Abstract
Background
Many studies have reported the predictive value of the atherogenic index of plasma (AIP) in the progression of atherosclerosis and the prognosis of percutaneous coronary intervention (PCI). However, the utility of the AIP for prediction is unknown after PCI among type 2 diabetes mellitus (T2DM).
Methods
2356 patients with T2DM who underwent PCI were enrolled and followed up for 4 years. The primary outcome was major cardiovascular and cerebrovascular adverse events (MACCEs), considered to be a combination of cardiogenic death, myocardial infarction, repeated revascularization, and stroke. Secondary endpoints included all-cause mortality, target vessel revascularization (TVR), and non-target vessel revascularization (non-TVR). Multivariate Cox proportional hazards regression modelling found that the AIP was correlated with prognosis and verified by multiple models. According to the optimal cut-off point of the ROC curve, the population was divided into high/low-AIP groups. A total of 821 pairs were successfully matched using propensity score matching. Then, survival analysis was performed on both groups.
Results
The overall incidence of MACCEs was 20.50% during a median of 47.50 months of follow-up. The multivariate Cox proportional hazards regression analysis before matching suggested that the AIP was an independent risk factor for the prognosis of T2DM after PCI (hazard ratio [HR] 1.528, 95% CI 1.100–2.123, P = 0.011). According to the survival analysis of the matched population, the prognosis of the high AIP group was significantly worse than that of the low AIP group (HR (95% CI) 1.614 (1.303–2.001), P < 0.001), and the difference was mainly caused by repeat revascularization. The low-density lipoprotein-cholesterol (LDL-C) level did not affect the prognosis of patients with T2DM (P = 0.169), and the effect of the AIP on prognosis was also not affected by LDL-C level (P < 0.001).
Conclusions
The AIP, a comprehensive index of lipid management in patients with T2DM, affects prognosis after PCI. The prognosis of diabetic patients with high levels of the AIP included more MACCEs and was not affected by LDL-C levels. It is recommended to monitor the AIP for lipid management in diabetic patients after PCI and ensure that the AIP is not higher than 0.318.
Trial registration This is an observational cohort study that does not involve interventions. So we didn’t register. We guarantee that the research is authentic and reliable, and hope that your journal can give us a chance.
Keywords: Atherogenic index of plasma, Type 2 diabetes mellitus, Major cardiovascular and cerebrovascular adverse events, Percutaneous coronary intervention
Background
Cardiovascular disease is one of the leading causes of death in patients with type 2 diabetes mellitus (T2DM) [1]. Despite adequate attention from clinicians, its prognosis in T2DM patients is still significantly worse than that in non-diabetic patients [2]. Abnormal lipid metabolism, including high triglyceride (TG), low high-density lipoprotein-cholesterol (HDL-C), and high low-density lipoprotein-cholesterol (LDL-C) levels and abnormal glucose metabolism (insulin resistance), is commonly present in diabetic patients and makes significant contributions to poor prognosis [3]. The atherogenic index of plasma (AIP) is calculated by log (TG/HDL-C) [4], which can reflect the characteristics of abnormal lipid metabolism in diabetic patients and can quantify the degree of abnormal lipid metabolism. In addition, the AIP is correlated with the degree of insulin resistance [5], which can also indicate the degree of abnormal glucose metabolism.
Studies [6] have shown that the AIP has predictive value for atherosclerosis, which indicates a significant positive correlation between diabetes mellitus and the AIP, as well as between carotid intima-media thickness (cIMT) progression and arterial stiffness. Furthermore, elevated AIP is a powerful independent predictor of all-cause mortality and subsequent cardiovascular disease after coronary revascularization [7]. In subpopulations of patients with diabetes, the AIP is related to the incidence of type 2 diabetes [8], while some studies have suggested that the AIP is a risk factor for coronary heart disease in patients with type 2 diabetes [9, 10]. However, whether the AIP still plays a role after percutaneous coronary intervention (PCI) in diabetic patients is still unknown. Therefore, we investigated the relationship between the AIP and long-term follow-up outcomes after PCI in patients with type 2 diabetes.
Methods
Patient population and study design
The present study is a large single-centre observational cohort study that mainly occurred in Beijing Anzhen Hospital, Capital Medical University (one of the largest interventional diagnostic and treatment centres in the world, with approximately 40,000 interventional diagnostic and treatment cases annually). A total of 10 968 consecutive patients underwent PCI with drug-eluting stents (DES) in our hospital from January to December 2014. Of those, 3068 patients with clearly diagnosed type 2 diabetes were enrolled. The exclusion criteria were as follow: patients with incomplete baseline and follow-up data; those with a previous history of CABG; or those with severe liver/kidney failure, cancer or other major diseases that affect long-term survival. Ultimately, 2356 patients were included in the statistical analysis. Patients underwent follow-up via telephone or outpatient service after the first 1/6/12 months after PCI and annually thereafter. All patients were followed-up for 4 years unless death occurred. Multivariate Cox proportional hazards regression modelling indicated that the AIP was correlated with prognosis and verified by multiple models. The optimal cut-off point of the AIP was found (AIP = 0.318) with the ROC curve, and the patients were divided into a high AIP group (AIP ≥ 0.318) and a low AIP group (AIP < 0.318). Patients in the two groups were matched according to the 1:1 propensity score, and 821 pairs of patients were successfully matched for survival analysis (see Fig. 1 for the detailed process). This study was approved by the Clinical Research Ethics Committee of Beijing Anzhen Hospital, Capital Medical University, and all patients were informed and agreed to participate in this study.
Fig. 1.
