Abstract
Rituximab (375 mg/m2) achieved remission of the first episode and six relapses of nephrotic syndrome (NS) in a young male patient with podocyte phospholipase A2 receptor (PLA2R)-related membranous nephropathy (MN) refractory to steroids and cyclosporine. Between-treatments interval averaged 17.4±4.2 months. The seventh infusion was complicated by delayed serum-sickness, which resolved with steroids. On subsequent relapse, the fully human anti-CD20 monoclonal antibody ofatumumab (300 mg) achieved remission of the NS, without significant side effects. Circulating CD19+ B cells were depleted, proteinuria decreased from 10.9 to 1.3 g/day, and serum albumin, immunoglobulin levels and glomerular filtration rate normalised. Twenty-eight months later, despite transient anti-PLA2R depletion, ofatumumab (100 mg) failed to induce remission of the eighth relapse. Remission was safely achieved 5 months later with repeated ofatumumab infusion (300 mg). This treatment (€723) was less expensive than rituximab (€1801). Ofatumumab could be a safe and cost/effective rescue therapy for patients with MN sensitised against rituximab.
Keywords: renal medicine, rheumatology, immunology
Background
Membranous nephropathy (MN) is the most common cause of nephrotic syndrome (NS) in adults and affects approximately 8–10 white adults per million population per year.1 MN is caused by the deposition of immunoglobulins and complement on the subepithelial layer of the glomerular capillary wall, which leads to loss of the glomerular sieving function and consequent proteinuria, with persistent NS in approximately one-third of affected patients.2 NS substantially reduces quality of life, exposes patients to an increased risk of serious cardiovascular and thromboembolic complications, and is almost invariably associated with relentless progression to end-stage renal disease.3
Current guidelines recommend that MN patients with persistent NS are treated with a combination of cyclophosphamide and steroids.4 This treatment induces remission of proteinuria in approximately two-thirds of patients, but is associated with severe complications, including myelotoxicity, infertility, diabetes, infections and cancer.5 Calcineurin inhibitors, previously considered as a second-line treatment, are scarcely effective and nephrotoxic.6 7
In recent years, the discovery of nephritogenic autoantibodies against the podocyte phospholipase A2 receptor (PLA2R) proved that MN is an autoimmune disease.8 9 Several groups consistently reported that higher autoantibody levels are associated with a more severe disease phenotype, and depletion of circulating anti-PLA2R precedes remission. Moreover, autoantibody reappearance into the circulation predicts relapse of the NS in patients who previously achieved remission.10 11
These findings also provided a clear pathophysiological rationale for B cell-targeted interventions aimed at preventing the production of nephritogenic autoantibodies and the glomerular deposition of immunocomplexes,2 which had been successfully used even when the nature of these autoantibodies was still unknown.12 Treatment with rituximab, an anti-CD20 monoclonal antibody that selectively depletes B cells, is well-tolerated and achieves remission of proteinuria in approximately two-thirds of MN patients.13 14 A recent randomised-controlled trial confirmed that rituximab induces remission more effectively than cyclosporine and is remarkably safer.15 Thus, rituximab may be a valuable alternative to less specific and more toxic medications such as steroid, calcineurin inhibitors and alkylating agents for first line therapy of MN patients.16
However, approximately one third of patients relapse after initial response to rituximab, and many experience multiple relapses requiring one or more retreatments. After repeated exposures to rituximab, these patients may become sensitised to the murine portion of the drug, and treatment can be complicated by acute or delayed serum-sickness, an immune-complex mediated hypersensitivity reaction (HSR) that may contraindicate additional rituximab infusions.2
Case presentation
An 18-year-old man was admitted on January 2003 to an Italian institution for overt NS. Kidney biopsy evaluation was consistent with MN, and the patient was treated with steroids and cyclosporine with transient reduction of proteinuria. This treatment was discontinued due to severe steroid-related side effects, and the patient was referred to our unit on July 2005.
On admission, diffuse oedema was evident, but physical examination was otherwise unremarkable. Laboratory workup confirmed NS (proteinuria 11.8 g/day, serum albumin 2.2 g/dL, total serum proteins 4.4 g/dL), and glomerular filtration rate (GFR) measured by the iohexol plasma clearance technique17 was 142.5 mL/min/1.73 m2. Secondary causes of MN were excluded, and the patient was treated with a course of rituximab (375 mg/m2).18 The patient received a total of four infusions, which were all well-tolerated and resulted in peripheral B-cell depletion (CD19+ <5 cells/μL). Urinary protein excretion progressively decreased to 2.7 g/day after 6 months, and clinical signs of the NS fully remitted.
