Abstract
Cervical spinal cord injury (SCI) impairs arm and hand function largely by interrupting descending tracts. Most SCI spare some axons at the lesion, including the corticospinal tract (CST), which is critical for voluntary movement. We targeted descending motor connections with paired electrical stimulation of motor cortex and cervical spinal cord in the rat. We sought to replicate the previously published effects of intermittent theta burst stimulation of forelimb motor cortex combined with trans-spinal direct current stimulation placed on the skin over the neck to target the cervical enlargement. We hypothesized that paired stimulation would improve performance in skilled walking and food manipulation (IBB) tasks. Rats received a moderate C4 spinal cord contusion injury (200 kDynes), which ablates the main CST. They were randomized to receive paired stimulation for 10 consecutive days starting 11 days after injury, or no stimulation. Behavior was assessed weekly from weeks 4-7 after injury, and then CST axons were traced. Rats with paired cortical and spinal stimulation achieved significantly better forelimb motor function recovery, as measured by fewer stepping errors on the horizontal ladder task (34±9% in stimulation group vs. 51±18% in control, p=0.013) and higher scores on the food manipulation task (IBB, 0-9 score; 7.2±0.8 in stimulated rats vs. 5.2±2.6 in controls, p=0.025). The effect size for both tasks was large (Cohen’s d=1.0 and 0.92, respectively). The CST axon length in the cervical spinal cord did not differ significantly between the groups, but there was denser and broader ipsilateral axons distribution distal to the spinal cord injury. The large behavioral effect and replication in an independent laboratory validate this approach, which will be trialed in cats before being tested in people using non-invasive methods.
Keywords: Electrical stimulation, spinal cord injury, corticospinal, motor recovery, motor cortex, iTBS, transcutaneous, spinal cord, direct current stimulation
Graphical Abstract
Introduction
Approximately 288,000 people in the United States live with spinal cord injury, and almost half (47.2%) of new injuries occur at the cervical level and spare some neurological function below the injury site (NSCISC 2018). For people with cervical injury, their top priority is the recovery of arm and hand function (Anderson 2004). Most spinal cord injuries spare some connections below the injury site, even in those who have no spared function (Sherwood, Dimitrijevic, and McKay 1992; Dimitrijevic et al. 1984; Dimitrijevic, Prevec, and Sherwood 1983; Bunge et al. 1993; Kakulas and Kaelan 2015). Our approach has been to electrically stimulate the descending motor connections spared by injury in order to promote connectivity.
Spared corticofugal connections can be targeted with phasic electrical stimulation applied to the motor cortex (Carmel and Martin 2014; Carmel, Kimura, and Martin 2014; Carmel et al. 2010) and/or with stimulation of the spinal cord (Gerasimenko et al. 2007) for functional recovery. Stimulating the spinal cord and brain in a coordinated fashion has the potential to selectively strengthen the connections between them (Harel and Carmel 2016). The rationale for pairing phasic cortical stimulation with tonic spinal cord stimulation is that the circuits at the intersection of the two sites of stimulation will have the largest plasticity effect.
In this study, we employed a paired motor cortex and spinal stimulation paradigm developed in the Martin laboratory to improve forelimb function after spinal cord injury in rats. The paradigm employs intermittent theta burst stimulation (iTBS) over the forelimb area of the motor cortex and cathodal transspinal direct current stimulation (tsDCS) over the cervical spinal cord. Motor evoked potentials (MEPs) from motor cortex are augmented by iTBS in humans (Huang et al. 2005) and animals (Song et al. 2016), and this effect in the rat lasts more than 30 minutes peaking at 15 minutes after iTBS (Song et al. 2016). tsDCS also augments motor cortex MEPs, but only when the current is being passed (Song et al. 2016). The combination of iTBS and tsDCS has a larger effect on motor responses than either form of stimulation alone (Song et al. 2016). Finally, combined iTBS and tsDCS was applied to rats 10 days after cervical spinal cord contusion and improved motor recovery. Rats treated with combined stimulation had improved performance of a food manipulation (IBB) task compared to the control group, and performance on a skilled walking task improved only in the paired stimulation group (Zareen et al. 2017).
A key advantage to the use of iTBS and tsDCS is that these paradigms can be applied non-invasively in people, and they have proven safe (Nierat et al. 2014; Huang et al. 2005). iTBS is applied over motor cortex via repetitive transcranial magnetic stimulation, and tsDCS is applied through skin electrodes on the neck. In addition, the combination of iTBS and tsDCS was applied for only 27 minutes, which is short enough to be applied daily in the clinic.
Before this therapy can be tested in people, however, it first must be validated in animal studies. Independent replication has been advocated as the best way to ensure that the original study was robust, because preclinical studies can be difficult to replicate, especially in SCI (Ioannidis 2005; Begley and Ioannidis 2015). The need for replication caused the National Institutes of Health to launch the Facility of Research Excellence in Spinal Cord Injury (FORE-SCI) program, specifically to replicate previous research. Surprisingly, only 1 out of 12 studies could be fully replicated, and half had no therapeutic effect. Some reasons for this low replication rate identified by the FORE-SCI investigators include lack of robustness of the original findings, differences in experimental details between replication and original studies, and insufficient experience of replication study experimenters (Steward et al. 2012).
We sought to replicate the findings of Zareen et al. by repeating the experiment in an independent laboratory. To improve the chances for success, we proceeded to the replication study only when individual procedures could be done with high inter-laboratory reliability. The replication study was then performed independently. Rats with combined iTBS and tsDCS had improved forelimb function on two skilled tasks compared to controls, and the effect size was large for each. In addition, paired stimulation caused denser and broader ipsilateral axons distribution distal to the spinal cord injury. The data support the advancement of this paired stimulation paradigm toward clinical application and identify a possible mechanism for how electrical stimulation might support neural connections weakened by injury.
Materials and methods
Overview
This study was conducted in two phases. In Phase 1, all of the methods required were validated between the Martin Laboratory, where the stimulation paradigm was developed, and the Carmel Laboratory, where the replication study was conducted. The surgical, physiological, and behavioral methods were compared between the laboratories, and consistency between experimenters determined. After the methods were found to be consistent between the laboratories, Phase 2, the independent replication, was performed. We conducted a double-blind, placebo-controlled trial of combined iTBS over motor cortex and tsDCS over the cervical spinal cord in rats after spinal cord injury. The study was powered to detect differences in forelimb motor function between the experimental and sham stimulation groups. All procedures were approved by the Animal Use and Care Committee of Weill Cornell Medicine.
Methods common to Phase 1 and Phase 2
Behavior
Food Manipulation Task (IBB)
We followed the methods of the Irvine, Beatties and Bresnahan task (Irvine et al. 2010; Irvine et al. 2014). We assessed the rat’s ability to manipulate differently shaped breakfast cereals: sphere-shaped (Reese’s Puffs, General Mills Sales Inc.) and donut-shaped (Froot Loops, Kellogg’s NA Co.). Briefly, the rats were acclimated to the testing environment for 15 minutes daily, for 10 days after cortical electrode implantation. Experimenters blinded to the paired stimulation treatment recorded the rats eating the cereals before injury and weekly from week 4 to week 7 after injury.
Horizontal ladder rung walking task
The horizontal ladder-walking task measured paw placement on the rungs, which were irregularly spaced (Carmel et al., 2010; Carmel et al., 2014; Metz & Whishaw, 2009). After cortical electrode implantation, we trained rats 20 minutes daily for 10 days to ensure rats walked across the ladder without distractions. We motivated rats to cross the ladder with puffs from a can of compressed air and by offering a cotton swab dipped into 20% sucrose after every successful trial. The pattern of the irregularly spaced rungs was altered every 6 trials and the walking direction was changed after 12 trials. We video recorded the behavior at 50 frames-per-second.