Flow chart of enrolled patients
Data collection
Data collection was mainly conducted by case report form (CRF), which mainly included patients’ demographic and clinical characteristics, age, sex, previous medical history, physical examination, laboratory examination, intervention-related data, types of drugs taken after PCI, follow-up information, etc. Blood samples were collected the next morning after an overnight fast. Lipid-related blood tests were performed for patients taking statins for more than 1 month. The calculation of the AIP is log (TG/HDL-C) on an empty stomach. The SYNTAX score of each patient was calculated using the online scoring system (www.syntaxscore.com) by scoring all coronary artery lesions with diameters ≥ 50% in vessels with reference diameters ≥ 1.5 mm [11].
Coronary angiography data at baseline and follow-up, such as the number of target vessels, minimum stent diameter, average stent length, and SYNTAX, were performed separately by two experienced interventional physicians. Follow-up was performed independently by 2 trained medical personnel (who were blinded to patient information).
Clinical end points and definitions
The primary outcome was major cardiovascular and cerebrovascular adverse events (MACCEs), considered to be a combination of cardiogenic death, myocardial infarction, repeated revascularization, and stroke. Secondary endpoints included all-cause mortality, target vessel revascularization (TVR), and non-target vessel revascularization (non-TVR). MACCEs were considered the first occurrence of an event during each patient’s follow-up. Cardiac death, myocardial infarction, repeat revascularization, and stroke were all events recorded for each patient during the 4-year follow-up period.
Diabetic patients were defined as patients who had a previous diagnosis of DM (treated with diet, oral agents or insulin) or a new diagnosis of DM (FBG ≥ 7.0 mmol/L on 2 occasions during hospitalization, HbA1c > 6.5% at baseline) based on the current guidelines [12]. Hypertension was defined by systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg and/or the use of antihypertensive treatment in the past 2 weeks [13]. Hyperlipidaemia was defined by an increase in plasma TG or TC but also included an increase in LDL cholesterol and a decrease in HDL cholesterol [14]. Adult LDL-C ranged from 2.07 to 3.37 mmol/L (80 to 130 mg/day). Smoking was defined as smoking at the time of PCI or having quit smoking for less than 1 year; target vessel revascularization was defined as any revascularization procedure, either percutaneous or surgical, involving the target vessel.
Statistical analysis
We performed Tests of Normality with Kolmogorov–Smirnov and Shapiro–Wilk to test. Continuous variables with a normal distribution, those with a non-normal distribution and categorical variables were expressed as the mean ± standard deviation, median/quartile and counts or percentages, respectively, and differences were detected with a t-test of two independent samples, rank sum test and Chi-square test, respectively. The incidence of events is expressed in terms of the number of incidents during the 4-year follow-up. Cox proportional hazards regression modelling was used to analyse independent risk factors associated with MACCE. The prognostic risk factors of each model adjustment include: Model: adjusted analysis. Model2: adjusted on Age, Sex, BMI, SBP, DBP, Smoking, drinking, DM time, hypertension, hyperlipidemia, stroke, MI, history of CAD, PCI, stable angina pectoris, UA/NSTEMI, STEMI. Model3: adjusted on model2 + HBC1, CREA, LVEF, TC, and LDL. Model4: adjusted on model3 + asprin, clopidigrel, b-blocker, statin, ACEI/ARB, oral antidiabetic agents, insulin. The ROC curve determined the optimal cut-off point of the AIP. Propensity score-matched analysis was used to conduct 1:1 matching between high AIP and low AIP. The Kaplan–Meier method was used for graphical evaluation of time-related events and was evaluated by log-rank tests. Statistical significance was accepted at the 95% confidence level (CI) (two-sided P ≤ 0.05). SPSS software for Windows (version 24.0, SPSS Inc., Chicago, Illinois) was used for statistical analyses.
Results
Differences in baseline clinical and angiographic characteristics among the MACE and non-MACE groups of the study population (total population)
The baseline clinical and angiographic characteristics of the total population are shown in Table 1.
Table 1.
Difference in baseline clinical and angiographic characteristics among the MACE and non-MACE group of study population (total population)
| Characteristics | MACE (n = 483) | Non-MACE (n = 1873) | P value |
|---|---|---|---|
| Demographic | |||
| Age, years | 58.03 ± 9.039 | 57.95 ± 9.182 | 0.867 |
| Male, n (%) | 353 (73.1) | 1385 (73.9) | 0.701 |
| Behavioral | |||
| Smoking, n (%) | 215 (44.5) | 832 (44.4) | 0.971 |
| Drinking, n (%) | 85 (17.6) | 331 (17.7) | 0.97 |
| Physical | |||
| BMI, kg/m2 | 26.07 ± 3.564 | 26.07 ± 3.504 | 0.985 |
| SBP, mmHg | 129.64 ± 14.982 | 130.5 ± 16.485 | 0.27 |
| DBP, mmHg | 77.67 ± 9.913 | 77.8 ± 10.614 | 0.81 |
| Medical history, n (%) | |||
| Hypertension | 299 (61.9) | 1208 (64.5) | 0.29 |
| Hyperlipidemia | 209 (43.3) | 812 (43.4) | 0.974 |
| History of MI | 50 (10.4) | 201 (10.7) | 0.81 |
| History of stroke | 29 (6.0) | 144 (7.7) | 0.206 |
| Family history of CAD | 62 (12.8) | 260 (13.9) | 0.551 |
| Previous PCI | 79 (16.4) | 317 (16.9) | 0.766 |
| Diagnosed DM, years | 7.82 ± 3.73 | 6.58 ± 3.942 | < 0.001 |
| Clinical presentation, n (%) | |||
| Stable CAD | 52 (10.8) | 249 (13.3) | 0.138 |