During the following months proteinuria increased again to the nephrotic range, thus the patient received a second rituximab course (single 375 mg/m2 infusion) in December 2006, which resulted again in B-cell depletion and partial remission of the NS. Between December 2007 and December 2011 there were four additional relapses, which were all treated with single infusions of rituximab. Pretreatment urinary protein excretion ranged from 3.1 to 14.5 g/day, while measured GFR remained stable (115–136 mL/min/1.73 m2). The treatment-free interval between each rituximab dose averaged 17.4±4.2 months. Therapy was generally well-tolerated, with occasional minor adverse events reported, including flushes and mild skin rashes during infusions that promptly resolved with temporary interruption of the infusion and/or steroid administration.
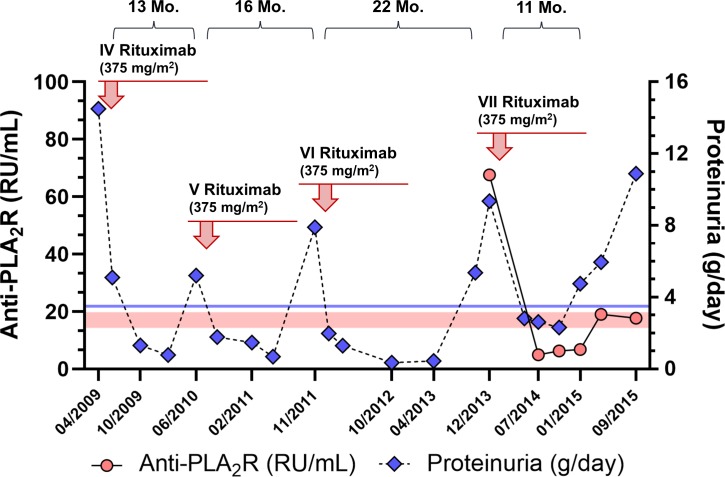
In December 2013 the patient experienced a new NS relapse associated with the emergence into the circulation of anti-PLA2R antibodies that could be detected through a commercially available assay (67.66 RU/mL—anti-PLA2R IgG ELISA, Euroimmun AG, Germany). A seventh course of rituximab was scheduled, but treatment was complicated by severe pruritus, cutaneous rash and hoarseness, which regressed with intravenous hydrocortisone and temporary interruption of the infusion. Rituximab administration was completed and the patient was discharged the next day. However, approximately 1 week later, he reported the onset of severe fatigue, fever and arthralgias, which recovered after treatment with low-dose oral steroids. Rituximab induced again partial remission of the NS and anti-PLA2R levels decreased below the positivity threshold (figure 1). Nevertheless, approximately 8 months after achieving remission, proteinuria increased again along with anti-PLA2R titres, serum albumin decreased to 2.0 g/dL and renal function started deteriorating (measured GFR 67.4 mL/min/1.73 m2). During this period, the patient had symptoms of worsening fatigue and malaise that severely limited his daily activities. Outcome was also complicated by recurrent respiratory tract infections requiring antibiotic therapy.
Figure 1.
Time course of proteinuria and anti-PLA2R antibody levels before and after the last four rituximab infusions. Red circles: anti-PLA2R levels (RU/mL); blue diamonds: 24 hours proteinuria (g/day); red-coloured area: borderline anti-PLA2R range (14–20 RU/mL); blue line: proteinuria threshold for partial remission (<3.5 g/day). Time to relapse (months) from each infusion is shown above each panel. PLA2R, podocyte phospholipase A2 receptor.
