Abstract
Objective
Management of heart failure is complex and multifaceted but adherence to medications remains the cornerstone of preventing avoidable readmissions, premature deaths, and unnecessary healthcare expenses. Despite of evidence-based efficacy on anti-failure drugs, poor adherence is pervasive and remains a significant barrier to improving clinical outcomes in heart failure population.
Results
We enrolled 459 patients with diagnosis of heart failure admitted at a tertiary cardiovascular hospital in Dar es Salaam, Tanzania. The mean age was 46.4 years, there was a female predominance (56.5%), 67.5% resided in urban areas and 74.2% had primary education. Of the 419 participants eligible for assessment of medication adherence, 313 (74.7%) had poor adherence and 106 (25.3%) had good adherence. Possession of a health insurance was found to be the strongest associated factor for adherence (adjusted OR 8.7, 95% CI 4.7–16.0, p < 0.001). Participants with poor adherence displayed a 70% increased risk for rehospitalization compared to their counterparts with good adherence (adjusted RR 1.7, 95% CI 1.2–2.9, p = 0.04). Poor adherence was found to be the strongest predictor of early mortality (HR 2.5, 95% CI 1.3–4.6, p < 0.01). In conclusion, Poor medication adherence in patients with heart failure is associated with increased readmissions and mortality.
Keywords: Heart failure, Nonadherence, Poor adherence, Low adherence, Drug adherence, Medication adherence, Medication compliance, Noncompliance, Tanzania
Introduction
Cardiovascular disorders (CVD) are responsible for about one-third of all global mortality with over three-quarters of deaths transpiring in the developing world [1]. In spite of the remarkable advances in novel screening techniques and therapeutic directions, the prognosis of heart failure (HF) remains strikingly poor around the globe particularly in the developing nations [2–7]. Owing to its chronic nature, clinical management of HF necessitate long-term use of several drugs to reduce morbidity [8–10] and mortality [11–13]. However, universally low prescription rates of such drugs among patients who require them is observed [14].
Despite of all developments in HF management, adherence plays a pivotal role in attaining maximal therapeutic benefits. Nevertheless, regardless of the assessment tool used or population studied, adherence rates are consistently suboptimal across studies making it a significant public health issue [15–25]. Poor adherence to prescribed regimens is pervasive and results in preventable hospitalizations, premature deaths and unnecessary health care expenditure regardless of the underlying cardiovascular etiology [15–26]. There is dearth of information regarding medication adherence among heart failure population in Tanzania and Sub-Saharan Africa at large. In this prospective cohort study, we sought to explore the adherence pattern, associated factors and outcomes among hospitalized heart failure patients in a tertiary hospital in Tanzania.
Main text
Methods
Recruitment process and definition of terms
All patients who were hospitalized at Jakaya Kikwete Cardiac Insitute (a tertiary care public teaching hospital) between March and October 2018 with established diagnosis of heart failure (for at least 3 months’ prior enrollment) were consecutively enrolled for this study. Sociodemographic, clinical, laboratory, echocardiographic, and adherence data were gathered using a structured questionnaire during the hospital admission of enrollment. Framingham criteria was used to screen participants for heart failure symptoms and a 2-dimensional echocardiography was utilized for diagnosis reconfirmation. Renal functions were estimated using the Modification of Diet in Renal Disease equation and estimated glomerular filtration rate (eGFR) value of < 60 mL/min/1.73 m2 was used to define renal dysfunction. Diagnosis of anemia utilized the WHO criteria i.e. Hemoglobin (Hb) concentration of < 13.0 g/dL and < 12.0 g/dL for males and females respectively. Diabetes was defined by fasting blood glucose levels ≥ 7.0 mmol/L or use of glucose lowering agents. Hypertension was defined as systolic blood pressure (SBP) > 140 mmHg and/or diastolic blood pressure (DBP) > 90 mmHg or use of antihypertensive medications. Total cholesterol level greater than 6.2 mmol/L was used to define dyslipidemia. Hyponatremia, hypokalemia, hypocalcemia, and hypomagnesemia were defined by concentrations < 135 mmol/L, < 3.5 mmol/L, < 2.1 mmol/L and < 0.7 mmol/L respectively. Potassium levels > 5.0 mmol/L was used to denote hyperkalemia. We assessed adherence based on the last time a participant last took her heart failure medications. For the purpose of this study, we defined good adherence as intake of all prescribed heart failure medications within 72 h before the admission of recruitment.
