Introduction
Purkinje cell cytoplasmic antibody type-2 (PCA2-IgG) was first defined as a paraneoplastic biomarker based on a unique immunofluorescence pattern seen on a mouse composite brain tissue assay.1 Lung and breast cancer are the most common malignancies associated with PCA2-IgG seropositivity.2 In 2017, microtubule-associated protein 1B (MAP1B) protein was identified as the autoantigen target for PCA2-IgG.2 Clinical presentation associated with MAP1B-IgG is variable including neuropathy, encephalopathy, cognitive dysfunction, brainstem syndrome, ophthalmic involvement and cerebellar ataxia.1 2 Despite neuropathy being the most common neurological accompaniment earlier reports lack details of those neuropathies.2 Here, we describe the neuropathy phenotypes in patients affected by MAP1B-IgG autoimmunity. Although rare, recognition of this paraneoplastic neuropathy phenotype may aid early detection of underlying malignancy.
Methods
We reviewed the Mayo Clinic Neuroimmunology laboratory database (1 January 1995 and 30 September 2018) for patients tested for paraneoplastic panel by indirect immunofluorescence assay (IFA) and western blot (WB) analysis of rat cerebellar and cortical extracts (online supplementary methods).2 All PCA2-IgG stored samples were also tested and confirmed positive on MAP1B fragment WB. Study inclusion criteria: (1) MAP1B-IgG seropositivity by IFA and MAP1B WB, (2) presence of somatic and autonomic peripheral neuropathies and (3) exclusion of alternative aetiologies including chemotherapy-induced neuropathies, diabetes mellitus, nutritional deficiency, systemic vasculitis, lymphoma and paraproteinemia as deemed clinically appropriate. Clinical outcomes were evaluated by improvement in modified Rankin Scale (≥1) and 5-year mortality rate. Anti-Neuronal Nuclear Antibody type-1 (ANNA1 aka anti-Hu-IgG) seropositive neuropathy cases evaluated at Mayo Clinic (2000–2018), were utilised to evaluate phenotype and survival outcome comparison. All patients with ANNA1-IgG neuropathy in the comparison group were negative for MAP1B-IgG.
jnnp-2019-322175supp001.pdf (62.6KB, pdf)
Results
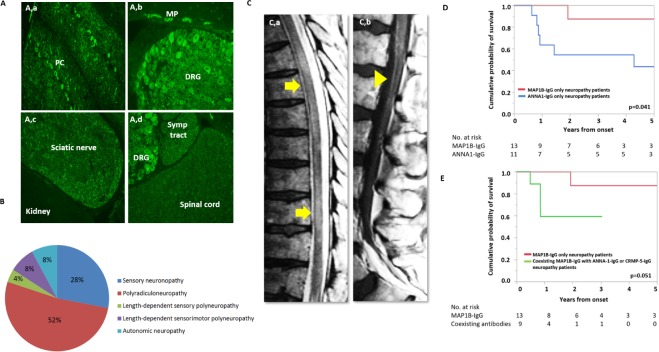
Indirect IFA using MAP1B-IgG neuropathy patients’ serum showed staining of rat dorsal root ganglia, sciatic nerves, sympathetic ganglia and spinal cord, along with typical staining of cerebellum and myenteric plexus (figure 1A), supporting expression of MAP1B protein in the peripheral nervous system. Clinical information was available on 95 of 211 MAP1B-IgG cases evaluated at Mayo Neuroimmunology lab Neuropathy was documented in 50 patients (53%), but phenotypic description of neuropathy was only available in 40 cases (34%) (online supplementary figure). Of all 40 MAP1B-IgG neuropathy patients, 23 (58%) were male, median age of onset was 68 years (range 22–82 years). Neuropathy presented as initial symptom in 29 (76%) cases. Median duration of follow-up was 13 months (2–163 months). Twenty-five patients had MAP1B-IgG positive as the only serological biomarker. Fifteen patients had coexisting collapsin response-mediator protein-5 (CRMP5)-IgG (n=12, 80%) and/or anti-neuronal nuclear antibody type-1 (ANNA1)-IgG (n=3, 20%).3 4
Figure 1.
Tissue expression, clinical presentations and outcomes of MAP1B neuropathy. Tissue-based immunofluorescence of serum from MAP1B neuropathy patient on rat tissue (A). Indirect immunofluorescence using serum of MAP1B-IgG seropositive patients demonstrating expression of protein in the cerebellum (Purkinje cells and dendrites in molecular layer (a) dorsal root ganglia and myenteric plexus (b), sciatic nerve (c), sympathetic tract and spinal cord (d)). Lack of rat kidney staining supports neural-specific tissue staining (c). Neuropathy phenotypes associated with MAP1B-IgG neuropathy phenotype (n=25, (B)). MRI of lumbar spine (C), demonstrating multifocal T2 hyperintensity involving the spinal cord (a) and gadolinium enhancement of lumbosacral roots (head arrow) on post-gadolinium T1 scan (b). Kaplan-Meier estimates of time to death from neurological symptom onset of neuropathy cases with MAP1B-IgG alone compared with those with MAP1B-IgG coexisting with ANNA1-IgG or CRMP5-IgG (D). Kaplan-Meier estimates of time to death from neurological symptom onset of neuropathy cases with MAP1B-IgG alone compared with those with ANNA1-IgG (E). ANNA1, anti-nuclear neuronal antibody type-1; CRMP5, collapsin response-mediator protein-5; DRG, dorsal root ganglia; MAP1B, microtubule-associated protein 1B; ML, molecular layer; MP, myenteric plexus; PC, Purkinje cell; Symp, sympathetic.
