Abstract
OBJECTIVE
To determine the prevalence of cognitive deficits and traditional diabetic complications and the association between metabolic factors and these outcomes.
RESEARCH DESIGN AND METHODS
We performed a cross-sectional study in severely obese individuals before bariatric surgery. Lean control subjects were recruited from a research website. Cognitive deficits were defined by the National Institutes of Health (NIH) Toolbox (<5th percentile for lean control subjects). Cardiovascular autonomic neuropathy (CAN) was defined by an expiration-to-inspiration (E-to-I) ratio of <5th percentile for lean control subjects. Retinopathy was based on retinal photographs and nephropathy on the estimated glomerular filtration rate (<60 mg/dL) and/or the albumin-to-creatinine ratio (ACR) (≥30 mg/g). NIH Toolbox, E-to-I ratio, mean deviation on frequency doubling technology testing, and ACR were used as sensitive measures of these outcomes. We used multivariable linear regression to explore associations between metabolic factors and these outcomes.
RESULTS
We recruited 138 severely obese individuals and 46 lean control subjects. The prevalence of cognitive deficits, CAN, retinopathy, and nephropathy were 6.5%, 4.4%, 0%, and 6.5% in lean control subjects; 22.2%, 18.2%, 0%, and 6.1% in obese participants with normoglycemia; 17.7%, 21.4%, 1.9%, and 17.9% in obese participants with prediabetes; and 25.6%, 31.9%, 6.1%, and 16.3% in obese participants with diabetes. Waist circumference was significantly associated with cognitive function (−1.48; 95% CI −2.38, −0.57) and E-to-I ratio (−0.007; 95% CI −0.012, −0.002). Prediabetes was significantly associated with retinal function (−1.78; 95% CI −3.56, −0.002).
CONCLUSIONS
Obesity alone is likely sufficient to cause cognitive deficits but not retinopathy or nephropathy. Central obesity is the key metabolic risk factor.
Introduction
Almost 40% of adults in the U.S. are obese, with 8% meeting criteria for severe obesity (1). Given that obesity and severe obesity have both increased substantially during the last 12 years, the adverse health consequences of this condition are likely to increase. In addition to increasing mortality, obesity is associated with a greater chance of developing type 2 diabetes (2). More recently, the relationship between obesity and traditional diabetic complications, such as peripheral neuropathy, cardiovascular autonomic neuropathy (CAN), retinopathy, and nephropathy, has been increasingly studied. Several studies have demonstrated that obesity is associated with neuropathy (3–9) and nephropathy (10,11). In contrast, a meta-analysis failed to show an association between obesity and retinopathy, although this is not consistent across all studies (12–15). While there are fewer CAN studies, waist circumference was correlated with a CAN outcome in a population with impaired glucose tolerance (16). Further clarification of the role of obesity in these complications is needed to inform future interventions to prevent these important adverse outcomes.
In addition to traditional diabetic complications, the effects of obesity on cognition have been an active source of investigation. Several large prospective studies revealed an association between obesity and cognitive function (17–22) and between obesity in midlife and dementia (23,24). However, the role of the distribution of obesity and other metabolic components is less well studied. One group demonstrated that central obesity, diabetes, and dyslipidemia were associated with mild cognitive impairment, but extensive anthropometric measurements were not performed (25). Therefore, further defining the role of the distribution of obesity on cognitive function is essential.
We have demonstrated in two separate cohorts that the prevalence of peripheral neuropathy is higher in obese individuals with normoglycemia than in lean control subjects (5,6). These results suggest that obesity alone is likely sufficient to cause peripheral neuropathy. Whether obesity alone is sufficient to cause other traditional diabetic complications and cognitive deficits is unclear. Furthermore, we have demonstrated that waist circumference, but not other anthropometric measurements, are significantly associated with peripheral neuropathy. Whether this is also true for other complications is unclear.
We aimed to determine the prevalence of cognitive deficits and traditional diabetic complications in obese participants, stratified by glycemic status, to inform whether obesity alone is sufficient to cause these complications. Furthermore, we investigated sensitive measures of these complications to see whether the earliest evidence of injury to these tissues can be seen in obese participants with normoglycemia. We also determined the association between metabolic syndrome (MetS) components and these complications, including the associations between different anthropometric measurements.
Research Design and Methods
Population
From March 2015 to June 2018, we recruited participants from the University of Michigan bariatric surgery clinic (before surgical intervention), as previously described (6). Inclusion criteria included age ≥18 years, BMI >35 kg/m2 with at least one comorbid medical condition or BMI >40 kg/m2, and being able/willing to provide written informed consent for the study. Full exclusion criteria are presented in our previous report (6). We recruited lean control subjects with no MetS components (see definition below) through a University of Michigan website (https://umhealthresearch.org/). Lean control subjects were excluded if they were taking medications for blood pressure, cholesterol, diabetes, or triglycerides.
The University of Michigan Institutional Review Board approved this study, and all participants signed informed consent documents for this study.
Anthropometric Measurements
Anthropometric measurements collected included the arm (midway between the acromion and olecranon process), forearm (maximal circumference), high waist (narrowest part of torso, above umbilicus and below xiphoid process), abdomen (greatest anterior extension of the abdomen), National Cholesterol Education Program (NCEP) waist (top of the iliac crest), buttocks/hips (maximal circumference of the buttocks), hips/thigh (maximal circumference of the hip/proximal thigh just below the gluteal fold), midthigh (midway between the inguinal crease and the proximal border of the patella), and calf (maximal circumference between the knee and ankle). Two measurements were collected at the same visit and averaged for each location.
Other Metabolic Phenotyping
Obese and lean participants underwent glucose tolerance testing (except for obese participants with a previous diagnosis of diabetes) and a fasting lipid panel. HbA1c was obtained on obese participants only. Participants also had blood pressure, height, weight, and BMI measurements at the time of study entry.
