Significance
Causes for congenital malformations and miscarriages can be genetic, environmental, or a combination of multiple factors; however, in most cases, the underlying reasons are unknown. This study shows that a dietary undersupply of tryptophan and vitamin B3, the metabolic precursors of nicotinamide adenine dinucleotide (NAD), during pregnancy is a cause for frequent multiple birth defects and miscarriages in wild-type mice. When the maternal malnutrition coincided with a genetic variant that impaired NAD synthesis or low oxygen levels as an environmental factor, embryos were more severely affected. Our findings in mice suggest NAD deficiency, due to environmental factors or gene–environment interactions, as a possible cause of congenital malformations in human cases in which no causative gene defect is present.
Keywords: congenital malformation, miscarriage, NAD, metabolism, embryonic development
Abstract
Causes for miscarriages and congenital malformations can be genetic, environmental, or a combination of both. Genetic variants, hypoxia, malnutrition, or other factors individually may not affect embryo development, however, they may do so collectively. Biallelic loss-of-function variants in HAAO or KYNU, two genes of the nicotinamide adenine dinucleotide (NAD) synthesis pathway, are causative of congenital malformation and miscarriage in humans and mice. The variants affect normal embryonic development by disrupting the synthesis of NAD, a key factor in multiple biological processes, from its dietary precursor tryptophan, resulting in NAD deficiency. This study demonstrates that congenital malformations caused by NAD deficiency can occur independent of genetic disruption of NAD biosynthesis. C57BL/6J wild-type mice had offspring exhibiting similar malformations when their supply of the NAD precursors tryptophan and vitamin B3 in the diet was restricted during pregnancy. When the dietary undersupply was combined with a maternal heterozygous variant in Haao, which alone does not cause NAD deficiency or malformations, the incidence of embryo loss and malformations was significantly higher, suggesting a gene–environment interaction. Maternal and embryonic NAD levels were deficient. Mild hypoxia as an additional factor exacerbated the embryo outcome. Our data show that NAD deficiency as a cause of embryo loss and congenital malformation is not restricted to the rare cases of biallelic mutations in NAD synthesis pathway genes. Instead, monoallelic genetic variants and environmental factors can result in similar outcomes. The results expand our understanding of the causes of congenital malformations and the importance of sufficient NAD precursor consumption during pregnancy.
Congenital malformations are a leading cause of death, morbidity, and disability, affecting 3 to 6% of all live births (1). Known causes include chromosomal aneuploidies, pathogenic genetic variants, and various environmental factors. In many cases, no obvious singular cause for the birth defect is identified because the etiologies are predominantly multifactorial and likely result from complex yet unidentified interactions between genes and the environment. Similarly, spontaneous miscarriages are very common, constituting about 15% of all clinically recognized pregnancies, and 1% of women trying to conceive experience recurrent miscarriage defined as three consecutive miscarriages (2). Similar to congenital malformations, causes for recurrent miscarriage are varied and complex, and 40 to 75% of cases are classified as idiopathic (2, 3).
Gene–environment interactions leading to miscarriage or birth defects are generally difficult to identify because implicated factors individually may not be overtly damaging but in combination with other specific factors, will disrupt the highly regulated processes of embryonic development (1, 4, 5). Aside from congenital malformations, gene–environment interactions are also involved in other processes and constitute, for example, the central concept of pharmacogenetics, which studies the effects of genetic heterogeneity on drug response (6). Similarly, diet is an environmental factor that has been implicated in gene–environment interactions and congenital malformations (7). The composition of the maternal diet has, for example, been linked to the causation of neural tube defects. In 1991, a randomized double-blind trial revealed that these defects are primarily a vitamin deficiency disorder, which can be prevented in 80% of cases by daily dietary supplementation with folic acid before and during the first trimester of pregnancy (8). Furthermore, neural tube defects are influenced by genetic factors, and single-nucleotide polymorphisms in genes involved in folate metabolism have been identified as potential risk factors (9).
Deficiency of nicotinamide adenine dinucleotide (NAD) during pregnancy, due to a genetic disruption of its synthesis pathway, was identified as a cause of multiple congenital malformations in families that also had recurrent miscarriages. In four individuals who exhibited a spectrum of similar congenital defects in multiple organs including the heart, vertebrae, kidneys, and limbs, biallelic loss-of-function variants in HAAO or KYNU were found. HAAO and KYNU encode 3-hydroxyanthraninlic acid oxygenase (HAAO) and kynureninase (KYNU), essential enzymes of the kynurenine pathway of NAD synthesis. The four affected individuals had strikingly lower serum NAD levels as a consequence of these loss-of-function variants compared with heterozygous family members who were asymptomatic (10). Their malformation phenotypes have been classified as Congenital NAD Deficiency Disorder (also called vertebral, cardiac, renal, and limb defects syndrome; Online Mendelian Inheritance in Man [OMIM] numbers 617660 and 617661, respectively). The findings were reproduced in mice, showing that, if maternal dietary NAD precursors were sufficiently limited, all embryos died regardless of whether they were homozygous null, heterozygous, or wild type for a loss-of-function allele. Furthermore, a level of NAD precursors could be identified where only homozygous null embryos were affected and developed similar combinations of malformations. NAD deficiency, embryo loss (miscarriage), and embryo abnormalities were completely prevented in these mouse models by supplementation with nicotinic acid (NA; niacin) during pregnancy, presumably boosting NAD synthesis via the Preiss–Handler pathway (10).
The biomolecule NAD (where NAD refers collectively to oxidized and reduced nicotinamide adenine dinucleotide [NAD+ and NADH, respectively]) is crucial to cellular energy metabolism, constituting an essential coenzyme of the cellular adenosine triphosphate (ATP) production system. NAD is also involved in cell signaling and numerous cellular processes, such as cell division, DNA damage repair, chromatin remodeling, and mitochondrial function (11, 12). NAD is synthesized de novo from tryptophan via the kynurenine pathway, which occurs primarily in the liver, and from vitamin B3. Vitamin B3 is a collective term for the NAD precursors NA, nicotinamide (NAM), and nicotinamide riboside (NR). NA and NAM are converted to NAD in the Preiss–Handler and salvage pathways, respectively. Cellular NAD content is regulated by its synthesis and consumption. Sirtuins, poly(adenosine diphosphate [ADP]-ribose) polymerases (PARPs), and cyclic ADP-ribose synthetases consume NAD, and the NAM generated in these processes can be recycled back to produce NAD (11–13).
Human carriers of loss-of-function variants in 17 NAD synthesis genes are present in the Genome Aggregation Database (gnomAD), an aggregate of human exome and genome sequencing data from 141,456 individuals without severe pediatric diseases (14). Within these genomes, there are 341 alleles with predicted loss-of-function variants across the 17 genes. Because congenital malformations frequently have multifactorial etiologies, individuals carrying these variants might be predisposed to developing NAD deficiency if they are exposed to other factors affecting NAD levels. Furthermore, over 3,000 alleles with missense variants in these genes are present in gnomAD, some of which might contribute to multifactorial scenarios. The actual proportion of individuals carrying such variants might be even higher given that the gnomAD cohort is enriched for healthy individuals.
Here, we aimed to show that NAD deficiency defects as seen with homozygous loss-of-function variants in Haao or Kynu can be induced by environmental factors and gene–environment interactions. We investigated whether NAD deficiency, embryo loss, and similar congenital malformations occur in wild-type mice or those with a heterozygous loss-of-function variant in an NAD synthesis gene. In the tested scenario, a limited dietary supply of NAD precursors during pregnancy represented the environmental factor, which was combined with either a heterozygous variant in Haao as a genetic factor or hypoxia as another environmental factor, presumed to affect NAD synthesis. Our findings support the hypothesis that some unsolved human cases of congenital malformations are due to NAD deficiency.
Results
Restricted Maternal Intake of NAD Precursors Leads to Embryo Loss and Multiple Congenital Malformations in Wild-Type Mice.
