Abstract
Peritoneal tuberculosis (TB) is one of the most challenging forms of extrapulmonary tuberculosis to diagnose. This challenge can be compounded in low incidence regions, and in patients with cirrhosis in whom the presence of ascites alone may not prompt further investigation. A delay in the diagnosis and treatment of peritoneal tuberculosis may lead to worse clinical outcomes. This case describes a 64-year-old Italian male with decompensated cirrhosis being evaluated for liver transplantation, who developed abdominal pain and a persistent inflammatory ascites with peritoneal thickening despite antibiotic therapy. Peritoneal tuberculosis was suspected, although non-invasive and invasive direct mycobacterial testing remained negative. A constellation of positive QuantiFERON-TB Gold In-Tube test, elevated ascitic adenosine deaminase and dramatic symptomatic and radiographic response to empiric anti-tuberculous therapy confirmed the diagnosis of peritoneal tuberculosis. This paper will review the approach to the diagnosis of peritoneal tuberculosis.
Keywords: infections, infection (gastroenterology), cirrhosis, global health, TB and other respiratory infections
Background
Peritoneal tuberculosis (TB) is a difficult diagnosis to render. Patients often present with non-specific symptoms and may not have clear epidemiological risk factors for Mycobacterium tuberculosis (MTB). Moreover, the sensitivity of microbiological diagnostic testing for peritoneal tuberculosis is poor, and invasive sampling is often required. Herein, we present a challenging case of peritoneal tuberculosis that offers insight into the diagnosis of this condition in patients with multiple concurrent diagnostic possibilities, and in whom direct microbiological testing remains negative.
Case presentation
A 64-year-old male with cirrhosis due to alcohol and fatty liver disease, and hepatocellular carcinoma treated with yttrium radioembolisation, presented with generalised abdominal pain for 3 days. He had recently been discharged from an outside hospital after treatment for spontaneous bacterial peritonitis (SBP) secondary to Klebsiella pneumoniae, for which he received ceftriaxone for 14 days with improvement in his ascitic fluid white blood cell count. Vital signs were within normal limits. Initial peripheral white blood cell count was 19.0×103/μL with a normal differential. Physical examination was notable for abdominal distention and diffuse tenderness. He was started on vancomycin and piperacillin-tazobactam. Paracentesis demonstrated 15 000 red blood cells/μL and 6425 nucleated cells/μL with 97% granulocytes (table 1). Five days after treatment, a repeat paracentesis showed 96 nucleated cells/μL (27% granulocytes, 52% lymphocytes), and he was changed to trimethoprim-sulfamethoxazole for SBP prophylaxis. Repeat paracentesis several days later showed 3475 nucleated cells/μL (86% granulocytes, 11% tissue cells), glucose of 2 mg/dL, lactate dehydrogenase 3052 U/L, protein 4.4 g/dL, adenosine deaminase (ADA) of 44 U/L. He was started on vancomycin, ceftriaxone and metronidazole. After failure of improvement in cell counts, piperacillin-tazobactam was substituted for ceftriaxone.
Table 1.
Ascitic fluid parameters and antibiotic regimens
| OSH paracentesis | YNHH first paracentesis (day 0) | YNHH second paracentesis (day 7) | YNHH third paracentesis (day 10) | YNHH fourth paracentesis (day 13, First Pocket) | YNHH fourth paracentesis (day 13, Second Pocket) | |
| Ascitic fluid WBC count and differential | 2213 nucleated cells/μL (79% granulocytes, 2% lymphocytes) | 6425 nucleated cells/μL (97% granulocytes) | 96 nucleated cells/μL (27% granulocytes, 52% lymphocytes | 3475 nucleated cells/μL (86% granulocytes) | 84 cells/µL (19% granulocytes, 55% lymphocytes) | 4030 cells/µL (90% granulocytes, 1% lymphocytes) |
| Ascitic fluid culture | Klebsiella pneumoniae | NG | NG | NG | NG | NG |
| Ascitic fluid ADA level | N/A | N/A | N/A | 44 U/L | 12.2 U/L | 42.0 U/L |
| Antibiotic treatment | Ceftriaxone for 14 days | Vancomycin and piperacillin-tazobactam | Trimethoprim-sulfamethoxazole for SBP prophylaxis | Vancomycin, ceftriaxone, and metronidazole | Vancomycin and piperacillin-tazobactam | Vancomycin and piperacillin-tazobactam |
ADA, adenosine deaminase; N/A, not available; NG, no growth; OSH, outside hospital; SBP, spontaneous bacterial peritonitis; WBC, white blood count; YNHH, Yale New Haven Hospital.
