Significance
Biological roles of inorganic phosphate require careful regulation of its transport across cell membranes, but mechanisms are poorly understood. We demonstrate that a major cellular phosphate export protein, XPR1, is regulated by the most “energetic” of cell signaling molecules—1,5-bisdiphosphoinositol 1,2,3,4-tetrakisphosphate (InsP8). We derive this conclusion by showing reduced XPR1-mediated phosphate efflux when InsP8 synthesis was attenuated by genetic or pharmacological techniques; phosphate efflux was rescued by using liposomes to deliver into cells metabolically resistant InsP8 analogues. Genetic elimination of InsP8 from an osteosarcoma cell line perturbed phosphate homeostasis and accelerated differentiation into a biomineralization phenotype. We propose that mutations in gene products that regulate InsP8 synthesis might compromise XPR1 function, with pathological consequences for bone maintenance and ectopic calcification.
Keywords: signaling, inositol, metabolism
Abstract
Homeostasis of cellular fluxes of inorganic phosphate (Pi) supervises its structural roles in bones and teeth, its pervasive regulation of cellular metabolism, and its functionalization of numerous organic compounds. Cellular Pi efflux is heavily reliant on Xenotropic and Polytropic Retrovirus Receptor 1 (XPR1), regulation of which is largely unknown. We demonstrate specificity of XPR1 regulation by a comparatively uncharacterized member of the inositol pyrophosphate (PP-InsP) signaling family: 1,5-bis-diphosphoinositol 2,3,4,6-tetrakisphosphate (InsP8). XPR1-mediated Pi efflux was inhibited by reducing cellular InsP8 synthesis, either genetically (knockout [KO] of diphosphoinositol pentakisphosphate kinases [PPIP5Ks] that synthesize InsP8) or pharmacologically [cell treatment with 2.5 µM dietary flavonoid or 10 µM N2-(m-trifluorobenzyl), N6-(p-nitrobenzyl) purine], to inhibit inositol hexakisphosphate kinases upstream of PPIP5Ks. Attenuated Pi efflux from PPIP5K KO cells was quantitatively phenocopied by KO of XPR1 itself. Moreover, Pi efflux from PPIP5K KO cells was rescued by restoration of InsP8 levels through transfection of wild-type PPIP5K1; transfection of kinase-dead PPIP5K1 was ineffective. Pi efflux was also rescued in a dose-dependent manner by liposomal delivery of a metabolically resistant methylene bisphosphonate (PCP) analog of InsP8; PCP analogs of other PP-InsP signaling molecules were ineffective. High-affinity binding of InsP8 to the XPR1 N-terminus (Kd = 180 nM) was demonstrated by isothermal titration calorimetry. To derive a cellular biology perspective, we studied biomineralization in the Soas-2 osteosarcoma cell line. KO of PPIP5Ks or XPR1 strongly reduced Pi efflux and accelerated differentiation to the mineralization end point. We propose that catalytically compromising PPIP5K mutations might extend an epistatic repertoire for XPR1 dysregulation, with pathological consequences for bone maintenance and ectopic calcification.
Inorganic phosphate (Pi) is an essential structural element of bones and teeth, and it is one of the most pervasive inhibitors of cellular enzymes (1, 2). Phosphate esters and anhydrides are also organic components of genomic material, they serve as an energy currency, and they are ubiquitous in cell signaling. Thus, it is not surprising that dysregulation of Pi homeostasis is acutely hazardous. For example, rickets and osteomalacia can emerge from a number of genetic disorders that promote hypophosphatemia in serum (1). Even when Pi levels in extracellular fluids are precisely maintained, dysregulation of Pi homeostasis can have pathological outcomes resulting, for example, in metabolic imbalance (1, 2). In addition, conditions that are permissive for excessive cellular accumulation of Pi are associated with ectopic calcification, most notably in the brain and cardiovascular system (3–6). Primary familial brain calcifications have been linked to loss-of-function variants of the human Xenotropic and Polytropic Retrovirus Receptor 1 (XPR1) (4, 6–9), the only human protein that has been characterized to transport Pi out of the cell (10). Such XPR1 malfunction manifests clinically as a heterogenous mix of psychiatric, cognitive, and movement disorders (4). In mouse models, conditional inactivation of Xpr1 in the renal tubule leads to hypophosphatemic rickets and the glycosuria, amino aciduria, and calciuria that typify human Fanconi syndrome (9); Xpr1-null mice exhibit embryonic lethality (9). These observations strongly imply that XPR1 activity in Pi homeostasis must normally be carefully regulated, but little is known about how this might be accomplished.
The inositol pyrophosphate (PP-InsP) signaling molecule, 5-diphosphoinositol 1,2,3,4,6-pentakisphosphate (5-InsP7) (Fig. 1A), has been the focus of previous studies into regulation of Pi transport by proteins related to XPR1 that are present in yeasts and trypanosomes (11–13). However, the PP-InsP family also includes 1,5-bisdiphosphoinositol 2,3,4,6-tetrakisphosphate (InsP8) (Fig. 1A), the molecule with the most dense three-dimensional array of phosphates found in nature (14). Yet, in animal cells, the biological actions of InsP8 are comparatively uncharacterized, with the consequence that little has been done to discriminate hierarchy of the actions of the different PP-InsPs in vivo. Clearly, identifying which member(s) of a signaling family regulates a particular biological process is fundamental to characterization of mechanisms and the design of therapeutic strategies. A major challenge to attributing functionality to one particular PP-InsP is that experimental manipulation of its synthesis inevitably alters levels of the others. This problem is well illustrated by the many published studies (ref. 15 describes biological effects of genetic and pharmacological manipulation of InsP6 kinase [IP6K]) (Fig. 1A); such approaches compromise synthesis of both InsP7 and InsP8 (Fig. 1A), either of which might be the active molecule. One especially pertinent study [published while this manuscript was under review (16)] described reduced XPR1-mediated Pi efflux from HCT116 cells following knockout (KO) of IP6K activity, but that study did not discern if it is InsP7 or InsP8 that regulates XPR1.