jnnp-2019-322175supp002.pdf (1.2MB, pdf)
Neuropathy phenotype
Among 25 neuropathy patients with MAP1B-IgG as the only autoantibody, most common neuropathy phenotype was polyradiculoneuropathy 13 (52%) (figure 1B), followed by sensory neuronopathy 7 (28%), length-dependent sensorimotor neuropathy 2 (8%), isolated autonomic neuropathy 2 (8%) and length-dependent sensory neuropathy 1 (4%). Neuropathic pain was documented in only five (20%) patients. Autoimmune gastrointestinal dysmotility was present among five cases (20%). In cases with detailed description of disease progression, onset of neuropathy was symmetrical 12/19 (63%) and progression was subacute 9/19 (47%) or chronic 10/19 (53%).
Of the 15 MAP1B-IgG positive patients with coexisting ANNA1-IgG and/or CRMP5-IgG, the most common phenotype was sensory neuronopathy 5/15 (33%), followed by length-dependent sensorimotor polyneuropathy 4/15 (27%), polyradiculoneuropathy 3/15 (20%), length-dependent sensory neuropathy 1/15 (7%), small fibre neuropathy 1 (7%) and autonomic neuropathy 1 (7%). A majority of the patients had subacute 5 (45%) or chronic 6 (55%) progression.
Compared with ANNA1 neuropathy (online supplementary table), MAP1B neuropathy had lower frequency of sensory neuronopathy (7/25 (28%) vs 13/17 (76%), p=0.004), neuropathic pain (5/25 (20%) vs 10/17 (63%), p=0.023) and autonomic dysfunction (5/25 (20%) vs 12/17 (75%), p=0.003).
jnnp-2019-322175supp003.pdf (15.4KB, pdf)
Cooccurring CNS disorders
Twelve MAP1B-IgG patients (48%) had coexisting central nervous system (CNS) involvement (online supplementary figure) which included one or more of the following brain-stem/cerebellar dysfunction 8 (67%), myelopathy 3 (25%, figure 1C), cognitive impairment 2 (17%) and seizure 1 (8%).
Among 15 patients with combination of MAP1B-IgG and ANNA1-IgG and/or CRMP5-IgG, 7 (47%) had coexisting CNS involvement. The most common cooccurring CNS manifestations were brain-stem/cerebellar dysfunction 5 (71%), myelopathy 2 (29%) and cognitive impairment 2 (29%).
Oncological association of MAP1B-IgG positive neuropathy patients
Sixteen of 18 MAP1B-IgG neuropathy patients (89%) who had cancer workup (at least CT chest, abdomen and pelvis, with/without PET scan) had detectable underlying malignancy. Among these 11 (69%) had small cell lung cell carcinoma. Other neoplasms were squamous cell carcinoma 2 (12%), renal cell carcinoma 1 (6%), large cell anaplastic lymphoma 1 (6%) and non-small cell lung cancer 1 (6%).
All 11 (100%) MAP1B-IgG seropositive neuropathy cases with coexisting CMRP5-IgG or ANNA1-IgG antibodies who underwent cancer evaluation had a detectable malignancy predominantly small cell cancer (lungs (n=10, 91%), pancreas (n=1, 9%)). In one patient, breast cancer was detected (9%).
Treatment and clinical outcomes
Clinical follow-up data were available on 10 MAP1B-IgG neuropathy cases (median duration: 17 months (range: 6–165 months)). Among these, three patients demonstrated clinical improvement following management of underlying cancer (surgical resection and/or chemotherapy), and five demonstrated clinical improvement after immunotherapy initiation. Immunotherapies utilised included high-dose intravenous corticosteroid (n=3), plasmapheresis (n=2), mycophenolate mofetil (n=2) and cyclophosphamide (n=1). Two patients with multifocal neurological involvement (encephalopathy (n=1), gastroparesis (n=1)) had a progressive refractory course.
Comparing MAP1B-IgG only and MAP1B-IgG with coexisting ANNA1-IgG and/or CRMP5-IgG neuropathy patients, MAP1B-IgG positive cases had a trend towards better survival rate, p=0.051 (figure 1E). MAP1B-IgG only seropositive patients had a significantly higher survival rate of ANNA1-IgG neuropathy4 patients, p=0.04 (figure 1D).
Discussion
We describe the phenotypic presentations of neuropathy associated with MAP1B-IgG. Two common neuropathy presentations include subacute/chronic polyradiculoneuropathy or sensory neuronopathy (81%). A considerable number of these patients also have coexisting central nervous system involvement (48%) in the form of rhombencephalitis or myelopathy. Autonomic dysfunction or gastrointestinal dysmotility was present only in a minority of cases. Malignancy (89%) is very common, most commonly small cell lung cancer. Neurological outcome and survival rates of paraneoplastic MAP1B-IgG neuropathy appear to be better than ANNA1-IgG associated neuropathy.