MetS Components
Diabetes (fasting glucose ≥126 mg/dL, 2-h glucose ≥200 mg/dL, HbA1c ≥6.5%, or previous diagnosis of diabetes) and prediabetes (fasting glucose ≥100 mg/dL, 2-h glucose ≥140 mg/dL, HbA1c ≥5.7%, or previous diagnosis of prediabetes) were defined according to the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (26). The updated NCEP/Adult Treatment Panel III (ATPIII) criteria were used to define the MetS and its individual components (27). Specifically, the MetS criteria were a waist circumference ≥102 cm in men and ≥88 cm in women, systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg, triglycerides ≥150 mg/dL, and HDL <40 mg/dL in men and <50 mg/dL in women. Participants taking medications for blood pressure, cholesterol, diabetes, or triglycerides were considered to have the corresponding MetS component.
Cognitive Deficits
Our primary cognitive outcome was an NIH Toolbox cognitive composite score of <5th percentile cutoff for lean control subjects. The primary sensitive cognitive measure was the NIH Toolbox cognitive composite score. The secondary cognitive outcome was the Rey Auditory Verbal Learning Test (AVLT) of delayed recall. All cognitive outcomes were adjusted for age and Wide Range Achievement Test 4 (WRAT4) testing, a measure of premorbid functioning.
CAN
Our primary clinical CAN outcome was an E-to-I ratio of <5th percentile cutoff for lean control subjects. Our primary sensitive CAN measure was the E-to-I ratio. Before CAN testing, participants were asked to fast, stop all medications for 12 h, and abstain from smoking, caffeine, alcohol, and vigorous exercise for 24 h. Participants with diabetes were advised to avoid activities or medication changes that could result in low serum glucose levels. Participants were placed supine, resting with the lights dimmed for 5 min before recording. Baseline heart rate and blood pressure were captured for 5 min. The patient then immediately completed a 1-min paced deep breathing exercise test consisting of six 5-s inspiration and 5-s expiration cycles. A secondary outcome was the Survey of Autonomic Symptoms (SAS), which is a validated instrument to measure autonomic symptoms in patients with early diabetic neuropathy (28).
Retinopathy
Our primary retinopathy clinical outcome was a diagnosis of any retinopathy based on review of nonmydriatic retinal photographs (CR-1 Mark II camera; Canon) by an ophthalmologist (T.W.G.). Our primary sensitive retinopathy measure was the mean deviation on frequency doubling technology (FDT) testing using the 24-2 program as described previously (29). Secondary outcomes included the pattern SD and foveal sensitivity measurements on FDT testing.
Nephropathy
The primary clinical nephropathy outcome was an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2 and/or a urine albumin-to-creatinine ratio (ACR) ≥30 mg/g. eGFR was calculated based on the MDRD formula (30). Our primary sensitive nephropathy measure was the ACR. The first urine sample of the day was used.
Peripheral Neuropathy
We have previously reported the prevalence of neuropathy in this population based on the Toronto consensus definition of probable neuropathy (6,31). Secondary outcomes included nerve fiber density (NFD) measured at the distal leg and the sural sensory amplitude. NFD was evaluated using brightfield immunohistochemistry. Fibers were labeled with rabbit anti-PGP 9.5 antibody, and individual nerve fibers that crossed into the epidermis were counted using an established protocol (32). Nerve conduction studies were performed using the CareFusion Viking on Nicolet EDX electrodiagnostic system.
Statistical Analysis
Descriptive statistics were used to describe the demographics, metabolic phenotyping, and outcome measures (cognitive, CAN, retinopathy, nephropathy, and peripheral neuropathy) of the obese and lean populations. The χ2 or Fisher exact tests were used to compare the two populations for categorical variables and t tests for continuous variables. We determined the prevalence of cognitive deficits, CAN, retinopathy, and nephropathy stratified by glycemic status. We compared the cognitive, CAN, retinopathy, nephropathy, and neuropathy measures between lean control subjects and normoglycemic obese participants using t tests and investigated for a trend across glycemic status using simple linear regression models.
We performed regression analyses to evaluate the associations between our primary sensitive cognitive (NIH Toolbox composite), CAN (E-to-I ratio), retinopathy (mean deviation from FDT testing), and nephropathy (ACR) measures and MetS components, restricted to the obese population. Multivariable linear regression was used to model the primary sensitive measures as a function of the MetS components (NCEP waist circumference, prediabetes, diabetes, HDL, triglycerides, and systolic blood pressure), after adjusting for demographic factors (age, sex, height). Our cognitive outcome was also adjusted for education level and WRAT4 testing, and the nephropathy outcome was transformed as log(ACR + 1) to meet regression assumptions. As a sensitivity analysis, we included medication use (diabetes, cholesterol, and hypertension) and obstructive sleep apnea in the CAN model. Because waist circumference was significantly associated with the cognitive and CAN outcomes, we performed nine additional models, with each of the other eight anthropometric measurements and weight replacing NCEP waist circumference and calculated the adjusted R2 for each resulting model. Additionally, to assess the effect of having multiple MetS components on our outcomes, we fit multivariable regression models for each outcome as a function of the ordinal number of nonhyperglycemic MetS components (out of four) after adjustment for prediabetes, diabetes, and demographic factors (age, sex, and height). We also included NFD of the distal leg as an outcome, but this required square root transformation to meet model assumptions.
For the NIH Toolbox composite outcome, we used multiple imputation by chained equations to deal with the missing outcome information for the four participants who were missing a single test component due to technical reasons. Specifically, we imputed using predictive mean matching through 50 imputations of the data and pooled the complete regression analysis using Rubin’s rules (33).
