Abstract
A 71-year-old woman with metastatic squamous cell carcinoma of the lung and insulin-dependent type 2 diabetes mellitus presented with a necrotic lesion on her lower abdomen. Further history revealed that this was the site of repeat insulin injections with reuse of the same needles. On investigation, biopsy of the site was positive for broad, aseptate, right-angle branching fungal hyphae consistent with mucormycosis. Studies have shown that insulin needle reuse is a common practice among diabetics for several reasons, including cost and convenience. While the current American Diabetes Association guidelines suggest that this is an acceptable practice among the general population of diabetics, they advise against it in patients who are actively ill or immunocompromised. Discussion about insulin needle reuse should be of utmost importance among providers and their diabetic patients, especially for patients who are immunocompromised.
Keywords: dermatology, infections, diabetes, infectious diseases
Background
Mucormycosis is an angioinvasive fungal infection that typically affects immunocompromised individuals, most commonly those with uncontrolled diabetes mellitus, haematological malignancies and recipients of solid organ or stem cell transplants.1 Primary cutaneous mucormycosis (PCM) is the clinical presentation of infection at sites of trauma that disrupt the skin barrier.2 Insulin needle reuse is a common practice among diabetic patients,3 and while it has not been proven to be associated with an increased risk of infection in the general diabetic population,4 few case reports of infection at the site of repeated insulin injections in both immunocompetent and immunocompromised patients exist.5–8 We present a case of PCM in a diabetic individual at the site of insulin injection in the context of insulin needle reuse. This case highlights the risk of cutaneous infection at sites of insulin injection, particularly in the context of confirmed needle reuse.
Case presentation
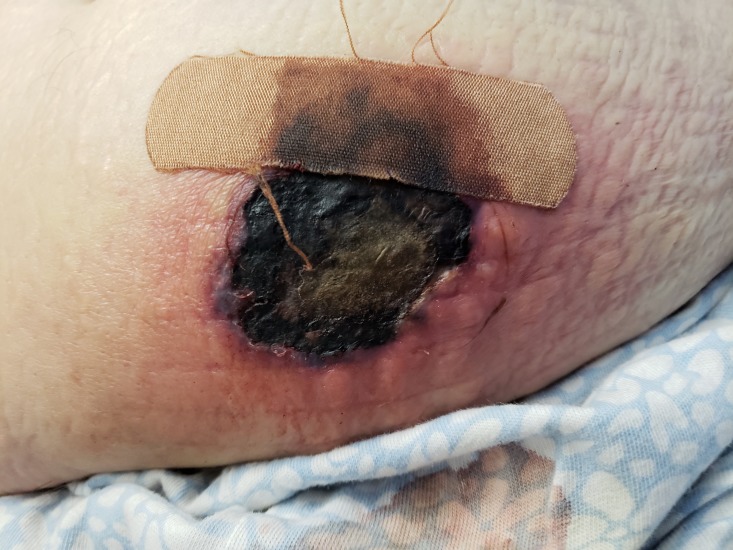
A 71-year-old Caucasian woman presented to the emergency department with a necrotic lesion of the left lower quadrant of her abdomen. The patient resided in the Northeast region of the USA and was retired at the time of presentation. Her medical history was significant for a right lower lobe lung squamous cell carcinoma, metastatic to the right humeral head and brain status post gamma knife and insulin dependent type 2 diabetes mellitus. She was not on any immunosuppressive agents at the time of admission, and her white cell counts were within normal limits on arrival. Seven days prior to admission the patient noted an erythematous non-pruritic eruption of the superficial abdomen. At that time, she was evaluated by an outpatient physician and subsequently completed a course of trimethoprim-sulfamethoxazole for suspected cellulitis. Despite the antibiotics, the rash progressed to a blister before erupting and forming an approximately 6 cm solitary stellate necrotic plaque with surrounding erythema, and haemorrhagic vesiculation at the borders (figure 1). Further history revealed that rash site was subjected to the reuse of insulin needles without altering the site of injection.
Figure 1.
Necrotic eschar on the left lower abdominal quadrant.
