Abstract
PURPOSE
Patients with indolent non-Hodgkin lymphoma typically respond well to first-line immunochemotherapy. At relapse, single-agent rituximab is commonly administered. Data suggest the immunomodulatory agent lenalidomide could increase the activity of rituximab.
METHODS
A phase III, multicenter, randomized trial of lenalidomide plus rituximab versus placebo plus rituximab was conducted in patients with relapsed and/or refractory follicular or marginal zone lymphoma. Patients received lenalidomide or placebo for 12 cycles plus rituximab once per week for 4 weeks in cycle 1 and day 1 of cycles 2 through 5. The primary end point was progression-free survival per independent radiology review.
RESULTS
A total of 358 patients were randomly assigned to lenalidomide plus rituximab (n = 178) or placebo plus rituximab (n = 180). Infections (63% v 49%), neutropenia (58% v 23%), and cutaneous reactions (32% v 12%) were more common with lenalidomide plus rituximab. Grade 3 or 4 neutropenia (50% v 13%) and leukopenia (7% v 2%) were higher with lenalidomide plus rituximab; no other grade 3 or 4 adverse event differed by 5% or more between groups. Progression-free survival was significantly improved for lenalidomide plus rituximab versus placebo plus rituximab, with a hazard ratio of 0.46 (95% CI, 0.34 to 0.62; P < .001) and median duration of 39.4 months (95% CI, 22.9 months to not reached) versus 14.1 months (95% CI, 11.4 to 16.7 months), respectively.
CONCLUSION
Lenalidomide improved efficacy of rituximab in patients with recurrent indolent lymphoma, with an acceptable safety profile.
INTRODUCTION
Non-Hodgkin lymphomas (NHLs) are mostly of B-cell origin1 and include low-grade, indolent histologies that usually respond to initial therapy but typically relapse.1-4 The most common indolent NHL types, follicular lymphoma (FL) and marginal zone lymphoma (MZL), account for 22% and 7% of adult NHL, respectively.5,6 Despite being distinct entities, recurrent FL and MZL are treated similarly.7,8 Single-agent rituximab is approved by the US Food and Drug Administration and is commonly used as treatment of these patients.
Lenalidomide is an immunomodulatory (IMiD) drug that binds to the cereblon E3 ubiquitin ligase complex, resulting in ubiquitination of the transcription factors Aiolos and Ikaros, leading to antilymphoma effects.9,10 Preclinically, lenalidomide restored the response of tumor-infiltrating lymphocytes in autologous T-cell conjugates11 and increased natural killer cell count and function in peripheral blood and natural killer cell lines.12,13 Adding lenalidomide to rituximab enhanced antibody-dependent cell-mediated cytotoxicity, immune synapse formation, monocyte-mediated killing, and direct cytotoxicity against FL cells.11,14-16
There are several treatment options, none considered curative, for patients with relapsed/refractory FL and MZL, including chemotherapy plus anti-CD20 monoclonal antibodies and targeted agents such as phosphatidylinositol 3-kinase inhibitors. Treatment choice is often based on duration of response to prior therapies, types of prior therapies, and patient comorbidities.3,17 Rituximab monotherapy is a treatment option in patients who had previously responded to rituximab, on the basis of observations that frequent responses can occur with rituximab retreatment.18,19 Rituximab monotherapy was commonly used in the second-line treatment of FL (25% to 47% of patients) according to studies in the United States and Europe.20-22 Lenalidomide plus rituximab combination showed clinical activity in patients with previously treated indolent NHL in a key phase II study23 and others24,25 demonstrating overall response rates of 65% to 77%, complete response (CR) rates of 35% to 41%, and median progression-free survival (PFS)/time to progression of 1 to 2 years. Recently, the lenalidomide plus rituximab combination also showed clinical activity in a phase III study of advanced untreated FL.26 The AUGMENT trial (ClinicalTrials.gov identifier: NCT01938001) prospectively compared efficacy and safety of lenalidomide plus rituximab to placebo plus rituximab (a standard of care, among several) in patients with relapsed or refractory indolent NHL who are appropriate for rituximab monotherapy (Appendix Table A1, online only).
METHODS
Patients
Eligible patients had MZL or FL (grades 1 to 3a) requiring treatment per investigator assessment; at least one prior chemotherapy, immunotherapy, or chemoimmunotherapy and two or more previous doses of rituximab; and relapsed, refractory, or progressive disease and not rituximab-refractory disease. Patients with neuropathy grade greater than one were excluded. Additional eligibility criteria are in the Appendix (online only).
Trial Design and Treatment
Patients were randomly assigned (1:1 ratio) to lenalidomide plus rituximab (lenalidomide plus rituximab group) or placebo plus rituximab (placebo plus rituximab group). Random assignment was stratified according to previous rituximab treatment (yes or no), time since last therapy (≤ 2 years v > 2 years), and histology (FL v MZL). Prior induction and maintenance treatment were considered one treatment line.
Treatment continued for 12 cycles or relapse, progressive disease, withdrawal of consent, or unacceptable toxicity. Lenalidomide plus rituximab dosing included oral lenalidomide 20 mg daily (10 mg for creatinine clearance 30 to 59 mL/min) on days 1 to 21 plus intravenous rituximab 375 mg/m2 days 1, 8, 15, and 22 of cycle 1 and day 1 of cycles 2 to 5 every 28 days. Placebo plus rituximab was administered similarly. The rituximab regimen was selected using efficacy and statistical assumptions from the LYM-3001 trial (ClinicalTrials.gov identifier: NCT00312845) in similar patients.18 Rationale for lenalidomide and rituximab dosing schedules are detailed in the Appendix. On treatment completion or discontinuation, patients were observed for progression, subsequent therapies, response to next therapies, and second malignancies for up to 5 years after the last patient was randomly assigned (Appendix).
Dose adjustments of lenalidomide were planned to manage toxicity (Appendix). In patients with thromboembolism risk, prophylactic anticoagulation or antiplatelet therapy at investigator discretion was recommended. Rituximab dose was not reduced, but if discontinued for toxicity, lenalidomide plus placebo continued per protocol (Data Supplement). Growth factor use was allowed per ASCO/European Society for Medical Oncology guidelines.27,28
This study was performed following Good Clinical Practice per International Conference on Harmonization Guideline E6 requirements under ethical principles in the Declaration of Helsinki. Study conduct followed guidance of each site’s institutional review board, independent ethics committee, and regulatory authorities. All patients provided written informed consent before trial enrollment.
Efficacy and Safety Assessments
Primary efficacy analyses were conducted in the intention-to-treat population, defined as all patients randomly assigned, regardless of receiving trial treatment. The primary end point was PFS assessed by the independent review committee (IRC) per 2007 International Working Group criteria,29 without positron emission tomographic imaging. Secondary end points included overall response rate, CR, duration of response, overall survival (OS), event-free survival, and time to next antilymphoma treatment. Time to next chemotherapy treatment and histologic transformation were exploratory end points.
Response and progression outcomes were assessed by a blinded, independent central review using 2007 International Working Group criteria on the basis of computed axial tomography/magnetic resonance imaging scans. Patients with gastric mucosa–associated lymphoid tissue lymphoma underwent endoscopy for response evaluation. Bone marrow biopsy was required to confirm CR.
The safety analysis population included all patients receiving at least one dose of study medication. Adverse event classification used National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03, except for tumor flare (graded by National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0) and tumor lysis (assessed per Cairo-Bishop Criteria).30
Statistical Analyses
The primary objective was to compare efficacy in the lenalidomide plus rituximab group versus the placebo plus rituximab group on the basis of the primary end point of PFS at one-sided α = 0.025 level. We hypothesized that median PFS for lenalidomide plus rituximab would be 17.6 months, and 11 months for placebo plus rituximab, on the basis of previous results of rituximab monotherapy (Appendix).18 For 90% power to detect this difference with one-sided α = 0.025 and one interim analysis for the futility at 50% information time, a total of 193 PFS events were required.
The distribution for PFS and other time-to-event data were estimated using the Kaplan-Meier procedure.31 A hazard ratio (HR) with a two-sided 95% CI was estimated using the stratified Cox proportional hazard regression model. For binary types of secondary efficacy end points, the number and percentage of patients were tabulated by treatment group, a stratified Cochran-Mantel-Haenszel test adjusting for possible confounding effects of the stratification factors was performed, and a P value was provided. Prespecified subgroup analysis of PFS was also performed and HR estimated from Cox proportional hazard regression model; P value was generated using Fisher’s exact t test for binary end points and log-rank test for time to event end points.
