeletal muscle imaging was performed in the left forearm in a transaxial plane 5 cm distal to the antecubital fossa. All images were analyzed using the QLAB software (Philips Medical Systems) to determine the MBV and MFV as previously described (16,20,21). MBF was calculated as the product of MBV and MFV. For cardiac muscle microvascular parameters, mean MBV, MFV, and MBF values from all three apical views are reported. The baseline coefficient of variability of MBV among three admissions were 31 ± 4% in skeletal muscle and 20 ± 3% in cardiac muscle.
Measurement of Brachial Artery Parameters
Brachial artery diameter and blood flow velocity were determined using a SONOS 7500 ultrasound as previously described (14,19–21). The left brachial artery was imaged ∼5 cm proximal to the antecubital crease using an L11-3 linear array transducer with a transmit frequency of 7.5 MHz. The diameter was measured during peak systole, and the flow velocity was determined using pulsed-wave Doppler. Brachial artery blood flow (Q.) was calculated from the averages of three diameter (d) and velocity (v) measurements using the equation (Q.) = vπ(d/2)2.
Measurement of PWV
Aortic arterial stiffness was assessed by carotid-femoral PWV. PWV was calculated from the time taken for the arterial pulse to propagate from the carotid to the femoral artery by applanation tonometry using the SphygmoCor system (AtCor Medical). The stiffer the aorta, the shorter the PWV.
Biochemical Analysis
Comprehensive metabolic panels, complete blood counts, lipid profiles, and pregnancy tests were assayed at the University of Virginia Clinical Chemistry Laboratory. Plasma glucose was measured using a YSI glucose analyzer (Yellow Spring Instruments). Plasma insulin concentrations were measured using an ELISA kit (ALPCO Diagnostics, Windham, NH). Plasma total GLP-1 concentrations were measured using an ELISA kit (Millipore, Watford, U.K.). Plasma FFA concentrations were measured using an in vitro enzymatic colorimetric assay with a Wako HR Series NEFA-HR kit (Wako Diagnostics, Richmond, VA).
Statistical Analysis
A priori calculation based on our prior studies in healthy humans (16,17) showed that a sample size of 14 would have 80% power (with α = 0.05) to detect ∼30% difference in muscle MBV. A total of 15 subjects were included in the study, and all data are presented as mean ± SEM. Statistical analyses were performed with software package SAS, version 9.4 (SAS Institute Inc., Cary, NC), as previously described (17). Changes in skeletal and cardiac muscle microvascular parameters from 0 to 30 min and from 30 to 150 min were analyzed via piecewise random coefficient regression. Brachial artery diameter, flow velocity, blood flow, and PWV were analyzed by way of random coefficient regression. A P value of <0.05 was considered statistically significant.
Results
Participant Characteristics and Biochemical Profiles
Supplementary Table 1 summarizes clinical parameters obtained at the screening visit. Participants were obese and normotensive and had normal lipid profiles. Insulin, GLP-1, or GLP-1 + insulin infusions did not alter subjects’ blood pressure but induced a small (statistically nonsignificant) increase in heart rate (Supplementary Table 2), similar to our prior observations in healthy young adults (17). Changes in plasma glucose, insulin, GLP-1, and FFA concentrations are summarized in Fig. 1. Insulin infusion raised plasma insulin concentrations by approximately ninefold (P < 0.001) and decreased plasma FFA concentrations by ∼90% (P < 0.01). GLP-1 infusion alone increased plasma GLP-1 levels by approximately threefold to fourfold to levels seen postprandially, and this was associated with a statistically significant decrease in plasma glucose concentrations at 30 min (from 5.2 ± 0.1 to 4.4 ± 0.2 mmol/L, P < 0.001), which remained lower than baseline during the entire study (Fig. 1B). Measurement of plasma insulin levels after 10 min of GLP-1 infusion demonstrated a statistically nonsignificant, transient increase (from mean ± SEM 56.8 ± 5.8 to 140.6 ± 32 pmol/L, P = 0.589), which rapidly returned back to baseline at 30 min (73.7 ± 8.8 pmol/L, P = 1.000). FFA concentrations declined slightly during GLP-1 infusion, but the reduction was statistically significant only at 60 min (P = 0.047). In GLP-1 + insulin protocol, GLP-1 infusion similarly increased total GLP-1 by threefold and decreased plasma glucose concentrations at 30 min (from 5.2 ± 0.1 to 4.4 ± 0.1 mmol/L, P < 0.001). While plasma insulin levels were also transiently increased at 10 min (from 52.7 ± 3.9 to 94.0 ± 9.7 pmol/L), this change was not statistically significant (P = 0.864) and the levels returned back to baseline (60.9 ± 5.4 pmol/L) at 30 min. Insulin and GLP-1 coinfusion raised plasma insulin concentrations and suppressed plasma FFA concentrations, similar to the insulin infusion study.
