Significance
Exotic infectious pathogens, like citrus huanglongbing (HLB), are increasingly introduced into agrosystems. Early detection is the key to mitigating their destructive effects. Human visual assessment is insufficiently sensitive to detect new plant infections in a responsive timeframe, and molecular assays are expensive and not easily deployable over large crop landscapes. We turned to detector dogs, an ancient technology, which can rapidly survey large plantings without laborious sample collection or laboratory processing. Dogs detected infections (>99% accuracy) weeks to years prior to visual survey and molecular methods and were highly specific, accurately discriminating target pathogens from other pathogens. Epidemiological models indicated that dogs were more effective and economical than current early detection methods for sustainable disease control.
Keywords: canine detection, early detection, huanglongbing, epidemic simulation, direct assay
Abstract
Early detection and rapid response are crucial to avoid severe epidemics of exotic pathogens. However, most detection methods (molecular, serological, chemical) are logistically limited for large-scale survey of outbreaks due to intrinsic sampling issues and laboratory throughput. Evaluation of 10 canines trained for detection of a severe exotic phytobacterial arboreal pathogen, Candidatus Liberibacter asiaticus (CLas), demonstrated 0.9905 accuracy, 0.8579 sensitivity, and 0.9961 specificity. In a longitudinal study, cryptic CLas infections that remained subclinical visually were detected within 2 wk postinfection compared with 1 to 32 mo for qPCR. When allowed to interrogate a diverse range of in vivo pathogens infecting an international citrus pathogen collection, canines only reacted to Liberibacter pathogens of citrus and not to other bacterial, viral, or spiroplasma pathogens. Canines trained to detect CLas-infected citrus also alerted on CLas-infected tobacco and periwinkle, CLas-bearing psyllid insect vectors, and CLas cocultured with other bacteria but at CLas titers below the level of molecular detection. All of these observations suggest that canines can detect CLas directly rather than only host volatiles produced by the infection. Detection in orchards and residential properties was real time, ∼2 s per tree. Spatiotemporal epidemic simulations demonstrated that control of pathogen prevalence was possible and economically sustainable when canine detection was followed by intervention (i.e., culling infected individuals), whereas current methods of molecular (qPCR) and visual detection failed to contribute to the suppression of an exponential trajectory of infection.
Delayed detection of exotic pathogens can lead to pronounced and widespread epidemics in human, animal, and plant populations. The increasing rate of introduction of exotic biota, microbial pathogens, and invertebrate pests threatens agricultural production and compromises our ability to produce sufficient food to feed the burgeoning human population (1, 2). The increase in introductions correlates with commensurate expansions in human travel and trade, resulting in a plethora of existing and new pathways of introduction and subsequent losses of billions of dollars in crops annually (1, 3). Regulatory agencies globally dedicate a majority of their manpower and fiscal resources surveilling for and attempting to mitigate exotic pests pre- and postestablishment (4, 5). Early detection of exotic introductions followed by a rapid response is the most efficient strategy to slow epidemics and minimize damage to agricultural and natural ecosystems (6). For plant diseases, the lack of postinfection therapy and inability to vaccinate due to the lack of an immune system in plants dictate that early detection and response are essential. One of the most severe pandemics in modern times, huanglongbing (HLB) of citrus, is caused by the bacterium Candidatus Liberibacter asiaticus (CLas), which has jumped from the animal to plant kingdom within the last 200 y (7). Proportionally, this epidemic has affected a larger proportion of the worldwide host population than most emerging zoonotic and botanical epidemics (8). Originating in Asia in the previous century (9, 10), HLB has spread to the majority of citrus-producing areas of the world, emerging throughout the Western Hemisphere with devastating results (11, 12) (SI Appendix, Fig. S1). Florida has lost >60% of citriculture acreage, >80% of production, and >70% of its juice plants and fresh fruit packinghouses. If not curtailed, the persistent decline could destroy the Florida citrus industry, previously the second largest globally (8, 13–15).
The CLas bacterium is believed to be an insect endophyte that has found an unfortunate alternative host in citrus (7, 11, 16). The bacterium is vectored within citrus populations by Diaphorina citri, the Asian citrus psyllid (ACP), another exotic pest introduced into North and Central America in the late 1990s. CLas multiplies within the insect, where it beneficially increases the reproduction rate and accelerates population growth of the insect (17). HLB was first described in India in the 1920s (9). CLas is believed to have first infected citrus after opportunistic introduction during psyllid feeding on this host plant within the last 200 y, and due to its recent introduction to citrus species, the lack of coevolution of pathogen with host has resulted in the absence of resistance or tolerance throughout the genus Citrus and its woody relatives. This new plant pathogen that has crossed from animal to plant kingdoms would likely result in a severe culling event in an indigenous outcrossing plant population. However, citriculture is composed of vast monocultures of genetically similar clones with no genetic reservoir for natural selection of resistance. As a consequence, over the last 13 y, citrus industries in the United States alone have dedicated over $100 million to research of this new pathosystem with no imminent solution for HLB control (18). Thus, for the remaining New World areas of citriculture that have largely escaped CLas introduction or currently have low CLas incidence, early detection and infected host removal (rapid response) augmented by ACP population suppression via insecticides to simultaneously suppress transmission are the primary mitigation strategies and are, at best, marginally effective.
The CLas pathogen is erratically distributed within the vascular system of infected trees (9, 11, 19–21) with an incubation period of a few months to 1 y or more prior to symptom expression depending on tree age and acuity of human visual detection. Regulatory agencies traditionally performed surveys by human visual assessment but increasingly augment them by confirmatory chemical, serological, and molecular assays, such as enzyme-linked immunoassay, PCR, assays for unique proteins (proteomics) or metabolites (metabolomics), and other assays of acute specificity related to infection. Currently, CLas detection relies heavily on human visual symptom detection followed by PCR confirmation, the regulatory standard (22) that requires considerable time, fiscal, and human resources for sampling, processing, and laboratory assay. No other molecular or chemical detection method has sufficient sensitivity or specificity nor has been scaled to field level (23, 24). Therefore, if a particular citrus-growing area or individual tree is suspected of CLas infection, PCR is frequently utilized as an early detection/confirmation tool for presymptomatic infections. However, PCR effectiveness is dependent on selection of infected tissue for assay, which can be scarce, erratic, and thus, elusive in newly infected trees. Systemic infections of CLas develop over time, often initiating from vector transmission to a few cells in a single shoot within a mature citrus tree composed of hundreds of thousands of leaves (SI Appendix, Fig. S2). Thus, CLas is not only incompletely distributed but also, rare within the canopy during the early stages of infection, leading to a propensity for false negative (FN) PCR assays (7, 11). In controlled inoculations, it is not uncommon for infections to remain subclinical to PCR for 6 to 32 mo (see below). As a consequence of delayed detection, trees can serve as an inoculum source for vector transmission to surrounding trees. Therefore, PCR is marginal to infeasible for early detection of CLas (7), especially for large-scale and/or rapid screening of commercial orchards. Recent studies have also been conducted to explore the possibility of profiling plant volatile organic compounds (VOCs) for disease detection. Unique VOC profiles that differentiate diseased from healthy plants can be evaluated by electronic odor detection utilizing an electronic nose system consisting of a series of gas sensors (25, 26). Each of the sensors has specific sensitivities to one or more VOCs, and the series of sensors is used to discriminate a complex of different compounds present in the atmosphere or sample (27). Gas chromatography/differential mobility spectrometry has shown potential to detect CLas from field samples (28, 29). Thus, early detection of incipient infections via pathogen-related VOCs has been shown to be feasible if scalable to the landscape scale.
