Abstract
A 76-year-old woman presented with a 6-week history of malaise, night sweats and recurrent fever. She had a background of dilated cardiomyopathy for which she had a cardiac resynchronisation device in situ. She had several hospital admissions across this time with differing diagnoses offered. She received multiple courses of antibiotics with short-term symptom resolution. Blood cultures grew Gram-negative rods and samples were sent to a specialist centre for subtype analysis. A transthoracic echocardiogram revealed thickening of the distal right ventricular lead. A transoesophageal echocardiogram demonstrated a clearer vegetation on this lead. It transpired that she had been scratched by her dog a fortnight before symptom onset. The causal bacterium was reported as Capnocytophaga canimorsus, a bacterium that exists almost exclusively in the saliva and claws of dogs and cats. She received an extended course of antibiotics with eventual removal of the infected device.
Keywords: valvar diseases, cardiovascular system
Background
This case details the first reported case of lead-related infective endocarditis from Capnocytophaga canimorsus (CCPC). The narrative from sentinel infection through diagnostic workup to resolving management is described. Cases of this type are rare, and case reports therefore play a crucial role in developing the evidence base further.
Case presentation
A 76-year-old woman presented to the acute medical take with a 1-week history of malaise, fever and night sweats. She had a background of dilated cardiomyopathy, a cardiac resynchronisation device in situ, left bundle branch block and coeliac disease. She was treated with intravenous meropenem 1 g three times a day, a carbapenem, over 5 days for an unspecified infection. Her symptoms resolved and she was discharged. Her chest X-ray (CXR), urine and blood cultures were reported as normal and she was diagnosed as having a suspected lower respiratory tract infection (LRTI). Her symptoms returned 2 weeks later this time accompanied by bloody diarrhoea. She was treated with intravenous tazocin 4.5 g three times a day, a combination drug including a ß-lactamase inhibitor and a penicillin, alongside intravenous metronidazole 500 mg three times a day, a member of the nitroimidazole class. This lasted for 5 days alongside rehydration therapy. A diagnosis was offered of suspected food poisoning. Again, her symptoms entirely resolved prior to discharge with a normal repeat CXR, urine, blood and stool cultures. Two weeks after discharge she again presented with fever, night sweats and rigours. A cardiac opinion was sought on the basis of possible cardiac infection. On specialist examination she was found to have splinter haemorrhages on her right middle and ring fingers with no history of trauma. The rest of her physical examination was normal. On extended history she described being scratched by her new puppy on her left forearm 2 weeks prior to her initial symptom onset. The emergency department clerking from the first admission described that the left forearm was demarcated by multiple 2 cm, narrow scratch marks which had scabbed over. There had been no erythema, discharge or pain associated with the wound. By her second admission the wound had healed completely. This was felt to be her sentinel event. On blood culture, for the first time, Gram-negative rods were grown. This proved consistent on repeated culture. The type of bacterium was not recognised by the hospital labs, and the samples had to be sent to a specialist centre. A week later this bacterium was reported as CCPC, which is predominantly found in the saliva and claws of dogs and cats. As culture was prolonged initial treatment was empirical with intravenous meropenem 1 g three times a day; however once sensitivities were known microbiology advised to continue this.
Investigations
Blood tests repeatedly demonstrated raised inflammatory markers, inclusive of white cell count (WCC), C-reactive protein (CRP) and erythrocyte sedimentation rate. Her initial biochemical profile was normal as was her renal, liver and coagulation function.
Her initial transthoracic echocardiogram (TTE) was initially felt to be inconclusive. The right ventricular (RV) lead was thickened but this is a common finding on devices that have been in situ for a long period of time (figure 1). In view of her risk profile, clinical suspicion and repeatedly positive blood cultures she was listed for transoesophageal echocardiogram (TOE) which demonstrated a vegetation on the RV lead (figure 2). A CT scan of her chest, abdomen and pelvis (CAP) was reported as normal.
Figure 1.