Experimenters blinded to the paired stimulation treatment analyzed the trials frame-by-frame to quantify the step quality. The start was defined when the rat placed all 4 limbs on the rungs and the end when it reached the last rung of the ladder. The criteria were used to establish when to begin and end scoring and also to measure the time taken to walk across the ladder. The steps were scored as a good step, overstep, understep, or a missed step based on the placement of the forepaw on the rung; for the main analysis, this was divided only into good steps or errors. We excluded one rat that did not meet the baseline average of 15% error rate.
C4 spinal cord contusion
All the surgeries (cortical implantation, spinal cord contusion, and BDA tracer injection) were performed using aseptic techniques. Rats were anesthetized with ketamine (90 mg/kg) and xylazine (10mg/kg) mixture. The anesthesia state was maintained with ketamine (1/3 of the initial dosage). Buprenorphine (0.05 mg/kg, Henry Schein, Melville, NY) was administered before and after surgery to alleviate pain. Anesthesia levels and the heart rate were continuously monitored throughout the surgeries. Rats were kept on heating pads to maintain the temperature at 37.5°C during surgery and for 24 hours post surgery.
The spinal cord contusion surgery was performed with the same method as the original study. Briefly, the C3 to C5 vertebrae were exposed by skin incision and muscle separation on the midline. Laminectomy of C4 was performed, taking care to ensure the dura remained intact. Following laminectomy, the rat was placed on the Infinite Horizon (IH) impactor stabilization platform, and the C3 and C5 spinous processes were clamped. Fine adjustment was made to the vertebral clamps to ensure that the exposed C4 spinal cord surface was level. The moderate contusion injury was made with a 3.5mm impactor tip raised to three complete turns ~2mm from the dura surface and was dropped with a preset 200 kdynes force on to the spinal cord with no dwell time. After injury, the rat was removed from the platform, and the wound was closed in layers. Topical antibiotic ointment was applied to the wound.
Rats received intense care after surgery. This included placing their cage on a heating pad for 12 hours, administering buprenorphine (4 dosages in total, 0.05mg/kg) every 4-12 hours, and Baytril (5mg/kg, Norbrook, Henry Schein, Melville, NY) daily for 5 days. For nutrition, Ringers Lactate solution (10ml, subcutaneous) was administered daily for 5-10 days, and Dietboost Gel (ClearH2O, Portland, ME) was placed within reach until rats regained the ability to reach the food and water dispensers. Weight was monitored daily. Bladder function was monitored closely; none of the rats showed bladder dysfunction.
Paired brain and spinal cord stimulation
iTBS was delivered through implanted cortical electrodes. All rats received bilateral electrode implantation once they were habituated to the testing environment, animal facility and experimenter handlers. Each electrode consisted of two stainless steel screws (1.19mm in diameter, PlasticOne). Insulated stainless steel wires connected the screws to a plastic connector. Under anesthesia, the rat was fixed on a stereotaxic frame and the skull was exposed. A hand-drill was used to make 4 holes over the forelimb areas of the motor cortex in both the hemispheres. The coordinates of the screws in both the left and right hemisphere relative to the bregma were 1mm rostral, 2mm lateral and 3mm rostral, 4mm lateral to bregma. The screw electrodes were placed so that the flat end rested upon the dura. The electrodes were tested during the surgery for all rats; this produced specific contralateral forelimb movement in all cases. An additional 4-6 screws (1.57mm in diameter, PlasticOne) were used to secure the head cap.
The paired stimulation parameters were the same as the original study. Before, during, and 10 days after iTBS+tsDCS treatment, the motor threshold (the minimal electrical intensity needed to elicit specific contralateral forelimb movement) was tested and confirmed for each rat on the stimulation group. Each rat in the stimulation group received iTBS epidurally through the screw electrodes. The iTBS delivered 5 epochs of stimulation (1630s total). Each epoch was 360s long, containing 20 repeats of stimulation (in total 200s stimulation, 160s interstimulus period). Each repeat was 10s long in total, consisted of 2s stimulation and 8s interstimulus period. Each 2s stimulation included 10 bursts of stimulation. Each burst was composed of 3 pulses (200μs, biphasic, interstimulus interval: 50ms). The intensity was set at 75% of the motor threshold. tsDCS was delivered using the same method as in the original study and described above. For paired stimulation treatment, tsDCS was delivered simultaneously with iTBS for 27 minutes.
Methods specific to Phase 1
We broke down the original study protocol into the individual procedures, which were adopted in the Carmel lab with the instruction of a member of the Martin lab. Typically, a procedure was first observed in the Martin lab and then performed in the Carmel lab under observation. Procedures were deemed sufficiently similar if they produced similar results (e.g. physiological effects of stimulation), or were submitted to formal assessment of interrater reliability (behavioral tasks). Inter-laboratory reliability was tested on: 1) skilled (ladder) walking and food manipulation (IBB), 2) acute physiology effects of iTBS/tsDCS , and 3) cervical spinal cord contusion injury model. In Phase 1, a total of 18 female Sprague Dawley rats (Charles River Laboratories, Wilmington, MA, USA) were used. We chose to focus our efforts on reliability on the behavioral tasks since they were both the main outcome measure and the most subjective. The validation process took approximately six months.
To ensure consistency in the way behavior performance was evaluated, video files taken and scored by the Martin lab were re-scored by members of the Carmel lab. Once the scoring system was consistent, the process was reversed: videos taken and scored the Carmel lab were re-scored by members of the Martin lab. We identified 2 important differences in the scoring systems in the two labs. For the ladder task, the paw position was initially assessed at different phases of the step cycle. For the IBB task, the scoring of manipulations without volar support (no palm involved) was inconsistent between the two labs. For the ladder, we assessed the step when the rat had only one forelimb on the rung, suggesting weight bearing. For the IBB, non-volar support received a score of 4 or less. Replication study personnel rescored previous videos recorded at different timepoints periodically to ensure that the scoring paradigm remains consistent throughout the duration of the study.
We tested whether the physiological effects of iTBS observed in the Martin laboratory (Song et al. 2016) was also observed in the Carmel lab. To test the effects of iTBS, we measured biceps EMG before, during, and after one epoch of iTBS. A pair of EMG electrodes was inserted into the biceps muscle as described in previous studies (Mishra et al. 2017; Song et al. 2016). Biceps EMG was elicited one of two ways: 1) before and after iTBS, a pair of biphasic, square wave pulses (0.1 ms each polarity; interstimulus interval 3ms) over motor cortex were used to elicit biceps EMG responses; 2) during the period of iTBS EMG responses were elicited by the bursts.
The original study used “L”-shaped wire electrodes to stimulate motor cortex, that were held in place with the dental cement of a head cap (Carmel et al. 2010, 2013; Carmel, Kimura, and Martin 2014). We changed this by using screw electrodes in the replication study, which could be placed more quickly and with a lower likelihood of injuring the underlying dura matter (Garcia-Sandoval et al. 2018; Mishra et al. 2017). We sought to determine if cortical stimulation using screw electrodes would have similar physiological effects to those elicited by L-shaped electrodes. We had previously observed no difference in the effects of stimulation with changes in the shape of the stimulating electrodes (Carmel, 2010).