Pregnant mice that genetically lack the ability to convert tryptophan to NAD produce offspring that die in utero or have multiple congenital malformations due to NAD deficiency when NAD precursor vitamins (vitamin B3) are limited (10). We addressed whether C57BL/6J mice without such genetic defects similarly have miscarriages and malformed embryos when their dietary intake of tryptophan and vitamin B3 is limited during pregnancy. From the start of pregnancy, mice were fed nicotinamide adenine dinucleotide precursor vitamin-depleted and tryptophan-free feed (NTF), and the drinking water was supplemented with defined concentrations of tryptophan.
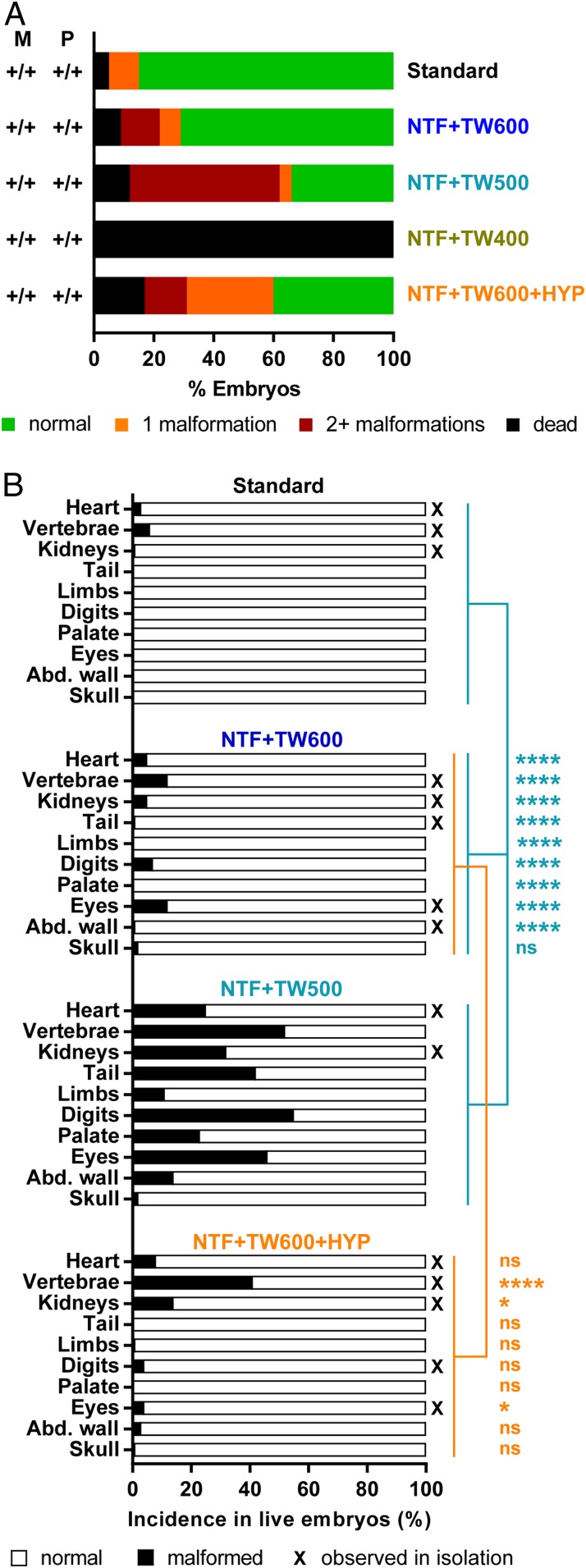
We first determined the amount of NAD precursors that pregnant mice require to sustain embryogenesis. Female mice that were put on NTF with 400 mg/L tryptophan in the drinking water (NTF + 400 mg/L tryptophan-supplemented water [TW400]) (Table 1) from conception were unable to sustain pregnancy. When dissected after 18.5 d of gestation (embryonic day [E] 18.5), embryos appeared as necrotic conceptuses 2 to 5 mm in size, indicative of death during early embryogenesis. Increasing the concentration of tryptophan in the water to 500 mg/L (NTF + TW500) resulted in a large proportion (66%) of affected (dead or malformed) embryos. Of the live embryos, 62% were malformed (Table 2), most of which exhibited multiple malformations in various organs (Fig. 1 and SI Appendix, Fig. S1). Digit malformations occurred most frequently (55%). Skeletal malformations were similarly common (52%),and included rib and vertebral anomalies, of which vertebral body fusions were predominant. Congenital heart defects were identified in 25% of embryos. Other malformations included underdeveloped eyes (46%), hypoplastic kidneys (29%), caudal agenesis (42%), and cleft palate (23%). Abdominal wall and skull defects were present at lower frequencies (Fig. 1B and SI Appendix, Table S1). Some of the malformations occurred in isolation in a subset of embryos. A further increase in tryptophan content to 600 mg/L (NTF + TW600) saw only 29% of embryos affected, and among the 91% of embryos that were alive, 22% were malformed (Fig. 1A and Table 2). The incidence of affected embryos significantly increased when comparing the data for standard, NTF + TW600, NTF + TW500, and NTF + TW400 diets with decreasing NAD precursor content (Table 2). Also, with reduction of NAD precursors in the diet, the frequency and diversity of malformations increased (Fig. 1B and SI Appendix, Table S1). The E18.5 embryos were significantly reduced in weight on NTF + TW500 and NTF + TW600 treatment compared with those on the standard diet (SI Appendix, Fig. S2). Generally, the embryo outcome was variable among litters, but litter sizes were comparable between dietary treatment groups, and the severity of embryo outcome did not seem to depend on litter size (SI Appendix, Fig. S3).
Table 1.
Overview of the mouse treatments and their abbreviations used throughout the text
| Diet/treatment | NA in feed (mg/kg) | Tryptophan in feed (mg/kg) | NA in water (mg/L) | Tryptophan in water (mg/L) | NAD precursors (µg/d)* | Hypoxia at E9.5 (8% O2, 8 h) |
| Standard | 31.4 | 2,700 | 0 | 0 | 298.0 | — |
| NTF + TW400 | 1.4 | 0 | 0 | 400 | 46.8 | — |
| NTF + TW500 | 1.4 | 0 | 0 | 500 | 57.1 | — |
| NTF + TW600 | 1.4 | 0 | 0 | 600 | 67.5 | — |
| NTF + TW600 + HYP | 1.4 | 0 | 0 | 600 | 67.5 | Yes |
| NTF + TW600 + NW15 | 1.4 | 0 | 15.0 | 600 | 160.5 | — |
| NF + NW6 | 1.4 | 1,800 | 6.0 | 0 | 159.7 | — |
Table 2.