Follow-up and study outcomes
Follow-up was conducted through scheduled weekly phone calls and continued through April 2019 with a predetermined stopping point providing a maximum of 180 days of follow-up for each patient after enrollment. Data was censored after completion of follow-up or death, whichever occurred first. A participant was deemed lost to follow-up when despite all attempts couldn’t be reached through phone numbers provided. Our primary outcome measures were rehospitalization and all-cause mortality. We defined rehospitalization as any cardiovascular-related hospital admission following a successful discharge from the hospitalization of enrollment. Early mortality was defined as death during the hospitalization of enrollment.
Statistical analysis
All statistical analyses utilized STATA v11.0 software. Pearson Chi square and Student’s T-test were used to compare categorical and continuous variables respectively. Logistic regression analyses was used to assess for factors associated with adherence and predictors of rehospitalization. Factors included in our logistic regression model included age, sex, education level, marital status, employment status, residence, comorbidities and possession of health insurance. Based on their adherence status, participants were compared with respect to survival using Cox proportional-hazards regression model. Differences in survival between the low- and high-adherence groups were compared using the log-rank test. We report Odds ratio (OR), Relative risk (RR) and Hazard ratio (HR) with 95% confidence intervals (CI) and p-values where appropriate. All tests were 2-sided and p < 0.05 was used to denote statistical significance.
Results
Study population
A total of 459 heart failure patients met the inclusion criteria and were enrolled into this study. During follow-up, 40 (8.7%) participants exited; 5 due to incomplete key data and 35 were lost to follow-up. Table 1 displays the baseline characteristics of participants. The mean age of our heart failure cohort was 46.4 ± 18.9 years, there was female preponderance (56.6%) and over two-thirds of all participants resided in urban areas. The mean BMI was 25.1 ± 5.2 and 39.4% of patients were overweight or obese. About 7.2% of participants were in NYHA functional class II while classes III and IV constituted 36.5% and 56.3% respectively. Heart failure with reduced ejection fraction (HFrEF) was present in 284 (67.8%) of participants while 135 (32.2%) had preserved systolic functions (HFpEF). Over a half (52.7%) of participants had a history of hypertension, 13.6% had diabetes, 6.7% were infected with HIV, 51.3% had renal insufficiency and 72.1% were anemic. Echocardiography revealed hypertensive heart disease was the predominant cause of HF (40.1%) followed by dilated cardiomyopathy (27.0%) and rheumatic heart disease (23.2%).
Table 1.
Baseline characteristics of participants (N = 419)
| Characteristic | All | Poor adherence | Good adherence | p-value |
|---|---|---|---|---|
| (N = 419) | (n = 313) | (n = 106) | ||
| Age | 46.4 (18.9) | 45.5 (19.0) | 49.1 (18.6) | 0.09 |
| Age groups | ||||
| < 30 | 103 (24.6%) | 82 (26.2%) | 21 (19.8%) | 0.19 |
| 30–50 | 129 (30.8%) | 96 (30.7%) | 33 (31.1%) | 0.94 |
| > 50 | 187 (44.6%) | 135 (43.1%) | 52 (49.1%) | 0.28 |
| Sex | ||||
| Male | 182 (43.4%) | 139 (44.4%) | 43 (40.6%) | 0.5 |
| Female | 237 (56.6%) | 174 (55.6%) | 63 (59.4%) | |
| Residence | ||||
| Urban | 283 (67.5%) | 197 (62.9%) | 86 (81.1%) | 0.001 |
| Rural | 136 (32.5%) | 116 (37.1%) | 20 (18.9%) | |
| Marital status | ||||
| Single | 100 (23.9%) | 82 (26.2%) | 18 (17.0%) | 0.05 |
| Married | 296 (70.6%) | 213 (68.1%) | 83 (78.3%) | 0.05 |
| Divorced/widowed | 23 (05.5%) | 18 (05.7%) | 5 (04.7%) | 0.67 |