Differential diagnosis
In the context of autoimmune diseases, the most common adverse effects due to rituximab administration are infusion reactions that range from flushing, chills and fever, to dyspnoea, vomiting and syncope. In most cases, however, these effects are not serious and resolve with temporary interruption of the infusion.13 Infusion reactions are due to cytokine release (eg, tumor necrosis factor alpha (TNFα), interleukin 6 (IL-6) and IL-1β) from target cells, occur more frequently during the first rituximab administration and can be usually prevented or mitigated by premedication with steroids and anti-histamine drugs.19
On the other hand, HSRs to rituximab imply an immune response towards the monoclonal antibody and can be classified according to their pathogenesis: type I anaphylactoid HSRs are associated with the release of mediators from mast cells and basophils (eg, histamine and prostaglandin) in response to IgE-induced FCε receptor engagement and cross-linking.20 These reactions are usually acute and may cause mild symptoms such as pruritus and urticaria, but could also precipitate potentially life-threatening complications such as laryngeal oedema and anaphylaxis.21
Type III reactions are instead caused by the formation of IgG/IgM antibodies that target the murine portion of rituximab (human anti-chimeric antibodies (HACA)), leading to immune-complex formation. Tissue deposition of immune-complexes may result in serum-sickness, a well-known complication of repeated rituximab infusions characterised by fever, arthralgia and rash.22 23 These symptoms may become evident as early as a few hours after rituximab administration (acute serum-sickness), but more often arise 1–2 weeks following drug exposure (delayed serum-sickness).24 Despite their relevance in the pathogenesis of these reactions, HACA are detected in 60% of patients only,23 and are more frequently observed in acute than in delayed serum-sickness cases.24
Our patient had received six rituximab courses that were either uneventful or were occasionally associated with mild infusional symptoms. These symptoms most likely reflected cytokine-induced infusion reactions, which were prevented or blunted by premedication. The reaction to the seventh infusion was different: aside from immediate symptoms, the delayed reaction after approximately 1 week from treatment was consistent with serum-sickness.
Treatment
Since the occurrence of serum-sickness after treatment with rituximab significantly increases the risk of severe adverse events on drug re-exposure,19 25 we decided not to repeat the previous treatment schedule.
After discussing with the patient the potential benefits and risks of treatment alternatives, we decided not to use steroids and cyclophosphamide because of the associated risk of severe side-effects.5 6 Indeed, the risk of infertility was one of the decisive factors for this young patient, who referred the desire of having children in the future. We also decided not to re-expose the patient to cyclosporine, which had been ineffective at disease onset and, more in general, is scarcely effective and nephrotoxic.6 7 Moreover, no data are available on the efficacy of these agents as second-line therapy in MN patients.
Thus, on September 2015 the patient was treated with 300 mg of the fully human anti-CD20 monoclonal antibody ofatumumab after premedication with hydrocortisone and chlorphenamine. The infusion was associated with mild flushing, which promptly resolved with supplementary steroids. Circulating CD19+ B cells were fully depleted and the patient was discharged the day after the infusion.
Outcome and follow-up
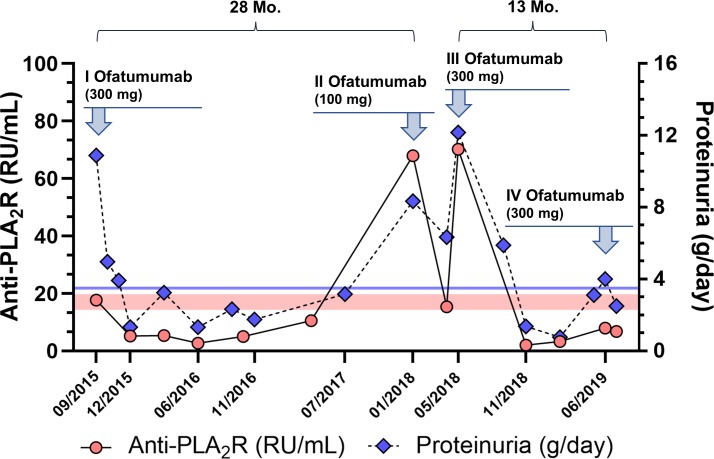
Over the next 3 months, we observed a rapid reduction of anti-PLA2R antibody levels up to full depletion from the circulation (figure 2), which was associated with partial NS remission (proteinuria 1.33 g/day). Consistently, at 9 months from treatment, urinary IgG excretion decreased from 220.5 to 49.4 mg/day, while serum albumin and IgG concentration increased from 2.0 to 3.1 g/dL and from 260 to 564 mg/dL, respectively. GFR (118.2 mL/min/1.73 m2) fully recovered at 6 months from the infusion and stabilised thereafter.