The mammalian olfactory system is quite ancient and likely evolved from chemotactic receptors in flatworms and early fish in the Precambrian Era over 600 to 800 million y ago to find food and mates, detect danger, avoid predators, etc. (30). Within canid evolution, dogs (Canis lupus familiaris) diverged from wolves (Canis lupus) ∼100,000 y ago (31), and studies on canine phylogenic variation suggest an East Asian origin of domestic canines ∼15,000 y ago (32, 33). Canines were used by early humans as chemical detectors dating back to their use as hunting dogs some 12,000 y ago through medieval times to the present (34, 35). Canines have demonstrated a proficiency at detecting and locating a wide array of organic and inorganic odors (e.g., explosives, drugs, tracking humans and game animals, finding cadavers, and identification of criminals by matching the scent of perpetrators to crime scenes) (36). Other canine inorganic odor detections include chemical accelerants; pollutants, such as aldrin, dieldrin, and DDT contamination; and toxins. In medicine, canines have been shown to detect unique VOCs of several types of cancer emanating from the skin or breath of cancer patients and the onset of epileptic and hypoglycemic events (36–39).
Canines have been used to locate and discriminate between domestic and endangered mammals and birds; find scat of rare animal species such as various bear Ursus species; track and locate foxes, coyotes, tigers, and ringed seals (Phoca hispida); find invasive brown tree snakes (Boiga irregularis) in cargo; and identify dairy domestic cattle in estrus (36). Canines have also been used to locate a number of invertebrate pests, including the red palm weevil, Rhynchophorus ferrugineus, that causes severe damage on date palms; egg masses of gypsy moths, Porthetria dispa, which damages tree crops, forests, and urban trees; eastern subterranean termites, Reticulitermes flavipes, and western subterranean termites, Reticulitermes hesperus; screwworm, Cochliomyia hominivorax, in animal wounds; and bedbug, Cimex lectularius, infestations in residences, hotels, and dormitories (30).
Canids are not unique in possessing sensitive and discriminatory olfactory ability as advanced chemotactic olfactory systems permeate all animal evolutionary lines. The use of other vertebrates (rats, swine, etc.) and recently, invertebrate animals (honeybees, fruit flies, wasps, moths, nematodes) as detectors is a burgeoning area of research with diverse practical applications (40). In all cases, the animal nondestructively interrogates its environment holistically (SI Appendix, Fig. S2) in contrast to molecular or biochemical assays, which often destructively subsample a small proportion of the host or environment. Here, we document early detection of the CLas exotic bacterial pathogen by the use of canine olfactory surveillance. Via a series of studies, we examine and evaluate the relevance and efficacy of canine detection as a viable and field-deployable detection technology.
Sensitization to Disease Pathogen Odor and Assessment of Performance
Twenty canines (breeds: Belgian Malinois [BM], German shepherd [GS], BM × GS hybrids, and springer spaniel) were selected from European breeders of detection canines based on assessment of “drive,” the instinct to hunt by odor, large stature to enable the canine to traverse long distances, and endurance (SI Appendix, Fig. S3). Initially, 10 canines were selected during the first year of the study and 10 more during the succeeding 2 y in anticipation of an expanded number of canine teams needed for commercial deployment for early detection of CLas. Following a 1- to 3- wk acclimation period, each canine received 8 to 10 wk of obedience and sensitization training. Because CLas is a nonculturable obligate bacterium that cannot be separated from the citrus host, canines were trained directly on a target odor via a self-discovery, heuristic training method to motivate discrimination between healthy vs. CLas-infected plants. Sensitivity training consisted of positive verbal reinforcement and reward (a few seconds of play with a toy rather than a food reward, which is sometimes used to motivate canines) when the canine trainee alerted on the proper target odor (infected plants), whereas correct nonresponses (no alert) following nontarget odors (noninfected plants) were not reinforced. Canines were trained to indicate a positive alert by sitting next to the target until rewarded (i.e., a positive alert consists of the canine smelling each suspect plant, sitting next to positive odor target plants, and being unwilling to move from the target until rewarded).
Target specimens were prepared from potted plants of Valencia orange (Citrus sinensis L.) or Ruby Red grapefruit (Citrus paradisi MacFad.) on rough lemon (Citrus jambhiri Lush) rootstock that were inoculated by graft transmission. Infection status was confirmed via qPCR as described below. Sensitization is composed of three general phases. In the first phase, each canine is allowed to interrogate four potted trees, one of which is infected with CLas. The canine soon recognizes that one tree is different, and this “self-discovery” of the CLas scent signature is encouraged verbally and rewarded with play. Simultaneously, the canine is trained and rewarded to sit (i.e., alert) when it has identified the correct CLas-infected tree. The process is repeated and reinforced until the trainer is confident that the canine has imprinted the scent signature and alerts reliably. In the second phase, the canine is presented with a row of 10 to 20 trees, 1 of which is CLas infected and randomly placed in the row. The canine is encouraged to interrogate each tree in the row in sequence and identify the infected tree by alerting, for which it is rewarded. This phase trains the canine to interrogate trees in rows as they will encounter in commercial setting, reinforces the imprinting of the target scent signature, and improves accuracy. When the trainer is confident that the canine is proficient at interrogating and recognizing CLas-infected trees in rows, training progresses to the third phase of evaluation of proficiency. For this phase, a field site was established in rural Volusia County, Florida in an area devoid of commercial citrus orchards and with few residential citrus trees. The site consists of a grid of 10 × 10 potted citrus trees with 3.05 m between trees within and across rows similar to a young citrus orchard. Empty plastic pots or sections of PVC pipe fitted into the ground at soil level serve as soil liners such that the potted citrus trees can be easily placed within the lined holes at ground level and easily moved/relocated. A random number generator is used to determine the location of CLas-positive trees (0 to 5% disease incidence) within the 100-tree grid; the remainder of the trees within the grid were all CLas negative. Initially, 10 canines were trained utilizing the grid for statistical analysis, and eventually, all 20 canines were trained via this method as they were acquired. For each trial replicate or “run,” a team (handler plus canine on a lead) trotted alongside each row, turned at the end of each row, and progressed along the next in a serpentine pattern until the canine team had sequentially interrogated each tree in the grid in succession, stopping at each tree on which the canine alerted. Canines are rewarded as described above for each correct alert on a CLas-positive tree.