Summary of the embryo counts and phenotypic outcomes at E18.5 with the tested treatments
| Row | Genotype | Of all embryos | Of all embryos | Of live embryos | |||||
| M | P | Treatment | Normal | Affected | Alive | Dead | Normal | Malformed | |
| a | +/+ | +/+ | Standard | 81 (85%) | 14 (15%) | 90 (95%) | 5 (5%) | 81 (90%) | 9 (10%) |
| b | +/+ | +/+ | NTF + TW600 | 72 (71%) | 29 (29%) | 92 (91%) | 9 (9%) | 72 (78%) | 20 (22%) |
| c | +/+ | +/+ | NTF + TW500 | 25 (34%) | 49 (66%) | 65 (88%) | 9 (12%) | 25 (38%) | 40 (62%) |
| d | +/+ | +/+ | NTF + TW400 | 0 (0%) | 36 (100%) | 0 (0%) | 36 (100%) | — | — |
| e | +/+ | +/+ | NTF + TW600 + HYP | 68 (40%) | 103 (60%) | 142 (83%) | 29 (17%) | 68 (48%) | 74 (52%) |
| f | +/− | +/− | Standard | 39 (89%) | 5 (11%) | 43 (98%) | 1 (2%) | 39 (91%) | 4 (9%) |
| g | +/− | +/− | NTF + TW600 | 44 (39%) | 68 (61%) | 65 (58%) | 47 (42%) | 44 (68%) | 21 (32%) |
| h | +/− | +/− | NTF + TW600 + NW15 | 51 (85%) | 9 (15%) | 57 (95%) | 3 (5%) | 51 (89%) | 6 (11%) |
| i | +/− | +/+ | NTF + TW600 | 36 (50%) | 36 (50%) | 51 (71%) | 21 (29%) | 36 (71%) | 15 (29%) |
| j | +/− | +/+ | NTF + TW600 + HYP | 40 (39%) | 62 (61%) | 74 (73%) | 28 (27%) | 40 (54%) | 34 (46%) |
| k | −/− | +/− | NF + NW6 | 24 (42%) | 33 (58%) | 50 (88%) | 7 (12%) | 24 (48%) | 26 (52%) |
Diet effect (comparing rows a–d): P < 0.0001 for all categories. Parental genotype effect with sufficient NAD precursors (comparing rows a and f): not significant (ns) for all categories. Parental genotype effect under NAD precursor restriction (comparing rows b and g): P < 0.0001 for normal/affected and alive/dead categories, ns for normal/malformed category. Hypoxia effect under NAD precursor restriction (comparing rows b and e): P < 0.0001 for normal/affected and normal/malformed categories, ns for alive/dead category. Effect of NA supplementation (comparing rows g and h): P < 0.0001 for normal/affected and alive/dead categories, P = 0.0044 for normal/malformed category. Hypoxia effect under NAD precursor restriction and maternal heterozygosity (comparing rows i and j): ns for all categories. Maternal genotype effect under NAD precursor restriction (comparing rows b and i): P = 0.0066 for normal/affected category, P = 0.0009 for alive/dead category, and ns for normal/malformed category. P values were calculated by two-sided Fisher’s exact test when comparing two groups or χ2 test when comparing multiple groups. M, maternal Haao genotype; P, paternal Haao genotype.
Fig. 1.
Phenotypic embryo outcomes at E18.5 in offspring from C57BL/6J wild-type mice show that, with a reduction of NAD precursors throughout pregnancy, the incidence of dead and malformed embryos increases. Mild hypoxia as an additional environmental factor leads to a further increase in the proportion of malformed embryos. (A) Percentages of normal embryos (green), embryos with isolated malformations (orange), embryos with more than one malformation (red), and dead embryos (black) within maternal diet treatment groups as indicated on the right. In this set of experiments, all mice were Haao+/+. Table 2 shows statistics and embryo numbers. M, maternal Haao genotype; P, paternal Haao genotype. (B) Incidence of specific organ defects. Black bars represent percentages of embryos among the respective treatment group that exhibited the indicated defect. Lines and asterisks indicate statistical comparison of malformation incidence between standard, NTF + TW600, and NTF + TW500 groups (light blue) by Fisher’s exact test with Freeman–Halton extension and NTF + TW600 and NTF + TW600 + HYP groups (orange) by two-sided Fisher’s exact test. The vertebrae category includes rib malformations. Abd. wall, abdominal wall; ns, not significant; X, malformation types observed in isolation in one or more embryos. *P < 0.05; ****P < 0.0001.
These data demonstrate that a maternal dietary deficit of NAD precursors during pregnancy constitutes an independent environmental factor causative of embryo loss as well as multiple congenital malformations reminiscent of genetic NAD deficiency models.
Mild Hypoxia during Gestation as an Additional Environmental Factor Increases the Likelihood of Embryo Malformations in Wild-Type Mice.
Aforementioned results show that a diet restricting NAD precursor intake is one way of inducing embryo loss and malformation. Given that NAD synthesis from tryptophan requires activity of the oxygenases tryptophan 2,3-dioxygenase, indoleamine 2,3-dioxygenase, kynurenine-3-mono-oxygenase, and HAAO, we then investigated whether limiting the activity of those oxygenases by hypoxia during pregnancy could also lead to NAD deficiency and the same embryo outcomes. NTF + TW600 diet was deemed a suitable treatment to test other environmental factors because it only mildly increased the malformation rate compared with standard diet (Table 2); therefore, a potential worsening due to other factors could be readily identified. Exposure of pregnant mice to 8% oxygen at E9.5 for 8 h has previously been shown to cause no embryo loss and only infrequent malformations when the diet is rich in NAD precursors (15, 16). Exposure to severe hypoxia (5.5% oxygen for 8 h) has been shown to cause the highest incidence of malformations when done at E9.5 (17).
Pregnant mice on NTF + TW600 diet exposed to 8% oxygen at E9.5 for 8 h had a more than twofold increase in the incidence of affected offspring compared with mice on NTF + TW600 without hypoxia, largely through a significant increase in the proportion of malformed embryos (Fig. 1A and Table 2). Interestingly, 50 of the 74 malformed embryos had isolated malformations. Hypoxia-exposed embryos exhibited significantly more vertebral and kidney malformations but fewer eye defects (Fig. 1B). These experiments show that hypoxic exposure during a key period in embryonic organogenesis can exacerbate the effect of the NAD precursor-restricted diets on early embryonic development.
A Maternal Haao Loss-of-Function Mutation Exacerbates the Effect of Dietary NAD Precursor Restriction on Embryonic Development.
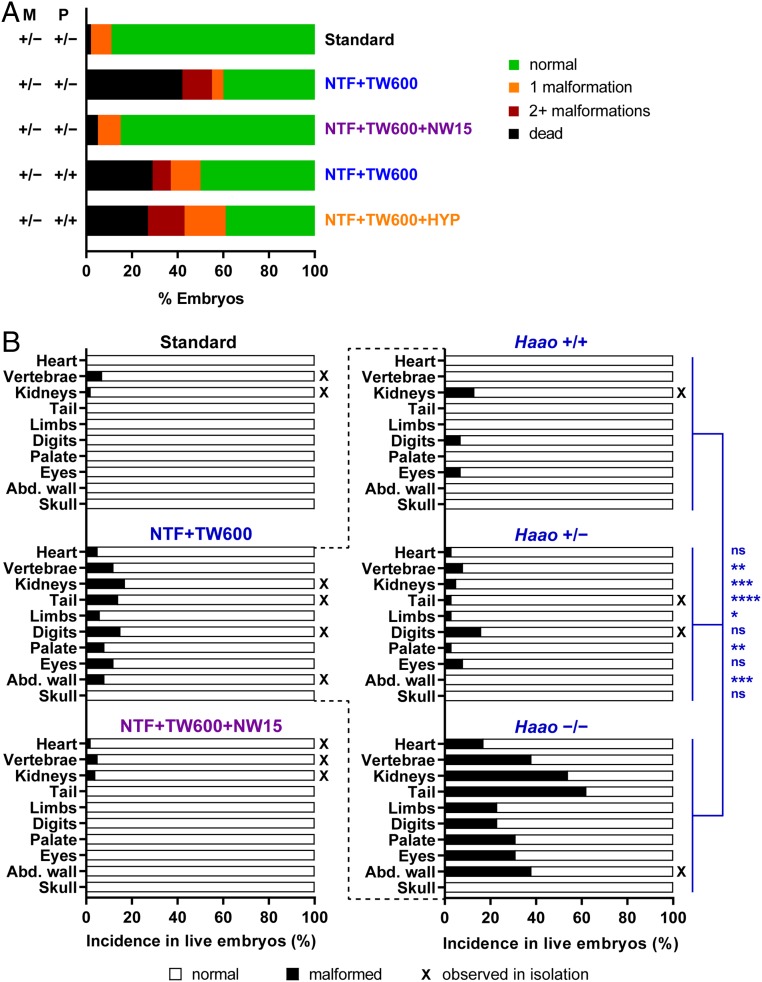
Next, we investigated potential gene–environment interactions between a parental heterozygous loss-of-function variant in Haao and the dietary restriction of tryptophan. Haao+/− females were mated with Haao+/− males. Female mice on standard diet throughout pregnancy had no higher incidence of embryo loss or malformations than wild-type mice (Fig. 2 and Table 2). This shows that, provided that a mother consumes an adequate amount of NAD precursors during pregnancy, a maternal Haao+/− genotype alone does not induce embryo loss or malformations independent of embryo genotype. With pregnant Haao+/− females maintained on NTF + TW600 diet until E18.5, we observed a significant increase in the incidence of affected embryos from 29 to 61% when compared with offspring of wild-type parents on the same diet. This is mainly attributed to a more than fourfold increase in embryo deaths from 9 to 42%, frequently of entire litters (Table 2 and SI Appendix, Fig. S4).