Figure 2.
Time course of proteinuria and anti-PLA2R antibody levels before and after treatment with ofatumumab. Red circles: anti-PLA2R levels (RU/mL); blue diamonds: 24 hours proteinuria (g/day); red-coloured area: borderline anti-PLA2R range (14–20 RU/mL); blue line: proteinuria threshold for partial remission (<3.5 g/day). Time to relapse (months) from each infusion is shown above each panel. PLA2R, podocyte phospholipase A2 receptor.
During follow-up, serum anti-PLA2R antibodies were persistently depleted and proteinuria remained below the partial remission threshold. The patient referred a fast, remarkable improvement in NS-associated symptoms and resumed his normal daily activities as early as 2 weeks after the infusion. No respiratory tract infection was reported any longer.
After more than 2 years from the first ofatumumab infusion, serum anti-PLA2R levels increased (67.9 RU/mL) and NS relapsed. In order to limit drug exposure and owing to evidence of efficacy with reduced ofatumumab doses in other cases,26 we treated this patient with 100 mg of ofatumumab. This regimen determined a complete depletion of peripheral CD19+ B cells and reduced circulating autoantibodies below the positivity threshold. Antibody levels however increased again up to 70.2 RU/mL and no effect could be detected on proteinuria during the next 5 months. Since immunological remission was not sustained, we decided to retreat the patient with 300 mg of ofatumumab, achieving again anti-PLA2R depletion and persistent remission of proteinuria without any acute or delayed reaction. Approximately 1 year later, the patient experienced a viral enteritis with high-grade fever; serum anti-PLA2R levels began to increase and nephrotic range proteinuria relapsed again (figure 2). Due to worsening symptoms, we decided to treat the patient again with a single infusion of ofatumumab (300 mg), which induced partial remission of proteinuria as early as 1 month after treatment. The time between ofatumumab infusions (excluding retreatment after the 100 mg infusion) was on average 20.5±10.6 months. The patient is currently well, without oedema and with normal GFR.
Discussion
We herein describe the case of an adult patient with multirelapsing MN requiring repeated rituximab infusions, who eventually developed delayed serum-sickness after the seventh, last exposure to this chimeric monoclonal antibody. Rescue treatment with the fully human anti-CD20 antibody ofatumumab was well tolerated, achieved antibody depletion and induced persistent remission of the NS for approximately 2 years. On relapse, retreatment with ofatumumab was uneventful and again effective in inducing disease remission. Notably, the time interval between treatments was similar for ofatumumab and rituximab (20.5±10.6 and 17.4±4.2 months, respectively). Within the limitations of intrapatient comparisons in the context of a single case report, these findings suggest that ofatumumab could be effective and safe in patients with MN who become intolerant to rituximab, and that its protective effect against relapse of the NS appears to be at least comparable to that of rituximab.
Several regimens of rituximab infusions have been described for the treatment of patients with MN. The original protocol employed 4 weekly doses, 375 mg/m2 each,12 but other strategies include two doses (either 375 mg/m2 or 1000 mg) administered every 1–2 weeks with or without a booster dose at 6 months.15 27 28
In our experience with MN patients, we observed that circulating B cells are fully depleted in most cases (>95%) after a single dose of rituximab (375 mg/m2). These observations questioned the indication to additional drug doses, which were expected not to confer additional benefit, expose patients to the risk of adverse effects and/or sensitisation, and to unnecessarily increase treatment costs. Consistently, in a prospective 1:2 matched-cohort study comparing the risk/benefit profile of the standard rituximab treatment (four 375 mg/m2 doses 1 week apart) with a B cell-driven rituximab protocol (a second dose was administered only if peripheral CD19+ B cells were >5/μL after the first infusion) in 36 patients with MN and NS, we found that only one patient allocated to the B cell-driven protocol required a second dose of the drug, and that both strategies promptly and persistently depleted circulating B cells in all patients, achieving a similar reduction in proteinuria over time.18 On the other hand, adverse events and hospitalisations were less frequent with the B cell-driven approach, which was also fourfold less expensive (table 1).
Table 1.