As a sensitivity analysis, LDL (mg/dL) and cholesterol medication use (yes/no) were added to the cognitive and nephropathy regression models to assess the adjusted associations between HDL and these outcomes.
All analyses were completed using R 3.4.2 software.
Results
We recruited 138 severely obese individuals and 46 lean control subjects. Our previous report provides further details of recruitment (6). Information was missing for several outcome variables: E-to-I ratio (n = 2), SAS (n = 1), FDT outcomes (n = 2), urine microalbumin-to-creatinine ratio (n = 2), NFD leg (n = 3), sural sensory amplitude (n = 2), and waist and buttocks/hips measurements (n = 1). NIH Toolbox and Rey testing was not completed for 15 participants. WRAT4 was not completed for 10 participants.
Demographics and metabolic phenotyping of the population are presented in Table 1. In addition to metabolic factors, race (P = 0.01), education level (P < 0.01), WRAT4 (P < 0.01), and alcohol consumption (P < 0.01) were different between the obese and lean control population. No differences in age and sex were observed. Of the obese participants, 33 (23.9%) were normoglycemic, 56 (40.6%) had prediabetes, and 49 (35.5%) had diabetes.
Table 1.
Demographics of the lean control group and the obese group
| Variable | Lean control group (n = 46) | Obese group | P value: Obese vs. lean | ||
|---|---|---|---|---|---|
| Normoglycemic (n = 33) | Prediabetes (n = 56) | Diabetes (n = 49) | |||
| Age, mean (SD) | 44.1 (12.1) | 40.2 (10.7) | 44.7 (11.4) | 48.9 (10.4) | 0.61 |
| Male, n (%) | 8 (17.4) | 8 (24.2) | 10 (17.9) | 15 (30.6) | 0.47 |
| Race, n (%) | 0.01 | ||||
| White | 40 (87.0) | 26 (78.8) | 39 (69.6) | 43 (87.8) | |
| Black | 1 (2.2) | 6 (18.2) | 15 (26.8) | 4 (8.2) | |
| Asian | 3 (6.5) | 0 (0.0) | 1 (1.8) | 1 (2.0) | |
| Other | 2 (4.4) | 1 (3.0) | 1 (1.8) | 1 (2.0) | |
| Hispanic, n (%) | 2 (4.4) | 1 (3.0) | 0 (0.0) | 1 (2.0) | 0.26 |
| Marital status, n (%) | 0.38 | ||||
| Single | 13 (28.3) | 11 (33.3) | 21 (37.5) | 8 (16.3) | |
| Married | 27 (58.7) | 18 (54.6) | 26 (46.4) | 33 (67.4) | |
| Divorced | 6 (13.0) | 4 (12.1) | 8 (14.3) | 7 (14.3) | |
| Widowed | 0 (0.0) | 0 (0.0) | 1 (1.8) | 1 (2.0) | |
| Smoking status, n (%) | 0.12 | ||||
| Current | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Never | 38 (82.6) | 24 (72.7) | 39 (69.6) | 33 (67.4) | |
| Former | 8 (17.4) | 9 (27.3) | 17 (30.4) | 16 (32.7) | |
| Education level, n (%) | <0.01 | ||||
| High school | 0 (0.0) | 2 (6.1) | 6 (10.9) | 6 (12.2) | |
| Some college | 4 (8.9) | 10 (30.3) | 16 (28.6) | 17 (34.7) | |
| College degree | 27 (60.0) | 16 (48.5) | 25 (44.6) | 19 (38.8) | |
| Graduate degree | 14 (31.1) | 5 (15.2) | 9 (16.1) | 7 (14.3) | |
| WRAT4, mean (SD)** | 101.7 (7.8) | 96.8 (9.3) | 95.5 (10.7) | 96.3 (10.7) | <0.01 |
| Alcohol (drinks/week during last 12 months), mean (SD) | 2.0 (2.2) | 0.9 (1.2) | 1.1 (2.4) | 0.6 (1.4) | <0.01 |
| Height (cm), mean (SD) | 167.4 (9.7) | 169.5 (11.8) | 167.2 (8.5) | 169.0 (10.1) | 0.58 |
| Weight (kg), mean (SD) | 64.6 (9.8) | 140.3 (33.7) | 131.7 (27.0) | 129.1 (25.8) | <0.01 |
| BMI (kg/m2), mean (SD) | 23.0 (2.0) | 48.5 (8.3) | 46.8 (7.1) | 45.0 (6.8) | <0.01 |
| HDL (mg/dL), mean (SD) | 68.1 (16.5) | 47.3 (12.9) | 45.5 (7.9) | 41.0 (12.9) | <0.01 |
| Blood pressure | |||||
| Systolic (mmHg), mean (SD) | 108.7 (10.2) | 126.2 (16.0) | 131.5 (15.6) | 131.2 (12.8) | <0.01 |
| Diastolic (mmHg), mean (SD) | 66.2 (9.5) | 72.9 (12.5) | 74.2 (13.3) | 72.9 (8.8) | <0.01 |
| Triglycerides (mg/dL), mean (SD) | 71.2 (22.7) | 104.5 (42.8) | 122.7 (48.2) | 160.5 (129.9) | <0.01 |
| Glucose | |||||
| Fasting (mg/dL), mean (SD) | 84.9 (6.3) | 88.2 (5.7) | 98.5 (10.3) | 129.5 (43.4) | <0.01 |
| 2-h (mg/dL), mean (SD)* | 89.0 (19.5) | 96.3 (17.8) | 126.6 (31.4) | 156.5 (64.3) | <0.01 |
| Insulin | |||||
| Fasting (mg/dL), mean (SD) | 6.1 (4.9) | 20.6 (16.5) | 26.5 (17.2) | 31.5 (18.3) | <0.01 |
| 2-h (mg/dL), mean (SD)* | 41.8 (26.7) | 67.8 (47.5) | 103.9 (70.9) | 136.2 (92.6) | <0.01 |
| HbA1c (%), mean (SD) | NA | 5.3 (0.3) | 5.7 (0.3) | 7.4 (1.5) | NA |
| HbA1c (mmol/mol), mean (SD) | NA | 34.4 (2.9) | 39.1 (3.6) | 57.7 (16.5) | NA |
| Waist circumference (cm), mean (SD) | 80.4 (7.1) | 132.9 (19.7) | 131.1 (20.4) | 129.9 (17.1) | <0.01 |
| MetS, n (%) | 0 (0.0) | 13 (39.4) | 39 (70.9) | 49 (100.0) | <0.01 |
NA, not applicable.