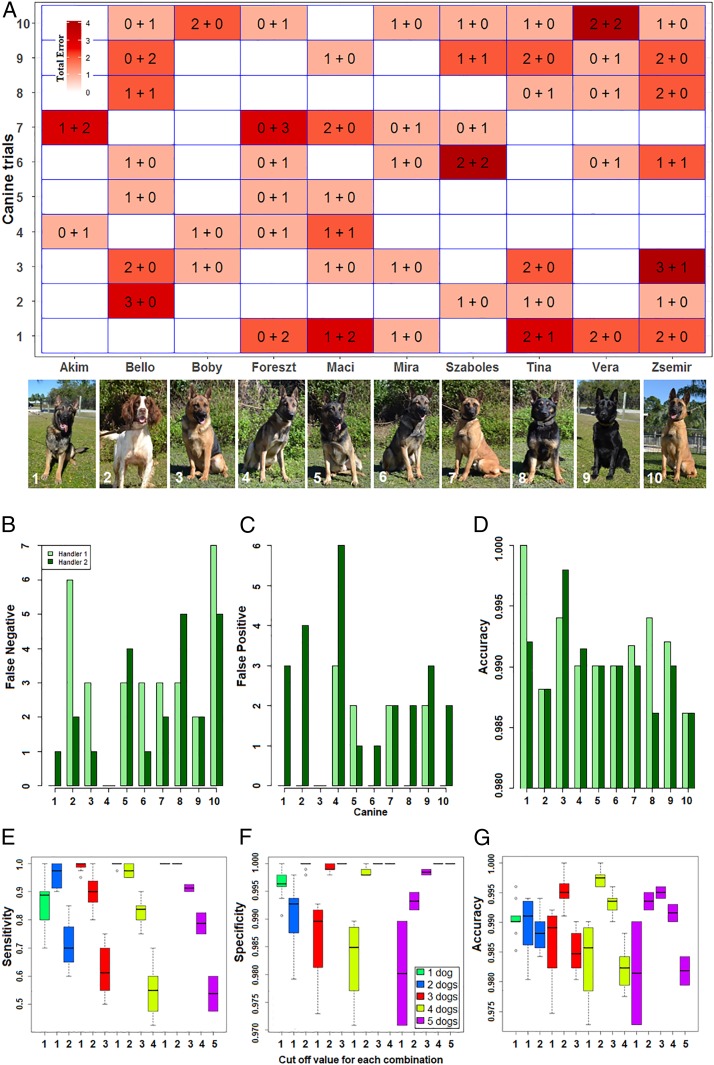
After fully trained to the CLas-infected tree scent signature, the initial 10 canines were repeatedly assessed for CLas detection performance in a simulated orchard grid of 100 trees with random placement of CLas-infected trees, and performance metrics were calculated (SI Appendix, section A and Movie S1). Performance measures demonstrated true positive (TP) rate (sensitivity) of 0.8579 (range = 0.7000 to 1.0000), true negative (TN) rate (specificity) of 0.9961 (range = 0.9906 to 1.0000), and high overall accuracy of 0.9905 (range = 0.9850 to 0.9960) (SI Appendix, section A and Table S1). In prior studies by others, canine detection sensitivity in different tasks ranged from 0.7500 to 1.0000, and specificity ranged from 0.8200 to 1.0000 (41). Performance differences among the 10 canines in this study were statistically insignificant, although some trends emerged. Examination of the error by canines across all trials demonstrated that the total error, FN + false positive (FP), ranged between 4 and 15 errors (0.0004 to 0.0015) per 950 to 1000 trees per canine. Over the study, there were slightly more FN (54) compared with FP (36) alerts, resulting in FP and FN rates of 0.0039 and 0.1421, respectively. The FP/FN ratio for the canine “Foreszt” was 9 to 0, whereas the same ratio for the canine “Boby” was 0 to 4, indicating the two canines were differentially conservative (i.e., Foreszt never missed a CLas-infected tree, whereas Boby never falsely identified a healthy tree). There was no improvement or degradation of performance across trials over time, although the overall error was lowest for trials 4 and 5 (Fig. 1A). There was no statistical difference in performance when pairing any of the 10 canines with two different handlers (Fig. 1 B–D and SI Appendix, section B). However, using two or more canines to gain consensus of detection raised accuracy to nearly 1.0000 (Fig. 1 E–G and SI Appendix, section C and Fig. S4). Thus, in subsequent surveillance of commercial orchards and residential properties (see below), alerts by one canine were always interrogated by at least one additional canine to ensure confirmation of infection. In subsequent studies, a smaller compliment of trained canines was selected for each study at the discretion of the handler such that a diversity of canines was used over the course of the studies.
Fig. 1.
Canine performance detecting CLas in citrus trees. Canines interrogated a 10 × 10 grid of potted citrus trees in which zero to five CLas-positive trees were randomly placed. For each of 10 replications (trial) by a canine, the placement of the infected trees was rerandomized. (A) White to red gradient indicates the total error (FN + FP; range = 0 to 4) associated with each canine for CLas detection over the 10 trials of 100 trees each. Numerical values in each cell indicate the number of FN errors (first number) + FP errors (second number) detections per replicate by individual canine. White blocks indicate that no errors occurred for that trial/canine. The canines with the fewest total errors were Akim, Boby, and Mira with four, six, and six errors, respectively. Canine–handler team performance assessment: (B) FN error, (C) FP error, and (D) overall accuracy. CLas detection metrics for consensus of two or more canines: (E) sensitivity, (F) specificity, and (G) overall accuracy.
Spatial Heterogeneity of CLas Detection Errors.
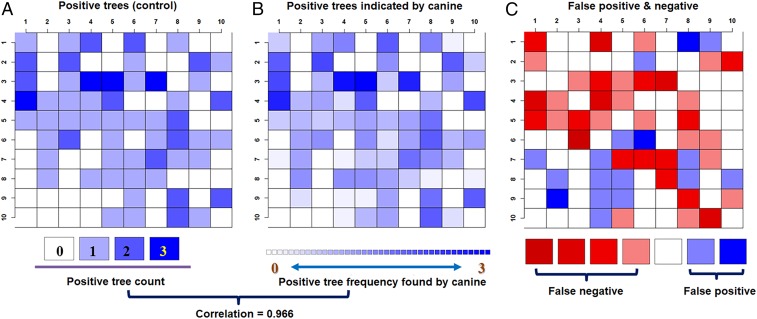
To address the concern of FN and FP canine alerts on CLas-infected and healthy trees, respectively, we analyzed the cumulative randomized placement of CLas-infected trees within the 100-tree test grid and compared it with correct CLas TP and TN tree positions (SI Appendix, section D). It is not uncommon for detector canines to acquire a target scent at some distance from the true target, occasionally alerting on a negative target within the scent plume (42, 43). However, for CLas detector canines, there was a high spatial correlation (0.966) between CLas-infected tree placement and TP alerts, with very few FN indications as seen by consistency of blue- to white-colored positions in Fig. 2 B and C.
Fig. 2.
Spatial heterogeneity of CLas detection. (A) Cumulative randomized placement of CLas-infected trees within the 100-tree test grid over all trials. (B) Frequency of TP CLas-infected tree identification by canine detection. (C) Spatial distribution of canine detection FN and FP errors.
The canines infrequently alerted on positions that contained CLas-infected trees in previous trials. This occasionally happens with detector canines, apparently due to the high sensitivity of the canines’ olfactory acuity reacting to residual volatiles from prior trials. These residual volatiles may have resulted in FP indications as indicated by blue positions in close proximity to the positions of CLas-infected trees in prior trials in Fig. 2. We investigated the effect of residual volatiles at prior CLas-infected tree positions by each canine (SI Appendix, Fig. S5). For example, 4 of 12, 4 of 8, and 4 of 8 FP alerts corresponded to prior CLas-infected tree locations vs. healthy tree locations (SI Appendix, Fig. S5). Additionally, for two of the spatial assessments, all three canines alerted to the tree at x–y coordinate 8–1 (SI Appendix, Fig. S5 B and C). Most remaining FP alerts were immediately proximal to prior CLas-infected TP positions. There were not enough data to correlate FP with previous TP locations nor was there any evidence of spatial patterns or edge effects (SI Appendix, Fig. S6).
Assessment of Canine Subclinical CLas Detection.