B cell-targeted treatment for glomerular diseases with NS: protocols, schedules and costs
| Regimen | Dose | Schedule | Costs* |
| Four-doses rituximab course in MN12 | 375 mg/m2 | Doses 1 week apart (four in total) | €7.202–€5.762† |
| Two-doses rituximab course in MN (GEMRITUX trial)27 |
375 mg/m2 | Doses 1 week apart (two in total) | €3.601–€2.881† |
| Two/four-doses rituximab course in MN (MENTOR trial)15 |
1000 mg | Dose every other week, with two additional identical doses at 6 months in case of partial response (four in total) | €11.102–€8.881† |
| B cell-driven rituximab course in MN18 | 375 mg/m2 | One single dose, B cell-driven‡ | €1.801–€1.440† |
| Ofatumumab in steroid-resistant NS32 | 300 mg/1.73 m2–2000 mg/1.73 m2 | One 300 mg/1.73 m2 dose for the first week, followed by 1 weekly 2000 mg/1.73 m2 dose for 5 weeks (total six doses) | €24.823 |
| Ofatumumab in rituximab-resistant NS35 | 300 mg/1.73 m2–700 mg/1.73 m2 | One 300 mg/1.73 m2 dose, followed by one 700 mg/1.73 m2 dose 2 weeks apart | €2.410 |
| Ofatumumab in rituximab-intolerant NS34 | 750 mg/1.73 m2 | One single 750 mg/1.73 m2 dose | €1.808 |
| Ofatumumab in rituximab-intolerant NS33 | 300 mg/m2 | One single 300 mg/m2 dose | €1.251 |
| B cell-driven ofatumumab course in rituximab-intolerant MN (current case) | 300 mg | One single 300 mg dose, B cell-driven‡ | €723 |
*In an average adult patient (1.73 m2 body surface area); cost calculations refer to ex-factory prices (before taxes), as reported by AIFA (Azienda Italiana del Farmaco, Italy).
†From rituximab originator to biosimilar approval.
‡A second 375 mg/m2 dose of rituximab or a second 300 mg dose of ofatumumab are administered 1 week apart only if >5 circulating B cells per mm3 persist after the first infusion.
MN, membranous nephropathy; NS, nephrotic syndrome.
Ofatumumab has been approved by both the US Food and Drug Administration and the European Medicines Agency for the treatment of chronic lymphocytic leukaemia, but has shown efficacy also in autoimmune diseases such as rheumatoid arthritis and autoimmune thrombotic thrombocytopenic purpura, especially when rituximab was contraindicated due to anaphylaxis.29–31
In the context of idiopathic NS (minimal change disease and focal segmental glomerulosclerosis), rescue therapy with ofatumumab was well tolerated and induced disease remission both in patients who were resistant to rituximab and in those who experienced anaphylaxis or delayed HSRs after repeated exposures to this chimeric drug.32–35 In these series, both single and multiple ofatumumab infusions were effective in inducing remission of the NS.
Indeed, standard ofatumumab treatment includes a first 300 mg dose followed by five 2000 mg doses 1 week apart from each other. However, we observed that, as previously reported for rituximab,18 B cells were fully depleted from the circulation within 1 week from the infusion of 300 mg of ofatumumab (P Ruggenenti, 2016 - personal communication). Thus, we decided to use a B cell-driven approach for ofatumumab treatment in our patient. Complete B-cell and anti-PLA2R depletion from the circulation was achieved after the first infusion in all relapses, but they were sustained over the following months only with the higher (ie, 300 mg) drug dose. We speculate that, in this patient, a lower ofatumumab dose (ie, 100 mg), although effective in inducing peripheral B cells lysis, may have not been sufficient to deplete autoreactive B cells in secondary lymphoid organs, leading to early B-cell reconstitution at 3 months from treatment, and possibly explaining the lack of sustained immunological remission.36 37 Of note, the use of a B cell driven protocol for ofatumumab (300 mg) resulted in further decrease in treatment costs as compared with the B cell-driven rituximab treatment protocol, even when the reduced cost of rituximab biosimilars was taken into account (table 1).
In summary, we provide the first, pilot evidence that ofatumumab rescue therapy may be safe and effective also for patients with MN in whom retreatment with rituximab is contraindicated due to drug-related HSRs. Should our pilot findings be confirmed in larger patient series, ofatumumab might emerge as a valuable alternative to rituximab because of its better risk/benefit and cost/effectiveness profile for the treatment of patients with MN and NS.
Patient’s perspective.