Only reported for those without prior diagnosis of diabetes.
WRAT4 scores are standardized according to patient age.
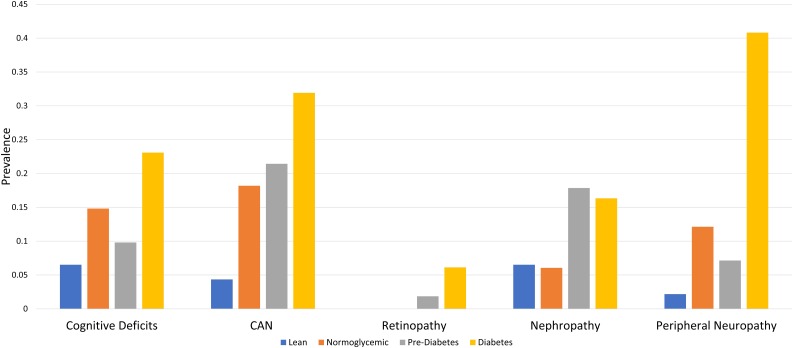
The prevalence of cognitive deficits was 6.5% in lean control subjects and 21.4% in obese participants (Fig. 1). Among the obese, the prevalence of cognitive deficits was 22.2% in those with normoglycemia, 17.7% in prediabetes, and 25.6% in diabetes. The prevalence of CAN was 4.4% in lean control participants and 24.3% in obese participants. Among the obese, the CAN prevalence was 18.2% in those with normoglycemia, 21.4% in prediabetes, and 31.9% in diabetes. The prevalence of retinopathy was 0% in lean control participants and 2.9% in obese participants. Among the obese, the retinopathy prevalence was 0% in those with normoglycemia, 1.9% in prediabetes, and 6.1% in diabetes. The prevalence of nephropathy was 6.5% in lean control participants and 14.5% in obese participants. Among the obese, the nephropathy prevalence was 6.1% in those with normoglycemia, 17.9% in prediabetes, and 16.3% in diabetes.
Figure 1.
Prevalence of cognitive deficits and traditional diabetic complications stratified by glycemic status. The prevalence of cognitive deficits (<5th percentile NIH Toolbox cognitive composite score), CAN (<5th percentile E-to-I ratio), retinopathy (retinal photographs), nephropathy (eGFR <60 mL/min/1.73 m2 and/or a urine ACR ≥30 mg/g), and peripheral neuropathy (Toronto consensus definition of probable neuropathy) stratified by glycemic status defined according to the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus.
For cognitive measures, obese participants with normoglycemia had significantly lower NIH Toolbox composite scores compared with lean control subjects (mean [SD] −0.1 [0.9] vs. 0.4 [0.8], P = 0.04) (Table 2). The Rey AVLT revealed no significant differences in delayed recall between obese participants with normoglycemia (0.04 [1.0]) and lean control participants (0.16 [1.1]) (P = 0.66). For CAN measures, no difference was seen for the E-to-I ratio between obese participants with normoglycemia compared with lean control participants (1.2 [0.1] vs. 1.2 [0.1], P = 0.67), but the SAS score was higher in obese participants with normoglycemia (5.3 [3.9] vs. 3.3 [4.8], P = 0.04). For retinopathy and nephropathy measures, no differences were observed between obese participants with normoglycemia and lean control subjects. For neuropathy measures, NFD leg (10.2 [7.5] fibers/mm vs. 13.7 [6.3] fibers/mm, P = 0.04) and sural amplitude (9.7 [6.5] μV vs. 20.7 [9.0] μV, P < 0.01) were lower in obese participants with normoglycemia compared with lean control subjects. Significant worsening with glycemic status was seen for the E-to-I ratio (P < 0.01), SAS (P = 0.01), fovea sensitivity measurements (P = 0.03), and NFD leg (P < 0.01).
Table 2.
