Abstract
Objective
To develop a practicable nomogram aimed at predicting the risk of severe exacerbations in COPD patients at three and five years.
Methods
COPD patients with prospective follow-up data were extracted from Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS) obtained from National Heart, Lung and Blood Institute (NHLBI) Biologic Specimen and Data Repository Information Coordinating Center. We comprehensively considered the demographic characteristics, clinical data and inflammation marker of disease severity. Cox proportional hazard regression was performed to identify the best combination of predictors on the basis of the smallest Akaike Information Criterion. A nomogram was developed and evaluated on discrimination, calibration, and clinical efficacy by the concordance index (C-index), calibration plot and decision curve analysis, respectively. Internal validation of the nomogram was assessed by the calibration plot with 1000 bootstrapped resamples.
Results
Among 1711 COPD patients, 523 (30.6%) suffered from at least one severe exacerbation during follow-up. After stepwise regression analysis, six variables were determined including BMI, severe exacerbations in the prior year, comorbidity index, post-bronchodilator FEV1% predicted, and white blood cells. Nomogram to estimate patients’ likelihood of severe exacerbations at three and five years was established. The C-index of the nomogram was 0.74 (95%CI: 0.71–0.76), outperforming ADO, BODE and DOSE risk score. Besides, the calibration plot of three and five years showed great agreement between nomogram predicted possibility and actual risk. Decision curve analysis indicated that implementation of the nomogram in clinical practice would be beneficial and better than aforementioned risk scores.
Conclusion
Our new nomogram was a useful tool to assess the probability of severe exacerbations at three and five years for COPD patients and could facilitate clinicians in stratifying patients and providing optimal therapies.
Keywords: severe exacerbations, COPD, nomogram, prediction model
Introduction
Severe exacerbation, one of the outcomes of chronic obstructive pulmonary disease (COPD) of most concern, refers to patients requiring hospitalization or visiting the emergency department (ED) because of worsening respiratory symptoms (cough, sputum, and dyspnea).1 It is estimated that severe episodes only accounted for less than 10% of all exacerbations, but hospitalizations approximately result in 60–70% of COPD-associated medical expenditure.2–4 Furthermore, severe exacerbations can not only increase short-term and long-term mortality,5,6 but also deteriorate lung function more rapidly7–9 and impair quality of life.10–12 Therefore, preventing or ameliorating the severity of exacerbation is deemed as an important target during the manangement of COPD.
COPD is a fairly heterogeneous disease13 and exacerbation modes vary greatly between patients and during follow-up.14 Future exacerbation risk was not well assessed with only the history of earlier treated events.15 With the use of composite prediction models, clinicians could make a more comprehensive evaluation of disease severity and gain more precise information about the risk of acute exacerbations. Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommended that different management strategies should be tailored on the basis of individualized assessment of symptoms and future risk of exacerbations.1 That is, clinicians should provide optimal therapeutic interventions after weighing up exacerbation risk and potential harm and economic load of treatment measures. Therefore, a good prediction model is needed.
However, there are limited tools for predicting the exacerbations in patients with COPD. Summing scores, such as ADO (age, dyspnea, obstruction),16 BODE (BMI, obstruction, dyspnea and exercise capacity)17 and DOSE (dyspnea, obstruction, smoking status, and exacerbations),18 were extensively validated as not very effective in predicting future exacerbations.15 Also, there are no reports about how these scores perform in predicting prospective severe exacerbations. One risk index, composed of age, percentage of predicted FEV1, oral steroids at entry, cardiovascular comorbidity and unscheduled clinic/ED visits for COPD in the prior year,19 was developed to forecast hospitalization due to COPD exacerbations. However, its population was from a clinical trial for tiotropium and confined to a single health-care system, which might not be representative of COPD patients. It was not externally validated in other independent populations, therefore, its clinical applicability was uncertain. A meta-analysis was also accomplished to appraise existing prediction models predicting exacerbations in COPD patients,20 which disappointingly drew the conclusion that most of the prediction models were at high risk of bias and none could be employed in clinical application currently.
Hence, we intend to develop and internally validate a practical tool to predict risk of severe exacerbations in COPD patients during long-term follow-up based on data derived from a multicenter prospective study. We present the results as a nomogram, user-friendly instrument with visualized prediction outcomes.
Methods
Under the guide of Transport Reporting of a Multivariable Prediction Model for Individual Prognosis of Diagnosis (TRIPOD) statement, a nomogram was developed and internally validated by bootstrapping. Detailed results were shown in Supplementary Table S1.
Study Design and Participants Selection
Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS)21 is funded by the National Health Lung and Blood Institute (ClinicalTrials.gov identifier: NCT01969344). Its research protocol was approved by the institutional review boards of all participating institutions and written informed consent was obtained from patients. This study was approved by the ethics committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (IRB ID: 20190310), which waived requirement for patients informed consent because of the anonymous nature of the data.
