Natural killer (NK) cells are key effector cells of innate resistance capable of destroying tumors and virus-infected cells through cytotoxicity and rapid cytokine production. The control of NK cell responses is complex and only partially understood. PD-1 is an inhibitory receptor that regulates T cell function, but a role for PD-1 in regulating NK cell function is only beginning to emerge.
KEYWORDS: human immunology, NK cells, malaria
ABSTRACT
Natural killer (NK) cells are key effector cells of innate resistance capable of destroying tumors and virus-infected cells through cytotoxicity and rapid cytokine production. The control of NK cell responses is complex and only partially understood. PD-1 is an inhibitory receptor that regulates T cell function, but a role for PD-1 in regulating NK cell function is only beginning to emerge. Here, we investigated PD-1 expression on NK cells in children and adults in Mali in a longitudinal analysis before, during, and after infection with Plasmodium falciparum malaria. We found that NK cells transiently upregulate PD-1 expression and interleukin-6 (IL-6) production in some individuals during acute febrile malaria. Furthermore, the percentage of PD-1 expressing NK cells increases with age and cumulative malaria exposure. Consistent with this, NK cells of malaria-naive adults upregulated PD-1 following P. falciparum stimulation in vitro. Additionally, functional in vitro studies revealed that PD-1 expression on NK cells is associated with diminished natural cytotoxicity but enhanced antibody-dependent cellular cytotoxicity (ADCC). These data indicate that PD-1+ NK cells expand in the context of chronic immune activation and suggest that PD-1 may contribute to skewing NK cells toward enhanced ADCC during infections such as malaria.
INTRODUCTION
Natural killer (NK) cells are innate immune cells that help protect against viral (1, 2) and parasitic (3, 4) infections, as well as tumor development (5, 6), through cytolytic activity and the production of cytokines such as gamma interferon (IFN-γ) and tumor necrosis factor (TNF). In addition, NK cells play a role in the regulation of adaptive immune responses by controlling the activity of antigen-presenting cells (APCs) and T cells (7–10). Lacking rearranged antigen-specific receptors, NK cells use multiple germ line-encoded receptors to detect changes in their environment and execute appropriate responses. NK cells respond to soluble factors such as chemokines or cytokines, especially interleukin-12 (IL-12), IL-18, IL-2, and IL-15 (11–13), and to cell-bound ligands that trigger activating or inhibitory receptors on NK cells (14, 15). NK cell activity is tightly controlled to avoid bystander cytotoxicity and overexuberant proinflammatory responses while still allowing for a high level of sensitivity in detecting and eliminating infected and diseased cells. The finely tuned regulation of NK cells is mediated by complex signaling pathways that integrate both activating and inhibitory ligands on target cells (16). Because the signaling pathways of some NK cell receptors remain unknown, it is not fully understood how signals from multiple receptors are spatially and temporally integrated to determine the functional response of NK cells.
Programmed death-1 (PD-1) is an inhibitory receptor expressed on T cells, B cells, NK cells, NKT cells, activated monocytes, and dendritic cells (17). The role of PD-1 in regulating T cell responses has been extensively described (18). After engaging one of its two ligands, PD-L1 or PD-L2, PD-1 delivers inhibitory signals to proximal T cell receptor (TCR) complexes, thereby downregulating T cell function (19–21). Antibodies blocking the interaction of PD-1 with its ligands have demonstrated therapeutic potential in the treatment of cancer (18, 22–24) and infectious diseases (25–28), and conversely, PD-1 agonists are being investigated as a potential treatment strategy for autoimmune diseases (29, 30).
It has been reported that PD-1-expressing NK cells are detectable in individuals with cancer (31–34) and chronic viral (35, 36) and bacterial infections (37) and in ∼25% of healthy European adults (32). In vitro studies suggest that blockade of PD-1 and other inhibitory receptors such as LAG3 and TIGIT may represent a strategy to increase NK cell function in cancer patients (34, 38–40). For example, in vitro studies of human NK cells show that blocking the PD-1 pathway with antibodies against PD-1/PD-L1 augments NK cell natural cytotoxicity against PD-L1+ multiple myeloma cells (34), enhances IFN-γ production but not cytotoxicity by NK cells from patients with posttransplantation lymphoproliferative disorders (PTLD) (41), and partially restores the degranulation capacity of PD-1+ NK cells against an ovarian carcinoma cell line (32).