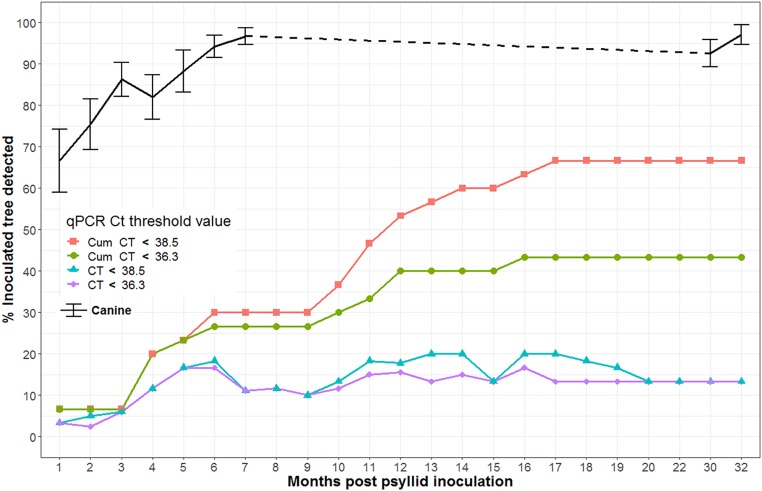
The regulatory standard for CLas detection/confirmation is qPCR (13); however, CLas has a prolonged subclinical period often months to years between vector transmission and detection by PCR (7). To assess canine detection of subclinical infection, 30 citrus trees inoculated via psyllid transmission of CLas were periodically interrogated by 4 to 10 canines and assayed by qPCR over 32 mo (SI Appendix, sections F and G). As a group, the canines discriminated all 30 CLas-infected trees within 30 d postinoculation (ΔTcanine = 30 d) with an average ∼0.6000 accuracy per canine when allowed to search mixed populations of 90 healthy and 10 infected trees (Fig. 3). Canine detection of CLas infections continued to improve over time, optimizing at 0.9900 detection accuracy 7 mo postinfection. In contrast, qPCR first began to detect ∼3% of infections at 2 mo and was only able to confirm 16 of 30 inoculated trees at 16 mo and 20 of 30 inoculated trees at 17 mo via cycle thresholds of 36.3 and 38.6, respectively. In addition, the variability of qPCR was problematic. At no single temporal assay did qPCR accurately detect more than 20% of the infected trees via leaf assay (Fig. 3 and SI Appendix, section G and Figs. S7 and S8).
Fig. 3.
Temporal assessment of canine subclinical detection of CLas infection (black) compared with qPCR detection/confirmation at two accepted regulatory qPCR thresholds for 30 psyllid-inoculated Valencia orange trees; 4 to 10 canines assayed each tree on each assay date (resulting in 0 to 10 detections indicated by variance bars). One sample for qPCR assay was selected from each tree consisting of four leaves split into two samples of two leaves; each was processed via qPCR (any positive subsample denoted a positive qPCR detection). CT, or cycle threshold, is the number of PCR cycles required for the fluorescent signal to cross the threshold and exceed background level and thereby denote a positive/negative assay.
The cumulative canine FP detection error was very low, and cumulative FN error was only slightly higher but decreased over the duration of the experiment and declined significantly over the duration of CLas infection (SI Appendix, Fig. S8). Thus, the greatest uncertainty of canine detection occurs in the initial stages of infection immediately following psyllid transmission of CLas, and uncertainty decreases rapidly as the infection persists and the bacteria multiply moving systemically. The qPCR results followed the commonly seen temporal detection pattern, reaching detectable CLas titer at different time points followed by intermittent detection over the 32-mo study. Most strikingly, 12 of 30 trees remained below the qPCR detection threshold throughout the study, clearly demonstrating the superior early detection capabilities of canines for CLas-infected trees.
Assessment of CLas Infection from Citrus Root Tissue.
When interrogating excised root tissue, overall detection accuracy was 0.9813, and total error (FN + FP) per canine did not exceed 0.0190 (i.e., slightly less than when canines interrogated entire trees). The cumulative FP detection error was very low, whereas cumulative FN error was slightly higher (SI Appendix, section H and Fig. S9). The FN/FP ratio indicates that canines were more likely to misidentify a CLas-infected root sample than implicate a healthy tree root sample as CLas infected. This result is similar to the differential detection of entire trees (see above) (Movie S2).
Discrimination of CLas from Other Citrus Pathogens and Other Liberibacter sp.
Four canines each interrogated a mixed population of 430 trees composed of healthy trees and trees infected by various citrus bacterial, viral, viroid, mycoplasma, and spiroplasma pathogens from the US Department of Agriculture (USDA) international citrus pathogen collection. Average total error per canine for CLas detection was very low, FN + FP = 0.0051, with an average accuracy of 0.9949 (SI Appendix, section I, Fig. S10, and Table S2 [list of pathogens interrogated]). Errors were not preferentially associated with any pathogen or pathogen combination. CLas-infected trees in the collection originated from eight countries, representing a diversity of CLas strains. Although canines were trained exclusively on CLas-infected trees, they were able to cross-identify trees containing Candidatus Liberibacter africanus from South Africa (a Liberibacter sp. with high homogeneity to CLas) with equivalent precision, indicating that trees infected with the two Liberibacter species both pathogenic to citrus likely have similar scent signatures to which the canines alert. Interestingly, all four canines alerted on a single tree previously inoculated with Candidatus Liberibacter americanus from Brazil (another Liberibacter sp. with high homogeneity to CLas), but repeated assays via PCR failed to confirm pathogen infection. Additionally, the canines accurately detected CLas isolates in mixed infections with citrus tristeza virus (CTV) but did not react to exclusively CTV-infected trees (Movie S3).
Two additional Liberibacter species potentially exist in crops in close proximity to cultivated citrus: Candidatus Liberibacter solanacearum, the causal agent of zebra chip disease of potato that also infects tomato, and Candidatus Liberibacter crescens, originally found in papaya and reportedly detected in residential citrus (44). In our replicated trials, the detector canines correctly identified control CLas-infected trees (1.0000 accuracy) with no alerts on Ca. L. solanacearum-infected tomato or the presumptive Ca. L. crescens-inoculated citrus, indicating that there was no cross-reaction of canines trained on CLas with other Liberibacter pathogens nonpathogenic to citrus (SI Appendix, section J).
Composition of the CLas-Infected Tree Volatilome.
Biological organisms give off unique complexes of VOCs that make up their volatilome (total VOC complement of an organism), providing a unique scent signature that can be interrogated by animals, including canines. The discipline of volatilomics is emerging, and only few whole-organism volatilomes are characterized. Little is understood of the relationship between VOCs and scent recognition or if unrelated diseases produce volatilomes with high similarity.
Because CLas is an obligate parasite and thus, inseparable from the citrus host plant (with exception of possible coculture; see below), it was not possible to train canines on CLas directly or on CLas volatilome VOC components. Rather, canines were trained on live CLas-infected biological hosts vs. noninfected plants, which required the canines to differentiate the associated infected vs. healthy volatilomes. Such training is considerably different from traditional canine training, where the target substance (e.g., drugs or explosives) can be isolated and has a definable VOC signature. In our case, the target was an entire plant system (i.e., a citrus plant infected with a bacterium).