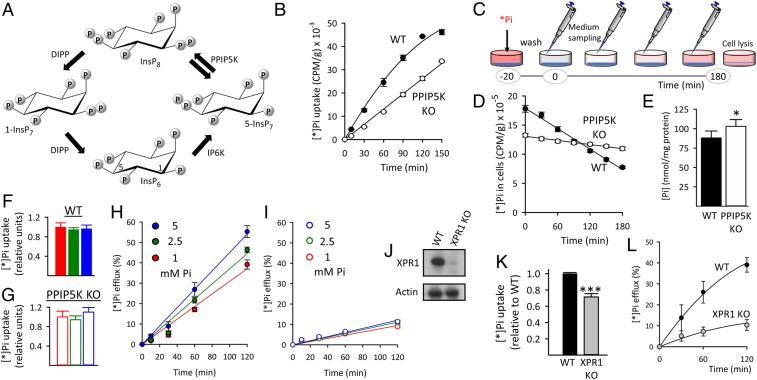
Fig. 1.
Reduced XPR1-mediated Pi efflux from HCT116 cells after either PPIP5K KO or XPR1 KO. (A) The proposed (17) cyclical pathway of PP-InsP turnover involving IP6K, PPIP5Ks (denoted by twin arrows because the enzyme has separate 1-kinase and 1-phosphatase activities), and diphosphoinositol polyphosphate phosphatases (DIPP). Carbons in InsP6 are labeled at positions 1 and 5. (B) Net [*]Pi uptake by WT and PPIP5K KO HCT116 cells. (C) Graphic depicting the timeline of the [*]Pi efflux assays. The [*]Pi efflux data are normalized (except in D) to total cell [*]Pi at zero time determined after cell lysis. (D) [*]Pi efflux from WT and PPIP5K KO HCT116 cells plotted as decreasing amounts of cell [*]Pi; CPM, counts per minute. (E) Total Pi in WT and PPIP5K HCT116 KO cells. (F and G) [*]Pi influx into WT and PPIP5K HCT116 KO cells, respectively, incubated with extracellular Pi at 1 mM (red), 2.5 mM (green), or 5 mM (blue). (H and I) [*]Pi influx into WT and PPIP5K HCT116 KO cells, respectively, incubated with extracellular Pi at 1 mM (red), 2.5 mM (green), or 5 mM (blue). (J) Western analysis of XPR1 expression in WT and XPR1 KO HCT116 cells. (K) WT and XPR1 KO HCT116 cells were labeled with [*]Pi for 20 min, and then, total [*]Pi was determined. (L) [*]Pi efflux from WT and XPR1 KO HCT116 cells prelabeled as in K. Statistical significance is indicated as follows. *P < 0.05; ***P < 0.001.
Here, we have determined the hierarchy of PP-InsP signaling in the regulation of XPR1-mediated cellular Pi efflux. We have used diphosphoinositol pentakisphosphate kinase (PPIP5K) KO cells, which cannot synthesize InsP8 (Fig. 1A); we now show that these cells exhibit greatly reduced Pi efflux. We also describe our rescue of Pi efflux by stable expression of PPIP5K1 activity in the KO background or by using liposomes to deliver into cells metabolically resistant analogs of PP-InsPs. We also created osteoblastic PPIP5K KO cells and found that dysregulation of Pi fluxes accelerates biomineralization. We propose that our work offers directions for studies into bone maintenance and the etiology of ectopic calcification.
Results and Discussion
PPIP5K KO Reduces the Rate of Cellular Pi Efflux.
InsP8 synthesis in mammalian cells is controlled by the competing 5-InsP7 1-kinase and InsP8 1-phosphatase activities of the type 1 and type 2 PPIP5Ks (Fig. 1A) (17). To investigate if InsP8 regulates Pi homeostasis, we first compared cellular Pi fluxes in wild-type (WT) and our previously described PPIP5K KO HCT116 human colonic epithelial cells (17). We found that PPIP5K KO cells accumulate extracellular radiolabeled inorganic phosphate (i.e., [*]Pi) at a 25% slower rate than do corresponding WT cells (Fig. 1B). Next, efflux of [*]Pi was determined after 20 min of [*]Pi labeling (Fig. 1C) as in previous Pi flux studies (10, 16). We found that the rate of [*]Pi efflux from the PPIP5K KO cells is 80% slower than that from WT cells (Fig. 1D); hereafter, [*]Pi efflux data are normalized to total [*]Pi accumulated during the prelabeling. Qualitatively similar effects of the PPIP5K KO on [*]Pi uptake and efflux were observed with HEK293 cells, although a larger proportion of [*]Pi efflux (60%) is insensitive to the PPIP5K KO (SI Appendix, Fig. S1). Total cell [Pi] is elevated in PPIP5K KO HCT116 cells (Fig. 1E), confirming that Pi homeostasis is compromised. A technique to specifically assay cytoplasmic [Pi] is not available, but in any case, excess Pi may also be accumulated into other molecules, such as adenosine triphosphate (ATP). Indeed, ATP levels are higher in both HCT116 and HEK293 PPIP5K KO cells compared with the corresponding WT cells (17).
