Abstract
CD27 deficiency is a rare primary immune deficiency which affects T cells, B cells and NK cells and is associated with hypogammaglobulinemia. Clinical presentation varies from asymptomatic disease to life-threatening Epstein Barr Virus (EBV)-driven complications including malignancy. Delay in diagnosis and late presentation adversely affects the clinical outcome and survival. We report a 10-year-old girl who had been symptomatic since 3 years of age with recurrent infections, developed bronchiectasis and was found to have hypogammaglobulinemia. Initially diagnosed as common variable immune deficiency, she had persistent lymphadenopathy, hepatosplenomegaly and pancytopenia, raising a clinical suspicion of a lymphoproliferative condition. On investigation, she was found to have a novel mutation involving the CD27 gene with very high EBV load. She was given rituximab injections to which she showed partial response and later developed non-Hodgkin’s lymphoma.
Keywords: immunology, immunological products and vaccines, paediatrics (drugs and medicines), paediatric oncology, congenital disorders
Background
CD27 deficiency is a newly diagnosed primary immune deficiency (PID), characterised by hypogammaglobulinemia, impaired specific antibody function, T cell function and persistent Epstein Barr Virus (EBV) viremia.1 Because of the rarity of the disease, the clinical features, treatment options and clinical outcome is not very well known.
Inherited in an autosomal recessive manner, CD27 deficiency leads to immune deficiency and dysregulation. EBV infection is ubiquitously seen in such patients due to survival of virus in B cell and their uncontrolled proliferation.2 The clinical phenotype may vary with asymptomatic borderline hypogammaglobulinemia to life-threatening EBV complications.1 2 In an immunocompetent person, primary EBV infection may be asymptomatic, presenting with mild non-specific complaints or with infectious mononucleosis (IM).3 In an immunocompromised individual, it may lead to chronic active EBV infection characterised by prolonged symptomatic phase of more than 6 months with fever, lymphadenopathy (LAP), hepatosplenomegaly and other atypical features like hepatitis, pneumonia or uveitis.4 Persistent EBV viremia leads to the various lymphoproliferative complications, including B-cell hyperplasia, polymorphic B-cell lymphoproliferative disorders (LPDs), indolent B-cell lymphomas, aggressive B-cell lymphomas and classic Hodgkin lymphoma (HL) like proliferations.5 6 Several other immune deficiency syndromes are known to be at high risk of developing EBV-associated LPD and these include SH2D1A/SLAM associated protein deficiency also known as X linked lymphoproliferative syndrome type 1, X linked inhibitor of apoptosis protein or X linked lymphoproliferative syndrome type 2, interleukin 2 inducible T cell kinase deficiency, magnesium transporter 1 deficiency, CD70 deficiency, cytidine triphosphate synthase deficiency and coronin 1 deficiency, all of which are genes that are involved in controlling EBV infection.4 7
About 19 cases with CD27 deficiency have been described so far, with variable clinical presentations ranging from asymptomatic hypogammaglobulinemia and mild EBV infection to repeated severe infections and LPDs including HL, malignant B cell lymphoma, haemophagocytic lympohistiocytosis (HLH) and aplastic anaemia.1 2 8–10 We report a case of a 10-year-old girl with the CD27 gene mutation, her clinical symptoms, course of illness and her treatment response to rituximab.
Case presentation
A 10-year-old girl born out of a non-consanguineous marriage, with uneventful birth and family history, presented to us at the age of 8 years with history of recurrent episodes of pneumonia and diarrhoea since 3 years of age. She had multiple admissions for lower respiratory tract infections till 6 years of age, about one to two times per year, requiring intravenous antibiotics and oxygen and recurrent episodes of loose stools in every 2 to 3 months. Some of these episodes were associated with oral ulcers and ear ache.
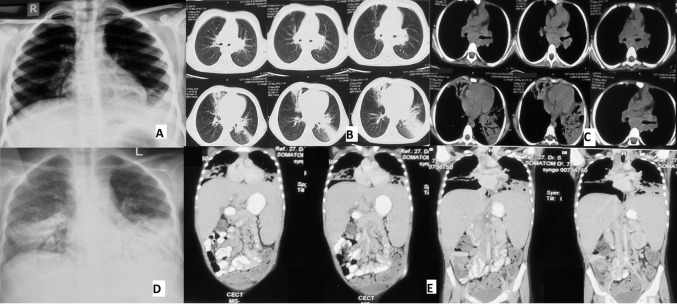
At six and a half year of age, she had a similar episode during which she was detected to have cervical LAP along with some opacities on chest X-ray (CXR, figure 1A). Fine needle aspiration cytology (FNAC) done from lymph node revealed reactive changes while the CT scan of the chest showed consolidation and mediastinal LAP (figure 1B, C). Bronchoalveolar lavage (BAL) culture grew Klebsiella pneumoniae and work up for tuberculosis was negative. However, she was started on antitubercular treatment (ATT) on clinical suspicion and was apparently well on ATT taken for 9 months. She had another hospitalisation at 8 years with dengue infection.