Our initial hypothesis was that the canines distinguish CLas-infected from noninfected citrus due to the complex physiological and metabolic host response changes caused by infection, resulting in an altered citrus volatilome. Our canines interrogate the HLB-associated volatilome in two main ways: as static objects in containers (e.g., unique sample headspace) in a laboratory environment or by actively searching for a target in an open environment (e.g., citrus orchard). We compared CLas-infected vs. healthy tree foliage (SI Appendix, section K and Fig. S11). The volatilomes of healthy and CLas-infected grapefruit leaves are composed of an array of VOCs (45) (SI Appendix, Table S3). In CLas-infected grapefruit foliage, some of the individual VOCs are amplified, while others are suppressed within the volatilome. It is known that canines can detect some VOCs below the general detection threshold of gas chromatography–mass spectrometry (GC-MS) (46). Therefore, it is unknown if other VOCs and how many that occur below the GC-MS detection threshold are part of the scent signature recognized by the canines as CLas infected. Additionally, it may not be the presence or absence of individual VOCs but also, their relative concentration one to another that is important for scent signature recognition. This has been likened to the volatile notes of perfumes or even of music, where variability in concentration or tone (flat or sharp), respectively, allows a perfume or piece of music to be easily recognized by smell or sound.
Canine Detection of CLas in Noncitrus Host Plants and Psyllid Vectors.
Catharanthus roseus (L.) G. Don–Apocynaceae (Madagascar periwinkle) and Nicotiana benthamiana Domin–Solanaceae are nonrutaceous indicator host plants for CLas that, when inoculated, display symptoms and test positive via PCR. Canine detection accuracy of CLas in C. roseus and N. benthamiana in mixed populations of infected and noninfected plants (SI Appendix, section L) was 0.9900 and 1.0000, respectively (SI Appendix, Fig. S12). These indicator plants are in divergent plant families and thus, ancestrally and genetically distant from citrus and each other. Therefore, the results made us question our hypothesis that the HLB scent signature is composed of unique VOCs resulting from a host response to CLas infection. How could such genetically divergent hosts produce the same or a highly similar response to CLas infection unless such a response is highly conserved? At this point, we also tested canine detection of CLas in bacterialiferous D. citri (ACP, the vector for CLas). In replicated studies (SI Appendix, section M), three canines alerted (sensitivity = 0.9200, specificity = 0.9837, accuracy = 0.9778) on caged CLas-infected psyllids (SI Appendix, Fig. S13 and Movie S4).
Thus, not only was it possible to train canines to discriminate CLas-infected citrus from noninfected citrus, but also, they were able to detect CLas infections in a wide range of plant families and insect vectors with no additional training. It is highly unlikely that such a diverse set of plant genera and families could all express similar host response VOCs and improbable that an animal host (i.e., psyllid vector) would also elicit a scent signature composed of similar VOCs as plants due to CLas infection. Therefore, could the canines be reacting to the pathogen itself rather than a host response?
Canine Detection of CLas with In Vitro Liquid Culture.
To answer this question, we utilized a recently developed in vitro coculture of CLas with other uncharacterized bacteria, an outgrowth of prior work (47). Because CLas has never been cultured axenically, the coculture was fed to psyllids that were subsequently fed on healthy citrus. Within a few weeks, the resulting vector-inoculated citrus became CLas infected (PCR positive) and expressed HLB symptoms. We used the CLas coculture randomized in a line with Bacillus megaterium, Pseudomonas sp., an uncharacterized bacterial isolate from the coculture, and sterile culture broth as negative controls (SI Appendix, section N). The canines alerted strongly on 1) undiluted CLas coculture (sensitivity = 0.9167, specificity = 1.0000, accuracy = 0.9917, P = 1 × 10−12); 2) 10−4 dilution (sensitivity = 0.8333, specificity = 0.9907, accuracy = 0.9750, P = 1 × 10−12), which is below the concentration in infected plants; and 3) 10−6 dilution (sensitivity = 0.4167, specificity = 0.9907, accuracy = 0.9333, P = 1 × 10−24), which is below the qPCR detection threshold. These results suggest that the canines respond directly to the CLas pathogen even at low concentration rather than a host response to infection, dispelling our hypothesis of a unique host response volatilome (SI Appendix, Fig. S14). However, this does not negate the possibility that host response volatiles perhaps augment CLas detection in infected citrus plants.
Field Validation and Deployment for Early Detection in an Emerging HLB Epidemic.
Field training of canines was undertaken in young citrus plantations <18 mo old in Florida with <10% CLas incidence confirmed via qPCR. To assess detection of subclinical infections in mature plantings, four and nine canines were transported to survey mature citrus blocks in the Rio Grande Valley of Texas on two multiday trips, respectively (SI Appendix, section O and Movie S5). Canines identified previously qPCR-confirmed CLas-infected trees (sensitivity = 0.7112, specificity = 0.9719, accuracy = 0. 9559) plus additional presumptive CLas-infected trees in the block (SI Appendix, Fig. S15). Had we been able to retest the trees on which the canines alerted through time, it is probable that many would have been confirmed CLas positive eventually, raising the true sensitivity and accuracy. One experimental orchard included multiple cultivars, species, and rootstocks, confirming the canine penchant for the CLas volatilome irrespective of citrus species, cultivar, etc.
Validation of CLas detection in residential environments was conducted in Southern California foci of infection composed of highly nonuniform properties (SI Appendix, section P) (sensitivity = 0.9024, specificity = 0.9394, accuracy = 0.9189) (SI Appendix, Fig. S16 and Movie S6). Canines also alerted on multiple residential trees that were unconfirmed by regulatory qPCR assays. Four trees were subsequently confirmed via PCR. The remainder will be periodically reassayed, and if confirmed, they will further improve the detection metrics and validate canine early detection capability.
CLas infection in commercial citrus is spatially heterogeneous with a prevalence of infections on orchard peripheries due to the initial accumulation of bacterialiferous vectors on planting edges (48). Simulation was used to determine the detection efficiency of various deployment strategies using data from 451 CLas low-incidence (0 to 5% infection) commercial citrus blocks (49) (SI Appendix, sections S and T and Figs. S17 and S18). Perimeter, stratified, and complete surveillance designs were tested. Results demonstrated that perimeter survey was superior to stratified designs for confirmation of CLas-infected tree presence and ranged from 76.6 to 99.5% reliability of detection when disease incidence is <2% for perimeters one to seven trees deep with 24% fewer trees interrogated and a commensurate savings in deployment time (SI Appendix, Table S5). Canine team duty cycles in commercial orchards are ∼30 min followed by a rest period of similar duration, during which a second team is deployed. When the second team is resting, the first team resumes a second surveillance cycle, etc. (SI Appendix, section R).
Throughout this study, canines were exposed to diverse environmental conditions. During different studies and deployments (Florida, Texas, and California), temperature, humidity, and wind speed ranged from 10 to 38 °C, from 50 to 98%, and from 0 to 32 km/h, respectively, with no variability in detection accuracy noted. Extremes of heat and humidity can also effect the number of duty cycles that a canine team can perform per day due to fatigue of not only the canines but human handlers as well. Canine teams avoided days with rain both due to discomfort and as a safety precaution to avoid slick conditions as orchards often have uneven ground where handlers and canines can slip and fall on mud or wet grass/weeds.
Comparative Efficacy of Human Visual Inspection, PCR, and Canine Detection When Integrated into CLas Control Programs.