Compared with the insulin infusion study, superimposing insulin onto GLP-1 infusion led to a significantly higher glucose infusion rate (GIR) required to maintain euglycemia at steady state (mean ± SEM 4.96 ± 0.47 vs. 4.08 ± 0.42 mg/kg/min, P = 0.019). Steady-state plasma insulin concentrations were higher in the GLP-1 + insulin protocol (630.4 ± 76.1 pmol/L) than those in the insulin protocol (470.3 ± 24.1 pmol/L). The M/I ratios, steady-state GIR corrected by plasma insulin concentrations, were comparable between these two protocols (P = 0.891).
Changes in Skeletal Muscle Microvascular Parameters
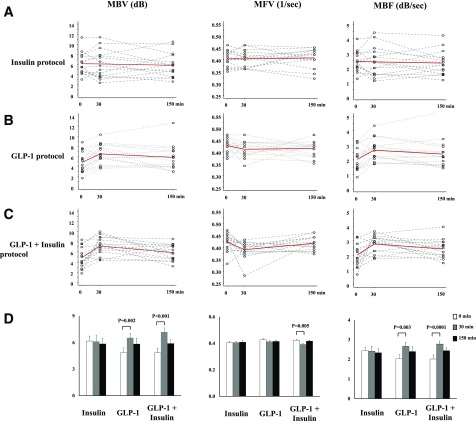
Figure 2 shows changes of skeletal muscle microvascular parameters. Insulin infusion alone did not change skeletal muscle MBV, MFV, or MBF, confirming the presence of skeletal muscle microvascular insulin resistance. On the contrary, GLP-1 infusion promptly increased skeletal muscle MBV from 4.85 ± 0.54 dB at 0 min to 6.53 ± 0.49 dB at 30 min (P = 0.002) without affecting MFV, leading to a significant increase in MBF (2.03 ± 0.21 vs. 2.66 ± 0.20 dB/sec for 0 vs. 30 min, respectively; P = 0.003). MBV and MBF remained elevated from 30 to 150 min during the GLP-1 infusion. In the GLP-1 + insulin protocol, GLP-1 infusion similarly increased skeletal muscle MBV and MBF at 30 min (P < 0.001 for both), which was associated with a slight, but statistically significant, reduction in MFV (0.422 ± 0.009 vs. 0.390 ± 0.009 1/s for 0 vs. 30 min, P = 0.005). Superimposition of insulin infusion onto GLP-1 infusion did not further increase MBV or MBF compared with GLP-1 infusion alone (P = 0.424 and 0.815, respectively).
Figure 2.
Changes of skeletal muscle microvascular parameters. A: Insulin protocol. B: GLP-1 protocol. C: GLP-1 + insulin protocol. Dotted lines, individual data; solid red lines, average. D: Quantitative changes.
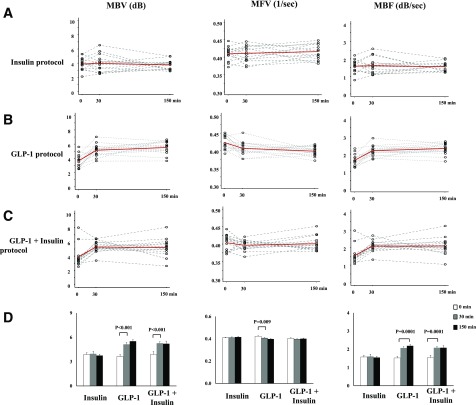
Changes in Cardiac Muscle Microvascular Parameters
Changes of cardiac muscle microvascular parameters were similar to those of skeletal muscle (Fig. 3). Insulin infusion alone had no effect on cardiac muscle MBV, MFV, or MBF. GLP-1 infusion significantly increased cardiac muscle MBV and MBF within 30 min (P < 0.001 for both). This was associated with a slight, but statistically significant, reduction in MFV (0.423 ± 0.005 vs. 0.406 ± 0.005 1/s for 0 vs. 30 min, respectively; P = 0.009). Cardiac muscle MBV and MBF remained elevated from 30 to 150 min during GLP-1 infusion. In GLP-1 + insulin protocol, GLP-1 infusion similarly increased cardiac muscle MBV and MBF within 30 min (P < 0.001 for both) with no significant alteration in MFV (0.404 ± 0.006 vs. 0.397 ± 0.004 1/s, P = 0.243). Addition of insulin infusion to GLP-1 infusion did not further increase MBV or MBF compared with GLP-1 infusion alone (P = 0.247 and 0.400 at 30 and 150 min, respectively).