Investigations
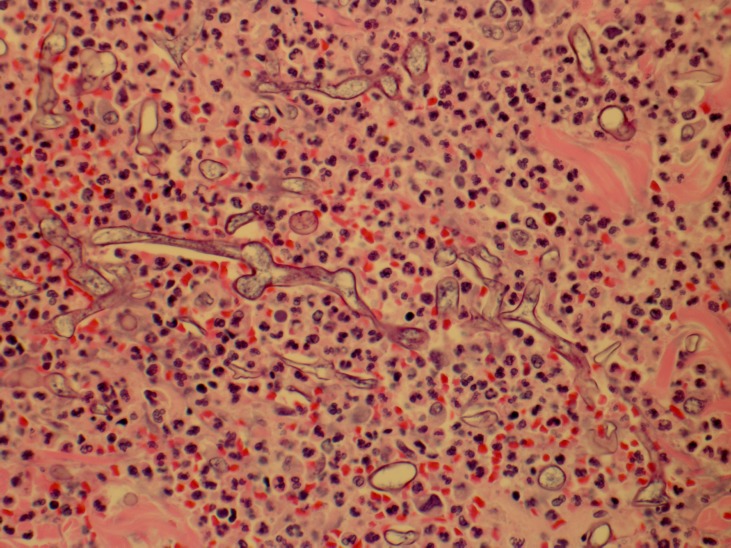
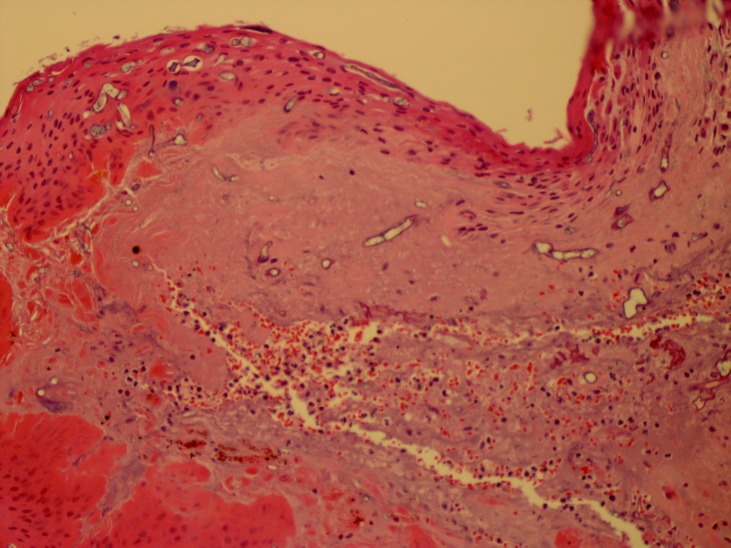
The patient was evaluated by the dermatology service and a punch biopsy was performed demonstrating numerous fungal hyphae, broad and aseptate, with right angle branching and prominent angioinvasion with associated microvascular thrombosis most consistent with mucormycosis (figures 2 and 3). Wound culture was positive for Mucor and methicillin-resistant Staphylococcus aureus.
Figure 2.
Broad aseptate hyphae with right angle branching in an acute inflammatory background (400×, H&E).
Figure 3.
Devitalised skin with Mucor hyphae in the epidermis and dermis (200S, H&E).
Treatment
Isavuconazole was administered and the patient underwent incision and debridement of the lesion. An 8×4×3.5 cm wound margin including skin and fat was incised. Following surgery, a wound vac was placed, and she completed a 14-day course of vancomycin; the plan was to complete a 1–2 months course of isavuconazole until the wound completely healed.
Outcome and follow-up
Prior to discharge, the patient became obtunded and was found to have increased intracranial swelling due to multiple cerebral metastases. She was transferred to the intensive care unit where she was intubated and had an external ventricular drain placed. She was transferred out of the unit 5 days later and ultimately the patient’s family elected home hospice until her death, declining further debridement or antifungal medication.
Discussion
Mucormycosis is an invasive fungal infection that typically infects immunocompromised individuals, particularly those with uncontrolled diabetes mellitus, haematological malignancies and organ transplant recipients.1 While aspergillosis and candidiasis are the more common invasive fungal infections infecting these patients, there is evidence that the incidence of invasive mucormycosis is increasing.9 Mucormycosis has five distinct clinical manifestations: rhinocerebral, pulmonary, cutaneous, gastrointestinal and disseminated.1 PCM is the third most common manifestation, accounting for 19% of cases in a review of published case reports, often infecting individuals with underlying disease, although multiple cases of patients with no underlying disease or immunosuppression have been published.10 The fungal spores are ubiquitious in nature, and transmission of the rhinocerebral and pulmonary forms typically occurs through ingestion and inhalation.2 In cutaneous mucormycosis, penetrating trauma is the most common mechanism by which patients are inoculated, where needles are a known source of infection; a review of published cases of cutaneous mucormycosis from 1940 to 2010 found that the majority had a preceding traumatic event that disrupted the skin barrier, with penetrating trauma such as that from a needle or sharp object accounting for 35% of infections.2 Treatment of PCM involves early diagnosis relying on clinical suspicion and biopsy, surgical debridement and administration of amphotericin B, yet mucormycosis can still be difficult to cure.11 It has been shown to have a mortality rate of up to 5.5% for localised disease and 43% for disease with deep tissue extension.2
Fewer than five case reports in the literature have reported cutaneous mucormycosis at sites of repeated insulin injection,5–7 however, to our knowledge, our case is the first to be in the setting of confirmed insulin needle reuse. In this case, we postulate that inoculation occurred through contamination of repeatedly used insulin needles, though the source of the contamination is unknown. Despite manufacturer guidelines that recommend single-use of needles and syringes for insulin injection,12 studies on needle reuse among diabetics show that it is a common practice, as patients find it more affordable and practical.3 A recent systematic review found no conclusive evidence to establish a causal between needle reuse and skin infection; there is little consensus among experts about whether it is safe to reuse needles or not.4 American Diabetes Association guidelines suggest that reusing needles is cost-effective and practical for patients, and that it may be acceptable as long as the needle is not visibly dull or deformed. They note, however, that reuse may carry an increased risk of infection and should not be practiced among those with ‘acute concurrent illness or decreased resistance to infection for any reason’.12 In the setting of underlying conditions that predispose patients to infections, needle reuse should be actively discouraged.
