Significance
Indoleamine 2,3-dioxygenase 1 (IDO1) is an immunoregulatory enzyme that transforms tryptophan into kynurenine, an endogenous agonist of the aryl hydrocarbon receptor (AhR) whose activation sustains IDO1 expression and activity over the long term in dendritic cells (DCs). Here we found that N-acetylserotonin (NAS), a tryptophan metabolite produced along the serotonin pathway, acts as a positive allosteric modulator (PAM) of IDO1 and thus increases kynurenine-mediated AhR activation in DCs. NAS effects on IDO1 translated into protection of mice from neuroinflammation and reinstallment of physiologic IDO1 activity in peripheral blood mononuclear cells from patients with relapsing-remitting multiple sclerosis.
Keywords: N-acetylserotonin (NAS); indoleamine 2,3-dioxygenase 1 (IDO1); aryl hydrocarbon receptor (AhR); neuroinflammation; dendritic cells
Abstract
l-tryptophan (Trp), an essential amino acid for mammals, is the precursor of a wide array of immunomodulatory metabolites produced by the kynurenine and serotonin pathways. The kynurenine pathway is a paramount source of several immunoregulatory metabolites, including l-kynurenine (Kyn), the main product of indoleamine 2,3-dioxygenase 1 (IDO1) that catalyzes the rate-limiting step of the pathway. In the serotonin pathway, the metabolite N-acetylserotonin (NAS) has been shown to possess antioxidant, antiinflammatory, and neuroprotective properties in experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS). However, little is known about the exact mode of action of the serotonin metabolite and the possible interplay between the 2 Trp metabolic pathways. Prompted by the discovery that NAS neuroprotective effects in EAE are abrogated in mice lacking IDO1 expression, we investigated the NAS mode of action in neuroinflammation. We found that NAS directly binds IDO1 and acts as a positive allosteric modulator (PAM) of the IDO1 enzyme in vitro and in vivo. As a result, increased Kyn will activate the ligand-activated transcription factor aryl hydrocarbon receptor and, consequently, antiinflammatory and immunoregulatory effects. Because NAS also increased IDO1 activity in peripheral blood mononuclear cells of a significant proportion of MS patients, our data may set the basis for the development of IDO1 PAMs as first-in-class drugs in autoimmune/neuroinflammatory diseases.
The metabolism of l-tryptophan (Trp) has evolved to be a primary regulatory node in the control of immune responses (1–3). Trp, an essential amino acid for mammals, is a substrate for indoleamine 2,3-dioxygenase 1 (IDO1), which catalyzes the first, rate-limiting step in the kynurenine pathway (SI Appendix, Fig. S1), leading to Trp depletion and the production of a series of immunoregulatory molecules collectively known as kynurenines (4–7). Both effects—namely Trp starvation and kynurenine production—are involved in the conversion of naïve CD4+ T cells into Foxp3+ regulatory T cells (5). Moreover, the main IDO1 catalytic product, l-kynurenine (Kyn), has immunoregulatory effects in the absence of Trp starvation, via activation of the aryl hydrocarbon receptor (AhR) (8–11). Highest IDO1 expression and catalytic activity occur in dendritic cells (DCs) (4, 12), the most potent antigen-presenting cells that, upon IDO1 up-regulation, acquire tolerogenic functions (13).
IDO1 represents an important drug target in neoplasia, where it is often overexpressed (8, 14, 15), and autoimmune/neuroinflammatory diseases, in which the enzyme has been found defective (16–19) and/or necessary to contain the pathology (20). Whereas IDO1 catalytic inhibitors are studied in clinical trials as antitumor drugs (21), enhancers of IDO1 enzymatic activity—to be used as therapeutic agents in autoimmunity and neuroinflammation—have not been developed so far.
N-acetylserotonin (NAS) is a Trp metabolite generated from serotonin along the serotonin pathway (SI Appendix, Fig. S1). NAS is endowed with several potential therapeutic effects, easy crossing of the blood–brain barrier, and no appreciable toxicity. For this reason, this natural Trp metabolite has been included in a library of 1,040 approved compounds by the Food and Drug Administration and selected by the National Institute of Neurological Disorders and Stroke (NIH) for further studies (22, 23). Previously considered to be simply an intermediate metabolite between the neurotransmitter serotonin and the hormone/chronobiotic melatonin, NAS exerts significant antidepressant (24), antiischemic (22), and antioxidant (25) effects in mice. More recently, administration of NAS was effective in restraining neuroinflammation in mice with experimental autoimmune encephalomyelitis (EAE) (23), a widely used model for human multiple sclerosis (MS). Besides the identification of the tropomyosin receptor kinase B (TrkB) receptor in both antidepressant and antioxidant effects (24, 25), NAS molecular targets operating in the control of immunity and inflammation have been unclear.
Prompted by the finding that NAS neuroprotective effects in EAE are lost in mice lacking IDO1 expression, we investigated the molecular/functional relationship between the Trp metabolite and IDO1. We found that NAS 1) is also inactive in EAE when mice lack AhR expression; 2) binds IDO1 directly at a previously identified allosteric site of the enzyme; 3) acts as a positive allosteric modulator (PAM) of IDO1 catalytic activity in vitro and in vivo; 4) exerts the most potent IDO1-enhancing effects in DCs; and 5) increases IDO1 catalytic activity in peripheral blood mononuclear cells (PBMCs) of patients with MS.
Results
NAS Protective Effects in EAE Are Lost in Ido1−/− and Ahr−/− Mice.
IDO1 may represent a major therapeutic target in MS. In fact, administration of 1-MT, the standard inhibitor of IDO1 catalytic activity, exacerbates the clinical course of EAE in mice (26, 27). Conversely, positive allosteric modulation of a metabotropic glutamate receptor shows therapeutic effects in EAE via activation of a signaling pathway in CD11c+ DCs (also known as conventional DCs; cDCs) involving IDO1 (20). Moreover, administration of stem cells ameliorates EAE via induction of IDO1 expression and activity in cDCs (28).
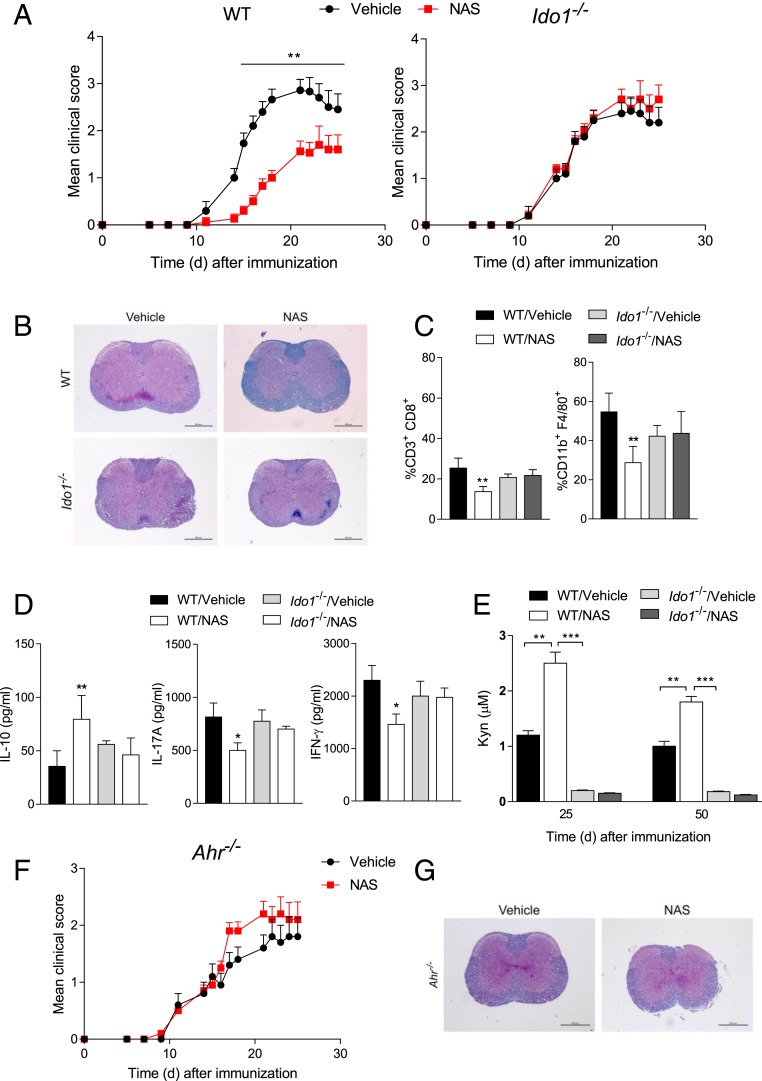
Because NAS exerts protective effects in EAE induced by vaccination with the myelin oligodendrocyte glycoprotein (MOG) (23), we investigated whether IDO1 could be involved in NAS effects in the same experimental model. Groups of IDO1-knockout (Ido1−/−) mice and their wild-type (WT) counterparts were immunized with the MOG35–55 peptide and injected intraperitoneally (i.p.) with NAS at the dose of 10 mg/kg every other day from 2 d until 24 d after vaccination. Control mice received vehicle alone. EAE clinical scores were recorded daily over a period of 25 d (Fig. 1A and SI Appendix, Table S1). As expected, NAS administration in WT mice resulted in a milder disease as compared with control mice (P = 0.003). In contrast, no protective effect of NAS could be observed in Ido1−/− mice. At 25 d postvaccination, white matter demyelination and inflammatory infiltrates present in control MOG-vaccinated mice were reduced by NAS in the spinal cord of WT but not Ido1−/− mice (Fig. 1B). Moreover, NAS in vivo treatment was associated with reduced infiltration of CD3+CD8+ and CD11b+F4/80+ cells in the spinal cords of WT but not Ido1−/− mice (Fig. 1C). NAS treatment of WT but not Ido1−/− mice also resulted in an increased production of antiinflammatory interleukin 10 (IL-10) by CD4+ T cells from cervical lymph nodes and reduced secretion of IL-17A (a cytokine produced by inflammatory Th17 cells) and interferon γ (IFN-γ) (Th1) by CD4+ T cells purified from spinal cords after restimulation with MOG in vitro (Fig. 1D).