Much less is known about the prevalence and function of PD-1+ NK cells in the context of human infectious diseases. In this study, we sought to determine if (i) PD-1 expression on NK cells is generalizable to pediatric and adult populations in West Africa, (ii) exposure to repeated malaria infections is associated with increased PD-1 expression, (iii) blood-stage Plasmodium falciparum parasites upregulate PD1 on NK cells in vitro, and (iv) PD-1 expression on NK cells in this context is associated with differences in natural cytotoxicity or ADCC. These questions are relevant to malaria, as recent studies demonstrate that NK cells inhibit P. falciparum growth in red blood cells via ADCC (42) and that an adaptive NK cell phenotype defined by the loss of transcription factor PLZF and Fc receptor γ-chain dominates ADCC responses in malaria-exposed individuals and correlates with lower parasitemia and resistance to febrile malaria (43). Earlier studies described direct lysis of P. falciparum-infected erythrocytes by NK cells in the absence of antibodies (44); however, other studies have not confirmed these findings (42, 45). It remains possible that under specific stimulatory conditions, human NK cells exhibit natural cytotoxic responses toward P. falciparum-infected erythrocytes. Finally, there is evidence that NK cells reduce levels of liver- and blood-stage Plasmodium parasites through proinflammatory cytokines and direct killing of infected cells (3, 4, 46–49) and, conversely, that NK cells may contribute to the immunopathology of severe malaria (50, 51).
We conducted a yearlong study in Mali in which NK cells were analyzed before, during, and after acute malaria. We found that PD-1 expression and IL-6 production are transiently upregulated by NK cells during acute malaria, in concert with increased expression of PD-L1 and to a lesser extent PD-L2 on other lymphocytes. Moreover, at homeostasis before the malaria season, age-stratified cross-sectional analysis showed that the percentage of PD-1-expressing NK cells increases with age—a surrogate for cumulative malaria exposure. That PD-1 upregulation is driven by malaria exposure is consistent with the observation that P. falciparum stimulation in vitro upregulated PD-1 on NK cells. Further studies in vitro revealed that PD-1 expression on NK cells is associated with diminished natural cytotoxicity but enhanced ADCC. Together, these data suggest that PD-1 may contribute to the regulation of NK cell effector functions during malaria and possibly other infections.
RESULTS
NK cells upregulate PD-1 expression and IL-6 production during acute malaria in children.
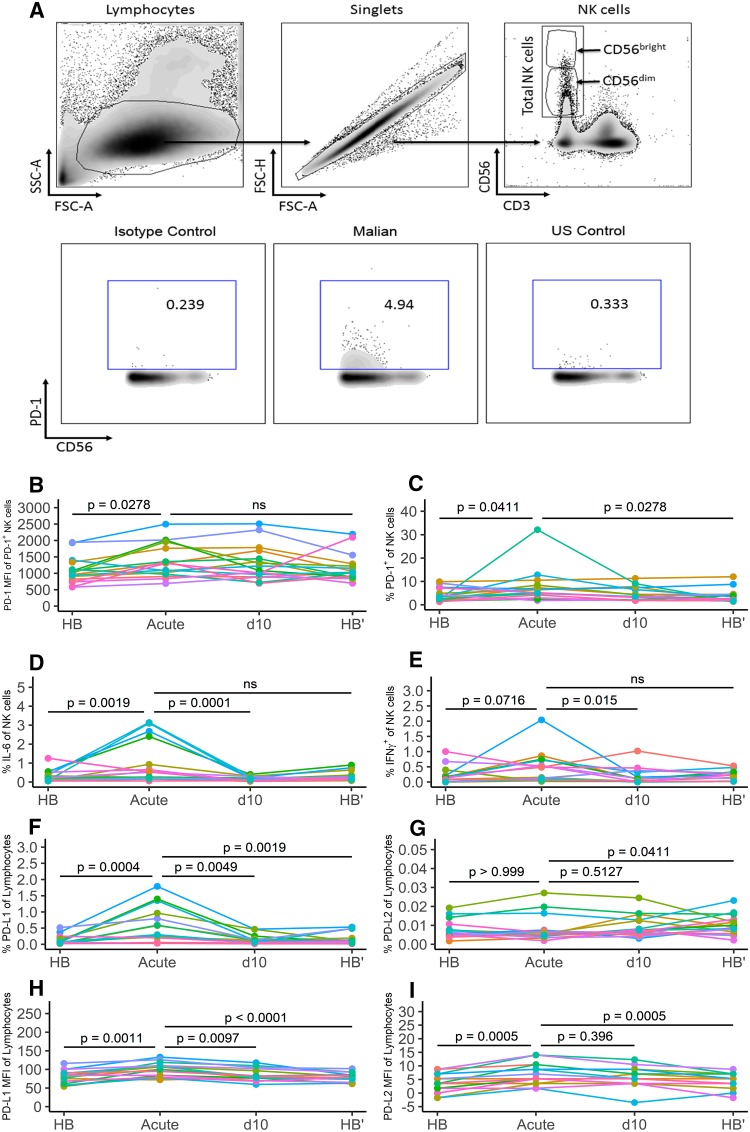
In a yearlong study, we first asked whether acute malaria in children is associated with changes in the expression of PD-1 on NK cells. Peripheral blood mononuclear cells (PBMCs) collected at the following four time points were analyzed: the healthy preinfection baseline (HB) at the end of the 6-month dry season (a period of negligible malaria transmission), at the first acute malaria episode of the ensuing 6-month malaria season (acute), 10 days later, after treatment (d10), and at the end of the subsequent 6-month dry season (HB′). PBMCs from all time points were thawed simultaneously and analyzed by flow cytometry. The gating strategy to identify PD-1-expressing NK cells is depicted in Fig. 1A. We found that acute malaria was associated with an increase in PD-1 expression on NK cells relative to the healthy preinfection baseline (Fig. 1B) (PD-1 median fluorescence intensity [MFI] for HB, 983.5; PD-1 MFI for acute, 1,305; P = 0.0278) and also an increase in the percentage of NK cells expressing PD-1 in some individuals (Fig. 1C) (HB median, 3.31%; acute median, 5.15%; P = 0.0411). Ten days after malaria treatment, there was a small, nonstatistically significant decrease in the percentage of PD-1+ NK cells relative to that for acute (Fig. 1C) (d10 median, 3.54%), which dropped further during the subsequent 6-month dry season to a point that resembled the healthy baseline (Fig. 1C) (HB′ median, 2.27%; P = 0.0278, acute versus HB′).