Spinal stimulation was delivered through skin electrodes (LGMedSupply; 0.5” x 1.5”) as in the original study. The anode was placed over the chest, while the cathode above C4-T2 vertebrae. The placement of the electrodes was confirmed with the original study laboratory (Zareen et al. 2017). The cathode was placed on the chest and the anode above the neck; this maximizes current delivered to the cervical enlargement (Song et al. 2015; Song et al. 2016). tsDCS was stepped up from 0 to 1.5mA over 2.5s, kept constant at 1.5mA throughout the testing period and stepped down to 0 in 2.5s at the end.
We measured biceps muscle electromyography (EMG) response to iTBS and tsDCS separately. EMG was acquired with a differential AC amplifier (AM Systems, Model 1700), amplified at a gain of 1000 and bandpass filtered between 1 and 1000Hz. EMG signal was recorded at 5000 Hz through Signal 5.08 (CED Ltd, CED Micro 1401, Cambridge Electronic Design Ltd, Cambridge, UK). Raw EMG signals were rectified to quantify peak to peak EMG, and area under the curve was analyzed using custom scripts in MATLAB (MathWorks, Natick, MA, USA).
Methods specific to Phase 2
General Procedures
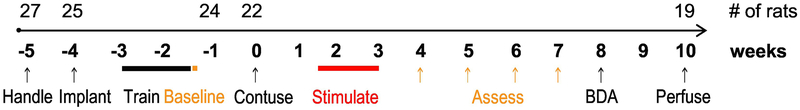
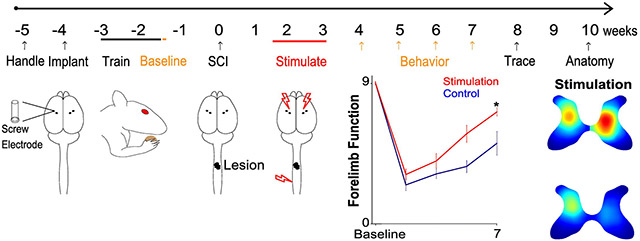
The timeline of the phase 2 experiment is shown in Figure 1. After one week of handling, all rats received cortical electrodes implantation. Rats recovered for one week and then were trained on the skilled walking task; a criterion of <15% errors within 2 weeks was set before the study began. After pre-injury assessment of skilled walking and food manipulation tasks, all rats received C4 midline moderate contusion injury. Then they were randomized into control and stimulation groups. Stimulation group rats received paired stimulation every day for 10 days beginning 11 days after contusion. Behavior (skilled walking and IBB) was assessed weekly from weeks 4 to 7 post injury. 27 female Sprague Dawley rats were used for this study. The replication study was performed in cohorts, with each cohort of 4-6 rats. Rat retention during Phase 2 is indicated in Figure 1 on top of the timeline.
Figure 1. Timeline.
Numbers above the line indicate the animals remaining in the study after the procedure indicated below it.
Randomization and blinding procedures
Rats were pseudorandomized with an attempt to balance each cohort into stimulation and control groups. Rats were randomized immediately after SCI surgery and without regard to baseline behavior performance. The replication phase was carried out with blinded measure. An experimenter (QY) randomized rats into different groups and performed paired stimulation treatment, but was not involved in behavior tasks training, testing or scoring process or in histology including, lesion reconstruction and histochemistry analyses. Experimenters (AR and SL) who performed behavior training, testing and scoring and histology, but they did not participate in randomization, electrophysiology testing, or paired stimulation. Control rats were wire-connected through the implanted cortical electrodes and skin electrodes similarly to the stimulation group, but no stimulation was delivered. Since all rats had cortical electrodes implanted, there was no way to distinguish by appearance whether rats received stimulation.
Anatomical methods and analyses
BDA tracer injection surgery and perfusion were performed identically to the original study. Briefly, the dental acrylic head cap was removed with an electric drill, and the electrode screws were extracted with a screwdriver. After the electrodes were taken out, a 3×3mm craniotomy was made above the right side forelimb area motor cortex in order to inject the tracer. 10% biotinylated dextran amine (BDA; 10,000 mw, Molecular Probes, Eugene, OR, USA) was injected at depth of 1.5mm at the following 7 sites, 300nl each site (mm rostral to bregma, mm lateral to bregma): (0.5, 2), (0.5, 2.8), (0.5, 3.9), (1.5, 3.5), (1.5, 2.5), (2.02, 2.5), (2.02, 3.5). After the injections were complete, a thin layer of GelFoam (Pfizer, NY) was placed over the dura, and the wound was closed. Two weeks later, rats were deeply anesthetized and transcardially perfused with ~400ml 0.1M phosphate buffered saline (PBS) containing 10,000IU/L of heparin and 600ml 4% paraformaldehyde. Brain and spinal cord (C1-T2) were dissected and post-fixed in 4% paraformaldehyde for 48 hours. The tissue was then transferred to 30% sucrose at −4°C until it was cut in the coronal section at 40μm thickness on a cryostat.
Tissue sections from C3 to C5 were processed for lesion reconstruction. Spinal cord sections were collected serially and mounted directly onto slides to preserve tissue integrity. The lesioned tissue was dried overnight and coverslipped the next day. The outline of the lesion, grey matter and white matter were traced using light microscopy and Neurolucida software (MBF Bioscience, Williston, Vermont, USA). The lesion was reconstructed by a blinded experimenter using the 3D visualization tool. The Cavalieri Estimator probe in Stereoinvestigator (MBF Bioscience, Williston, Vermont, USA) was used to determine lesioned and total tissue area for sections rostral, caudal, and at the epicenter of the lesion. Spared tissue area was computed by subtracting lesioned tissue area from total tissue area. To compare the extent of injury in the two groups, spared tissue area data was quantified every 3 slices for the whole injury length. The data was then averaged and converted to spared tissue area percentage (average spared tissue area/average total tissue area X 100%).
For axon quantification, BDA histology was performed on sections above (C3) and below (C7) the lesion site as described previously (Wen et al. 2018). Briefly, sections from spinal cord levels C3 and C7 were incubated in 0.3% hydrogen peroxide solution, rinsed with 0.05 M Tris-HCL buffer, then incubated into 0.05 M Tris-HCL buffer containing 1% avidin-biotin complex reagent (ABC kit, catalogue # PK6100; Vector Laboratories) and 0.2% Triton-X 100 (Sigma-Aldrich) for 90 minutes, followed by diaminobenzidine and nickel (Peroxidase substrate kit DAB, catalogue # SK4100; Vector Laboratories) solution after rinsing. After mounting and dehydrating with increasing concentrations of ethanol solution and incubation into xylene (Sigma-Aldrich), sections were coverslipped.
At spinal cord level C3, the spaceballs probe in Stereoinvestigator was used to estimate axon length in the grey matter contralateral to the BDA injection site (Zareen et al. 2017). For the side of the spinal cord ipsilateral to BDA injection (sparse CST projections) and for both sides of the spinal cord at C7, the axons were hand traced using Neurolucida (MBF Bioscience, Williston, Vermont, USA). For each rat, data were collected from two sections at C3 and two sections at C7, and then axon length was averaged for each of the two spinal cord levels. To correct for differences in the efficacy of the tracer, axon length was normalized by the efficiency of tract tracing, as in the original study and our previous publications (Carmel et al. 2010; Carmel and Martin 2014; Carmel et al. 2013). The Optical Fractionator probe in Stereoinvestigator was used to determine an estimate of the number of labelled axons in the contralateral dorsal column at C3. A correction factor was calculated by dividing each rat’s averaged dorsal column axon number by the average number of axons in the dorsal column. Two rats were excluded from the BDA quantification analysis because of too little BDA staining to be quantified.