Fig. 1.
NAS protects from EAE in an IDO1- and AhR-dependent fashion. (A) Clinical EAE scores (mean ± SD) over time of WT and Ido1−/− mice, treated with NAS or vehicle alone. (B) Histopathological analysis of spinal cord sections from representative WT and Ido1−/− mice as in A at 25 d after MOG immunization. (C) Percentages of CD3+CD8+ and CD11b+F4/80+ cells in the spinal cord of mice as in A at 25 d after MOG immunization. (D) Cytokine production by CD4+ T cells sorted at 25 d after MOG immunization from cervical lymph nodes from mice as in A and restimulated with MOG or medium alone in vitro for 24 h. (E) Kyn levels measured at 25 and 50 d after MOG immunization in sera of mice as in A. (F) Clinical EAE scores (mean ± SD) over time of Ahr−/− mice, treated as in A. One experiment is representative of 3 (A) and 2 (F). (G) Histopathological analysis of spinal cord sections from representative Ahr−/− mice as in F at 25 d after MOG immunization. *P < 0.05, **P < 0.01, ***P < 0.001. Data (C–E; analyzed in triplicate) are from 3 experiments. Error bars indicate means ± SD. (Scale bars: B and G, 600 μm.)
Prompted by the IDO1 critical role in NAS neuroprotective effects, we measured systemic levels of Kyn during (i.e., 25 d) and after the end (50 d) of NAS treatment. A significant increase (almost 2.5-fold at 25 d and 2-fold at 50 d) in Kyn concentration could be observed only in the blood of MOG-vaccinated WT mice treated with NAS (Fig. 1E).
Because Kyn is an endogenous agonist of immunoregulatory AhR (9) and NAS increases Kyn levels in mice competent for IDO1, we evaluated whether AhR could also be involved in NAS effects. AhR-knockout (Ahr−/−) mice were immunized with the MOG peptide and treated with NAS or vehicle alone, according to the protocol used in Fig. 1A. Evaluation of the clinical score of the 2 groups over time indicated that the NAS administration in Ahr−/− mice did not modify the EAE course observable in vehicle-treated mice (Fig. 1F). Lack of NAS effects was also evident in studies of histopathology (Fig. 1G), percentages of immune cell subsets in leukocytes infiltrating spinal cords, and the cytokine profile of CD4+ T cells in cervical lymph nodes (SI Appendix, Fig. S2).
Because we found very high levels of endogenous NAS but not serotonin in cervical lymph nodes of mice in the recovery phase of EAE (SI Appendix, Fig. S3), these data would support the therapeutic and physiologic value of NAS in the control of neuroinflammation. Perhaps more importantly, these data suggested that NAS, by increasing IDO1 catalytic activity, turns on the immunoregulatory IDO1–AhR axis in vivo.
NAS Confers Immunosuppressive Effects on DCs via Increased Catalytic Activity but Not Expression of IDO1.
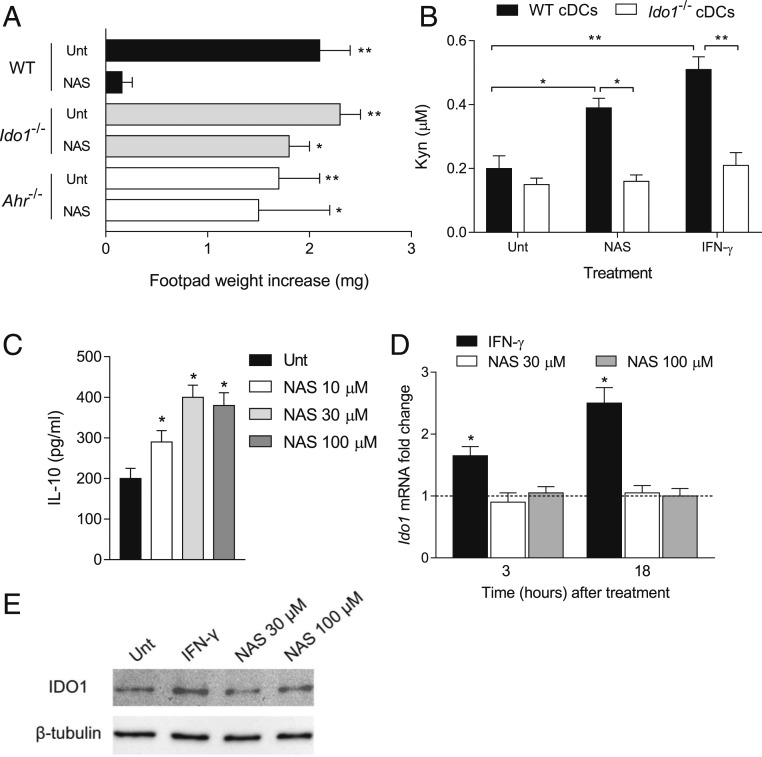
CD11c+ DCs often constitute the cell target of immunosuppressive treatments acting in EAE (29–31). We thus investigated whether cDCs could represent the cell target of NAS effects. To do so, we resorted to the skin test assay, an established protocol for measuring the in vivo induction of antigen-specific immunoreactivity versus tolerance in DCs (32). WT female mice were sensitized with the HY peptide (containing the H-2Db epitope of male minor transplantation antigen) presented by WT CD8− DCs (constituting an immunostimulatory, splenic cDC subset) (32) administered in combination with a minority fraction of the same cells (5%) purified from either WT, Ido1−/−, or Ahr−/− animals after conditioning with NAS at 30 µM or medium alone for 24 h. After priming the mice, we assessed immune reactivity at 2 wk by intrafootpad challenge with the HY peptide in the absence of DCs, as described (32). As expected, the default priming ability of immunostimulatory DCs was not affected by the presence of untreated cells (Fig. 2A). Yet, sensitization with NAS-pretreated WT DCs caused suppression of HY-specific reactivity, an effect not detectable in mice sensitized with Ido1−/− or Ahr−/− cDCs.
Fig. 2.