Figure 3.
Changes of cardiac muscle microvascular parameters. A: Insulin protocol. B: GLP-1 protocol. C: GLP-1 + insulin protocol. Dotted lines, individual data; solid red lines, average. D: Quantitative changes.
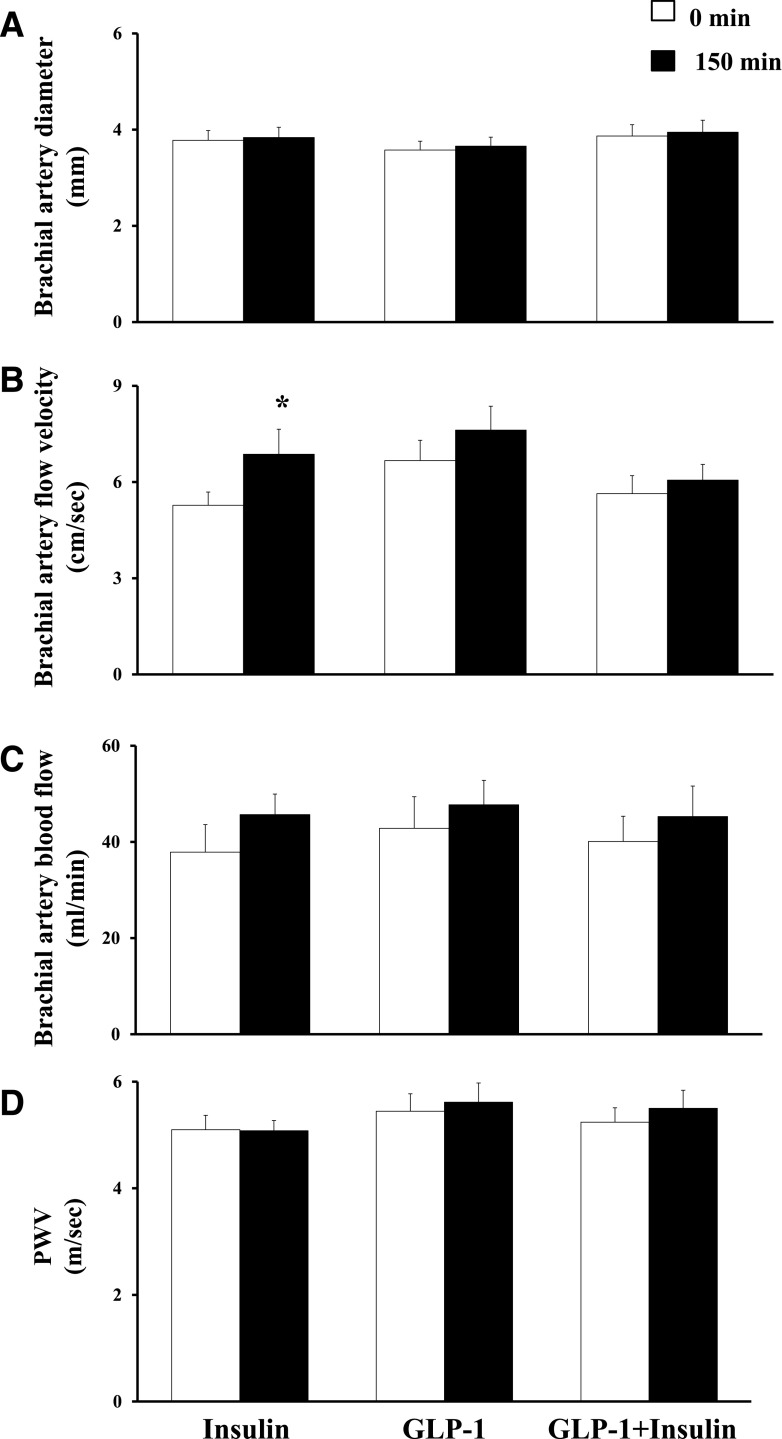
Changes in Brachial Artery Diameter, Flow Velocity, and Blood Flow
The ultrasound images of one participant from the insulin protocol and another from the GLP-1 + insulin protocol were excluded from analysis due to poor image quality. Insulin infusion had no effect on brachial artery diameter (3.78 ± 0.20 vs. 3.84 ± 0.21 mm for 0 vs. 150 min, respectively; P = 0.304) but resulted in a statistically significant increase in flow velocity (5.27 ± 0.42 vs. 6.87 ± 0.78 cm/s for 0 vs. 150 min, P = 0.008). Overall, the changes in brachial artery blood flow were not statistically significant (37.88 ± 5.69 vs. 45.68 ± 4.26 mL/min for 0 vs. 150 min, P = 0.082). Neither GLP-1 alone nor GLP-1 and insulin coinfusions had a significant effect on brachial artery parameters (Fig. 4).