[*]Pi uptake was not affected by increasing total [Pi] from 1 to 5 mM (Fig. 1 F and G). An earlier study noted that the rate of [*]Pi efflux from HEK293 cells rises ∼50% when extracellular [Pi] is increased from 1 to 10 mM (10). We observed a similar phenomenon: a 70% increase in [*]Pi efflux when extracellular [Pi] is increased from 1 to just 5 mM (SI Appendix, Fig. S2A). This effect is PPIP5K dependent (SI Appendix, Fig. S2). Similar data were obtained when comparing the effects of [Pi] on [*Pi] fluxes in WT and PPIP5K KO HCT116 cells (Fig. 1 H and I).
The knockdown of XPR1 by RNA interference (RNAi) has shown that it promotes Pi efflux (10, 16). In the current work, we utilized a more efficient strategy: knockout of XPR1 in HCT116 cells using CRISPR-Cas9 (Fig. 1J). The XPR1 KO cells exhibited a 30% slower initial rate of [*]Pi uptake (Fig. 1K), which was unexpected, as a previous study did not identify any effect of XPR1 on Pi uptake (6). Significantly, the rate of Pi efflux in XPR1 KO cells was reduced by 75% compared with WT cells (Fig. 1L). In control experiments, we found that PPIP5K knockout did not alter the degree of XPR1 expression (SI Appendix, Fig. S3). Thus, the XPR1 KO (Fig. 1L) phenocopies the reduced [*]Pi efflux from PPIP5K KO cells (Fig. 1 D, H, and I). Our data also reveal that both HEK293 and HCT116 cells possess a second pathway for cellular Pi efflux that is independent of both XPR1 (Fig. 1L) and PPIP5Ks (Fig. 1D and SI Appendix, Fig. S2B).
PPIP5K-Dependent Effects on Pi Efflux Are Mediated by InsP8.
We next used several complementary experimental approaches to determine if the regulation of cellular Pi fluxes by PPIP5Ks involves either catalytic or scaffolding mechanisms. We first found that both [*]Pi uptake and [*Pi] efflux in PPIP5K KO HCT116 cells were partly rescued by stable transfection of WT PPIP5K1 [which restores InsP8 levels (17)] and more so when InsP8 levels were further elevated by transfection of the phosphatase-dead PPIP5K1R399A mutant (Fig. 2 A–C) (17). Significantly, there was no rescue of [*]Pi flux in PPIP5K KO cells stably transfected with the kinase-dead PPIP5K1D332A mutant of PPIP5K1, which does not rescue InsP8 synthesis (Fig. 2 A–C) (17). Thus, we conclude that the reduction in Pi efflux from PPIP5K KO cells is a consequence of loss of the enzyme’s catalytic activity: either the elimination of InsP8 (in which case Pi efflux must require InsP8) or the accompanying two- to threefold accumulation of 5-InsP7 (17, 18) (in which case elevated 5-InsP7 must inhibit Pi efflux). To distinguish between these two possibilities, we used our previously described pharmacological approach (17). We treated WT and PPIP5K KO HCT116 cells with 10 µM IP6K inhibitor N2-(m-trifluorobenzyl), N6-(p-nitrobenzyl) purine (TNP) (Fig. 2A), which reduces levels of both InsP7 (17) and InsP8 (Fig. 2D). In WT cells, TNP treatment did not alter Pi uptake, but it inhibited [*]Pi efflux by ∼40% (Fig. 2 E and F); the latter value is obtained by subtracting the contribution from the minor PPIP5K-independent Pi efflux pathway (depicted by the open circles in Fig. 2C). In PPIP5K KO cells, the TNP treatment had no effect on [*]Pi fluxes (Fig. 2 E and G). Such data indicate that the elevated [5-InsP7] in PPIP5K KO cells (17) does not inhibit Pi efflux. Overall, our demonstration that TNP inhibits [*]Pi efflux from WT cells without affecting [*Pi] efflux from the corresponding PPIP5K KO cells is entirely consistent with Pi efflux being regulated by InsP8 and not by 5-InsP7. Similar results were obtained following TNP treatment of HEK293 cells (SI Appendix, Fig. S1).
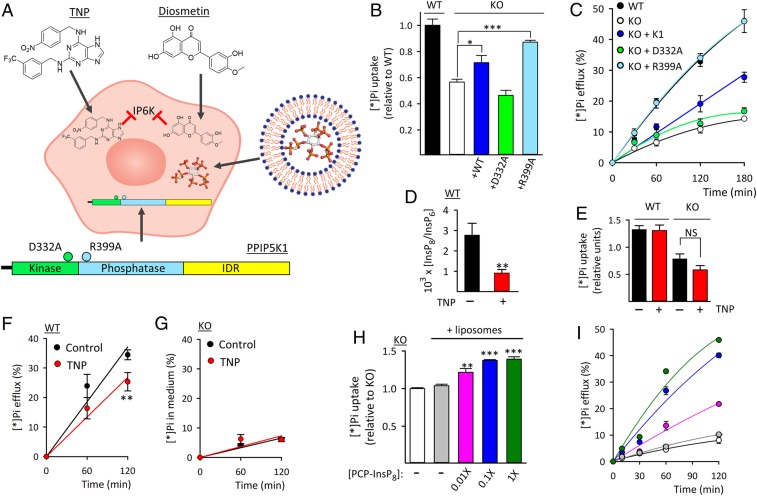
Fig. 2.
