Abstract
Dengue is an arboviral infection that classically presents with fever, headache, joint pain, skin flush and morbilliform rashes. Neurological manifestations are well recognised but their exact incidence is unknown. Though myalgias are common in dengue virus infection, myositis and/or elevated serum creatine kinase is an uncommon complication. Guillain-Barré syndrome is another rare neurological manifestation associated with dengue fever. Here, we report the case of a 21-year-old man with serologically confirmed dengue fever presenting with severe myalgia, bilateral lower and upper limb weakness with raised creatine kinase, MRI suggestive of myositis and myonecrosis and nerve conduction velocity showing bilateral lower limb and axillary sensory motor neuropathy. He was managed conservatively and made an uneventful recovery.
Keywords: infectious diseases, infection (neurology), muscle disease, peripheral nerve disease
Background
Dengue is a common arboviral infection in tropical and subtropical areas of the world. The WHO estimates an annual incidence of approximately 100 million infections, with approximately 500 000 people with dengue haemorrhagic fever (DHF) requiring hospitalisation and a large proportion of them being children.1 The clinical presentation of dengue virus infection has a wide spectrum, ranging from mild clinical febrile illness to severe life-threatening situations like dengue haemorrhagic fever and dengue shock syndrome. With the recent large epidemics, there has been an increase in reported cases of unusual manifestation of dengue fever. The neurological complications associated with dengue include encephalitis, myositis, myelitis, Guillain-Barré syndrome (GBS) and mononeuropathies.2 Most of these manifestations are under-reported, under-recognised or not casually linked with dengue fever due to lack of suspicion among physicians. These complications if detected early can be managed promptly to reduce morbidity and financial burden.
Case presentation
A 21-year-old man, presented to us with complaint of intermittent fever for 5 days duration, associated with chills and rigours with no diurnal variation and not associated with any skin rash, body swelling or bleeding. Fever was associated with severe myalgia and painful joint movements. After 4 days of fever onset, he gradually developed weakness in lower limbs, which progressed to involve the upper limbs. There was no history of any trauma, loss of consciousness, seizures, respiratory distress and bladder or bowel involvement. There was no history of any diarrhoea, dog bite, snake bite or any recent vaccinations. There was no history of muscle fatigability and no similar episodes in the past.
On examination, he was conscious, oriented, febrile with pulse rate of 90/min, blood pressure of 100/70 mm Hg with no postural drop and respiratory rate of 20/min. No petechiae, ecchymosis, eschar, lymphadenopathy, mucocutaneous bleed or any rash were discernible. Respiratory, cardiovascular and abdominal examinations were unremarkable. Neurological examination revealed weakness of all four limbs with power of 3/5 at all joints in upper limbs and lower limbs. The tendon reflexes were absent even with reinforcement in the lower limbs. Sensory modalities were intact. Active and passive stretching of the muscles was associated with marked tenderness with decreased range of motion especially at left shoulder and elbow joint. No muscle atrophy or fasciculation was detected. Higher mental functions and cranial nerve examination were normal, also there were no meningeal signs.
Investigations
Investigations at presentation (on day 5 of fever) revealed thrombocytopaenia and haemoconcentration with haemoglobin of 12.3 gm%, haematocrit of 38%, total leucocyte count of 3.8×109/L, platelet count of 50×109/L. As per protocol for investigation of acute febrile illness with thrombocytopaenia, dengue antigen detection and serology were obtained which were positive for dengue NS1 antigen as well as IgM antibodies by ELISA. Search for other tropical infections like malaria, scrub typhus, leptospirosis, Chikungunya and Brucella were negative. Liver function tests, renal function tests, serum electrolytes and other routine biochemistry tests were within normal range. However, creatine kinase (MM isotype) was markedly elevated with an absolute value of 838 IU/L (ref range 46–171 IU/L). Routine urinary analysis was normal and there was no haemoglobinuria or myoglobinuria (no evidence of red or cola coloured urine). Blood culture was sterile. Cerebrospinal fluid study was not done in view of thrombocytopaenia and also as the relatives did not give written consent for the procedure.
Nerve conduction studies done on major nerves of upper and lower limbs showed motor neuropathy in lower limbs and axillary neuropathy in upper limbs (prolonged F waves and absent H reflexes, increased distal latencies with marked decrease in compound muscle action potential (CMAP) amplitude) suggestive of Guillain-Barré syndrome.