| Unstable angina/NSTEMI | 352 (72.9) | 1267 (67.6) | 0.027 |
| STEMI | 79 (16.3) | 357 (19.1) | 0.172 |
| Medical treatment, n (%) | |||
| Asprin | 479 (99.2) | 1862 (99.4) | 0.785 |
| Clopidogrel | 476 (98) | 1832 (97.8) | 0.305 |
| β-Blocker | 391 (81) | 1498 (80) | 0.632 |
| Statins | 473 (97.9) | 1827 (97.5) | 0.62 |
| ACEI/ARB | 275 (56.9) | 1028 (54.9) | 0.419 |
| Oral hypoglycemic drugs | 353 (72.9) | 1322 (70.6) | 0.321 |
| Insulin | 183 (37.9) | 649 (34.7) | 0.184 |
| Laboratory results | |||
| TC, mmol/L | 4.25 ± 1.111 | 4.11 ± 1.091 | 0.015 |
| LDL-C, mmol/L | 2.49 ± 0.841 | 2.47 ± 0.89 | 0.643 |
| AIP | 0.3 ± 0.356 | 0.22 ± 0.292 | < 0.001 |
| HbA1c, % | 8.1 ± 1.269 | 7.4 ± 1.299 | < 0.001 |
| hs-CRP, mg/L | 4.18 ± 6.708 | 4.01 ± 6.877 | 0.614 |
| Creatinine, μmol/L | 71.98 ± 17.595 | 71.46 ± 19.905 | 0.604 |
| GFR, mL/min | 95.13 ± 20.680 | 95.79 ± 19.612 | 0.515 |
| LVEF, % | 61.44 ± 8.231 | 62.09 ± 8.15 | 0.118 |
| Number of target vessels | 0.229 | ||
| One, n (%) | 206 (45.1) | 856 (45.7) | |
| Multivessel, n (%) | 277 (57.3) | 1017 (54.3) | |
| Target vessels | |||
| LM, n (%) | 17 (3.5) | 75 (4) | 0.624 |
| LAD, n (%) | 290 (60) | 1121 (59.9) | 0.939 |
| LCX, n (%) | 720 (34.8) | 200 (41.4) | 0.233 |
| RCA, n (%) | 212 (43.9) | 782 (41.8) | 0.396 |
| SYNTAX score | 14.61 ± 6.971 | 12.48 ± 7.162 | < 0.001 |
| Minimal stent diameter, mm | 2.92 ± 0.457 | 2.92 ± 0.46 | 0.931 |
| Average stent length, mm | 22.27 ± 6.748 | 22.16 ± 6.43 | 0.749 |
| Types of stent, n (%) | 0.338 | ||
| First generation DES | 225 (46.6) | 827 (44.2) | |
| Second generation DES | 258 (53.4) | 1046 (55.8) | |
Continuous variables were expressed as mean () ± standard deviation (SD) in case of normal distribution and compared between two groups by two-independent samples t-test. Data were expressed as medians (interquartile ranges) in case of skewed distribution and compared using the Mann–Whitney U-test. Categorical variables are presented as counts (percentages) and compared by Chi-square test
MACCE major adverse cardiac and cerebrovascular event, BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, DM diabetes mellitus, MI myocardial infraction, CAD coronary artery disease, PCI percutaneous coronary intervention, NSTEMI non-ST-segment–elevation myocardial infarction, STEMI ST-segment–elevation myocardial infarction, TG triglyceride, TC total cholesterol, LDL-C low density lipoprotein cholesterol, HDL-C high density lipoprotein cholesterol, AIP atherogenic index of plasma, HbA1c glycosylated hemoglobin, hs-CRP high-sensitivity C-reactive protein, LVEF left ventricular ejection fraction, ACEI angiotensin converting enzyme inhibitor, ARB angiotensin receptor blocker, LM left main, LAD left anterior descending, LCX left circumflex artery, RCA right coronary artery, SYNTAX synergy between PCI with taxus and cardiac surgery, DES drug-eluting stent
There was no significant difference between the MACCE group and the non-MACCE group in age, sex, medical history, or medical treatment. There were significant differences in the duration of diabetes, HbA1c, TG, TC, HDL-C, the AIP, and SYNTAX score. In Cox proportional hazards regression modelling, the AIP was one of the independent predictors of prognosis in patients with diabetes after adjusting for other confounding factors, and multiple models were used for verification (Table 2). ROC curve analysis showed that the AIP had good predictive accuracy for prognosis. Baseline AIP was identified at 0.318 as the optimal cut-off point to predict the risk of prognosis.
Table 2.
Independent predictors of MACCES in patients with DM after baseline PCI
| Variables | HR | 95% CI | P values |
|---|---|---|---|
| Model1 | 2.137 | 1.600–2.856 | < 0.001 |
| Model2 | 1.619 | 1.170–2.241 | 0.004 |
| Model3 | 1.618 | 1.169–2.239 | 0.004 |
| Model4 | 1.528 | 1.100–2.123 | 0.011 |
Model1: age, male, BMI, SBP, DBP, smoking, drinking, diagnosed DM, hypertension, hyperlipidemia, history of MI, history of stroke, family history of CAD, previous PCI, clinical presentation
Model2: Model1 + HbA1c, CRP, CREA, LVEF, TC-C, LDL-C
Model3: Model2 + medical treatment (aspirin, clopidogrel, β-blocker, statins, ACEI/ARB, oral hypoglycemic drugs, insulin)
Model4: Model3 + number of target vessels, target vessels (LM, LAD, LCX, RCA), minimal stent diameter, average stent length, types of stent, SYNTAX score
Baseline clinical and angiographic characteristics and Kaplan–Meier curve (propensity score-matched population)
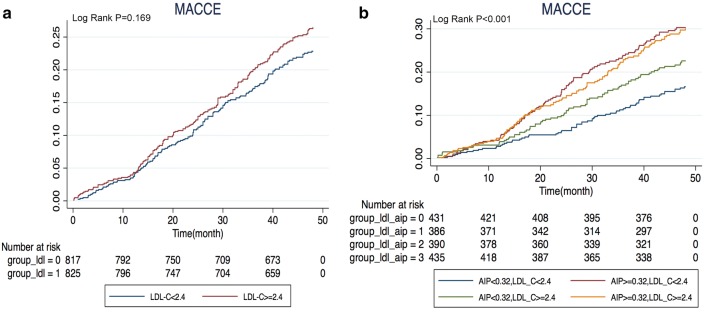
The clinical baseline characteristics before and after propensity score matching are shown in Fig. 2. There was no significant difference between the low AIP group and the high AIP group in age, sex, BMI, medical history, duration of diabetes, HbA1c, TC, LDL-C, drug use, other biomarkers, angiography and process characteristics. The C statistic value of the propensity score model was 0.01. We tabulated the events that occurred in matched pre/post populations according to MACCE/all-cause mortality/cardiogenic death/myocardial infarction/stroke, etc. (see Table 3 for details). The log-rank test was used to compare the Kaplan–Meier curve of the matched population for overall survival analysis, and the influence of high/low AIP group on prognosis was evaluated, as well as the influence of a combination of the SYNTAX score and HbA1c on prognosis (see Fig. 3). In addition, given that LDL-C is a traditional classic risk factor for arteriosclerosis, the population was divided into high/low LDL-C groups to assess the influences on the MACCE rate and on prognosis under the influence of high/low AIP (see Fig. 4).