| Education | ||||
| None | 16 (03.8%) | 12 (03.9%) | 4 (03.8%) | 0.96 |
| Primary | 295 (70.4%) | 248 (79.2%) | 47 (44.3%) | <0.001 |
| Secondary | 68 (16.2%) | 36 (11.5%) | 32 (30.2%) | <0.001 |
| University | 40 (09.6%) | 17 (05.4%) | 23 (21.7%) | <0.001 |
| Occupation | ||||
| None | 76 (18.1%) | 47 (15.0%) | 29 (27.3%) | <0.01 |
| Employed/self-employed | 311 (74.3%) | 250 (79.9%) | 61 (57.6%) | <0.001 |
| Retired | 32 (07.6%) | 16 (05.1%) | 16 (15.1%) | 0.001 |
| Body mass index | 25.1 (05.2) | 24.8 (04.2) | 26.0 (07.4) | 0.04 |
| BMI categories | ||||
| Underweight | 11 (02.6%) | 7 (02.2%) | 4 (03.8%) | 0.37 |
| Normal | 243 (58. 0%) | 188 (60.1%) | 55 (51.9%) | 0.14 |
| Overweight | 105 (25.1%) | 79 (25.2%) | 26 (24.5%) | 0.89 |
| Obese | 60 (14.3%) | 39 (12.5%) | 21 (19.8%) | 0.06 |
| Health insured | ||||
| Yes | 93 (22.2%) | 32 (10.2%) | 61 (57.6%) | <0.001 |
| No | 326 (77.8%) | 281 (89.8%) | 45 (42.4%) | |
| HF etiology | ||||
| DCM | 113 (27.0%) | 78 (24.9%) | 34 (32.1%) | 0.15 |
| HHD | 168 (40.1%) | 134 (42.8%) | 35 (33.0%) | 0.08 |
| RHD | 97 (23.2%) | 72 (23.0%) | 25 (23.6%) | 0.9 |
| Others | 41 (09.8%) | 29 (09.3%) | 12 (11.3%) | 0.55 |
| Comorbidities | ||||
| Hypertension | 221 (52.7%) | 171 (54.6%) | 50 (47.2%) | 0.19 |
| Diabetes | 57 (13.6%) | 39 (12.5%) | 18 (17.0%) | 0.24 |
| HIV/AIDS | 28 (06.7%) | 15 (04.8%) | 13 (12.3%) | 0.01 |
| Renal insufficiency | 215 (51.3%) | 163 (52.1%) | 52 (49.1%) | 0.59 |
| eGFR < 15 | 100 (23.9%) | 80 (25.6%) | 20 (18.9%) | 0.16 |
| Anemia | 302 (72.1%) | 234 (74.8%) | 68 (64.2%) | 0.04 |
| Hb < 8 g/dL | 99 (23.6%) | 75 (24.0%) | 24 (22.6%) | 0.77 |
| NYHA class | ||||
| II | 30 (07.2%) | 19 (06.0%) | 11 (10.4%) | 0.13 |
| III | 153 (36.5%) | 112 (35.8%) | 41 (38.7%) | 0.59 |
| IV | 236 (56.3%) | 182 (58.2%) | 54 (50.9%) | 0.19 |
| Systolic functions | ||||
| Preserved (HFpEF) | 135 (32.2%) | 96 (71.1%) | 39 (28.9%) | 0.24 |
| Reduced (HFrEF) | 284 (67.8%) | 217 (76.4%) | 67 (23.6%) | |
| Admission days | 14.0 (13.3) | 13.8 (13.4) | 14.3 (12.8) | 0.74 |
| HF-related hospitalization | ||||
| 1st | 211 (50.4%) | 167 (53.3%) | 44 (41.5%) | 0.04 |
| > 1 | 208 (49.6%) | 146 (46.7%) | 62 (58.5%) | |
Medication adherence
Overall, 337 (73.4%) were on angiotensin converting enzyme inhibitors (ACEI), 122 (26.6%) on angiotensin receptor blockers (ARB), 386 (84.1%) on beta-blockers, 432 (94.1%) on diuretics, 395 (86.1%) on aldosterone antagonists, 166 (36.2%) on inotropes and 36 (7.8%) were on digoxin. Of the 419 participants eligible for assessment of medication adherence, 313 (74.7%) had poor adherence and 106 (25.3%) had good adherence. The mean number of days’ participants last took medications before the index hospitalization was 17.7 (± 6.9) days. Among participants with poor adherence, 254 (81.2%) had not taken any of their anti-failure medications within the past 1-week prior admission. Inability to afford medications was the most (87.3%) reported reason for nonadherence. Other reported factors affecting adherence in this cohort included; medication side effects (8.1%), forgetfulness (53.9%), negligence (26.0%), local unavailability of drugs (18.9%) and pill burden (34.4%). Differences in age, sex, marital status, and BMI displayed similar medication adherence patterns, Table 1. However, during bivariate analyses four characteristics including education level, residence, employment status, and health insurance possession showed significant associations with adherence, Table 2. Significant variables then underwent multivariate logistic regression analysis where possession of a health insurance was found to be the strongest associated factor for adherence (OR 8.7, 95% CI 4.7–16.0, p < 0.001), Table 2.
Table 2.