Fig. 2.
Phenotypic embryo outcomes at E18.5 in offspring from mice with an Haao+/− mutation show a high incidence of embryo mortality with NTF + TW600 diet and indicate a gene–environment effect. With additional supply of NA in the water (NTF + TW600 + NW15), embryo mortality is reduced. (A) Summarized embryo outcomes. The dietary treatments throughout pregnancy are indicated on the right. Table 2 shows statistics and embryo numbers. M, maternal Haao genotype; P, paternal Haao genotype. (B) Incidence of specific organ defects occurring in the respective treatment condition (Left) and of the different embryo Haao genotypes within the NTF + TW600 group (Right). Asterisks indicate significant differences between the three embryo Haao genotypes by Fisher’s exact test with Freeman–Halton extension. The vertebrae category includes rib malformations. Abd. wall, abdominal wall; ns, not significant; X, malformation types observed in isolation in one or more embryos. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Categorizing embryos with mothers that were on NTF + TW600 diet by their Haao genotype revealed a significantly higher incidence of malformations in Haao−/− embryos compared with all of the other genotypes (SI Appendix, Fig. S5A). It was not possible to genotype dead embryos due to death early in development and complete necrosis of conceptuses by E18.5. The genotypic distribution of surviving embryos at E18.5 conformed to the expected Mendelian ratio (SI Appendix, Fig. S5C), indicating that the parental Haao+/− genotype increased the mortality of all three embryo genotypes. Therefore, embryonic deaths seemed to occur independently of the embryo genotype and dependent on the maternal genotype.
To strengthen the hypothesis that the observed embryo loss and malformations are due to deficiency of NAD and not of tryptophan, we performed experiments in which the NTF + TW600 diet was supplemented with 15 mg/L NA in the drinking water (NTF + TW600 + 15 mg/L nicotinic acid-supplemented water [NW15]). The percentage of affected embryos of all genotypes was considerably lower with NTF + TW600 + NW15 than with the nonsupplemented NTF + TW600 diet (15 to 61%) and similar to the baseline rate observed with standard diet (Table 2). Embryo loss and malformation were, therefore, not caused by restriction of dietary tryptophan per se but rather, by general limitation of NAD precursors.
As a positive control, we mated Haao−/− females with Haao+/− males as done previously (10), and as in our experiments with wild-type C57BL/6J mice, we kept pregnant females on a precursor-restricted diet (nicotinamide adenine dinucleotide precursor vitamin-depleted feed [NF] + NW6) throughout pregnancy. At E18.5, Haao−/− embryos from this treatment exhibited very similar types of malformations to those seen in embryos from wild-type parents treated with NTF + TW500 or NTF + TW600 diets (SI Appendix, Fig. S6 and Table S1). Complete genetic disruption of the NAD de novo pathway resulted in the same malformations as a restriction of dietary tryptophan and vitamin B3 supply.
We also examined the impact of combining two environmental factors, maternal diet and oxygen supply during pregnancy, with the genetic component of maternal heterozygosity for Haao. Pregnant Haao+/− females that had been mated with wild-type males were fed NTF + TW600 diet until embryo collection at E18.5. They were either exposed to 8% oxygen at E9.5 for 8 h (NTF + TW600 + pregnant mouse exposed to hypoxia [8 h of 8% oxygen at E9.5; HYP]) or remained exclusively in a normoxic environment. Similar to the Haao+/− intercrosses, embryos from mating with only a maternal Haao+/− mutation exhibited a higher rate of affected embryos and reduced embryo survival compared with embryos from wild-type mating with NTF + TW600 diet (Table 2). This gene–environment effect was irrespective of embryo genotype (SI Appendix, Fig. S7). The added hypoxia treatment resulted in a nonsignificant increase in proportion of embryos with congenital malformations compared with the normoxic controls on the same diet (Fig. 2A and Table 2).
In summary, these results evidence a strong gene–environment interaction between maternal heterozygosity for NAD synthesis genes and NAD precursor-restricted diets. Although the maternal NTF + TW600 diet affected embryos only mildly and the maternal Haao+/− mutation had no overt effect under adequate NAD precursor supply, with both factors combined the proportion of dead and affected embryos was significantly elevated (Table 2).
Maternal and Embryonic NAD Levels Are Lowered under Maternal Treatment Conditions That Cause Embryo Loss and Congenital Malformations in Mouse Embryos.
We collected embryos at E9.5 and E11.5 to assess survival rates during embryonic organogenesis. With a restriction of maternal NAD precursor intake, we observed both higher rates of embryo death as well as developmental delay at E9.5, whereby embryos morphologically resembled E8.5 embryos (SI Appendix, Fig. S8). At E11.5, the mortality of embryos was similarly elevated with a reduction of maternal dietary NAD precursors (SI Appendix, Fig. S9 and Table S2).
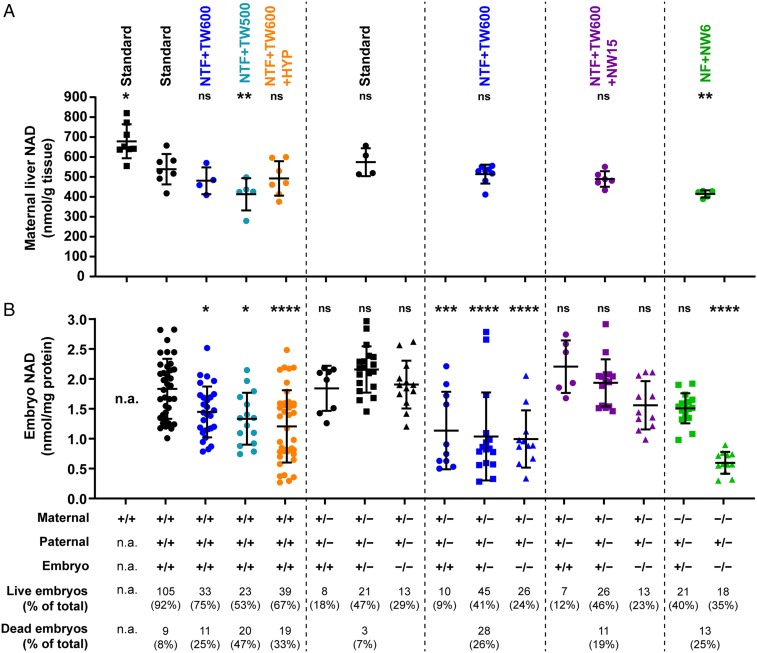
We then aimed to directly measure NAD levels in pregnant mice and their offspring. We collected maternal livers and corresponding embryos at E11.5 as at this timepoint, structures in which we observed malformations, specifically the heart (18) and kidney (19), are in a key stage of organogenesis. We first compared maternal NAD stores. Liver NAD levels in nonpregnant control females maintained on standard diet were 673 ± 86 nmol/g. By contrast, pregnant females had significantly lower NAD levels (561 ± 65 nmol/g), and all other cases were compared with this group (Fig. 3 and SI Appendix, Table S3). Haao−/− females maintained on the NF + NW6 diet, which provides ample supply of tryptophan but restricts supply of NAD precursor vitamins, produce a large proportion of Haao−/− embryos with malformations at E18.5 (SI Appendix, Fig. S6). At E11.5, pregnant Haao−/− mothers on NF + NW6 had significantly lower liver NAD levels than pregnant wild types on standard diet (Fig. 3 and SI Appendix, Table S3). Pregnant wild-type mice on NTF + TW500 diet, which frequently had offspring with multiple malformations, had liver NAD levels similar to those of Haao−/− mice on NF + NW6 diet. By contrast, liver NAD levels of wild types on the comparatively more tryptophan-rich NTF + TW600 diet were only insignificantly lower than those of the standard diet group. These data indicate that lowered maternal NAD levels cause embryo demise irrespective of the NAD deficiency being caused by limited NAD precursors in the diet or genetic blockage of NAD synthesis. They also show that pregnancy alone leads to a reduction in maternal NAD liver stores.