Rescue of Pi efflux from PPIP5K KO HCT116 cells. (A) Graphic depicting the various procedures used to rescue Pi efflux from PPIP5K KO cells: inhibition of IP6K by either TNP or flavonoids; liposomal delivery of PCP analogs of PP-InsPs; and stable transfection of WT PPIP5K1 (InsP7 kinase/InsP8 phosphatase plus an intrinsically disordered region [IDR]), the D332A mutant (no kinase activity), or the “hyperkinase” R399A mutant (no phosphatase activity). (B) Total [*]Pi uptake after 20 min of labeling of WT cells, PPIP5K KO cells, and the KO cells stably transfected with either PPIP5K1 or one of the indicated mutants. (C) [*]Pi efflux from cells prelabeled with [*]Pi as described in B. (D) InsP8 levels in WT cells plus or minus 24 h of treatment with 10 µM TNP. (E) Following TNP treatment (as in D), WT and PPIP5K KO cells were incubated with [*]Pi for 20 min, and then, total [*]Pi was determined. (F) Cells were first treated with TNP for 24 h and then, prelabeled with [*]Pi. (G) Cells were treated with TNP and prelabeled with [*]Pi as described in F, and then [*]Pi efflux was determined. (H) PPIP5K KO cells were incubated for 3 h with either empty liposomes or liposomes containing PCP-InsP8 at the indicated dilution. Then, [*]Pi was added for 20 min, and total [*]Pi was determined. (I) Pi efflux from PPIP5K KO cells treated with liposomes (plus or minus PCP-InsP8) and prelabeled with [*]Pi, all as described in H. Statistical significance is indicated as follows. *P < 0.05; **P < 0.02; ***P < 0.001; NS, not significant.
For a complementary pharmacological approach, we treated HCT116 cells with 2.5 µM one of three dietary flavonoids (diosmetin, quercetin, and luteolin) (SI Appendix, Fig. S4 A–E) that we (19) previously identified as IP6K inhibitors. In these experiments, there were reductions in cellular levels of both 5-InsP7 (19), and InsP8 (SI Appendix, Fig. S4F); [*]Pi influx trended lower in the treated cells but was not statistically significant. More importantly, inhibition of IP6K by flavonoids reduced [*]Pi efflux from WT cells and did not significantly alter [*]Pi efflux from PPIP5K KO (i.e., InsP8-null) cells (SI Appendix, Fig. S4 B–E). Again, we conclude that Pi efflux is regulated by InsP8 and not by 5-InsP7.
The PPIP5K KO cells have elevated levels of ATP (17); we used antimycin plus rotenone to inhibit mitochondrial energy production (17), and ATP levels fell in both WT and PPIP5K KO cells, but there was no effect on [*]Pi fluxes (SI Appendix, Fig. S5). Thus, we conclude that the hypermetabolic state of the PPIP5K KO cells does not affect Pi fluxes.
Assays of Cellular Pi Efflux Following Loading of Metabolically Resistant PP-InsPs into HCT116 Cells.
We next directly investigated the effects of individual PP-InsPs on Pi efflux. Previously, we used liposomes to deliver ATP into HCT116 cells (17). We now used this methodology to load methylene bisphosphonate (PCP) analogs of PP-InsPs (20–22) into PPIP5K KO cells (Fig. 2A); unlike the natural pyrophosphates, the PCP bonds are metabolically resistant. Delivery efficiency was determined by loading “1×” PCP-InsP8 (Materials and Methods), following which cell extracts were prepared and analyzed by polyacrylamide gel electrophoresis (23) (SI Appendix, Fig. S6A). This analysis cannot determine what proportion of the internalized PCP-InsP8 is within the cytoplasm, but under our 1× loading conditions, its nominal average concentration is approximately equivalent to that of InsP6 (SI Appendix, Fig. S6A) (i.e., 29 µM) based on NMR assays of our WT HCT116 cells (18). Liposomal delivery into PPIP5K KO cells of a 0.1× dose of PCP-InsP8 (nominally 2.9 µM) was sufficient to rescue both [*]Pi uptake and [*]Pi efflux; even a 10-fold lower dose was functional (Fig. 2 H and I). Neither 1-PCP-InsP7 nor 5-PCP-InsP7 rescued [*]Pi fluxes (SI Appendix, Fig. S6 B and C). These data are strong testimony to the signaling specificity of InsP8 in vivo, and the rescue of [*]Pi efflux by PCP-InsP8 reveals that protein pyrophosphorylation (24) is not involved.
InsP8 Is a High-Affinity Ligand for the SYG1/Pho81/XPR1 (SPX) Domain of XPR1.
Interactions of PP-InsPs with electropositive SPX domains are thought to allosterically regulate protein function; XPR1 has an N-terminal SPX domain (11). A previous study (16) used a polyethylene glycol pulldown/ligand competition assay to show that the XPR1-SPX domain binds 5-InsP7 (Kd = 4.2 µM); InsP8 affinity was not determined. We examined the binding of chemically synthesized PP-InsPs (25–27) to the XPR1-SPX domain using the more direct binding methodology of isothermal titration calorimetry (Fig. 3 A–C). We found InsP8 to be a higher-affinity ligand (Kd = 0.18 µM) than 5-InsP7 (Kd = 0.34 µM) and 1-InsP7 (Kd = 0.28 µM). While averaged cellular levels of 5-InsP7 are 5- to 10-fold higher than those of InsP8 (14, 15), the specificity with which InsP8 regulates Pi fluxes in intact cells (see above) could also be reinforced by interactions of the InsP8/SPX domain complexes with additional proteins (11, 16). Furthermore, InsP8 concentration may be locally elevated through compartmentalization of its synthesis (14, 16) and by electrostatic interactions that likely restrict its rate of diffusion (14).