Cognitive, retinopathy, and CAN measures stratified by glycemic status
| Variable | Lean control group (n = 46) | Obese group | P value: Obese-normoglycemic vs. lean | P value: Glycemic trend | ||
|---|---|---|---|---|---|---|
| Normoglycemic (n = 33) | Prediabetes (n = 56) | Diabetes (n = 49) | ||||
| Cognitive outcomes | ||||||
| NIH Toolbox composite* | 0.4 (0.8) | −0.1 (0.9) | −0.2 (1.1) | −0.3 (1.0) | 0.04 | 0.42 |
| Rey AVLT delayed recall* | 0.16 (1.1) | 0.04 (1.0) | −0.0001 (1.0) | −0.21 (0.9) | 0.66 | 0.25 |
| Autonomic outcomes | ||||||
| E-to-I ratio | 1.2 (0.1) | 1.2 (0.1) | 1.2 (0.1) | 1.1 (0.1) | 0.67 | <0.01 |
| SAS | 3.3 (4.8) | 5.3 (3.9) | 6.5 (6.1) | 9.0 (8.6) | 0.04 | 0.01 |
| Retinopathy outcomes | ||||||
| Mean deviation | −0.3 (2.8) | −0.4 (2.7) | −2.1 (4.0) | −1.4 (4.4) | 0.86 | 0.36 |
| PSD | 2.9 (0.6) | 3.2 (0.9) | 3.4 (1.1) | 3.2 (1.0) | 0.14 | 0.94 |
| Fovea | 28.9 (4.2) | 28.9 (4.8) | 26.0 (4.3) | 26.3 (5.1) | 0.97 | 0.03 |
| Kidney outcomes | ||||||
| ACR, mg/g | 34.7 (221.6) | 136.4 (729.4) | 130.9 (420.1) | 26.8 (122.3) | 0.46 | 0.24 |
| eGFR, mL/min/1.73 m2 | 87.9 (14.2) | 95.2 (21.9) | 92.4 (22.0) | 87.2 (24.1) | 0.10 | 0.11 |
| Neuropathy outcomes | ||||||
| NFD leg, fibers/mm | 13.7 (6.3) | 10.2 (7.5) | 8.6 (6.3) | 5.8 (6.4) | 0.04 | <0.01 |
| Sural amplitude, μV | 20.7 (9.0) | 9.7 (6.5) | 11.7 (6.8) | 8.6 (6.4) | <0.01 | 0.30 |
Data are mean (SD). PSD, pattern SD.
NIH Toolbox and Rey AVLT outcomes are standardized according to patient age and WRAT4 score.
For cognitive measures, multivariable linear regression revealed that NCEP waist circumference (−1.48; 95% CI −2.38, −0.57), HDL (−3.38; 95% CI −6.38, −0.37), and systolic blood pressure (2.30; 95% CI 0.12, 4.48) were the only metabolic variables significantly associated with the NIH Toolbox composite score (Table 3). For CAN measures, NCEP waist circumference (−0.01; 95% CI −0.01, −0.002) was the only metabolic variable significantly associated with the E-to-I ratio. The sensitivity analysis adjusting for medication use and obstructive sleep apnea did not significantly change the results. For retinopathy measures, prediabetes (−1.78; 95% CI −3.56, −0.002) was the only metabolic variable significantly associated with mean deviation from FDT testing. For nephropathy measures, triglycerides (0.21; 95% CI 0.01, 0.41) was the only metabolic variable significantly associated with ACR.
Table 3.
Linear regression evaluating the association of MetS components with traditional diabetic complications and mild cognitive impairment
| Variable | NIH Toolbox composite^† | E-to-I ratio | Mean deviation | ACR** |
|---|---|---|---|---|
| Age | −0.004 (−0.01, −0.002)* | 0.06 (−0.005, 0.13) | −0.02 (−0.05, 0.01) | |
| Female (reference male) | 8.49 (−1.84, 18.82) | 0.02 (−0.04, 0.08) | 0.96 (−1.27, 3.19) | −0.56 (−1.69, 0.57) |
| Height (unit = 5 cm) | 0.23 (−0.23, 0.68) | −0.001 (−0.01, 0.01) | 0.29 (−0.18, 0.75) | −0.10 (−0.34, 0.14) |
| Glycemic status | ||||
| Prediabetes (reference normal) | −2.58 (−10.57, 5.40) | −0.01 (−0.06, 0.03) | −1.78 (−3.56, −0.002)* | 0.54 (−0.37, 1.45) |
| Diabetes (reference normal) | −3.49 (−12.31, 5.34) | −0.03 (−0.09, 0.02) | −1.42 (−3.39, 0.55) | 0.01 (−1.00, 1.02) |
| SBP (unit = 10 mmHg) | 2.30 (0.12, 4.48)* | 0.004 (−0.01, 0.02) | −0.24 (−0.72, 0.24) | −0.03 (−0.27, 0.21) |
| Triglycerides (unit = 50 mg/dL) | −1.02 (−2.80, 0.76) | −0.01 (−0.02, 0.003) | 0.13 (−0.27, 0.53) | 0.21 (0.01, 0.41)* |
| HDL (unit = 10 mg/dL) | −3.38 (−6.38, −0.37)* | −0.01 (−0.02, 0.01) | 0.09 (−0.58, 0.75) | −0.02 (−0.36, 0.31) |
| Waist circumference (unit = 5 cm) | −1.48 (−2.38, −0.57)* | −0.01 (−0.01, −0.002)* | −0.02 (−0.21, 0.18) | 0.04 (−0.06, 0.14) |
Data are presented with the 95% CI. SBP, systolic blood pressure. *P < 0.01.
Adjusted for age, WRAT4, and education level.
Used Multiple Imputation using Chained Equations (MICE) with predictive mean matching and 50 imputations. Effect estimates are pooled using Rubin’s rules.
Log-transformed (ACR + 1).
Multivariable linear regression revealed that NCEP waist circumference and abdominal circumference (−0.22; 95% CI −0.42, −0.02) were the only anthropometric variables significantly associated with the NIH Toolbox composite score. The model including NCEP waist circumference had the highest adjusted R2 (0.29), followed by abdominal circumference (0.25), compared with 0.22–0.23 for the other eight models, including weight or the other anthropometric measures. Multivariable linear regression revealed that NCEP waist circumference, high waist (−0.003; 95% CI −0.004, −0.001), abdomen (−0.001; 95% CI −0.003, −0.0001), and weight (−0.001; 95% CI −0.002, −0.00003) were the anthropometric variables significantly associated with the E-to-I ratio. The model including high waist circumference had the highest adjusted R2 (0.20), followed by NCEP waist circumference (0.18), abdomen (0.17), and weight (0.16), compared with 0.14–0.15 for the other six models including the other anthropometric measures.