Factors associated with adherence
| Control group | Comparative group | OR | 95% CI | p-value | Adj. OR | Adj. 95% CI | Adj. p-value |
|---|---|---|---|---|---|---|---|
| Age < 50 | Age ≥ 50 | 0.8 | 0.5–1.2 | 0.3 | – | – | – |
| Female | Male | 1.2 | 0.7–1.8 | 0.5 | – | – | – |
| ≥ Secondary education | ≤ Primary education | 5.3 | 3.3–8.6 | <0.001 | 1.9 | 0.9–4.0 | 0.07 |
| Married | Single | 1.7 | 1.0–2.8 | 0.05 | – | – | – |
| Employed | No employment | 0.3 | 0.2–0.5 | <0.001 | 1.2 | 0.6–2.4 | 0.6 |
| Urban | Rural | 2.5 | 1.5–4.3 | 0.001 | 2.0 | 1.1–3.7 | 0.03 |
| No comorbidity | ≥ 1 comorbidity | 0.9 | 0.5–1.4 | 0.56 | – | – | – |
| Health insurance | Not insured | 11.9 | 7.0–20.2 | <0.001 | 8.7 | 4.7–16.0 | <0.001 |
| HFpEF | HFrEF | 1.3 | 0.8–2.1 | 0.28 | – | – | – |
Rehospitalization and mortality
Overall, 208 (49.6%) patients had a history of a prior cardiovascular-related hospitalization. Despite of similar rehospitalization rates between poor and good-adherence participants at 30-days (35.4% vs 27.2%, p = 0.12) and 90-days (51.8% vs 40.2%, p = 0.07), patients with poor adherence had significantly higher rates of rehospitalization at 180 days (57.5% vs 43.5%, p = 0.03). Overall, participants with poor adherence displayed a 70% increased risk for rehospitalization compared to their counterparts with good adherence (RR 1.7, 95% CI 1.2–2.9, p = 0.04).
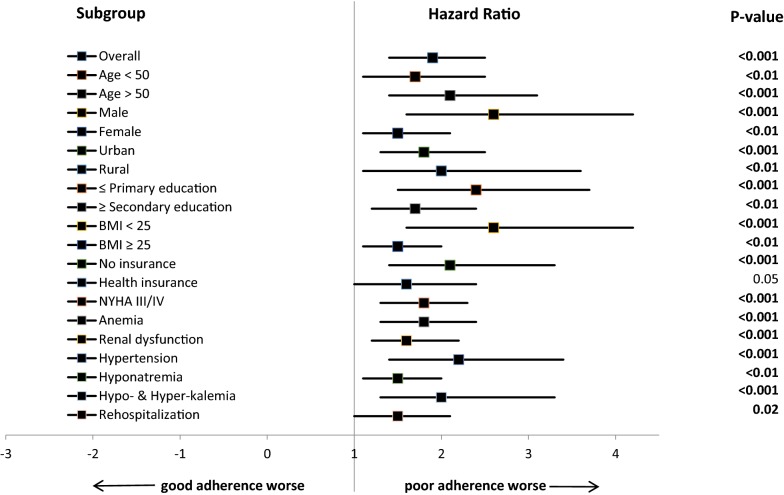
177 (42.2%) patients survived the 180-days of follow-up. The mean survival days was 103.3 ± 74.8 days and participants with good adherence (140.5 ± 63.1 days) displayed a longer survival compared to their poor adherence (90.8 ± 74.3 days) counterparts, p < 0.001. Regardless of the assessment time, participants with poor adherence displayed superior mortality compared to those with good adherence i.e. 37.1% vs 12.3%, 56.6% vs 25.5%, and 65.5% vs 34.9% at 30, 90, and 180 days respectively; all p < 0.001). Additionally, we performed subgroup analyses to assess for all-cause mortality by adherence status. In all 19 characteristics involved in subgroup analyses, participants with poor adherence had inferior survival rates compared to their counterparts with good adherence, Fig. 1. More interestingly, even within the subgroup of those who possessed a health insurance, it was observed that poor adherence participants fared worse compared to good adherence controls, (HR 1.6, 95% CI 1.0–2.4, p = 0.05).
Fig. 1.
Hazard Ratios for All-cause Mortality by Adherence status. This forest plot shows the hazard ratios (black squares), 95% CIs (horizontal lines), and p-values for the interaction between the All-cause mortality and any subgroup variable by Adherence status
Discussion
Management of heart failure is complex and multifaceted but adherence to medications remains a fundamental measure to prevent acute exacerbations [27, 28]. Despite of unwavering evidence on the efficacy of anti-failure drugs, poor adherence is common and remains a significant barrier to improving clinical outcomes in heart failure population. Estimates of nonadherence in heart failure patients have varied widely (22–90%) [18, 25, 29–36] in the literature. In this present study, less than one-fifth of participants were categorized as having high adherence. Our rate of nonadherence is skewed to the extreme undesired end of the reported range in the literature.