Figure 1.
(A) CXR at 6.5 years of age. (B) CT chest at 6.5 years, lung window showing areas of consolidation and bronchiectasis. (C) CT chest, mediastinal window showing subcarinal lymphadenopathy. (D) CXR at 9 years showing bilateral lower lobe consolidation with bronchiectasis. (E) CT chest and abdomen at 9 years showing bronchiectasis in bilateral lower lobes, hepatosplenomegaly and lymph nodes at coeliac and porta hepatis. CXR, chest X-ray.
With this history, she presented in our outpatient department, and on examination was found to have clubbing, crepitations and bronchial breath sounds on auscultation of the chest. She also had cervical lymph nodes (1.5×1.5 cm) and hepatosplenomegaly, with the liver palpable 4 cm below right costal margin and spleen palpable 5 cm below left costal margin along its axis.
Investigations
She was negative for HIV infection. Immunoglobulins (Ig) profile, showed decreased IgM (19.79 mg/dL) and IgA level (24.23 mg/dL), while IgG was within normal range for age (878 mg/dL). Flow cytometry report showed decreased CD4 and increased CD8 cells (CD4:CD8 0.18). The blood cell counts were normal and investigations for chronic granulomatous disease and leucocyte adhesion were normal.
Diagnosis
A provisional diagnosis of PID with bronchiectasis was made and she was started on cotrimoxazole and antifungal prophylaxis.
At eight and half years, she was admitted with acute respiratory exacerbation. Her haemogram, metabolic prolife, including liver function, renal function and electrolytes were normal except for anaemia with haemoglobin (Hb) of 10 g/dL. A repeat Ig levels revealed pan-hypogammaglobulinemia with IgG of 369 mg/dL, IgM of 18 mg/dL and IgA of 22 mg/dL (normal range- IgG: 633–1280 mg/dL, IgM: 48–207 mg/dL and IgA: 33–202 mg/dL). A diagnosis of common variable immune deficiency (CVID) was made, and she was started on monthly intravenous immunoglobulins (IVIG) infusions and chest physiotherapy for bronchiectasis.
She remained well for a period of 3 months but had to be readmitted at 9 years with progressively worsening abdominal distension, fever and productive cough for 2 months, not responding to oral medications. On examination, LAP and hepatosplenomegaly had increased (liver 6 cm below costal margin, with span of 10 cm and spleen 9 cm along its axis, reaching up to umbilicus). Investigations revealed anaemia and thrombocytopenia (Hb 8.8 g/dL and platelet count of 0.9×109/L). At this stage, a possibility of autoimmune lymphoproliferative syndrome (ALPS), malignancy and HLH were also considered in view of chronic hepatosplenomegaly, LAP and bicytopenia. Infective work was done along with bronchoscopy and BAL for bacteria, tuberculosis, fungus and other opportunistic organisms which revealed a normal anatomy and BAL reports were noncontributory. CXR and CT chest and abdomen (figure 1D, E) was repeated and revealed bilateral lower lobe consolidation with bronchiectasis, mediastinal lymph node enlargement and hepatosplenomegaly with nodes at porta hepatis and coeliac region. HLH work up was negative (triglyceride: 201 mg/dL, ferritin: 155 ug/L and fibrinogen: 220 mg/dL). Peripheral smear examination did not show any atypical cells and FNAC from cervical lymph nodes was reactive. For ALPS, vitamin B12 level, direct Coombs test (DCT) and markers for double negative T cells (DNT, CD3+CD4- CD8-TCRαβ+) were sent. B12 level was found to be low (24.6 pg/mL). and DCT was also negative But DNT cells were increased (44.9% of total vs 0.15% in control) and switched memory B cells were also low (1.02% vs 11.2% in control). Keeping a probable diagnosis of APLS as two of the required criteria (chronic LAP/splenomegaly and increased DNT cells) and one secondary criteria (cytopenia), were being met, she was started on oral steroids at 1 mg/kg/day.11 Whole exome sequencing was sent to look for genetic mutations responsible.