Infectious Diseases was asked to consult for liver pre-transplant evaluation and work-up of refractory peritonitis. Further social history revealed the patient was born in Italy, emigrated to the USA at the age of 20 and last travelled to Italy 4 years prior. He lived on a farm as a child and drank unpasteurised milk many years before.
Investigations
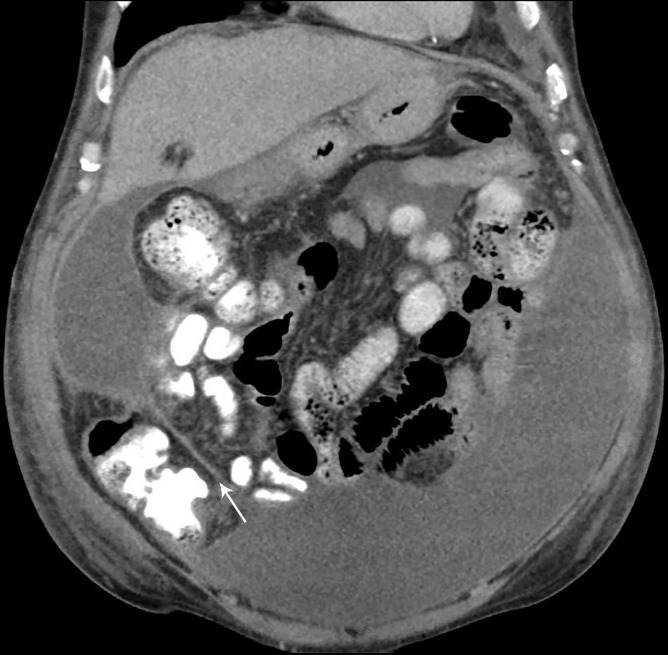
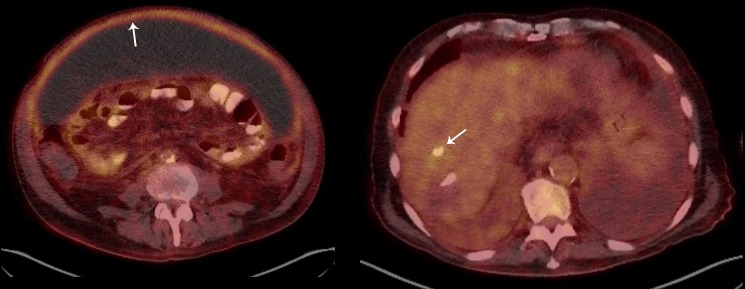
CT of the abdomen and pelvis with intravenous and oral contrast showed loculated ascites with no evidence of perforation (figure 1), and all antibiotics were held. QuantiFERON-TB Gold testing was positive. Repeat paracentesis from different loculated collections performed on the same day had neutrocytic ascites with nucleated cell counts of 84 and 4030/μL, with ADA levels of 12.2 and 42.0 U/L, respectively, suggesting loculated ascites with variable levels of inflammation. An F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET)-CT scan was performed showing hypermetabolism of the peritoneum and a non-specific area of inflammation at prior Transarterial chemoembolisation therapy (TACE) site (figure 2A, B). Cytology repeated multiple times showed abundant acute inflammation and inflammatory debris. A third ascitic fluid ADA level was 39.1 U/L.
Figure 1.
CT of the abdomen and pelvis (coronal view) demonstrating densely-loculated ascites (arrow).
Figure 2.
A and B. CT of the abdomen and pelvis (transverse view) with peritoneal enhancement and thickening consistent with peritonitis (left arrow), and non-specific hypermetabolism at the prior site of embolisation (right arrow).
