Abstract
Diaphragm disease (DD) of the small bowel is a rarely reported complication of non-steroidal anti-inflammatory drug (NSAID) use, characterised by diaphragm-like strictures, most commonly in the ileum, causing varying degrees of obstruction. It typically presents in the elderly, over many years with non-specific symptoms. Diagnosis is challenging, the majority of cases relying on histopathology for confirmation. Treatment involves NSAID cessation and surgery through a combination of stricturoplasties and/or segmental resection. Very rarely DD presents as a surgical emergency. A case presenting as acute small bowel obstruction (SBO) is described, initially diagnosed as adhesions, later confirmed to be DD of the terminal ileum following histopathological examination. Given the widespread use of NSAIDs and an ageing population, it is likely the incidence of DD will increase. It is, therefore, important that surgeons are aware of this disease entity and consider it as a potential diagnosis in patients presenting with acute SBO.
Keywords: gastrointestinal surgery, rheumatoid arthritis, unwanted effects/adverse reactions, drugs: gastrointestinal system
Background
Following the synthesis of aspirin in 1899, non-steroidal anti-inflammatory drugs (NSAIDs) have grown in popularity to become the most prescribed class of drugs in the world. The annual consumption of aspirin alone is thought to be in excess of 40 million kg.1 This is unlikely to change significantly in the foreseeable future given NSAIDs’ efficacy, particularly in treating inflammatory conditions such as osteoarthritis and rheumatoid arthritis (RA). The adverse effects of NSAIDs, including gastrointestinal inflammation, ulceration, bleeding and perforation, are well known.2 However, small bowel enteropathy associated with NSAID use is infrequently reported in the literature.3
Diaphragm disease (DD) was first described by Lang et al. in 1988 following the histopathological identification of diaphragm-like strictures in the small bowel resections of seven patients that were directly attributed to NSAID use.4 These diaphragm-like mucosal projections can narrow the small bowel lumen to a ‘pinhole’ and result in obstructive symptoms. DD is more common in middle-aged to elderly patients and typically presents insidiously over many years with a variety of non-specific symptoms/signs including abdominal pain, change in bowel habit, weight loss and anaemia.5 Rarely DD presents as a surgical emergency and is difficult to diagnose preoperatively with definitive diagnosis often being made following histopathological examination of the resected specimen.3
We report a case of DD that presented as acute small bowel obstruction (SBO) requiring emergency laparotomy and resection, initially diagnosed as being secondary to adhesions via contrast-enhanced CT, however, later confirmed as DD following histopathological opinion.
Case presentation
A 73-year-old woman presented to the emergency department with a 4-day history of worsening colicky upper abdominal pain, bloating, anorexia and bilious vomiting for 12 hours. She had been discharged from hospital 4 days prior, having been treated for faecal impaction with laxatives. Since being discharged, she had opened her bowels daily but only small amounts and had stopped the prescribed laxatives due to exacerbation of her pain. She could not recall passing flatus in the hours preceding presentation.
Her previous medical history included hypertension, diverticular disease and RA for which she had been taking etanercept for 6 months (due to inadequate response to disease-modifying antirheumatic drugs), methotrexate, hydroxychloroquine, prednisolone and paracetamol as required. She initially denied any significant NSAID usage but later confirmed she may have been prescribed it by her general practitioner. Her surgical history included a prior tonsillectomy and uterine polypectomy.
On examination, the patient was apyrexial and haemodynamically stable. Her abdomen was distended with generalised tenderness, hypoactive bowel sounds but no signs of peritonitis.
Investigations
Laboratory tests revealed raised C-reactive protein (7.05 mg/L; reference range 0–6), raised white cell count (13.04×109/L; reference range 3.6–10.5), raised alanine aminotransferase (62.6 iU/L; reference range 7–40), low haemoglobin (118 g/L; reference range 125–172) and normal lactate (1.2 mmol/L; reference range 0.6–1.4).
Contrast-enhanced CT of the abdomen and pelvis demonstrated multiple dilated small loops of bowel with a transition point seen in the pelvis (figure 1). The distal terminal ileal loops were collapsed and angulated, suggestive of adhesions. Trace perihepatic-free fluid was noted but no evidence of perforation.
Figure 1.