SPIROMICS is a multicenter prospective cohort study, enrolling participants from 2010 to 2015 at six clinical centers with a total of 11 recruitment locations. Details of the SPIROMICS study design, data collection, data quality, and eligibility criteria have been reported previously.21 Briefly, participants, aged 40–80 years, were enrolled and distributed across four strata according to smoking status and spirometry. In this study, clinical data were extracted from selected stratum 3 and 4 (mild/moderate and severe COPD), a wide range in the severity of COPD. These patients were identified as current or former smokers (history of smoking >20 pack-year), with post-bronchodilator FEV1/FVC ratio <70%. Principal exclusion criteria were: 1) failed to consent/complete the study; 2) BMI >40 kg/m2; 3) inability to fulfill pulmonary function testing; 4) diagnosed with myocardial infarction and uncontrolled congestive failure in the past six weeks.
Information about frequency and severity of prospective COPD exacerbations, offered by patients self-reported and identified or verified by medical records, were collected every three months during longitudinal follow-up visits. Any exacerbation of COPD was a report of worsening respiratory symptoms needing additional therapy. Severe exacerbation was defined as a patient suffering from an acute episode of respiratory symptoms required to be admitted to hospital or visit the ED. Participants were treated by their usual health-care providers and this research did not interfere.
Predictors and Definitions
At baseline, sociodemographic and clinical data were collected in the SPIROMICS study. To develop a practicably predictive tool, we summarized factors related to severe exacerbations in published literature and considered clinical ready accessibility. Candidate predictors contained: age19,22,24 BMI, (calculated as weight (kg)/[height (m)2]),23,24 severe exacerbations history,19,24 comorbidities (coronary artery disease, congestive heart failure, diabetes mellitus, gastro-esophageal reflux disease, asthma and sleep apnea),19,24–27 post-bronchodilator FEV1% predicted,19,24 questionnaires: modified Medical Research Council dyspnea scale (mMRC)28 measuring degree of dyspnea and COPD assessment test (CAT)24,29 evaluating health status, recent using of oral corticosteroids19 and white blood cell count.24 All of these predictors were assessed as long as participants were recruited in this cohort.
Severe exacerbations history, which was defined as COPD patients need medical care in hospital or ED because of intolerant respiratory symptoms in the 12 months prior to enrollment, was gathered through their recall of exacerbation treatments. Comorbidities were diagnosed by a health professional and reported by participants. There have already been scoring systems quantifying significance of comorbidities in COPD, such as the Charlson comorbidity index (CCI),30 COPD specific comorbidity test (COTE)31 and comorbidities in chronic obstructive lung disease (COMCOLD).32 However, all of them were too complicated and difficult to be applied in our model. Subsequently, we only considered comorbid diseases reported to seriously influence outcomes of COPD patients.
Statistical Analysis
Data were described as mean ± SD or median (interquartile) for continuous variables, and as frequency or percentage for categorical variables. Patients with and without future severe exacerbations were compared with respect to demographic characteristics and clinical data using t-test or Mann–Whitney U-test for continuous data, chi squared test for categorical data.
Post-bronchodilator FEV1% predicted was divided into ≥80%; 50–79%; 30–49% and <30%, according to GOLD stage.1 White blood cells count ≥10*109/L was defined as leukocytosis. We used the X-tile 3.6.1 (Yale University, New Haven, CT, USA) to identify the best cut-off for age (65 years) and BMI (21 kg/m2). Independent association between each of candidate variables and severe exacerbations was assessed through multivariable Cox proportional hazard regression model. Results were presented as HR and 95%CI. To make the best use of prognostic value of predictive models, we selected one optimal combination of parameters based on the smallest Akaike Information Criterion (AIC). A nomogram to forecast risk of severe exacerbations at three and five years was established with determined predictors.
Our nomogram, as well as those widely validated risk scores (ADO, BODE and DOSE), were estimated and compared in discrimination (the concordance index), calibration (calibration plot) and clinical efficacy (decision curve analysis). Decision curve analysis could ascertain whether the implementation of the nomogram in clinical practice would be beneficial and which of alternative models should be chosen. Calibration plots were conducted with 1000 bootstrapped resamples for internal validation.
Statistical analyses were performed by R software (version 3.4.3, http://www.R-project.org), and EmpowerStats software (www.empowerstats.com, X&Y Solutions, Inc. Boston MA, USA). A two-tailed P<0.05 was considered to be statistically significant.