FIG 1.
In some children, acute malaria induces PD-1 expression and IL-6 production in NK cells and PD-L1 and PD-L2 expression on other lymphocytes. PBMCs were collected from the same children (n = 17) at four time points over 12 months: at the healthy uninfected baseline before the 6-month malaria season (HB), just prior to treatment of the first febrile malaria episode of the ensuing malaria season (acute), 10 days after malaria treatment, when symptoms had resolved (d10), and at the healthy uninfected baseline after the subsequent 6-month dry season (HB′). Surface and intracellular staining of PBMCs was done directly ex vivo without prior culturing or restimulation. (A) Upper panel, gating strategy to identify CD56bright, CD56dim, and total NK cells. Lower panel, representative plots of PD-1-expressing NK cells: left, PD-1 isotype control staining of NK cells; middle, PD-1-expressing NK cells of a Malian adult; right, PD-1-expressing NK cells of a U.S. adult. (B and C) Median fluorescence intensity (MFI) of PD-1 on PD-1-expressing NK cells (B) and percentage of PD-1-expressing NK cells of total NK cells (C) at the indicated time points. (D and E) Ex vivo intracellular cytokine staining for IL-6 (D) and IFN-γ (E) in NK cells at the indicated time points. (F to I) Percentage of lymphocytes expressing PD-L1 (F) and PD-L2 (G) and MFI of PD-L1 (H) and PD-L2 (I) on lymphocytes at the indicated time points. (B to I) P values were determined using the Friedman test, with a comparison of results for HB, d10, and HB′ to those at the acute time point. Individual subjects are color coded. ns, not significant.
Ex vivo intracellular staining of NK cells at the same time points revealed a statistically significant increase in the percentage of IL-6-producing NK cells during acute malaria relative to baseline (Fig. 1D) (HB median, 0.19%; acute median, 0.52%; P = 0.0019), as well as an increase in the percentage of IFN-γ-producing NK cells during acute malaria relative to baseline, although this did not reach statistical significance (Fig. 1E) (HB median, 0.14%; acute mean, 0.32%; P = 0.0716). There were no statistically significant correlations between PD-1 expression and the percentage of IFN-γ-producing cells or between PD-1 expression and the percentage of IL-6-producing cells (data not shown).
Because PD-1 requires the presence of its ligands PD-L1 (52) and PD-L2 (53) to transmit inhibitory signals, we also assessed the expression of these ligands in the same PBMC samples. A detailed analysis of PD-L1 and PD-L2 expression by cell type is shown in Fig. S1 in the supplemental material. We found that the percentage of PD-L1-expressing lymphocytes increased during acute malaria relative to baseline (Fig. 1F) (HB median, 0.06%; acute median, 0.29%; P = 0.0004), whereas the percentage of lymphocytes expressing PD-L2 did not change (Fig. 1G) (HB median, 0.007%; acute median, 0.006%; P > 0.999). However, the expression level of PD-L1 increased on lymphocytes during acute malaria (Fig. 1H) (HB MFI, 81.55; acute MFI, 100; P = 0.0011), as did the expression level of PD-L2 (Fig. 1I) (HB MFI, 4.38; acute MFI, 6.14; P = 0.0005). Of note, the expression of PD-L1 and PD-L2 on NK cells did not correlate within individuals (Fig. S2). Together, these data indicate that in some children, acute malaria is associated with an increase in PD-1 expression and IL-6 production by NK cells, in concert with upregulation of PD-L1 and PD-L2 on other lymphocytes, suggesting a possible role for PD-1 in regulating NK cell responses during acute malaria.
PD-1-expressing NK cells increase with age and cumulative malaria exposure.