RESULTS
Patients and Trial Treatment
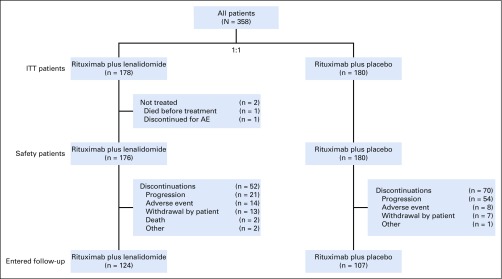
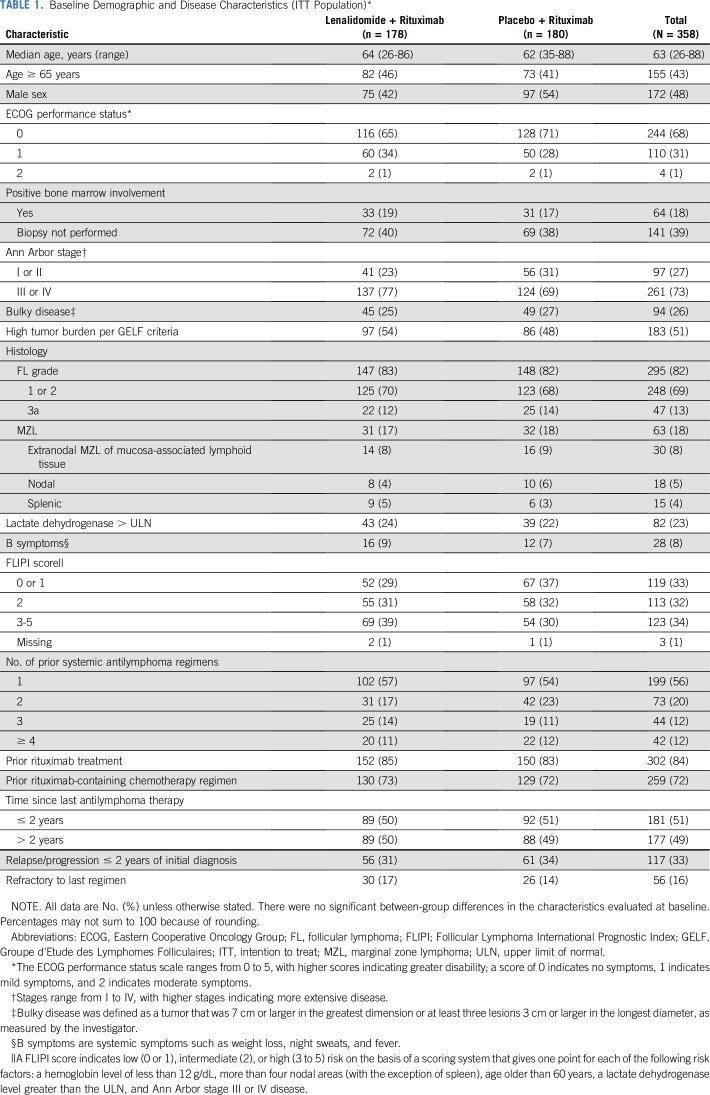
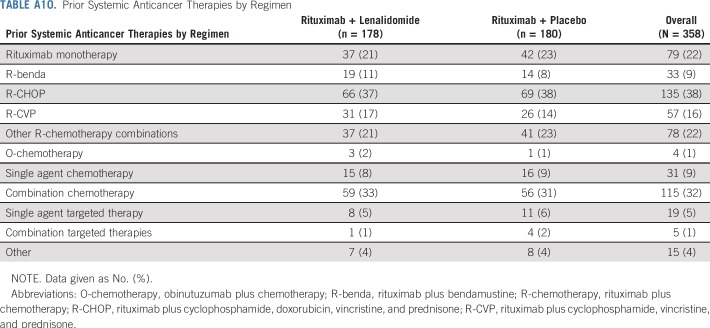
From February 13, 2014 through January 26, 2017, 358 patients enrolled from 97 centers in 15 countries were randomly assigned 1:1 to lenalidomide plus rituximab (n = 178) or placebo plus rituximab (n = 180; Fig 1). Baseline characteristics were similar (Table 1). Median age was 63 years (range, 26 to 88 years); 295 patients (82%) had FL, and 63 (18%) had MZL. Overall, 51% had high tumor burden per Groupe d’Etude des Lymphomes Folliculaires (GELF) criteria. The median number of prior therapies was one (range, one to 12), and 86 patients (24%) had three or more prior systemic treatments. Relapse or progression within 2 years of initial diagnosis had occurred in 117 patients (33%), and 56 (16%) were refractory to their last regimen. Four patients had been lost to follow-up.
FIG 1.
Lenalidomide plus rituximab and placebo plus rituximab group CONSORT diagram (flow of patients from enrollment to analysis). AE, adverse event; ITT, intention to treat.
TABLE 1.
Baseline Demographic and Disease Characteristics (ITT Population)*
In the safety population, the full treatment course was completed in 71% of patients with lenalidomide plus rituximab and 61% with placebo plus rituximab. Adverse events leading to dose interruptions were more common with lenalidomide plus rituximab than lenalidomide plus placebo (64% v 26%), reductions (26% v 3%), and discontinuations (9% v 5%). Neutropenia was the most common adverse event leading to reduction (18%) and interruption (39%) of lenalidomide. Dose modifications successfully addressed neutropenia, with only five patients (3%) discontinuing lenalidomide because of neutropenia. Disease progression led to treatment discontinuations in 21 patients (12%) and 54 patients (30%) in the lenalidomide plus rituximab and placebo plus rituximab groups, respectively. Type 1 error was not controlled for secondary and exploratory end points, and P values were descriptive in nature.
Efficacy
Primary end point.
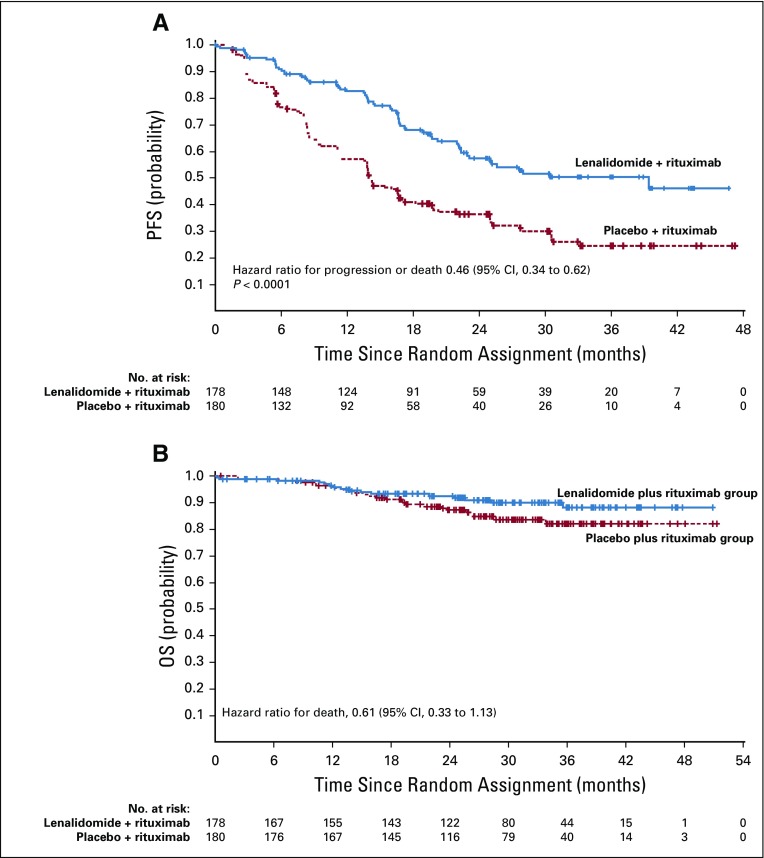
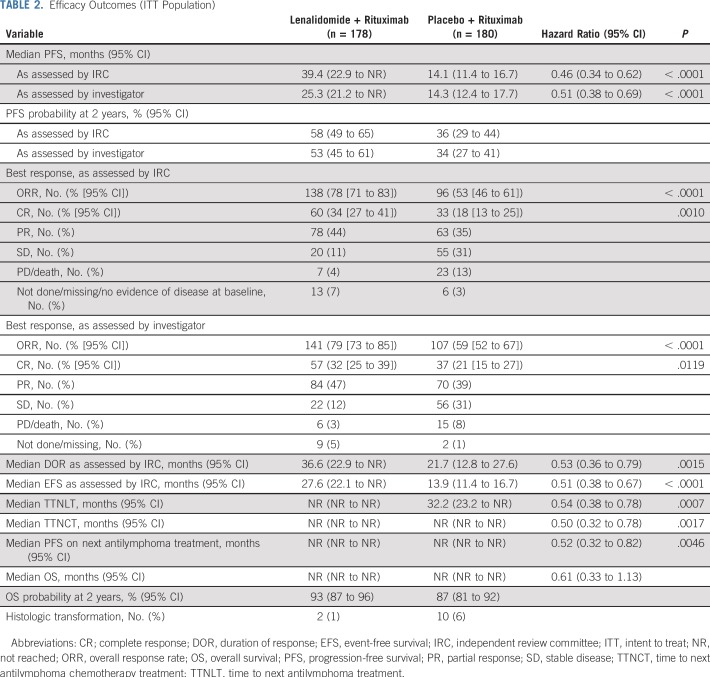
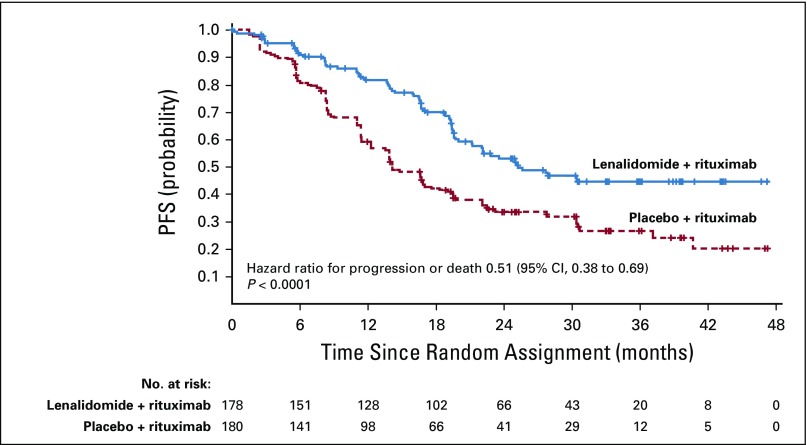
At the final analysis, median follow-up was 28.3 months, and 185 events total (progression or death) were assessed by IRC (200 events per investigator assessment) before censoring. The primary end point of PFS assessed by IRC was significantly superior in the lenalidomide plus rituximab group (HR, 0.46; 95% CI, 0.34 to 0.62; P < .001; Fig 2A; Table 2). Median PFS assessed by IRC was 39.4 months (95% CI, 22.9 months to not reached) with lenalidomide plus rituximab versus 14.1 months (95% CI, 11.4 to 16.7 months) with placebo plus rituximab. PFS assessed by investigator also showed superiority with lenalidomide plus rituximab versus placebo plus rituximab (HR, 0.51; 95% CI, 0.38 to 0.69; P < .0001; the median PFS was 25.3 months; 95% CI, 21.2 months to not reached v 14.3 months; 95% CI, 12.4 to 17.7 months; Appendix Fig A1, online only; Table 2). PFS probability at 2 years also favored lenalidomide plus rituximab (Table 2). Post hoc subgroup analyses for PFS on the basis of prior rituximab plus bendamustine or plus cyclophosphamide, doxorubicin, vincristine, and prednisone showed results consistent with those of the overall population (Appendix Table A2, online only).