Results
Incidence of Severe Exacerbations and Baseline Characteristic
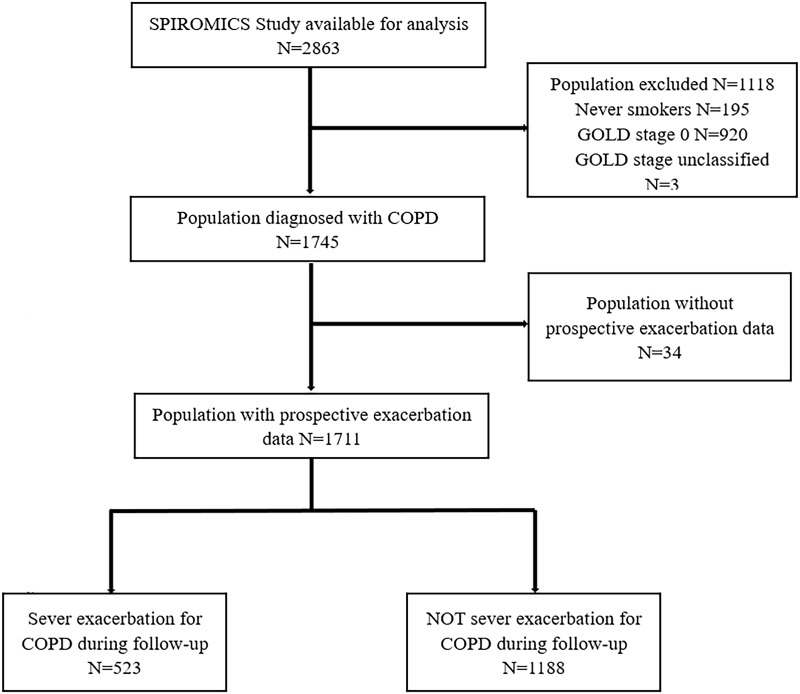
Figure 1 showed the flow diagram of our study. A total of 2863 participants were enrolled in SPIROMICS study. There were 1745 COPD patients after excluding 1118 cases, which included 195 healthy never-smokers, 920 smokers without airflow obstruction and 3 GOLD stage unclassified. Finally, a cohort of 1711 patients was eligible for this study excluding 34 cases without prospective exacerbations data. During follow-up, there were 983 (74%) patients who encountered ≥1 any exacerbations of COPD, 523 (30.6%) who had severe exacerbations. Median duration of follow-up was 50 months. Mean age of study population was 65 years and around half was male and mean BMI was 27.3 kg/m2, meeting or even exceeding threshold of overweight. The severity of COPD measuring by GOLD stage was widespread, from mild to very severe, and the major (68.6%) patients were moderate to severe airflow limitation. Participants who experienced prospective severe exacerbations showed more frequent severe exacerbations in the prior year along with worse airflow limitation, higher level of inflammation biomarkers, worsen dyspnea symptoms, poorer health status and more need in therapy, including oral and inhaled corticosteroids, inhaled bronchodilator (Table 1).
Figure 1.
Study flow chart. SPIROMICS: Subpopulations and Intermediate Outcome Measures in COPD Study.
Table 1.
Baseline Characteristic of Study Population
| Total COPD Cohort (N=1711) | Severe Exacerbations During Follow-up | P-value | ||
|---|---|---|---|---|
| No (N=1188) | Yes (N=523) | |||
| Demographics | ||||
| Age (years) | 65.2 ± 8.0 | 65.6 ± 7.9 | 64.4 ± 8.2 | 0.005 |
| Male | 984 (57.5%) | 713 (60.0%) | 271 (51.8%) | 0.002 |
| BMI (kg/m2) | 27.3 ± 5.3 | 27.4 ± 5.2 | 27.0 ± 5.5 | 0.176 |
| Clinical Data | ||||
| GOLD Stage | <0.001 | |||
| 1 | 367 (21.4%) | 315 (26.5%) | 52 (9.9%) | |
| 2 | 764 (44.7%) | 571 (48.1%) | 193 (36.9%) | |
| 3 | 409 (23.9%) | 224 (18.9%) | 185 (35.4%) | |
| 4 | 171 (10.0%) | 78 (6.6%) | 93 (17.8%) | |
| Current smoker | 572 (34.0%) | 388 (33.2%) | 184 (35.9%) | 0.286 |
| Smoking pack-years, median (IQR) | 46.0 (35.0–62.0) | 45.0 (35.0–60.0) | 48.0 (36.2–63.8) | 0.235 |
| Severe exacerbations history | 259 (15.4%) | 99 (8.5%) | 160 (31.1%) | <0.001 |
| Comorbidities | ||||
| History of coronary artery disease | 179 (10.6%) | 121(10.3%) | 58(11.3%) | 0.548 |
| History of congestive heart failure | 53 (3.1%) | 28 (2.4%) | 25 (4.9%) | 0.007 |
| History of GERD | 534 (31.6%) | 355 (30.2%) | 179 (34.9%) | 0.054 |
| History of diabetes | 230 (13.6%) | 145 (12.3%) | 85 (16.5%) | 0.020 |
| History of asthma | 381 (22.8%) | 220 (19.0%) | 161 (31.3%) | <0.001 |
| History of sleep apnea | 321 (19.9%) | 212 (18.9%) | 109 (22.3%) | 0.115 |
| Physiology | ||||
| Post-bronchodilator FEV1 (% predicted) | 60.8 ± 23.0 | 65.4 ± 22.3 | 50.5 ± 21.1 | <0.001 |
| CAT | 15.5 ± 8.0 | 14.0 ± 7.8 | 18.7 ± 7.7 | <0.001 |
| mMRC score | 1.3 ± 1.0 | 1.1 ± 1.0 | 1.6 ± 1.0 | <0.001 |
| Inflammation Biomarker | ||||
| White blood cell, median (IQR), (109/L) | 7.0 (5.9–8.4) | 6.9 (5.8–8.2) | 7.2 (6.0–8.7) | <0.001 |
| Treatment | ||||
| Currently using oral corticosteroids | 60 (3.6%) | 25 (2.1%) | 35 (6.8%) | <0.001 |
| Inhaled bronchodilators | 1120 (66.2%) | 691 (58.7%) | 429 (83.5%) | <0.001 |
| Inhaled corticosteroids | 793 (46.8%) | 465 (39.4%) | 328 (63.7%) | <0.001 |
Abbreviations: GERD, gastroesophageal reflux disease; CAT, COPD assessment test; mMRC, modified Medical Research Council.