Fig. 3.
Maternal liver and embryo total NAD levels (NAD+ and NADH) and phenotypic embryo outcomes at E11.5. Dots represent NAD levels, and bars indicate the mean ± SD. (A) Maternal liver NAD levels. Treatment groups are indicated on the top. The parental and embryo Haao genotypes are indicated at the bottom. Liver tissue was collected at E11.5 along with the embryos. The first column represents liver NAD levels of nonpregnant females maintained on standard diet for at least 3 wk prior to dissection, which were of similar age to the pregnant females. (B) Whole-embryo NAD levels. Analyzed embryos were offspring of the mothers with liver NAD levels that were measured. The total numbers and percentages of live and dead embryos observed with each treatment condition are indicated at the bottom. Note that not every collected embryo underwent NAD measurement. Asterisks indicate NAD levels that are significantly different to those of the pregnant C57BL/6J wild-type standard diet group (second column) by one-way ANOVA with Dunnett’s multiple comparisons test with *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. A summary of NAD level values is in Table 3 and SI Appendix, Table S3. n.a., not applicable (no embryos in nonpregnant females); ns, not significant.
Next, we compared NAD levels in whole E11.5 embryos. Embryo NAD levels were significantly lower compared with the standard diet group when wild-type mothers were fed NTF + TW600 diet, even lower with NTF + TW500, and further reduced when NTF + TW600 was combined with hypoxia (Fig. 3 and Table 3). In experiments involving Haao+/− mothers on NTF + TW600 diet, maternal liver NAD levels were not significantly affected by the genetic variant, but the embryos had significantly lower NAD levels. Comparing the embryo NAD levels of the three embryo genotypes between standard and NTF + TW600 diet revealed that the diet effect is significant, whereas the embryo genotype effect is not (Table 3). These data indicate the NAD precursor-restricted diet has a general impact on all embryos at this stage independent of their genotype (Fig. 3). The NA-supplemented NTF + TW600 + NW15 diet restored the embryo NAD levels, which was consistent with the lack of malformations at E18.5.
Table 3.
Embryo NAD levels at E11.5 with different maternal treatments during pregnancy
| Row | Maternal genotype | Paternal genotype | Embryo genotype | Treatment | NAD (nmol/mg protein ± SD) | n | P |
| a | +/+ | +/+ | +/+ | Standard | 1.84 ± 0.501 | 36 | |
| b | +/+ | +/+ | +/+ | NTF + TW600 | 1.46 ± 0.424 | 28 | 0.0219 |
| c | +/+ | +/+ | +/+ | NTF + TW500 | 1.34 ± 0.433 | 15 | 0.0116 |
| d | +/+ | +/+ | +/+ | NTF + TW600 + HYP | 1.21 ± 0.604 | 39 | <0.0001 |
| e | +/− | +/− | +/+ | Standard | 1.85 ± 0.377 | 8 | >0.9999 |
| f | +/− | 2.16 ± 0.387 | 19 | 0.2049 | |||
| g | −/− | 1.91 ± 0.400 | 13 | 0.9995 | |||
| h | +/− | +/− | +/+ | NTF + TW600 | 1.14 ± 0.648 | 10 | 0.0010 |
| i | +/− | 1.04 ± 0.737 | 16 | <0.0001 | |||
| j | −/− | 1.00 ± 0.478 | 11 | <0.0001 | |||
| k | +/− | +/− | +/+ | NTF + TW600 + NW15 | 2.21 ± 0.440 | 6 | 0.6201 |
| l | +/− | 1.94 ± 0.395 | 15 | 0.9991 | |||
| m | −/− | 1.57 ± 0.405 | 11 | 0.6894 | |||
| n | −/− | +/− | +/− | NF + NW6 | 1.52 ± 0.252 | 16 | 0.2596 |
| o | −/− | 0.60 ± 0.181 | 12 | <0.0001 |
P values were calculated by ANOVA followed by Dunnett’s multiple comparisons test comparing all treatment groups with the pregnant wild type on standard diet control group (row a). Additional ANOVAs were done between selected groups. Diet effect (comparing rows a–c): P = 0.0004. Hypoxia effect under NAD precursor restriction (comparing rows b and d): P = 0.0423. Two-way ANOVA followed by Tukey’s multiple comparison test to evaluate diet and genotype effects as well as potential interactions (comparing rows e, f, g and h, i, j): P = 0.3977 (interaction), P = 0.5489 (genotype effect), P < 0.0001 (diet effect). Two-way ANOVA to evaluate diet and genotype effects with NA supplementation (comparing rows h, i, j and k, l, m): P = 0.3530 (interaction, P = 0.1037 (genotype effect), P < 0.0001 (diet effect).
We measured embryo NAD levels at E9.5 to further evaluate the impact of the 8-h hypoxia treatment. Similar to the observations at E11.5, the NTF + TW600 diet resulted in a significant reduction in embryo NAD levels at E9.5 compared with standard diet. Embryos exposed to hypoxia (NTF + TW600 + HYP) and collected directly after the treatment had NAD levels that were lowered compared with NTF + TW600 without reaching statistical significance, concordant with the results obtained with E11.5 embryos (SI Appendix, Fig. S10 and Table S4).
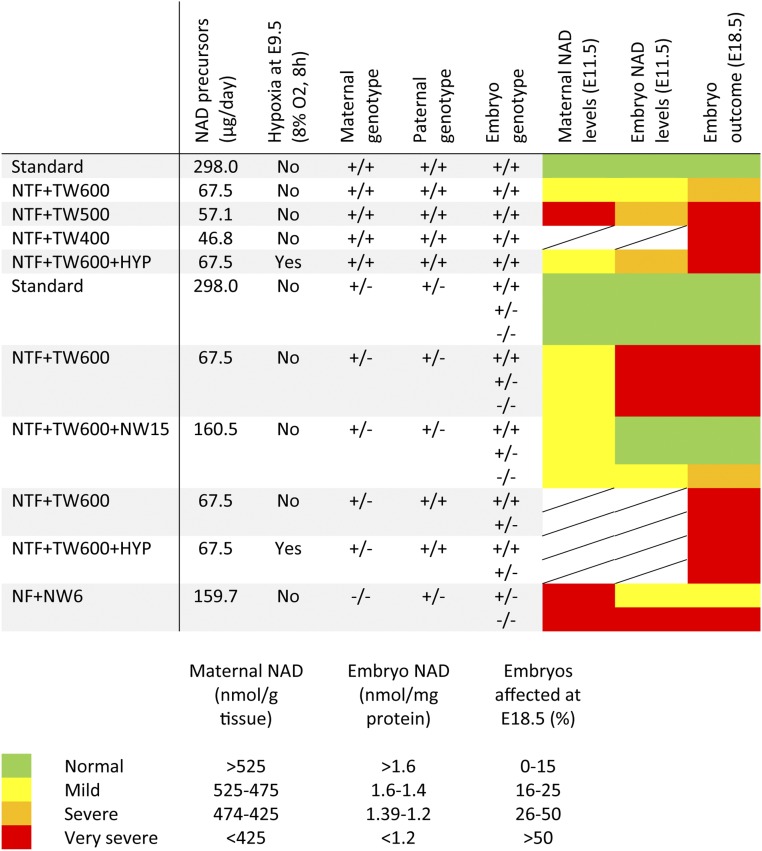
Overall, the embryo NAD levels at E9.5 and E11.5 correlate with observed embryo outcomes at E18.5 and confirm the hypothesized gene–environment effect. Severe NAD deficiency, defined as <1.39 nmol NAD per 1 mg protein in the embryos (Fig. 4), and disrupted embryonic development could be induced by modifying the gestational environment solely by restricting the maternal intake of NAD precursors using NTF + TW500 (providing ∼57.1 µg NAD precursors per day). In the presence of a maternal Haao+/− variant, the less restrictive NTF + TW600 diet (providing 67.5 µg/d of NAD precursors) caused very severe NAD deficiency (<1.2 nmol/mg protein). Pregnant mice that genetically lack the ability to convert tryptophan to NAD, in our case due to a homozygous Haao−/− variant, have severely NAD-deficient embryos with a high incidence of malformations under a diet comparatively more abundant in NAD precursors, namely NF + NW6 providing 159.7 µg of NAD precursors per day (Fig. 4).