Statistical analysis
The primary endpoint of the study was behavior performance at week 7 after injury, the end of the study. An independent t-test was computed for the horizontal ladder task and food manipulation (IBB) task at week 7 post-injury. All data were assessed for normality using the Shapiro-Wilk test. For non-normally distributed data non-parametric tests were used. For all tests, two-tailed significance was reported, and the p value threshold set at 0.05. Since two behavioral tests were used as the primary endpoint, a Bonferroni correction was performed, and the significance was set at p=0.025. A power analysis performed before the study found that 10 rats in each group were sufficient to meet the primary endpoints with a power of 0.8 (G*Power). Cohen’s d was calculated to measure effect size (Cohen 1988). We defined the effect size is large if the d value is greater than 0.8 (Sawilowsky 2009).
All other analyses were considered secondary. For behavior, a secondary analysis was performed on time to cross the ladder and error rate using multivariate ANOVA. For physiology, a Welch ANOVA was used to test whether motor thresholds changed before and after SCI. For spinal cord lesions, comparison of the spared tissue area between groups was tested using an independent t-test. Analyses for the length of axons rostral and caudal to injury were computed using the Mann-Whitney U test, a non-parametric equivalent of the independent t-test. To calculate the spatial distribution of axon densities in a group of spinal cord sections, the sections were transformed into a common coordinate system. This was achieved by performing image registration of each section to a corresponding traced rat spinal cord atlas image (Wen et al. 2018).
Axon density spatial distributions were averaged after registration to obtain representative heatmaps for each group. To test if there were statistical differences between groups, we used permutation testing (Wen et al. 2018). The chosen statistic was the Euclidean distance between the means of the two groups. This statistic was evaluated for 1000 permutations with a significance threshold set at 95%. All data are expressed as Mean+/− SEM.
Results
The study was divided into two phases. In the first phase, we tested each method used in the Martin laboratory by performing it in the Carmel laboratory with the involvement of the original study authors. In the second phase, we performed an independent replication of the original study without the involvement of the original study authors.
Phase 1: Replication of methods
We tested the individual methods of the original study, including surgeries (electrode implantation and spinal contusion), physiology (cortical and cervical stimulation) and behavior (skilled walking and manipulation tasks). The spinal cord injuries performed in the Carmel laboratory (n=11) were similar to those from the original study according to three metrics: contusion force, spared tissue in the spinal cord and recovery during the first 10 days after injury, a period during which rats cannot perform the ladder or IBB tasks. Rats were able to right themselves, raise their heads and upper body, and began to move around using hindlimbs between 5 and 10 days after SCI. At this stage, rats’ forelimbs began to regain function, but still exhibited flaccid paralysis or spastic contractures as in the original study, and consistent with moderate cervical injury (Anderson, Sharp, and Steward 2009).
The physiological effects of cortical iTBS and tsDCS were similar between the two laboratories (Zareen et al. 2017; Song et al. 2016). We determined that the screw electrodes produced similar movements (mostly wrist extension, elbow flexion, and some digit movements) to the L-shaped electrodes (Zareen et al. 2017) and at similar cortical stimulation intensities (usually 1-1.5mA). During the iTBS stimulation in the awake intact animal, the muscle responses to the stimulation showed a progressive increase with each stimulation burst. We tested the change in cortical motor evoked potentials (MEPs) before and after 15 minutes of iTBS. The peak-to-peak EMG amplitude increased from 0.18±0.01mV to 1.53±0.15mV (7.5-fold increase, n=10 sessions) after iTBS. We also tested MEPs before and during tsDC. The peak-to-peak EMG amplitudes increased from 0.07±0.01mV to 0.19mV±0.01mV (1.7-fold increase, n=5 sessions).
Behavioral tasks were performed and scored consistent with the original study. For the two behavior tasks, the Bland-Altman test was used to assess the reliability of the scores between the scorers of the original study and this study (Bland and Altman 1999). The Bland-Altman test indicated that the scorers were consistent with the original study scorers. The Bland-Altman plots throughout the duration of the study indicated that all of the differences were within the 95% limits of agreement (1.46 and −1.60; 1.53 and −1.53) with a low mean difference between the two scores (0 & 0.01).
Phase 2: Randomized, blinded replication study
The timeline of the study is shown in Figure 1. The number of rats in the study is shown above the timeline. Five rats died during surgeries (2 rats in cortical electrodes implantation, 2 rats in spinal cord contusion, 1 rat in BDA injection). One rat was removed because it did not achieve a 15% error rate before injury on the skilled walking task. Two rats had poor health after spinal cord injury and were euthanized as suggested by the veterinarian. Due to BDA staining efficacy, two rat’s histochemistry results were not available post-mortem. Thus, we used 27 rats in total in the study and report data of behavior performance of 22 rats (n=12 for the stimulation group, n=10 for the control group), spinal cord lesion of 19 rats, and axon quantification of 17 rats.
Primary outcome: improved behavior performance with stimulation
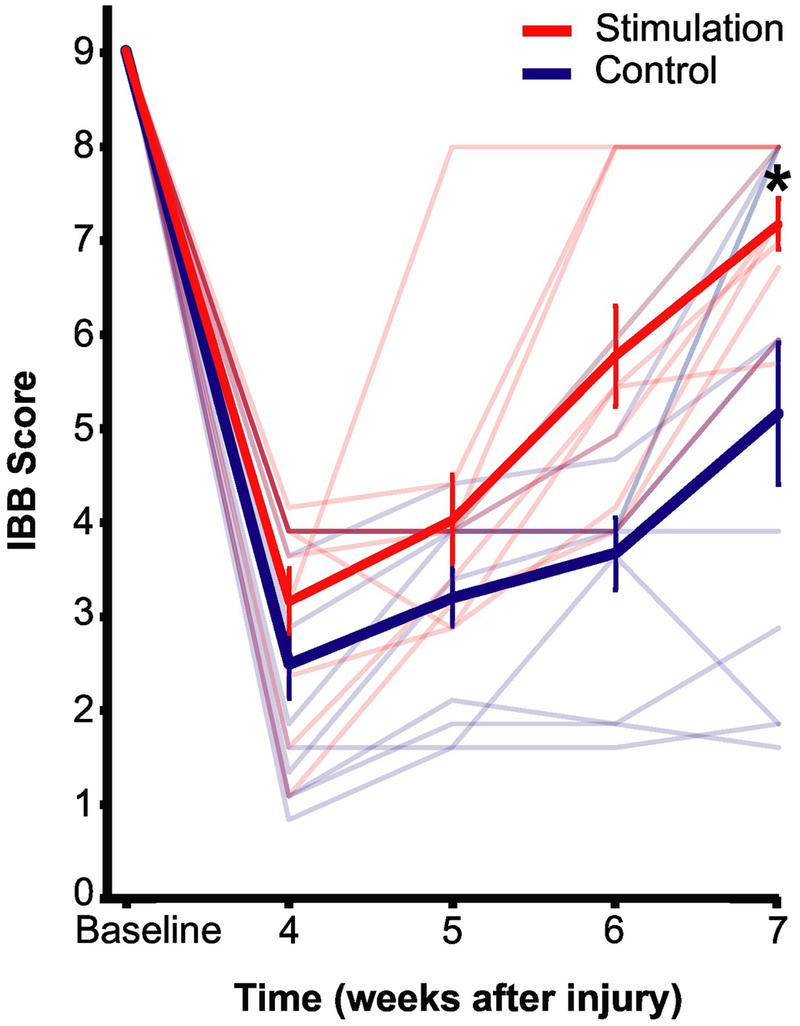
We assessed performance on a food manipulation task (Figure 2, Supplement Video 2). All rats had optimal cereal manipulation and were scored 9 before injury. At week 7, control group rats on an average had a grasping method different from baseline wherein their forepaws could not conform to the cereal shape, only one digit contributed to manipulation, and were thus rated an IBB score of 5.2±2.6. In contrast, the stimulation group rats on an average were rated a score of 7.2±0.8 as their forepaws could conform to the shape of the cereal and had a grasp similar to that before injury. The stimulation group rats performed significantly better on the food manipulation task at the end of the assessment compared to rats in the control group, t(13.7)=−2.5, p=0.025. The effect size (Cohen’s d =1.0) is considered large to very large (Cohen 1988; Sawilowsky 2009).