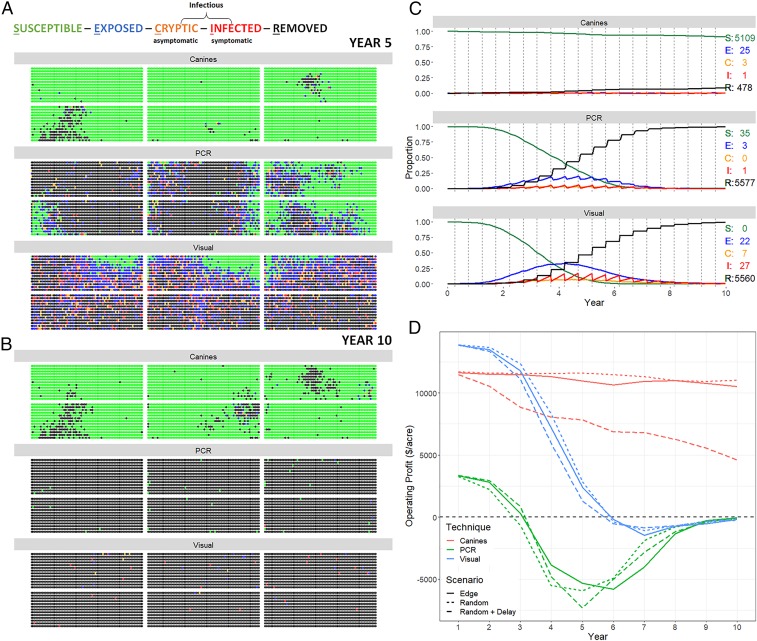
We used an SECIR (individual plants are partitioned according to the associated disease status: susceptible [i.e., uninfected], exposed, cryptic [asymptomatic], infected [symptomatic], and removed [i.e., culled]) modeling framework to simulate spatiotemporal dynamics of CLas infection (49) and examine the efficacy and sustainability of disease control programs using commonly utilized detection methodologies approved by the USDA, APHIS [e.g., visual inspection (50), PCR, and canine detection] (Fig. 4 and SI Appendix, section U and Figs. S19 and S20). Simulations focused on initial low incidence levels, detection (two full surveys each year), and removal of CLas-infected trees (within 30 d) in a 16-ha (40-acre) orchard constructed of six contiguous blocks.
Fig. 4.
SECIR simulation model comparison. Scenario-based simulations integrating disease control strategies of HLB via canine, PCR, and visual survey to detect CLas infections over a 10-y period in a 16.2-ha (40-acre) orchard with 10 initial edge infections. Fiscal outcomes are based on actual orchard management costs for 180-d survey intervals and removal of infected trees postdetection (SI Appendix, Figs. S19–S22 shows full 10-y simulations). (A) Five- and (B) 10-y grove snapshots from a single simulation run where the hosts are color coded (susceptible [S; green], exposed [E; blue], cryptic [C; orange], infected [I; red], and removed [R; black]) dots indicating the spatial location and individual tree disease status within each of six contiguous citrus planting blocks. Cryptic denotes infectious CLas individuals that are asymptomatic, while infected denotes CLas hosts that are both infectious and showing symptoms. (C) Disease dynamics and resulting tree numbers for SECIR partition for each detection method when integrated into control over 10 y. (D) Predicted dynamics of operating profit per acre for additional scenarios involving initial introduction settings (edge or random) and removal protocols (within 30 d or delayed removal up to 90 d postdetection). Profits decline steadily when deploying PCR or visual detection methods, leading to losses early in all scenarios. PCR detection is infeasible throughout due to the high cost of assays, whereas canine detection sustains both viable plantings and long-term profits when deployed twice a year, particularly when paired with prompt removal (SI Appendix, Figs. S23 and S24 shows additional removal delay and replanting effects when deploying canines).
Results demonstrate that, over a 10-y duration, canine detection resulted in removal of <8% of the trees due to CLas infection in the orchard, maintaining over 92% healthy tree population and viable orchard production, whereas the delayed detection when using PCR and visual surveillance resulted in removal of ∼97 and ∼99% CLas infection, respectively, and <2 and 0% healthy trees remaining, respectively. Visual surveillance detected less of the CLas-infected tree population over time, providing inferior disease control, but it resulted in some cryptically infected and marginally productive trees remaining for a longer duration with commensurate higher profits in early years (Fig. 4, SI Appendix, section U and Figs. S21 and S23, and Movie S7). Comparisons of the three control strategies demonstrate improved orchard health and positive cost benefit of canine early detection. In particular, simulations suggest that using canine detection linked with CLas-infected tree removal results in sustainable production and profit over the 10-y period simulated, whereas when using PCR detection linked with tree removal, profits dropped immediately and substantially within the first year due to the high cost of PCR assays. PCR detection also led to fiscal losses in years 2 to 10 when operating costs exceeded earnings, which lessened over the remaining years because the majority of trees were removed over time, requiring fewer PCR assays. Conversely, operating profit considering detection via visual surveillance linked with tree removal was slightly higher than canine detection for the first 2 y, decreased for years 3 to 5, and dropped into fiscal losses by year 6 when costs exceeded earnings (SI Appendix, Figs. S21 and S23 and Movie S7).
Additional scenarios were simulated to investigate different initial infections (edge vs. random), delays in the removal timeframe (30, 60, 90, 120 d) postdetection, and tree replacement (Fig. 4, SI Appendix, Figs. S21–S24, and Movie S8). Incorporation of annual tree replacement of any culled hosts increases the cost of orchard management, causing a slight fluctuation but sustainable profit with canine detection (with replanted trees of various age coming into increasing production) compared with significant profit erosion with visual and PCR detection over a 10-y period (Fig. 4, SI Appendix, Figs. S23 and S24, and Movie S8). Comparisons indicate that canine detection paired with relatively swift removal timelines is necessary for long-term grove sustainability without a tree replacement protocol.
Discussion
Holistic Canine Detection vs. Sampling Error of Molecular Assay.
These collective studies clearly demonstrate the early subclinical capacity of canine detection with high sensitivity and specificity for an exotic phytobacterial pathogen. Holistic canine detection vs. the sampling error associated with molecular assay has considerable implications for early detection. Over the course of the study, we assayed numerous excised parts of CLas-infected trees (leaves, twigs, stems, fruit, roots) with consistent results (i.e., canines could detect CLas infection in all citrus tissues), whereas qPCR results were inconsistent. PCR is a robust and highly accurate assay with negligible FN/FP error if the tissue selected for assay contains even a few copies of the target DNA. The issue of sampling trees for CLas is the unavoidable undersampling error. Mature citrus trees can have 1 × 105 or more leaves. When surveilling citrus orchards with thousands of trees, fiscal, manpower, and laboratory resources can be the limiting factor constraining the number of samples. If only a few leaves are selected per tree for assay, selecting tissue that is sufficiently infected from recent vector-transmitted CLas or even from old systemic infections is highly improbable (7).