Learning points.
We present the case of a patient with severe, necrotic, abdominal primary cutaneous mucormycosis due to repeated insulin needle reuse.
Mucormycosis is an invasive fungal infection that most often affects immunocompromised individuals, particularly patients with uncontrolled diabetes mellitus, through penetrating trauma to the skin.
Although insulin needle reuse may be a viable option in select patients, patient education around needle reuse is paramount, particularly in patients who are immunosuppressed.
Footnotes
Contributors: AP: conception, planning, analysis and data interpretation, manuscript drafting and editing, final draft approval. GM: conception, planning, analysis and data interpretation, manuscript drafting and editing, final draft approval. EF: conception, planning, analysis and data interpretation, manuscript drafting and editing, final draft approval. JW: conception, planning, acquisition of data, analysis and data interpretation, manuscript editing, final draft approval. WR: conception, planning, acquisition of data, analysis and data interpretation, manuscript editing, final draft approval.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Next of kin consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis 2012;54:S23–34. 10.1093/cid/cir866 [DOI] [PubMed] [Google Scholar]
- 2. Skiada A, Rigopoulos D, Larios G, et al. Global epidemiology of cutaneous zygomycosis. Clin Dermatol 2012;30:628–32. 10.1016/j.clindermatol.2012.01.010 [DOI] [PubMed] [Google Scholar]
- 3. Poteet GW, Reinert B, Ptak HE. Outcome of multiple usage of disposable syringes in the insulin-requiring diabetic. Nurs Res 1987;36:350–2. 10.1097/00006199-198711000-00008 [DOI] [PubMed] [Google Scholar]
- 4. Zabaleta-del-Olmo E, Vlacho B, Jodar-Fernández L, et al. Safety of the reuse of needles for subcutaneous insulin injection: a systematic review and meta-analysis. Int J Nurs Stud 2016;60:121–32. 10.1016/j.ijnurstu.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 5. Clark R, Greer DL, Carlisle T, et al. Cutaneous zygomycosis in a diabetic HTLV-I–seropositive man. J Am Acad Dermatol 1990;22:956–9. 10.1016/0190-9622(90)70134-4 [DOI] [PubMed] [Google Scholar]
- 6. Chambers CJ, Reyes Merin M, Fung MA, et al. Primary cutaneous mucormycosis at sites of insulin injection. J Am Acad Dermatol 2011;64:e79–81. 10.1016/j.jaad.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 7. Delie A, Vlummens P, Creytens D, et al. Cutaneous mucormycosis as result of insulin administration in an AML patient: case report and review of the literature. Acta Clin Belg 2017;72:352–6. 10.1080/17843286.2016.1266802 [DOI] [PubMed] [Google Scholar]
- 8. Sousa TS, Matherne RJ, Wilkerson MG. Case report: cutaneous nontuberculous mycobacterial abscesses associated with insulin injections. J Drugs Dermatol 2010;9:1439–42. [PubMed] [Google Scholar]
- 9. Chayakulkeeree M, Ghannoum MA, Perfect JR. Zygomycosis: the re-emerging fungal infection. Eur J Clin Microbiol Infect Dis 2006;25:215–29. 10.1007/s10096-006-0107-1 [DOI] [PubMed] [Google Scholar]
- 10. Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005;41:634–53. 10.1086/432579 [DOI] [PubMed] [Google Scholar]
- 11. Katragkou A, Walsh TJ, Roilides E. Why is mucormycosis more difficult to cure than more common mycoses? Clin Microbiol Infect 2014;20:74–81. 10.1111/1469-0691.12466 [DOI] [PubMed] [Google Scholar]
- 12. American Diabetes Association Insulin administration. Diabetes Care 2004;27:S106–7. 10.2337/diacare.27.2007.S106 [DOI] [PubMed] [Google Scholar]