Multivariable linear regression revealed that the number of MetS components, other than hyperglycemia, was significantly associated with NFD (−0.49; 95% CI −0.73, −0.24) but not with the E-to-I ratio (−0.02; 95% CI −0.05, 0.004), NIH Toolbox composite score (−0.23; 95% CI −5.09, 4.64), mean deviation from FDT testing (−0.49; 95% CI −1.44, 0.47), or ACR (0.47; 95% CI −0.01, 0.96).
Conclusions
In a well-phenotyped population with severe obesity, we were able to demonstrate that in addition to peripheral neuropathy, cognitive deficits and CAN were the most common clinical complications. Cognitive deficits and peripheral neuropathy were also the only clinical complications that were seen in obese participants with normoglycemia, indicating that obesity alone is likely sufficient to cause these complications. In contrast, retinopathy and nephropathy were not seen in obese participants with normoglycemia more than in lean control subjects, even when looking at sensitive measures of function. Similar to our previous findings in peripheral neuropathy (6), central obesity is the main metabolic factor associated with cognitive deficits and CAN, indicating the importance of the distribution of obesity.
Peripheral neuropathy and CAN are the only common traditional diabetic complications found in this severely obese population. This is in contrast to previous studies looking at the prevalence of these complications in populations with diabetes where retinopathy and nephropathy were also frequently present. Although the definitions of peripheral neuropathy, CAN, retinopathy, and nephropathy are not uniform, the prevalence of these conditions in populations with diabetes have usually been reported to be quite similar (34–37). The study that used definitions closest to ours indicated a prevalence of 16.2% for peripheral neuropathy, 23.5% for retinopathy, and 22.3% for nephropathy (ACR ≥30 mg/g) compared with our study, which revealed a prevalence of 20.3%, 2.9%, and 14.5%, respectively (37). In contrast to the prevalence of peripheral neuropathy and CAN, the prevalence of retinopathy and nephropathy are quite low to nonexistent in obese participants with normoglycemia. One possible explanation for the difference in prevalence between these different complications is that peripheral neuropathy and CAN are caused by obesity itself, in addition to hyperglycemia, whereas retinopathy and nephropathy may be primarily caused by hyperglycemia. Further studies are needed to confirm these observations, which would have implications for understanding the underlying mechanisms of injury and for potential therapeutic interventions.
In addition to peripheral neuropathy and CAN, cognitive deficits were also more common in this severely obese population. These results are in concert with the extensive literature, including large prospective studies that have demonstrated an association between obesity and cognitive function and/or dementia (17–24). In contrast to previous studies, we were able to investigate peripheral neuropathy, CAN, and cognitive deficits in the same population. We demonstrated that all three complications are common in the severely obese. While peripheral neuropathy patients commonly present with numbness, tingling, and/or pain, early CAN and cognitive deficits are often unnoticed by patients. Therefore, peripheral neuropathy may be the first clue to patients and clinicians of obesity-mediated nerve injury. Given the similar associations between central obesity and peripheral and central nerve injury, similar interventions may work for all three of these important outcomes (38). Of note, we saw differences in the NIH Toolbox composite score but not with Rey AVLT delayed recall. Possible explanations include that obesity results in small, diffuse changes to the brain without predilection to memory structures or that we were underpowered to detect changes in memory.
Similar to our recent study that revealed that central obesity is one of the key metabolic risk factors for peripheral neuropathy (6), we demonstrate here similar findings for CAN and cognitive function. Although previous studies have also revealed that central obesity is associated with both of these conditions, our study is the first to look at extensive anthropometric measurements (16,25). Despite looking at nine anthropometric measures, only NCEP waist and abdominal circumferences were associated with cognitive function, which is similar to peripheral neuropathy (6). CAN was associated with all three central obesity measures (NCEP waist, abdomen, and high waist) but none of the other anthropometric measures. CAN was the only one of these three outcomes where there was also an association with weight. Taken together, our results indicate that central obesity is much more important than general obesity. Future studies need to focus on the mechanisms by which central obesity leads to peripheral and central nerve injury, including the potential difference in roles between subcutaneous and visceral adiposity. Interventions that shift adipose storage preferentially to noncentral locations may help reduce many different complications of obesity.
Distinct from CAN and cognitive deficits, retinopathy and nephropathy were not associated with central obesity. Moreover, these two complications were not seen in participants with severe obesity and normoglycemia more so than in lean control subjects. Even when highly sensitive measures of retinal and kidney function were used, no significant differences were seen between obese participants with normoglycemia and lean control participants. In contrast to peripheral and central nerve injury, these results provide evidence that obesity is unlikely to be a significant risk factor for retinopathy and nephropathy. Despite conflicting previous studies, our results are in agreement with a meta-analysis detailing the lack of association between obesity and retinopathy (15). However, a previous meta-analysis revealed a significant association between obesity and nephropathy (11). While it is possible that our study was underpowered to detect an association between obesity and nephropathy, our study clearly highlights the relative importance of hyperglycemia compared with obesity for this complication. Evidence supporting the importance of hyperglycemia is that retinopathy and nephropathy were seen in participants with prediabetes and diabetes and that prediabetes was the only significant metabolic risk factor for retinopathy. Although peripheral and autonomic neuropathy have traditionally been lumped in with retinopathy and nephropathy as diabetic complications, our results indicate significant differences in the metabolic risk factors for these different conditions, which may also have therapeutic implications. Our clinical data are in agreement with a recent series of preclinical studies revealing distinct bioenergetics profiles between the nervous system, the retina, and kidney in animal models of obesity and diabetes (39,40).