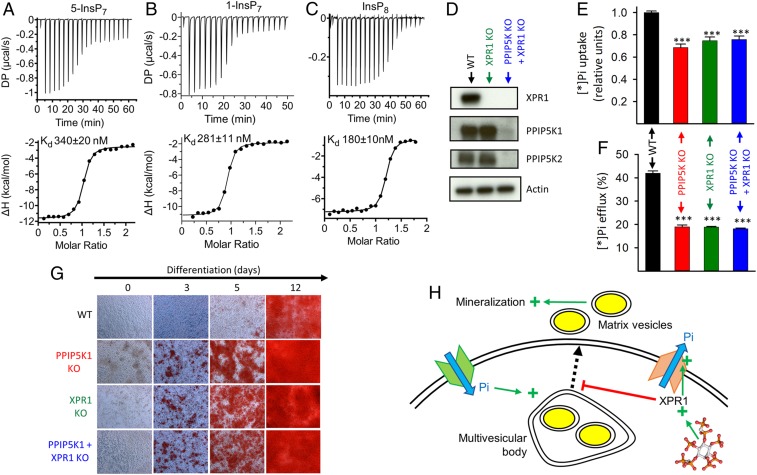
Fig. 3.
A regulatory interaction of InsP8 with XPR1 contributes to the effects of PPIP5K KO on Pi fluxes and biomineralization by Saos-2 cells. (A–C) Representative (of three to four) thermograms for titration of the XPR1-SPX domain with (A) 5-InsP7 (stoichiometry = 1.11 ± 0.05), (B) 1-InsP7 (stoichiometry = 0.88 ± 0.01), or (C) InsP8 (stoichiometry = 1.07 ± 0.05). (D) Western analysis of WT, XPR1 KO, and XPR1 KO + PPIP5K KO Saos-2 cells. (E) Saos-2 cells were incubated with [*]Pi for 20 min, and then, net [*Pi] uptake was determined. (F) [*]Pi efflux from WT and PPIP5K KO Saos-2 cells prelabeled as in E. (G) Alizarin Red S staining is shown for WT, PPIP5K KO cells, XPR1 KO cells, or PPIP5K KO + XPR1 KO cells cultured in osteoblastic medium for the indicated times. The area covered by each individual field of view is 0.15 mm2. (H) Graphic describing InsP8-mediated regulation of XPR1-dependent Pi efflux in the context of XPR1 restraining the availability of cellular Pi for stimulation of Pi-dependent matrix vesicle secretion for biomineralization as previously reported (28). Statistical significance is indicated as follows. ***P < 0.001.
PPIP5K KO Promotes Mineralization by Saos-2 Cells.
Biomineralization is one of several biological activities that require careful regulation of Pi homeostasis (28). We investigated if biomineralization is affected by the PPIP5K KO. We used CRISPR-Cas9 to knockout both PPIP5Ks in the Saos-2 osteosarcoma line (Fig. 3D and SI Appendix, Fig. S7A), an osteoblastic cell model (29, 30). The PPIP5K KO cells showed reductions in both [*]Pi influx and efflux (Fig. 3 E and F), mirroring the effects of the PPIP5K KO on HCT116 and HEK293 cells (see above). This Pi flux phenotype of PPIP5K KO Saos-2 cells was quantitatively mimicked by the XPR1 KO and by the PPIP5K + XPR1 KO (Fig. 3 E and F); these data are consistent with our hypothesis that PPIP5K-produced InsP8 is required for XPR1-mediated Pi efflux.
Two osteoblastic marker genes, osteocalcin and osteonectin (30), are more highly expressed in the PPIP5K KO Saos-2 cells than in WT cells, particularly after we induced differentiation (31) into an osteoblastic phenotype (SI Appendix, Fig. S7 B and C); a separate osteogenic marker, alkaline phosphatase (ALP), was also elevated in the PPIP5K KO cells (SI Appendix, Fig. S7D). Mineralization is considered the end point of this Saos-2 differentiation phenotype (32). This terminal stage, assayed by Alizarin red S staining (32), was attained in an accelerated manner in the PPIP5K KO cells (Fig. 3G). This faster timeline to mineralization is phenocopied by the XPR1 KO; the effects of the XPR1 KO and PPIP5K KO are not additive (Fig. 3G). Finally, we created further Saos-2 lines in which PPIP5K1 constructs were stably expressed in the PPIP5K KO background (SI Appendix, Fig. S8A). We found that transfection of either WT PPIP5K1 or the phosphatase-dead PPIP5K1R399A mutant rescued [*]Pi fluxes and restored the slower mineralization timeline to that of WT cells (SI Appendix, Fig. S8 B–D). The kinase-dead mutant, PPIP5KD332A, had no effect (SI Appendix, Fig. S8 B–D), thereby demonstrating a requirement for PPIP5K1 kinase activity. These experiments reinforce our conclusion that Pi homeostasis relies on InsP8 to regulate XPR1 and further indicate that dysregulation of this process may contribute to disorders of mineralization (Fig. 3H).
Concluding Comments.
Our study breaks ground by describing regulation of Pi efflux by human XPR1 through hierarchy in signaling by the PP-InsP family: InsP8 is functionally dominant over both 5-InsP7 and 1-InsP7. Three experimental approaches were of particular value for reaching this conclusion. First, we used CRISPR-Cas9 to create multiple PPIP5K KO and XPR1 KO cell lines; in particular, our demonstration that InsP8 has unique signaling functionality arises out of our interrogating PP-InsP function by intervening in PPIP5K activity. The targeting of IP6K is a less specific experimental approach as it inhibits synthesis of both 5-InsP7 and InsP8 (Fig. 1A), which each have different regulatory roles. Second, we used complementary genetic and pharmacological tools to help distinguish functionality of InsP8 from that of InsP7. Third, we used liposomes to deliver metabolically resistant PCP analogs of PP-InsPs directly into cells. Although our study primarily focuses on regulation of cellular Pi efflux, we also found that the rate of Pi uptake is reduced by 30% in XPR1 KO cells (Fig. 1K); these data imply that XPR1 also has some previously unsuspected influence over Pi uptake.