NAS-conditioned cDCs express an IDO1-dependent, immunosuppressive phenotype. (A) In vivo suppression of the activity of HY-pulsed, WT CD8− DCs in combination with a minority fraction (5%) of WT, Ido1−/−, or Ahr−/− CD8− DCs with no conditioning (Unt) or conditioned in vitro with NAS at 30 µM for 24 h. Analysis of skin reactivity of recipient mice to the eliciting peptide at 15 d is presented as change in footpad weight (experimental versus control footpads). (B) Kyn release by WT and Ido1−/− cDCs after a 24-h incubation with NAS at 30 µM, IFN-γ at 200 U/mL, or medium alone (Unt). (C) IL-10 production by WT cDCs after a 24-h incubation with NAS at different concentrations or medium alone. (D) Real-time PCR analysis of Ido1 transcripts in WT cDCs incubated with NAS (30 or 100 µM) or IFN-γ as in B for 3 or 18 h. Transcript expression was normalized to the expression of Gapdh and presented relative to results in untreated cells (dotted line, 1-fold). (E) Immunoblotting of the IDO1 protein in WT cDCs incubated with NAS at different concentrations, IFN-γ as in B, or medium alone for 24 h. *P < 0.05, **P < 0.01. All data are representative of 3 experiments. Experiments in B–D were performed in triplicate. Error bars indicate means ± SD.
Kyn levels were measured in 24-h culture supernatants of WT and Ido1−/− cDCs, incubated with NAS, IFN-γ, or medium alone. Data showed that both NAS and IFN-γ significantly increased Kyn release in WT but not Ido1−/− cDCs (Fig. 2B). In order to better comprehend the overall effects of NAS on cDCs, we determined the cytokine profile of those cells by measuring IL-6, IL-10, IL-12 p70, IL-27, and transforming growth factor β (TGF-β) in culture supernatants at 24 h of cell incubation with NAS at 10, 30, and 100 µM. The results showed that the DC conditioning with NAS does not greatly modify the overall cytokine profile, although a modest, yet significant, increase in antiinflammatory IL-10 could be observed at all tested concentrations of NAS (Fig. 2C).
Given its critical role in the control of immune responses, modulation of IDO1 expression and activity is stringently controlled. High-rate transcription of IDO1 in cDCs can occur in response to IFN-γ, TGF-β, Toll-like receptor ligands, and polyamines (1, 3, 33). We thus investigated whether NAS could modulate IDO1 transcript and protein expression in cDCs by real-time PCR and Western blotting experiments, respectively. cDCs were incubated with NAS at 30 and 100 µM. After 3 and 18 h (real-time PCR) or 24 h (Western blotting), IDO1 transcripts and protein were measured, respectively. IFN-γ was used as positive control of IDO1 up-regulation. Results showed that IFN-γ but not NAS increases IDO1 expression in cDCs, at both the transcript (Fig. 2D) and protein (Fig. 2E) level.
As a whole, our data indicated that NAS, a Trp metabolite of the serotonin pathway, up-regulates IDO1 catalytic activity in cDCs and confers IDO1-dependent immunosuppressive effects on the same cells without modulating IDO1 transcript and protein expression.
NAS Requires IDO1 to Activate AhR.
In the control of immune responses, IDO1 and AhR have been found to be functionally interconnected (34). Kyn represents a major endogenous ligand in the activation of immunoregulatory AhR (11), yet activated AhR can increase IDO1 expression and activity in cDCs, thus creating a positive functional circuitry (35).
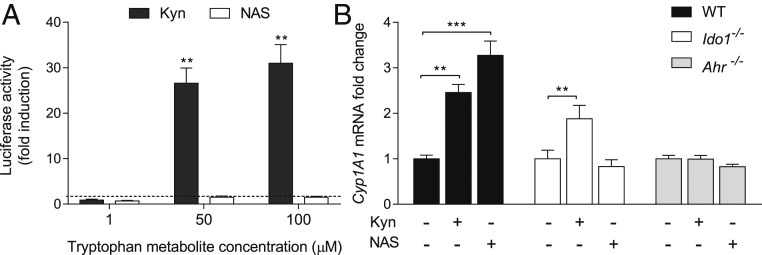
Given the critical role of IDO1 and AhR in NAS protective effects in EAE (Fig. 1), we investigated whether NAS may directly activate AhR. To do so, we first evaluated the ability of a range of NAS concentrations (1, 50, and 100 µM) to induce AhR transcriptional activity in hepatoma cells constitutively expressing AhR but not IDO1 and stably transfected with a firefly luciferase reporter plasmid containing an upstream enhancer of mouse Cyp1a1, a gene up-regulated by AhR activation (36). As a positive control, Kyn was used at the same range of concentrations. After 12 h, Cyp1a1 transcriptional activity increased in a dose-dependent fashion in cells incubated with Kyn but not NAS (Fig. 3A).
Fig. 3.
NAS requires IDO1 expression to activate AhR. (A) AhR transactivation activity by Kyn and NAS in IDO1−/− H1L1 hepatoma cells. Data (mean ± SD from 3 experiments in triplicate) are presented as fold change in normalized transactivation activity in cells incubated with Trp metabolites relative to medium alone (in which fold change =1; dotted line). (B) Real-time PCR analysis of Cyp1a1 transcripts in WT and Ido1−/− cDCs incubated with Kyn or NAS at different concentrations for 12 h, using Gapdh for normalization. Data are presented as in A. **P < 0.01, ***P < 0.001. One representative experiment of 3 is shown.
Although AhR expression is essentially ubiquitous in mammals, the nature of the ligand as well as that of the cell, which may provide a specific set of nuclear coactivators, may condition AhR activation (37). We thus investigated whether NAS could activate AhR in cDCs, that is, cells in which the Trp metabolite increases IDO1 catalytic activity (Fig. 2). WT and Ido1−/− cDCs were incubated with NAS or Kyn at 30 µM for 12 h. Ahr−/− cDCs were used as a control. Kyn significantly increased Cyp1a1 transcriptional activity, regardless of IDO1 expression by the cDCs (Fig. 3B). NAS also increased Cyp1a1 transcriptional activity in WT cDCs. However, no detectable levels of AhR activation by NAS could be observed in Ido1−/− cDCs. Neither Kyn nor NAS exerted effects on Ahr−/− cDCs. In order to investigate whether the effect could be due to melatonin (SI Appendix, Fig. S1), possibly generated from NAS by DCs (38) and also previously shown to be protective in EAE (23, 39), Cyp1a1 transcriptional activity was evaluated in WT cDCs incubated with 50 to 100 µM melatonin. However, no melatonin effect could be observed, in accordance with previous data indicating that melatonin does not constitute an AhR ligand (40).
As a whole, these data are consistent with the hypothesis that NAS, but not its metabolite, turns on the IDO1–AhR axis in cDCs by acting on IDO1 to increase production of Kyn that in turn activates AhR transcriptional activity. Our data also indicated that NAS is not an endogenous agonist of AhR.
NAS Acts as an IDO1 PAM.
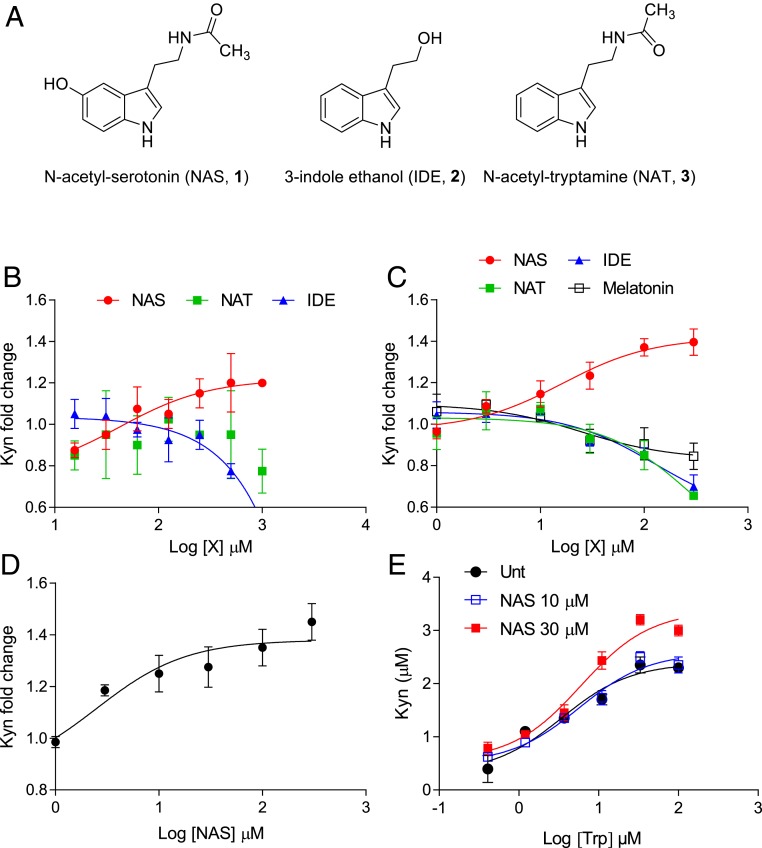
3-Indole ethanol (IDE), an indole derivative (compound 2; Fig. 4A) also known as tryptophol and produced by gut bacteria (41), has been considered an IDO1 modulator (i.e., catalytic enhancer or PAM) (42, 43). Studies with this compound indeed led to the identification of an allosteric site that, when bound by IDE, increases Trp affinity versus the primary catalytic site of IDO1 and thus potentiates IDO1-mediated transformation of Trp into Kyn (42, 43). We thus investigated whether NAS could act as a PAM of the IDO1 enzyme.