With regards to reasons for poor adherence, numerous factors have predominated in various studies. For instance, in studies by Toh et al. (71%) and Mujtaba et al. (72.7%), poor medication instructions was the most reported factor [25, 36]. On the other hand, studies by Aggarwal et al. and Dickson et al. found forgetfulness and comorbidities respectively as the leading factors for nonadherence [29, 32]. In this present study, nearly 90% of nonadherent participants reported medication cost as the major barrier to their adherence. These findings are in unison with Dunlay et al. study as far as cost being the most reported factor is concerned, however it was a barrier in a significantly lesser proportion (22%) compared to what we observed [37]. While majority of known risk factors for nonadherence are potentially modifiable, inability to comply due to poverty is not. Owing to this, improving medication adherence in impoverished societies continues to be a very difficult undertaking. It should not be forgotten that such poor societies and their already overwhelmed health sectors continue to struggle with prevention and management of the ever present infectious diseases.
Several studies have demonstrated the repercussions of poor adherence on prognosis of heart failure [16, 32, 33, 38, 39]. Moreover, numerous studies have established the prognostic benefits of interventions to improve adherence [24, 40–50]. In this present study, nearly 60% of participants with poor adherence were rehospitalized within 6-months of enrollment. Our findings are in consonance with several other prospective studies which have produced rehospitalization rates ranging between 20 and 69% [16, 32, 33, 38, 39]. Additionally, intervention studies have uniformly shown that improved adherence is associated with reduction (3–96%) in readmission risk [40, 41, 43, 44, 46–49]. Furthermore, systematic reviews and meta-analyses by Ruppar et al. and Unverzagt et al. revealed a 21% and 10% decreased odds of rehospitalization respectively in the adherence intervention arm [24, 50].
Survival prospects among heart failure patients remain poor all over the globe. Overall, less than half of patients in this study survived the 6-months of follow-up. Nonadherent participants displayed about three times mortality hazard compared to their adherent counterparts. Similar to our findings, intervention studies have shown mortality reduction (2–84%) in favor of adherent participants [40–42, 44–46, 48]. Moreover, two meta-analyses that included over 50 studies each showed a 2% and 11% mortality reduction in favor of the adherence intervention arm [24, 50]. Poor adherence was found to be the strongest predictor of early mortality in this study. To solidify on the significance of adherence in heart failure prognostication, participants with low adherence displayed significantly higher rates of primary outcomes compared to their high adherence counterparts in all subgroup analyses we conducted.
Conclusions
In conclusion, findings of this present study provide important insight pertaining to medication adherence and its potential in dictating the prognosis of heart failure patients residing in resource-limited settings. Poor adherence in patients with heart failure contributes to a considerable burden on the healthcare system above all increased rehospitalizations and mortality. These findings call for deliberate efforts to ensure that measures to assess and improve adherence are incorporated and become an integral component in routine clinical practice. Furthermore, strategies to improve health insurance acquisition including endeavours to make it a right rather than a privilege is fundamental in improving adherence especially among persons living in impoverished societies.
Limitations
Medication adherence was ascertained by self-report and thus reporting bias and recall bias could have in some way affected our findings. Prospective comparison of patients receiving adherence intervention versus control would allow a more rigorous evaluation of adherence potential in prognosticating heart failure and should be considered in the future studies in this setting.
Acknowledgements
We thank the nursing and medical staff of the Jakaya Kikwete Cardiac Institute for their cooperation and active participation during this study. We extend our gratitude to all the study participants and their relatives for their willingness, tolerance and cooperation offered during the entire study duration.
Abbreviations
- ACE
Angiotensin converting enzyme
- ARB
Angiotensin receptor blocker
- BMI
Body mass index
- CI
Confidence interval
- CVD
Cardiovascular disorders
- DBP
Diastolic blood pressure
- eGFR
Estimated glomerular filtration rate
- FBG
Fasting blood glucose
- Hb
Hemoglobin
- HF
Heart failure
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- HR
Hazard ratio
- MDRD
Modification of diet in renal disease
- MMAS-8
8-Item Morisky Medication Adherence Scale
- NYHA
New York Heart Association
- OR
Odd ratio
- RR
Relative risk
- SBP
Systolic blood pressure
- WHO
World Health Organization
Authors’ contributions
PP conceived the study. JM, ZM, NM, HJS and NRH conducted all the interviews and physical examinations. Echocardiography was performed by MJ, AK, and SB. PP performed data entry and analysis. The corresponding author wrote the first draft of the manuscript, and other authors contributed to and approved it. All authors made the decision to submit the manuscript for publication. All authors assume responsibility for the accuracy and integrity of the analysis. All authors read and approved the final manuscript.