Fig. 2.
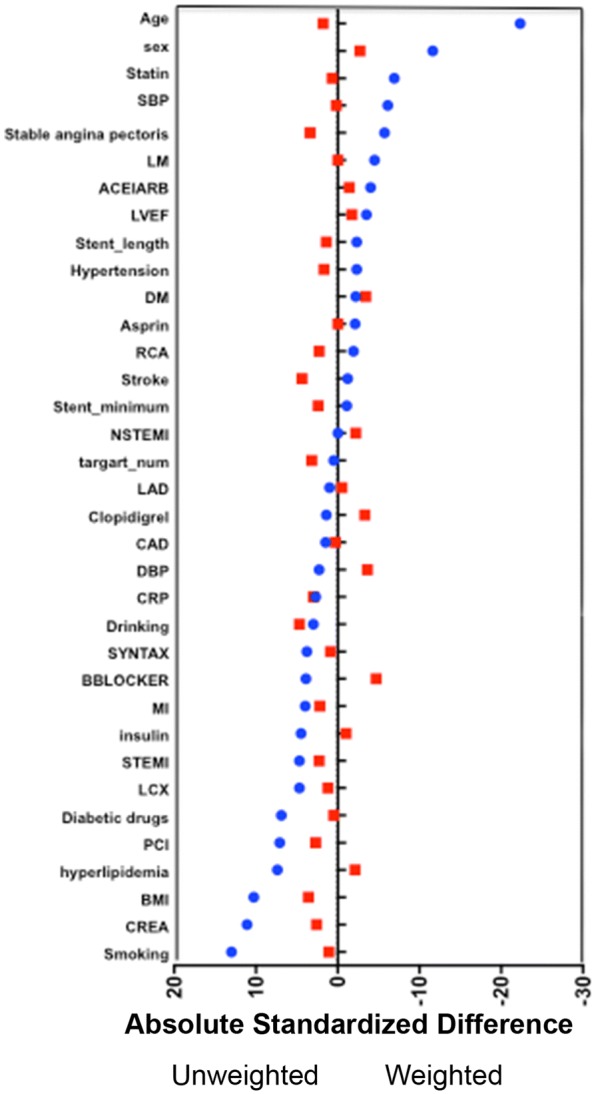
The baseline clinical and angiographic characteristics before and after propensity score-matched. HDL-C high density lipoprotein cholesterol, ARB angiotensin receptor blocker, LDL-C low density lipoprotein cholesterol, ACEI angiotensin converting enzyme inhibitor, LAD left anterior descending, SYNTAX synergy between PCI with taxus and cardiac surgery, LVEF left ventricular ejection fraction, DBP diastolic blood pressure, AIP atherogenic index of plasma
Table 3.
Estimated Kaplan–Meier events rates of 4-year follow-up
| Adverse events | Overall population | Propensity score-matched population | ||||||
|---|---|---|---|---|---|---|---|---|
| High AIP (n = 937) | Low AIP (n = 1419) | Adjusted HRa (95% CI) | P Value | High AIP (n = 821) | Low AIP (n = 821) | HR (95% CI) | P value | |
| MACCE | 253 (27.0) | 230 (16.2) | 1.638 (1.363–1.969) | < 0.001 | 214 (26.1) | 145 (17.7) | 1.614 (1.303–2.001) | < 0.001 |
| All-cause death | 40 (4.3) | 33 (2.3) | 1.690 (1.049–2.724) | 0.031 | 34 (4.1) | 21 (2.6) | 1.750 (1.003–3.054) | 0.049 |
| Cardiac death | 28 (3.0) | 19 (1.3) | 2.184 (1.197–3.984) | 0.011 | 24 (2.9) | 11 (1.3) | 2.615 (1.251–5.470) | 0.011 |
| Cardiac death/MI | 71 (7.58) | 50 (3.52) | 2.199 (1.531–3.158) | < 0.001 | 58 (7.06) | 30 (3.65) | 1.968 (1.266–3.058) | 0.003 |
| MI | 58 (6.3) | 41 (2.9) | 1.962 (1.301–2.960) | 0.001 | 47 (5.8) | 25 (3.1) | 2.080 (1.267–3.415) | 0.004 |
| Repeat revascularization | 210 (22.9) | 190 (13.5) | 1.644 (1.343–2.013) | < 0.001 | 177 (22.0) | 119 (14.6) | 1.628 (1.285–2.062) | < 0.001 |
| TVR | 125 (13.6) | 99 (7.0) | 1.768 (1.347–2.322) | < 0.001 | 105 (13.1) | 60 (7.4) | 1.809 (1.310–2.498) | < 0.001 |
| Non-TVR | 85 (9.4) | 91 (6.5) | 1.413 (1.043–1.916) | 0.026 | 72 (9.0) | 59 (7.3) | 1.314 (0.927–1.865) | 0.125 |
| Stroke | 23 (2.5) | 27 (1.9) | 1.124 (0.628–2.014) | 0.694 | 20 (2.5) | 17 (2.1) | 1.191 (0.610–2.325) | 0.608 |
Values expressed are n (%) or hazard ratio (95% confidence interval). The percentages shown are Kaplan–Meier estimates from the intention to treat analysis
Major adverse cardiac and cerebrovascular events included cardiac death, myocardial infarction, repeat revascularization, and stroke
AIP atherogenic index of plasma, CI indicates confidence interval, HR hazard ratio, MACCE major adverse cardiac and cerebrovascular event, MI myocardial infarction, TVR target vessel revascularization
aHazard ratio was adjusted with age, body mass index, sex, medical history, medical treatment
Fig. 3.