MAP1B is an intracellular cytoskeletal protein that is associated with axonal transport, neurite outgrowth and regeneration in peripheral nerve. Its intracellular localisation likely precludes a direct role of MAP1B-IgG in neural dysfunction. In this regard, it is similar to previously described classic onconeural antibodies.1 Its robust expression in the dorsal root ganglia and peripheral nerves (figure 1A) would support the hypothesis of MAP1B-specific T-cells contributing to observed paraneoplastic neuropathy phenotype.1 MAP1B-IgG staining of the myenteric plexus or enteric nervous system in the rat gut mucosa may explain autonomic dysfunction or gastrointestinal (GI) dysmotility in a subset of these patients.
Asymmetrical axonal polyradiculoneuropathy has been reported with CRMP5-IgG; however, the majority of those cases were associated with pain which may be a distinguishable feature compared with painless MAP1B-IgG polyradiculoneuropathy.4 Additionally, ANNA1-IgG paraneoplastic neuropathy more frequently presents as sensory neuronopathy with prominent autonomic dysfunction compared with MAP1B-IgG seropositive cases.
Prior studies have highlighted importance of antibody cluster in prediction of underlying cancer.5 This is further supported by our findings of 100% cancer association among patients with MAP1B-IgG coexisting CRMP5 or ANNA1-IgG.
Limitations of this study include its retrospective design. Furthermore, long-term follow-up data were available in very few PCA2 and ANNA1 neuropathy cases, limiting our assessment of clinical outcomes.
MAP1B-IgG evaluation should be considered among patients with subacute or chronic painless polyradiculoneuropathy or sensory neuronopathy, especially among patients with coexisting CNS involvement and/or significant weight loss. The detection of MAP1B-IgG may help early diagnosis of underlying occult malignancy (especially small cell cancer) and in turn affect long-term outcomes.
Footnotes
Contributors: JJ was involved in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, and acquisition of data. CJK, SJP, AG, AM and JRM were involved in revising the manuscript for content and analysis and interpretation of data. DD was involved study concept and design, analysis and interpretation of data, acquisition of data and study supervision.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: CJK reports honorarium from Ackea for teaching on TTR amyloid and Fabry and consultation with Pfizer pharmaceuticals on TTR amyloidosis. SJP is a named inventor on filed patents that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker; has a patent pending for MAP1B, Septin 5, Klech like protein 11, GFAP and MAP1B as markers of neurological autoimmunity and paraneoplastic disorders; consulted for Alexion and Medimmune; and received research support from Grifols, Medimmune and Alexion. All compensation for consulting activities is paid directly to Mayo Clinic. AG has a patent pending for MAP1B as markers of neurological autoimmunity and paraneoplastic disorders. AM has a patent pending for MAP1B, Klech like protein 11, GFAP and MAP1B as markers of neurological autoimmunity and paraneoplastic disorders; consulted for Grifols, Medimmune and Euroimmun; and received research support from Medimmune and Euroimmun but has not received personal compensation. DD has a patent pending for Klech like protein 11 as a marker of neurological autoimmunity and paraneoplastic disorders; has received grant support for centre of multiple sclerosis and autoimmune neurology, and has consulted for UCB and Astellas.
Patient consent for publication: Not required.
Ethics approval: The Mayo Clinic Institutional Review Board approved the study (08–0 06 647).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Vernino S, Lennon VA. New Purkinje cell antibody (PCA-2): marker of lung cancer-related neurological autoimmunity. Ann Neurol 2000;47:297–305. [DOI] [PubMed] [Google Scholar]
- 2. Gadoth A, Kryzer TJ, Fryer J, et al. . Microtubule-Associated protein 1B: novel paraneoplastic biomarker. Ann Neurol 2017;81:266–77. 10.1002/ana.24872 [DOI] [PubMed] [Google Scholar]
- 3. Lucchinetti CF, Kimmel DW, Lennon VA. Paraneoplastic and oncologic profiles of patients seropositive for type 1 antineuronal nuclear autoantibodies. Neurology 1998;50:652–7. 10.1212/WNL.50.3.652 [DOI] [PubMed] [Google Scholar]
- 4. Dubey D, Lennon VA, Gadoth A, et al. . Autoimmune CRMP5 neuropathy phenotype and outcome defined from 105 cases. Neurology 2018;90:e103–10. 10.1212/WNL.0000000000004803 [DOI] [PubMed] [Google Scholar]
- 5. Pittock SJ, Kryzer TJ, Lennon VA. Paraneoplastic antibodies coexist and predict cancer, not neurological syndrome. Ann Neurol 2004;56:715–9. 10.1002/ana.20269 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2019-322175supp001.pdf (62.6KB, pdf)
jnnp-2019-322175supp002.pdf (1.2MB, pdf)
jnnp-2019-322175supp003.pdf (15.4KB, pdf)