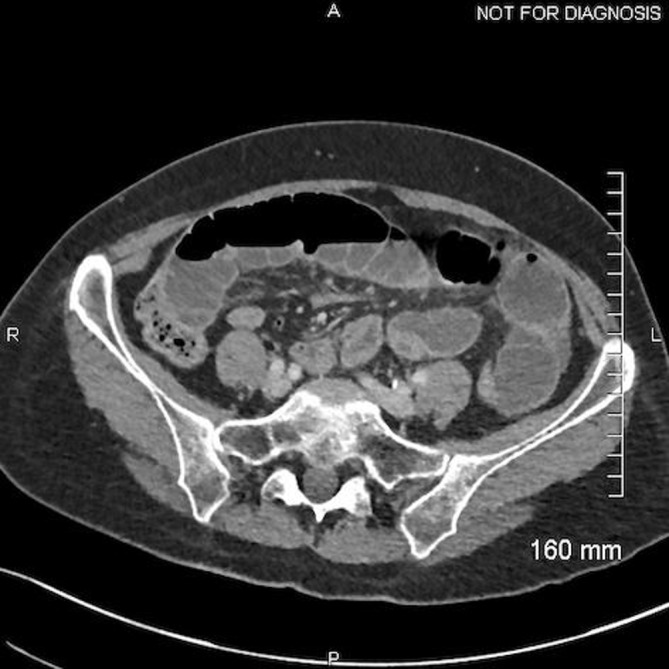
Contrast-enhanced CT of the abdomen and pelvis demonstrating multiple-dilated small loops of bowel with a transition point (A=anterior; P=posterior; R=right; L=left).
Differential diagnosis
Initial clinical impression was that of SBO secondary to adhesions and consequently the patient was consented for emergency laparotomy to relieve her obstructive symptoms and confirm the diagnosis. The calculated P-POSSUM score conveyed a mortality risk of 8.56% and morbidity risk of 76.13%.
Treatment
On admission, the patient was kept nil by mouth, a wide bore nasogastric tube was inserted on free drainage and appropriate analgesia and antiemetics were prescribed. At laparotomy, dilated loops of small bowel were noted from the duodenojejunal flexure to approximately 60 cm from the ileocaecal valve where there was an apparent cut-off without evidence of an adhesional band internal hernia. Three circumferential intraluminal strictures were noted. An enterotomy was made proximal to the affected bowel and a 1 cm ball bearing introduced. There was no evidence of proximal stricture but it was not possible to pass the ball bearing through the distal stricture. Following colorectal specialist opinion, approximately 20 cm of small bowel containing the strictures was resected and a primary anastomosis formed with staples.
The patient was reviewed postoperatively by the rheumatology team who advised withholding methotrexate and etanercept for the duration of admission and to consider increasing the dose of prednisolone should she deteriorate. Postoperatively she progressed well on the ward and was discharged home 8 days later, tolerating a normal oral diet, opening her bowels and with agreement from the physiotherapy and occupational therapy teams.
Four days after discharge the patient represented with symptoms suggestive of a superficial surgical site infection. CT of the abdomen and pelvis with contrast excluded a collection. The infection was treated with a course of antibiotics and resolved without sequalae.
Etanercept was restarted following resolution of the surgical site infection and a repeat blood test showing satisfactory inflammatory markers as per rheumatology advice. Due to personal concerns from the patient that methotrexate might have had a role in the development of her SBO, she did not restart methotrexate until 8 months after discharge, triggered by a worsening of the symptoms of her RA.
Outcome and follow-up
Macroscopic examination of the resected terminal ileum revealed a 10 mm stricture with the mucosa forming a fibrous band with focal haemorrhage. The background mucosa was mildly oedematous but otherwise unremarkable. 20 mm from the first stricture there was a second and a further 5 mm from this a third. No discernible mass focal lesion was seen. Microscopically, there were transmural chronic inflammatory cell infiltrates including eosinophils. This was more prominent at the sites of stricture where there was also fibrosis and oedema in the submucosa with mucosal ulceration (figures 2 and 3). Granulomas were not identified. Reactive/degenerative changes were also noted at the edges of the ulcers but no dysplasia or evidence of malignancy was seen. Resection margins were deemed viable. The appearances were reported as being consistent with NSAID-associated DD.
Figure 2.
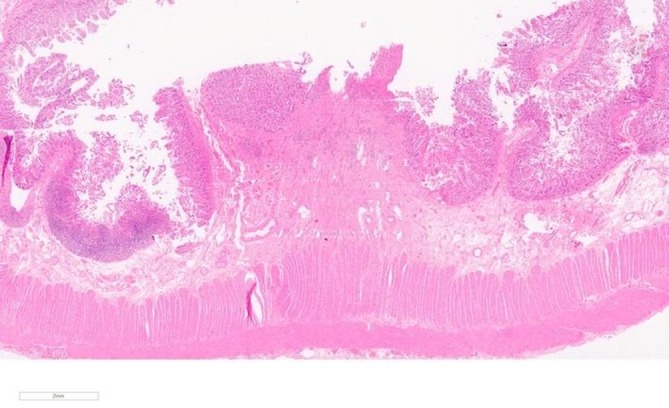
(H&E 10×)—microscopic overview of an area of stricture showing mucosal ulceration with submucosal fibrosis.