Fig. 4.
Summary of NAD levels and phenotypic outcomes of all treatment conditions tested in this study. Four thresholds for maternal liver and embryo NAD levels were set to specify normal, mild, severe, and very severe NAD deficiency. Similarly, four levels for the incidence of affected embryos at E18.5 were set. For the classification of embryo outcomes at E18.5 involving Haao genotypes, the assumption was made that the genotypes of dead (resorbed) embryos, which could not be genotyped, were distributed in a normal Mendelian ratio (25:50:25) based on the finding that the genotypes of surviving embryos did not significantly deviate from a normal Mendelian ratio.
Discussion
Biallelic loss-of-function variants in either Haao or Kynu (Haao−/− or Kynu−/−) in mice, which genetically block the synthesis pathway from tryptophan to NAD, cause congenital malformations and embryo loss as a consequence of NAD deficiency (10). Similar to mice, an HAAO−/− or KYNU−/− genotype in humans causes reduced NAD levels and congenital malformations in multiple organs, classified as Congenital NAD Deficiency Disorder. To date, four unrelated human cases of this disorder have been described, with each of them displaying heart, vertebral, and renal defects. Additional clinical features were reported for these individuals, including talipes, syndactyly, and cleft palate. Miscarriage was also a feature in three of the families (10).
This study demonstrates that NAD deficiency during pregnancy results in embryo loss and congenital malformations irrespective of it being caused by environmental factors, genetic variants, or a combination of both. We first showed that an NAD precursor-restricted diet fed to mice during pregnancy is a causative environmental factor for congenital malformations. Reduction of dietary tryptophan and NAD precursor vitamins throughout pregnancy caused NAD deficiency in wild-type mice and resulted in similar malformations and embryo loss as seen with the biallelic variants. The severity of embryo outcome correlated with the extent of NAD precursor restriction in the diet (Figs. 1A and 4). In addition to combinations of malformations, embryos also had isolated abnormalities in various organs such as the heart, skeleton, and kidney (Fig. 1B), indicating that NAD deficiency during embryonic development results in a variable spectrum of phenotypes. When dissected at E9.5, embryos from NAD precursor-restricted treatment groups frequently appeared developmentally delayed and resembled E8.5 embryos, indicating a perturbation of normal embryonic development (SI Appendix, Fig. S8).
The NAD de novo pathway involves oxygenases requiring oxygen as cosubstrate (20). Exposure of pregnant mice to mild hypoxia for 8 h at E9.5 has been shown to only insignificantly affect embryonic development when the diet is rich in NAD precursors (15, 16). Here, we show that, when the hypoxia coincides with dietary NAD precursor deficiency, the malformation incidence is increased, with a distinct rise in frequency of isolated defects (Fig. 1A and Table 2). Similarly, while a maternal heterozygous loss-of-function variant in Haao alone confers no overt phenotypic effect in the offspring, embryos were significantly more likely to become malformed or die when mothers were given NAD precursor-restricted diets throughout pregnancy, indicating a gene–environment interaction (Figs. 2A and 4 and Table 2). These data demonstrate that genetic and environmental factors, which individually are benign with respect to NAD synthesis and embryonic development, can disrupt the highly regulated embryo development processes and adversely affect pregnancy when occurring in combination. Essentially, the stronger the genetic component, the lesser the degree of a dietary/environment component required to cause NAD deficiency and impair embryonic development.
The essential amino acid tryptophan is not only catabolized for NAD synthesis in the kynurenine pathway but is also used for protein synthesis and biotransformations leading to serotonin and other chemical messengers. Quantitatively, the kynurenine pathway is the most important metabolic pathway and accounts for over 90% of tryptophan catabolism (21). Two of our findings suggest that the embryonic malformations and deaths caused by the tryptophan- and vitamin B3-restricted diets are a result of NAD deficiency and not due to tryptophan deficiency per se. The NA-supplemented NTF + TW600 + NW15 diet contains the same limited amount of tryptophan as NTF + TW600 but significantly reduced the proportion of dead and malformed embryos to values resembling the standard diet with adequate tryptophan content (Fig. 2). Furthermore, there is a striking similarity to the types of malformations seen in embryos of Haao−/− mothers maintained on NF + NW6 diet, which is vitamin B3 restricted but contains 1.8 g/kg tryptophan (SI Appendix, Table S1).
A relatively narrow margin was discovered between sufficient NAD precursors and levels that cause complete loss of embryos. With NTF + TW400 diet, all embryos died early in embryogenesis, whereas with a moderately higher maternal NAD precursor supply (NTF + TW600), the majority survived, and less than 50% of embryos were malformed (Fig. 1 and Table 2). To put the dietary treatments used in this study into a context of human requirements, the daily NAD precursor dosage that an average human would consume should they follow diets equivalent to those fed to our pregnant mice (Table 1) can be calculated (22) and related to the recommended daily intake (RDI) for a healthy individual of a particular life stage and gender group. The RDI for a pregnant woman of 14 to 50 y of age is 18 mg/d of NAD precursors (23, 24). Such calculation shows that diets that caused malformations in the offspring of wild-type and Haao+/− female mice would be below the RDI when converted to the human equivalents (i.e., they potentially also represent a nutritional deficiency in humans) (SI Appendix, Table S5). Conversely, the amount of NAD precursors that a mouse receives by consuming standard and NTF + TW600 + NW15 diets theoretically would be sufficient (above the RDI) when converted to the human equivalents. According to data of a survey conducted in the United States in 2015 to 2016, the usual NAD precursor intake of females between ages 12 to 49 is in the range of 20.9 (±0.63) to 24.2 (±0.87) mg/d (25), and data from a previous survey indicate that only 1% of adults had intakes of vitamin B3 from foods and beverages below the estimated average requirement (EAR), the daily nutrient level estimated to meet the requirements of half the healthy individuals in a particular life stage and gender group (26). While these population-based statistical data indicate that the majority of the US population receives sufficient supply of NAD precursors with their diet, the gene–environment interactions observed in mice likely also apply to humans, and pregnant women with a deleterious variant in a gene required for the absorption or conversion of NAD precursors to NAD might have an actual dietary requirement that is considerably higher than the EAR.
We investigated the relationship between maternal liver and whole-embryo total NAD levels, embryo phenotype, and embryo survival. Pregnant mice with an Haao−/− genotype maintained on NF + NW6 diet had significantly lower liver NAD levels than wild-type (Haao+/+) mice on standard diet (Figs. 3 and 4). Pregnant wild-type mice on NTF + TW500 diet had similarly low liver NAD levels, indicating that dietary restriction of NAD precursors including tryptophan has similar consequences on maternal NAD stores as a genetic disruption of NAD de novo synthesis. With NTF + TW500, both maternal liver and embryos were NAD deficient at E11.5, consistent with the very high incidence of multiple malformations observed in embryos at E18.5 (Figs. 1 and 4).