Ultrasound of right lower limb was performed which demonstrated wet echotexture with net hypoechoic appearance of the muscles of adductor compartment suggestive of myositis. Ultrasound of left thigh showed mild subcutaneous oedema, collection of size 20×1 cm on the medial aspect of left thigh, 14×7 mm collection on anteromedial aspect of lower 1/3 of thigh in the intermuscular planes and a large collection with moving internal echoes along the entire length of the thigh on the anteromedial aspect suggestive of myositis with multiple collections. Ultrasound of left elbow showed a collection of size 5.3×16 cm with internal echoes in the intermuscular planes in the region of cubital fossa with subcutaneous oedema. Electromyography of the involved muscles revealed small, low-amplitude polyphasic motor unit potentials, bizarre high-frequency repetitive discharges and fibrillation potentials at rest consistent with the diagnosis of myositis. The findings on ultrasound were confirmed on MRI which showed myositis with myonecrosis (figure 1). Aspirate from the collection was sterile.
Figure 1.
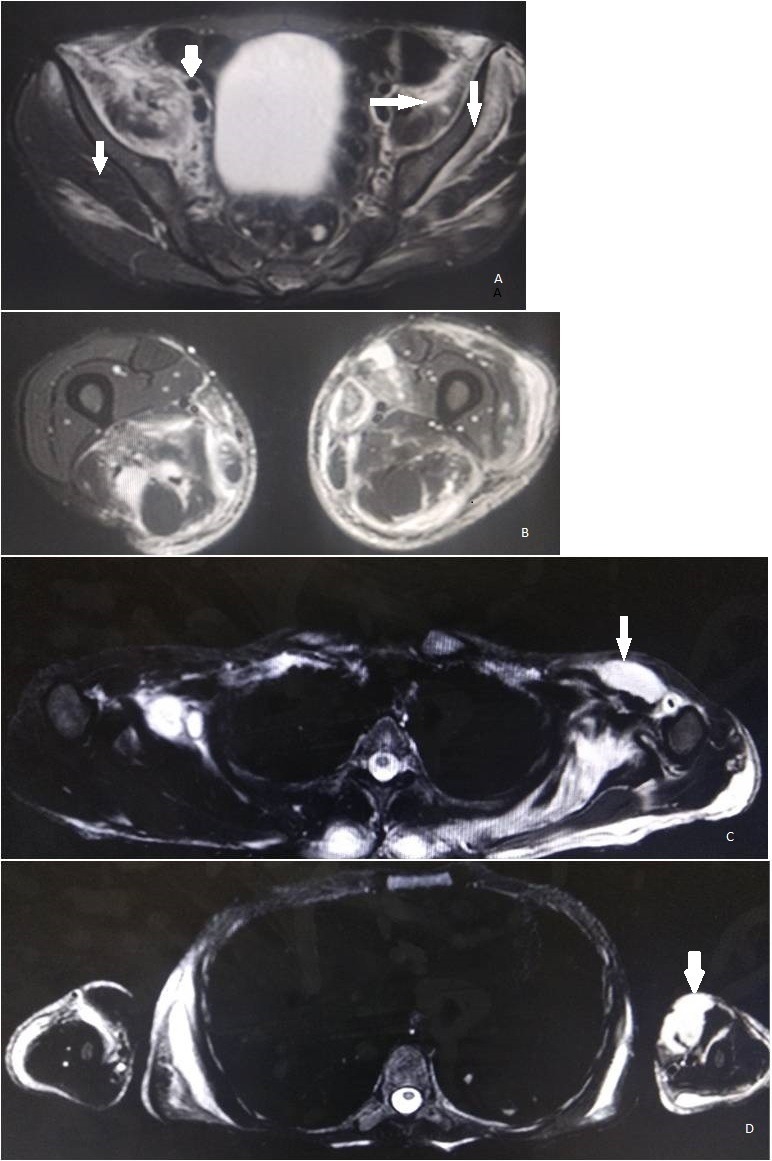
MRI images. (A) Lower limbs: oedema in bilateral iliacus, right psoas, bilateral gluteus maximus and minimus (arrows). (B) Oedema in quadriceps femoris and adductor group of muscles, left vastus lateralis and left tensor fascia lata. (C) Upper limb: oedema seen in the pectoral girdle muscles (arrow), left subscapularis, bilateral supraspinatous, trapezius, deltoid and flexor compartment. (D) Collections along left biceps and brachialis muscle (arrow).
Differential diagnosis
Our patient presented with fever followed by gradually progressive weakness of all four limbs. For a patient living in tropical area, various infectious aetiologies like poliomyelitis, rabies, diptheria (early oropharyngeal disturbances), Lyme’s disease and other tick borne paralyses, cytomegalovirus polyradiculitis (in immunocompromised individuals) need to be considered in the differential diagnosis.