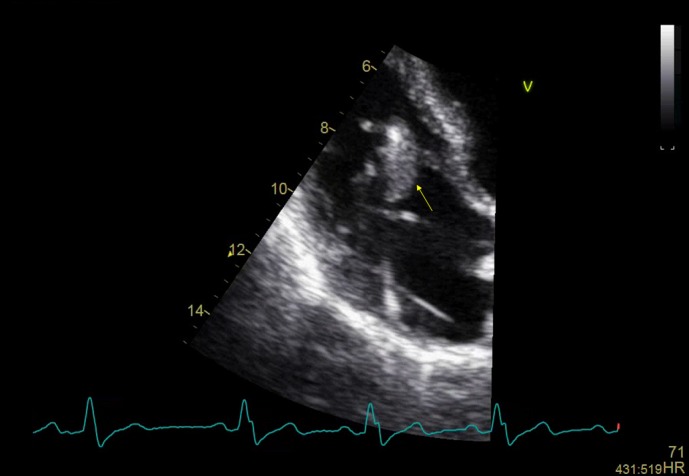
Transthoracic echocardiogram, 4-chamber apical view, close up of right atrium, distal right ventricular lead thickening.
Figure 2.
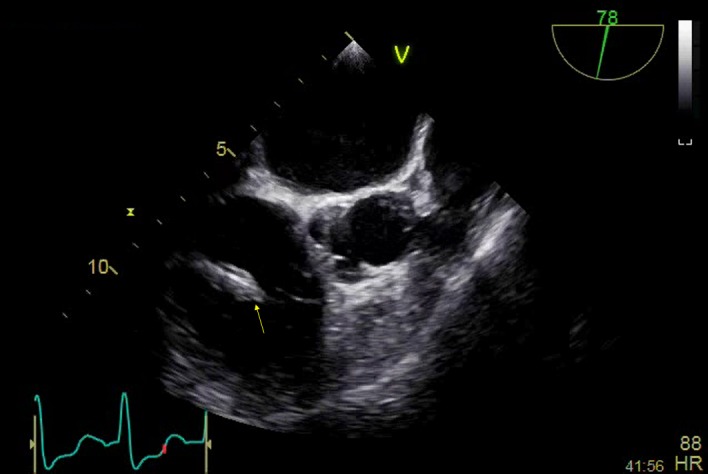
Transoesophageal echocardiogram, mid-oesophageal, right ventricular (RV) inflow outflow view. An amorphous mass envelops the RV lead on the atrial side of the tricuspid valve; there is soft echo-density attached to it, which is independently mobile and consistent with a vegetation.
Differential diagnosis
Despite initially being treated for an LRTI and subsequently food poisoning, it is clear with hindsight that the patient had an infection of unknown origin. Suspected occult infection or infection of an unknown source remains a common problem to the general physician. Classical deep-seated infection such as abscess, collection, discitis and osteomyelitis need to be screened for. One must remain cognisant to device-related infection when an intracardiac prosthesis is present. The possibility also exists of an atypical presentation of a more routine infection, and there is merit in repeated cultures of all types and regular clinical reassessment. Adding further uncertainty are the broad differentials of both autoimmune and neoplastic disease which can present in a similar way with regard to timeline and symptomology. Keeping an open mind is perhaps the physician’s best response in the face of significant diagnostic uncertainty. In this patient, there were features on peripheral examination suggestive of infective endocarditis (IE); however the aforementioned differentials were still assessed for. An autoimmune screen was negative as was her CT-CAP for both malignancy and deep-seated infection. Her urine culture was negative with blood film and wider lymphoma screen also being normal. She described no joint pain and displayed no joint swelling, skin breaks, localised erythema or lymphadenopathy.
Treatment
She was commenced on the broad spectrum antibiotic meropenem 1 g three times a day intravenous. Initial discussions regarding lead extraction and replacement were undertaken with the local tertiary centre a short time after the diagnosis was confirmed on TOE. A conservative approach was advised in the first instance, with a prolonged antibiotic course of 12 weeks and repeat TOE to assess efficacy. Repeat TOE demonstrated resolution of her vegetation. She completed a total of 12 weeks of antibiotic therapy and was monitored for a further week with clinical observations, blood tests to assess her inflammatory markers and repeated cultures. At discharge she had been apyrexial for over 6 weeks, with a normal WCC and CRP for 5 weeks. She was subsequently discharged. Unfortunately, she was readmitted 1 month later with recurrent fever. A repeat TTE revealed a new vegetation (figure 3) and Gram-negative rods were again grown on blood culture; this was again subtyped as CCPC and meropenem was restarted. The patient was rediscussed with the tertiary centre and transferred for lead extraction.
Figure 3.