Figure 4.
Changes in brachial artery diameter (A), flow velocity (B), blood flow (C), and PWV (D). Compared with 0 min, *P = 0.008. sec, second.
Changes in PWV
PWV remained unchanged during insulin infusion (5.10 ± 0.27 vs. 5.08 ± 0.19 m/s for 0 vs. 150 min, respectively; P = 0.931). GLP-1 and GLP-1 + insulin infusions resulted in a small, statistically nonsignificant, increase in PWV (0.17 ± 0.15 m/s, P = 0.341, and 0.25 ± 0.22 m/s, P = 0.157, respectively) (Fig. 4).
Conclusions
In addition to confirming the presence of significant insulin resistance in conduit artery and skeletal and cardiac muscle microvasculature in otherwise healthy, obese humans, the current study convincingly demonstrated that in the obesity state the vasodilatory action of GLP-1 is blunted in the conduit artery but preserved in the skeletal and cardiac muscle microvasculature. This GLP-1–induced microvascular recruitment (within 30 min) was clearly independent of insulin, as GLP-1 infusion only slightly raised plasma insulin concentrations (from 56.8 ± 5.8 to 73.7 ± 8.8 pmol/L during GLP-1 admission and from 52.7 ± 3.9 to 60.9 ± 5.4 pmol/L during GLP-1 + insulin admission, P = 0.07 and 0.10 respectively). On the contrary, insulin infusion alone increased plasma insulin concentrations by ∼10-fold within 30 min (P < 0.001) but failed to recruit skeletal and cardiac muscle microvasculature. As muscle microvasculature provides the endothelial surface area for substrate and hormone entry into muscle interstitium, GLP-1 may play a vital role in maintaining skeletal and cardiac muscle health via enhancing microvascular perfusion in the obese state.
While it is known that obesity is associated with endothelial dysfunction (22) and vascular insulin resistance (5,23), our study confirms that insulin resistance is present in all arterial segments in young adults with only class I obesity and no other features of insulin resistance or cardiovascular risk factors. This is consistent with our prior report that insulin-mediated muscle microvascular recruitment and increase in brachial artery blood flow seen in lean humans were blunted in obese individuals (5). These findings are of major clinical and socioeconomic importance. Indeed, our study cohort consisted of subjects with only class I obesity (average BMI 33.7 kg/m2) who had no first-degree relatives with diabetes and who had normal blood pressure and normal fasting plasma glucose levels and fasting lipid profile. We would traditionally consider this population in general healthy and at only slightly increased cardiovascular risks. However, the clear presence of vascular insulin resistance in these subjects argues strongly that even class I obesity engenders a significant cardiovascular risk and lifestyle intervention should be initiated well before the appearance of traditional, modifiable cardiovascular risk factors.