FIG 2.
Progression-free survival (PFS) and overall survival (OS) as assessed by independent review committee in the intention-to-treat population: (A) progression-free survival; (B) overall survival.
TABLE 2.
Efficacy Outcomes (ITT Population)
Secondary and exploratory end points.
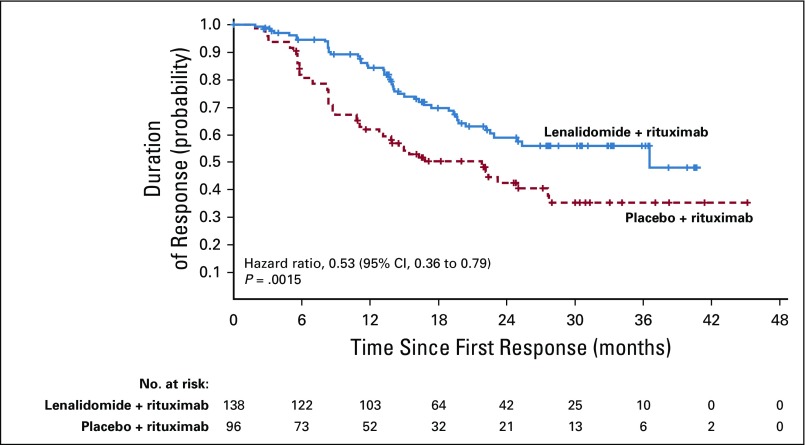
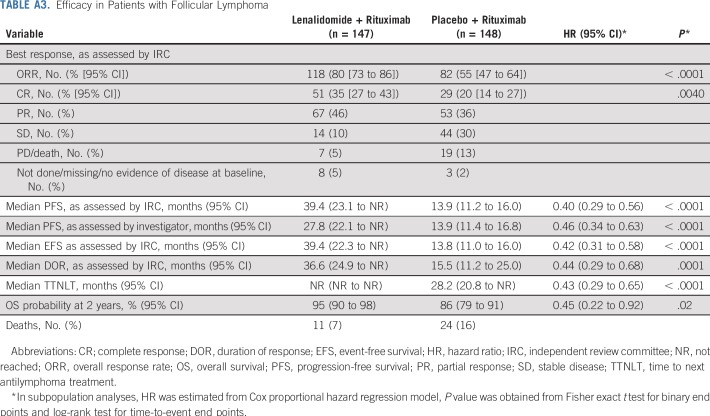
Among patients in the lenalidomide plus rituximab versus placebo plus rituximab groups, the rate of best overall response assessed by IRC was 78% (95% CI, 71% to 83%) versus 53% (95% CI, 46% to 61%; P < .0001), with 34% (95% CI, 27% to 41%) versus 18% (95% CI, 13% to 25%) of patients achieving CR (P = .001; Table 2), with similar investigator-assessed results. Other secondary and exploratory time-to-event end points assessed by IRC also showed superior results with lenalidomide plus rituximab—response duration (Appendix Fig A2, online only; Table 2), event-free survival (Appendix Fig A3, online only; Table 2), time to next antilymphoma treatment (Appendix Fig A4, online only; Table 2), time to next chemotherapy treatment (Appendix Fig A5, online only; Table 2), and PFS on next antilymphoma treatment (Appendix Fig A6, online only; Table 2).
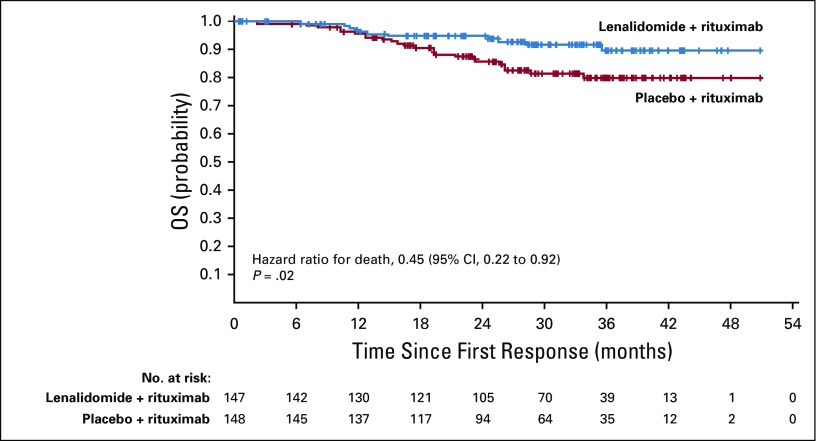
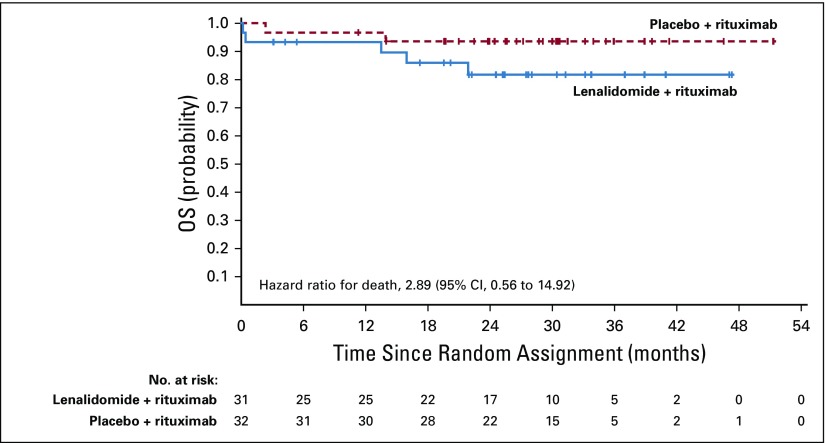
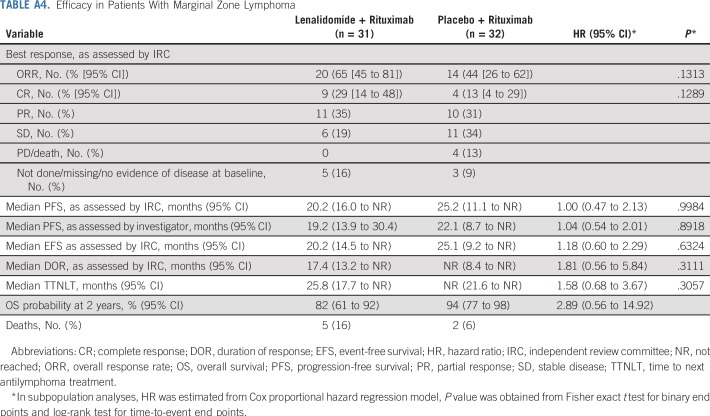
Overall survival results are maturing, with an HR of 0.61 (95% CI, 0.33 to 1.13; Fig 2B; Table 2). Numerically fewer deaths in treated patients have been observed with lenalidomide plus rituximab versus placebo plus rituximab (15 v 26), although the trial was not powered to detect OS differences. Estimated 2-year survival in the lenalidomide plus rituximab group was 93% (95% CI, 87% to 96%) versus 87% (95% CI, 81% to 92%) in the placebo plus rituximab group. Survival for FL and MZL subgroups are shown in Appendix Figures A7 and A8 (online only). In patients with FL, OS results favored lenalidomide plus rituximab (HR, 0.45; 95% CI, 0.22 to 0.92; P = .02; Appendix Table A3, online only), with 11 deaths with lenalidomide plus rituximab versus 24 with placebo plus rituximab. No difference was seen between groups in patients with MZL (HR, 2.89; 95% CI, 0.56 to 14.92), but with few events—five with lenalidomide plus rituximab versus two with placebo plus rituximab (Appendix Table A4, online only). Two patients with MZL in the lenalidomide plus rituximab arm died early after random assignment (3 days [did not receive any study treatment] and 13 days). Estimated 2-year survival probability in FL was 95% (95% CI, 90% to 98%) with lenalidomide plus rituximab and 86% (95% CI, 79% to 91%) with placebo plus rituximab (Appendix Table A3). Estimated 2-year survival in MZL was 82% (95% CI, 61% to 92%) with lenalidomide plus rituximab and 94% (95% CI, 77% to 98%) with placebo plus rituximab (Appendix Table A4).