Figure 3.
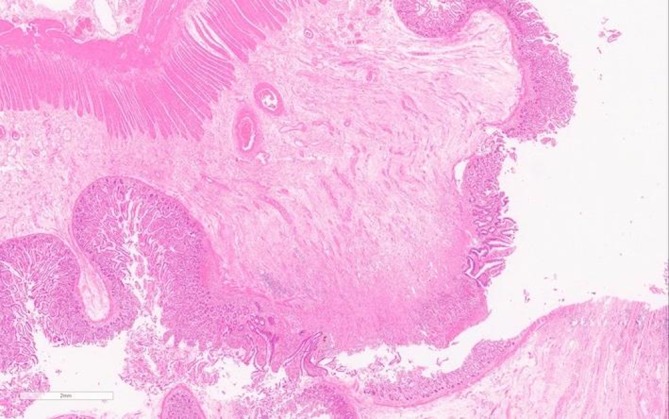
(H&E 140×)—microscopic overview of another area of stricture showing ulceration with submucosal fibrosis. The mucosa adjacent to the ulcer also shows reactive changes.
At 1-month follow-up the wound was healing well and the patient was starting to put on weight and increase her physical activities. Her abdominal pain had resolved.
Discussion
DD is a rare complication of NSAID use and is characterised by the presence of multiple, thin, concentric, circumferential strictures of the small bowel lumen causing varying degrees of obstruction.2 The current consensus regarding the mechanism of injury is that NSAIDs cause a reduction in villous microcirculation resulting in increased permeability of the small intestinal mucosa. This effect is potentiated by the enterohepatic recirculation of some of the drugs. As a result of this increased permeability, enteral bacteria, their products and bile gain access to the mucosa causing localised inflammation. Neutrophil infiltration and endothelial cell injury cause the production of reactive oxygen species which leads to tissue damage.6 If the tissue damage results in circumferential ulceration, the subsequent healing process can cause collagenous rings of scar tissue to form. Over time these rings can contract, like draw strings across the bowel lumen, eventually producing the diaphragm-like strictures reported.2
The majority of DD occurs in the ileum with the terminal ileum often being spared, unlike in our case where the terminal ileum was affected. DD is characterised histopathologically by focal lesions, a lack of severe inflammation and the reactive appearance of the changes. Submucosal fibrosis is observed with usual sparing of the muscularis propria, serosa and mesentery. Eosinophilia has been reported, as was seen in our case, but granulomata are absent.6
The relative risk of specific NSAIDs in relation to the development of DD is unclear, however, several studies have shown selective cyclooxygenase-2 inhibitors to be less injurious to the gastrointestinal tract compared with less selective NSAIDs.7 DD seems to be particularly associated with high doses of NSAIDs taken over a prolonged period of time. A systemic review by Wang et al. in 2016 found that 96% of documented cases had been taking NSAIDs for greater than 1 year.
Since DD of the small bowel is rare, there are currently no accurate prevalence data for the UK. A study of 120 patients using long-term NSAIDs demonstrated a 2% incidence of small bowel DD using capsule endoscopy.8 The incidence of patients presenting acutely and requiring emergency surgery is likely to be significantly less than this. There is a female preponderance with a ratio of 3:1 but this is thought to reflect the increased incidence of chronic diseases in females requiring NSAID prescription.2
Preoperative diagnosis is difficult given its often insidious presentation, non-specific symptoms and greater incidence in older patients who are more likely to have associated comorbidities and polypharmacy. However, a history of long-term NSAID use with obstructive symptoms should raise suspicion. Blood tests may reveal normocytic or microcytic anaemia and hypoalbuminaemia. Contrast-enhanced CT lacks the resolution to identify the thin diaphragms, plan radiographs are unhelpful and other contrast studies are non-specific as the features may be misidentified as plicae circulares. Conventional endoscopy does not have the coverage.3 Capsule endoscopy has shown some promise but must be used with caution as the capsule is prone to retention by a diaphragm and can precipitate SBO.9 The majority of cases are, therefore, initially diagnosed at laparotomy when the small bowel can be palpated, however, histopathology confirmation is usually still required.5
As DD is a benign condition, it can either be treated conservatively by the cessation of NSAIDs, or operatively through a combination of stricturoplasties and/or segmental resection. Approximately 50% of patients may experience recurrence following resection, attributed to the failure of complete resection at initial surgery or due to continued use of NSAIDs.2 Following withdrawal of NSAIDs the prognosis is good, as seen in our case. However, in a subset of patients the benefit of continuing NSAID therapy will outweigh the risks of small bowel enteropathy and these instances need to be considered on a case-by-case basis.3
A literature review revealed a systemic review published in 2016 which identified a total of 95 cases of small bowel DD over a 42-year period. Of these only 49 were diagnosed by laparotomy.2 It is unclear which cases presented acutely with SBO requiring emergency laparotomy but it is likely to be considerably less than 49, given that most patients present insidiously over many years. The same review also identified only five cases involving the terminal ileum, the site of the lesions in our case.2 Our case, therefore, fits with the reported literature with the exception that it is an exceedingly rare acute presentation requiring emergency surgery and involving the terminal ileum.