Fig. 4.
NAS acts as an IDO1 PAM. (A) Chemical structures of NAS, IDE, and NAT. (B) Enzymatic activity of recombinant human IDO1 in the presence of NAS, NAT, or IDE at different concentrations. (C) Effect of NAS, NAT, IDE, and melatonin at different concentrations on the 24-h Kyn release by P1.HTR (P1) cells transfected with mouse WT IDO1. Trp was at 10 µM (B) and 50 µM (C). (D) Effect of NAS at different concentrations on the 24-h Kyn release by WT cDCs. (E) Effect of NAS on the 24-h Kyn release by P1 cells transfected with mouse WT IDO1 in the presence of different Trp concentrations. For all curves, r2 was >0.91. In B–D, results are the mean ± SD of the Kyn fold change (relative to the buffer in B or medium in C and D without Trp metabolites) of 3 independent experiments, each performed in triplicate. Control absolute values of Kyn (buffer or medium without Trp metabolites) were 8.82 ± 2.56 µM (B), 4.82 ± 1.92 µM (C), and 0.24 ± 0.03 µM (D).
To do so, we first evaluated NAS effects over a range of concentrations on the catalytic activity of purified recombinant human IDO1 (rhIDO1) by using a reaction mixture as described (44) containing a fixed concentration of 10 µM Trp (Fig. 4B). In addition to IDE, we also evaluated the effects of N-acetyltryptamine (NAT), an indole derivative similar to NAS but lacking the hydroxyl group in the 5′ position (compound 3; Fig. 4A), to get insights into the structure–activity relationship of NAS. After 75 min of incubation, Kyn levels were measured and the results showed that NAS, but not NAT, increased Kyn production with a half-maximal effective concentration (EC50) of 20.95 ± 3.56 µM (mean ± SD) at 10 µM Trp. Unexpectedly, IDE did not increase but rather reduced IDO1 catalytic activity, with a half-maximal inhibitory concentration (IC50) equal to 0.4 ± 0.06 µM.
IDO1 catalytic activity requires incorporation of the heme prosthetic group and agents reducing heme Fe3+ into Fe2+, in addition to the presence of oxygen or superoxide anion (4, 45, 46). Although the biochemical assay of IDO1 catalytic activity does include the use of agents capable of reducing heme Fe3+ into Fe2+, IDO1 inhibitors such as 4-amino-1,2,3-triazoles and BMS-986205, which respectively bind IDO1 in its heme Fe2+ and apo-form, have been shown to display their IDO1-inhibitory effects better or solely in live cells (47, 48). We therefore analyzed the effects of NAS, IDE, NAT, and melatonin over a range of concentrations in P1.HTR cells [a highly transfectable clonal variant of mouse mastocytoma P815 that does not express IDO1 (49, 50)] transfected with mouse IDO1 (mIDO1) or hIDO1. NAS effects were also evaluated in P1.HTR cells transfected with mouse tryptophan 2,3-dioxygenase (mTDO), an enzyme mainly expressed in the liver and greatly contributing to the catabolism of Trp taken by diet into circulating Kyn (1). Results showed that NAS increases Kyn production in cells transfected with mIDO1 (Fig. 4C; EC50 19.56 ± 2.09 µM) or hIDO1 (SI Appendix, Fig. S4A; EC50 20.95 ± 6.06 µM) but not with mTDO, in which NAS instead appeared to inhibit rather than increase Kyn production (SI Appendix, Fig. S4B; IC50 0.4 ± 0.02 µM). In contrast, melatonin, NAT, and IDE exhibited inhibitory effects on Kyn production by cells transfected with mIDO1 (Fig. 4C). We finally measured the EC50 of NAS in enhancing IDO1 activity in freshly purified mouse cDCs. Results showed that NAS is capable of increasing Kyn production in cDCs (as in Fig. 2B) with an EC50 of 2.48 ± 0.3 µM (Fig. 4D).
In order to get insights into the mechanism of NAS in potentiating IDO1 catalytic activity, we incubated P1.HTR cells stably expressing mIDO1 with different concentrations of Trp in the presence or absence of NAS. Results showed that the presence of NAS at the highest concentration increased the efficacy, namely the maximal Kyn production, by almost 30%, whereas the EC50 (the Trp concentration at which half of the efficacy is observed) of the enzyme was not reduced (3.43 ± 1.28 µM with no NAS to 5.50 ± 1.10 with NAS at 30 µM), thus suggesting that NAS would act as an IDO1 PAM without increasing the enzyme affinity for its substrate, namely Trp (Fig. 4E).
As a whole, our data indicated that NAS may represent a true and selective IDO1 PAM in both mice and humans, an effect that appears to be more pronounced (as suggested by decreasing NAS EC50) when passing from artificial (use of recombinant protein) to semiartificial (cell transfectants) and to physiological (mouse cDCs) experimental settings. Our data would also suggest that the reported NAS antioxidant effects (25) (possibly occurring in P1.HTR cells and more so in primary cDCs) may provide additional help in the enhancing catalytic effects of NAS on IDO1—whose enzymatic activity is favored by reducing conditions—by means of NAS mechanisms not characterized yet. Finally, it is interesting to note that our data indicated a failure of IDE in enhancing IDO1 catalytic activity, which, however, are in accordance with very recent data obtained by others (51), as discussed in the next paragraph.
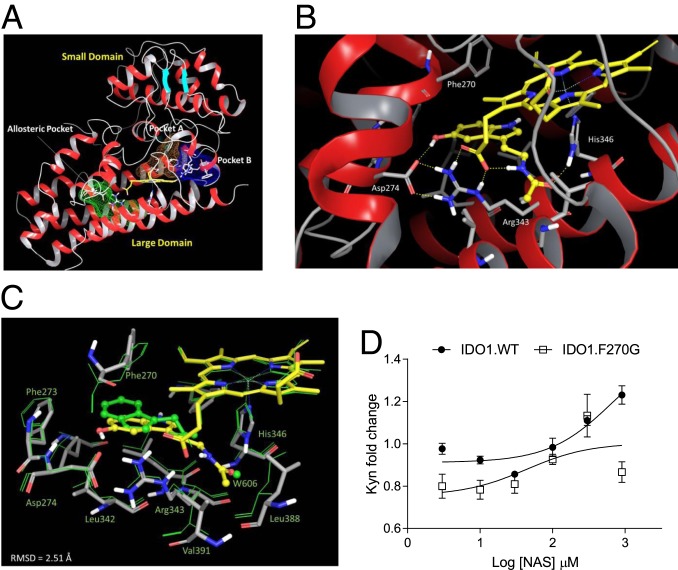
NAS Directly Binds the IDO1 Allosteric Site.