We report below a brief patient interview, which was conducted to gain his perspective on the disease and treatment.
Q: How did you feel when your disease relapsed after the last rituximab infusion?
A: My face and leg swelling was unacceptable; I also had chronic fatigue, which was obscure to me for my age and I often had bronchitis in that period. I felt sick. This also had a significant impact on my job, since I was unable to work for more than two hours consecutively. I was depressed. I had the impression that rituximab was not working as well as after the previous infusions.
Q: What was your reaction when your doctors told you that some of your symptoms such as weakness, articular pain and fever could be caused by rituximab and therefore rituximab could not be used any longer to treat your relapses?
A: I was terrified because I thought no other treatment would be available. My doctors told me about cyclophosphamide, but that option was off the table for me, especially because I did not want to risk not being able to have children in the future. I was worried about my fate, because I was afraid I would not ever get rid of the disease again. I started to think that I would have ended up on dialysis.
Q: And what was your reaction when your doctors told you that ofatumumab could be used in alternative to rituximab?
A: Initially I was disoriented because I had been told that this drug had never been used in patients with my disease. On the other hand I was enthusiastic, since I realized that there were other options, and that I could still hope to recover and go back to my daily life. When my doctors reassured me that this was a safe drug and that they would have carefully managed any possible reaction, I trusted them and decided to accept the new treatment.
Q: How would you describe your experience with ofatumumab?
A: When they put the needle in my arm I was terribly anxious. But nothing serious happened during the infusion. I took heart again and started to think that I had taken the right decision. I almost immediately felt better, even before my proteinuria started to decrease. All the symptoms related to my disease gradually disappeared and I did not have fever or pain to my fingers and joints such as after the last dose of rituximab. I restarted working and having fun with my friends very soon after the infusion.
Q: How do you feel now?
A: My job and my two kids keep me busy! I know my disease may relapse again in the future, but now I feel like I have another chance to have a normal life.
Learning points.
Patients with membranous nephropathy (MN) and persistent nephrotic syndrome (NS) are at risk of progression to end stage renal disease, as well as cardiovascular, thromboembolic and infectious complications.
B-cell targeted therapy with the anti‐CD20 monoclonal antibody rituximab is safe and achieves remission of proteinuria in approximately two‐thirds of patients with MN. In those with podocyte phospholipase A2 receptor (PLA2R)‐related disease, remission can be predicted by anti‐PLA2R antibody depletion and relapse by antibody re‐emergence into the circulation.
After initial remission, some patients may experience one or more relapses of NS and thus require retreatment. Repeated treatment with rituximab may increase the risk of developing serum sickness, which is a relative contraindication to further exposure to the drug.
The fully human anti-CD20 monoclonal antibody ofatumumab was safe and achieved NS remission in a rituximab-intolerant patient with MN.
If confirmed, our preliminary findings may indicate that ofatumumab could be a valuable alternative to rituximab for the treatment of patients with MN and NS because of its better risk/benefit and cost/effectiveness profile.
Acknowledgments
The authors would like to thank Drs Alessia Gennarini and Valentina Portalupi, as well as all the staff from the Nephrology Unit, for their outstanding professionalism in patient care. The authors are also grateful to Nadia Rubis for her assistance with data retrieval and organisation.