Figure 2. Food manipulation (IBB) task.
Average and individual rat’s IBB scores. At each time point, the IBB score is averaged over four pieces of cereals. Thick red and blue line are the average IBB scores for all stimulation group rats and controls respectively. Thin light red and blue lines indicate individual stimulation and control group rats respectively. Error bar represents SEM. *-unpaired t test, p=0.025. N=10 for stimulation rats, n=12 for controls.
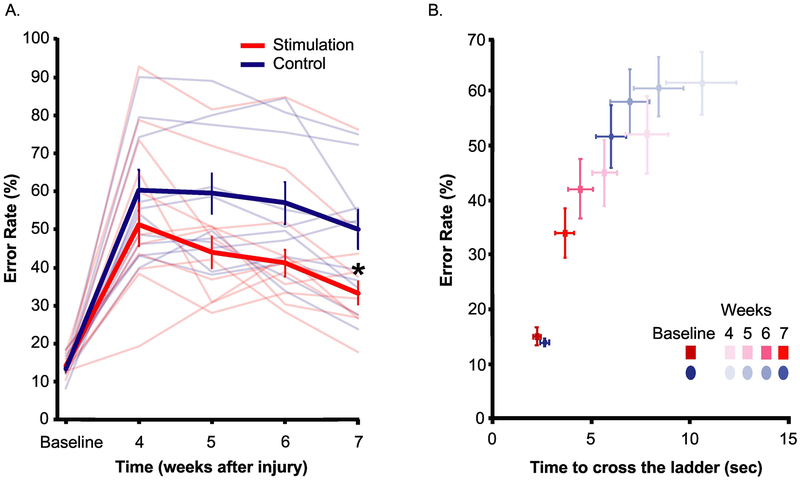
In Figure 3A, we quantified the error rate on the horizontal ladder walking task before injury and from week 4 to 7 after injury. The stimulation group rats made significantly fewer errors (34±9%) 7 weeks after spinal cord injury compared to control rats (51±18%, t(16.9)=2.8, p=0.013). Similar to the food manipulation task, the effect of stimulation in the performance of skilled walking task was large (Cohen’s d=0.92).
Figure 3. Horizontal ladder walking task.
A) Error rate rats made while walking across the horizontal ladder over 20-24 trials at each time point, averaged over left and right forelimbs. Thick red and blue line are the average error rate for all stimulation group rats and controls respectively. Thin light red and blue lines indicate individual stimulation and control group rats respectively. B) Error rate vs. time taken to cross the ladder. *-unpaired t test, p=0.013. N=10 for stimulation rats, n=12 for controls. (Note the difference in y axis of Figure A & B.)
Secondary Outcomes
In health, gains in accuracy are often at the expense of speed, known as the speed-accuracy trade-off (Fitts 1955). We assessed if the stimulation group rats prioritized accuracy over speed to gain paw placement precision. In addition to error rate, we measured time to cross the ladder of all rats on the skilled walking task (Figure 3B, Supplement Video 3). We found that stimulation group rats not only made fewer errors (F (1,20)=6.961, one way MANOVA, p=0.016, partial η2=0.258), but also crossed the ladder faster (time to cross is 3.67±1.50s) than control group rats (6.01±2.48s), F(1,20)=6.806, p=0.017, partial η2=0.254. This partial η2is considered a large effect size (Cohen 1988). Thus, rats improved both in accuracy and speed.
Rats in control and stimulation groups received similar injuries
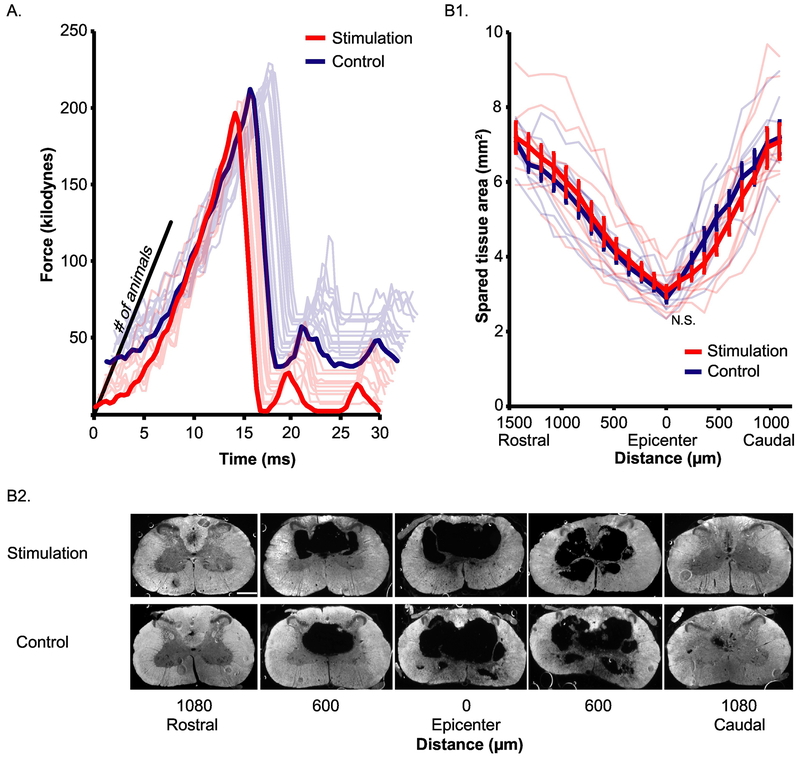
We compared the amount of contusion force rats received and the spared tissue preserved at the injury site. The amount of force that rats received is shown in Figure 4A. There is no significant difference between stimulation group (207.00±1.92kdynes, n=10) and control group (207.75±1.25kdynes, n=12, independent t test, t(20)=0.338, p=0.739).
Figure 4. Stimulation and control animals did not differ in their injuries.
A) Force applied on rats’ spinal cord through the 30ms time course during spinal cord contusion injury for stimulation and control groups. N=10 for stimulation rats, n=12 for controls. B) Spared tissue after SCI. 1) Area of spared tissue left through the whole length of lesioned spinal cord. Thick red and blue line are the average spared tissue area for all stimulation group rats and controls respectively. Thin light red and blue lines indicate individual stimulation and control group rats respectively. Error bar: standard error. N=10 for stimulation rats, n=9 for controls. 2) Montage showing sections used in B1) quantification from one representative animal in each group. Scale bar: 1mm.
We also compared the amount of tissue spared by the injury throughout the area of the spinal cord injury. Reconstruction of the contusion injury shows a central spherical cavity with rostral and caudal diminishing “tails” as illustrated in the three-dimensional lesion reconstructions (Supplement Video 1) and spared tissue area plotted by distance relative to the epicenter of the lesion (Figure 4B1,2). Rats in both the control (n=9) and the stimulation (n=10) groups exhibit this similar pattern of injury. We quantified the area of spared tissue area throughout the lesion and expressed it as a percentage of the cross sectional area of the perimeter of the tissue (Fig. 4B). There was no significant difference between the control (46.7% ± 3.7%, mean ± SEM) and the stimulation group (45.3% ± 3.6%, t(17) = 0.268, p = 0.792). The injury abolished almost all the CST axons. We concluded that the injury rats received are similar between stimulation and control groups.