Identifying Predictors and Developing Nomogram
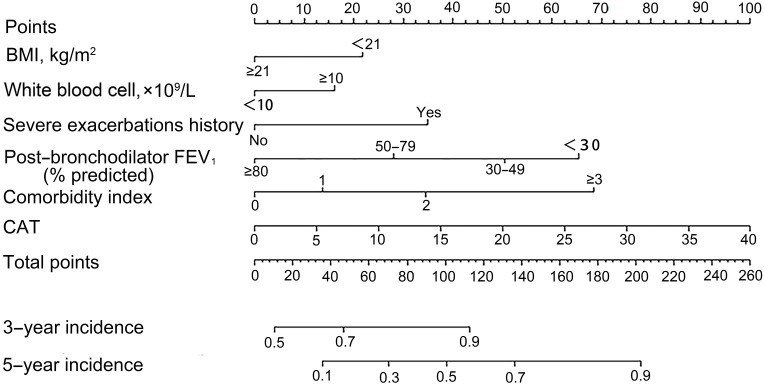
After stepwise regression selection based on the smallest AIC, following predictors were recognized as the best combination: BMI, severe exacerbations history, congestive heart failure, GERD, diabetes, asthma, post-bronchodilator FEV1% predicted, CAT and white blood cells count. Coefficient of variable was showed in Table S2. To improve the nomogram’s simplicity, we created a variable as comorbidity index ranging from 0 to 4, with directly counting numbers of congestive heart failure, GERD, diabetes and asthma one patient had. Results of regression analysis with comorbidity index were performed repeatedly and presented in Table 2. For comparison, regression model was conducted with all variables in their original form (Table S3). Identified predictors in the Table S2 were the same as Table S3. Therefore, we developed a nomogram for predicting risk of COPD severe exacerbations at 3- and 5-year using these selected predictors (Figure 2).
Table 2.
Identified Predictors of Severe Exacerbations from Cox Proportional Hazards Model, N=1438
| HR | 95%CI | P-value | |
|---|---|---|---|
| BMI, <21 kg/m2 | 1.49 | (1.11–2.00) | 0.0076 |
| Severe exacerbations history | 1.90 | (1.54–2.35) | <0.0001 |
| Post-Bronchodilator FEV1 (% predicted) | |||
| ≥80 | |||
| 50–79 | 1.68 | (1.20–2.35) | 0.0024 |
| 30–49 | 2.53 | (1.79–3.58) | <0.0001 |
| <30 | 3.33 | (2.26–4.92) | <0.0001 |
| Comorbidity Index | |||
| 0 | |||
| 1 | 1.29 | (1.04–1.59) | 0.0194 |
| 2 | 1.89 | (1.45–2.47) | <0.0001 |
| ≥3 | 3.53 | (2.09–5.97) | <0.0001 |
| CAT | 1.05 | (1.03–1.06) | <0.0001 |
| White blood cell, ≥10×109/L | 1.35 | (1.03–1.77) | 0.0318 |
Figure 2.
A nomogram to predict the risk of severe exacerbations in stable COPD patients. To use the nomogram, draw a vertical line to identify corresponding points of each variable according to their actual status. Then sum up the points of all variables and find the position on the total points axis. With the same line mentioned above, you can determine the risk of severe exacerbations of 3- and 5-year at the lower line of the nomogram. Comorbidity index is identified through counting the number of comorbid diseases one had among congestive heart failure, GERD, diabetes, and asthma.