Crystallographic studies and site-directed mutagenesis experiments of IDO1 have shed light on the overall architecture, key residues, and structural features of small-molecule–binding pockets of the enzyme (Fig. 5A), as reviewed elsewhere (52). These studies unveiled that IDO1 folds into a globular structure comprising a small noncatalytic domain and a large catalytic domain. The catalytic site is located above the distal position of the iron heme, spanning 2 regions as defined by residues Ser167, Phe163, and Phe164 (pocket A) and Phe226, Phe227, and Arg231 (pocket B). A distinct allosteric site was recently identified below the proximal position of the heme plane and defined by residues Phe270, Asp274, and Arg343 (51, 53). This allosteric pocket was shown to bind IDE and suggested to account for the substrate inhibition behavior of the enzyme occurring at high concentrations of Trp. In view of the structural similarity between IDE and NAS (Fig. 4A), docking studies were carried out to investigate whether NAS could bind the allosteric pocket of IDO1 (SI Appendix, Fig. S5 and Table S2). According to the best energy-scored binding mode (Fig. 5B), the hydroxyl group of NAS would make a hydrogen bond with Asp274, whereas the acetamide group is involved in 2 hydrogen bonds with His346 and the propionate group of the heme cofactor. The indole ring is packed through an edge-to-face π interaction with the side chain of Phe270. Specifically, the hydrogen bond of the hydroxyl group with Asp274 supports a key role for such a group in the structure–activity relationship of NAS with respect to IDE and NAT that, lacking this polar substituent, are devoid of catalytic enhancing activity. Compared with the IDE-binding mode, the indole ring of NAS is flipped by 180°, and its carbonyl atom is placed in a region wherein a water molecule (W606) is found in the crystal structure of IDE-bound IDO1 (Protein Data Bank [PDB] ID code 5WMV) (51), supporting the presence of a favorable polar interaction near His346 (Fig. 5C).
Fig. 5.
NAS binds the allosteric pocket in IDO1, enhancing the catalytic activity of the enzyme. (A) IDO1 structure with indications of the small and large domains as well as of the catalytic site (A and B pockets, colored in orange and blue mesh surfaces) and the allosteric pocket (colored in green mesh surface). (B) Best energy-scored binding mode of NAS in the allosteric pocket of IDO1 (ligand and protein are colored by atom types, with yellow carbon the former and gray carbon the latter). Key hydrogen-bond interactions are shown with yellow dashed lines. (C) Structural superposition of the energy-refined structure of NAS-bound IDO1 (ligand and protein are colored as in B), and the crystal structure of IDE-bound IDO1 (PDB ID code 5WMV; ligand and protein are shown as green sticks). The position of water molecule W606 is shown as a green ball. (D) Effect of NAS at different concentrations as in Fig. 4C on the 24-h Kyn release by P1 cells transfected with mouse WT IDO1 (IDO1.WT) or the F270G mutant (IDO1.F270G). Trp was at 10 µM. Results are represented as Kyn fold change as in Fig. 4 of 3 independent experiments, each performed in triplicate. The control absolute value of Kyn (medium without NAS) was 4.82 ± 1.92 µM for P1 cells transfected with IDO1.WT and 1.19 ± 0.19 µM for P1 cells transfected with IDO1.F270G. Error bars indicate means ± SD.
In order to experimentally prove the proposed binding mode of NAS, mutagenesis experiments were carried out by engineering the Phe270Gly (F270G) mutant of mouse IDO1. The comparative evaluation of the enzymatic activity between WT and the IDO1 mutant showed that NAS loses its Kyn-enhancing activity when tested in tumor transfectants expressing IDO1.F270G (Fig. 5D). This confirmed the key role of Phe270 in the molecular recognition of NAS, providing evidence on the location of its binding site.
Because the IDO1 allosteric pocket is quite distant from the catalytic site (Fig. 5A) but very close to heme (54), these data may also explain why NAS increases the catalytic efficacy but not the binding affinity of IDO1 toward its substrate Trp.
NAS Increases IDO1 Catalytic Activity in PBMCs from Patients with MS.
MS can manifest in different forms, mainly represented by relapsing-remitting MS (RRMS; affecting about 85% of MS patients and marked by flare-ups of symptoms followed by periods of remission) and primary progressive MS (PPMS; a disease that affects ∼10% of MS patients and continues to worsen gradually from the beginning). In a recent study, IDO1 expression and Kyn levels were significantly reduced in PBMCs from RRMS patients as compared with healthy control subjects (19). In contrast, TDO expression was similar in PBMCs from RRMS patients and healthy subjects (19). Interestingly, whereas several pieces of evidence would indicate IDO1 as a protective player in RRMS (55, 56), lack of TDO expression will protect mice with EAE from neuronal loss in the spinal cord (57).
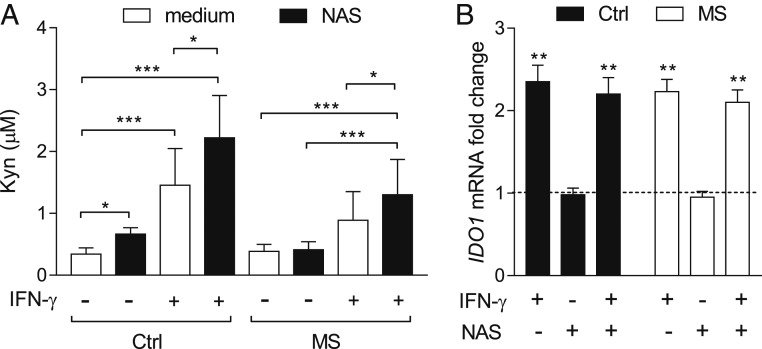
Because of the protective effects of NAS in WT mice with EAE, we investigated whether the Trp metabolite could modulate IDO1 activity in PBMCs from RRMS patients. We evaluated the Kyn release in culture supernatants of PBMCs, either unstimulated or stimulated for 24 h with IFN-γ, from RRMS patients as compared with healthy subjects (SI Appendix, Table S3), in the presence or absence of NAS. No significant difference could be observed for the basal Kyn release between the 2 groups. The Kyn production significantly increased in IFN-γ–stimulated PBMCs from healthy subjects but not RRMS patients (Fig. 6A). However, incubation with NAS resulted in a highly significant increase of IDO1 activity in RRMS PBMCs stimulated with the cytokine but not in unstimulated cells (Fig. 6A). In PBMCs from healthy subjects, NAS increased the Kyn release in both unstimulated and IFN-γ–stimulated conditions. No modulation by NAS could be observed in IDO1 transcript expression in unstimulated or IFN-γ–treated PBMCs from both RRMS patients and healthy subjects (Fig. 6B).
Fig. 6.
NAS increases IDO1 activity in PBMCs from MS patients. (A) Kyn levels in supernatants of PBMCs, either unstimulated (medium) or stimulated for 24 h with IFN-γ at 500 U/mL, from control subjects (n = 32) or RRMS patients (n = 59), in the presence or absence (medium) of NAS at 30 µM. (B) Real-time PCR analysis of IDO1 transcripts in PBMCs from RRMS patients, either unstimulated or stimulated with 500 U/mL IFN-γ, incubated with NAS at 30 µM for 12 h, normalized to the expression of ACTB (encoding β-actin), and presented relative to results in untreated cells (dotted line, 1-fold). All samples were analyzed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001. Error bars indicate means ± SD.
We previously showed that the majority of patients with autoimmune diabetes are characterized by a defect in IDO1 expression and activity, which is associated with specific genotypes at the IDO1 rs7820268 single-nucleotide polymorphism (SNP) (58). We thus compared the frequency of 5 haplotype-tagging SNPs (tagSNPs) in the IDO1 gene (SI Appendix, Table S4), including the rs7820268 SNP, using genomic DNA samples from a cohort of MS patients and matched control subjects (n = 200 in each group). In line with our previous report on autoimmune diabetes, the rs7820268 SNP, but not other IDO1 SNPs, displayed a significantly different allele (P = 0.007; SI Appendix, Table S5) and genotype (P = 0.006) distribution among MS cases and control subjects (Table 1). Upon correction of the association test results for age of onset, gender, and MS severity and classification, the CC genotype at rs7820268 was found to confer a 1.5-fold (95% CI, 1.04 to 2.12; P = 0.035) increased risk of developing MS. When the MS patients were stratified according to the type of MS, namely RRMS or PPMS, the genotype distribution of rs7820268 SNP tended to be different among RRMS versus PPMS patients (Table 2).
Table 1.