Even though CLas infections take place in the tree canopy, canine detectors interrogate (sniff) both foliage and soil under trees simultaneously. The volatile CLas scent signature presumably simultaneously emanating from tree canopy and infected roots. The scent signature VOCs are transported spatially and temporally by complex fluid dynamics, forming an odor plume gradient with an epicenter that is the source: in this case, an infected tree (51). Canines interrogate the tree holistically by alerting on the CLas scent signature regardless of its origin (i.e., a single leaf, root, stem, or the entire tree if it is systemically infected) (SI Appendix, Fig. S2). Thus, early detection via canines, even of initial infections of a few cells as discussed above, is devoid of sampling issues, such as the ability to select and process only a small amount of tissue from an extensive canopy. Although canines and PCR are nearly equivalent in detection capability, the holistic assay of entire trees within 1 to 2 s (rapid real-time detection/assay large commercial or residential areas) clearly outweighs the inherent and enormous undersampling problems associated with deploying PCR as a field assay. Intuitively, any early detection method (molecular, serological, chemical, or otherwise) that relies on subsampling will not approach the detection accuracy of a method that can holistically assay an entire tree. Additionally, canine detection is essentially instantaneous, avoiding subsampling, sample processing, and chemical or molecular assay, which can require protracted time between survey and confirmation. Our data suggest that canines detect the CLas pathogen directly rather than indirectly (i.e., via a host response to infection scent signature), making canines equivalent to PCR or other direct assays from a regulatory perspective. However, it is possible that VOCs generated by a host response to infection may augment the CLas scent signature and aid canine detection.
One limitation in the deployment of the detector canines is infected host incidence. Low CLas prevalence (i.e., 5 to 10%) is optimal. As CLas incidence increases, the canines begin to alert on a high proportion of targets, requiring more reward time, which substantially slows down the search pattern. If it becomes obvious that the canines have been deployed in a high-incidence situation (i.e., the canine is alerting on a high proportion of trees), it is best to remove the canine from that particular planting. Operationally, canines are best suited to early detection scenarios rather than the identification of all infected individuals in a population of high disease prevalence. High-prevalence situations should be referred to the proper regulatory agency for appropriate regulatory action. However, canines can be useful in high-incidence residential settings where the early identification of infected trees can delimit an outbreak and rapid removal of all canine detected CLas-infected trees can curtail further pathogen spread.
Early detection and rapid regulatory response are imperative to limit exotic pathogen infection and spread. Therefore, we want to minimize the time between infection and detection of the pathogen in the host population regionally and within individual plantings to a few weeks rather than months or years as is the current delay in detection. Canines can also be trained to recognize multiple targets, which could be useful for deployment to detect introduction of any of several target exotic pathogens or pests simultaneously. Because canine detection is direct and immediate, this method has potential for use in real-time detection of bacterial, viral, and fungal exotic and endemic pathogens in field situations. Deployment of canine teams for detection of CLas in commercial and research orchards began in Southern California in 2018 to 2019.
Data Availability.
Supporting data and executable models for this study currently reside with the USDA, Agricultural Research Service. These resources are considered to be in the public domain and can be accessed at https://agcros-usdaars.opendata.arcgis.com/.
Supplementary Material
Acknowledgments
We thank F1-K9 and Coast to Coast K9 Teams, specifically Jerry Bishop and William Moraitis, for their deep collaboration on this project. We also thank Daniel Scott, David Bartels, Mamoudou Sétamou, Evan Johnson, and Georgios Vidalakis for assistance with field validations in Florida, Texas, and California; Mike Irey, Jack Williams, Tim Riley, and Holly Chamberlin for citrus production costs and human survey estimates; Neil McRoberts for editorial suggestions; and the following research technicians for assistance with studies and qPCR assessments: Len Therrien, Joanne Hodge, Cristina Gouin, Leigh Sitler, Tom Ingram, Greg Brock, Frank Albano, Michelle Preston, and Nick Rotindo. We thank the numerous technicians in the California Department of Food and Agriculture for help locating field and residential study sites and for help sampling trees for PCR confirmation and Phil Berger (USDA, Animal and Plant Health Inspection Service [APHIS]) for valuable consultation. Finally, we thank Greg Para, Mary Palm, and Angela Miller-Branagan for administrative oversight and support. This work was funded by USDA, APHIS HLB Mac Grant 14-8130-0313-CA and USDA Farm Bill Grant 13-8130-0313. Mention of a trademark, warranty, proprietary product, or vendor does not constitute a guarantee by the USDA and does not imply its approval to the exclusion of other products or vendors that may also be suitable.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited on the publicly accessible US Department of Agriculture (USDA), Agricultural Research Service (ARS), Agricultural Collaborative Research Outcomes System website, a US government website for USDA, ARS public data, models, etc., and can be directly accessed at https://gpsr.ars.usda.gov/caninehlb.
2Retired.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1914296117/-/DCSupplemental.
References
- 1.Mastin A. J., van den Bosch F., Gottwald T. R., Alonso Chavez V., Parnell S. R., A method of determining where to target surveillance efforts in heterogeneous epidemiological systems. PLoS Comput. Biol. 13, e1005712 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilkinson K., et al. , Infectious diseases of animals and plants: An interdisciplinary approach. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 1933–1942 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapin F. S., 3rd, et al. , Consequences of changing biodiversity. Nature 405, 234–242 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Aukema J. E., et al. , Historical accumulation of nonindigenous forest pests in the continental United States. Bioscience 60, 886–897 (2010). [Google Scholar]
- 5.Gottwald T., et al. , A probabilistic census-travel model to predict introduction sites of exotic plant, animal and human pathogens. Philos. Trans. R Soc. Lond. B Biol. Sci. 374, 20180260 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Invasive Species Council , General guidelines for the establishment and evaluation of invasive species early detection and rapid response systems. Version 1. https://www.doi.gov/sites/doi.gov/files/migrated/invasivespecies/edrr/upload/Guidelines-for-Early-Detection-Rapid-Response.pdf. Accessed 1 November 2019.
- 7.Gottwald T. R., McCollum T. G., Huanglongbing solutions and the need for anti-conventional thought. J. Citrus Pathol. 4, 1–8 (2017). [Google Scholar]
- 8.Zhang C., Citrus greening is killing the world’s orange trees. Scientists are racing to help. Chemical & Engineering News, 9 June 2019. https://cen.acs.org/biological-chemistry/biochemistry/Citrus-greening-killing-worlds-orange/97/i23. Accessed 11 September 2019. [Google Scholar]
- 9.Husain M. A., Nath D., The citrus psylla (Diaphorina citri Kuw.) [Psyllidae: Homoptera]. Mem. Dept. Agric. India. Entomol. Ser. 10, 1–27 (1927). [Google Scholar]
- 10.Lin K. H., Observations on yellow shoot of citrus: Etiological studies of yellow shoot of citrus. Acta Phytopathologica Sin. 2, 1–42 (1956). [Google Scholar]
- 11.Gottwald T. R., Current epidemiological understanding of citrus Huanglongbing. Annu. Rev. Phytopathol. 48, 119–139 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Bové J., Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. Plant Pathol. 88, 7–37 (2006). [Google Scholar]
- 13.Singerman A., Burani-Arounca M., Hutch S. H., The profitability of new citrus plantings in Florida in the era of huanglongbing. HortScience 53, 1655–1663 (2018). [Google Scholar]
- 14.Farnsworth D., et al. , The potential economic cost and response to greening in Florida citrus. Choices 29, 1–6 (2014). [Google Scholar]
- 15.US Department of Agriculture , National Agricultural Statistics Service, Citrus Fruits 2018 Summary (August 2018). https://downloads.usda.library.cornell.edu/usda-esmis/files/j9602060k/4t64gq48w/s7526f365/CitrFrui-08-28-2018.pdf. Accessed 11 August 2018.