Interestingly, high HDL and systolic blood pressure were associated with a decline in cognitive function. Interpreting these findings is difficult because our sample size makes it difficult to adjust for covariates such as LDL and cholesterol treatment while maintaining power. In a sensitivity analysis, adjusting for both additional covariates made the association between HDL and cognitive function nonsignificant. Larger studies are needed to confirm or refute the relationship of HDL and systolic blood pressure with cognitive function. Furthermore, the number of MetS components, other than hyperglycemia, was associated with peripheral neuropathy but not the other complications tested. This may indicate that other MetS components are more important for somatic nerve injury than central and autonomic nerve injury, retinal injury, and kidney injury, but future studies are needed to confirm this finding.
Limitations of our study include the cross-sectional design of the study, which makes causal inferences difficult. Future longitudinal studies are needed to confirm the results of this study, and we are currently monitoring this population for 2 years after bariatric surgery. The small sample size limits the power of our study to detect associations; however, we were able to determine significant relationships between metabolic factors and traditional diabetic complications and cognitive function. Missing data were assumed to be missing at random, but the possibility exists that the missing data were skewed. How our results generalize to other populations, including those with less severe obesity and those not planning on bariatric surgery, is unclear and warrants further investigation. Our lean control participants differed from the obese population in race, education level, alcohol consumption, and potentially in unmeasured ways, which may impact the comparisons with this group; however, these factors were unlikely to account for the observed differences in our study. The low physical fitness of our obese participants could also confound our CAN measures. Our use of ACR as a nephropathy outcome in our mixed population of those with and without diabetes is a potential limitation. However, the main conclusions would be unchanged if the eGFR was the only nephropathy outcome.
In summary, cognitive deficits, CAN, and peripheral neuropathy are the most prevalent complications in severely obese persons and are common even in those with normoglycemia. Therefore, obesity is likely sufficient to cause these complications. In contrast, the fact that retinopathy and nephropathy are almost exclusively seen in those obese participants with prediabetes and diabetes indicates that hyperglycemia is an important determinant in these conditions. Outside of hyperglycemia, central obesity is the most important risk factor for cognitive function, CAN, and peripheral neuropathy.
Article Information
Acknowledgments. Dr. Justin Dimick (University of Michigan, Ann Arbor, MI) aided study recruitment and interactions with the University of Michigan bariatric surgery team.
Funding. The project described was supported by NIH National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant P30-DK-020572 to the Michigan Diabetes Research Center. B.C.C. was supported by NIH National Institute of Neurological Disorders and Stroke grant K23-NS-079417 and is currently funded by NIDDK R01-DK-115687. B.C.C., E.L.R., and E.L.F. receive support from the Program for Neurology Research and Discovery and the A. Alfred Taubman Medical Research Institute. T.W.G. was supported by NIDDK DP3-DK-094292 and is currently funded by R24-DK-082841, NIH National Eye Institute R01-EY-20582, Research to Prevent Blindness, and the A. Alfred Taubman Medical Research Institute. S.P. was supported by NIDDK P30-DK-081943, P30-DK-89503, and R24-DK-082841. E.L.F. was supported by NIDDK DP3-DK-094292 and is currently funded by R24-DK-082841, National Institute of Neurological Disorders and Stroke R21-NS-102924, and the Novo Nordisk Foundation Center for Basic Metabolic Research (NNF14°C0011633).
Duality of Interest. B.C.C. consults for a Patient-Centered Outcomes Research Institute (PCORI) grant, DynaMed, and the Immune Tolerance Network and performs medical legal consultations, including consultations for the Vaccine Injury Compensation Program. T.W.G. receives research funding from Zebra Biologicals. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. B.C.C. was involved in the study design and interpretation of the statistical analysis and wrote the manuscript. E.L.R., M.B., T.W.G., K.V., B.G., R.P.-B., S.P., and E.L.F. were integrally involved in the study design, interpretation of the data, and critical revisions of the manuscript. E.C. and E.V.-U. were involved in the study design and critical revisions of the manuscript. B.C.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Hales CM, Fryar CD, Carroll MD, Freedman DS, Aoki Y, Ogden CL. Differences in obesity prevalence by demographic characteristics and urbanization level among adults in the United States, 2013-2016. JAMA 2018;319:2419–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 2006;355:763–778 [DOI] [PubMed] [Google Scholar]
- 3.Callaghan BC, Gao L, Li Y, et al. Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann Clin Transl Neurol 2018;5:397–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callaghan BC, Xia R, Banerjee M, et al.; Health ABC Study . Metabolic syndrome components are associated with symptomatic polyneuropathy independent of glycemic status. Diabetes Care 2016;39:801–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callaghan BC, Xia R, Reynolds E, et al. Association between metabolic syndrome components and polyneuropathy in an obese population. JAMA Neurol 2016;73:1468–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callaghan BCRE, Banerjee M, Chant E, Villegas-Umana E, Feldman EL. Distribution of obesity is a key differentiator of neuropathy status. Presented at the Peripheral Nerve Society Annual Meeting, 22-26 June 2019, Genoa, Italy [Google Scholar]
- 7.Hanewinckel R, Drenthen J, Ligthart S, et al. Metabolic syndrome is related to polyneuropathy and impaired peripheral nerve function: a prospective population-based cohort study. J Neurol Neurosurg Psychiatry 2016;87:1336–1342 [DOI] [PubMed] [Google Scholar]