The genetic work up revealed a novel homozygous CD27 gene mutation (c.95A>G/p.Tyr32Cys). EBV titers by PCR were then sent and was found to be very high (474 188 copies/mL).
Treatment
A diagnosis of EBV-driven lymphoproliferative disease with pan hypogammaglobulinemia and bronchiectasis due to CD 27 gene mutation was made, with EBV viremia as the likely cause of current symptoms. Treatment options were discussed with the parents and she was given four doses of rituximab at 375 mg/m2 at weekly interval after ruling out active infection. There were no adverse effects noted except transient febrile reaction with tachypnea after first infusion, managed symptomatically.
Outcome and follow-up
Post rituximab injections, there was slight improvement in LAP and hepatosplenomegaly. Repeat EBV virus titers had decreased to 5608 copies per mL. But after a period of 1 month, left-sided lymph nodes again increased in size. She had persistent LAP and the FNAC from the node was repeated after 2 months which revealed non-Hodgkin’s lymphoma. Therapeutic options were discussed with parents and hematopoietic stem cell transplant (HSCT) has been offered to them.
Discussion
CD27 gene is located on chromosome 12 in humans, at 12p13.31, and is a member of TNF receptor super-family that encodes for the CD27 transmembrane receptor protein.12 It binds to its ligand, CD70, and has a role in the survival and differentiation of T, B, NK and plasma cells.13 14 In T cells, this interaction costimulates the cell with effects on T cell proliferation, survival, antiviral, antitumor and allogenic activities.15–17 For the B cells, it leads to T cell-dependent B cell activation with increased plasma cell formation and IgG production.18 19 CD27 is also used as a marker for memory B cells that has been used as a diagnostic and prognostic marker in CVID.20 21 It is also involved in NK cell maturation into cytolytic cells and defect in this function leads to loss of control of EBV infection and is attributed to cause EBV-driven complications and lymphomas.22
Around 19 cases with CD27 deficiency have been described so far (table 1).
Table 1.
Characteristics of patient with CD27 deficiency
| Ethnicity | Sex | Age of onset of symptoms/age of diagnosis (years) | Clinical features | Treatment | Outcome | Mutation | |
| 1 | Moroccan (F1) | M | 2.5/21 | Fever, LAP, HSM, primary HGG, | IVIG | Alive | HOM, NS c.G24A/p.W8X, Exon 1 |
| 2 | Moroccan (F1) | M | 3/PM | Fever, LAP, HSM, uveitis, aplastic anaemia, sepsis | Acyclovir | Died aged 4 years | “ |
| 3 | Turkish (F2) | F | 1/1 | IM, HGG, Recurrent EBV-related LPD, HLH | Steroids, IVIG, rituximab, 375 mg/m2×4, repeat course after 1 y |
Alive with borderline 20 HGG |
HOM, MS c.G158A/p.C53Y, Exon 2 |
| 4 | Turkish (F2) | F | 4/14 | Severe varicella (age 4 years), borderline HGG | None | Alive | “ |
| 5 | Turkish (F2) |
M | NA/ 4 | Subclinical EBV, CMV, borderline HGG | Prophylactic IVIG |
Alive | “ |
| 6 | Lebanese (F3) | M | 1.5/4 | HLH, recurrent infections, oral ulcers, EBV-related LPD, secondary HGG |
Rituximab and HLH-2004, repeat treatment and cord HSCT on relapse | Alive | “ |
| 7 | Lebanese (F3) | F | 1/1 | Oral and anal ulcers, uveitis, chronic EBV viremia, EBV meningitis, recurrent infections, secondary HGG |
IVIG, rituximab, HSCT |
Alive | “ |
| 8 | Lebanese (F4) | M | 15/15 | Oral ulcers, uveitis, chronic EBV viremia, recurrent infections, EBV-related LPD, T-cell lymphoma |
IVIG, rituximab, R-CHOP, cord HSCT |
Alive | “ |
| 9 | Lebanese (F4) | F | 2/PM | EBV-driven lymphoma (DLBCL) | Prednisolone | Died aged 2 years | |
| 10 | Lebanese (F4) | F | 22/PM | EBV-driven lymphoma (DLBCL) | CHOP | Died aged 22 years | |