Assessment of Nomogram
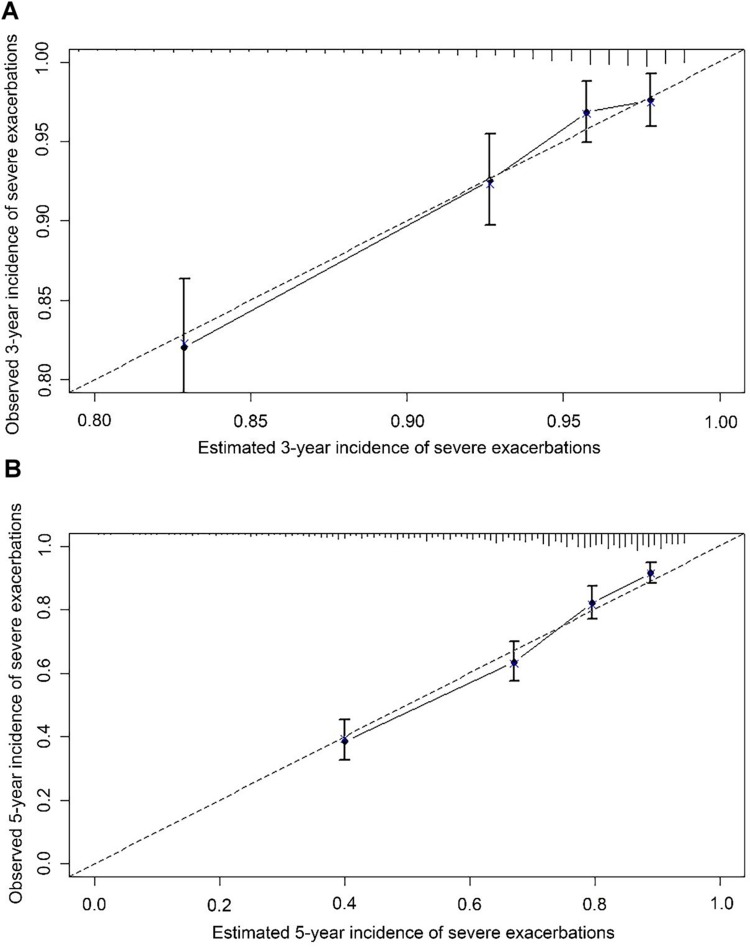
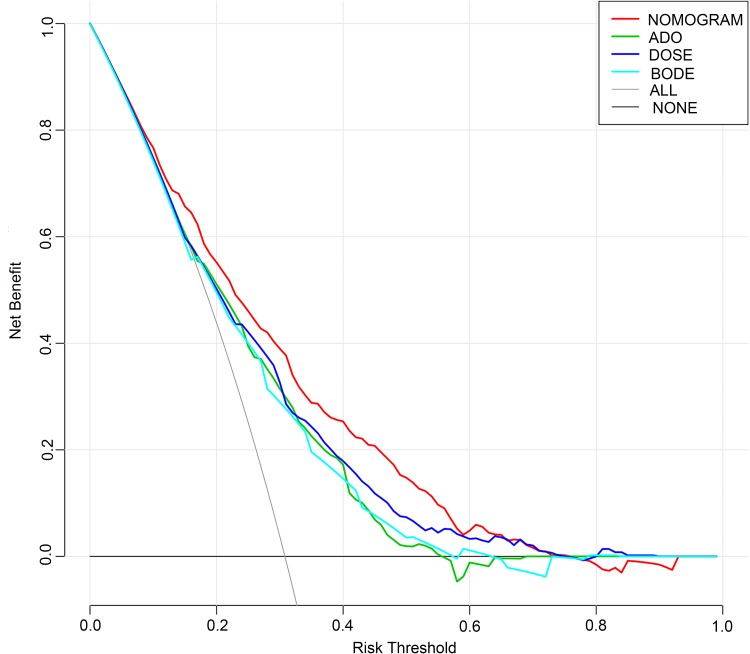
The C-index of established nomogram was 0.74 (95% CI: 0.71–0.76), the same as model with all variables in their original form and model with congestive heart failure, GERD, diabetes and asthma. Table 3 exhibited C-index and 95% CI of the nomogram, BODE, ADO and DOSE, which revealed that the nomogram had better discrimination than other scores for predicting severe exacerbations in our cohort. The internal calibration plots in Figure 3 showed great consistency between the risk predicted by nomogram and the observed occurrence of severe exacerbations at 3- and 5-year. By decision curve analysis, net benefit of applying the nomogram to guide clinical decision-making was beneficial and better than the other 3 risk scores between risk threshold of 10% and 60% (Figure 4).
Table 3.
C-Index of Nomogram, ADO, BODE and DOSE Risk Scores
| Prediction Models | C-Index | 95%CI |
|---|---|---|
| Nomogram | 0.74 | 0.71–0.76 |
| ADO | 0.69 | 0.66–0.71 |
| BODE | 0.69 | 0.66–0.72 |
| DOSE | 0.70 | 0.68–0.73 |
Figure 3.
Calibration plot showing predicted probability of severe exacerbations against the observed proportion of outcomes. The dashed line is the ideal calibration line. Vertical lines in grouped observation represents 95%CI. All groups of predicted probabilities fitting close to the ideal calibration line show perfect calibration. (A) 3-year incidence; (B) 5-year incidence.
Figure 4.
Decision curves.
Discussion
From a large multicenter prospective cohort of COPD patients during long term follow-up, we constructed a practical nomogram to predict severe exacerbations at 3- and 5-year incorporating easily available clinical data and inflammation biomarkers, which performed better than ADO, BODE and DOSE and had great internal validity.