Differential diagnosis
Given that investigation for common diagnoses associated with neutrocytic ascites had been unrevealing, a work-up for tuberculosis and other atypical infections was undertaken. A commercial TB PCR assay (Quest Diagnostics) of the peritoneal fluid was negative as were multiple Acid-fast bacilli (AFB) cultures. Histoplasma urine antigen, cryptococcal serum antigen, Brucella antibody and HIV antigen/antibody were negative. The patient then underwent peritonoscopy, which showed a significantly sclerosed peritoneum, and multiloculated ascites with dense adhesions, with poor views of the peritoneum as a result, leading to the procurement of blind peritoneal biopsies. Microbiological cultures of peritoneal tissue were negative. Pathology revealed organising granulation tissue and overlying fibrin deposition, but no evidence of malignancy or granulomas. A laboratory-developed TB PCR of peritoneal tissue was negative.
Treatment
Given his epidemiological risk factors, lack of an alternative diagnosis and laboratory evidence of an elevated peritoneal ADA level with positive QuantiFERON-TB Gold testing, the decision was made to treat him empirically for peritoneal tuberculosis with rifampin (RIF), isoniazid (INH), pyrazinamide, ethambutol and vitamin B6.
Outcome and follow-up
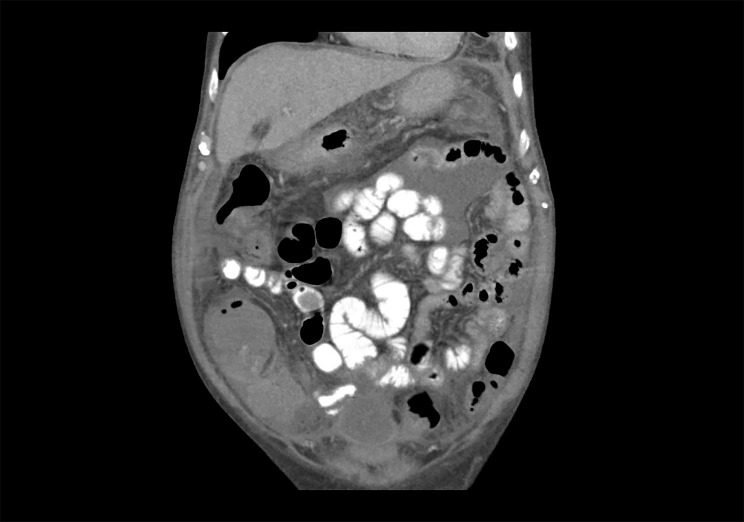
After 1 week of anti-tuberculous therapy, the patient noted a substantial improvement in his previously diuretic-refractory ascites. The mild peripheral leukocytosis (usually 10 to 12×103/μL) present for over a month resolved. After 2 weeks of therapy, a repeat attempt at ultrasound-guided paracentesis revealed only a small residual amount of highly loculated ascites unsafe for sampling. After 2 months of therapy, a repeat CT abdomen and pelvis demonstrated resolution of loculated ascites and improvement in peritoneal thickness from 8.1 cm to 2.5 cm (figure 3). He was narrowed to INH/RIF after 2 months and completed a total of 9 months of therapy without evidence of hepatotoxicity. He had no recurrence of symptoms at 6 weeks post-completion of therapy, and is undergoing evaluation for transplant candidacy.
Figure 3.
CT of the abdomen and pelvis (coronal view) showing near-total resolution of previously noted loculated ascites.