| 11 | German (F5)- NCM | F | 4/9 | Fever, HSM pancytopenia, EBV encephalitis, 20 HLH, extra nodal LPD orbit | HLH 2004 (poor response), rituximab (275 g/m2), IVIG repeat rituximab (for LPD) | Alive with 20 HGG | HET, NS, c.C30A/p.C10X, Exon 1 |
| 12 | German (F6)- NCM |
F | 13/17 | Chronic active EBV infection with ulcer, uveitis, LN, fever, mixed cellularity HD, HLH | EuroNet PHL-C1 protocol. IVIG for20 HGG | Alive with postchemotherapy peripheral neuropathy (VCR) and 20 HGG | Compound HET c.C319T/p.R107C and c.G24A/p.W8X, Exon 1, 3 |
| 13 | Turkish (F7) | F | 7/7 | Recurrent pneumonia, LAP, splenomegaly | Rituximab (2×500 mg/m2), 3 times | Alive with 20 HGG requiring IVIG | HOM, MS, c.G287A/p.C96Y, Exon 3 |
| 14 | Turkish (F7) | F | 6/13 | Primary EBV infection | Symptomatic management | Alive, asymptomatic | “ |
| 15 | Iranian (F8) | F | 5/PM | CVID/HGG at 13 years, recurrent pneumonia/infections, bronchiectasis, eczema, skin abscess, LAP, HSM, chronic hepatitis, HD nodular sclerosis, infections, hepatic failure | IVIG | Died aged 20 years | HOM, MS c.G287A/p.C96Y, Exon 3 |
| 16 | Iranian (F8) | M | 8/32 | Recurrent influenza, EBV infection, HD nodular sclerosis, decreased IgA, infections | Chemotherapy (ABVD) and radiotherapy | Alive | “ |
| 17 | Iranian (F8) | F | 16/PM | EBV, nodular sclerosis classical HD. Infections. | Chemotherapy and radiotherapy | Died (age not known) | Not tested |
| 18 | Iranian (F9) | F | 8/PM | Recurrent URI, bronchitis, sinusitis, pneumonia, HGG/CVID, IM, chronic diarrhoea, giardiasis, EBV pneumonia | IVIG | Died aged 25 years | HOM, MS c.C232T/p.R78W Exon 3 |
| 19 | Tunisian (F10) |
NK | NK | Recurrent EBV lymphoproliferative disease | NK | NK | HOM, c.G329A/p.W110X |
ABVD, adriamycin, bleomycin, vinblastine, dacarbazine; CHOP-R, rituximab and chemotherapy; CMV, cytomegalovirus; CVID, common variable immune deficiency; DLBCL, diffuse large B cell lymphoma; EBV, Epstein Barr Virus; EuroNet PHL-C1, vincristine, adriamycin, etoposide, prednisolone; F, female; F1 to F9, family 1 to family 9; HD, Hodgkin’s disease; HET, heterozygous; HGG, hypogammaglobulinemia; HLH, haemophagocytic lymphohistiocytosis; HOM, homozygous; HSCT, haematopoietic stem cell transplant; HSM, hepatosplenomegaly; IM, infectious mononucleosis; IVIG, intravenous immunoglobulin; LAP, lymphadenopathy; LPD, lymphoproliferative disorder; M, male; MS, missense mutation; NA, not applicable (as detected on screening); NCM, non-consanguineous marriage; NK, not known; NS, nonsense mutation; PM, postmortem; URI, upper respiratory tract infections; VCR, vincristine.
It was first reported by van Montfras et al in 2012 in two brothers of Moroccan descent who suffered from persistent EBV viremia.1 The first case (#1) was symptomatic since 2 years of age with fever, LAP, hepatosplenomegaly, EBV seropositivity and hypogammaglobulinemia that required replacement IVIG. His elder brother (#2) had a documented EBV infection at 3 years of age with fever, LAP, hepatosplenomegaly, EBV-associated uveitis, developed aplastic anaemia and fulminant gram-negative sepsis during his course and ultimately expired. Both the siblings were found to be CD27 deficient and mutation analysis revealed a homozygous mutation (c.G24A/p.W8X) in the CD27 gene resulting in a premature stop codon.1
The second case report came from Seidel the same year, in a 15-month-old girl (#3) of Turkish origin who presented with hypogammaglobulinemia, HLH, systemic inflammatory response and LPD, 2 months after an IM fever.9 She was initially managed with IVIG and steroids for LPD but on recurrence, was given rituximab with favourable response. She required a repeat course of rituximab after a period of 1 year due to recurrence.