Corticospinal axon length and distribution
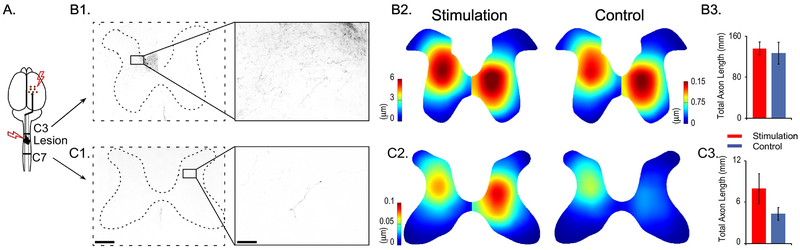
We measured CST axon length and distribution in the grey matter (Figure 5). rostral and caudal to the injury. Rostral to the injury, the control group had 126.7±21.0mm (n=7) of total axon length and the stimulation group had 135.8±12.3mm (n=10) of axon length within each 40 micron section (Figure 5B1 and 5B3, U=32, p=0.770). We also tested whether the distribution and density of axons in the grey matter differed between the two groups. We created heat maps that depicted the density of axons (Figure 5B2). Stimulation and control animals did not differ from each other regarding axon density and distribution (p = 0.340 for contralateral to the side of BDA injection, p = 0.297 for ipsilateral side). Caudal to the injury at C7, control group rats had 4.3±0.94 mm of axon and the stimulation group had 7.9±2.2 mm of axon (Figure 5C1 and 5C3, U=23.5, p=0.261). Axon density and distribution analysis required that the two sides of the spinal cord be compared separately. Contralateral to the side of BDA injection, areas of high axon density and length of axons were located in the dorsal horn of the grey matter in both groups (Figure 5C2). There were no significant differences between the two groups in their distributions of axon length (p = 0.474). Ipsilateral to the side of BDA injection, both the stimulation and control groups showed axons projecting to the intermediate zone. However, the stimulation group showed significantly denser and broader axon distribution in the spinal cord ipsilateral to the BDA injection side (p = 0.037, Figure 5C2).
Figure 5. Axon length and density above and below the lesion site.
A) Cartoon illustration indicates BDA labeled axon number and length quantification sites (C3 and C7), relative to the stimulation and lesion sites. B, C) Axons length and density in stimulation and control animals. BDA labeled axons at B1) C3 and C1) C7. Left panels: 4× magnification, scale bar: 0.5 mm. Right panels: magnified image as in the black rectangles shown in the left panel, 20× magnification, scale bar: 0.1 mm. Heatmap showing the axon density and distribution at B2) C3 and C2) C7 in stimulation and control groups. Note the difference in scale bars for B2) the left and right sides of the spinal cord and for C2) C7. Total axon length of both sides of the spinal cord at B3) C3 and C3) C7 levels. N=10 for stimulation rats, n=7 for controls.
Motor threshold was not significantly altered by spinal cord injury
At various phases of the experiment, the motor threshold for producing a forepaw movement after cortical stimulation was measured. While the motor threshold was not a prespecified outcome measure, it was notable that it did not change significantly after spinal cord injury. The average motor threshold for movement was 1.31±0.08mA (1.39±0.15mA for stimulation group, 1.29±0.13mA for control group) before spinal cord injury. In the early post-injury period, 4-10 days after injury the threshold was 1.26±0.03mA, and 11-20 days after spinal cord injury it was 1.34±0.07mA (1.35±0.06mA for stimulation group, 1.23±0.14mA for control group). The motor thresholds between the stimulation and control group were not different before injury (unpaired t test, t(20)=0.512, p=0.614) or after injury (t(52)=0.634, p=0.529). The threshold was unchanged among these three testing periods; Welch’s F (2, 48.715)=0.546, p=0.583.
Discussion
This replication study of paired stimulation after contusion SCI in rats met the pre-certified primary outcome; stimulation rats had significant improvement on the two forelimb specific behavior tasks compared with controls. In addition, we observed a large effect size, with rats receiving stimulation making 17% fewer errors crossing the ladder and achieving a 2 point greater score in the 9 point IBB manipulation scale. A secondary analysis of the ladder walking task showed that rats with stimulation crossed faster than controls, in addition making fewer errors. The study did not observe significant difference in axon lengths between stimulation vs. control groups, consistent with the original study’s result. However, below the lesion, the axon length in the rats with stimulation was double that of the control rats, and there was a change in distribution, suggesting an association of CST sprouting and movement recovery. On the other hand, the cortical stimulation intensity required to produce a movement did not change with the C4 contusion injury, which ablated the main CST. This suggested that other descending motor pathways might mediate the effects of cortical stimulation, in addition to the CST. The robust behavioral results warranted a trial of paired stimulation in a large animal model as the next step in translation.
Rats with paired stimulation recovered more forelimb skill than unstimulated controls. We assert that this was due to recovery, rather than compensation, because they were able to cross with fewer errors and were faster. This makes changes due to a more careful walking strategy (which would likely be slower) or increased motivation (which might be faster but with more errors) unlikely. The data in Fig. 3B show a clear shift in the speed and accuracy curve with stimulation. More careful kinematic assessment might reinforce this point, but to us the effects demonstrated can only be seen with improved skill.
The biggest limitation of this study is that forelimb function was continuing to change at the end of the study period, 7 weeks after injury, so the long-term functional differences between the groups is not known. It will be important to follow the function longer in the next study to understand if the large functional differences observed in this study persist at the time that function hits its asymptote on the recovery curve.
To validate a scientific finding, one wants to ensure good knowledge transfer and also to maintain independence. We tried to strike this balance by dividing the study in two phases. The goal of Phase 1 was to ensure that the techniques were performed the same in the two laboratories. We found it quite helpful to involve original study authors in this phase. Another benefit of dedicating phase 1 to methods validation is time saving. Dividing and validating one procedure could be done in parallel with another procedure, making it time efficient and easier to identify and correct for errors.
The goal of Phase 2 was to perform an independent validation study. To ensure independence, there was no interaction between the two laboratories about the substance of the project. Limiting interaction in this phase was meant to enable the hypothesis to be tested independently. It also helps to ensure that the methods are robust enough that once they are acquired, they do not need further updating. The analyses and conclusions were drawn independently before being reviewed by the original study’s authors.
Two observations in the study suggest possible mechanisms by which paired stimulation exerted a beneficial effect on functional recovery. The first is axon sprouting of the CST, the principal pathway for dexterity in people. We and others have observed a strong association between axon sprouting and functional recovery (Liebscher et al. 2005; Starkey et al. 2011; Maier et al. 2008). There was no significant difference in axon length between the two groups either above or below the lesion. However, rats with stimulation had more than double the axon length than controls below the lesion, and the distribution of density was significantly different on the half of the spinal cord ipsilateral to the tracer (BDA) injection. These findings suggest the CST may participate in repair with this protocol.
A second observation suggests a different possible pathway. The threshold for producing a forelimb movement did not change significantly from before injury to after, even though our C4 contusion injury ablated the main CST. This suggests that cortical responses are mediated by other descending motor connections to the degree that the loss of CST does not change the threshold. A prime candidate for this is the cortico-reticulospinal tract, that has been shown to mediate recovery from spinal cord injury in mice (Asboth et al. 2018). A crucial question for the neuromodulation field to address in future studies is whether this is the right circuit to target for repair.