In most prior studies into PP-InsP regulation of Pi homeostasis, InsP7 has been the focus of attention. For example, 1-InsP7 was proposed to mediate adaptations to Pi starvation in Saccharomyces cerevisiae (33, 34); 5-InsP7 is the dominant activator of inorganic polyphosphate synthesis by the vacuolar transporter chaperone in S. cerevisiae (11, 13), and 5-InsP7 stimulates Na+/Pi cotransport by TbPho91 in Trypanosoma brucei (12). However, very recent work with plants has drawn attention to InsP8 as a regulator of Pi homeostasis (35, 36); this signaling process centers on InsP8 functionalizing a standalone SPX domain to inhibit a transcriptional activator (PHR1) of genes induced by phosphate starvation (35). Our study describes a very different aspect of Pi homeostasis in animals that is regulated by InsP8. Our data also offer a functional context for previously puzzling, stimulus-dependent decreases in cellular InsP8 levels when cells undergo relatively mild bioenergetic stress (37). We now show that loss of InsP8 in PPIP5K KO cells inhibits Pi efflux (Fig. 1D) such that Pi availability is increased (Fig. 1E), likely supporting a compensatory up-regulation of ATP synthesis. Indeed, a drop in [InsP8] is associated with elevated [ATP] (17). Such conclusions raise the profile of InsP8 as a second messenger acting at the interface of signaling and metabolism.
Our study has human health relevance. The concentrations of dietary flavonoids used in our experiments (2.5 µM) are equivalent to those attained in the human gut (38, 39), and therefore, their actions on Pi fluxes in HCT116 cells (SI Appendix, Fig. S4) could have biological relevance to Pi homeostasis in the gastrointestinal tract. Furthermore, flavonoids enhance mineralization by Saos-2 cells and therefore, have been advocated as being of potential therapeutic value for treating osteoporosis (40). The latter observation dovetails with our own data, which show that bone cell mineralization is enhanced on loss of InsP8 synthesis (Fig. 3) due to inhibition of IP6Ks by flavonoids (SI Appendix, Fig. S4). Nevertheless, we identify PPIP5Ks as separate bona fide therapeutic targets because these enzymes more directly control InsP8 synthesis (Fig. 1A). Our data further suggest that enzymatically compromising missense mutations in PPIP5Ks might expand the repertoire of genetic factors that underlie the pathology of both defective bone maintenance and ectopic mineralization.
Materials and Methods
Cells and Cell Culture.
The origins of the WT and PPIP5K KO lines of HCT116 and HEK293 cells are as previously described (17). The Saos-2 cells were purchased from ATCC, and the PPIP5K KO lines were derived exactly as previously described for HCT116 cells (17). The XPR1 KO lines of HCT116 cells were generated using a guide messenger RNA that targets exon 1 of XPR1 (5′-GCCGTGCCGGTACCTCATAC-3′). All KO lines were verified by genomic sequencing. All data from each KO line have been recapitulated with a second independent clone. HEK293 and Saos-2 were cultured in Dulbecco’s modified Eagle medium (DMEM), and HCT116 cells were cultured in DMEM/F12. During propagation, all cell cultures were supplemented with 10% fetal bovine serum (FBS) (Gemini Bio-product) and 100 U/mL Penicillin-Streptomycin (ThermoFisher Scientific) at 37 °C with 5% CO2. Osteoblastic differentiation of Saos-2 cells seeded at 1 × 105 per well in 12-well plates was induced as described previously (31). In some experiments, cells were radiolabeled with [3H]inositol to determine intracellular levels of InsP6 and the PP-InsPs as previously described (17).
Phosphate Uptake and Efflux Assays.
Monolayer cultures were seeded at 5 × 105 cells per well in 1.5 mL medium in six-well plates; Pi transport assays were performed 24 h later at 37 °C. For Pi uptake assays, a previously published protocol was used (10) (i.e., the culture medium was replaced with phosphate-free DMEM [Gibco catalog number: 11971–025] plus 10% FBS). In early experiments, we labeled cells with 0.5 μCi/mL [32P]-Pi (Perkin-Elmer; NEZ080001MC), but for health and safety reasons, we subsequently switched to 0.5 μCi/mL [33P]-Pi (ARC; ARP 0153) without any impact on the data obtained. Thus, radiolabeled Pi is indicated as [*]Pi throughout this study. To assay [*]Pi uptake, cells were separated from medium by three rapid washes in 1 mL ice-cold inorganic phosphate-buffered saline (PBS). Cells were then lysed in 1 mL PBS plus 1% Triton X-100, and the accumulated [*]Pi was determined using a liquid scintillation counter. Uptake was normalized to cell protein as determined using a BCA protein assay (Pierce). For [*]Pi efflux assays, cells were loaded with [*]Pi for 20 min; then, cells were washed three times with 1 mL Pi-free DMEM and incubated with 1 mL DMEM supplemented with Pi (as an NaH2PO4/Na2HPO4 mixture, pH 7.4; 1 mM unless otherwise indicated). Aliquots (10% [vol/vol]) of medium were taken at the indicated times, and [*]Pi therein was determined. Experiments were terminated by cell lysis (see above), and residual cell-associated [*]Pi was determined; [*]Pi efflux is depicted as a percentage of total cellular [*]Pi at zero time.
Liposomal Delivery of PP-InsP Analogs into Cells.