Salzer et al in 2013 reported follow-up of this patient, and two of her other siblings (#4 and #5), who on screening were also found to be CD27 deficient, with borderline hypogammaglobulinemia with one requiring replacement IVIG.2 They also described five other patients from two different families. The third family was of Lebanese origin with two affected siblings, 18 months old boy (#6) with EBV LPD and HLH, treated according to HLH 2004 protocol and rituximab, developed secondary hypogammaglobulinemia and relapsed after 4 months requiring repeat treatment followed by unrelated cord blood transplant.23 His younger sister (#7) was diagnosed later with CD27 deficiency and EBV infection. The fourth family, also of Lebanese descent, had three affected members. The index case (#8) presented at 15 years with EBV LPD, responded to rituximab but had recurrence of EBV viremia 3 months later with secondary hypogammaglobulinemia. He later progressed to T cell lymphoma, was treated with rituximab and chemotherapy followed by matched unrelated cord blood transplantation. His two elder sisters (#9 and #10) had died at age of 2 and 22 years with suspected EBV-driven lymphomas. All these three families were found to have the same missense mutation (c.G158A/p.C53Y) by whole exome sequencing.
The rest of the eight cases were described by Alkhairy et al in 2015.8 The next case (#11) presented at 4 years of age with fever, hepatosplenomegaly, pancytopenia, EBV encephalitis and secondary HLH. He was treated with HLH 2004 protocol with poor response, followed by rituximab to which he responded well. He developed extra nodal EBV LPD of the orbit 5 months later, treated with rituximab and received IVIG for secondary hypogammaglobulinemia. Sequencing revealed a novel heterozygous c.C30A/p.C10X non-sense mutation. The second case described by them was a 13-year-old girl with chronic active EBV infection, recurrent fever, oral ulcers, uveitis, LAP, diagnosed with mixed cellularity HL, treated with chemotherapy (EuroNet PHL-C1 protocol) leading to complete remission. IVIG was given postchemotherapy for persistent hypogammaglobulinemia. A novel compound heterozygous mutation, c.C319T/p.R107C and c.G24A/p.W8X was detected. Both these cases were born to non-consanguineous parents of German descent.
The rest six cases were from three families with three different novel missense mutations (c.G287A/p.C96Y, c.G287A/p.C96Y and c.C232T/p.R78W). Their third case (#13) was a 7-year-old girl of Turkish descent with recurrent pneumonia, LAP and splenomegaly with high EBV load, treated successfully with rituximab while her sister (#14) had an EBV infection at 6 years, treated symptomatically and was currently well. The next case (#15) was an Iranian girl, symptomatic since 5 years of age, diagnosed as CVID at 13 years, had recurrent pneumonia treated with IVIG and later developed bronchiectasis, eczema, skin abscess and stage 1 nodular sclerosis HL. She died at age of 20 years due to lymphoma, infections and hepatic failure. Both her older brother (#16) and younger sister (#17) developed nodular sclerosis classical HL at 8 and 16 years of age. While early stage treatment with radiotherapy and chemotherapy led to remission in his brother, the sister succumbed to the disease. The last case (#18) was also an Iranian descent girl, symptomatic since 8 years of age, with recurrent upper respiratory infections and giardiasis, developed bronchitis and pneumonia at 19 years with hypogammaglobulinemia requiring IVIG initiation and later developed IM, chronic diarrhoea and expired at 25 years due to EBV pneumonia. The 19th patient was mentioned by Izawa et al in a patient of Tunisian origin with consanguineous parents carrying homozygous mutation c.G392A/pW110X who had recurrent EBV-driven LPD.10
Our case is the 20th case detected with this disease to our knowledge, and is homozygous for a novel mutation c.95A>G/p.Tyr32Cys. This single nucleotide variation wherein adenine is replaced by guanine, lies within the 75 base pairs of the coding exon and leads to a missense mutation wherein amino acid tyrosine is replaced by cysteine and has an allele frequency of 0.000004042.24 This mutation is likely to be deleterious as revealed by the combined annotation-dependent depletion score (raw score-3.733444, PHRED- 26.1), sorting intolerant from tolerant score (damaging with score 0) and PolyPhen-2 scores (probably damaging with a score of 1.000).25–27 The parents have not yet been tested for the mutation though it is planned. Hence, we could not provide the information currently if they are heterozygous carriers of the mutation as expected.