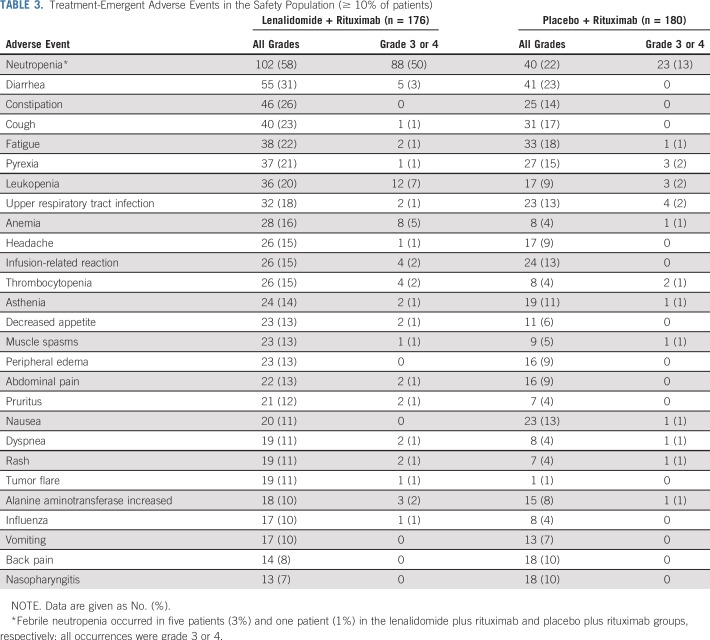
Histologic transformation occurred in two patients (incidence per 100 person-years: 0.5%) with lenalidomide plus rituximab and 10 patients (incidence per 100 person-years: 2.5%) with placebo plus rituximab (Table 3). After transformation, one patient in the lenalidomide plus rituximab group and six in the placebo plus rituximab group died.
TABLE 3.
Treatment-Emergent Adverse Events in the Safety Population (≥ 10% of patients)
Subgroup analyses.
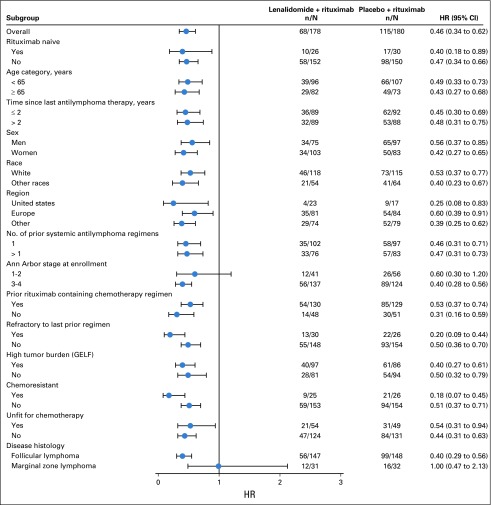
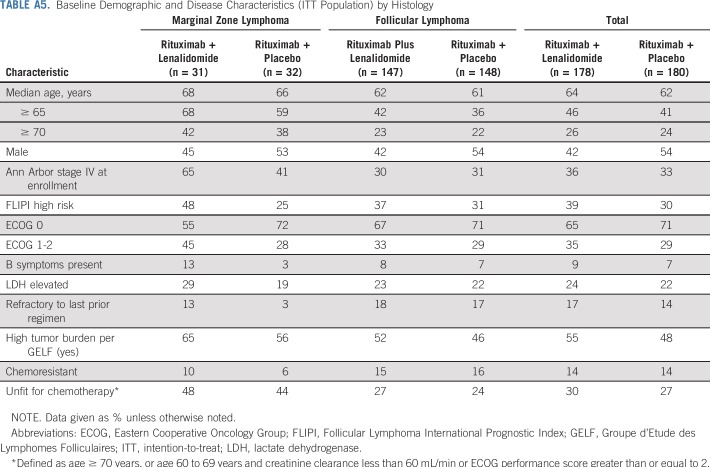
PFS improvements were consistent with those of the overall population in all prespecified subgroups except the MZL subgroup (Fig 3). In this subset (n = 63), PFS was not significantly different between the two groups, with an unstratified HR of 1.00 (95% CI, 0.47 to 2.13), and the wide confidence intervals imply the data are uninformative. PFS results in the MZL subgroup are difficult to interpret because of the small sample size and imbalance in baseline prognostic factors (Appendix Table A5, online only).
FIG 3.
Forest plot: subgroup analyses of progression-free survival. GELF, Groupe d’Etude des Lymphomes Folliculaires; HR, hazard ratio.
Safety
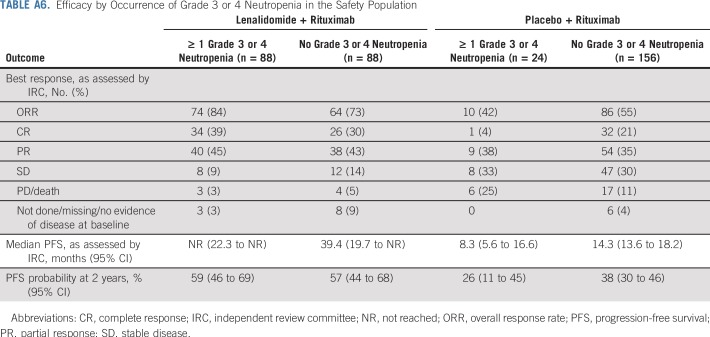
The safety population included 176 patients who received lenalidomide plus rituximab and 180 patients who received placebo plus rituximab. Of these, 174 patients who received lenalidomide plus rituximab (99%) and 173 who received placebo plus rituximab (96%) had any-grade adverse events within 28 days after last dose. Most common adverse events are in Table 3. Adverse events of any grade that occurred more frequently (≥ 10% difference) with lenalidomide plus rituximab versus placebo plus rituximab included neutropenia (58% v 22%), constipation (26% v 14%), leukopenia (20% v 9%), anemia (16% v 4%), thrombocytopenia (15% v 4%), and tumor flare (11% v 1%), respectively. More patients who received lenalidomide plus rituximab (69%) had at least one grade 3 or 4 adverse event compared with placebo plus rituximab (32%). The increased rates of grade 3 or 4 adverse events with lenalidomide plus rituximab were attributable primarily to increased grade 3 or 4 neutropenia (50% v 13%) and leukopenia (7% v 2%). No other grade 3 or 4 adverse events occurred in 5% or more patients in either group. Febrile neutropenia occurred in 3% of patients with lenalidomide plus rituximab versus 1% with placebo plus rituximab. Growth factors were administered to 36% of the lenalidomide plus rituximab group versus 12% in the placebo plus rituximab group. Neutropenia was primarily managed through dose interruptions and/or reductions and growth-factor support. Only five patients had neutropenia leading to lenalidomide discontinuation. All incidences of grade 3 or 4 neutropenia in the lenalidomide plus rituximab group recovered to grade 1 or less, with a median time of 9.0 days. Efficacy was similar regardless of occurrence of grade 3 or 4 neutropenia in the lenalidomide plus rituximab group on the basis of post hoc analysis (Appendix Table A6, online only).
Fatal adverse events (grade 5) occurred in four patients (1%), two in each group (lenalidomide plus rituximab: arrhythmia and cardiopulmonary failure; placebo plus rituximab: general physical health deterioration and pneumonia). Among treated patients, 15 deaths (9%) occurred in the lenalidomide plus rituximab group (five deaths attributed to lymphoma) versus 26 (14%) with placebo plus rituximab (18 deaths attributed to lymphoma).
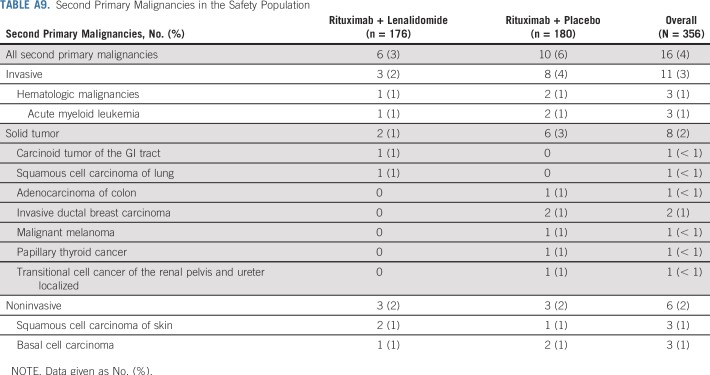
With lenalidomide plus rituximab, 45 patients (26%) had at least one serious adverse event versus 25 (14%) with placebo plus rituximab (Appendix Table A7, online only). Pneumonia was the most common serious adverse event, occurring in five patients (3%) in each group. Deep vein thrombosis occurred in three patients (2%) in the lenalidomide plus rituximab and one patient (1%) in the placebo plus rituximab groups; only one incidence (lenalidomide plus rituximab group) was a serious adverse event. Antiplatelet and anticoagulant medication use is listed in Appendix Table A8 (online only). Second primary cancers were reported in six patients (3%) in the lenalidomide plus rituximab group and 10 patients (6%) with placebo plus rituximab (Appendix Table A9, online only). Two patients died of second primary cancers (both placebo plus rituximab arm).