Footnotes
Contributors: Supervised by PR and GR. The patient was under the care of BR, the report was written by MAP and PR, and the final version of the manuscript was revised and approved by all contributors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Keri KC, Blumenthal S, Kulkarni V, et al. Primary membranous nephropathy: comprehensive review and historical perspective. Postgrad Med J 2019;95:23–31. 10.1136/postgradmedj-2018-135729 [DOI] [PubMed] [Google Scholar]
- 2. Ruggenenti P, Fervenza FC, Remuzzi G. Treatment of membranous nephropathy: time for a paradigm shift. Nat Rev Nephrol 2017;13:563–79. 10.1038/nrneph.2017.92 [DOI] [PubMed] [Google Scholar]
- 3. Cattran DC, Kim ED, Reich H, et al. Membranous nephropathy: quantifying remission duration on outcome. J Am Soc Nephrol 2017;28:995–1003. 10.1681/ASN.2015111262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. KDIGO clinical practice guidelines for glomerulonephritis. Kidney International 2012;2:139–274. [Google Scholar]
- 5. van den Brand JAJG, Ruggenenti P, Chianca A, et al. Safety of rituximab compared with steroids and cyclophosphamide for idiopathic membranous nephropathy. J Am Soc Nephrol 2017;28:2729–37. 10.1681/ASN.2016091022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howman A, Chapman TL, Langdon MM, et al. Immunosuppression for progressive membranous nephropathy: a UK randomised controlled trial. Lancet 2013;381:744–51. 10.1016/S0140-6736(12)61566-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Y, Schieppati A, Cai G, et al. Immunosuppression for membranous nephropathy: a systematic review and meta-analysis of 36 clinical trials. Clin J Am Soc Nephrol 2013;8:787–96. 10.2215/CJN.07570712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beck LH, Bonegio RGB, Lambeau G, et al. M-Type Phospholipase A 2 Receptor as Target Antigen in Idiopathic Membranous Nephropathy. N Engl J Med 2009;361:11–21. 10.1056/NEJMoa0810457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tomas NM, Beck LH, Meyer-Schwesinger C, et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 2014;371:2277–87. 10.1056/NEJMoa1409354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beck LH, Fervenza FC, Beck DM, et al. Rituximab-Induced Depletion of Anti-PLA2R Autoantibodies Predicts Response in Membranous Nephropathy. J Am Soc Nephrol 2011;22:1543–50. 10.1681/ASN.2010111125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ruggenenti P, Debiec H, Ruggiero B, et al. Anti-Phospholipase A2 Receptor Antibody Titer Predicts Post-Rituximab Outcome of Membranous Nephropathy. JASN 2015;26:2545–58. 10.1681/ASN.2014070640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Remuzzi G, Chiurchiu C, Abbate M, et al. Rituximab for idiopathic membranous nephropathy. Lancet 2002;360:923–4. 10.1016/S0140-6736(02)11042-7 [DOI] [PubMed] [Google Scholar]
- 13. Ruggenenti P, Cravedi P, Chianca A, et al. Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol 2012;23:1416–25. 10.1681/ASN.2012020181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fervenza FC, Cosio FG, Erickson SB, et al. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int 2008;73:117–25. 10.1038/sj.ki.5002628 [DOI] [PubMed] [Google Scholar]
- 15. Fervenza FC, Appel GB, Barbour SJ, et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med 2019;381:36–46. 10.1056/NEJMoa1814427 [DOI] [PubMed] [Google Scholar]
- 16. Ruggenenti P, Remuzzi G. A first step toward a new approach to treating membranous nephropathy. N Engl J Med 2019;381:86–8. 10.1056/NEJMe1906666 [DOI] [PubMed] [Google Scholar]
- 17. Gaspari F, Perico N, Ruggenenti P, et al. Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate. J Am Soc Nephrol 1995;6:257–63. [DOI] [PubMed] [Google Scholar]
- 18. Cravedi P, Ruggenenti P, Sghirlanzoni MC, et al. Titrating rituximab to circulating B cells to optimize lymphocytolytic therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 2007;2:932–7. 10.2215/CJN.01180307 [DOI] [PubMed] [Google Scholar]
- 19. Kumar A, Khamkar K, Gopal H. Serum sickness and severe angioedema following rituximab therapy in RA. Int J Rheum Dis 2012;15:e6–7. 10.1111/j.1756-185X.2011.01645.x [DOI] [PubMed] [Google Scholar]