She shared similar features of recurrent infections/pneumonia, ulcers, persistent LAP with hepatosplenomegaly, primary hypogammaglobulinemia and bronchiectasis as the above-mentioned cases. She also showed a decreased CD4/CD8 ratio, and Alkhairy et al too found that CD8 T cells were expanded in four of the seven patients, leading to an inversion of the CD4/CD8 ratio.8 He also found that all the mutations led to near-absent CD27 expression on T-cell and B-cell surface in these patients or undetectable CD27 protein by western blotting. We were unable to document the absence of CD27 expression in our patient though due to unavailability of testing facilities.
Treatment was guided by the clinical presentation and included IVIG for hypogammaglobulinemia, primary or secondary, rituximab and chemotherapy for malignancy. Rituximab, an anti-CD 20 monoclonal antibody, is effective for EBV LPD, HLH and lymphoma. It showed a favourable response in these patients though the response to conventional chemotherapy was variable. In our patient though, complete remission was not achieved after four doses of rituximab. She went onto develop non-Hodgkin’s lymphoma and is currently planned for HSCT, which is the definitive treatment and was done in three of the patients, all of whom are alive.
The disease carries a high mortality rate (30%, 6/18) and a delay in diagnosis is associated with worse outcome. Cause of death included malignancy (4/18), sepsis (2/18), aplastic anaemia (1/18), hepatic failure (1/18) and severe EBV pneumonia (1/18).8
To conclude, CD27 deficiency is a rare PID with a high mortality and morbidity. Early diagnosis leads to a better chance of survival and should be suspected in patients with a recurrent infection, CVID like presentation with hypogammaglobulinemia, chronic LAP with hepatosplenomegaly and very high EBV titers. CD27 expression on lymphocytes by flow cytometry can be used as a screening test.
Learning points.
CD27 deficiency is a rare combined immune deficiency associated with Epstein Barr Virus (EBV) viremia.
Recurrent infections, hypogammaglobulinemia, persistent lymphadenopathy, hepatosplenomegaly and atypical EBV infections should raise a suspicion of CD27 deficiency.
Rituximab has a role in EBV-driven complications and malignancy in these patients.
Footnotes
Contributors: RK was involved in acquisition, analysis and interpretation of the data and writing the case report. AG was involved in the acquisition of data and analysis of the report. AKG and SKK were involved in conception, design and analysis of data. All authors were involved in revision, provided critical inputs and approved the final version of the manuscript. AKG would be the guarantor for the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. van Montfrans JM, Hoepelman AIM, Otto S, et al. Cd27 deficiency is associated with combined immunodeficiency and persistent symptomatic EBV viremia. J Allergy Clin Immunol 2012;129:787–93. 10.1016/j.jaci.2011.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Salzer E, Daschkey S, Choo S, et al. Combined immunodeficiency with life-threatening ebv-associated lymphoproliferative disorder in patients lacking functional CD27. Haematologica 2013;98:473–8. 10.3324/haematol.2012.068791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Odumade OA, Hogquist KA, Balfour HH. Progress and problems in understanding and managing primary Epstein-Barr virus infections. Clin Microbiol Rev 2011;24:193–209. 10.1128/CMR.00044-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen JI. Epstein-Barr virus infection. N Engl J Med 2000;343:481–92. 10.1056/NEJM200008173430707 [DOI] [PubMed] [Google Scholar]
- 5. Natkunam Y, Gratzinger D, Chadburn A, et al. Immunodeficiency-associated lymphoproliferative disorders: time for reappraisal? Blood 2018;132:1871–8. 10.1182/blood-2018-04-842559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crombie JL, LaCasce AS. Epstein Barr virus associated B-cell lymphomas and iatrogenic lymphoproliferative disorders. Front Oncol 2019;9:109 10.3389/fonc.2019.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Latour S, Winter S. Inherited immunodeficiencies with high predisposition to Epstein-Barr virus-driven lymphoproliferative diseases. Front Immunol 2018;9:1103 10.3389/fimmu.2018.01103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alkhairy OK, Perez-Becker R, Driessen GJ, et al. Novel mutations in TNFRSF7/CD27: clinical, immunologic, and genetic characterization of human CD27 deficiency. J Allergy Clin Immunol 2015;136:703–12. 10.1016/j.jaci.2015.02.022 [DOI] [PubMed] [Google Scholar]