Comparison of estimated event rates in the propensity score-matched population. Kaplan–Meier curve of the matched population for overall survival analysis, and the influence of high/low AIP group on prognosis was evaluated, as well as combined the influence of SYNTAX score and HbA1c on prognosis, respectively. a–c MACCE rates, d–f cardiac death/MI rates, g–i repeat revascularization rate, j–l Stroke rates. Target incidence of observation considering AIP level and SYNTAX score (b, e, h, k) or HbA1c (c, f, i, l). MACCE major adverse cardiac and cerebrovascular events, AIP atherogenic index of plasma, SYNTAX synergy between PCI with taxus and cardiac surgery, HbA1c glycosylated hemoglobin, PCI percutaneous coronary intervention
Fig. 4.
Comparison of estimated event rates in the propensity score-matched population. a Comparison of MACCE rate considering LDL-C level; b comparison of MACCE rate considering LDL-C and AIP level. MACCE major adverse cardiac and cerebrovascular events, LDL-C low-density lipoprotein cholesterol, AIP atherogenic index of plasma, PCI percutaneous coronary intervention
Discussion
Main findings in this study
In the present single-centre observational study, we found that the AIP, a parameter related to abnormal lipid and glucose metabolism, plays an important role in the long-term prognosis of type 2 diabetes patients undergoing percutaneous coronary intervention. MACCEs were significantly reduced in the group with lower AIP after PCI compared to the group with higher AIP. This benefit was mainly due to a reduction in the rate of repeated revascularization. In the subgroup analysis, we found that the effect of the AIP on prognosis was independent of LDL-C level.
Patients with T2DM have a poor prognosis, which is mainly related to abnormal metabolism
The mechanism of atherosclerosis is complex, and epidemiological studies suggest that the internationally recognized risk factors for coronary heart disease include dyslipidaemia, hypertension, diabetes and smoking [15]. In type 2 diabetes, atherosclerosis has the same mechanism—lipids and inflammation can also cause damage to cardiovascular organs [16]. There is a direct association between inflammatory cytokines, interleukins and matrix-metalloproteinase 12 (MMP-12), as well as an inverse association between MMP-12 and HDL, both in T2DM patients and in non-T2DM subjects. However, patients with type 2 diabetes also have a unique mechanism of atherosclerosis. For example, in patients with elevated total cholesterol, LDL-C or non-HDL-C, an increase in LP(a) can lead to additional adverse events in patients with T2DM [17]. Abnormal lipid metabolism and abnormal glucose metabolism are present in T2DM patients. Under the combined influence of these two factors, the lesions are more severe, and the prognosis is worse [18]. Therefore, the management of blood lipids and glucose after PCI is of great significance for the prognosis of diabetic patients.
The AIP-A comprehensive index of specific dyslipidaemia in diabetic patients
At present, a single lipid index cannot fully reflect cardiovascular disease. The triglyceride-glucose index is positively associated with a higher prevalence of symptomatic CAD and metabolic and behavioural risk factors and could also be used as a marker for atherosclerosis [19]. Similarly, the AIP, calculated by log (TG/HDL-C), which is a comprehensive index of blood lipids [20], is almost always associated with other CVD risk factors [21], and a higher AIP value is positively and strongly associated with obesity [22]. Hyperlipidaemia is closely related to atherosclerosis, of which LDL-C is the most important component. However, there was no significant difference in LDL-C levels between diabetic and non-diabetic patients in previous studies on the prognosis of PCI [23]. In the prognostic analysis of the diabetes subgroup, LDL-C levels were also not increased in patients with poor prognosis [4]. Therefore, the classic single lipid index (LDL-C) can no longer explain the severe lesions and poor prognosis in diabetic patients. Abnormal lipid metabolism in diabetic patients is often manifested as high TG and low HDL-C, while TC and LDL-C are usually normal or slightly elevated [3].
LDL-C is the core component of atherosclerosis in blood lipids. However, LDL-C is mostly normal in diabetic patients with abnormal lipid metabolism, which cannot explain the theory of atherosclerosis caused by blood lipids. Currently, sLDL-C, which is a subcomponent of LDL-C, does not easily bind to LDL receptors in circulation, reducing the clearance rate. Moreover, sLDL-C is easily oxidized and thus engulfed by macrophages to form foam cells, which then disintegrate and release a large amount of cholesterol [24]. sLDL-C is a more important and sensitive indicator of atherosclerosis [25]. The average diameter of LDL-C can indirectly reflect the number of sLDL-C particles, but it is not widely available in the clinic due to the complexity and high cost of measurement. Studies have shown that the AIP is negatively correlated with the particle size of LDL [3, 26]. To some extent, the AIP can be used to replace the number of sLDL-C particles.
Previous studies [27] have also confirmed that the AIP is more suggestive than LDL-C in atherosclerosis. In diabetic patients with normal LDL-C, the AIP can better predict the degree of atherosclerosis than LDL [28]. All the above evidence confirms that the AIP can comprehensively represent blood lipids in diabetic patients. In this study, the AIP also played an important role in predicting prognosis. MACCEs were significantly increased in patients with an AIP greater than 0.318 and were not affected by LDL-C levels. The AIP is an independent indicator that can reflect the comprehensive situation of blood lipids in diabetic patients. We believe that our study provides a new indicator for the management of blood lipids in diabetic patients after PCI.