DISCUSSION
The AUGMENT study met its primary end point; lenalidomide plus rituximab demonstrated statistically significant and clinically relevant superiority in PFS over placebo plus rituximab in patients with relapsed or refractory indolent FL/MZL who are considered appropriate for rituximab monotherapy. Lenalidomide plus rituximab, administered over 1 year, reduced risk of progression by 54% (HR, 0.46; 95% CI, 0.34 to 0.62; P < .0001) and increased median PFS by more than 2 years compared with rituximab monotherapy. Efficacy of the combination was also reflected by improvements in secondary and exploratory end points—response rates, response duration, time to next treatment, and time to next chemotherapy. Furthermore, PFS improved in all prespecified subgroups (prior rituximab, age, time since last therapy, sex, race, region, number of prior antilymphoma regimens, stage, refractory to last regimen, high tumor burden, chemoresistant, and unfit for chemotherapy [defined as age ≥ 70 years, or age 60 to 69 years and creatinine clearance < 60 mL/min or Eastern Cooperative Oncology Group performance status ≥ 2]) except for the MZL subgroup. No difference in PFS was observed between treatment groups in the MZL subgroup. Although this could relate to lack of effect in this subset, the small number of patients and imbalance in prognostic factors between arms limit interpretation for this histology.
One limitation of this study was the differences in median PFS seen when assessed by IRC (39.4 months) compared with investigator assessment (25.3 months); however, HRs and P values were similar between assessments, indicating consistency in PFS results between IRC and investigator assessments. Another limitation was that longer treatment duration occurred in the lenalidomide plus rituximab group; however, this likely did not influence efficacy results. Notably, separation of PFS Kaplan-Meier curves began early, and PFS benefits and durability of responses persisted beyond 1 year of study treatment duration and were consistently observed throughout the study follow-up period. In addition, schedule of rituximab (eight doses given over 5- to 28-day cycles) is not likely to have influenced results; several studies showed benefit of extended treatment with rituximab (ie, beyond standard 4 weekly infusions) in a manner that is independent of the number or schedule of extended rituximab dosing (ie, four or more doses every 1, 2, 3, or 6 months).18,32-35 Importantly, the control group performed as expected on the basis of historical rituximab data, with results similar to the 11-month estimate used in the statistical plan.18,19,36
Current treatment options for recurrent FL include rituximab monotherapy, bendamustine, or other chemotherapy alone or with obinutuzumab37 or rituximab.38 In addition, the phosphatidylinositol 3-kinase inhibitors idelalisib39 and copanlisib are approved for patients who are more heavily pretreated than the patients in the AUGMENT study.40 For many patients, the efficacy/toxicity tradeoffs of rituximab versus chemotherapy-based or kinase inhibitor regimens are major issues. There is clear rationale for adding lenalidomide to rituximab in relapsed patients with FL,26 and in a previous study in recurrent FL, lenalidomide plus rituximab was superior to lenalidomide alone.23 Our data demonstrate that lenalidomide plus rituximab offers clinically meaningful efficacy advantages over single-agent rituximab in the context of the safety profile. Adverse events were more common in the lenalidomide plus rituximab group, largely due to higher rates of grade 3 or 4 neutropenia, which was successfully managed in most patients through dose modifications and growth factors. Neutropenia was predictable and manageable; the modest increase in infections and cutaneous reactions must be considered in light of the significantly greater efficacy of the lenalidomide plus rituximab combination. In fact, more patients in the lenalidomide plus rituximab group completed therapy than those in the placebo plus rituximab group (71% v 61%) because of a lower rate of progression. The magnitude of efficacy differences between the two treatments is clinically meaningful and suggests that lenalidomide plus rituximab should replace rituximab monotherapy as a standard of care for patients with relapsed or refractory indolent NHL.
ACKNOWLEDGMENT
We thank the patients, families, caregivers, and investigators who participated in the AUGMENT clinical trial; the numerous research and trial groups who participated; the international board of expert pathologists who provided histopathological review (Maria Calaminici, MD, Christiane Copie-Bergman, MD); the independent expert hematologists who validated efficacy data for clinical assessments and imaging review; the scientific steering committee, including John P. Leonard, MD, John G. Gribben, MD, Marek Trneny, MD, Koji Izutsu, MD, and Phillip Scheinberg, MD; the data monitoring committee that served as an independent expert advisory group to evaluate safety and efficacy data during the trial, including Umberto Vitolo, MD, Chair, Armando López-Guillermo, MD, Owen O’Connor, MD, Michael Williams, MD, Stefan Suciu, PhD, Statistician, and Benjamin Levine, PhD; and Julie Kern, PhD, CMPP of Bio Connections (funded by Celgene Corporation) for editorial support in the preparation of an earlier version of the manuscript.
Appendix
Methods
The hypothesized median progression-free survival (PFS) for lenalidomide plus rituximab was 17.6 months, on the basis of a reasonable risk reduction (hazard ratio [HR] = 0.0625) from a median PFS expected of rituximab monotherapy (11 months). In single-arm studies of lenalidomide plus rituximab in previously treated settings, the median PFS has ranged from approximately 12 months to 24 months, making 17.6 months a reasonable assumption. We hypothesized that median PFS for placebo plus rituximab would be 11 months, on the basis of previous results of rituximab monotherapy.
The choice of the rituximab schedule took into consideration studies showing that extended dosing of rituximab with four additional infusions (total eight infusions) further improves efficacy with acceptable toxicity, which has led to the approval of four or eight doses of rituximab in certain countries, including the United States. The totality of published studies showed that extended dosing (ie, additional doses of rituximab beyond standard four weekly infusions) further improves benefit in a manner that is independent of the number or schedule of extended rituximab dosing (ie, four or more doses; every 1, 2, 3, or 6 months; Hainsworth JD, et al: J Clin Oncol 23:1088-1095, 2005; Piro LD, et al: Ann Oncol 10:655-661, 1999).18,32,33 Lenalidomide 20 mg was chosen based on published studies indicating that the combination of lenalidomide 20 mg with rituximab is well tolerated and active in relapsed/refractory indolent non-Hodgkin lymphoma.23,35 In a previous study, 25 mg lenalidomide in combination with rituximab was not tolerated because of grade 3 tumor lysis syndrome in two out of four patients.23
Key inclusion criteria included: age ≥ 18 years; histologically confirmed marginal zone lymphoma or grade 1, 2, or 3a follicular lymphoma (FL; CD20+; histology was retrospectively reviewed by an independent panel of expert hematopathologists); previous treatment with one or more prior systemic chemotherapy, immunotherapy, or chemoimmunotherapy and two or more previous doses of rituximab; documented relapsed, refractory, or progressive disease after prior systemic therapy, and not rituximab refractory (refractory defined as no response or PD < 6 months after last rituximab dose); Eastern Cooperative Oncology Group performance status less than or equal to 2; and bidimensionally measurable disease with at least one nodal lesion greater than 1.5 cm in diameter or at least one extranodal lesion greater than 1.0 cm in both long and short diameters. Key exclusion criteria included: histology other than FL or marginal zone lymphoma or clinical evidence of transformed lymphoma, grade 3b FL, systemic therapy within 28 days before cycle 1 day 1 dosing, prior use of lenalidomide, neuropathy grade greater than one, presence or history of CNS involvement by lymphoma, or unwillingness to take venous thromboembolism prophylaxis if considered at risk for a thromboembolic event.
Dose reductions and interruptions of lenalidomide were planned for management of toxic effects associated with the drug. No more than one dose reduction from one cycle to the next was permitted, and no dose escalation was permitted at any time unless as specified. If lenalidomide or placebo was discontinued, rituximab treatment could continue as per protocol. The dose of rituximab could not be reduced; if rituximab was discontinued because of toxicity, lenalidomide or placebo was continued as per protocol. Lenalidomide was held and restarted at the next lower dose (5-mg increments) if the event resolved to a lower grade or discontinued as specified in the protocol. For all grade 4 neutropenia or grade 3 neutropenia that was sustained for 7 or more days or associated with fever (38.5°C), complete blood counts were monitored every 7 days, growth factor use was permitted, and lenalidomide was restarted if resolved to grade 2 or less. For thrombocytopenia grade 3 or greater (platelets < 50,000 cells/mm3), complete blood counts were monitored every 7 days, and lenalidomide was restarted if resolved to grade 2 or less. For grade 3 nondesquamating or nonblistering rash, lenalidomide was restarted if resolved to grade 1 or less. For grade 4 rash or any-grade desquamating rash, lenalidomide was discontinued. For grade 3 or greater tumor flare reaction, investigators could initiate therapy with nonsteroidal anti-inflammatory drugs, limited-duration corticosteroids, and/or narcotic therapy, and lenalidomide was restarted if resolved to grade 1 or less. For grade 2 allergic reaction or hypersensitivity, lenalidomide was restarted if resolved to grade 1 or less. For grade 3 or greater allergic reaction or hypersensitivity, lenalidomide was discontinued. For grade 3 or greater constipation, lenalidomide was restarted if resolved to grade 2 or less. For grade 3 or greater vascular access complication, anticoagulation was started and lenalidomide was restarted at investigator’s discretion. For grade 3 peripheral neuropathy, lenalidomide was restarted if resolved to grade 1 or less. For grade 4 peripheral neuropathy, lenalidomide was discontinued. For incidences of ALT or AST elevation to grade 3 or greater (> 5 × upper limit of normal) or total bilirubin grade 2 or greater (> 1.5 × upper limit of normal), ALT, AST, and total bilirubin were monitored weekly, and lenalidomide was resumed at the same dose if levels returned to baseline in 14 days or less or resumed at next lower dose when returned to baseline if recovery was at greater than 14 days. In the instance of clinical tumor lysis syndrome grade 2 or greater, lenalidomide was held and restarted when resolved to grade 0.