Funding
This work was funded by the Commission for Science and Technology (COSTECH) of Tanzania. The funder had no role in the design of this study, collection of data, data analysis, interpretation of results or writing of this manuscript.
Availability of data and materials
The final version of data set supporting the findings of this paper is submitted together with this manuscript to the editorial committee. All the raw data is included in this manuscript. There are no ethics restrictions preventing the sharing of the raw data.
Ethics approval and consent to participate
Participants gave written informed consent to participate in the study. The study protocol was approved by the local ethics committees (Muhimbili University of Health and Allied Sciences) and was conducted in accordance with the Declaration of Helsinki.
Consent to publish
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pedro Pallangyo, Email: pedro.pallangyo@gmail.com.
Jalack Millinga, Email: jalackmillinga2@gmail.com.
Smita Bhalia, Email: sbhaliaa@gmail.com.
Zabella Mkojera, Email: zabellaseif@gmail.com.
Nsajigwa Misidai, Email: nsajmisidai@gmail.com.
Happiness J. Swai, Email: hajuswa@gmail.com
Naairah R. Hemed, Email: neebrah20@gmail.com
Alice Kaijage, Email: kaijagealice@gmail.com.
Mohamed Janabi, Email: moddyyakubu@gmail.com.
References
- 1.World Health Organization. Cardiovascular diseases Fact sheet reviewed September 2016. http://www.who.int/mediacentre/factsheets/fs317/en/.
- 2.Kraus S, Ogunbanjo G, Sliwa K, Ntusi NAB. Heart failure in sub-Saharan Africa: a clinical approach. S Afr Med J. 2016;106(1):23–31. doi: 10.7196/SAMJ.2016.v106i1.10325. [DOI] [PubMed] [Google Scholar]
- 3.Ntusi NB, Mayosi BM. Epidemiology of heart failure in sub-Saharan Africa. Expert Rev Cardiovasc Ther. 2009;7(2):169–180. doi: 10.1586/14779072.7.2.169. [DOI] [PubMed] [Google Scholar]
- 4.Makubi A, Hage C, Lwakatare J, et al. Contemporary aetiology, clinical characteristics and prognosis of adults with heart failure observed in a tertiary hospital in Tanzania: the prospective Tanzania Heart Failure (TaHeF) study. Heart. 2014;100(16):1235–1241. doi: 10.1136/heartjnl-2014-305599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pallangyo P, Fredrick F, Bhalia S, et al. Cardiorenal anemia syndrome and survival among heart failure patients in Tanzania: a prospective cohort study. BMC Cardiovasc Disord. 2017;17:59. doi: 10.1186/s12872-017-0497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parmar KR, Xiu PY, Chowdhury MR, et al. In-hospital treatment and outcomes of heart failure in specialist and non-specialist services: a retrospective cohort study in the elderly Open. Heart. 2015;2:e000095. doi: 10.1136/openhrt-2014-000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karaye KM, Sani MU. Factors associated with poor prognosis among patients admitted with heart failure in a Nigerian tertiary medical centre: a cross-sectional study. BMC Cardiovasc Disord. 2008;8:16. doi: 10.1186/1471-2261-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gheorghiade M, Patel K, Filippatos G, et al. Effect of oral digoxin in high-risk heart failure patients: a pre-specified subgroup analysis of the DIG trial. Eur J Heart Fail. 2013;15(5):551–559. doi: 10.1093/eurjhf/hft010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 10.Metra M, Teerlink JR, Voors AA, et al. Vasodilators in the treatment of acute heart failure: what we know, what we don’t. Heart Fail Rev. 2009;14:299–307. doi: 10.1007/s10741-008-9127-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konstam MA, Rousseau MF, Kronenberg MW, Udelson JE, Melin J, Stewart D, et al. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dysfunction in patients with heart failure. SOLVD Investigators. Circulation. 1992;86(2):431–438. doi: 10.1161/01.CIR.86.2.431. [DOI] [PubMed] [Google Scholar]
- 12.Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362(9386):759–766. doi: 10.1016/S0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 13.Haas SJ, Vos T, Gilbert RE, Krum H. Are beta-blockers as efficacious in patients with diabetes mellitus as in patients without diabetes mellitus who have chronic heart failure? A meta-analysis of large-scale clinical trials. Am Heart J. 2003;146:848–853. doi: 10.1016/S0002-8703(03)00403-4. [DOI] [PubMed] [Google Scholar]
- 14.Yusuf S, Islam S, Chow CK, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet. 2011;378(9798):1231–1243. doi: 10.1016/S0140-6736(11)61215-4. [DOI] [PubMed] [Google Scholar]