The AIP is also correlated with abnormal glucose metabolism
Previous studies [29, 30] confirmed that blood glucose management in patients with diabetes after PCI is very important, and MACCEs were significantly reduced in patients with strict blood glucose control. However, a few studies [31] refuted the prognostic benefits of blood glucose management.
Theoretically, hyperglycaemia, insulin resistance and hyperinsulinaemia can all cause lipid metabolism disorders, oxidative stress and vascular endothelial damage, ultimately leading to the aggravation of coronary atherosclerosis [32]. Most patients with T2DM have insulin resistance, which gradually worsens. Insulin resistance reduces the activity of lipoprotein esterase, leading to a decrease in the clearance rate of very low-density lipoprotein, while an increase in hepatic lipase activity leads to the acceleration of the catabolism of high-density lipoprotein and eventually leads to abnormal lipid metabolism. Hyperinsulinaemia caused by insulin resistance can lead to the proliferation of smooth muscle and the formation of foam cells. These factors all accelerate hardening of the arteries. Blood insulin levels and insulin resistance reinforce each other in a vicious circle that eventually leads to hyperglycaemia. A hyperglycaemic environment can promote the production of early and late glycation products of the Amadori type, which leads to the development of vascular (coronary heart disease) and microvascular (diabetic nephropathy) diseases in patients with T2DM [33, 34].
Previous studies suggested that the AIP was negatively correlated with insulin sensitivity in diabetic patients [5]. Therefore, the use of the AIP in diabetic patients can not only describe the comprehensive situation of blood lipids but also reflect the degree of insulin resistance. In addition, propensity score matching was performed to adjust baseline characteristics and differences in subgroup analysis to verify that the AIP effects were consistent across subgroups. In addition, we adjusted for baseline characteristics between the two groups by propensity score matching to make the results more reliable.
In this study, we found a correlation between the AIP and clinical outcomes in diabetic patients. Low values of the AIP can significantly reduce MACCEs in diabetic patients after PCI, providing a new clinical indicator for secondary prevention of coronary heart disease in diabetic patients. Although the limitations of the study itself prevent us from finding accurate reference values of the AIP for clinical guidance, persistent management of blood lipids and blood glucose is a matter of constant attention.
Limitations
Some limitations and strengths of the present study have to be acknowledged. First, this study was a single-centre observational study, not a randomized controlled trial. The severity of CHD was calculated based on the clinical manifestations of CHD (stable/unstable/NSTEMI/STEMI) and the SYNTAX score. Hypertension/hyperlipidaemia was classified by prior medical history, but there is no detailed breakdown of severity. Although the sample size was large and the propensity score was matched, there were still unforeseeable confounding factors affecting the experimental results. Second, we only included stable blood lipid levels after statin use and did not monitor the blood lipid levels 4 years after PCI. Repeated measurement of the AIP during follow-up may be of further value for prediction of MACCEs. Third, we monitored HbA1c while the patients were in the hospital. Although it can reflect the short-term glycaemic control of patients, it cannot represent the long-term glycaemic control of patients after PCI.
Conclusions
The AIP is related to MACCEs after PCI in patients with T2DM: a low AIP can improve prognosis. Optimizing blood lipid and blood glucose management according to the value of the AIP is a new choice for secondary prevention of coronary heart disease in diabetic patients. More rigorous studies with larger sample sizes are expected in the future to verify our conclusions.
Acknowledgements
Not applicable.
Abbreviations
- AIP
atherogenic index of plasma
- ACEI
angiotensin-converting enzyme inhibitor
- ARB
angiotensin receptor blocker
- BMI
body mass index
- BP-DESs
biodegradable polymer drug-eluting stent
- CAD
coronary artery disease
- cIMT
carotid intima-media thickness
- CRF
case report form
- DBP
diastolic blood pressure
- DM
diabetes mellitus
- ECG
electrocardiograph
- FBG
fasting blood glucose
- G2-DESs
second-generation drug-eluting stent
- GFR
glomerular filtration rate
- HDL-C
high-density lipoprotein cholesterol
- HPLC
high-performance liquid chromatography
- hs-CRP
high-sensitivity C-reactive protein
- ISR
in-stent restenosis
- LAD
left anterior descending
- LCX
left circumflex artery
- LDL-C
low-density lipoprotein cholesterol
- LM
left main
- LVEF
left ventricular ejection fraction
- MI
myocardial infraction
- MMP
matrix metalloproteinases
- MVD
multivessel disease
- non-HDL-C
non-high-density lipoprotein cholesterol
- PCI
percutaneous coronary intervention
- RCA
right coronary artery
- ROC
receiver operating characteristics
- RLP-C
remnant-like particle cholesterol
- SBP
systolic blood pressure
- SYNTAX
synergy between PCI with taxus and cardiac surgery
- TC
total cholesterol
- TG
triglyceride
- UA
uric acid
Authors’ contributions
ZQ as the first author made substantial contributions to the conception and design, acquisition of data, analysis and interpretation of data, and drafting of the manuscript. YJZ, as the corresponding author, made substantial contributions to conception and design and critical revision of the manuscript for important intellectual content. KZ, YPL, WJC, ZJW, JLW, et al. made substantial contributions to the acquisition of data and many other aspects. All authors read and approved the final manuscript.