A main advantage of this study was the population derived from the SPIROMICS, a large prospective observational cohort study. Its COPD patients enrolled with broad criteria resemble the population for whom the nomogram will be applied to, whose obstruction severity ranging from mild to very severe with moderate and severe being the principal part. To the best of our knowledge, this is the first study to develop a nomogram for predicting severe exacerbations in longitudinal follow-up. There were relatively few prediction models focused on severe exacerbations and existing reports were associated with the risk of hospitalization.19,33 However, in our study regarded, severe exacerbations were main target, whose definition included exacerbations requiring hospitalization or ED visits, accordant with the GOLD guideline,1 rather than only focused on hospitalized exacerbations. Statistically, there were 3206 emergency department visits (weighted estimates of 13,938,778 visits) made by patients with exacerbations of obstructive airway diseases during 2007–2015 in 50 states and district of Columbia, of whom 42% had COPD exacerbation.34 Also, in the Confronting COPD International Survey, 12.8% of patients reported at least one hospitalization and 13.8% required ED care due to COPD in the prior year.35 Therefore, emergency department visits because of COPD exacerbations should not be ignored. Last, we externally validated that ADO, BODE and DOSE scores could predict prospect risk of severe exacerbations and DOSE performed better.
Some of the predictors in our study independently associated with severe exacerbations agreed with previously published data: advancing age,19,22,24 low BMI,23 severe exacerbations in the prior year19,24 and leukocytosis.24 FEV1 impairment was related with frequency of both moderate and severe exacerbations, hospitalized exacerbations and readmission after discharging from hospital.24,36–38 And in our study, results of the regression model demonstrated that occurrence of severe exacerbations grew with increasing severity of airflow limitation. Some prior studies have identified higher risk, especially for severe exacerbations, occurred in those patients with COPD and significant comorbidities (e.g. diabetes mellitus, cardiac insufficiency, ischemic heart disease) when compared with those with COPD only.39 As is shown in Table S2, four important comorbidities were demonstrated as risk factors in COPD patients which were consistent with previous studies.19,24–27 Congestive heart failure was the strongest predictor of severe exacerbations, followed by diabetes, asthma and GERD, that indicated clinicians should take these diseases’ therapy into consideration during COPD management. These coexisting diseases, except for GERD, shared similar risk factors, like smoking, and common pathophysiological mechanism with COPD.40 CAT, covering multidimensional aspects of COPD burden,41 had been certified as a brief and valid instrument to thoroughly evaluate health-related quality of life.42,43 CAT score was reported as a predictor of the time to first exacerbation, any exacerbation and moderate-severe exacerbation.44 We also confirmed that the CAT score was an independent risk factor for severe exacerbations in a large cohort of COPD patients during long-term follow-up. mMRC assessment was not included in the nomogram after stepwise regression based on the smallest AIC. This may correspond with the accepted concept that COPD is a systemic disease rather than impairment confined in the lung and we should make a comprehensive evaluation of all components during clinical management.45 Compared to mMRC only involving dyspnea, CAT seemed to be more suitable for the nomogram as it incorporates disease symptoms (cough, phlegm, and chest tightness) and activity levels.46
With adoption of the proposed nomogram, health-care professionals could make a detailed and individualized assessment of future severe exacerbations in each COPD patient. Such accurate risk stratification would aid professionals in tailoring more reasonable drug treatments and provide more cost effective precautionary measures to improve patients prognosis.47 Maybe clinicians could combine the nomogram with other summing scores of predicting exacerbations and supply intensified treatments if patients have higher risk of severe exacerbations on the basis of evaluation by scores. And it would be easier to communicate between clinicians and patients about the severity of COPD using this visualized tool. In addition to it can be used to risk-stratify participants in COPD therapeutic clinical trials.47
This study also had some limitations. First, as lack of external validation in other population, the generalization of our predicting nomogram should be further validated. Second, in order to ensure simplicity and practicability, we did not take all associated comorbidities into account, but simply counted the number of those coexisting diseases. However, despite that, our nomogram had great performance in predicting severe exacerbations with these limited comorbid diseases.
Conclusion
Herein, we established a nomogram to predict severe exacerbations in COPD patients at three- and five-year follow-up, which has great internal validation and performed well in discrimination, calibration and clinical efficacy. It might assist clinicians to precisely stratify patients and make salutary prevention management in clinical settings.