- 20. Levin AS, Otani IM, Lax T, et al. Reactions to rituximab in an outpatient infusion center: a 5-year review. J Allergy Clin Immunol 2017;5:107–13. 10.1016/j.jaip.2016.06.022 [DOI] [PubMed] [Google Scholar]
- 21. Isabwe GAC, Garcia Neuer M, de las Vecillas Sanchez L, et al. Hypersensitivity reactions to therapeutic monoclonal antibodies: phenotypes and endotypes. J Allergy Clin Immunol 2018;142:159–70. 10.1016/j.jaci.2018.02.018 [DOI] [PubMed] [Google Scholar]
- 22. Khan DA. Hypersensitivity and immunologic reactions to biologics: opportunities for the allergist. Ann Allergy Asthma Immunol 2016;117:115–20. 10.1016/j.anai.2016.05.013 [DOI] [PubMed] [Google Scholar]
- 23. Karmacharya P, Poudel DR, Pathak R, et al. Rituximab-induced serum sickness: a systematic review. Semin Arthritis Rheum 2015;45:334–40. 10.1016/j.semarthrit.2015.06.014 [DOI] [PubMed] [Google Scholar]
- 24. Vendramin C, Thomas M, Westwood J-P, et al. Rituximab-induced acute and delayed serum sickness in thrombotic thrombocytopenic purpura: the role of anti-rituximab antibodies. Br J Haematol 2019;184:858–61. 10.1111/bjh.15177 [DOI] [PubMed] [Google Scholar]
- 25. Isoda A, Ishikawa T, Sato N, et al. Repeated rituximab-induced serum sickness with anaphylaxis. Rinsho Ketsueki 2016;57:771–3. 10.11406/rinketsu.57.771 [DOI] [PubMed] [Google Scholar]
- 26. Podestà MA, Gennarini A, Portalupi V, et al. Accelerating the Depletion of Circulating Anti-Phospholipase A2 Receptor Antibodies in Patients with Severe Membranous Nephropathy: Preliminary Findings with Double Filtration Plasmapheresis and Ofatumumab. Nephron 2019:1–6. 10.1159/000501858 [DOI] [PubMed] [Google Scholar]
- 27. Dahan K, Debiec H, Plaisier E, et al. Rituximab for severe membranous nephropathy: a 6-month trial with extended follow-up. J Am Soc Nephrol 2017;28:348–58. 10.1681/ASN.2016040449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seitz-Polski B, Dahan K, Debiec H, et al. High-Dose rituximab and early remission in PLA2R1-Related membranous nephropathy. Clin J Am Soc Nephrol 2019;14:1173–82. 10.2215/CJN.11791018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wierda WG, Padmanabhan S, Chan GW, et al. Ofatumumab is active in patients with fludarabine-refractory CLL irrespective of prior rituximab: results from the phase 2 international study. Blood 2011;118:5126–9. 10.1182/blood-2011-04-348656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taylor PC, Quattrocchi E, Mallett S, et al. Ofatumumab, a fully human anti-CD20 monoclonal antibody, in biological-naive, rheumatoid arthritis patients with an inadequate response to methotrexate: a randomised, double-blind, placebo-controlled clinical trial. Ann Rheum Dis 2011;70:2119–25. 10.1136/ard.2011.151522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crowley MP, McDonald V, Scully M. Ofatumumab for TTP in a patient with anaphylaxis associated with rituximab. N Engl J Med 2018;378:92–3. 10.1056/NEJMc1714146 [DOI] [PubMed] [Google Scholar]
- 32. Basu B. Ofatumumab for rituximab-resistant nephrotic syndrome. N Engl J Med 2014;370:1268–70. 10.1056/NEJMc1308488 [DOI] [PubMed] [Google Scholar]
- 33. Fujinaga S, Sakuraya K. Single infusion of low-dose ofatumumab in a child with complicated nephrotic syndrome with anti-rituximab antibodies. Pediatr Nephrol 2018;33:527–8. 10.1007/s00467-017-3866-2 [DOI] [PubMed] [Google Scholar]
- 34. Vivarelli M, Colucci M, Bonanni A, et al. Ofatumumab in two pediatric nephrotic syndrome patients allergic to rituximab. Pediatr Nephrol 2017;32:181–4. 10.1007/s00467-016-3498-y [DOI] [PubMed] [Google Scholar]
- 35. Bonanni A, Rossi R, Murtas C, et al. Low-Dose ofatumumab for rituximab-resistant nephrotic syndrome. BMJ Case Rep 2015;2015:bcr2015210208 10.1136/bcr-2015-210208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ramwadhdoebe TH, van Baarsen LGM, Boumans MJH, et al. Effect of rituximab treatment on T and B cell subsets in lymph node biopsies of patients with rheumatoid arthritis. Rheumatology 2019;58:1075–85. 10.1093/rheumatology/key428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Theil D, Smith P, Huck C, et al. Imaging mass cytometry and single-cell genomics reveal differential depletion and repletion of B-cell populations following ofatumumab treatment in cynomolgus monkeys. Front Immunol 2019;10:1340 10.3389/fimmu.2019.01340 [DOI] [PMC free article] [PubMed] [Google Scholar]