Discussion
Peritoneal TB, caused by agents of the M. tuberculosis complex, can be one of the most challenging infectious diseases to diagnose, owing to a classically insidious onset, non-specific symptoms and the limitations of diagnostic testing.1 Involvement of nearly every organ has been described in extrapulmonary TB (EPTB) infection including lymphatic, pleural, bone and joint, genitourinary and meningeal sites. Peritoneal TB is the sixth most common site of EPTB, accounting for 4.9% of all EPTB cases.2 3
TB may reach the peritoneum haematogenously, through the lymphatic system, from ingestion of contaminated sputum from pulmonary TB, contaminated food (especially unpasteurised dairy in the case of Mycobacterium bovis), or through direct extension from adjacent foci of infection.3–5 Liver disease, and alcoholic liver disease in particular is a significant risk factor for development of peritoneal TB, as are HIV infection, peritoneal dialysis, malignancy and anti-Tumor necrosis factor (TNF) therapy, due to impairment of protective cellular immune responses.3 6 7
Peritoneal tuberculosis is a form of abdominal tuberculosis that most often presents as an insidious progression of abdominal pain (50% to 100%) and distention due to ascites (40% to 73%).1 8–11 Constitutional symptoms including weight loss (50% to 61%), fever (13% to 59%) and night sweats (6%) may also be present.1 8 11 Because symptoms are subtly progressive, patients may be symptomatic for months before seeking medical care.3 8 An additional challenge is posed in patients with cirrhosis, where ascites may be attributed to underlying liver disease.3 In patients with ascites and abdominal pain or fever, SBP is often first suspected. The persistence of inflammation on cytological evaluation of ascitic fluid after treatment with conventional antibiotics should prompt evaluation for secondary peritonitis (eg, gastrointestinal perforation), resistant pathogens (eg, vancomycin-resistant Enterococci), malignancy (including hepatocellular carcinoma) and atypical infections such as TB, cryptococcosis and brucellosis among others. Peritoneal TB demonstrates a lymphocytic-predominant ascitic fluid in around 68% of cases, with white cell counts typically between 500 to 1500 white cells/mm3 but other patterns including neutrophilic predominance can be seen as in this case.3 4 10 The ascitic protein is almost invariably >25 g/L and the serum albumin-ascites gradient less than 1.1 g/L, with cirrhotic patients being the exception to these rules.4 10 12 Laboratory evaluation reveals non-specific findings, most often anaemia, elevated erythrocyte sedimentation rate and hypoalbuminaemia.1 8
Ultrasound and CT may reveal free, loculated or localised ascites (36% to 67%), lymphadenopathy (14% to 47%) and peritoneal thickening (23% to 32%).8 13 CT classically reveals high attenuation ascites (20 to 45 Hounsfield units), attributed to significant inflammatory debris.7 10 Interestingly, CT imaging may demonstrate findings consistent with peritoneal carcinomatosis, though histopathology revealing granulomas in these cases should prompt immediate evaluation for TB or sarcoidosis.8 10 14 15 Unfortunately when infection is not initially suspected, cultures are unlikely to be sent.14 PET imaging is not routinely used, and differentiating between the avid uptake seen in peritoneal carcinomatosis and peritoneal TB is typically not possible.16 Findings of avid uptake in anatomical areas unusual for the metastasis of peritoneal carcinomatosis should raise suspicion for TB.
Making a direct microbiological diagnosis of peritoneal TB can be challenging, owing to suboptimal sensitivity of diagnostic testing, the paucibacillary nature of the disease and a dilutional effect of ascites.5 9 A Ziehl-Neelsen stain of the ascitic fluid is positive in 0% to 6% of patients, and culture positive in 16% to 58% of cases, though yield may be improved by centrifugation and culture of a large volume (1 litre) of ascites.1 9 10 12 14 17–20 Molecular diagnostics such as TB PCR are highly specific but lack sensitivity. A study examining Xpert MTB/RIF on ascitic fluid showed a sensitivity ranging from 8% to 50%, with higher sensitivities seen when a combination of smear, culture, histology and ADA were already suggestive of the diagnosis.19 In a meta-analysis of peritoneal TB patients, the sensitivity of Xpert MTB/RIF on ascitic fluid was 59% with a specificity of 97.9%, while only 16% of these patients had a positive ascitic fluid culture.21