Liposomes were prepared as a lipid film inside a round-bottom glass flask (17). As needed, the lipid film was hydrated (with vortexing) in 3.5 mL of 5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.2 with NaOH) with 1-PCP-InsP7, 5-PCP-InsP7, or 1,5-PCP-InsP8 [all chemically synthesized (20–22)], which were added at various concentrations, “n×,” where n = 1 (i.e., 1×) describes a ratio of 0.1 µmol PCP analog per 1 mg lipid. Empty liposomes were also prepared. The liposomal dispersion was subjected to five cycles of freezing at −80 °C for 30 min and thawing at 45 °C for 5 min. Liposomes were then sequentially extruded through two membrane filters, with pore sizes of 0.45 μm and then, 0.2 μm. The liposomes were stored in aliquots at 4 °C for up to 2 wk. For cell treatment, 1 μL liposomes were added to 1 mL culture medium (Pi-free DMEM plus 10% FBS) for 3 h. To assay the efficiency of delivery by gel electrophoresis (23), liposomes were added to two 150-mm plates, each containing ∼2 × 107 cells in 20 mL medium.
Isothermal Titration Calorimetry.
The SPX domain of human XPR1 (residues 1 to 219) was subcloned into pDest-566 vector and expressed in DE3 competent Escherichia coli (Stratagene) transformed with the chaperone plasmid pGro7 (Takara Clontech). The 6xHis tag was exploited for purification and then, removed along with the maltose binding protein tag by tobacco etch virus-protease cleavage. The protein was further purified using a HiTrap Heparin affinity column and then, size exclusion chromatography (Superdex 200 gel filtration column; GE Healthcare) using 500 mM NaCl and 20 mM Tris⋅HCl, pH 7.2. Purified protein was concentrated to 15 to 30 mg/mL and stored in aliquots at −80 °C. Calorimetry experiments were performed using a Microcal PEAQ-ITC (Malvern Panalytical) with 25 to 60 µM recombinant Xpr1 and 350 to 500 µM of each PP-InsP at 25 °C in buffer containing 20 mM Tris⋅HCl, pH 7.2, 150 mM KCl, and 0.8 mM MgCl2. Data were fitted to a single-binding site model.
Alizarin Red S Staining.
Approximately 1 × 105 cells per well were seeded in 24-well plates and cultured in DMEM plus 10% FBS for a “wait time” of 2 d prior to initiation of differentiation by a medium change to fresh DMEM/10% FBS plus 50 μg/mL ascorbic acid, 5 mM Pi, 5 mM β-glycerol phosphate, and 1 μM dexamethasone. Cells were then cultured for a further 7 d, with medium changes every 2 to 3 d. Alizarin Red S staining was then performed (32) and imaged using a phase contrast microscope. For studies into the time course of differentiation, the total number of days of culture was made identical for each time point by extending the wait time prior to addition of differentiation medium.
Other Assays and Reagents.
Total cellular Pi was determined as previously described (41). Alkaline phosphatase (ALP) activity was measured using a kit that was purchased from AnaSpec (catalog no. AS-72146); according to the vendor, 1 unit of activity is nominally 1 ng ALP. For western analyses, the sources of the antibodies are as follows: PPIP5K1, Sigma, SAB1401487; PPIP5K2, Abcam, ab154046; β-Actin (C4), Santa Cruz Biotechnology, sc-47778; and XPR1, Proteintech, 14174–1-AP. We added 10 µM TNP (Sigma) or flavonoids to cells in dimethyl sulfoxide (0.5% final concentration) as previously described (17, 19).
Data Availability.
All data, associated protocols, methods, and sources of materials can be accessed in the text or SI Appendix.
Statistics.
Each experiment was performed at least three times. All data are presented as means and SEs. Following Student’s t test, P values of less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences; the German Research Foundation under Germany’s Excellence Strategy CIBSS–EXC-2189 Project 390939984; and Swiss National Foundation Sinergia Grant CRSII5_170925 (to S.H.). We thank Dr. Zhenzhen Wang for assistance with liposome delivery. We also acknowledge helpful discussions with Drs. Vincent Tagliabracci and Anju Sreelatha.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1908830117/-/DCSupplemental.
References
- 1.Chande S., Bergwitz C., Role of phosphate sensing in bone and mineral metabolism. Nat. Rev. Endocrinol. 14, 637–655 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam M. T., et al. , The self-inhibitory nature of metabolic networks and its alleviation through compartmentalization. Nat. Commun. 8, 16018 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quintáns B., Oliveira J., Sobrido M. J., Primary familial brain calcifications. Handb. Clin. Neurol. 147, 307–317 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Legati A., et al. , Mutations in XPR1 cause primary familial brain calcification associated with altered phosphate export. Nat. Genet. 47, 579–581 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hortells L., Sur S., St Hilaire C., Cell phenotype transitions in cardiovascular calcification. Front. Cardiovasc. Med. 5, 27 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.López-Sánchez U., et al. , Characterization of XPR1/SLC53A1 variants located outside of the SPX domain in patients with primary familial brain calcification. Sci. Rep. 9, 6776 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo X. X., et al. , Spectrum of SLC20A2, PDGFRB, PDGFB, and XPR1 mutations in a large cohort of patients with primary familial brain calcification. Hum. Mutat. 40, 392–403 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Yao X. P., et al. , Analysis of gene expression and functional characterization of XPR1: A pathogenic gene for primary familial brain calcification. Cell Tissue Res. 370, 267–273 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Ansermet C., et al. , Renal fanconi syndrome and hypophosphatemic rickets in the absence of xenotropic and polytropic retroviral receptor in the nephron. J. Am. Soc. Nephrol. 28, 1073–1078 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giovannini D., Touhami J., Charnet P., Sitbon M., Battini J. L., Inorganic phosphate export by the retrovirus receptor XPR1 in metazoans. Cell Rep. 3, 1866–1873 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Wild R., et al. , Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science 352, 986–990 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Potapenko E., et al. , 5-Diphosphoinositol pentakisphosphate (5-IP7) regulates phosphate release from acidocalcisomes and yeast vacuoles. J. Biol. Chem. 293, 19101–19112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerasimaite R., et al. , Inositol pyrophosphate specificity of the SPX-dependent polyphosphate polymerase VTC. ACS Chem. Biol. 12, 648–653 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Shears S. B., Intimate connections: Inositol pyrophosphates at the interface of metabolic regulation and cell signaling. J. Cell. Physiol. 