Paired stimulation using iTBS over motor cortex and tsDCS over the neck is poised for the next step toward translation. The salutary effects of paired stimulation were replicated by an independent laboratory, and the effect size was large. Testing of a preclinical approach with a robust effect is more likely to become a clinical therapy than one with a more moderate effect (Hempel et al. 2011).
Our next step plan for translation is to test iTBS and tsDCS in a cat model of spinal cord injury. The SCI research community has strongly endorsed the use of a large animal model for preclinical testing (Kwon et al. 2015). This is particularly true for therapies using electrical stimulation, since current flow in the body depends so critically on the body morphology and composition (Bikson et al. 2015). Although iTBS and tsDCS have been shown to be safe to use in people and could be applied non-invasively, the logic of exactly how to stimulate is still in progress. The next experiments can help to determine whether this promising therapy will move forward.
Supplementary Material
Supplement Video 1. Spinal cord lesion 3D representation.
The cervical spinal cord was sectioned at 40μm, and every 10th section was taken for reconstruction. The outline of spinal cord was labeled in purple, the grey matter in blue, the lesion in red for each section. The sections were combined in Neurolucida, and a 3D representation was created. The 3D rendering was then rotated from the rostral part to the cervical spinal cord to the caudal part.
Supplement Video 2. Stimulation and control animals’ performance on the food manipulation (IBB) task
4 videos are shot in each session angled towards a single forepaw for a single cereal, each video is scored individually and an average score is obtained per session.
A) Control animal’s performance on the food manipulation (IBB) task before SCI and 7 weeks post injury.
B) Stimulation animal’s performance on the food manipulation (IBB) task 7 weeks post injury.
Supplement Video 3. Stimulation and control animals’ performance on the horizontal ladder walking task
A) Control animal’s performance on the skilled walking task before SCI and 7 weeks post injury. The same animal’s error rate averaged over all trials in each week was indicated in black line in the inserted figure. Other light blue lines were performance of all other control animals.
B) Stimulation animal’s performance on the skilled walking task 7 weeks post injury. The same animal’s error rate averaged over all trials in each week was indicated in black line in the inserted figure. Other light orange lines were performance of all other stimulation animals.
Acknowledgement
NYS Department of Health Spinal Cord Injury Board, NIH 5R01NS064004 (JHM), NYS SCIRB C30606GG, C31291GG (JHM)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson Kim D. 2004. “Targeting Recovery: Priorities of the Spinal Cord-Injured Population.” Journal of Neurotrauma 21 (10): 1371–83. [DOI] [PubMed] [Google Scholar]
- Anderson Kim D., Sharp Kelli G., Steward Oswald. 2009. “Bilateral Cervical Contusion Spinal Cord Injury in Rats.” Experimental Neurolog 220 (1): 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asboth, Leonie, Friedli Lucia, Beauparlant Janine, Martinez-Gonzalez Cristina, Anil Selin, Rey Elodie, Baud Laetitia, et al. 2018. “Cortico-Reticulo-Spinal Circuit Reorganization Enables Functional Recovery after Severe Spinal Cord Contusion.” Nature Neuroscience 21 (4): 576–88. [DOI] [PubMed] [Google Scholar]
- Begley, Glenn C, Ioannidis John P. A.. 2015. “Reproducibility in Science: Improving the Standard for Basic and Preclinical Research.” Circulation Research 116 (1): 116–26. [DOI] [PubMed] [Google Scholar]
- Bikson, Marom, Truong Dennis Q., Mourdoukoutas Antonios P., Aboseria Mohamed, Khadka Niranjan, Adair Devin, Rahman Asif. 2015. “Modeling Sequence and Quasi-Uniform Assumption in Computational Neurostimulation.” Progress in Brain Research 222 (September): 1–23. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. 1999. “Measuring Agreement in Method Comparison Studies.” Statistical Methods in Medical Research 8 (2): 135–60. [DOI] [PubMed] [Google Scholar]
- Bunge RP, Puckett WR, Becerra JL, Marcillo A, and Quencer RM. 1993. “Observations on the Pathology of Human Spinal Cord Injury. A Review and Classification of 22 New Cases with Details from a Case of Chronic Cord Compression with Extensive Focal Demyelination.” Advances in Neurology 59: 75–89. [PubMed] [Google Scholar]
- Carmel, Jason B, Berrol Lauren J., Brus-Ramer Marcel, Martin John H.. 2010. “Chronic Electrical Stimulation of the Intact Corticospinal System after Unilateral Injury Restores Skilled Locomotor Control and Promotes Spinal Axon Outgrowth.” The Journal of Neuroscience: The Official Journal of the Society: for Neuroscience 30 (32): 10918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel, Jason B, Kimura Hiroki, Berrol Lauren J., Martin John H.. 2013. “Motor Cortex Electrical Stimulation Promotes Axon Outgrowth to Brain Stem and Spinal Targets That Control the Lorelimb Impaired by Unilateral Corticospinal Injury.” The European Journal of Neuroscience 37 (7): 1090–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel, Jason B, Kimura Hiroki, and Martin John H.. 2014. “Electrical Stimulation of Motor Cortex in the Uninjured Hemisphere after Chronic Unilateral Injury Promotes Recovery of Skilled Locomotion through Ipsilateral Control.” The Journal of Neuroscience: The Official Journal of the Society: for Neuroscience 34 (2): 462–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel, Jason B, and Martin John H.. 2014. “Motor Cortex Electrical Stimulation Augments Sprouting of the Corticospinal Tract and Promotes Recovery of Motor Function.” Frontiers in Integrative Neuroscience 8 (June): 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Jacob. 1988. “Statistical Power Analysis for the Behavioral Sciences 2nd Edn.” Erlbaum Associates, Hillsdale. [Google Scholar]
- Dimitrijevic MR, Dimitrijevic MM, Faganel J, and Sherwood AM. 1984. “Suprasegmentally Induced Motor Unit Activity in Paralyzed Muscles of Patients with Established Spinal Cord Injury.” Annals of Neurology: 16 (2): 216–21. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic MR, Prevec TS, Sherwood AM. 1983. “Somatosensory Perception and Cortical Evoked Potentials in Established Paraplegia.” Journal of the Neurological Sciences 60 (2): 253–65. [DOI] [PubMed] [Google Scholar]
- Fitts Paul M. 1955. “The Information Capacity of the Human Motor System in Controlling the Amplitude of Movement.” Journal of Experimental Psychology: 47 (6): 381. [PubMed] [Google Scholar]
- Garcia-Sandoval Aldo, Pal Ajay, Mishra Asht M., Sherman Sydney, Parikh Ankit R., Alexandra Joshi-Imre David Arreaga-Salas, et al. 2018. “Chronic Softening Spinal Cord Stimulation Arrays.” Journal of Neural Engineering 15 (4): 045002. [DOI] [PubMed] [Google Scholar]
- Gerasimenko Yury P., Ichiyama Ronaldo M., Lavrov Igor A., Courtine Gregoire, Cai Lance, Zhong Hui, Roy Roland R., and Edgerton V. Reggie. 2007. “Epidural Spinal Cord Stimulation plus Quipazine Administration Enable Stepping in Complete Spinal Adult Rats.” Journal of Neurophysiology: 98 (5): 2525–36. [DOI] [PubMed] [Google Scholar]