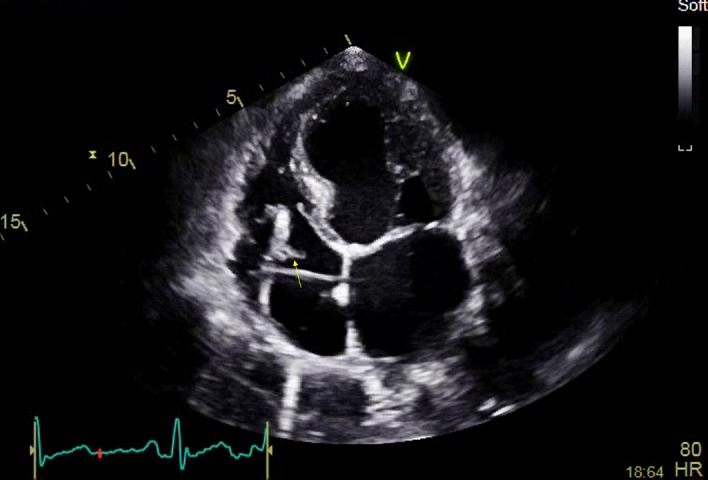
Transthoracic echocardiogram, 4-chamber apical view, close up of right atrium, clear vegetation on distal right ventricular lead.
Outcome and follow-up
Post-lead extraction she completed another 4 weeks of intravenous meropenem in combination with intravenous vancomycin, a glycopeptide, with a further week of monitoring of antibiotics before being discharged. On 2-year follow-up there has been no further recurrence. A new device has not been sited as her left ventricular function is now only mildly impaired and the patient declined further device therapy.
Discussion
CCPC is a Gram-negative rod of the genus Capnocytophaga. It has a fusiform appearance on Gram stain. It is capnophilic, microaerophilic and has a characteristic gliding motility visible on microscopy.1 It was first identified in 1976 by Bobo and Newton as dysgonic fermenter 2 and subsequently renamed in 1989. It is found predominantly in the saliva and claws of cats and dogs. It its normal environment it forms part of the animal’s commensal flora; however on transmission to humans it proves a serious threat to life. Typically transmitted by a bite or scratch from an animal vector it is associated with severe forms of infection including meningitis, cellulitis and IE.2 It is a fastidious slow growing organism, with blood cultures often proving negative. Cases are reported that needed as many as 38 blood cultures before a confirming diagnostic culture was achieved.3 For this reason its incidence is likely under-reported globally. This will likely rise as better, more selective media and PCR become more widely available.4
On introduction to the human blood stream CCPC releases far fewer proinflammatory cytokines than other comparative organisms. This generates a far reduced immune response to the infection. An international review into cases of lab confirmed diagnosis reported to national Centers for Disease Control and Prevention from 1961 to 2014 described 484 cases in total. There was a ratio of 2:1 for male to female patients, respectively. 60% of the cohort reported being bitten by a dog, with an additional 27% reporting being scratched or licked. The median age for infection was 55 years. IE was reported in only 12 patients.5 A separate review of 12 cases of CCPC IE described an 80% male population with mean age of 53, with a high level of contact with dogs at 66%. Three patients died from fulminant sepsis within this cohort.6 Overall CCPC is an uncommon cause of IE but correlates highly with associated animal injury as the causal event. The typical incubation period is 7–14 days before symptom onset. Symptoms are typical of those traditionally associated with IE, namely night sweats, rigours and malaise. Fever is the most common symptom with 78% presenting with this. 26% of patients reported diarrhoea, which is unusual for IE. CCPC is widely treatable with antibiotics, offering a broad spectrum of antibiotic sensitivity. However, often extensive courses are required, as symptom resolution and negative cultures are not particularly reassuring in the acute period. The overall fatality rate for CCPC infection lies at around 25%. In associated IE, rates are higher at between 40% and 50%. Patients with a degree of immune dysfunction are at higher risk again. Patients with prosthetic material within the body are traditionally considered higher risk for infection, though whether this is equivalent to immune dysfunction is debatable.7 A high-risk comorbid profile for device implantation would include diabetes, renal failure, prior infection, immune dysfunction and heart failure.