- 8.Lu B, Hu J, Wen J, et al. Determination of peripheral neuropathy prevalence and associated factors in Chinese subjects with diabetes and pre-diabetes - ShangHai Diabetic neuRopathy Epidemiology and Molecular Genetics Study (SH-DREAMS). PLoS One 2013;8:e61053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlesinger S, Herder C, Kannenberg JM, et al. General and abdominal obesity and incident distal sensorimotor polyneuropathy: insights into inflammatory biomarkers as potential mediators in the KORA F4/FF4 cohort. Diabetes Care 2019;42:240–247 [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med 2004;140:167–174 [DOI] [PubMed] [Google Scholar]
- 11.Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol 2011;6:2364–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porta M, Sjoelie AK, Chaturvedi N, et al.; EURODIAB Prospective Complications Study Group . Risk factors for progression to proliferative diabetic retinopathy in the EURODIAB Prospective Complications Study. Diabetologia 2001;44:2203–2209 [DOI] [PubMed] [Google Scholar]
- 13.van Leiden HA, Dekker JM, Moll AC, et al. Risk factors for incident retinopathy in a diabetic and nondiabetic population: the Hoorn study. Arch Ophthalmol 2003;121:245–251 [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Wang C, Shi K, Yin X. Relation of metabolic syndrome and its components with risk of diabetic retinopathy: a meta-analysis of observational studies. Medicine (Baltimore) 2018;97:e12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Zhang Y, Shi K, Wang C. Body mass index and risk of diabetic retinopathy: a meta-analysis and systematic review. Medicine (Baltimore) 2017;96:e6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laitinen T, Lindström J, Eriksson J, et al. Cardiovascular autonomic dysfunction is associated with central obesity in persons with impaired glucose tolerance. Diabet Med 2011;28:699–704 [DOI] [PubMed] [Google Scholar]
- 17.Cournot M, Marquié JC, Ansiau D, et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology 2006;67:1208–1214 [DOI] [PubMed] [Google Scholar]
- 18.Dahl AK, Hassing LB, Fransson EI, Gatz M, Reynolds CA, Pedersen NL. Body mass index across midlife and cognitive change in late life. Int J Obes 2013;37:296–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Obesity, diabetes and cognitive deficit: the Framingham Heart Study. Neurobiol Aging 2005;26(Suppl. 1):11–16 [DOI] [PubMed] [Google Scholar]
- 20.Hassing LB, Dahl AK, Pedersen NL, Johansson B. Overweight in midlife is related to lower cognitive function 30 years later: a prospective study with longitudinal assessments. Dement Geriatr Cogn Disord 2010;29:543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien PD, Hinder LM, Callaghan BC, Feldman EL. Neurological consequences of obesity. Lancet Neurol 2017;16:465–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabia S, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A. Body mass index over the adult life course and cognition in late midlife: the Whitehall II Cohort Study. Am J Clin Nutr 2009;89:601–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev 2011;12:e426–e437 [DOI] [PubMed] [Google Scholar]
- 24.Pedditzi E, Peters R, Beckett N. The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing 2016;45:14–21 [DOI] [PubMed] [Google Scholar]
- 25.Ng TP, Feng L, Nyunt MS, et al. Metabolic syndrome and the risk of mild cognitive impairment and progression to dementia: follow-up of the Singapore Longitudinal Ageing Study Cohort. JAMA Neurol 2016;73:456–463 [DOI] [PubMed] [Google Scholar]
- 26.Genuth S, Alberti KG, Bennett P, et al.; Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–3167 [DOI] [PubMed] [Google Scholar]
- 27.Grundy SM, Cleeman JI, Daniels SR, et al.; American Heart Association; National Heart, Lung, and Blood Institute . Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement [published correction appears in Circulation 2005;112:e297; Circulation 2005;112:e298] Circulation 2005;112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 28.Zilliox L, Peltier AC, Wren PA, et al. Assessing autonomic dysfunction in early diabetic neuropathy: the Survey of Autonomic Symptoms. Neurology 2011;76:1099–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joltikov KA, de Castro VM, Davila JR, et al. Multidimensional functional and structural evaluation reveals neuroretinal impairment in early diabetic retinopathy. Invest Ophthalmol Vis Sci 2017;58:BIO277–BIO290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Coresh J, Greene T, et al.; Chronic Kidney Disease Epidemiology Collaboration . Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate [published correction appears in Ann Intern Med 2008;149:519] Ann Intern Med 2006;145:247–254 [DOI] [PubMed] [Google Scholar]
- 31.Dyck PJ, Albers JW, Andersen H, et al.; Toronto Expert Panel on Diabetic Neuropathy . Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev 2011;27:620–628 [DOI] [PubMed] [Google Scholar]
- 32.Lauria G, Hsieh ST, Johansson O, et al.; European Federation of Neurological Societies; Peripheral Nerve Society . European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 2010;17:903–912, e44-9 [DOI] [PubMed] [Google Scholar]
- 33.van Buuren Sv, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw 2011;45:1–67 [Google Scholar]
- 34.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) [published correction appears in Lancet 1999;354:602] Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 35.Ismail-Beigi F, Craven T, Banerji MA, et al.; ACCORD trial group . Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raman R, Gupta A, Krishna S, Kulothungan V, Sharma T. Prevalence and risk factors for diabetic microvascular complications in newly diagnosed type II diabetes mellitus. Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study (SN-DREAMS, report 27). J Diabetes Complications 2012;26:123–128 [DOI] [PubMed] [Google Scholar]
- 37.Yokoyama H, Araki SI, Kawai K, et al. Declining trends of diabetic nephropathy, retinopathy and neuropathy with improving diabetes care indicators in Japanese patients with type 2 and type 1 diabetes (JDDM 46). BMJ Open Diabetes Res Care 2018;6:e000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gottesman RF, Albert MS, Alonso A, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol 2017;74:1246–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sas KM, Kayampilly P, Byun J, et al. Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight 2016;1:e86976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sas KM, Lin J, Rajendiran TM, et al. Shared and distinct lipid-lipid interactions in plasma and affected tissues in a diabetic mouse model. J Lipid Res 2018;59:173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]