A positive ascitic fluid ADA can be highly suggestive. Numerous studies have been performed looking at the optimal cut-off for sensitivity and specificity, with most studies suggesting an ADA level of >30 IU/L as yielding sensitivities of close to 100% and specificity generally greater than 95% for peritoneal tuberculosis.10 A meta-analysis of ascitic fluid ADA revealed a pooled sensitivity and specificity of 93% and 96% for peritoneal TB, respectively, with 14 of the 18 studies using an ADA cut-off value >30 IU/L.22 In one study of patients with peritoneal TB, the median ADA was substantially higher at 61.6 IU/L compared with those with malignancy (medians were 12.8 and 16.2 IU/L, respectively, for those with or without significant malignant hepatic involvement), and those with liver disease and cirrhosis (6.5 IU/L).23 ADA values in cirrhotic patients with peritoneal TB are lower than for their non-cirrhotic counterparts.10 As such, an ADA value lowered to 27 IU/L in a cirrhotic cohort yielded a sensitivity of 100% and specificity of 93.3% in one study.24 In this study, the mean ascitic ADA value was 58 IU/L in patients with peritoneal TB and cirrhosis, and 7 IU/L in non-infected patients with cirrhosis.24 ADA is very useful in the cirrhotic population, with a caveat of a mild decrease in sensitivity compared with non-cirrhotic patients with peritoneal TB. Interferon-gamma (IFN-γ) concentrations in ascitic fluid have also been shown to be highly accurate for peritoneal TB, with values being significantly higher for peritoneal TB (6.70 U/mL) compared with malignancy (3.10 U/mL) or cirrhosis (3.08 U/mL), yielding a sensitivity of 93% and specificity of 98% when a cut-off of 3.2 U/mL was used.18
Tuberculin skin testing (TST) and interferon-gamma release assays (IGRAs) may be supportive but cannot differentiate between latent and active TB. The TST has the added limitation of cross-reacting with prior BCG vaccine. A review of the TST for peritoneal TB yielded sensitivity of 53%.10 Data on peripheral blood IGRAs specifically in peritoneal TB is limited, though a meta-analysis in patients with EPTB showed a pooled sensitivity and specificity of 72% and 82% for the QuantiFERON-TB Gold or QuantiFERON-TB Gold In-Tube Test (ESAT-6, CFP-10 and TB7.7 antigens), and 90% and 68% for T-SPOT.TB (ESAT-6 and CFP-10 antigens), respectively, after excluding indeterminate results, compared with a reference standard of positive culture, nucleic acid amplification or histopathological findings.25
IGRAs from ascitic fluid have shown some promise. A case report of peritoneal TB showed 40-fold higher levels of IFN-γ production at the site of infection (ascites) compared with peripheral blood using the QuantiFERON-TB Gold In-Tube Test.26 It is hypothesised that TB antigen-specific CD4 +T cells are present in higher concentrations in ascitic fluid than in peripheral blood and are responsible for the difference. Another small prospective study showed a six-fold increase in ascitic fluid concentrations of IFN-γ-producing CD4 +T cells at baseline in patients with TB compared with those without.27 On short in-vitro stimulation using the TB antigens purified protein derivative (PPD), HBHA and ESAT-6, significantly higher levels of IFN-γ were noted in the ascitic fluid of patients with TB, with a sensitivity of 100% and specificity of 92% based on proposed cut-offs. Another case report measured peritoneal fluid concentrations of TB antigen-specific CD4 +T cells by cytokine flow cytometry in a patient on peritoneal dialysis. A substantial increase in dialysate (ascitic) fluid concentrations of stimulated T-cells (8.72%) compared with peripheral blood (0.22%) was noted when stimulated overnight by the TB antigens PPD and ESAT-6.28 The above approaches may be particularly useful for paucibacillary disease as detection is dependent on the concentration of T-cells rather than the abundance of mycobacteria at the site of infection.
For a definitive diagnosis, invasive diagnostic testing may be required. This can be challenging in patients with cirrhosis, coagulopathy, encephalopathy and high perioperative risk. However, the benefits of achieving a diagnosis are significant. Peritonoscopy with peritoneal biopsy is the gold standard for diagnosis. Three patterns of peritoneal TB have been described on peritonoscopy: a thickened peritoneum with yellow-white tubercles, a thickened peritoneum without tubercles and substantial peritoneal thickening with dense adhesions that may extend to adjacent organs.4 17 The appearance may be so characteristic as to have accuracy >95% in some series.17 A peritoneal biopsy specimen is positive on acid-fast stain in 50% to 63% of cases, and culture is typically positive in about 70% of cases.3 12 14 18 Histopathological evaluation of the tissue may reveal the presence of caseating granulomas in 70% to 95% of patients as an essential diagnostic clue, while PCR of the tissue is positive in 25% to 70% of cases.3 8–10 12 14 17 18
Despite an aggressive workup, the diagnosis may remain elusive. If the suspicion for peritoneal TB remains high despite negative testing, empiric anti-tuberculosis treatment is indicated.1 A response to therapy manifests as normalisation of ascitic fluid pleocytosis (cytologic response), resolution or significant decrease in ascites, decreased peritoneal thickening on repeat imaging and/or improvement in symptoms.