The implant rate for all types of cardiac device is increasing. From 2008 to 2011, pacemaker, implantable cardiac defibrillators (ICD) and complex resynchronisation therapy (CRT) implantation rates rose from 77, 11 and 3 per 100 000 patients to 108, 16 and 4.6 per 100 000 patients, respectively.8 Indications for both ICD and CRT have expanded over time as the evidence base for these treatment modalities has evolved. Device infection rates are fairly low in all patients at around 1%.8 Lead endocarditis generally is a far rarer cause of infection than that which effects native or prosthetic valves. The mortality rate for device infection is high at 35%. A firm diagnosis of the condition is also much harder to establish. In most types of cardiac device, the majority of the associated prosthetic material is placed in the right side of the heart which is much harder to image with both TTE and TOE. Indeed, devices that have been in situ for a long period of time can become embedded within the cardiac tissue and the leads are seen to thicken. Other difficulties arise with ultrasound imaging as the metallic leads reverberate producing artefact. There is also an aspect of the lead which lies flat to the endocardium which is impossible to assess accurately. This combination of factors reduces the sensitivity of obtaining a confirmatory echocardiographic diagnosis. TTE generally is considered to have a 25% sensitivity to vegetations sized less than 5 mm and a 70% sensitivity at 6–10 mm. TOE in comparison is 95% sensitive at this size.9 There is also a role for positive emission tomography scanning in cases where clinical suspicion remains high and TTE/TOE have proven unhelpful; this imaging modality is recommended at class IIb level C evidence. There is also a role for intracardiac echo, again recommended at class IIb level C, although access to this modality is sparse nationally. IE remains a clinical diagnosis and time needs to be taken to establish safe decision making. Repeated blood cultures prior to antibiotics and a thorough physical exam remain crucial diagnostic tools. More common causative organisms for IE include Strep Viridans, Strep Bovis, Staph Aureus, Enterococci and HACEK group. This includes the groups Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella and Kingela.9
Perhaps surprisingly, removal of the infected lead or device remains controversial and there are few guidelines on this. Lead removal is a high-risk procedure and is undertaken as last resort in specialist centres with onsite cardiothoracic surgical cover. A percutaneous approach to extraction is recommended in the first instance. After extraction the need for repeat implantation needs to be reassessed and ideally should be postponed as long as possible to ensure sterility. The gold standard of treatment for IE of all subtypes is an multi-disciplinary team(MDT) led approach consisting of microbiologists, cardiac imaging, cardiac anaesthetics, cardiac surgeons and valve specialists all offering input.9
Learning points.
An injury sustained from an animal vector should prompt consideration of subsequent atypical infection.
Endocarditis remains an important differential of infection of unknown source.
Intracardiac prostheses and devices increase a patient’s risk of developing cardiac infection.
Capnocytophaga canimorsus is challenging to culture and exposure to an animal injury should prompt an increase in suspicion.
Footnotes
Contributors: GS wrote the manuscript, SH acted as senior author.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Sandoe JAT. Capnocytophaga canimorsus endocarditis. J Med Microbiol 2004;53:245–8. 10.1099/jmm.0.05274-0 [DOI] [PubMed] [Google Scholar]
- 2. Guillaume C, Olivier L, Christophe B, et al. . Capnocytophaga canimorsus endocarditis with root abscess in a patient with a bicuspid aortic valve. Heart International 2009;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kooter AJ, Derks A, Vasmel WLE. Rapidly progressive tricuspid valve endocarditis caused by Capnocytophaga canimorsus infection in an immunocompetent host. Clin Microbiol Infect 1999;5:173–5. 10.1111/j.1469-0691.1999.tb00533.x [DOI] [PubMed] [Google Scholar]
- 4. Gaastra W, Lipman LJA. Capnocytophaga canimorsus. Vet Microbiol 2010;140:339–46. 10.1016/j.vetmic.2009.01.040 [DOI] [PubMed] [Google Scholar]
- 5. Butler T. Capnocytophaga canimorsus: an emerging cause of sepsis, meningitis, and post-splenectomy infection after dog bites. Eur J Clin Microbiol Infect Dis 2015;34:1271–80. 10.1007/s10096-015-2360-7 [DOI] [PubMed] [Google Scholar]
- 6. Nishioka H, Kozuki T, Kamei H. Capnocytophaga canimorsus bacteremia presenting with acute cholecystitis after a dog bite. J Infect Chemother 2015;21:215–7. 10.1016/j.jiac.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 7. Decoster H, Snoeck J, Pattyn S. Capnocytophaga canimorsus endocarditis. Eur Heart J 1992;13:140–2. 10.1093/oxfordjournals.eurheartj.a060035 [DOI] [PubMed] [Google Scholar]
- 8. Krahn AD, Longtin Y, Philippon F, et al. . Prevention of arrhythmia device infection trial: the PADIT trial. J Am Coll Cardiol 2018;72:3098–109. 10.1016/j.jacc.2018.09.068 [DOI] [PubMed] [Google Scholar]
- 9. Habib G, Lancellotti P, Iung B. 2015 ESC guidelines on the management of infective endocarditis: a big step forward for an old disease. Heart 2016;102:992–4. 10.1136/heartjnl-2015-308791 [DOI] [PubMed] [Google Scholar]