Other causes that may enter into the differential diagnosis include electrolyte imbalance, porphyria (abdominal pain, seizures, psychosis), neuromuscular disorders like myasthenia gravis (ptosis and proximal muscle weakness) and botulism (pupillary reactivity lost and dysarthria), autoimmune causes (like polymyositis/dermatomyositis, systemic lupus erythematosus and vasculitic neuropathy), drug induced myopathy/myositis, poisoning with organophosphates, arsenic or severe hypophosphatemia (rare). Tropical pyomyositis is an important entity in the tropical areas characterised by suppuration within skeletal muscles, manifesting as single or multiple abscesses, aetiological organism being Staphylococcus aureus. The list of differential diagnoses is quite extensive, therefore clinical and diagnostic acumen is essential.
Treatment
Our patient was managed conservatively with five cycles of plasmapheresis (as the patient was not able to afford immunoglobulins), fluids, analgesics and antibiotics. The collections were aspirated and sent for culture sensitivity which was sterile. Over the course of hospital stay, the patient became afebrile and platelet count improved (platelet count was 4.63×109/L on discharge).
Outcome and follow-up
The weakness in lower limbs improved (power in lower limbs was 5/5 at all the joints); however, there was residual weakness in left upper limb (power was 3/5 at all joints in left upper limb) at the time of discharge (total duration of hospital stay was 36 days). At 4-week follow-up, the patient was symptomatically better (power was 4/5in left upper limb and 5/5 in right upper limb and bilateral lower limbs at all the joints).
Discussion
Dengue fever is an arboviral disease transmitted by the bite of Aedes aegypti mosquitoe. There are four serotypes of the virus; all can exhibit the full spectrum of disease including subclinical infection to a mild self-limiting disease to severe DHF/dengue shock syndrome.3According to Centers for Disease Control and Prevention guidelines, dengue should be suspected in a patient presenting with acute onset of fever, headache, body aches and sometimes rash spreading from the trunk. Laboratory confirmation can be made from a single acute-phase serum specimen obtained early (≤7 days after fever onset) in the illness by detecting viral genomic sequences with reverse transcription polymerase chain reaction (RT-PCR) or dengue nonstructural protein 1 (NS1) antigen by immunoassay. The sensitivity of RT-PCR assays in serologically confirmed cases ranges from 40% to 80% and may decrease as the interval from symptom onset to specimen collection increases.4 For patients presenting >1 week after fever onset, IgM detection is most useful. Our patient presented to the hospital on day 5 of illness and due to financial constraints and non-availability of RT-PCR in our hospital setting, it was not performed. The diagnosis of dengue fever was made on the basis of clinical profile, thrombocytopaenia and positive NS1 antigen and IgM serology (performed on day 5 of illness).
Neurological complications are most frequently reported with serotype-2 and serotype-3.5 The spectrum includes encephalitis, myositis, myelitis, GBS and mononeuropathies.5 GBS or acute inflammatory demyelinating polyradiculopathy is a postinfectious ascending, usually demyelinating, polyradiculoneuropathy.6 Well-established associations with GBS are recent infections with Campylobacter jejuni, cytomegalovirus, Epstein-Barr virus, Mycoplasma pneumonie and HIV.
Dengue fever as an antecedent infection in GBS was uncommon a decade back; however, several authors have increasingly described GBS in dengue, majority of these being children and a few cases of postdengue GBS in adults.6 7 It is suggested that the clinical manifestations of GBS in dengue result from an abnormal immune response to non-self antigens which can cross-react with the myelin or axons of peripheral nerves via molecular mimicry.8 This process is facilitated by activated T cells which disrupts the blood–brain barrier with the help of proinflammatory cytokines like tumour necrosis factor (TNF), complements and interleukins and allows antibodies to act on endoneural compartment and on Schwann cells. Several randomised clinical trials indicate that plasma exchange is more effective than supportive treatments in reducing the median time taken for recovery in GBS.7 9Corticosteroids alone do not alter the outcome of GBS, and there is insufficient evidence to support their use in combination with immunoglobulin.6 9 Dengue virus is a rare but a potential cause for acute myositis. Viral myositis and its complications are well described with several acute viral infections, most notably influenza A and B virus, HIV, coxsackie viruses and cytomegalovirus. Similar to viruses well known to cause myositis, dengue is also associated with a viraemic phase with prostration and myalgia. Myositis due to dengue virus can be mainly due to production of various inflammatory cytokines such as TNF and interferon alpha released in response to viral infection. Direct invasion of muscle by the virus has also been demonstrated in some studies. Study by Warke et al showed a high efficiency infection and replication of the virus in human primary skeletal muscles.10 Another study by Salgado et al demonstrated the presence of dengue virus in the striated muscles.11 Dengue-associated myositis can be of varying severity, ranging from self-limiting mild muscle weakness to severe dengue myositis resulting in complete quadriplegia and respiratory insufficiency. Deaths were also noted in severe cases.12 Laboratory findings include elevated creatine phosphokinase (CPK) levels, electromyography showing features of myositis like fibrillation potentials, positive sharp waves and complex repetitive discharges and muscle biopsy. Dengue myositis is differentiated from other causes of neuromuscular weakness by the presence of calf and thigh muscle tenderness on stretching, relatively preserved muscle power and deep tendon reflexes and an elevated CPK. In medical literature, myositis following dengue illness is rare, which may be due to low level of suspicion of myositis in cases presenting with myalgia. Thus, it is suggested that in dengue illness with weakness and myalgia, serum creatine kinase and myoglobinuria should be done for early diagnosis of myositis.