In healthy humans, both insulin and GLP-1 at physiological concentrations are able to dilate brachial artery and recruit muscle microvasculature to increase skeletal and cardiac muscle perfusion (16,17). Vascular insulin resistance not only blunts insulin-mediated perfusion but may even reduce tissue blood flow due to insulin-stimulated vasoconstriction (24). This makes our findings that insulin-resistant obese subjects have a robust microvascular response to GLP-1 particularly important in that GLP-1 may contribute to skeletal and cardiac muscle health in the obese state by enhancing skeletal and cardiac muscle perfusion. Indeed, we have previously observed in rats that GLP-1 infusion can acutely increase muscle microvascular perfusion along with muscle interstitial oxygenation in both insulin-sensitive and insulin-resistant rodents (6,7). It is of interest to note that while the slopes of MBV increases (0–30 min) were similar in skeletal and cardiac muscle in response to GLP-1 infusion, there was no statistically significant correlation between the percentage of responses of the two muscle beds (r = 0.285, P = 0.31), likely due to the semiquantitative nature of the microbubble signal and the heterogeneity of the responses (95% CI 0.0208–0.0913 vs. 0.0320–0.0680 for skeletal muscle vs. cardiac muscle, respectively).
In the current study, 11 out of 15 subjects were women. While we saw no difference between obese men and women, the study design was not powered to detect a sex difference, and thus more study is needed. In addition, whether the current findings can be extrapolated to humans with metabolic derangement such as T2DM or used to explain the vascular effects of chronic GLP-1 receptor stimulation remains to be examined. This is important, as T2DM patients exhibit vascular insulin resistance, are prone to developing cardiovascular complications including diastolic dysfunction and heart failure (25,26), and have reduced functional exercise capacity (27,28), and improvement in cardiac and skeletal muscle perfusion may enhance their functions. Studies have already demonstrated that GLP-1 receptor agonists improve cardiovascular outcomes in T2DM patients with high cardiovascular risks (29,30) and rescue insulin-mediated muscle microvascular perfusion and oxygen content in insulin-resistant rodents (18).
In healthy humans, GLP-1 causes vasodilation of the microvasculature to increase tissue perfusion and of the conduit artery to increase total tissue blood flow. While GLP-1 infusion can acutely increase brachial artery flow in healthy humans (16,17), it failed to do so in obese humans in the current study. One possible explanation is the short duration of GLP-1 infusion. In humans with diabetes, endoplasmic reticulum stress contributes to vascular insulin resistance and endothelial dysfunction, and stimulation of the GLP-1 receptor with liraglutide can ameliorate endoplasmic reticulum stress and restore insulin‐mediated NO synthase activation in endothelial cells isolated from patients with diabetes (31). However, it may take much longer to reach such an effect in vivo. Six months of treatment with liraglutide markedly improved brachial artery flow-mediated dilation and reduced oxidative stress markers in newly diagnosed subjects with T2DM (32), and treating HFD-fed rats with liraglutide for 4 weeks restored endothelial function; both support this possibility (18).
Our observation that superimposition of insulin infusion on top of GLP-1 infusion induced no change in either brachial arterial diameter and blood flow or skeletal and cardiac muscle microvascular perfusion is certainly not surprising given the presence of significant vascular insulin resistance in the study population. However, we noted a significant increase in insulin-mediated whole-body glucose disposal during combined insulin and GLP-1 infusions compared with insulin infusion alone. This increase appears to be due to an increased plasma insulin concentration, as when the GIRs were corrected with plasma insulin concentrations, the M/I ratios (i.e., GIRs per unit of insulin) between the two admissions were comparable. This pattern is similar to that seen in our recent observations in healthy humans (17). The higher plasma insulin levels seen during GLP-1 and insulin coinfusion are most likely secondary to reduced insulin clearance after GLP-1 administration (33). In mice with double deletion of GLP-1 and glucose-dependent insulinotropic polypeptide receptors, insulin clearance is also increased (34). Higher plasma insulin concentrations may lead to higher muscle interstitial insulin concentrations due to GLP-1–mediated microvascular recruitment, which increases muscle insulin delivery (35), and it is the interstitial insulin concentrations that correlate with insulin-mediated muscle glucose disposal (36).
Similar to our prior observations in healthy humans (17), insulin, GLP-1, and GLP-1 + insulin infusion did not alter PWV in obese humans in the current study. As arterial stiffness reflects arterial elasticity and vascular aging, it may take a much longer treatment time to observe a change. In overweight patients with type 1 diabetes, addition of liraglutide to insulin treatment for 24 weeks had no impact on PWV (37), but similar lengths of liraglutide treatment did reduce arterial stiffness and improve left ventricular myocardial deformation in subjects with newly diagnosed T2DM (32). Furthermore, treatment of early T2DM with dipeptidyl peptidase 4 inhibitor linagliptin for 26 weeks also significantly improved PWV (38). Whether these effects were related to reduction in weight and plasma insulin levels remains unclear, as both were associated with improved PWV in the Slow the Adverse Effects of Vascular Aging (SAVE) trial (39). The lack of GLP-1 effects on PWV, as well as on conduit flow, could also be false negatives owing to different sensitivity for detection of an actual effect that might be present but small. Further studies are warranted given the high prevalence of increased PWV in people with diabetes (40).