- Harel Noam Y., and Carmel Jason B.. 2016. “Paired Stimulation to Promote Lasting Augmentation of Corticospinal Circuits.” Neural Plasticity: 2016 (October): 7043767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel Susanne, Suttorp Marika J., Miles Jeremy N. V., Wang Zhen, Maglione Margaret, Morton Sally, Johnsen Breanne, Valentine Diane, and Shekelle Paul G.. 2011. Empirical Evidence of Associations Between Trial Quality and Effect Size. Rockville (MD): Agency for Healthcare Research and Quality (US). [PubMed] [Google Scholar]
- Huang Ying-Zu, Edwards Mark J., Rounis Elisabeth, Bhatia Kailash P., and Rothwell John C.. 2005. “Theta Burst Stimulation of the Human Motor Cortex.” Neuron 45 (2): 201–6. [DOI] [PubMed] [Google Scholar]
- Ioannidis John P. A. 2005a. “Contradicted and Initially Stronger Effects in Highly Cited Clinical Research.” JAMA: The Journal of the American Medical Association 294 (2): 218–28. [DOI] [PubMed] [Google Scholar]
- Ioannidis John P. A. 2005b. “Why Most Published Research Findings Are False.” PLoS Medicine 2 (8): e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine Karen-Amanda, Ferguson Adam R., Mitchell Kathleen D., Beattie Stephanie B., Beattie Michael S., and Bresnahan Jacqueline C.. 2010. “A Novel Method for Assessing Proximal and Distal Forelimb Function in the Rat: The Irvine, Beatties and Bresnahan (IBB) Forelimb Scale.” Journal of Visualized Experiments: JoVE, no. 46 (December), 10.3791/2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine, Karen-Amanda, Ferguson Adam R., Mitchell Kathleen D., Beattie Stephanie B., Lin Amity, Stuck Ellen D., Russell Huie J, et al. 2014. “The Irvine, Beatties, and Bresnahan (IBB) Forelimb Recovery Scale: An Assessment of Reliability and Validity.” Frontiers in Neurology 5 (July): 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakulas Byron A., and Kaelan Cahyono. 2015. “The Neuropathological Foundations for the Restorative Neurology of Spinal Cord Injury.” Clinical Neurolog and Neurosurgery 129 Suppl 1 (February): S1–7. [DOI] [PubMed] [Google Scholar]
- Kwon Brian K., Streijger Femke, Hill Caitlin E., Anderson Aileen J., Bacon Mark, Beattie Michael S., Blesch Armin, et al. 2015. “Farge Animal and Primate Models of Spinal Cord Injury for the Testing of Novel Therapies.” Experimental Neurology 269 (July): 154–68. [DOI] [PubMed] [Google Scholar]
- Liebscher Thomas, Schnell Lisa, Schnell Dina, Scholl Jeannette, Schneider Regula, Gullo Miijam, Fouad Karim, et al. 2005. “Nogo-A Antibody Improves Regeneration and Focomotion of Spinal Cord-Injured Rats.” Annals of Neurology 58 (5): 706–19. [DOI] [PubMed] [Google Scholar]
- Maier Irin C., Baumann Kaspar, Thallmair Michaela, Weinmann Oliver, Scholl Jeannette, and Schwab Martin E.. 2008. “Constraint-Induced Movement Therapy in the Adult Rat after Unilateral Corticospinal Tract Injury.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 28 (38): 9386–9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz Gerlinde A., Whishaw Ian Q.. 2009. “The Fadder Rung Walking Task: A Scoring System and Its Practical Application.” Journal of Visualized Experiments: JoVE, no. 28 (June). 10.3791/1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra Asht M., Pal Ajay, Gupta Disha, and Carmel Jason B.. 2017. “Paired Motor Cortex and Cervical Epidural Electrical Stimulation Timed to Converge in the Spinal Cord Promotes Lasting Increases in Motor Responses.” The Journal of Physiology, July 10.1113/JP274663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierat M-C, Nierat M-C, Similowski T, Lamy J-C. 2014. “Does Trans-Spinal Direct Current Stimulation Alter Phrenic Motoneurons and Respiratory Neuromechanical Outputs in Humans? A Double-Blind, Sham-Controlled, Randomized, Crossover Study.” Journal of Neuroscience. 10.1523/jneurosci.1288-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NSCISC. 2018. “Spinal Cord Injury Facts and Figures at a Glance”. https://www.nscisc.uab.edu.
- Sawilowsky, Shlomo S 2009. “New Effect Size Rules of Thumb.”. Journal of Modern Applied Statistical Methods: JMASM 8 (2): 26. [Google Scholar]
- Sherwood AM, Dimitrijevic MR, and McKay WB. 1992. “Evidence of Subclinical Brain Influence in Clinically Complete Spinal Cord Injury: Discomplete SCI.” Journal of the Neurological Sciences 110(1-2): 90–98. [DOI] [PubMed] [Google Scholar]
- Song Weiguo, Amer Alzahraa, Ryan Daniel, and Martin John H.. 2016. “Combined Motor Cortex and Spinal Cord Neuromodulation Promotes Corticospinal System Functional and Structural Plasticity and Motor Function after Injury.” Experimental Neurology 277 (March): 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Weiguo, Truong Dennis Q., Bikson Marom, and Martin John H.. 2015. “Transspinal Direct Current Stimulation Immediately Modifies Motor Cortex Sensorimotor Maps.” Journal of Neurophysiology 113 (7): 2801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey, Michelle L, Bleul Christiane, Maier Irin C., and Martin E Schwab. 2011. “Rehabilitative Training Following Unilateral Pyramidotomy in Adult Rats Improves Forelimb Function in a Non-Task-Specific Way.” Experimental Neurology. https://doi.Org/10.1016/j.expneurol.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Steward Oswald, Popovich Phillip G., Dietrich W. Dalton, and Kleitman Naomi. 2012. “Replication and Reproducibility in Spinal Cord Injury Research.” Experimental Neurology 233 (2): 597–605. [DOI] [PubMed] [Google Scholar]
- Wen Tong-Chun, Lall Sophia, Pagnotta Corey, Markward James, Gupta Disha, Ratnadurai-Giridharan Shivakeshavan, Bucci Jacqueline, et al. 2018. “Plasticity in One Hemisphere, Control From Two: Adaptation in Descending Motor Pathways After Unilateral Corticospinal Injury in Neonatal Rats.” Frontiers in Neural Circuits 12 (April): 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zareen N, Shinozaki M, Ryan D, Alexander H, Amer A, Truong DQ, Khadka N, et al. 2017. “Motor Cortex and Spinal Cord Neuromodulation Promote Corticospinal Tract Axonal Outgrowth and Motor Recovery after Cervical Contusion Spinal Cord Injury.” Experimental Neurology 297 (November): 179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Video 1. Spinal cord lesion 3D representation.
The cervical spinal cord was sectioned at 40μm, and every 10th section was taken for reconstruction. The outline of spinal cord was labeled in purple, the grey matter in blue, the lesion in red for each section. The sections were combined in Neurolucida, and a 3D representation was created. The 3D rendering was then rotated from the rostral part to the cervical spinal cord to the caudal part.
Supplement Video 2. Stimulation and control animals’ performance on the food manipulation (IBB) task
4 videos are shot in each session angled towards a single forepaw for a single cereal, each video is scored individually and an average score is obtained per session.
A) Control animal’s performance on the food manipulation (IBB) task before SCI and 7 weeks post injury.
B) Stimulation animal’s performance on the food manipulation (IBB) task 7 weeks post injury.
Supplement Video 3. Stimulation and control animals’ performance on the horizontal ladder walking task
A) Control animal’s performance on the skilled walking task before SCI and 7 weeks post injury. The same animal’s error rate averaged over all trials in each week was indicated in black line in the inserted figure. Other light blue lines were performance of all other control animals.
B) Stimulation animal’s performance on the skilled walking task 7 weeks post injury. The same animal’s error rate averaged over all trials in each week was indicated in black line in the inserted figure. Other light orange lines were performance of all other stimulation animals.