- 16.Beattie G. A. C., et al. , “Aspects and insights of Australia-Asia collaborative research on huanglongbing” in Proceedings of the International Workshop for the Prevention of Citrus Greening Disease in Severely Infected Areas (Multilateral Research Network for Food and Agricultural Safety, Japanese Ministry of Agriculture, Forestry and Fisheries, Tokyo, Japan, 2006), pp. 47–64. [Google Scholar]
- 17.Pelz-Stelinski K. S., Killiny N., Better together: Association with ‘Candidatus liberibacter asiaticus’ increases the reproductive fitness of its insect vector, Diaphorina citri (Hemiptera: Liviidae). Ann. Entomol. Soc. Am. 109, 371–376 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Academies of Sciences, Engineering, and Medicine , A Review of the Citrus Greening Research and Development Efforts Supported by the Citrus Research and Development Foundation: Fighting a Ravaging Disease (National Academies Press, Washington, DC, 2018). [Google Scholar]
- 19.Aubert B., Citrus greening disease, a serious limiting factor for citriculture in Asia and Africa. Proc. Int. Soc. Citricult 2, 817–820 (1992). [Google Scholar]
- 20.da Graça J. V., Korsten L., “Citrus huanglongbing: Review, present status and future strategies” in Diseases of Fruits and Vegetables, Naqvi S. A. M. H., Ed. (Kluwer Academic Press, Dordrecht, the Netherlands, 2004), vol. 1, pp. 229–245. [Google Scholar]
- 21.Gottwald T. R., da Graça J. V., Bassanezi R. B., Citrus huanglongbing: The pathogen, its epidemiology, and impact. Plant Health Progress, 10.1094/PHP-2007-0906-01-RV (2007).
- 22.Anonymous , “Multiplex TaqMan real-time PCR for screening detection of citrus Huanglongbing (HLB) in citrus tissues” (Work Instruction WI-B-T-D-2, US Department of Agriculture, Washington, DC, 2013).
- 23.McGuire B., McRoberts N., An update from the early detection technologies task force. Citrograph 10, 40–43 (2019). [Google Scholar]
- 24.McGuire B., McRoberts N., California-based EDT experiment–Baseline data collection and quarantine zone testing. Citrograph 10, 44–49 (2019). [Google Scholar]
- 25.Li C., Krewer G., Kays S. J., “Blueberry postharvest disease detection using an electronic nose” in American Society of Agricultural and Biological Engineers Annual International Meeting (American Society of Agricultural and Biological Engineers, St. Joseph, MI, 2009), paper no. 096783.
- 26.Laothawornkitkul J., et al. , Discrimination of plant volatile signatures by an electronic nose: A potential technology for plant pest and disease monitoring. Environ. Sci. Technol. 42, 8433–8439 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Sankarana S., et al. , A review of advanced techniques for detecting plant diseases. Comput. Electron. Agric. 72, 1–13 (2010). [Google Scholar]
- 28.Aksenov A. A., et al. , Detection of huanglongbing disease using differential mobility spectrometry. Anal. Chem. 86, 2481−2488 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Dala-Paula B. M., et al. , Effect of huanglongbing or greening disease on orange juice quality, a review. Front. Plant Sci. 9, 1976 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leffingwell J. C., Olfaction–Update no. 5 (Leffingwell & Associates, Canton, GA, 2002). http://www.leffingwell.com/download/Olfaction5.pdf. Accessed 7 November 2019.
- 31.Vilà C., et al. , Multiple and ancient origins of the domestic dog. Science 276, 1687–1689 (1997). [DOI] [PubMed] [Google Scholar]
- 32.Savolainen P., Zhang Y. P., Luo J., Lundeberg J., Leitner T., Genetic evidence for an East Asian origin of domestic dogs. Science 298, 1610–1613 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Leonard J. A., et al. , Ancient DNA evidence for Old World origin of New World dogs. Science 298, 1613–1616 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Lorenzo N., et al. , Laboratory and field experiments used to identify Canis lupus var. familiaris active odor signature chemicals from drugs, explosives, and humans. Anal. Bioanal. Chem. 376, 1212–1224 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Galibert F., Quignon P., Hitte C., André C., Toward understanding dog evolutionary and domestication history. C. R. Biol. 334, 190–196 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Browne C., et al. , The use of scent-detection dogs. Ir. Vet. J. 59, 97–104 (2006). [Google Scholar]
- 37.Moser E., McCulloch M., Canine scent detection of human cancers: A review of methods and accuracy. J. Vet. Behav. 5, 145–152 (2010). [Google Scholar]
- 38.Willis C. M., et al. , Olfactory detection of human bladder cancer by dogs: Proof of principle study. BMJ 329, 712–714 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordon R. T., et al. , The use of canines in the detection of human cancers. J. Altern. Complement. Med. 14, 61–67 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Leitch O., Anderson A., Kirkbride K. P., Lennard C., Biological organisms as volatile compound detectors: A review. Forensic Sci. Int. 232, 92–103 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Helton W. S., The Science of Working Dogs (CRC Press, Boca Raton, FL, 2009), pp. 83–97. [Google Scholar]
- 42.Cablk M. E., Sagebiel J. C., Heaton J. S., Valentin C., Olfaction-based Detection Distance: A quantitative analysis of how far away dogs recognize tortoise odor and follow it to source. Sensors (Basel) 8, 2208–2222 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wells D. L., Hepper P. G., Directional tracking in the domestic dog, Canis familiaris. Appl. Anim. Behav. Sci. 84, 297–305 (2003). [Google Scholar]
- 44.Rascoe J., et al. , Candidatus Liberibacter crescens detected in citrus (abstract). J. Cit. Pathol. 4, 35 (2017). [Google Scholar]
- 45.Cevallos-Cevallos J. M., Futch D. B., Shilts T., Folimonova S. Y., Reyes-De-Corcuera J. I., GC-MS metabolomic differentiation of selected citrus varieties with different sensitivity to citrus huanglongbing. Plant Physiol. Biochem. 53, 69–76 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Furton K. G., Myers L. J., The scientific foundation and efficacy of the use of canines as chemical detectors for explosives. Talanta 54, 487–500 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Davis M. J., Mondal S. N., Chen H., Rogers M. E., Brlansky R. H., Co-cultivation of ‘Candidatus liberibacter asiaticus’ with Actinobacteria from Citrus with Huanglongbing. Plant Dis. 92, 1547–1550 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Anco D. J., Gottwald T. R. Within-orchard edge effects of the azimuth of the sun on Diaphorina citri adults in mature orchards. J. Cit. Pathol. 2, https://escholarship.org/uc/item/14w3d91j (2015).
- 49.Parry M., et al. , Bayesian inference for an emerging arboreal epidemic in the presence of control. Proc. Natl. Acad. Sci. U.S.A. 111, 6258–6262 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bassanezi R. B., et al. , Yield loss caused by huanglongbing in different sweet orange cultivars in São Paulo, Brazil. Eur. J. Plant Pathol. 130, 577–586 (2011). [Google Scholar]
- 51.Moore P. A., “Aerodynamics of odor plums and odor plum structures in different habitats” in Canine Olfaction Science and Law, Advances in Forensic Science, Medicine, Conservation, and Environmental Remediation, Jezierski T., Ensminger J., Papet L. E., Eds. (CRC Press, Boca Raton, FL, 2016), pp. 87–102. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supporting data and executable models for this study currently reside with the USDA, Agricultural Research Service. These resources are considered to be in the public domain and can be accessed at https://agcros-usdaars.opendata.arcgis.com/.