In conclusion, obese humans display insulin resistance in both conduit artery and skeletal and cardiac muscle microvasculature, but their responses to GLP-1 are preserved in both skeletal and cardiac muscle microvasculature, which may contribute to improving metabolic insulin responses, tissue health, and cardiovascular outcomes in obesity.
Supplementary Material
Article Information
Funding. This work was supported by National Institutes of Health grants R01HL094722 and R01DK102359 and American Diabetes Association grant 1-17-ICTS-059 (to Z.L.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. N.W., A.W.K.T., L.A.J., L.H., E.J.B., K.W.A., and Z.L. researched data. N.W. and Z.L. wrote the manuscript. J.T.P. performed statistical analyses. S.L. contributed to discussion. Z.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
This article contains Supplementary Data online at https://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-1465/-/DC1.
References
- 1.Li G, Barrett EJ, Wang H, Chai W, Liu Z. Insulin at physiological concentrations selectively activates insulin but not insulin-like growth factor I (IGF-I) or insulin/IGF-I hybrid receptors in endothelial cells. Endocrinology 2005;146:4690–4696 [DOI] [PubMed] [Google Scholar]
- 2.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz S-S, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 2008;117:2340–2350 [DOI] [PubMed] [Google Scholar]
- 3.Vincent MA, Clerk LH, Lindner JR, et al. . Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 2004;53:1418–1423 [DOI] [PubMed] [Google Scholar]
- 4.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 1994;94:1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 2006;55:1436–1442 [DOI] [PubMed] [Google Scholar]
- 6.Chai W, Zhang X, Barrett EJ, Liu Z. Glucagon-like peptide 1 recruits muscle microvasculature and improves insulin’s metabolic action in the presence of insulin resistance. Diabetes 2014;63:2788–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chai W, Dong Z, Wang N, et al. . Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes 2012;61:888–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong Z, Chai W, Wang W, et al. . Protein kinase A mediates glucagon-like peptide 1-induced nitric oxide production and muscle microvascular recruitment. Am J Physiol Endocrinol Metab 2013;304:E222–E228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sjøberg KA, Holst JJ, Rattigan S, Richter EA, Kiens B. GLP-1 increases microvascular recruitment but not glucose uptake in human and rat skeletal muscle. Am J Physiol Endocrinol Metab 2014;306:E355–E362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clerk LH, Rattigan S, Clark MG. Lipid infusion impairs physiologic insulin-mediated capillary recruitment and muscle glucose uptake in vivo. Diabetes 2002;51:1138–1145 [DOI] [PubMed] [Google Scholar]
- 11.Wallis MG, Wheatley CM, Rattigan S, Barrett EJ, Clark ADH, Clark MG. Insulin-mediated hemodynamic changes are impaired in muscle of Zucker obese rats. Diabetes 2002;51:3492–3498 [DOI] [PubMed] [Google Scholar]
- 12.Zhao L, Fu Z, Wu J, et al. . Inflammation-induced microvascular insulin resistance is an early event in diet-induced obesity. Clin Sci (Lond) 2015;129:1025–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baron AD. Hemodynamic actions of insulin. Am J Physiol 1994;267:E187–E202 [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Jahn LA, Fowler DE, Barrett EJ, Cao W, Liu Z. Free fatty acids induce insulin resistance in both cardiac and skeletal muscle microvasculature in humans. J Clin Endocrinol Metab 2011;96:438–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Liu J, Jahn LA, Fowler DE, Barrett EJ. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J Clin Endocrinol Metab 2009;94:3543–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subaran SC, Sauder MA, Chai W, et al. . GLP-1 at physiological concentrations recruits skeletal and cardiac muscle microvasculature in healthy humans. Clin Sci (Lond) 2014;127:163–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan AWK, Subaran SC, Sauder MA, et al. . GLP-1 and insulin recruit muscle microvasculature and dilate conduit artery individually but not additively in healthy humans. J Endocr Soc 2018;2:190–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chai W, Fu Z, Aylor KW, Barrett EJ, Liu Z. Liraglutide prevents microvascular insulin resistance and preserves muscle capillary density in high-fat diet-fed rats. Am J Physiol Endocrinol Metab 2016;311:E640–E648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z. Insulin at physiological concentrations increases microvascular perfusion in human myocardium. Am J Physiol Endocrinol Metab 2007;293:E1250–E1255 [DOI] [PubMed] [Google Scholar]