A maternal Haao+/− mutation combined with the NTF + TW600 diet resulted in embryo NAD levels that were lower than those of offspring from wild-type mating on the same diet (Figs. 3 and 4). This gene–environment effect was also reflected at E18.5, at which stage pregnant mice of the Haao+/− genotype had a significantly higher rate of affected embryos than wild-type mice (Fig. 4 and Table 2). Embryos of all three Haao genotypes had similarly low NAD levels at E11.5, suggesting that at this embryonic stage the embryos were not reliant on their own NAD de novo pathway, and the maternal genotype was the main determinant for the embryo outcome. This is likely because the mother predominantly provides circulating intermediates that feed into the salvage pathway, primarily NAM, to the embryos (12). Also, the de novo pathway is primarily active in the liver and kidney (12, 13), and the embryos are already affected (malformed/developmentally delayed) before liver and kidney tissues have developed this metabolic activity (19, 27).
Whole-blood NAD levels of Haao−/− mice maintained on NF without any NAD precursors added to the water (Table 1) remained unchanged for at least 4 d and then, started to decline to about 50% of initial levels within 2 wk (SI Appendix, Fig. S11). Conversely, the amount of NAD available to the conceptus appears to be reduced very quickly because embryos will not survive if their Haao−/− mothers were given the NAD precursor vitamin-depleted NF in the first 5 d of pregnancy (from E0.5 to 5.5) (10). This implies that the diet has a greater effect on embryogenesis than the NAD store in the body.
Energy and nutrient requirements are generally higher during pregnancy to allow for fetal growth, and studies in humans and rats suggest that the production of NAM from tryptophan gradually becomes more efficient, peaking late in pregnancy (28, 29). At the later stage of E18.5 when the mother generated NAD more efficiently from tryptophan, the Haao−/− embryos of the NAD precursor-restricted NTF + TW00 group had a significantly higher malformation incidence compared with the other genotypes, but the embryo mortality was similar across all genotypes (SI Appendix, Fig. S5 A and C). In late pregnancy, the embryos likely also rely on their own NAD de novo synthesis for growth and development because they require more NAD, and their liver and kidneys have formed. However, the insufficient NAD precursor supply during early stages of embryogenesis (prior to E9.5) presumably has the biggest impact given the rates of embryo death at this stage.
Individual embryo NAD levels were variable among dietary treatment groups (Fig. 3), consistent with the variability of embryo outcomes at E18.5. The number of embryos per litter did not have an influence on litter outcome (SI Appendix, Figs. S3 and S4). Inconsistent rates of mouse feed and water consumption could have contributed to this variability, but the relatively consistent maternal liver NAD levels suggest a relatively consistent NAD precursor supply from the diet. Despite this, the amount of NAD precursors that the embryos receive may still differ between litters. Larger mice with bigger livers might buffer their circulating NAD precursor levels more effectively than lower-weight mice. We tried to minimize this variable by using female mice of a similar age range and giving them standard diet with defined and standardized contents for 3 wk before mating.
An interesting finding from the liver NAD measurements is the distinct reduction of NAD levels due to pregnancy (Fig. 3). Similar observations have been reported for humans, and reduced levels of NAD precursors in the blood during all trimesters of pregnancy seem to be common, even with nondeficient diets and ample dietary vitamin intake (30). These findings indicate that the risk of developing NAD deficiency is elevated during pregnancy, the time when sufficient NAD supply is required to ensure normal embryonic development.
In humans, a variety of factors can impair NAD synthesis and potentially lead to NAD deficiency. While insufficient dietary supply of vitamin B3 is rare today, NAD synthesis has also been shown to be impaired by pathophysiological factors, such as inflammation, type 2 diabetes, and obesity (31–33). Kynurenine aminotransferases (KATs; KATI/II/III/IV) and KYNU, enzymes of the kynurenine pathway, require pyridoxal 5′-phosphate, a form of vitamin B6, as a cofactor, and its deficiency affects tryptophan metabolism (34). Vitamin B6 deficiency is rare in developed countries, but low-plasma vitamin B6 status has been reported in cases of obesity and diabetes and linked to oral contraceptive use, smoking, excessive alcohol consumption, and certain drugs (35, 36). Pregnant women are also predisposed to having depressed plasma vitamin B6 levels (37). Deficiency of vitamin B6 can, therefore, be a confounding factor on NAD synthesis, especially in the context of pregnancy.
Our data show that a genetic factor (i.e., a heterozygous variant of a gene related to NAD synthesis) can cause severe NAD deficiency when combined with other factors, even if the variant alone does not lead to an overt phenotype. Numerous genes are involved with NAD synthesis, including those encoding amino acid transporters, NA transporters, and enzymes of the de novo and salvage pathways, and mutations in these genes are potentially implicated in NAD deficiency. Gene variant databases, such as gnomAD, indicate that there are numerous individuals carrying heterozygous variants in these genes. Together with the many environmental factors that affect vitamin B3, vitamin B6, and NAD levels, NAD deficiency might occur in more pregnancies than expected from dietary intake survey data.
In summary, our data show that NAD deficiency leading to miscarriage and congenital malformation is not restricted to rare cases of biallelic loss-of-function mutations in NAD synthesis pathway genes but can also be provoked by combinations of other, presumably more common factors. Also, depending on the cause, NAD deficiency can result in isolated congenital defects, which are more common than syndrome-like combinations of malformations. This study supports the notion that NAD deficiency is clinically relevant. It would be interesting to determine the range of serum NAD levels before and during pregnancy in families with a history of miscarriage or congenital malformations and investigate the prevalence of NAD deficiency. In patients with nonsyndromic malformations for which no causative genetic variant or obvious other factor is detectable, NAD deficiency is a possible cause for considering. Therefore, the findings of this study expand our understanding of the causes of congenital malformations and the importance of adequate maternal intake of NAD precursors during pregnancy.
Materials and Methods
Animal Experiments.
All animal experiments were performed in accordance with protocols approved by the Garvan Institute of Medical Research/St. Vincent’s Animal Experimentation Ethics Committee, Sydney, Australia (approvals 15/27 and 18/27). The Haao loss-of-function mouse line (allele Haaoem1Dunw) has been described previously (10).
Female mice to be used in timed mating were fed a “standard” feed with defined composition (Table 1) (AIN93G; Specialty Feeds) for at least 3 wk prior to mating. From the start of pregnancy, their food was replaced with a feed depleted in NA, NAM, and NR as well as tryptophan (NTF; SF16-097; Specialty Feeds), and drinking water contained defined concentrations of tryptophan (TW) or NA (NW) (Table 1). Pregnant mice were maintained on these diets until embryo collection at E11.5 for NAD quantification or E18.5 for embryo phenotyping.
For gestational hypoxia experiments (HYP), pregnant mice at E9.5 were exposed to 8% oxygen at normal atmospheric pressure for 8 h as described (15). After exposure, the mice were either dissected immediately or returned to normoxia for embryo harvest at E11.5 or E18.5.
A detailed description of mouse genotyping, dietary treatments, and embryo phenotyping is in SI Appendix, Supplementary Materials and Methods.
NAD Quantification.
Total NAD levels (NAD+ and NADH) of whole-embryo lysates were measured with an enzymatic cycling assay that utilizes diaphorase-catalyzed conversion of resazurin to the fluorescent resorufin.
A detailed description of the protocol for NAD measurements is in SI Appendix, Supplementary Materials and Methods.
Statistical Analysis.
Two-sided Fisher’s exact test was used to compare numbers of normal and affected embryos, dead and alive embryos, and normal and malformed embryos between two treatment groups. The χ2 test was used for comparing multiple groups. In experiments involving mouse mating with Haao loss-of-function mutations, malformation incidence among the embryo genotypes was compared using Fisher’s exact test with Freeman–Halton extension (2 × 3 contingency table). This test was also used to compare observed embryo numbers for each genotype with expected Mendelian genotype distribution. One-way ANOVA with Dunnett’s multiple comparisons was used to compare NAD levels and embryo weights between different treatment groups (using wild types on standard diet as control group), and unpaired two-tailed t test was used to compare two groups. In experiments involving mice with Haao loss-of-function mutations, the main effects of embryo genotype and diet and their interaction were analyzed using a two-way ANOVA followed by Tukey’s multiple comparison test to assess effects of genotype within the diet groups. NAD levels are displayed as mean ± SD. P values < 0.05 were considered significant. All statistical analyses were performed with Prism (version 8; GraphPad Software) except for Fisher’s exact test with Freeman–Halton extension, for which an online tool was used (38).