Patient’s perspective.
I was shocked to experience that dengue fever can be so devastating and could affect my nerves. I could not come for visit to hospital as I had recovered although I have some pain in my shoulder muscles when I do hard labour.
Learning points.
Dengue fever can manifest with a wide spectrum of unusual complications, thus a high index of suspicion for dengue should be kept.
Viral myositis and Guillain-Barré syndrome may occur as a consequence of many viral illnesses, and dengue fever may be included in the list of viral aetiologies for both these debilitating illnesses.
As dengue and dengue haemorrhagic fever are assuming global proportions in terms of numbers, more and more atypical manifestations appear which might be under-reported, therefore awareness is the key in establishing early diagnosis and for early therapeutic intervention.
Footnotes
Contributors: All the authors have provided substantial contributions in the clinical management of the case and literature review on the topic in question. MG and PD have drafted the manuscript, and MG and DK have revised it critically for important intellectual content. All the authors have read the final version and approved it. All the authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. World Health Organization, Regional Office for South-East Asia Comprehensive guideline for prevention and control of dengue and dengue haemorrhagic fever, 2011. Available: https://apps.who.int/iris/handle/10665/204894
- 2. Carod-Artal FJ, Wichmann O, Farrar J, et al. . Neurological complications of dengue virus infection. Lancet Neurol 2013;12:906–19. 10.1016/S1474-4422(13)70150-9 [DOI] [PubMed] [Google Scholar]
- 3. Bhatt S, Gething PW, Brady OJ, et al. . The global distribution and burden of dengue. Nature 2013;496:504–7. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raengsakulrach B, Nisalak A, Maneekarn N, et al. . Comparison of four reverse transcription-polymerase chain reaction procedures for the detection of dengue virus in clinical specimens. J Virol Methods 2002;105:219–32. 10.1016/S0166-0934(02)00104-0 [DOI] [PubMed] [Google Scholar]
- 5. Solomon T, Dung NM, Vaughn DW, et al. . Neurological manifestations of dengue infection. Lancet 2000;355:1053–9. 10.1016/S0140-6736(00)02036-5 [DOI] [PubMed] [Google Scholar]
- 6. Qureshi NK, Begum A, Saha PR, et al. . Guillain-Barre syndrome following dengue fever in adult patient. J Med 2012;13:246–9. 10.3329/jom.v13i2.12772 [DOI] [Google Scholar]
- 7. Gulati S, Maheshwari A. Atypical manifestations of dengue. Trop Med Int Health 2007;12:1087–95. 10.1111/j.1365-3156.2007.01891.x [DOI] [PubMed] [Google Scholar]
- 8. Hahn AF. Guillain-Barré syndrome. Lancet 1998;352:635–41. 10.1016/S0140-6736(97)12308-X [DOI] [PubMed] [Google Scholar]
- 9. Chew NK, Goh KJ, Omar S, et al. . Guillain–Barre syndrome with antecedent dengue infection: a report of two cases. Neurol J Southeast Asia 1998;3:85–6. [Google Scholar]
- 10. Warke RV, Becerra A, Zawadzka A, et al. . Efficient dengue virus (DENV) infection of human muscle satellite cells upregulates type I interferon response genes and differentially modulates MHC I expression on bystander and DENV-infected cells. J Gen Virol 2008;89:1605–15. 10.1099/vir.0.2008/000968-0 [DOI] [PubMed] [Google Scholar]
- 11. Salgado DM, Eltit JM, Mansfield K, et al. . Heart and skeletal muscle are targets of dengue virus infection. Pediatr Infect Dis J 2010;29:238–42. 10.1097/INF.0b013e3181bc3c5b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paliwal VK, Garg RK, Juyal R, et al. . Acute dengue virus myositis: a report of seven patients of varying clinical severity including two cases with severe fulminant myositis. J Neurol Sci 2011;300:14–18. 10.1016/j.jns.2010.10.022 [DOI] [PubMed] [Google Scholar]