Acknowledgments
This manuscript was prepared using SPIROMICS Research Materials obtained from NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the SPIROMICS of the NHLBI. This work was supported by National Key Technologies R&D Program (2016YFC1304700, 2016YFC1303900).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi: 10.1183/13993003.00164-2019 [DOI] [PubMed] [Google Scholar]
- 2.Donaldson GC, Seemungal TAR, Patel IS, Lloyd-Owen SJ, Wilkinson TMA, Wedzicha JA. Longitudinal changes in the nature, severity and frequency of COPD exacerbations. Eur Respir J. 2003;22(6):931–936. doi: 10.1183/09031936.03.00038303 [DOI] [PubMed] [Google Scholar]
- 3.Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31(2):416–469. doi: 10.1183/09031936.00099306 [DOI] [PubMed] [Google Scholar]
- 4.Sullivan SD, Strassels SA, Smith DH. Characterization of the costs of chronic obstructive pulmonary disease (COPD) in the US. Eur Respir J. 2016;9(suppl23):412S. [Google Scholar]
- 5.Hartl S, Lopez-Campos JL, Pozo-Rodriguez F, et al. Risk of death and readmission of hospital-admitted COPD exacerbations: European COPD Audit. Eur Respir J. 2015;47(1):113–121. [DOI] [PubMed] [Google Scholar]
- 6.Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halpin DMG, Decramer M, Celli BR, Mueller A, Metzdorf N, Tashkin DP. Effect of a single exacerbation on decline in lung function in COPD. Respir Med. 2017;128:85–91. [DOI] [PubMed] [Google Scholar]
- 8.Dransfield MT, Kunisaki KM, Strand MJ, et al. Acute exacerbations and lung function loss in smokers with and without COPD. Am J Respir Crit Care Med. 2017;195(3):324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celli BR, Thomas NE, Anderson JA, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178(4):332–338. [DOI] [PubMed] [Google Scholar]
- 10.WANG Q, BOURBEAU J. Outcomes and health-related quality of life following hospitalization for an acute exacerbation of COPD. Respirology. 2005;10(3):334–340. doi: 10.1111/res.2005.10.issue-3 [DOI] [PubMed] [Google Scholar]
- 11.Andersson I, Johansson K, Larsson S, Pehrsson K. Long-term oxygen therapy and quality of life in elderly patients hospitalised due to severe exacerbation of COPD. A 1 year follow-up study. Respir Med. 2002;96(11):944–949. doi: 10.1053/rmed.2002.1370 [DOI] [PubMed] [Google Scholar]
- 12.Spencer S, Jones PW. Time course of recovery of health status following an infective exacerbation of chronic bronchitis. Thorax. 2003;58(7):589–593. doi: 10.1136/thorax.58.7.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 14.Han MK, Quibrera PM, Carretta EE, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626. doi: 10.1016/S2213-2600(17)30207-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertens LC, Reitsma JB, Moons KG, et al. Development and validation of a model to predict the risk of exacerbations in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2013;8:493–499. doi: 10.2147/COPD.S49609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puhan MA, Garcia-Aymerich J, Frey M, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. LANCET. 2009;374(9691):704–711. doi: 10.1016/S0140-6736(09)61301-5 [DOI] [PubMed] [Google Scholar]
- 17.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Eng J Med. 2004;350(10):05–12. doi: 10.1056/NEJMoa021322 [DOI] [PubMed] [Google Scholar]
- 18.Jones RC, Donaldson GC, Chavannes NH, et al. Derivation and validation of a composite index of severity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(12):1189–1195. doi: 10.1164/rccm.200902-0271OC [DOI] [PubMed] [Google Scholar]
- 19.Niewoehner DE, Lokhnygina Y, Rice K, et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007;131(1):20–28. doi: 10.1378/chest.06-1316 [DOI] [PubMed] [Google Scholar]
- 20.Guerra B, Gaveikaite V, Bianchi C, Puhan MA. Prediction models for exacerbations in patients with COPD. Eur Respir Rev. 2017;26(143):160061. doi: 10.1183/16000617.0061-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Couper D, LaVange LM, Han M, et al. Design of the subpopulations and intermediate outcomes in COPD study (SPIROMICS): table 1. Thorax. 2014;69(5):492–495. doi: 10.1136/thoraxjnl-2013-203897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gadoury M. Self-management reduces both short- and long-term hospitalisation in COPD. Eur Respir J. 2005;26(5):853–857. doi: 10.1183/09031936.05.00093204 [DOI] [PubMed] [Google Scholar]
- 23.Pouw EM, Velde GPM, Croonen BHPM, et al. Early non-elective readmission for chronic obstructive pulmonary disease is associated with weight loss. Clin Nutr. 2000;19(2):95–99. doi: 10.1054/clnu.1999.0074 [DOI] [PubMed] [Google Scholar]
- 24.Müllerova H, Maselli DJ, Locantore N, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147(4):999–1007. doi: 10.1378/chest.14-0655 [DOI] [PubMed] [Google Scholar]