- 9. Seidel MG. Cd27: a new player in the field of common variable immunodeficiency and ebv-associated lymphoproliferative disorder? J Allergy Clin Immunol 2012;129:1175–6. 10.1016/j.jaci.2012.01.053 [DOI] [PubMed] [Google Scholar]
- 10. Izawa K, Martin E, Soudais C, et al. Inherited CD70 deficiency in humans reveals a critical role for the CD70-CD27 pathway in immunity to Epstein-Barr virus infection. J Exp Med 2017;214:73–89. 10.1084/jem.20160784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oliveira JB, Bleesing JJ, Dianzani U, et al. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (Alps): report from the 2009 NIH International workshop. Blood 2010;116:e35–40. 10.1182/blood-2010-04-280347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prasad KV, Ao Z, Yoon Y, et al. Cd27, a member of the tumor necrosis factor receptor family, induces apoptosis and binds to SIVA, a proapoptotic protein. Proc Natl Acad Sci U S A 1997;94:6346–51. 10.1073/pnas.94.12.6346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Denoeud J, Moser M. Role of CD27/CD70 pathway of activation in immunity and tolerance. J Leukoc Biol 2011;89:195–203. 10.1189/jlb.0610351 [DOI] [PubMed] [Google Scholar]
- 14. Nolte MA, van Olffen RW, van Gisbergen KPJM, et al. Timing and tuning of CD27-CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol Rev 2009;229:216–31. 10.1111/j.1600-065X.2009.00774.x [DOI] [PubMed] [Google Scholar]
- 15. Hendriks J, Gravestein LA, Tesselaar K, et al. Cd27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol 2000;1:433–40. 10.1038/80877 [DOI] [PubMed] [Google Scholar]
- 16. Schildknecht A, Miescher I, Yagita H, et al. Priming of CD8+ T cell responses by pathogens typically depends on CD70-mediated interactions with dendritic cells. Eur J Immunol 2007;37:716–28. 10.1002/eji.200636824 [DOI] [PubMed] [Google Scholar]
- 17. Peperzak V, Veraar EAM, Keller AM, et al. The PIM kinase pathway contributes to survival signaling in primed CD8+ T cells upon CD27 costimulation. J Immunol 2010;185:6670–8. 10.4049/jimmunol.1000159 [DOI] [PubMed] [Google Scholar]
- 18. Borst J, Hendriks J, Xiao Y. Cd27 and CD70 in T cell and B cell activation. Curr Opin Immunol 2005;17:275–81. 10.1016/j.coi.2005.04.004 [DOI] [PubMed] [Google Scholar]
- 19. Agematsu K, Hokibara S, Nagumo H, et al. Plasma cell generation from B-lymphocytes via CD27/CD70 interaction. Leuk Lymphoma 1999;35:219–25. 10.3109/10428199909145724 [DOI] [PubMed] [Google Scholar]
- 20. Klein U, Rajewsky K, Küppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med 1998;188:1679–89. 10.1084/jem.188.9.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wehr C, Kivioja T, Schmitt C, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood 2008;111:77–85. 10.1182/blood-2007-06-091744 [DOI] [PubMed] [Google Scholar]
- 22. Jang Y-S, Kang W, Chang D-Y, et al. Cd27 engagement by a soluble CD70 protein enhances non-cytolytic antiviral activity of CD56bright natural killer cells by IFN-γ secretion. Clin Immunol 2013;149:379–87. 10.1016/j.clim.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 23. Henter J-I, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007;48:124–31. 10.1002/pbc.21039 [DOI] [PubMed] [Google Scholar]
- 24. Single nucleotide variant: 12-6554356-A-G (GRCh37). Available: https://gnomad.broadinstitute.org/variant/12-6554356-A-G?dataset=gnomad_r2_1 [Accessed Dec 2019].
- 25. Rentzsch P, Witten D, Cooper GM, et al. Cadd: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res 2019;47:D886–94. 10.1093/nar/gky1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vaser R, Adusumalli S, Leng SN, et al. SIFT missense predictions for genomes. Nat Protoc 2016;11:1–9. 10.1038/nprot.2015.123 [DOI] [PubMed] [Google Scholar]
- 27. Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods 2010;7:248–9. 10.1038/nmeth0410-248 [DOI] [PMC free article] [PubMed] [Google Scholar]