Genotype distribution of IDO1 genotypes among cases of MS and matched controls
| SNP rs no. | Alleles: Status | Genotype, n (%) | P value | ||
| A/A | A/a | a/a | |||
| rs9657182 | T>C | ||||
| MS | 59 (33.3) | 84 (47.5) | 34 (19.2) | 0.680 | |
| Controls | 144 (31.8) | 231 (51.0) | 78 (17.2) | ||
| rs3808606 | C>T | ||||
| MS | 50 (27.6) | 98 (54.1) | 33 (18.2) | 0.791 | |
| Controls | 130 (28.6) | 232 (51.2) | 91 (20.1) | ||
| rs10089078 | G>A | ||||
| MS | 65 (35.5) | 96 (52.5) | 22 (12.0) | 0.463 | |
| Controls | 180 (39.7) | 213 (47.0) | 60 (13.2) | ||
| rs7820268 | C>T | ||||
| MS | 78 (44.6) | 81 (46.3) | 16 (9.1) | 0.006 | |
| Controls | 158 (34.9) | 212 (46.8) | 83 (18.3) | ||
| rs3739319 | G>A | ||||
| MS | 57 (32.4) | 81 (46.0) | 38 (21.6) | 0.456 | |
| Controls | 123 (27.3) | 224 (49.7) | 104 (23.1) | ||
The major and minor alleles are represented by the first and second nucleotides, respectively. A and a indicate distinct alleles of the same gene. P value is for Fisher’s exact t test. Significant associations are reported in bold. Individual numbers may not add to the total number of patients due to missing patient data or genotypes.
Table 2.
Association between IDO1 genotypes and MS disease phenotype
| SNP rs no. | Alleles: Status | Genotype, n (%) | P value | ||
| A/A | A/a | a/a | |||
| rs9657182 | T>C | ||||
| PPMS | 10 (55.6) | 7 (38.9) | 1 (5.6) | 0.110 | |
| RRMS | 33 (31.1) | 52 (49.1) | 21 (19.8) | ||
| rs3808606 | C>T | ||||
| PPMS | 8 (42.1) | 10 (52.6) | 1 (5.3) | 0.179 | |
| RRMS | 28 (25.7) | 59 (54.1) | 22 (20.2) | ||
| rs10089078 | G>A | ||||
| PPMS | 9 (50.0) | 6 (33.3) | 3 (16.7) | 0.257 | |
| RRMS | 38 (33.9) | 60 (53.6) | 14 (12.5) | ||
| rs7820268 | C>T | ||||
| PPMS | 6 (33.3) | 12 (66.7) | 0 (0.0) | 0.059 | |
| RRMS | 54 (51.9) | 40 (38.5) | 10 (9.6) | ||
| rs3739319 | G>A | ||||
| PPMS | 7 (38.9) | 8 (44.4) | 3 (16.7) | 0.793 | |
| RRMS | 33 (31.1) | 53 (50.0) | 20 (18.9) | ||
The major and minor alleles are represented by the first and second nucleotides, respectively. A and a indicate distinct alleles of the same gene. P value is for Fisher’s exact t test. Individual numbers may not add to the total number of patients due to missing patient data or genotypes.
Because human PBMCs do not up-regulate TDO or IDO2 (the IDO1 paralog) in response to IFN-γ (58), our data indicated that, at least in inflammatory conditions, 1) IDO1 activity is deficient in RRMS patients; 2) the defect is associated with a differential distribution of the IDO1 rs7820268 SNP, mainly occurring in RRMS patients; and 3) deficient IDO1 activity can be increased by NAS in PBMCs from patients with RRMS to levels comparable to those of healthy controls.
Discussion
Allosteric modulation occurs when the functional activity of a protein is altered by the binding of an effector at a site topographically distinct from the orthosteric, active site. Allosteric modulators do not possess intrinsic efficacy but instead augment (positive allosteric modulators) or diminish (negative allosteric modulators; NAMs) the activity of orthosteric agonists (on receptors) or the catalytic transformation of substrates (by enzymes). Therefore, because their action is limited by the concentration of the endogenous ligand, allosteric drugs generally possess important advantages over orthosteric drugs, such as fewer side effects and lower toxicity. Despite much progress having been made in understanding the mechanisms of allosteric modulation, the development of allosteric drugs for therapeutic targets is still very limited, particularly in the enzymology field. This is a stark contrast to our knowledge on enzyme inhibition and the wealth of studies describing enzyme orthosteric inhibitors and their mechanisms of action. In fact, whereas the number of orthosteric catalytic inhibitors developed as drugs is significant, drug-like compounds acting as PAMs, namely potentiating the reaction efficacy of the enzyme, are scarce (59, 60).
Autoimmune diseases, including MS, are conditions involving breakdown of tolerogenic circuitries and consequent activation of autoreactive immune cells (61). Immune checkpoints are molecular regulators of the immune system and are thus crucial for self-tolerance, which prevents autoimmunity (62). The IDO1 enzyme is emerging as a novel type of immune checkpoint molecule, with multiple effects on effector as well as regulatory arms of the immune response (63). Because IDO1 deficiency seems to occur in several autoimmune pathologies (64), the development of IDO1-selective PAMs may be beneficial for a significant number of patients. Although an allosteric site in the enzyme structure was recently described (51), no IDO1 PAM capable of enhancing Trp metabolism in vivo and thus keeping autoimmunity in check has ever been reported.
By investigating the mechanism of action of NAS in protecting mice from EAE, we found that the Trp metabolite 1) directly binds IDO1 at a recently identified allosteric site; 2) acts as a PAM for both mouse and human IDO1 but not TDO; 3) increases the production of Kyn that in turn activates immunoregulatory AhR in DCs; and 4) rescues Trp metabolism in PBMCs from patients with MS.
The relevance of our data could be manifold. From a molecular perspective, the broad hydrogen-bond network of NAS with Asp274, His346, and the heme cofactor might provide a clue on the mechanism of the catalytic enhancing activity for such compounds. Indeed, it has been previously reported that IDO1 may adopt a heme-free apo-form within cells that can be catalytically activated upon addition of heme (65). A more recent study showed that the apo-form of IDO1 is the predominant state of the enzyme over the heme-bound form in cellular conditions, with an equilibrium between the 2 forms being dependent on the redox state of the enzyme and substrate concentrations (48). In the same study, inhibitors have been reported that specifically bind the apo-form of IDO1, competing with heme recruitment and occupying both allosteric and orthosteric pockets of the enzyme. By interacting with Asp274 through its key hydroxyl group in the allosteric pocket of IDO1, NAS may favor heme binding by promoting an equilibrium shift in the pool of intracellular IDO1 from the apo-form to the heme-bound form of the enzyme that will become catalytically active upon redox reaction of the heme cofactor (SI Appendix, Fig. S6). It is noteworthy of mention that this allosteric pocket is not present in the structure of TDO, thereby accounting for the lack of enhancing effects of NAS on this enzyme (53).
From a biologic perspective, our data provided evidence that an indole derivative of the serotonin pathway acts as an endogenous IDO1 PAM, that is, opposed to Trp, that, when present at high concentrations, is known to act as an IDO1 NAM (53). Therefore, although kynurenines acting as endogenous PAMs for the enzymes of the serotonin pathway have not been identified yet, we might hypothesize that products downstream of the kynurenine and the serotonin pathways may guarantee an appropriate equilibrium between the 2 main metabolic routes of Trp metabolism by allosteric mechanisms. These observations may have important therapeutic consequences. In fact, the therapeutic success of cancer immunotherapy based on the use of potent orthosteric inhibitors of IDO1 could be hampered by the induction of a skewing toward the serotonin pathway and thus an excess production of immunoregulatory NAS. Moreover, our data may indicate that the immunoregulatory effects of serotonin, namely such as the induction of IL-10, observed in human PBMCs from MS patients could be ascribed to NAS, the serotonin metabolite (66). However, although we did find high levels of NAS in cervical lymph nodes of mice, particularly in the EAE recovery phase, the origin of endogenous NAS production in the periphery is still discussed. In fact, aralkylamine N-acetyltransferase (SI Appendix, Fig. S1), the enzyme producing NAS from serotonin, is expressed not only in the pineal gland for melatonin synthesis but also in thymus, spleen, and bone marrow (67).