- 20.Sauder MA, Liu J, Jahn LA, Fowler DE, Chai W, Liu Z. Candesartan acutely recruits skeletal and cardiac muscle microvasculature in healthy humans. J Clin Endocrinol Metab 2012;97:E1208–E1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chai W, Liu J, Jahn LA, Fowler DE, Barrett EJ, Liu Z. Salsalate attenuates free fatty acid-induced microvascular and metabolic insulin resistance in humans. Diabetes Care 2011;34:1634–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 1996;97:2601–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest 1990;85:1844–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev 2007;28:463–491 [DOI] [PubMed] [Google Scholar]
- 25.Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord 2013;14:5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ofstad AP, Atar D, Gullestad L, Langslet G, Johansen OE. The heart failure burden of type 2 diabetes mellitus-a review of pathophysiology and interventions. Heart Fail Rev 2018;23:303–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauer TA, Reusch JEB, Levi M, Regensteiner JG. Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes Care 2007;30:2880–2885 [DOI] [PubMed] [Google Scholar]
- 28.Brandenburg SL, Reusch JE, Bauer TA, Jeffers BW, Hiatt WR, Regensteiner JG. Effects of exercise training on oxygen uptake kinetic responses in women with type 2 diabetes. Diabetes Care 1999;22:1640–1646 [DOI] [PubMed] [Google Scholar]
- 29.Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marso SP, Bain SC, Consoli A, et al.; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 31.Bretón-Romero R, Weisbrod RM, Feng B, et al. . Liraglutide treatment reduces endothelial endoplasmic reticulum stress and insulin resistance in patients with diabetes mellitus. J Am Heart Assoc 2018;7:e009379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambadiari V, Pavlidis G, Kousathana F, et al. . Effects of 6-month treatment with the glucagon like peptide-1 analogue liraglutide on arterial stiffness, left ventricular myocardial deformation and oxidative stress in subjects with newly diagnosed type 2 diabetes. Cardiovasc Diabetol 2018;17:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahrén B, Thomaseth K, Pacini G. Reduced insulin clearance contributes to the increased insulin levels after administration of glucagon-like peptide 1 in mice. Diabetologia 2005;48:2140–2146 [DOI] [PubMed] [Google Scholar]
- 34.Tura A, Bizzotto R, Yamada Y, Seino Y, Pacini G, Ahrén B. Increased insulin clearance in mice with double deletion of glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide receptors. Am J Physiol Regul Integr Comp Physiol 2018;314:R639–R646 [DOI] [PubMed] [Google Scholar]
- 35.Barrett EJ, Eggleston EM, Inyard AC, et al. . The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 2009;52:752–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castillo C, Bogardus C, Bergman R, Thuillez P, Lillioja S. Interstitial insulin concentrations determine glucose uptake rates but not insulin resistance in lean and obese men. J Clin Invest 1994;93:10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dejgaard TF, Johansen NB, Frandsen CS, et al. . Effects of liraglutide on cardiovascular risk factors in patients with type 1 diabetes. Diabetes Obes Metab 2017;19:734–738 [DOI] [PubMed] [Google Scholar]
- 38.de Boer SA, Heerspink HJL, Juárez Orozco LE, et al. . Effect of linagliptin on pulse wave velocity in early type 2 diabetes: a randomized, double-blind, controlled 26-week trial (RELEASE). Diabetes Obes Metab 2017;19:1147–1154 [DOI] [PubMed] [Google Scholar]
- 39.Hughes TM, Althouse AD, Niemczyk NA, Hawkins MS, Kuipers AL, Sutton-Tyrrell K. Effects of weight loss and insulin reduction on arterial stiffness in the SAVE trial. Cardiovasc Diabetol 2012;11:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 2005;25:932–943 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.