This patient’s case is atypical due to the presence of neutrocytic rather than typically lymphocytic ascites, with a significantly elevated white blood cell count. It is possible that the prior episode of SBP influenced the type of pleocytosis noted. Additionally, while granulomas are typically noted on peritoneal biopsy, the limited views at laparoscopy due to the dense loculations limited the attainment of a high-quality biopsy specimen.
No single test can effectively rule out the diagnosis of peritoneal TB, and a combination of socio-epidemiological history (eg, travel, homelessness, incarceration, sick contacts, drug use) and immunologic risk assessment is essential. Classical ‘B-symptoms’ of fever, weight loss and night sweats may not be present. Supportive diagnostics may include TST/IGRA, ascitic fluid studies including ADA, Ziehl-Neelsen stain and culture, peritoneal biopsy and molecular techniques. Ultimately the diagnosis may be confirmed with clinical, radiographic and cytologic response to therapy.
Learning points.
Peritoneal tuberculosis should be suspected in patients with refractory inflammatory ascites, particularly those from endemic areas.
Diagnosis may be delayed in patients with underlying cirrhosis, which might otherwise explain the presence of ascites. Additionally, the adenosinedeaminase (ADA) may be falsely low in this population.
Diagnostic testing for peritoneal tuberculosis is often insensitive, and a combination of interferon -gammarelease assay/purified protein derivative, ADA testing, molecular testing, direct culture, histopathology and response to treatment may be needed to establish a diagnosis.
Treatment duration is not well defined, but commonly ranges from 6 to 12 months.
Footnotes
Contributors: AK contributed to literature review, writing the initial draft of the manuscript and processing of edits and suggestions by co-author in the subsequent drafts. MA contributed to literature review, substantial edits of the manuscript with respect to format and content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Uygur-Bayramicli O, Dabak G, Dabak R. A clinical dilemma: abdominal tuberculosis. World J Gastroenterol 2003;9:1098–101. 10.3748/wjg.v9.i5.1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peto HM, Pratt RH, Harrington TA, et al. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis 2009;49:1350–7. 10.1086/605559 [DOI] [PubMed] [Google Scholar]
- 3. Sophia De Saram JSF. Gastrointestinal and Peritoneal Tuberculosis : ASaH E, Extrapulmonary tuberculosis. 1 ed Springer International Publishing, 2019: 25–42. [Google Scholar]
- 4. Sharma MP, Bhatia V. Abdominal tuberculosis. Indian J Med Res 2004;120:305–15. [PubMed] [Google Scholar]
- 5. Norbis L, Alagna R, Tortoli E, et al. Challenges and perspectives in the diagnosis of extrapulmonary tuberculosis. Expert Rev Anti Infect Ther 2014;12:633–47. 10.1586/14787210.2014.899900 [DOI] [PubMed] [Google Scholar]
- 6. Marshall JB. Tuberculosis of the gastrointestinal tract and peritoneum. Am J Gastroenterol 1993;88:989–99. [PubMed] [Google Scholar]
- 7. Hulnick DH, Megibow AJ, Naidich DP, et al. Abdominal tuberculosis: CT evaluation. Radiology 1985;157:199–204. 10.1148/radiology.157.1.4034967 [DOI] [PubMed] [Google Scholar]
- 8. Fillion A, Ortega-Deballon P, Al-Samman S, et al. Abdominal tuberculosis in a low prevalence country. Med Mal Infect 2016;46:140–5. 10.1016/j.medmal.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 9. Salgado Flores L, Hernández Solís A, Escobar Gutiérrez A, et al. Peritoneal tuberculosis: a persistent diagnostic dilemma, use complete diagnostic methods. Revista Médica Del Hospital General De México 2015;78:55–61. 10.1016/j.hgmx.2015.03.009 [DOI] [Google Scholar]
- 10. Sanai FM, Bzeizi KI. Systematic review: tuberculous peritonitis--presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther 2005;22:685–700. 10.1111/j.1365-2036.2005.02645.x [DOI] [PubMed] [Google Scholar]