Although in the current work we focused our studies on Kyn, the main IDO1 product, we cannot exclude that other metabolites produced downstream of Kyn may contribute to the overall effect of increased activation of AhR by NAS. In fact, kynurenic acid (68, 69), cinnabarinic acid (a naturally condensed form of 3-hydroxyanthranilic acid) (70), and also xanthurenic acid (a lateral product of the pathway generated from 3-hydroxykynurenine) (71) are also agonists of AhR. Moreover, 3-hydroxyanthranilic acid, although not capable of acting as an AhR agonist on its own, can engage nuclear coactivator 7 and thus enhance AhR activation by Kyn in DCs (72). Along the same line, although metabolites downstream of Kyn are in general produced at lower levels as compared with Kyn, we cannot exclude that NAS may operate as a PAM also of enzymes downstream of IDO1 along the kynurenine pathway.
Perhaps most importantly, our data may have far-reaching therapeutic implications. In fact, the main therapeutic approach in containing MS has been immunosuppression. As per their definition, that is, drugs that lower adaptive immune responses, immunosuppressive agents impair activation and proliferation of T and B lymphocytes. However, given their nonselective mode of action, such drugs are unavoidably accompanied by significant adverse effects. Our data would indicate that the development of PAMs selective for the IDO1 enzyme and therapeutically active in vivo is feasible and therefore may provide unprecedented opportunities to develop first-in-class therapeutic agents for MS but possibly also for other autoimmune diseases. Of course, the identification of peripheral cells producing NAS in humans and of the conditions of the administration of NAS or drug-like compounds with NAS effects will be mandatory for the optimal therapeutic exploitation of IDO1 PAMs.
Materials and Methods
For detailed information and protocols, see SI Appendix.
EAE Induction and Treatment with NAS In Vivo.
Induction and evaluation of MOG-induced EAE were done as outlined in SI Appendix and as described (73). NAS (10 mg/kg) was administered i.p. every other day starting 1 d after MOG immunization for 24 d. Mice were monitored daily, and neurological effects were scored as outlined in SI Appendix. The in vivo experiments performed in this work were approved by the Italian Ministry of Health (Authorizations 482/2016-PR and 641/2016-PR).
Subject Recruitment and Purification of PBMCs.
The study was approved by the Ethics Committee of Health Agencies of Umbria (Reference 2925/16 of December 12, 2016), and all subjects provided informed written consent for the collection of samples and subsequent analysis.
Statistical Analysis.
Data were analyzed using Prism version 6.0 (GraphPad Software) by 2-tailed unpaired Student’s t test (datasets meeting normality) or Mann–Whitney test (no normality). Association between 2 variables was analyzed by least-squares regression analysis. EAE data were analyzed by 2-way ANOVA with 2 variables, time and treatment. To compare mean day of onset and maximal score, Mann–Whitney’s rank-sum test was used. All n values were computed by power analysis to yield a power of at least 80% with an alpha level of 0.05.
Data Availability.
All data are included in the manuscript and SI Appendix.
Supplementary Material
Acknowledgments
We thank the patients and subjects who participated in this study as well as nurses and staff of the Neurology Clinic of S. Maria della Misericordia Hospital, Perugia, Italy. We also thank Dr. Giovanni Ricci for histologies. This work was supported by the European Research Council (338954-DIDO and 780807-DIDO-MS; both to U.G. and A.M.) and the Italian Ministry of Education, University, and Research (PRIN 20155C2PP7 to C. Volpi). A. Carvalho, C.C., and P.M. were supported by the Northern Portugal Regional Operational Programme (NORTE 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (FEDER) (NORTE-01-0145-FEDER-000013), and the Fundação para a Ciência e Tecnologia (CEECIND/04601/2017 to C.C.; CEECIND/03628/2017 to A. Carvalho).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1918215117/-/DCSupplemental.
References
- 1.Grohmann U., Bronte V., Control of immune response by amino acid metabolism. Immunol. Rev. 236, 243–264 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Murray P. J., Amino acid auxotrophy as a system of immunological control nodes. Nat. Immunol. 17, 132–139 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mondanelli G., Ugel S., Grohmann U., Bronte V., The immune regulation in cancer by the amino acid metabolizing enzymes ARG and IDO. Curr. Opin. Pharmacol. 35, 30–39 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Mellor A. L., Munn D. H., IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat. Rev. Immunol. 4, 762–774 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Puccetti P., Grohmann U., IDO and regulatory T cells: A role for reverse signalling and non-canonical NF-kappaB activation. Nat. Rev. Immunol. 7, 817–823 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Platten M., Nollen E. A. A., Röhrig U. F., Fallarino F., Opitz C. A., Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 18, 379–401 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Cervenka I., Agudelo L. Z., Ruas J. L., Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 357, eaaf9794 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Platten M., Wick W., Van den Eynde B. J., Tryptophan catabolism in cancer: Beyond IDO and tryptophan depletion. Cancer Res. 72, 5435–5440 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Bessede A., et al. , Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 511, 184–190 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutiérrez-Vázquez C., Quintana F. J., Regulation of the immune response by the aryl hydrocarbon receptor. Immunity 48, 19–33 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Opitz C. A., et al. , An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478, 197–203 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Grohmann U., Fallarino F., Puccetti P., Tolerance, DCs and tryptophan: Much ado about IDO. Trends Immunol. 24, 242–248 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Banchereau J., Steinman R. M., Dendritic cells and the control of immunity. Nature 392, 245–252 (1998). [DOI] [PubMed] [Google Scholar]
- 14.van Baren N., Van den Eynde B. J., Tryptophan-degrading enzymes in tumoral immune resistance. Front. Immunol. 6, 34 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz J. B., Muller A. J., Prendergast G. C., Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol. Rev. 222, 206–221 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Mondanelli G., et al. , The proteasome inhibitor bortezomib controls indoleamine 2,3-dioxygenase 1 breakdown and restores immune regulation in autoimmune diabetes. Front. Immunol. 8, 428 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llamas-Velasco M., et al. , Immune cells from patients with psoriasis are defective in inducing indoleamine 2,3-dioxygenase expression in response to inflammatory stimuli. Br. J. Dermatol. 176, 695–704 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Cribbs A. P., et al. , Treg cell function in rheumatoid arthritis is compromised by ctla-4 promoter methylation resulting in a failure to activate the indoleamine 2,3-dioxygenase pathway. Arthritis Rheumatol. 66, 2344–2354 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Negrotto L., Correale J., Amino acid catabolism in multiple sclerosis affects immune homeostasis. J. Immunol. 198, 1900–1909 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Volpi C., et al. , Allosteric modulation of metabotropic glutamate receptor 4 activates IDO1-dependent, immunoregulatory signaling in dendritic cells. Neuropharmacology 102, 59–71 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheong J. E., Sun L., Targeting the IDO1/TDO2-KYN-AhR pathway for cancer immunotherapy—Challenges and opportunities. Trends Pharmacol. Sci. 39, 307–325 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Zhou H., et al. , N-acetyl-serotonin offers neuroprotection through inhibiting mitochondrial death pathways and autophagic activation in experimental models of ischemic injury. J. Neurosci. 34, 2967–2978 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen J., et al. , Efficacy of N-acetylserotonin and melatonin in the EAE model of multiple sclerosis. J. Neuroimmune Pharmacol. 11, 763–773 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Jang S. W., et al. , N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc. Natl. Acad. Sci. U.S.A. 107, 3876–3881 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoo J. M., Lee B. D., Sok D. E., Ma J. Y., Kim M. R., Neuroprotective action of N-acetyl serotonin in oxidative stress-induced apoptosis through the activation of both TrkB/CREB/BDNF pathway and Akt/Nrf2/antioxidant enzyme in neuronal cells. Redox Biol. 11, 592–599 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwidzinski E., et al. , Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. FASEB J. 19, 1347–1349 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Sakurai K., Zou J. P., Tschetter J. R., Ward J. M., Shearer G. M., Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 129, 186–196 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Matysiak M., et al. , Stem cells ameliorate EAE via an indoleamine 2,3-dioxygenase (IDO) mechanism. J. Neuroimmunol. 