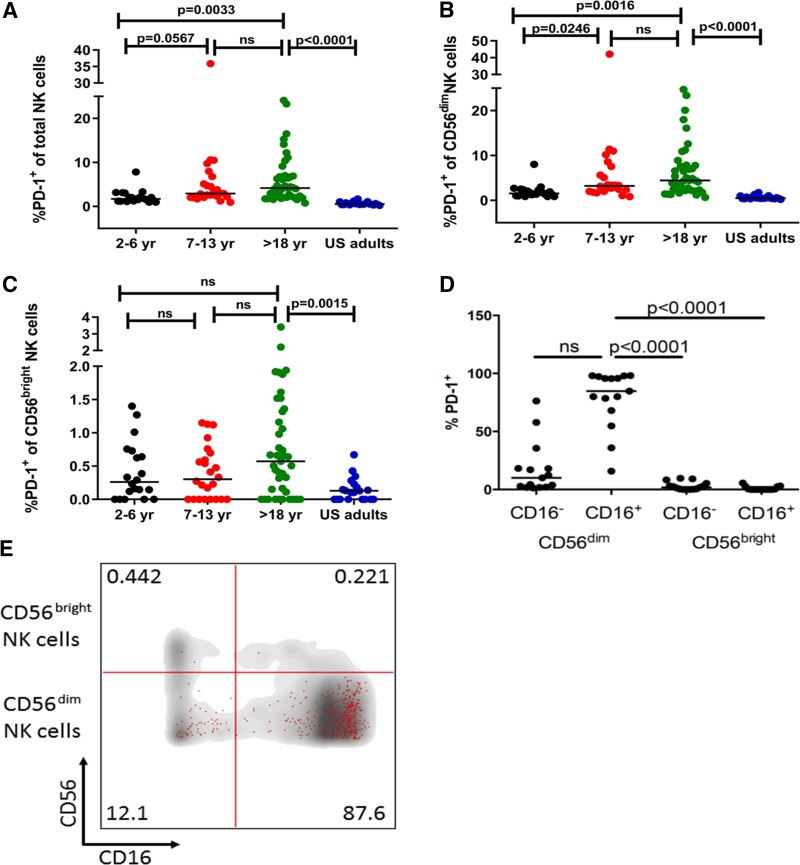
Given that the MFI of PD-1 expression on NK cells increased with acute malaria (Fig. 1B), we hypothesized that PD-1 expression on NK cells would increase incrementally with age, a surrogate for cumulative malaria exposure in regions of intense transmission. Indeed, age-stratified cross-sectional analysis at the end of the dry season showed that the percentage of PD-1-expressing NK cells increased with age (Fig. 2A) (median for 2- to 6-year-olds, 1.7%; median for 7- to 13-year-olds, 2.92%; median for >18-year-olds, 4.16%) and that the percentage of PD-1-expressing NK cells was significantly higher in Malian adults than in U.S. adults (Fig. 2A) (median for U.S. adults, 0.54%; P < 0.0001 for Malian versus U.S. adults). Together, these data suggest that cumulative malaria exposure (or other factors associated with malaria transmission) drives the gradual increase in PD-1 expression on NK cells.
FIG 2.
PD-1-expressing NK cells increase with age in the Mali cohort and are disproportionately CD16+ CD56dim. PBMCs collected from children and adults in Mali at the healthy baseline before the malaria season, and from healthy U.S. adults, were surface stained ex vivo for CD3, CD56, CD16, and PD-1. (A to C) Percentages of PD-1-expressing total NK cells (A), CD56dim NK cells (B), and CD56bright NK cells (C) in Malian subjects stratified by age (2 to 6 years, n = 20; 7 to 13 years, n = 24; >18 years, n = 41), and in healthy U.S. adults (n = 21). (D) Distribution of PD-1-expressing NK cells on the basis of CD16 and CD56 expression (n = 15 Malian adults). (E) Overlay of PD-1-expressing NK cells (red dots) on total NK cells (gray background) on the basis of CD16 and CD56 expression (one representative donor). P values were determined by the Kruskal-Wallis test (A to C) and the Friedman test (D), adjusted for multiple comparisons. Data are shown as medians.
The majority of PD-1-expressing NK cells are CD16+ CD56dim.
On the basis of CD56 expression, NK cells can be divided into CD56bright and CD56dim populations. CD56bright NK cells are more abundant in tissues and represent <10% of NK cells in the peripheral blood. They are activated by cytokines such as IL-12 and IL-18 and respond by producing high levels of cytokines and chemokines (16). Conversely, CD56dim NK cells comprise the majority of NK cells in peripheral blood, respond more efficiently to cell-bound ligands, display higher cytotoxic potential, and secrete cytokines such as IFN-γ when activated (16). Although Malian adults had a higher percentage of PD-1-expressing CD56bright and CD56dim NK cells than U.S. adults, the difference was more pronounced for CD56dim NK cells (Fig. 2B; Malian adult CD56dim median, 4.45%; U.S. adult CD56dim median, 0.5%; P < 0.0001) (Fig. 2C; Malian adult CD56bright median, 0.57%; U.S. adult CD56bright median, 0.13%; P = 0.0015). The age-dependent increase in PD-1-expressing total NK cells (Fig. 2A) and CD56dim NK cells (Fig. 2B) was also seen as a trend in the CD56bright population (Fig. 2C), but it did not reach statistical significance.
We then examined PD-1 expression on NK cells on the basis of CD16 expression. CD16 is an Fc receptor that allows NK cells to detect and directly kill antibody-coated target cells through antibody-dependent cellular cytotoxicity (ADCC) and cytokine production. While most activating receptors on NK cells act synergistically to activate NK cells, CD16 is the only receptor that is known to trigger degranulation of NK cells independently of other activating receptors (54). We found that the majority of PD-1-expressing NK cells in adults are CD16+ CD56dim NK cells (Fig. 2D and E).