- 11. Vaid U, Kane GC. Tuberculous peritonitis. Microbiol Spectr 2017;5 10.1128/microbiolspec.TNMI7-0006-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shakil AO, Korula J, Kanel GC, et al. Diagnostic features of tuberculous peritonitis in the absence and presence of chronic liver disease: a case control study. Am J Med 1996;100:179–85. 10.1016/S0002-9343(97)89456-9 [DOI] [PubMed] [Google Scholar]
- 13. Kedar RP, Shah PP, Shivde RS, et al. Sonographic findings in gastrointestinal and peritoneal tuberculosis. Clin Radiol 1994;49:24–9. 10.1016/S0009-9260(05)82909-5 [DOI] [PubMed] [Google Scholar]
- 14. Cavalli Z, Ader F, Valour F, et al. Clinical presentation, diagnosis, and bacterial epidemiology of peritoneal tuberculosis in two university hospitals in France. Infect Dis Ther 2016;5:193–9. 10.1007/s40121-016-0113-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee SW, Lee MH, Lee JE, et al. Peritoneal sarcoidosis: a case report. Medicine 2019;98:e16001 10.1097/MD.0000000000016001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jeffry L, Kerrou K, Camatte S, et al. Peritoneal tuberculosis revealed by carcinomatosis on CT scan and uptake at FDG-PET. BJOG 2003;110:1129–31. 10.1111/j.1471-0528.2003.03070.x [DOI] [PubMed] [Google Scholar]
- 17. Bhargava DK, Chopra P, Nijhawan S, et al. Peritoneal tuberculosis: laparoscopic patterns and its diagnostic accuracy. Am J Gastroenterol 1992;87:109–12. [PubMed] [Google Scholar]
- 18. Sathar MA, Simjee AE, Coovadia YM, et al. Ascitic fluid gamma interferon concentrations and adenosine deaminase activity in tuberculous peritonitis. Gut 1995;36:419–21. 10.1136/gut.36.3.419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sharma SK, Kohli M, Chaubey J, et al. Evaluation of Xpert MTB/RIF assay performance in diagnosing extrapulmonary tuberculosis among adults in a tertiary care centre in India. Eur Respir J 2014;44:1090–3. 10.1183/09031936.00059014 [DOI] [PubMed] [Google Scholar]
- 20. Singh MM, Bhargava AN, Jain KP. Tuberculous peritonitis. An evaluation of pathogenetic mechanisms, diagnostic procedures and therapeutic measures. N Engl J Med 1969;281:1091–4. 10.1056/NEJM196911132812003 [DOI] [PubMed] [Google Scholar]
- 21. Kohli M, Schiller I, Dendukuri N, et al. Xpert® MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev 2018;8:CD012768 10.1002/14651858.CD012768.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shen Y-C, Wang T, Chen L, et al. Diagnostic accuracy of adenosine deaminase for tuberculous peritonitis: a meta-analysis. Arch Med Sci 2013;9:601–7. 10.5114/aoms.2013.36904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burgess LJ, Swanepoel CG, Taljaard JJ. The use of adenosine deaminase as a diagnostic tool for peritoneal tuberculosis. Tuberculosis 2001;81:243–8. 10.1054/tube.2001.0289 [DOI] [PubMed] [Google Scholar]
- 24. Liao Y-J, Wu C-Y, Lee S-W, et al. Adenosine deaminase activity in tuberculous peritonitis among patients with underlying liver cirrhosis. World J Gastroenterol 2012;18:5260–5. 10.3748/wjg.v18.i37.5260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fan L, Chen Z, Hao X-H, et al. Interferon-Gamma release assays for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. FEMS Immunol Med Microbiol 2012;65:456–66. 10.1111/j.1574-695X.2012.00972.x [DOI] [PubMed] [Google Scholar]
- 26. Lorenz R, Würl P, Haerter G, et al. Interferon-Gamma release assay in the ascites: early hint for diagnosis of abdominal tuberculosis. Infection 2010;38:69–72. 10.1007/s15010-009-9469-5 [DOI] [PubMed] [Google Scholar]
- 27. Henrard S, Corbière V, Schandené L, et al. Proportions of interferon-γ-producing ascites lymphocytes in response to mycobacterial antigens: a help for early diagnosis of peritoneal tuberculosis in a low TB incidence country. PLoS One 2019;14:e0214333 10.1371/journal.pone.0214333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Müller C, Puttinger H, Winnicki W, et al. Rapid T-cell-based immunodiagnosis of tuberculous peritonitis in a peritoneal dialysis patient. Scand J Urol Nephrol 2012;46:314–6. 10.3109/00365599.2012.659206 [DOI] [PubMed] [Google Scholar]