- 15.Simpson SH, Eurich DT, Majumdar SR, et al. A metaanalysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333:18–26. doi: 10.1136/bmj.333.7557.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 17.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119:3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 18.Oosterom-Calo R, van Ballegooijen AJ, Terwee CB, et al. Determinants of adherence to heart failure medication: a systematic literature review. Heart Fail Rev. 2013;18:409–427. doi: 10.1007/s10741-012-9321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juarez DT, Williams AE, Chen C, et al. Factors affecting medication adherence trajectories for patients with heart failure. Am J Manag Care. 2015;21(3):197–205. [PMC free article] [PubMed] [Google Scholar]
- 20.Molloy GJ, O’Carroll RE, Witham MD, et al. Interventions to enhance adherence to medications in patients with heart failure: a systematic review. Circ Heart Fail. 2012;5:126–133. doi: 10.1161/CIRCHEARTFAILURE.111.964569. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald AA, Powers JD, Ho PM, et al. Impact of medication nonadherence on hospitalizations and mortality in heart failure. J Card Fail. 2011;17(8):664–669. doi: 10.1016/j.cardfail.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Shah D, Simms K, Barksdale DJ, Wu JR. Improving medication adherence of patients with chronic heart failure: challenges and solutions. Res Rep Clin Cardiol. 2015;6:87–95. [Google Scholar]
- 23.Ruppar TM, Delgado JM, Temple J. Medication adherence interventions for heart failure patients: a meta-analysis. Eur J Cardiovasc Nurs. 2015;14(5):395–404. doi: 10.1177/1474515115571213. [DOI] [PubMed] [Google Scholar]
- 24.Ruppar TM, Cooper PS, Mehr DR, et al. Medication adherence interventions improve heart failure mortality and readmission rates: systematic review and meta-analysis of controlled trials. J Am Heart Assoc. 2016;5(6):e002606. doi: 10.1161/JAHA.115.002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toh CT, Jackson B, Gascard DJ, et al. Barriers to medication adherence in chronic heart failure patients during home visits. J Pharm Pract Res. 2010;40:27–30. doi: 10.1002/j.2055-2335.2010.tb00721.x. [DOI] [Google Scholar]
- 26.Sabate´ E. Adherence to long-term therapies: evidence for action. Geneva: World Health Organization; 2003. [Google Scholar]
- 27.Kemp C, Conte J. The pathophysiology of heart failure. Cardiovasc Pathol. 2012;21(5):365–371. doi: 10.1016/j.carpath.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 28.O’Connor CM, Stough WG, Gallup DS, Hasselblad V, Gheorghiade M. Demographics, clinical characteristics, and outcomes of patients hospitalized for decompensated heart failure: observations from the IMPACT-HF registry. J Card Fail. 2005;11(3):200–205. doi: 10.1016/j.cardfail.2004.08.160. [DOI] [PubMed] [Google Scholar]
- 29.Aggarwal B, Pender A, Mosca L, Mochari-Greenberger H. Factors associated with medication adherence among heart failure patients and their caregivers. J Nurs Educ Pract. 2015;5(3):22–27. doi: 10.5430/jnep.v5n3p22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Wu SH, Fendrick AM, Baicker K. Variation in medication adherence in heart failure. JAMA Intern Med. 2013;173(6):468–470. doi: 10.1001/jamainternmed.2013.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knafl GJ, Riegel B. What puts heart failure patients at risk for poor medication adherence? Patient Prefer Adherence. 2014;17(8):1007–1018. doi: 10.2147/PPA.S64593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickson VV, Knafl GJ, Riegel B. Predictors of medication nonadherence differ among black and white patients with heart failure. Res Nurs Health. 2015;38(4):289–300. doi: 10.1002/nur.21663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu JR, Frazier SK, Rayens MK, et al. Medication adherence, social support, and event-free survival in patients with heart failure. Health Psychol. 2013;32(6):637–646. doi: 10.1037/a0028527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alakhali KM, Daniel PS, Noohu AM, Sirajudeen SA. Patient medication adherence and physician prescribing among congestive heart failure patients of Yemen. Indian J Pharm Sci. 2013;75(5):557–562. [PMC free article] [PubMed] [Google Scholar]
- 35.Lee D, Mansi I, Bhushan S, Parish R. Non-adherence in at-risk heart failure patients: characteristics and outcomes. J Nat Sci. 2015;1(5):e95. [Google Scholar]