Frequency of follow-up visits was based on disease status. For patients who completed treatment or discontinued treatment for reasons other than PD or relapse, overall survival and second malignancies were assessed every 6 months, and follow-up computed tomography or magnetic resonance imaging scans occurred every year. For patients who discontinued treatment because of PD or relapse, overall survival, subsequent antilymphoma therapies, and second malignancies were assessed every 6 months.
An interim futility analysis was planned when approximately 96 patients had developed PD per independent review committee (IRC) assessment or died (ie, PFS) during the study. The purpose of the interim analysis was to stop the trial in case of futility.
At the time of the interim analysis (data cutoff of March 20, 2017), approximately 96 IRC-assessed events were expected. However, after the IRC had completed review, 137 events were identified. The futility boundary was adjusted based on the actual number of events.
A data monitoring committee (DMC) meeting was held on May 30, 2017, in which DMC members reviewed unblinded efficacy and safety data. The DMC concluded that the results from the primary end points, PFS per IRC assessment under US Food and Drug Administration censoring rule, remained within the protocol-directed futility boundaries, and the adverse event profile did not raise any unexpected safety concerns. Therefore, the DMC recommended that the AUGMENT study continue as planned.
Results
The primary analysis of PFS was based on a total of 183 events assessed by IRC after censoring per rule suggested in US Food and Drug Administration guidance (185 events before censoring; 200 events were assessed by investigator, 199 events after censoring). The proportional hazards assumption was checked by introducing a constructed time-dependent variable, that is, add to the model interaction term for treatment and time. The effect of interaction term is not significant (data not shown). This suggests that the assumption of proportional hazards is not violated. Prior systemic anticancer therapies by regimen for both arms are listed in Appendix Table A10 (online only).
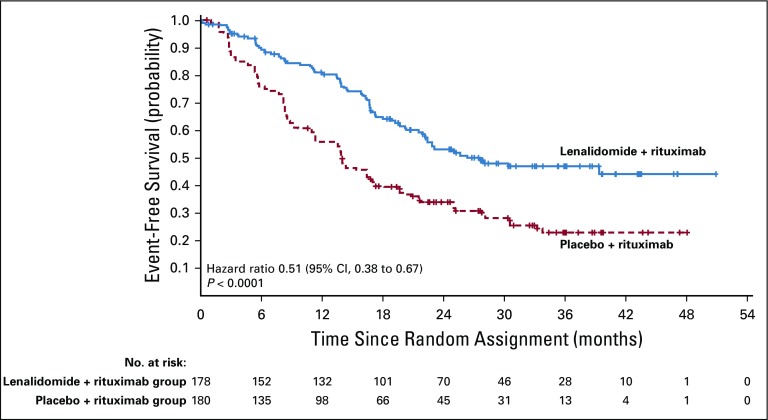
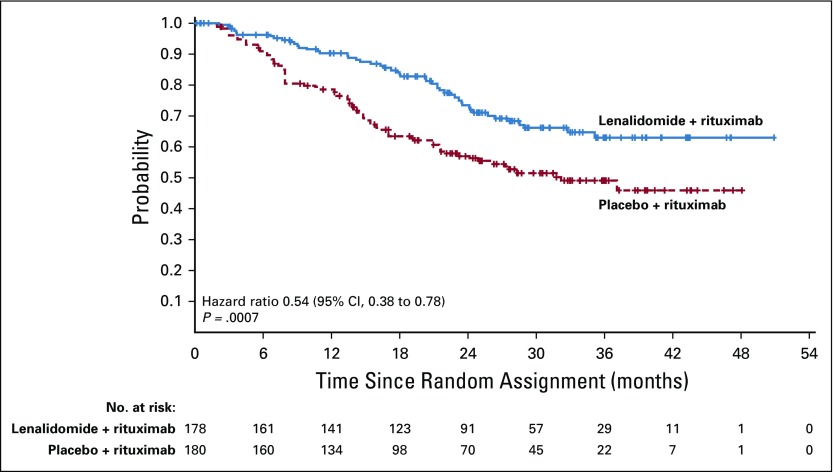
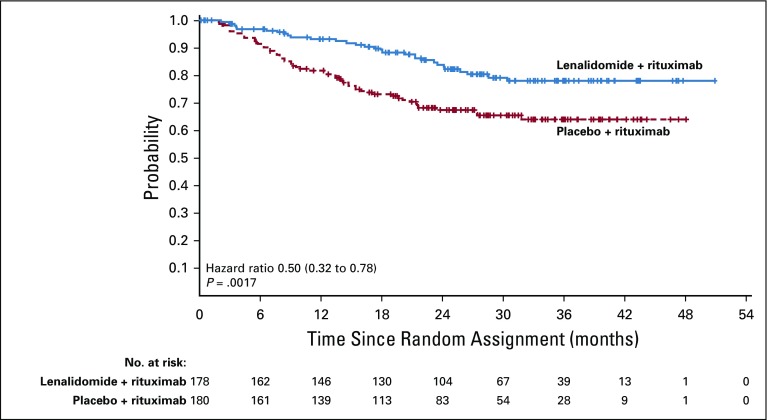
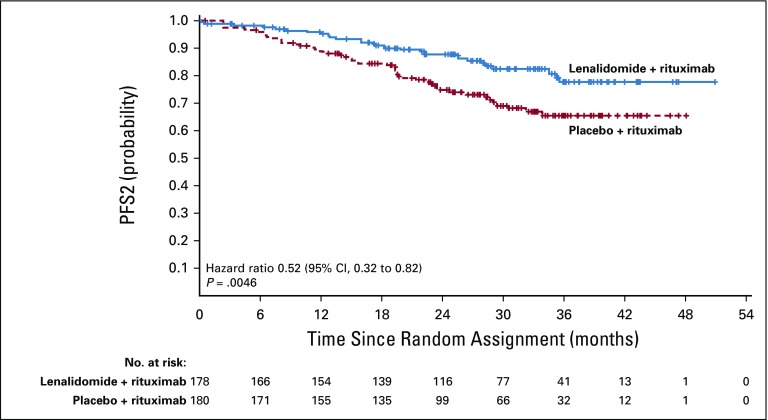
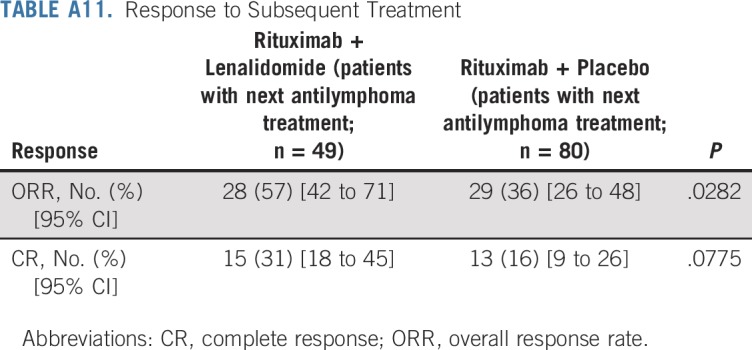
Duration of response was superior for lenalidomide plus rituximab (HR, 0.53; 95% CI, 0.36 to 0.79; P = .015). Median duration of response was 36.6 months (95% CI, 22.9 months to not reached) versus 21.7 months (95% CI, 12.8 to 27.6 months; Appendix Fig A2, online only). Event-free survival was significantly improved for lenalidomide plus rituximab compared with placebo plus rituximab, with HR of 0.51 (95% CI, 0.38 to 0.67; P < .0001). Median event-free survival was 27.6 months (95% CI, 22.1 months to not reached) versus 13.9 months (Appendix Fig A3, online only). Time to next lymphoma treatment was significantly improved for lenalidomide plus rituximab compared with placebo plus rituximab, with HR of 0.54 (95% CI, 0.38 to 0.78; P = .0007). Median time to next lymphoma treatment was not reached versus 32.2 months (95% CI, 23.2 months to not reached; P = .0007; Appendix Fig A4, online only). Time to next antilymphoma chemotherapy treatment was significantly improved for lenalidomide plus rituximab compared with placebo plus rituximab, with HR of 0.50 (95% CI, 0.32 to 0.78; P < .0017). Median time to next antilymphoma chemotherapy treatment was not reached in either group (Appendix Fig A5, online only). PFS on next antilymphoma treatment (PFS2) was significantly improved for lenalidomide plus rituximab compared with placebo plus rituximab, with HR of 0.52 (95% CI, 0.32 to 0.82; P < .0046). Post hoc analysis has shown that response to subsequent treatment was better with lenalidomide plus rituximab compared with placebo plus rituximab (Appendix Table A11, online only). Median PFS2 was not reached in either group (Appendix Fig A6, online only).
FIG A1.
Progression-free survival (PFS) as assessed by investigator in the intention-to-treat population.
FIG A2.
Duration of response as assessed by independent review committee in the intention-to-treat population.
FIG A3.
Event-free survival as assessed by independent review committee in the intention-to-treat population.
FIG A4.
Time to next antilymphoma treatment in the intention-to-treat population.
FIG A5.
Time to next antilymphoma chemotherapy in the intention-to-treat population.
FIG A6.
Progression-free survival incorporating next antilymphoma treatment (PFS2) in the intention-to-treat population.
FIG A7.
Overall survival (OS) in patients with follicular lymphoma in the intention-to-treat population.