These data indicate that PD-1 is preferentially expressed on CD16+ CD56dim NK cells, suggesting that PD-1 may regulate NK cell cytotoxicity and cytokine production that is triggered by cell-bound ligands.
NK cells of malaria-naive adults upregulate PD-1 following P. falciparum stimulation in vitro.
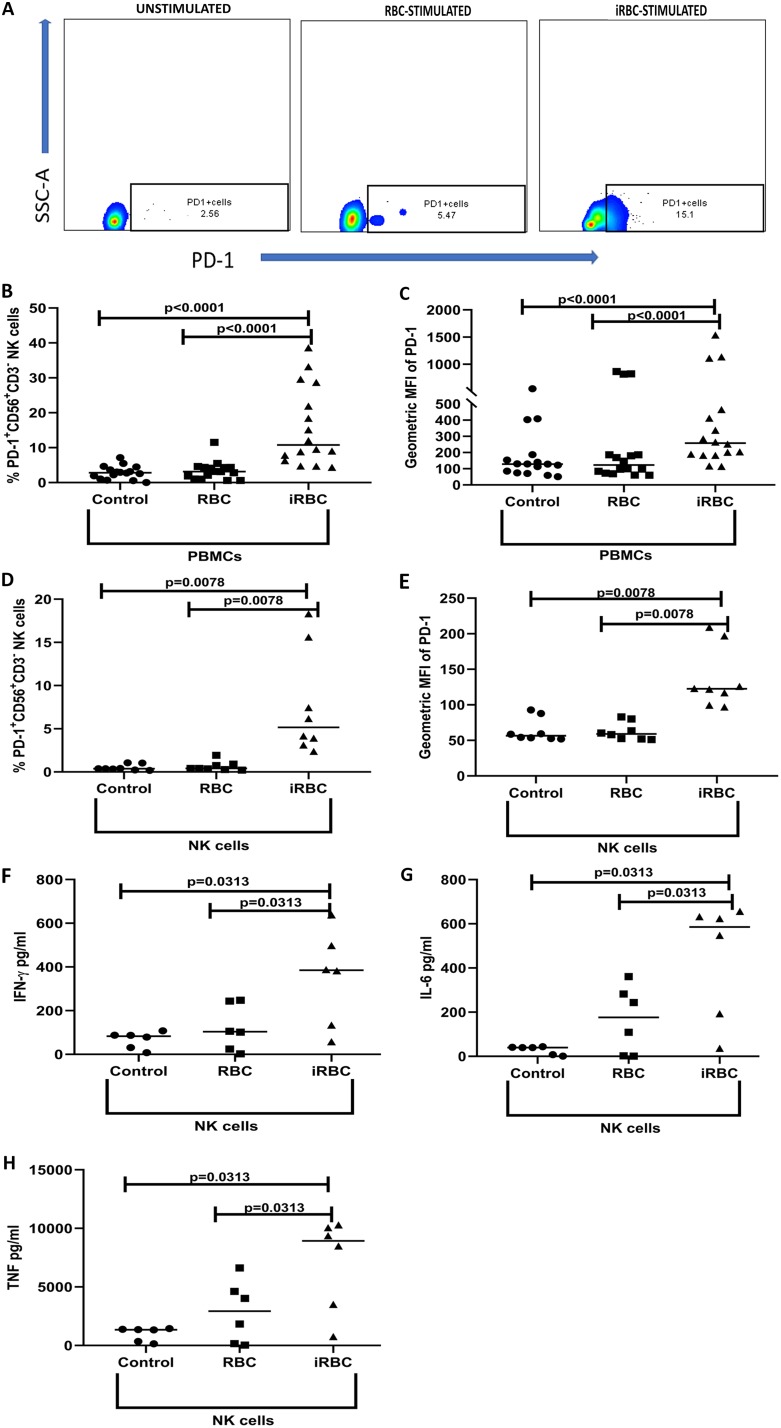
To more directly test the hypothesis that P. falciparum induces PD-1 expression on NK cells, PBMCs from healthy, malaria-naive U.S. adult donors were incubated for 3 days with P. falciparum-infected red blood cells (iRBCs), uninfected red blood cells (RBCs), or medium, stained for PD-1, and gated on CD3− CD56+ NK cells (Fig. 3A). On average, 10.5% of NK cells expressed PD-1 after 3 days of iRBC stimulation, which was significantly higher than that of either control (Fig. 3B) (median % CD3− CD56+ PD-1+ NK cells with medium alone, 3.1%, P < 0.0001 versus iRBCs; median % CD3− CD56+ PD-1+ NK cells with RBCs, 4.7%, P < 0.0001 versus iRBCs). Similarly, the MFI of PD1 on NK cells increased with iRBC stimulation compared to that of controls (Fig. 3C), consistent with the hypothesis that repeated malaria exposure contributes to the expansion of PD-1+ NK cells observed in the Mali cohort.