193, 12–23 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H. Y., et al. , Estrogen inhibition of EAE involves effects on dendritic cell function. J. Neurosci. Res. 70, 238–248 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Legge K. L., et al. , On the role of dendritic cells in peripheral T cell tolerance and modulation of autoimmunity. J. Exp. Med. 196, 217–227 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei H. J., Pareek T. K., Liu Q., Letterio J. J., A unique tolerizing dendritic cell phenotype induced by the synthetic triterpenoid CDDO-DFPA (RTA-408) is protective against EAE. Sci. Rep. 7, 9886 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mondanelli G., et al. , A relay pathway between arginine and tryptophan metabolism confers immunosuppressive properties on dendritic cells. Immunity 46, 233–244 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mondanelli G., Iacono A., Allegrucci M., Puccetti P., Grohmann U., Immunoregulatory interplay between arginine and tryptophan metabolism in health and disease. Front. Immunol. 10, 1565 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaronen M., Quintana F. J., Immunological relevance of the coevolution of IDO1 and AHR. Front. Immunol. 5, 521 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen N. T., et al. , Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc. Natl. Acad. Sci. U.S.A. 107, 19961–19966 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denison M. S., Nagy S. R., Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 43, 309–334 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Veldhoen M., Duarte J. H., The aryl hydrocarbon receptor: Fine-tuning the immune-response. Curr. Opin. Immunol. 22, 747–752 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Carrillo-Vico A., et al. , Evidence of melatonin synthesis by human lymphocytes and its physiological significance: Possible role as intracrine, autocrine, and/or paracrine substance. FASEB J. 18, 537–539 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Chen S. J., et al. , Melatonin enhances interleukin-10 expression and suppresses chemotaxis to inhibit inflammation in situ and reduce the severity of experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 31, 169–177 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Heath-Pagliuso S., et al. , Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry 37, 11508–11515 (1998). [DOI] [PubMed] [Google Scholar]
- 41.Dai Z. L., Wu G., Zhu W. Y., Amino acid metabolism in intestinal bacteria: Links between gut ecology and host health. Front. Biosci. 16, 1768–1786 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Sono M., Enzyme kinetic and spectroscopic studies of inhibitor and effector interactions with indoleamine 2,3-dioxygenase. 2. Evidence for the existence of another binding site in the enzyme for indole derivative effectors. Biochemistry 28, 5400–5407 (1989). [DOI] [PubMed] [Google Scholar]
- 43.Lu C., Lin Y., Yeh S. R., Inhibitory substrate binding site of human indoleamine 2,3-dioxygenase. J. Am. Chem. Soc. 131, 12866–12867 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Austin C. J., et al. , Optimised expression and purification of recombinant human indoleamine 2,3-dioxygenase. Protein Expr. Purif. 37, 392–398 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Macchiarulo A., Nuti R., Bellocchi D., Camaioni E., Pellicciari R., Molecular docking and spatial coarse graining simulations as tools to investigate substrate recognition, enhancer binding and conformational transitions in indoleamine-2,3-dioxygenase (IDO). Biochim. Biophys. Acta 1774, 1058–1068 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Romani L., et al. , Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature 451, 211–215 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Alexandre J. A. C., et al. , New 4-amino-1,2,3-triazole inhibitors of indoleamine 2,3-dioxygenase form a long-lived complex with the enzyme and display exquisite cellular potency. ChemBioChem 19, 552–561 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Nelp M. T., et al. , Immune-modulating enzyme indoleamine 2,3-dioxygenase is effectively inhibited by targeting its apo-form. Proc. Natl. Acad. Sci. U.S.A. 115, 3249–3254 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pallotta M. T., et al. , Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat. Immunol. 12, 870–878 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Albini E., et al. , Distinct roles of immunoreceptor tyrosine-based motifs in immunosuppressive indoleamine 2,3-dioxygenase 1. J. Cell. Mol. Med. 21, 165–176 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis-Ballester A., et al. , Structural insights into substrate and inhibitor binding sites in human indoleamine 2,3-dioxygenase 1. Nat. Commun. 8, 1693 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coletti A., et al. , Advances in indoleamine 2,3-dioxygenase 1 medicinal chemistry. MedChemComm 8, 1378–1392 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewis-Ballester A., Karkashon S., Batabyal D., Poulos T. L., Yeh S. R., Inhibition mechanisms of human indoleamine 2,3 dioxygenase 1. J. Am. Chem. Soc. 140, 8518–8525 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Littlejohn T. K., Takikawa O., Truscott R. J., Walker M. J., Asp274 and His346 are essential for heme binding and catalytic function of human indoleamine 2,3-dioxygenase. J. Biol. Chem. 278, 29525–29531 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Zhu W. H., Lu C. Z., Huang Y. M., Link H., Xiao B. G., A putative mechanism on remission of multiple sclerosis during pregnancy: Estrogen-induced indoleamine 2,3-dioxygenase by dendritic cells. Mult. Scler. 13, 33–40 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Amirkhani A., et al. , Interferon-beta affects the tryptophan metabolism in multiple sclerosis patients. Eur. J. Neurol. 12, 625–631 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Lanz T. V., et al. , Tryptophan-2,3-dioxygenase (TDO) deficiency is associated with subclinical neuroprotection in a mouse model of multiple sclerosis. Sci. Rep. 7, 41271 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orabona C., et al. , Deficiency of immunoregulatory indoleamine 2,3-dioxygenase 1 in juvenile diabetes. JCI Insight 3, 96244 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nussinov R., Tsai C. J., Allostery in disease and in drug discovery. Cell 153, 293–305 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Fenton A. W., Allostery: An illustrated definition for the ‘second secret of life.’ Trends Biochem. Sci. 33, 420–425 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Theofilopoulos A. N., Kono D. H., Baccala R., The multiple pathways to autoimmunity. Nat. Immunol. 18, 716–724 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baumeister S. H., Freeman G. J., Dranoff G., Sharpe A. H., Coinhibitory pathways in immunotherapy for cancer. Annu. Rev. Immunol. 34, 539–573 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Orabona C., Mondanelli G., Puccetti P., Grohmann U., Immune checkpoint molecules, personalized immunotherapy, and autoimmune diabetes. Trends Mol. Med. 24, 931–941 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Mondanelli G., et al. , Amino acid metabolism as drug target in autoimmune diseases. Autoimmun. Rev. 18, 334–348 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Thomas S. R., et al. , Antioxidants inhibit indoleamine 2,3-dioxygenase in IFN-gamma-activated human macrophages: Posttranslational regulation by pyrrolidine dithiocarbamate. J. Immunol. 166, 6332–6340 (2001). [DOI] [PubMed] [Google Scholar]
- 66.Sacramento P. M., et al. , Serotonin decreases the production of Th1/Th17 cytokines and elevates the frequency of regulatory CD4+ T-cell subsets in multiple sclerosis patients. Eur. J. Immunol. 48, 1376–1388 (2018). [DOI] [PubMed] [Google Scholar]
- 67.Gómez-Corvera A., et al. , Evidence of immune system melatonin production by two pineal melatonin deficient mice, C57BL/6 and Swiss strains. J. Pineal Res. 47, 15–22 (2009). [DOI] [PubMed] [Google Scholar]
- 68.DiNatale B. C., et al. , Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci. 115, 89–97 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.García-Lara L., Pérez-Severiano F., González-Esquivel D., Elizondo G., Segovia J., Absence of aryl hydrocarbon receptors increases endogenous kynurenic acid levels and protects mouse brain against excitotoxic insult and oxidative stress. J. Neurosci. Res. 93, 1423–1433 (2015). [DOI] [PubMed] [Google Scholar]
- 70.Joshi A. D., Carter D. E., Harper T. A. Jr, Elferink C. J., Aryl hydrocarbon receptor-dependent stanniocalcin 2 induction by cinnabarinic acid provides cytoprotection against endoplasmic reticulum and oxidative stress. J. Pharmacol. Exp. Ther. 353, 201–212 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Novikov O., et al. , An aryl hydrocarbon receptor-mediated amplification loop that enforces cell migration in ER−/PR−/Her2− human breast cancer cells. Mol. Pharmacol. 90, 674–688 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gargaro M., et al. , Engagement of nuclear coactivator 7 by 3-hydroxyanthranilic acid enhances activation of aryl hydrocarbon receptor in immunoregulatory dendritic cells. Front. Immunol. 10, 1973 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fallarino F., et al. , Metabotropic glutamate receptor-4 modulates adaptive immunity and restrains neuroinflammation. Nat. Med. 16, 897–902 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript and SI Appendix.