FIG A8.
Overall survival (OS) in patients with marginal zone lymphoma in the intention-to-treat population.
TABLE A1.
List of AUGMENT Trial Investigators
TABLE A2.
Post hoc Subgroup Analyses for Progression-Free Survival on the Basis of Prior Treatment as Assessed by IRC
TABLE A3.
Efficacy in Patients with Follicular Lymphoma
TABLE A4.
Efficacy in Patients With Marginal Zone Lymphoma
TABLE A5.
Baseline Demographic and Disease Characteristics (ITT Population) by Histology
TABLE A6.
Efficacy by Occurrence of Grade 3 or 4 Neutropenia in the Safety Population
TABLE A7.
Serious Adverse Events Occurring in Three or More Patients in Either Group in the Safety Population
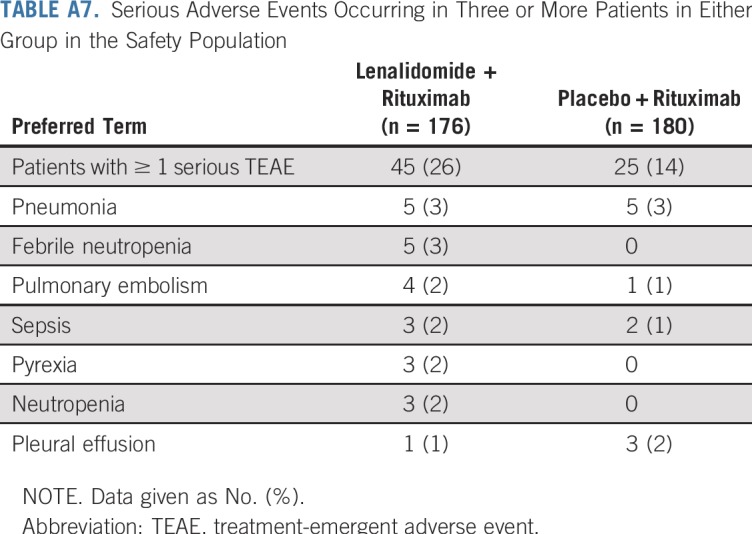
TABLE A8.
On-Study Use of Antiplatelet or Anticoagulant Concomitant Medications in the Safety Population
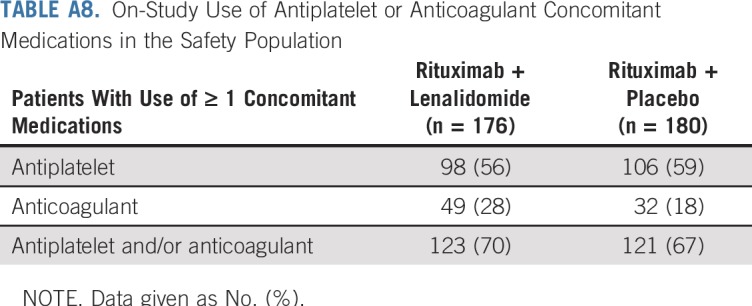
TABLE A9.
Second Primary Malignancies in the Safety Population
TABLE A10.
Prior Systemic Anticancer Therapies by Regimen
TABLE A11.
Response to Subsequent Treatment
Footnotes
Clinical trial information: NCT01938001, EudraCT 2013-001245-14.
Presented in part at the 60th annual meeting of the American Society of Hematology, San Diego, CA, December 1-4, 2018.
Supported by Celgene Corporation, Summit, NJ.
See accompanying Editorial on page 1151
AUTHOR CONTRIBUTIONS
Administrative support: Jun Zhu
Conception and design: John P. Leonard, Marek Trneny, Nathan H. Fowler, Xiaonan Hong, José Cabeçadas, Pierre Fustier, John G. Gribben
Provision of study material or patients: John P. Leonard, Jun Zhu, Fritz Offner, Grzegorz S. Nowakowski, Francesca Re, Laura Maria Fogliatto
Collection and assembly of data: John P. Leonard, Marek Trneny, Koji Izutsu, Nathan H. Fowler, Xiaonan Hong, Jun Zhu, Huilai Zhang, Fritz Offner, Adriana Scheliga, Grzegorz S. Nowakowski, Antonio Pinto, Francesca Re, Ian W. Flinn, Claudia Moreira, José Cabeçadas, David Liu, Stacey Kalambakas, Pierre Fustier
Data analysis and interpretation: John P. Leonard, Marek Trneny, Koji Izutsu, Nathan H. Fowler, Xiaonan Hong, Fritz Offner, Grzegorz S. Nowakowski, Laura Maria Fogliatto, Phillip Scheinberg, Ian W. Flinn, José Cabeçadas, David Liu, Stacey Kalambakas, Pierre Fustier, Chengqing Wu, John G. Gribben
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
AUGMENT: A Phase III Study of Lenalidomide Plus Rituximab Versus Placebo Plus Rituximab in Relapsed or Refractory Indolent Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
John P. Leonard
Consulting or Advisory Role: Celgene, Biotest, Sunesis Pharmaceuticals, Bristol-Myers Squibb, Gilead Sciences, Epizyme, Pfizer, Bayer, Genentech, ADC Therapeutics, MEI Pharma, AstraZeneca, Novartis, Merck, Sutter Medical Group, MorphoSys, BeiGene, Nordic Nanovector, United Therapeutics, Karyopharm Therapeutics, Sandoz
Research Funding: Celgene (Inst), Alliance for Clinical Trials in Oncology (Inst), Takeda (Inst), Pfizer (Inst), National Cancer Institute (Inst)
Travel, Accommodations, Expenses: BeiGene
Marek Trneny
Honoraria: Janssen, Gilead Sciences, Takeda, Bristol-Myers Squibb, Amgen, AbbVie, Roche, MorphoSys, Incyte
Consulting or Advisory Role: Takeda, Bristol-Myers Squibb, Incyte, AbbVie, Amgen, Roche, Gilead Sciences, Janssen, Celgene, MorphoSys
Travel, Accommodations, Expenses: Gilead Sciences, Takeda, Bristol-Myers Squibb, Roche, Janssen, Abbvie
Koji Izutsu
Honoraria: Takeda, Chugai Pharma, Eisai, Kyowa Hakko Kirin, Janssen, Ono Pharmaceutical, Gilead Sciences, AbbVie, Novartis, MSD, Otsuka Pharmaceutical, Dainippon Sumitomo Pharma, Nihon Medi-Physics, Bayer, Bristol-Myers Squibb
Consulting or Advisory Role: Bayer, Celgene, AstraZeneca
Research Funding: Eisai, Chugai Pharma
Nathan H. Fowler
Consulting or Advisory Role: Genentech, TG Therapeutics, Verastem, Bayer, Celgene, Novartis
Research Funding: Roche, Celgene, Gilead Sciences, TG Therapeutics, Novartis, AbbVie, BeiGene
Grzegorz S. Nowakowski
Consulting or Advisory Role: Celgene (Inst), MorphoSys (Inst), Genentech (Inst)
Research Funding: Celgene (Inst), NanoString Technologies (Inst), MorphoSys (Inst)
Antonio Pinto
Honoraria: Genentech, Merck Sharp & Dohme, Bristol-Myers Squibb, Celgene, Servier
Consulting or Advisory Role: Servier, Genentech
Patents, Royalties, Other Intellectual Property: Mundipharma EDO
Phillip Scheinberg
Honoraria: Novartis
Consulting or Advisory Role: Novartis, AbbVie, Alexion Pharmaceuticals, Janssen, Celgene
Speakers' Bureau: Novartis, Pfizer
Ian W. Flinn
Consulting or Advisory Role: AbbVie (Inst), Seattle Genetics (Inst), TG Therapeutics (Inst), Verastem Oncology (Inst)
Research Funding: Acerta Pharma (Inst), Agios Pharmaceuticals (Inst), Calithera Biosciences (Inst), Celgene (Inst), Constellation Pharmaceuticals (Inst), Genentech (Inst), Gilead Sciences (Inst), Incyte (Inst), Infinity Pharmaceuticals (Inst), Janssen (Inst), Karyopharm Therapeutics (Inst), Kite Pharma (Inst), Novartis (Inst), Pharmacyclics (Inst), Portola Pharmaceuticals (Inst), Roche (Inst), TG Therapeutics (Inst), Trillium Therapeutics (Inst), AbbVie (Inst), ArQule (Inst), BeiGene (Inst), Curis (Inst), FORMA Therapeutics (Inst), Forty Seven (Inst), Merck (Inst), Pfizer (Inst), Takeda (Inst), Teva (Inst), Verastem (Inst), Gilead Sciences (Inst), AstraZeneca (Inst), Juno Therapeutics (Inst), Unum Therapeutics (Inst), MorphoSys (Inst), AbbVie (Inst)
José Cabeçadas
Honoraria: Novartis, AstraZeneca
Consulting or Advisory Role: Roche Diagnostics
Research Funding: Gilead Sciences (Inst)
Patents, Royalties, Other Intellectual Property: InVisoScribe Technologies: member of the board of a scientific group receiving royalties
Travel, Accommodations, Expenses: Novartis, Takeda, Gilead Sciences
David Liu
Employment: Celgene, Bristol-Myers Squibb (I)
Leadership: 3D Medicines
Stock and Other Ownership Interests: Celgene, 3D Medicines, Bristol-Myers Squibb (I)
Patents, Royalties, Other Intellectual Property: Receiving royalties from patents filed more than 15 years ago with Massachusetts Institute of Technology
Stacey Kalambakas
Employment: Celgene
Stock and Other Ownership Interests: Celgene, Novartis
Pierre Fustier
Employment: Celgene
Stock and Other Ownership Interests: Celgene
Travel, Accommodations, Expenses: Celgene
Chengqing Wu
Employment: Celgene
Stock and Other Ownership Interests: Celgene
John G. Gribben
Honoraria: AbbVie, Acerta Pharma/AstraZeneca, Celgene, Janssen, Gilead Sciences, Genentech, TG Therapeutics
Consulting or Advisory Role: AbbVie, Acerta Pharma/AstraZeneca, Celgene, Janssen
Research Funding: Acerta Pharma/AstraZeneca, Janssen, Celgene
No other potential conflicts of interest were reported.