- 25.Mitsiki E, Alexopoulos E, Gourgoulianis K, Bania E, Varounis C, Malli F. Frequency and risk factors of COPD exacerbations and hospitalizations: a nationwide study in Greece (Greek Obstructive Lung Disease Epidemiology and health ecoNomics: GOLDEN study). Int J Chronic Obstruct Pulmon Dis. 2015;10:2665–2674. doi: 10.2147/COPD.S91392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benson VS, Müllerová H, Vestbo J, Wedzicha JA, Patel A, Hurst JR. Associations between gastro-oesophageal reflux, its management and exacerbations of chronic obstructive pulmonary disease. Respir Med. 2015;109(9):1147–1154. doi: 10.1016/j.rmed.2015.06.009 [DOI] [PubMed] [Google Scholar]
- 27.Tzanakis N, Hillas G, Perlikos F, Tsiligianni I. Managing comorbidities in COPD. Int J Chronic Obstruct Pulmon Dis.. 2015;10:95–109. doi: 10.2147/COPD.S54473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes N, Calverley PM, Kaplan A, Rabe KF. Chronic obstructive pulmonary disease and exacerbations: patient insights from the global hidden depths of COPD survey. BMC Pulm Med. 2013;13:54. doi: 10.1186/1471-2466-13-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jo YS, Yoon HI, Kim DK, Yoo C, Lee C. Comparison of COPD assessment test and clinical COPD questionnaire to predict the risk of exacerbation. Int J Chronic Obstructive Pulm Dis. 2018;13:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montserrat-Capdevila J, Godoy P, Marsal JR, Barbé F, Galván L. Risk factors for exacerbation in chronic obstructive pulmonary disease: a prospective study. Int J Tuberculosis Lung Dis. 2016;20(3):389–395. doi: 10.5588/ijtld.15.0441 [DOI] [PubMed] [Google Scholar]
- 31.Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–161. [DOI] [PubMed] [Google Scholar]
- 32.Frei A, Muggensturm P, Putcha N, et al. Five comorbidities reflected the health status in patients with chronic obstructive pulmonary disease: the newly developed COMCOLD index. J Clin Epidemiol. 2014;67(8):904–911. doi: 10.1016/j.jclinepi.2014.03.005 [DOI] [PubMed] [Google Scholar]
- 33.Yii AC, Loh CH, Tiew PY, et al. A clinical prediction model for hospitalized COPD exacerbations based on “treatable traits”. Int J Chron Obstruct Pulmon Dis. 2019;14:719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goto T, Camargo CA, Faridi MK, Yun BJ, Hasegawa K. Machine learning approaches for predicting disposition of asthma and COPD exacerbations in the ED. Am J Emerg Med. 2018;36(9):1650–1654. [DOI] [PubMed] [Google Scholar]
- 35.Rennard S, Decramer M, Calverley PMA, et al. Impact of COPD in North America and Europe in 2000: subjects’ perspective of confronting COPD international survey. Eur Respir J. 2002;20(4):799–805. [DOI] [PubMed] [Google Scholar]
- 36.Hurst JR, Vestbo J, Anzueto A, et al. Evaluation of COPD longitudinally to identify predictive surrogate endpoints (ECLIPSE) investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Aymerich J, Serra Pons I, Mannino DM, Maas AK, Miller DP, Davis KJ. Lung function impairment, COPD hospitalisations and subsequent mortality. Thorax. 2011;66(7):585–590. [DOI] [PubMed] [Google Scholar]
- 38.GARCIA-AYMERICH J, MONSO E, MARRADES RM, et al. Risk factors for hospitalization for a chronic obstructive pulmonary disease exacerbation. EFRAM study. Am J Respir Crit Care Med. 2001;164(6):1002–1007. [DOI] [PubMed] [Google Scholar]
- 39.Miravitlles M, Guerrero T, Mayordomo C, et al. Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. Respiration. 2000;67(5):495–501. [DOI] [PubMed] [Google Scholar]
- 40.Eriksson B, Lindberg A, Müllerova H, Rönmark E, Lundbäck B. Association of heart diseases with COPD and restrictive lung function – results from a population survey. Respir Med. 2013;107(1):98–106. [DOI] [PubMed] [Google Scholar]
- 41.Ghobadi H, Ahari SS, Kameli A, Lari SM. The relationship between COPD Assessment Test (CAT) scores and severity of airflow obstruction in stable COPD patients. Tanaffos. 2012;11(2):22–26. [PMC free article] [PubMed] [Google Scholar]
- 42.Lari SM, Ghobadi H, Attaran D, Mahmoodpour A, Shadkam O, Rostami M. COPD assessment test (CAT): simple tool for evaluating quality of life of chemical warfare patients with chronic obstructive pulmonary disease. Clin Respir J. 2014;8(1):116–123. [DOI] [PubMed] [Google Scholar]
- 43.Jones PW, Brusselle G, Dal Negro RW, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J. 2011;38(1):29–35. [DOI] [PubMed] [Google Scholar]
- 44.Lee S, Huang M, Kang J, et al. The COPD assessment test (CAT) assists prediction of COPD exacerbations in high-risk patients. Respir Med. 2014;108(4):600–608. [DOI] [PubMed] [Google Scholar]
- 45.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–1185. [DOI] [PubMed] [Google Scholar]
- 46.Lari S, Attaran D, Tohidi M. Improving communication between the physician and the COPD patient: an evaluation of the utility of the COPD assessment test in primary care. Patient Relat Outcome Meas. 2014;5:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moons KG, Royston P, Vergouwe Y, et al. Prognosis and prognostic research: what, why, and how? BMJ. 2009;338:b375. [DOI] [PubMed] [Google Scholar]