FIG 3.
NK cells of malaria-naive adults upregulate PD-1 following P. falciparum exposure in vitro. PBMCs (n = 16) or NK cells (n = 8) from malaria-naive U.S. adults were incubated at 37°C for 3 days with medium or with iRBCs or RBCs at a ratio of 1:30 (1 PBMC or NK cell to 30 iRBCs or RBCs) and analyzed by flow cytometry. Supernatants were removed at 24 h from the purified NK cell culture to quantify secreted cytokines (n = 6). (A) Representative flow cytometry plots show the gating strategy to identify PD-1+ CD3− CD56+ NK cells within FSC-SSC lymphocytes after gating on singlet live cells. (B) Percentage of total NK cells expressing PD-1. (C) MFI values of PD-1 on NK cells. (D) Percentage of purified NK cells expressing PD-1. (E) MFI values of PD-1 on purified NK cells. (F to H) Levels of IFN-γ (F), IL-6 (G), and TNF (H) in supernatants. P values were determined using the Wilcoxon signed rank test. Data are shown as medians (B to H).
We then asked whether P. falciparum-induced upregulation of PD-1 on NK cells depends on the indirect effects of PBMC simulation. To this end, NK cells were isolated from PBMCs through negative selection and were incubated for 3 days with iRBCs, RBCs, or medium, stained for PD-1, and analyzed by flow cytometry. On average, 5.2% of NK cells expressed PD-1 after 3 days of iRBC stimulation, significantly higher than the percentage of either control (Fig. 3D) (median % CD3− CD56+ PD-1+ NK cells with medium alone, 0.41%, P = 0.0078 versus iRBCs; median % CD3− CD56+ PD-1+ with RBCs, 0.74%, P = 0.0078 versus iRBCs). Similarly, the MFI of PD-1 on NK cells increased with iRBC stimulation compared to that of controls (Fig. 3E). Furthermore, iRBCs induced the isolated NK cells to secrete IFN-γ, TNF, and IL-6 (Fig. 3F to H), potentially contributing to a microenvirment that upregulates PD-1 expression on NK cells.
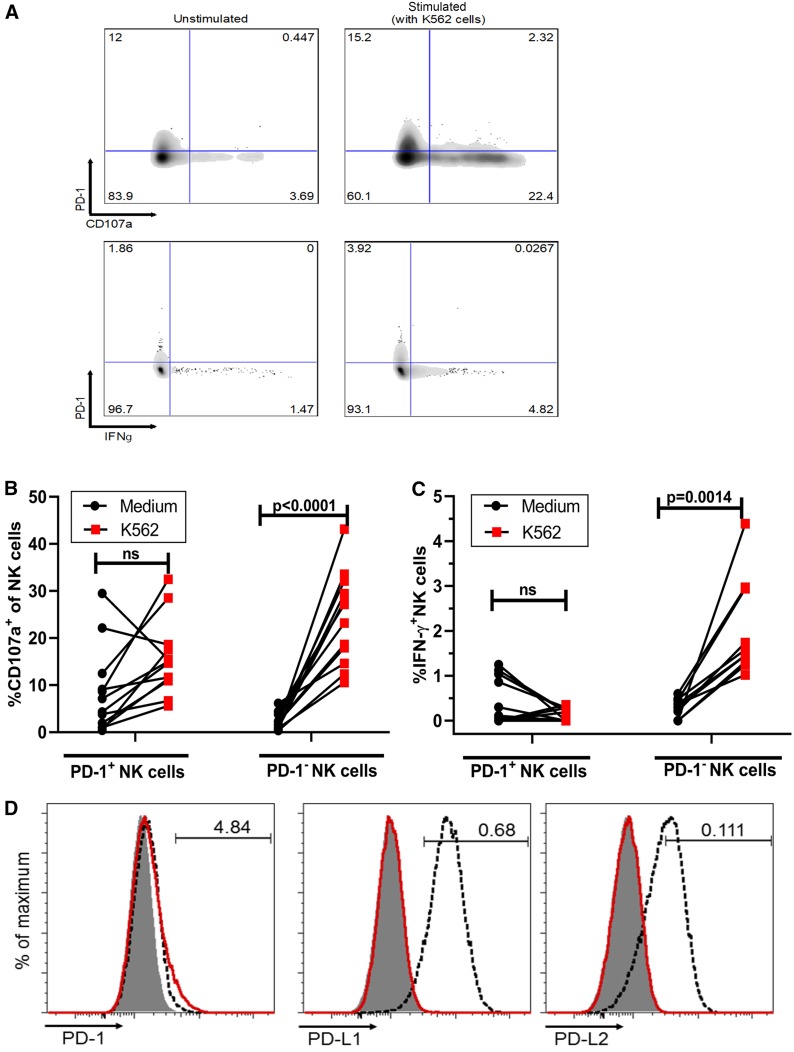
PD-1 expression on NK cells is associated with diminished activation by K562 cells.