Authors’ information
Yujie Zhou M.D., Ph.D. is the Vice President and Chief Physician of Beijing Anzhen Hospital, Capital Medical University, Professor of Medicine and doctoral supervisor of Cardiology, Capital Medical University, Deputy Director of Beijing Institute of Heart, Lung and Blood Vessel Diseases. Prof. Zhou is head of the National Center for Clinical Research of Cardiovascular Diseases, the National Key Clinical Specialties for Cardiovascular and Geriatric Diseases, Key Laboratory for Precision Medicine of Coronary Artery Disease, Centre for Clinical Management of Coronary Artery Disease of Beijing Anzhen Hosptial, “Dengfeng” Talent Development Program and “Shiming” Talent Planning Team. Prof. Zhou was rewarded with Leading Personnel in Beijing Health System, Excellent Health and Technology Talent, New Century Talents Project, National Outstanding Contribution Expert, State Council Expert for Special Allowance, Honoured Doctor and Beijing Scholar.
Prof. Zhou served as the President of the College of SINO-EURO Coronary Heart Disease, Fellowship Member of the Society for Cardiovascular Angiography and Interventions (SCAI), Heart Rhythm Society (HRS), American College of Cardiology (ACC) and European Society of Cardiology (ESC). Prof. Zhou was also the previous Chairperson of the Chinese Medical Association, the Association of Interventional Cardiology, China Medical and Health International Exchange Promotion Committee, Honorary Chairman of China Geriatric Health Association (a branch of the Cardiovascular Disease Committee), Chairman of China Council for Prevention and Treatment of Chronic Diseases of Geriatric Health Care Research, Chairman of the Chinese Society of Geriatrics, the Committee on Precise Medical Treatment of Cardiovascular Diseases, Vice-Chairman of Chinese Medical Doctor’s Association, Vice-Chairman of Geriatrics Committee of Chinese Gerontology Society, Vice-Chairman of Beijing Society of Cardiology, Chinese Medical Association, Vice-Chairman of China Society of Geriatric Cardiology and Cerebrovascular Disease Specialized Committee, Secretary-General of Chinese Society of Cardiology and Expert Consultation of Central Health Committee.
Prof. Zhou was the Editor of Angiology, Editor-in-Chief of Journal of Cardiovascular and Pulmonary Diseases, Associate Editor of Chinese Journal of Interventional Cardiology, and Associate Editor of European Heart Journal (Chinese). Prof. Zhou published more than 280 papers in peer-reviewed SCI journals with cumulative impact factors greater than 500 and has undertaken National Keypoint Research and Interventional Programs of the Thirteenth “Precise Diagnosis and Treatment of Coronary Heart Disease and Atrial Fibrillation”. Dr. Zhou was the National Intervention Advisor for coronary artery disease and structural heart disease as a specialist in percutaneous coronary intervention for complex and higher-risk patients (total PCI volume > 20,000).
Funding
This work was supported by the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (code: ZYLX201303, XMLX201601), a grant from the National Key Research and Development Program of China (2017YFC0908800), the Beijing Municipal Administration of Hospitals “Mission plan” (SML20180601), the National Key Research and Development Program of China (2017YFC0908800), and the Beijing Municipal Health Commission “Project of Science and Technology Innovation Center” (PXM2019_026272_000006 and PXM2019_026272_000005).
Availability of data and materials
The datasets generated and analysed for this study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
This study was approved by the Clinical Research Ethics Committee of Beijing Anzhen Hospital, Capital Medical University, and all patients were informed and agreed to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Diabetes Association (8) Cardiovascular disease and risk management. Diabetes Care. 2015;38(Suppl):S49–S57. doi: 10.2337/dc15-S011. [DOI] [PubMed] [Google Scholar]
- 2.Koskinas KC, Siontis GC, Piccolo R, Franzone A, Haynes A, Rat-Wirtzler J, et al. Impact of diabetic status on outcomes after revascularization with drug-eluting stents in relation to coronary artery disease complexity: patient-level pooled analysis of 6081 patients. Circ Cardiovasc Interv. 2016;9:e003255. doi: 10.1161/CIRCINTERVENTIONS.115.003255. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Singh V. Atherogenic dyslipidemia and diabetes mellitus: what’s new in the management arena? Vasc Health Risk Manag. 2010;6:665–669. doi: 10.2147/VHRM.S5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobiasova M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)) Clin Biochem. 2001;34:583–588. doi: 10.1016/S0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 5.Tan MH, Johns D, Glazer NB. Pioglitazone reduces atherogenic index of plasma in patients with type 2 diabetes. Clin Chem. 2004;50:1184–1188. doi: 10.1373/clinchem.2004.031757. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu Y, Nakazato M, Sekita T, Kadota K, Yamasaki H, Takamura N, et al. Association of arterial stiffness and diabetes with triglycerides-to-HDL cholesterol ratio for Japanese men: the Nagasaki Islands study. Atherosclerosis. 2013;228:491–495. doi: 10.1016/j.atherosclerosis.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Wan K, Zhao J, Huang H, Zhang Q, Chen X, Zeng Z, et al. The association between triglyceride/high-density lipoprotein cholesterol ratio and all-cause mortality in acute coronary syndrome after coronary revascularization. PLoS ONE. 2015;10:e0123521. doi: 10.1371/journal.pone.0123521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu XW, Deng FY, Lei SF. Meta-analysis of atherogenic index of plasma and other lipid parameters in relation to risk of type 2 diabetes mellitus. Prim Care Diabetes. 2015;9:60–67. doi: 10.1016/j.pcd.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Mazidi M, Katsiki N, Mikhailidis DP, Banach M. Association of ideal cardiovascular health metrics with serum uric acid, inflammation and atherogenic index of plasma: a population-based survey. Atherosclerosis. 2019;284:44–49. doi: 10.1016/j.atherosclerosis.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Yan PJ, Xu Y, Wan Q, Feng J, Li H, Gao CL, et al. Decreased plasma neuregulin 4 concentration is associated with increased high-sensitivity C-reactive protein in newly diagnosed type 2 diabetes mellitus patients: a cross-sectional study. Acta Diabetol. 2017;54:1091–1099. doi: 10.1007/s00592-017-1044-4. [DOI] [PubMed] [Google Scholar]