Data Availability.
All data are available in the text and SI Appendix.
Supplementary Material
Acknowledgments
We thank Joelene Greasby and Kavitha Iyer for technical assistance, Eleni Giannoulatou and Loïc Thibaut for statistical genetics assistance, and Gavin Chapman and Paul Mark for critically assessing the manuscript. This work was supported by National Health and Medical Research Council Project Grant 1162878 (S.L.D.) and Fellowship 1042002 (S.L.D.), Office of Health and Medical Research of the New South Wales Government (S.L.D.), The Key Foundation (S.L.D.), and St. Vincent’s Clinic Foundation (S.L.D.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1916588117/-/DCSupplemental.
References
- 1.Hobbs C. A., et al. , Genetic epidemiology and nonsyndromic structural birth defects: From candidate genes to epigenetics. JAMA Pediatr. 168, 371–377 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rai R., Regan L., Recurrent miscarriage. Lancet 368, 601–611 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Kaiser J., Branch D. W., Recurrent pregnancy loss: Generally accepted causes and their management. Clin. Obstet. Gynecol. 59, 464–473 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Baldacci S., et al. , Environmental and individual exposure and the risk of congenital anomalies: A review of recent epidemiological evidence. Epidemiol. Prev. 42 (suppl. 1), 1–34 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Zhu H., Kartiko S., Finnell R. H., Importance of gene-environment interactions in the etiology of selected birth defects. Clin. Genet. 75, 409–423 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Meyer U. A., Pharmacogenetics–Five decades of therapeutic lessons from genetic diversity. Nat. Rev. Genet. 5, 669–676 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Ordovas J. M., Mooser V., Nutrigenomics and nutrigenetics. Curr. Opin. Lipidol. 15, 101–108 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Wald N., Sneddon J., Densem J., Frost C., Stone R., Prevention of neural tube defects: Results of the medical research council vitamin study. MRC Vitamin Study Research Group. Lancet 338, 131–137 (1991). [PubMed] [Google Scholar]
- 9.Boyles A. L., et al. ; NTD Collaborative Group , Neural tube defects and folate pathway genes: Family-based association tests of gene-gene and gene-environment interactions. Environ. Health Perspect. 114, 1547–1552 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi H., et al. , NAD deficiency, congenital malformations, and niacin supplementation. N. Engl. J. Med. 377, 544–552 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Yang Y., Sauve A. A., NAD(+) metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim. Biophys. Acta 1864, 1787–1800 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houtkooper R. H., Cantó C., Wanders R. J., Auwerx J., The secret life of NAD+: An old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 31, 194–223 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matasic D. S., Brenner C., London B., Emerging potential benefits of modulating NAD+ metabolism in cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 314, H839–H852 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karczewski K. J., et al. , Variation across 141,456 human exomes and genomes reveals the spectrum of loss-of-function intolerance across human protein-coding genes. bioRxiv:10.1101/531210 (30 January 2019).
- 15.Sparrow D. B., et al. , A mechanism for gene-environment interaction in the etiology of congenital scoliosis. Cell 149, 295–306 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Moreau J. L. M., et al. , Gene-environment interaction impacts on heart development and embryo survival. Development 146, dev172957 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Shi H., et al. , Gestational stress induces the unfolded protein response, resulting in heart defects. Development 143, 2561–2572 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Jensen B., Wang T., Christoffels V. M., Moorman A. F., Evolution and development of the building plan of the vertebrate heart. Biochim. Biophys. Acta 1833, 783–794 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Little M. H., McMahon A. P., Mammalian kidney development: Principles, progress, and projections. Cold Spring Harb. Perspect. Biol. 4, 1–18 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badawy A. A., Kynurenine pathway of tryptophan metabolism: Regulatory and functional aspects. Int. J. Tryptophan Res. 10, 1178646917691938 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sainio E. L., Pulkki K., Young S. N., L-Tryptophan: Biochemical, nutritional and pharmacological aspects. Amino Acids 10, 21–47 (1996). [DOI] [PubMed] [Google Scholar]
- 22.Nair A. B., Jacob S., A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 7, 27–31 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Health and Medical Research Council; Australian Government Department of Health and Ageing, New Zealand Ministry of Health, Nutrient reference values for Australia and New Zealand including recommended dietary intakes (2006). https://www.nhmrc.gov.au/about-us/publications/nutrient-reference-values-australia-and-new-zealand-including-recommended-dietary-intakes. Accessed 27 August 2019.
- 24.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and Its Panel on Folate; Other B Vitamins; and Choline , Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline (National Academies Press, Washington, DC, 1998). [PubMed] [Google Scholar]
- 25.Agricultural Research Service - US Department of Agriculture, What we eat in America, NHANES 2015-2016 (2016). https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables/. Accessed 27 August 2019.
- 26.Blumberg J. B., Frei B. B., Fulgoni V. L., Weaver C. M., Zeisel S. H., Impact of frequency of multi-vitamin/multi-mineral supplement intake on nutritional adequacy and nutrient deficiencies in U.S. adults. Nutrients 9, 1–15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaestner K. H., The making of the liver: Developmental competence in foregut endoderm and induction of the hepatogenic program. Cell Cycle 4, 1146–1148 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Ftukijwatari T., et al. , Changes in the urinary excretion of the metabolites of the tryptophan-niacin pathway during pregnancy in Japanese women and rats. J. Nutr. Sci. Vitaminol. (Tokyo) 50, 392–398 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Fukuwatari T., Shibata K., Nutritional aspect of tryptophan metabolism. Int. J. Tryptophan Res. 6 (suppl. 1), 3–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker H., DeAngelis B., Holland B., Gittens-Williams L., Barrett T. Jr, Vitamin profile of 563 gravidas during trimesters of pregnancy. J. Am. Coll. Nutr. 21, 33–37 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Oxenkrug G., Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways. Mol. Neurobiol. 48, 294–301 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aasheim E. T., Hofsø D., Hjelmesaeth J., Birkeland K. I., Bøhmer T., Vitamin status in morbidly obese patients: A cross-sectional study. Am. J. Clin. Nutr. 87, 362–369 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Allegri G., et al. , Metabolism of tryptophan along the kynurenine pathway in alloxan diabetic rabbits. Adv. Exp. Med. Biol. 527, 387–393 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Bender D. A., Njagi E. N., Danielian P. S., Tryptophan metabolism in vitamin B6-deficient mice. Br. J. Nutr. 63, 27–36 (1990). [DOI] [PubMed] [Google Scholar]
- 35.Ueland P. M., Ulvik A., Rios-Avila L., Midttun Ø., Gregory J. F., Direct and functional biomarkers of vitamin B6 status. Annu. Rev. Nutr. 35, 33–70 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lussana F., Zighetti M. L., Bucciarelli P., Cugno M., Cattaneo M., Blood levels of homocysteine, folate, vitamin B6 and B12 in women using oral contraceptives compared to non-users. Thromb. Res. 112, 37–41 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Lumeng L., Cleary R. E., Wagner R., Yu P.-L., Li T.-K., Adequacy of vitamin B6 supplementation during pregnancy: A prospective study. Am. J. Clin. Nutr. 29, 1376–1383 (1976). [DOI] [PubMed] [Google Scholar]
- 38.Soper D. S., Fisher’s exact test calculator for a 2x3 contingency table (2019). https://www.danielsoper.com/statcalc/. Accessed 27 August 2019.
- 39.Bachmanov A. A., Reed D. R., Beauchamp G. K., Tordoff M. G., Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav. Genet. 32, 435–443 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldsmith G. A., Niacin-tryptophan relationships in man and niacin requirement. Am. J. Clin. Nutr. 6, 479–486 (1958). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the text and SI Appendix.