We then asked whether PD-1 expression on NK cells is associated with altered NK cell effector function. We used two assays that represent distinct mechanisms by which NK cells are activated through cell-bound ligands. First, we activated PBMCs in vitro with target cells that lack the major histocompatibility complex class I (MHC I) (human erythroblast cell line K562) and quantified NK cell degranulation by CD107a expression and IFN-γ production by intracellular staining (representative flow cytometry results are shown in Fig. 4A). We found that in the presence of K562 cells, NK cell degranulation significantly increased in PD-1− NK cells (P = 0.0001) but not in PD-1+ NK cells (Fig. 4B). In some individuals, higher basal levels of CD107a+ NK cells were observed in the PD-1+ subset. Similarly, in the presence of K562 cells, PD-1− NK cells showed a significant increase in IFN-γ production (P = 0.0014), whereas there was no significant increase in IFN-γ production by PD-1+ NK cells (Fig. 4C). Of note, K562 cells do not express PD-1, PD-L1, or PD-L2 (Fig. 4D). Similar experiments were performed in which PD-L1 blocking antibodies were added to PBMCs before stimulation with K562 cells, but no significant changes in degranulation or IFN-γ production were observed (Fig. S3A to D).
FIG 4.
PD-1 expression on NK cells is associated with diminished activation by K562 cells. (A) Representative flow cytometry plots of CD107a (upper panels) and IFN-γ (lower panels) producing PD-1+/− NK cells (total NK cells, gating as shown in Fig. 1A) among PBMCs with or without 5 h of stimulation with K562 targets cells. (B and C) Percentage of CD107a-positive (B) and IFN-γ-positive (C) NK cells stratified by PD-1 expression following stimulation of PBMCs (n = 12) with medium alone or K562 cells. (D) K562 and .221 cells stained for PD-1, PD-L1, and PD-L2. K562 cells with the isotype control are indicated by a gray background, K562 cells stained with the indicated antibody are indicated by a red line, and .221 cells stained with the indicated antibody are indicated by a dashed line. The .221 cell line control is HLA null and expresses PDL1 and PDL2. P values were determined by Friedman’s matched pairs test with Dunn’s test adjustment. Data are shown as matched pairs.
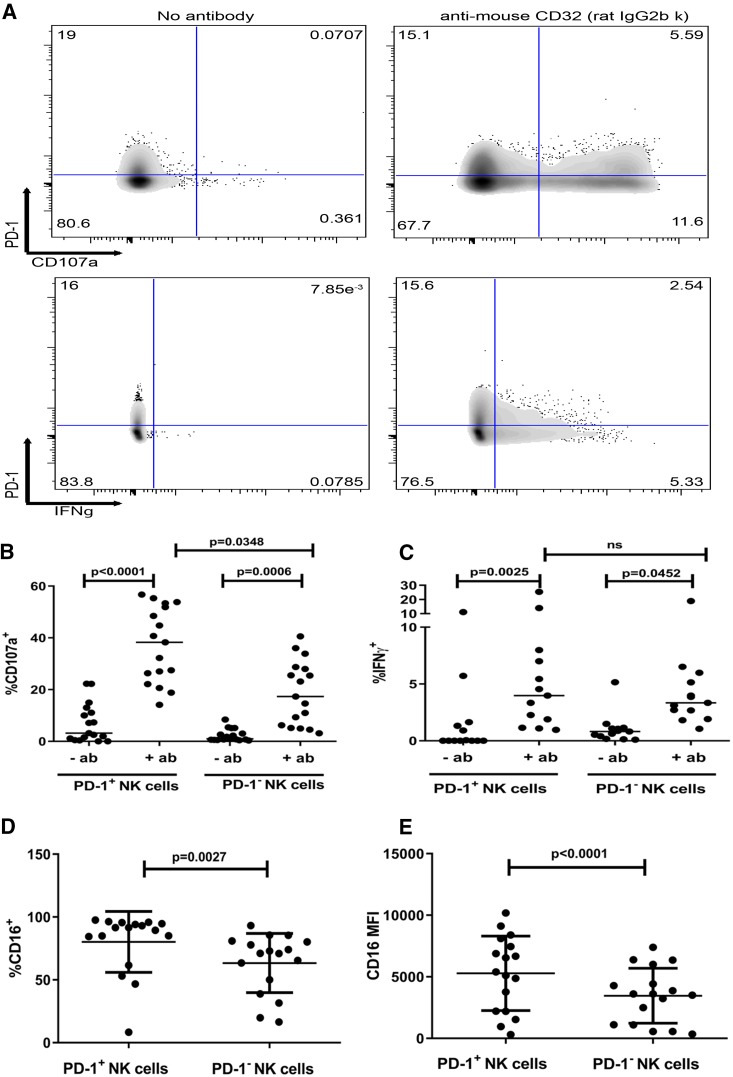
PD-1 expression on NK cells is associated with increased degranulation in an assay of ADCC.