- 36.Mujtaba SF, Masood T, Saad M. Reasons of medical noncompliance in heart failure patients. Pak Heart J. 2010;43:3–4. [Google Scholar]
- 37.Dunlay SM, Eveleth JM, Shah ND, et al. Medication adherence among community-dwelling patients with heart failure. Mayo Clin Proc. 2011;86(4):273–281. doi: 10.4065/mcp.2010.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albert NM. Improving medication adherence in chronic cardiovascular disease. Crit Care Nurse. 2008;28(5):54–64. [PubMed] [Google Scholar]
- 39.Leventhal MJ, Riegel B, Carlson B, De GS. Negotiating compliance in heart failure: remaining issues and questions. Eur J Cardiovasc Nurs. 2005;4:298–307. doi: 10.1016/j.ejcnurse.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Antonicelli R, Testarmata P, Spazzafumo L, et al. Impact of telemonitoring at home on the management of elderly patients with congestive heart failure. J Telemed Telecare. 2008;14:300–305. doi: 10.1258/jtt.2008.071213. [DOI] [PubMed] [Google Scholar]
- 41.Antonicelli R, Mazzanti I, Abbatecola AM, Parati G. Impact of home patient telemonitoring on use of b-blockers in congestive heart failure. Drugs Aging. 2010;27:801–805. doi: 10.2165/11538210-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 42.Assyag P, Renaud T, Cohen-Solal A, et al. RESICARD: East Paris network for the management of heart failure: absence of effect on mortality and rehospitalization in patients with severe heart failure admitted following severe decompensation. Arch Cardiovasc Dis. 2009;102:29–41. doi: 10.1016/j.acvd.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Bisharat B, Hafi L, Baron-Epel O, et al. Pharmacist counselling to cardiac patients in Israel prior to discharge from hospital contribute to increasing patient’s medication adherence closing gaps and improving outcomes. J Transl Med. 2012;10:34. doi: 10.1186/1479-5876-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bocchi EA, Cruz F, Guimaraes G, et al. Long-term prospective, randomized, controlled study using repetitive education at six-month intervals and monitoring for adherence in heart failure outpatients: the REMADHE trial. Circ Heart Fail. 2008;1:115–124. doi: 10.1161/CIRCHEARTFAILURE.107.744870. [DOI] [PubMed] [Google Scholar]
- 45.DeWalt DA, Schillinger D, Ruo B, et al. Multisite randomized trial of a single-session versus multisession literacy sensitive self-care intervention for patients with heart failure. Clin Trial Regist. 2012;125:2854–2862. doi: 10.1161/CIRCULATIONAHA.111.081745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falces C, Lopez-Cabezas C, Andrea R, et al. Intervencion educativa para mejorar el cumplimiento del tratamiento y prevenir reingresos en pacientes de edad avanzada con insuficiencia cardıaca [An educative intervention to improve treatment compliance and to prevent readmissions of elderly patients with heart failure] Med Clin (Barc). 2008;131:452–456. doi: 10.1157/13126954. [DOI] [PubMed] [Google Scholar]
- 47.Ferrante D, Varini S, Macchia A, on behalf of the GESICA Investigators et al. Long-term results after a telephone intervention in chronic heart failure: DIAL (randomized trial of phone intervention in chronic heart failure) follow-up. J Am Coll Cardiol. 2010;56:372–378. doi: 10.1016/j.jacc.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 48.Powell LH, Calvin JE, Jr, Richardson D, et al. Self-management counselling in patients with heart failure: the heart failure adherence and retention randomized behavioral trial. JAMA. 2010;304:1331–1338. doi: 10.1001/jama.2010.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shively MJ, Gardetto NJ, Kodiath MF, et al. Effect of patient activation on self-management in patients with heart failure. J Cardiovasc Nurs. 2013;28:20–34. doi: 10.1097/JCN.0b013e318239f9f9. [DOI] [PubMed] [Google Scholar]
- 50.Unverzagt S, Meyer G, Mittmann S, et al. Improving Treatment Adherence in Heart Failure: a Systematic Review and Meta-analysis of Pharmacological and Lifestyle Interventions. Dtsch Arztebl Int. 2016;113(25):423–430. doi: 10.3238/arztebl.2016.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The final version of data set supporting the findings of this paper is submitted together with this manuscript to the editorial committee. All the raw data is included in this manuscript. There are no ethics restrictions preventing the sharing of the raw data.