233, 1897–1912 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakraborty A., The inositol pyrophosphate pathway in health and diseases. Biol. Rev. Camb. Philos. Soc. 93, 1203–1227 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson M. S., Jessen H. J., Saiardi A., The inositol hexakisphosphate kinases IP6K1 and -2 regulate human cellular phosphate homeostasis, including XPR1-mediated phosphate export. J. Biol. Chem. 294, 11597–11608 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu C., et al. , KO of 5-InsP7 kinase activity transforms the HCT116 colon cancer cell line into a hypermetabolic, growth-inhibited phenotype. Proc. Natl. Acad. Sci. U.S.A. 114, 11968–11973 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harmel R. K., et al. , Harnessing 13C-labeled myo-inositol to interrogate inositol phosphate messengers by NMR. Chem. Sci. (Camb.) 10, 5267–5274 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu C., et al. , Inhibition of inositol polyphosphate kinases by quercetin and related flavonoids: A structure-activity analysis. J. Med. Chem. 62, 1443–1454 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hager A., et al. , Cellular cations control conformational switching of inositol pyrophosphate analogues. Chemistry 22, 12406–12414 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu M., Dul B. E., Trevisan A. J., Fiedler D., Synthesis and characterization of non-hydrolysable diphosphoinositol polyphosphate second messengers. Chem. Sci. (Camb.) 4, 405–410 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu M., et al. , Elucidating diphosphoinositol polyphosphate function with nonhydrolyzable analogues. Angew. Chem. Int. Ed. Engl. 53, 7192–7197 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Wilson M. S., Bulley S. J., Pisani F., Irvine R. F., Saiardi A., A novel method for the purification of inositol phosphates from biological samples reveals that no phytate is present in human plasma or urine. Open Biol. 5, 150014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhandari R., et al. , Protein pyrophosphorylation by inositol pyrophosphates is a posttranslational event. Proc. Natl. Acad. Sci. U.S.A. 104, 15305–15310 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capolicchio S., Wang H., Thakor D. T., Shears S. B., Jessen H. J., Synthesis of densely phosphorylated bis-1,5-diphospho-myo-inositol tetrakisphosphate and its enantiomer by bidirectional P-anhydride formation. Angew. Chem. Int. Ed. Engl. 53, 9508–9511 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavlovic I., et al. , Cellular delivery and photochemical release of a caged inositol-pyrophosphate induces PH-domain translocation in cellulo. Nat. Commun. 7, 10622 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capolicchio S., Thakor D. T., Linden A., Jessen H. J., Synthesis of unsymmetric diphospho-inositol polyphosphates. Angew. Chem. Int. Ed. Engl. 52, 6912–6916 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Chaudhary S. C., et al. , Phosphate induces formation of matrix vesicles during odontoblast-initiated mineralization in vitro. Matrix Biol. 52–54, 284–300 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prideaux M., et al. , SaOS2 Osteosarcoma cells as an in vitro model for studying the transition of human osteoblasts to osteocytes. Calcif. Tissue Int. 95, 183–193 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Li W., Deng Y., Feng B., Mak K. K., Mst1/2 kinases modulate glucose uptake for osteoblast differentiation and bone formation. J. Bone Miner. Res. 33, 1183–1195 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Wiens M., et al. , Osteogenic potential of biosilica on human osteoblast-like (SaOS-2) cells. Calcif. Tissue Int. 87, 513–524 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Hoemann C. D., El-Gabalawy H., McKee M. D., In vitro osteogenesis assays: Influence of the primary cell source on alkaline phosphatase activity and mineralization. Pathol. Biol. (Paris) 57, 318–323 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Lee Y. S., Mulugu S., York J. D., O’Shea E. K., Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates. Science 316, 109–112 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulugu S., et al. , A conserved family of enzymes that phosphorylate inositol hexakisphosphate. Science 316, 106–109 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Dong J., et al. , Inositol pyrophosphate InsP8 acts as an intracellular phosphate signal in Arabidopsis. Mol. Plant 12, 1463–1473 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Zhu J., et al. , Two bifunctional inositol pyrophosphate kinases/phosphatases control plant phosphate homeostasis. eLife 8, e43582 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi K., Mollapour E., Choi J. H., Shears S. B., Cellular energetic status supervises the synthesis of bis-diphosphoinositol tetrakisphosphate independently of AMP-activated protein kinase. Mol. Pharmacol. 74, 527–536 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Z., Zheng S., Li L., Jiang H., Metabolism of flavonoids in human: A comprehensive review. Curr. Drug Metab. 15, 48–61 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Jenner A. M., Rafter J., Halliwell B., Human fecal water content of phenolics: The extent of colonic exposure to aromatic compounds. Free Radic. Biol. Med. 38, 763–772 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Wang X., et al. , Isoquercitrin and polyphosphate co-enhance mineralization of human osteoblast-like SaOS-2 cells via separate activation of two RUNX2 cofactors AFT6 and Ets1. Biochem. Pharmacol. 89, 413–421 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Gu C., et al. , The significance of the bifunctional kinase/phosphatase activities of PPIP5Ks for coupling inositol pyrophosphate cell-signaling to cellular phosphate homeostasis. J. Biol. Chem. 292, 4544–4555 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data, associated protocols, methods, and sources of materials can be accessed in the text or SI Appendix.