REFERENCES
- 1.Swerdlow SH CE, Harris NL, Jafie ES, et al. World Health Organization Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon France: IARC Press; 2017. [Google Scholar]
- 2.Casulo C, Burack WR, Friedberg JW. Transformed follicular non-Hodgkin lymphoma. Blood. 2015;125:40–47. doi: 10.1182/blood-2014-04-516815. [DOI] [PubMed] [Google Scholar]
- 3.Johnson PW, Rohatiner AZ, Whelan JS, et al. Patterns of survival in patients with recurrent follicular lymphoma: A 20-year study from a single center. J Clin Oncol. 1995;13:140–147. doi: 10.1200/JCO.1995.13.1.140. [DOI] [PubMed] [Google Scholar]
- 4.Kahl BS, Hong F, Williams ME, et al. Rituximab extended schedule or re-treatment trial for low-tumor burden follicular lymphoma: Eastern Cooperative Oncology Group protocol e4402. J Clin Oncol. 2014;32:3096–3102. doi: 10.1200/JCO.2014.56.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 6.Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66:443–459. doi: 10.3322/caac.21357. [DOI] [PubMed] [Google Scholar]
- 7.Dreyling M, Thieblemont C, Gallamini A, et al. ESMO Consensus conferences: Guidelines on malignant lymphoma. Part 2: Marginal zone lymphoma, mantle cell lymphoma, peripheral T-cell lymphoma. Ann Oncol. 2013;24:857–877. doi: 10.1093/annonc/mds643. [DOI] [PubMed] [Google Scholar]
- 8.Izutsu K. Treatment of follicular lymphoma. J Clin Exp Hematop. 2014;54:31–37. doi: 10.3960/jslrt.54.31. [DOI] [PubMed] [Google Scholar]
- 9.Gandhi AK, Kang J, Havens CG, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.) Br J Haematol. 2014;164:811–821. doi: 10.1111/bjh.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. doi: 10.1038/leu.2012.119. Lopez-Girona A, Mendy D, Ito T, et al: Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 26:2326-2335, 2012 [Erratum: Leukemia 26:2445, 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramsay AG, Clear AJ, Kelly G, et al. Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: Implications for the tumor microenvironment and immunotherapy. Blood. 2009;114:4713–4720. doi: 10.1182/blood-2009-04-217687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler NH, Davis RE, Rawal S, et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: An open-label, phase 2 trial. Lancet Oncol. 2014;15:1311–1318. doi: 10.1016/S1470-2045(14)70455-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu L, Adams M, Carter T, et al. Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14:4650–4657. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez-Ilizaliturri FJ, Reddy N, Holkova B, et al. Immunomodulatory drug CC-5013 or CC-4047 and rituximab enhance antitumor activity in a severe combined immunodeficient mouse lymphoma model. Clin Cancer Res. 2005;11:5984–5992. doi: 10.1158/1078-0432.CCR-05-0577. [DOI] [PubMed] [Google Scholar]
- 15.Lagrue K, Carisey A, Morgan DJ, et al. Lenalidomide augments actin remodeling and lowers NK-cell activation thresholds. Blood. 2015;126:50–60. doi: 10.1182/blood-2015-01-625004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, et al. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol. 2008;140:36–45. doi: 10.1111/j.1365-2141.2007.06841.x. [DOI] [PubMed] [Google Scholar]
- 17.Smith SM. Dissecting follicular lymphoma: High versus low risk. Hematology (Am Soc Hematol Educ Program) 2013;2013:561–567. doi: 10.1182/asheducation-2013.1.561. [DOI] [PubMed] [Google Scholar]
- 18.Coiffier B, Osmanov EA, Hong X, et al. Bortezomib plus rituximab versus rituximab alone in patients with relapsed, rituximab-naive or rituximab-sensitive, follicular lymphoma: A randomised phase 3 trial. Lancet Oncol. 2011;12:773–784. doi: 10.1016/S1470-2045(11)70150-4. [DOI] [PubMed] [Google Scholar]
- 19.Davis TA, Grillo-López AJ, White CA, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin’s lymphoma: Safety and efficacy of re-treatment. J Clin Oncol. 2000;18:3135–3143. doi: 10.1200/JCO.2000.18.17.3135. [DOI] [PubMed] [Google Scholar]
- 20.Link BK, Miller TP, Byrtek M, et al. Second-line therapy in follicular lymphoma in the United States: Report of NLCS observational study. J Clin Oncol. 2011;29(15_suppl; abstr 8049) [Google Scholar]
- 21.Onukwugha E, Nagarajan M, Albarmawi H, et al. Treatment patterns among elderly follicular lymphoma patients diagnosed between 2000 and 2011: An analysis of linked SEER-Medicare data. J Clin Oncol. 2017;35(15_suppl; abstr 7563) [Google Scholar]
- 22.Sortais C, Lok A, Gastinne T, et al. Progression of disease within 2 years (POD24) is a clinically significant endpoint to identify follicular lymphoma patients with high risk of death. Ann Oncol. 2018;29(suppl 8):359–371. [Google Scholar]
- 23.Leonard JP, Jung SH, Johnson J, et al. Randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma: CALGB 50401 (Alliance) J Clin Oncol. 2015;33:3635–3640. doi: 10.1200/JCO.2014.59.9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chong EA, Ahmadi T, Aqui NA, et al. Combination of lenalidomide and rituximab overcomes rituximab resistance in patients with indolent B-cell and mantle cell lymphomas. Clin Cancer Res. 2015;21:1835–1842. doi: 10.1158/1078-0432.CCR-14-2221. [DOI] [PubMed] [Google Scholar]
- 25.Tuscano JM, Dutia M, Chee K, et al. Lenalidomide plus rituximab can produce durable clinical responses in patients with relapsed or refractory, indolent non-Hodgkin lymphoma. Br J Haematol. 2014;165:375–381. doi: 10.1111/bjh.12755. [DOI] [PubMed] [Google Scholar]
- 26.Morschhauser F, Fowler NH, Feugier P, et al. Rituximab plus lenalidomide in advanced untreated follicular lymphoma. N Engl J Med. 2018;379:934–947. doi: 10.1056/NEJMoa1805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crawford J, Caserta C, Roila F. Hematopoietic growth factors: ESMO Clinical Practice Guidelines for the applications. Ann Oncol. 2010;21(suppl 5):v248–v251. doi: 10.1093/annonc/mdq195. [DOI] [PubMed] [Google Scholar]
- 28.Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33:3199–3212. doi: 10.1200/JCO.2015.62.3488. [DOI] [PubMed] [Google Scholar]
- 29.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 30.Cairo MS, Bishop M. Tumour lysis syndrome: New therapeutic strategies and classification. Br J Haematol. 2004;127:3–11. doi: 10.1111/j.1365-2141.2004.05094.x. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 32.Ardeshna KM, Qian W, Smith P, et al. Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: An open-label randomised phase 3 trial. Lancet Oncol. 2014;15:424–435. doi: 10.1016/S1470-2045(14)70027-0. [DOI] [PubMed] [Google Scholar]
- 33.Ghielmini M, Schmitz SF, Cogliatti SB, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood. 2004;103:4416–4423. doi: 10.1182/blood-2003-10-3411. [DOI] [PubMed] [Google Scholar]
- 34.Hainsworth JD, Litchy S, Burris HA, III, et al. Rituximab as first-line and maintenance therapy for patients with indolent non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20:4261–4267. doi: 10.1200/JCO.2002.08.674. [DOI] [PubMed] [Google Scholar]
- 35.Taverna C, Martinelli G, Hitz F, et al. Rituximab maintenance for a maximum of 5 years after single-agent rituximab induction in follicular lymphoma: Results of the randomized controlled phase III trial SAKK 35/03. J Clin Oncol. 2016;34:495–500. doi: 10.1200/JCO.2015.61.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLaughlin P, Grillo-López AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 37.Sehn LH, Chua N, Mayer J, et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): A randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol. 2016;17:1081–1093. doi: 10.1016/S1470-2045(16)30097-3. [DOI] [PubMed] [Google Scholar]
- 38.Rummel M, Kaiser U, Balser C, et al. Bendamustine plus rituximab versus fludarabine plus rituximab for patients with relapsed indolent and mantle-cell lymphomas: A multicentre, randomised, open-label, non-inferiority phase 3 trial. Lancet Oncol. 2016;17:57–66. doi: 10.1016/S1470-2045(15)00447-7. [DOI] [PubMed] [Google Scholar]
- 39.Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370:1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dreyling M, Morschhauser F, Bouabdallah K, et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann Oncol. 2017;28:2169–2178. doi: 10.1093/annonc/mdx289. [DOI] [PMC free article] [PubMed] [Google Scholar]