Given the widespread use of NSAIDs and an ageing population, it is likely that the incidence of small bowel DD will increase. It is, therefore, important for surgeons to familiarise themselves with this disease entity and to consider it as a potential diagnosis in patients with a long history of NSAID use presenting with obstructive symptoms in the emergency setting.
Learning points.
Non-steroidal anti-inflammatory drugs (NSAIDs) are the most prescribed class of drugs in the world; although their adverse effects are well known, small bowel enteropathy is infrequently reported in the literature.
Diaphragm disease (DD) of the small bowel results in the formation of diaphragm-like mucosal projections that can narrow the lumen and cause obstructive symptoms.
It typically presents insidiously over many years with a variety of non-specific symptoms but rarely can present as an emergency with acute small bowel obstruction (SBO).
Given the widespread use of NSAIDs and an ageing population, it is likely that the incidence of DD will increase.
It is, therefore, important that surgeons are familiar with this disease entity and consider it as a potential diagnosis in patients with a history of NSAID use presenting with acute SBO.
Footnotes
Contributors: SG and JM were responsible for the surgical management of the case and review of the case report. BMA was responsible for the examination of the histology specimens and review of the case report. SB wrote the case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Zeino Z, Sisson G, Bjarnason I. Adverse effects of drugs on small intestine and colon. Best Pract Res Clin Gastroenterol 2010;24:133–41. 10.1016/j.bpg.2010.02.008 [DOI] [PubMed] [Google Scholar]
- 2. Wang Y-Z, Sun G, Cai F-C, et al. . Clinical features, diagnosis, and treatment strategies of gastrointestinal diaphragm disease associated with nonsteroidal anti-inflammatory drugs. Gastroenterol Res Pract 2016;2016:1–8. 10.1155/2016/3679741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pilgrim S, Velchuru V, Waters G, et al. . Diaphragm disease and small bowel enteropathy due to nonsteroidal anti-inflammatory drugs: a surgical perspective. Colorectal Dis 2011;13:463–6. 10.1111/j.1463-1318.2009.02176.x [DOI] [PubMed] [Google Scholar]
- 4. Lang J, Price AB, Levi AJ, et al. . Diaphragm disease: pathology of disease of the small intestine induced by non-steroidal anti-inflammatory drugs. J Clin Pathol 1988;41:516–26. 10.1136/jcp.41.5.516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Slesser AAP, Wharton R, Smith GV, et al. . Systematic review of small bowel diaphragm disease requiring surgery. Colorectal Dis 2012;14:804–13. 10.1111/j.1463-1318.2011.02741.x [DOI] [PubMed] [Google Scholar]
- 6. De Petris G, López JI. Histopathology of diaphragm disease of the small intestine: a study of 10 cases from a single institution. Am J Clin Pathol 2008;130:518–25. 10.1309/7DDT5TDVB5C6BNHV [DOI] [PubMed] [Google Scholar]
- 7. Ishihara M, Ohmiya N, Nakamura M, et al. . Risk factors of symptomatic NSAID-induced small intestinal injury and diaphragm disease. Aliment Parmacol Ther 2014;40:538–47. [DOI] [PubMed] [Google Scholar]
- 8. Maiden L. Capsule endoscopic diagnosis of nonsteroidal antiinflammatory drug-induced enteropathy. J Gastroenterol 2009;44:64–71. 10.1007/s00535-008-2248-8 [DOI] [PubMed] [Google Scholar]
- 9. Kelly ME, McMahon LE, Jaroszewski DE, et al. . Small-bowel diaphragm disease: seven surgical cases. Arch Surg 2005;140:1162–6. 10.1001/archsurg.140.12.1162 [DOI] [PubMed] [Google Scholar]