- 11.Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–227. [PubMed] [Google Scholar]
- 12.American Diabetes Association Standards of medical care in diabetes–2014. Diabetes Care. 2014;37(Suppl 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 13.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 14.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 15.Liao J, Farmer J. Arterial stiffness as a risk factor for coronary artery disease. Curr Atheroscler Rep. 2014;16:387. doi: 10.1007/s11883-013-0387-8. [DOI] [PubMed] [Google Scholar]
- 16.Kozakova M, Morizzo C, Goncalves I, Natali A, Nilsson J, Palombo C. Cardiovascular organ damage in type 2 diabetes mellitus: the role of lipids and inflammation. Cardiovasc Diabetol. 2019;18:61. doi: 10.1186/s12933-019-0865-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen Y, Chen S, Dai Y, Wang XQ, Zhang RY, Yang ZK, et al. Lipoprotein (a) interactions with cholesterol-containing lipids on angiographic coronary collateralization in type 2 diabetic patients with chronic total occlusion. Cardiovasc Diabetol. 2019;18:82. doi: 10.1186/s12933-019-0888-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landes U, Kornowski R, Assali A, Vaknin-Assa H, Greenberg G, Lev EI, et al. Predictors of long term outcomes in 11,441 consecutive patients following percutaneous coronary interventions. Am J Cardiol. 2015;115:855–859. doi: 10.1016/j.amjcard.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Da Silva A, Caldas APS, Hermsdorff HHM, Bersch-Ferreira AC, Torreglosa CR, Weber B, et al. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovasc Diabetol. 2019;18:89. doi: 10.1186/s12933-019-0893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Xu C, Liu J, Bai X, Li R, Wang L, et al. Baseline plasma fibrinogen is associated with haemoglobin A1c and 2-year major adverse cardiovascular events following percutaneous coronary intervention in patients with acute coronary syndrome: a single-centre, prospective cohort study. Cardiovasc Diabetol. 2019;18:52. doi: 10.1186/s12933-019-0858-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bo MS, Cheah WL, Lwin S, Moe Nwe T, Win TT, Aung M. Understanding the relationship between atherogenic index of plasma and cardiovascular disease risk factors among staff of an University in Malaysia. J Nutr Metab. 2018;2018:7027624. doi: 10.1155/2018/7027624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu X, Yu L, Zhou H, Ma Q, Zhou X, Lei T, et al. Atherogenic index of plasma is a novel and better biomarker associated with obesity: a population-based cross-sectional study in China. Lipids Health Dis. 2018;17:37. doi: 10.1186/s12944-018-0686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin Z, Zhou K, Li YP, Wang JL, Cheng WJ, Hu CP, et al. Remnant lipoproteins play an important role of in-stent restenosis in type 2 diabetes undergoing percutaneous coronary intervention: a single-centre observational cohort study. Cardiovasc Diabetol. 2019;18:11. doi: 10.1186/s12933-019-0819-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobiasova M. Atherogenic index of plasma [log(triglycerides/HDL-cholesterol)]: theoretical and practical implications. Clin Chem. 2004;50:1113–1115. doi: 10.1373/clinchem.2004.033175. [DOI] [PubMed] [Google Scholar]
- 25.Nishikura T, Koba S, Yokota Y, Hirano T, Tsunoda F, Shoji M, et al. Elevated small dense low-density lipoprotein cholesterol as a predictor for future cardiovascular events in patients with stable coronary artery disease. J Atheroscler Thromb. 2014;21:755–767. doi: 10.5551/jat.23465. [DOI] [PubMed] [Google Scholar]
- 26.Yan L, Xu MT, Yuan L, Chen B, Xu ZR, Guo QH, et al. Prevalence of dyslipidemia and its control in type 2 diabetes: a multicenter study in endocrinology clinics of China. J Clin Lipidol. 2016;10:150–160. doi: 10.1016/j.jacl.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Cai G, Liu W, Lv S, Wang X, Guo Y, Yan Z, et al. Gender-specific associations between atherogenic index of plasma and the presence and severity of acute coronary syndrome in very young adults: a hospital-based observational study. Lipids Health Dis. 2019;18:99. doi: 10.1186/s12944-019-1043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onat A, Can G, Kaya H, Hergenc G. “Atherogenic index of plasma” (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J Clin Lipidol. 2010;4:89–98. doi: 10.1016/j.jacl.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 29.UK Prospective Diabetes Study (UKPDS) group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 30.Hwang JK, Lee SH, Song YB, Ahn J, Carriere K, Jang MJ, et al. Glycemic control status after percutaneous coronary intervention and long-term clinical outcomes in patients with type 2 diabetes mellitus. Circ Cardiovasc Interv. 2017;10:e004157. doi: 10.1161/CIRCINTERVENTIONS.116.004157. [DOI] [PubMed] [Google Scholar]
- 31.Ike A, Nishikawa H, Shirai K, Mori K, Kuwano T, Fukuda Y, et al. Impact of glycemic control on the clinical outcome in diabetic patients with percutaneous coronary intervention–from the FU-registry. Circ J. 2011;75:791–799. doi: 10.1253/circj.CJ-10-0474. [DOI] [PubMed] [Google Scholar]
- 32.Beckman JA, Paneni F, Cosentino F, Creager MA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Eur Heart J. 2013;34:2444–2452. doi: 10.1093/eurheartj/eht142. [DOI] [PubMed] [Google Scholar]
- 33.Cohen MP. Intervention strategies to prevent pathogenetic effects of glycated albumin. Arch Biochem Biophys. 2003;419:25–30. doi: 10.1016/j.abb.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Wolf G. New insights into the pathophysiology of diabetic nephropathy: from haemodynamics to molecular pathology. Eur J Clin Invest. 2004;34:785–796. doi: 10.1111/j.1365-2362.2004.01429.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed for this study are available from the corresponding author upon reasonable request.