Given that the majority of PD-1+ NK cells express the Fc receptor CD16 (Fig. 2D), we examined the function of PD-1+ NK cells in an assay of ADCC that involves target cells coated with antibody (CD32+ mouse mastocytoma cell line P815 pretreated with rat IgG2b anti-mouse CD32 antibody [Ab]). Among PBMCs stimulated with P815 cells, NK cell degranulation was quantified by intracellular CD107a expression and IFN-γ production by intracellular staining (representative flow cytometry results are shown in Fig. 5A). In comparison to the unstimulated control, we observed a significant increase in degranulation and IFN-γ production in both PD-1+ and PD-1− NK cells (Fig. 5B and C). However, in contrast to stimulation with K562 cells, we found that stimulation with Ab-coated P815 cells induced a higher percentage of PD-1+ NK cells to degranulate than of PD-1− NK cells (Fig. 5B) (% CD107a+ of PD-1− NK cell median, 17.32%; % CD107a+ of PD-1+ NK cell median, 38.26%; P = 0.0348), whereas no significant difference in the percentage of IFN-γ-producing cells was observed between PD-1+ and PD-1− NK cells (Fig. 5C). Although the majority of both PD-1− and PD-1+ NK cells expressed CD16, a higher percentage of PD-1+ NK cells were CD16+ (Fig. 5D) (% CD16+ PD-1+ cell mean, 80.20%; 95% confidence interval [CI], 67.75 to 92.65%; % CD16+ PD-1− cell mean, 63.37%; 95% CI, 51.26 to 75.48; P = 0.0027), and the CD16 MFI was higher for PD-1+ NK cells (Fig. 5E) (CD16 MFI on PD-1+ cells, 5,276; 95% CI, 3,721 to 6,831; CD16 MFI on PD-1− cells, 3,461; 95% CI, 2,316 to 4,607; P < 0.0001), potentially explaining the increased degranulation of PD-1+ NK cells and suggesting that PD-1 expression may be associated with differences in the repertoire of activating and inhibitory receptors.
FIG 5.
PD-1 expression is associated with enhanced NK cell degranulation following stimulation of CD16 by ADCC. (A) Representative flow cytometry plots of CD107a (upper panels)- and IFN-γ (lower panels)-producing NK cells among PBMCs after 5 h of stimulation with antibody-coated (anti-mouse CD32) versus uncoated (no antibody) P815 cells. (B and C) Percentage of CD107a-positive (B) and IFN-γ-positive (C) NK cells stratified by PD-1 expression following stimulation of PBMCs with antibody-coated P815 cells. (D) Percentage of CD16-expressing PD-1+ and PD-1− NK cells. (E) CD16 MFI on PD-1+ and PD-1− NK cells. (B and C) Data are shown as medians. P values were determined by the Friedman test, adjusted for multiple comparisons. (D and E) Data are shown as means ± SD. P values were determined by a paired t test.
DISCUSSION
Here, we show in a longitudinal study that acute malaria in some individuals is associated with increased production of proinflammatory cytokines and PD-1 expression on NK cells, in concert with upregulation of the PD-1 ligands PD-L1 and PD-L2 on a variety of lymphocyte populations. Ten days after malaria treatment, cytokine-producing NK cells were not detectable, while PD-1 expression on NK cells continued to decrease slowly, not returning to preinfection levels until the end of the subsequent 6-month dry season. Given the upregulation of PD-1 expression during acute malaria, we speculated that cumulative malaria exposure over years would be associated with an accumulation of PD-1 on NK cells. Indeed, at the healthy baseline before the malaria season, PD-1 expression on NK cells increased with age and reached levels in Malian adults that were higher than levels of PD-1+ NK cells in U.S. adults. Circulating PD-1+ NK cells appear to be relatively stable and not simply an indicator of recent NK cell activation, since Malian adults had increased levels of PD-1+ NK cells in the absence of recent febrile illnesses.
Previous studies have shown that exposure to pathogens such as cytomegalovirus (CMV) modulates the expression of activating and inhibitory receptors on CD56dim NK cells (55–57). Lacking control samples from U.S. children or adolescents, we cannot exclude the possibility that similar age-dependent changes occur in individuals who are not exposed to malaria. However, increased PD-1 expression in Malian adults versus U.S. adults suggests that PD-1 is upregulated through exposure to intense malaria transmission and/or factors associated with malaria transmission in this region, such as differences in pathogen exposure, microbiota composition, and diet. In addition, differential PD-1 expression due to differences in biospecimen processing in the United States and Mali cannot be ruled out. This hypothesis is supported by in vitro findings that P. falciparum-infected red blood cells induce PD-1 upregulation in NK cells of malaria-naive adults. If repeated exposure to Plasmodium or other pathogens is causing the age-dependent increase in PD-1 expression on NK cells, these cells, originally classified as components of the innate immune system, are “remembering” preceding infections. Immunological memory, allowing for rapid and improved effector functions in response to a secondary infection, is a quality that defines the adaptive immune system. Recent NK cell studies showed that NK cells in fact have features of both innate and adaptive immunity (for reviews, see references 58 and 59), including the ability to generate long-lived memory cells (60, 61) that can mount robust recall responses (62–64). Indeed, we recently reported that malaria exposure is associated with a high proportion of adaptive NK cells, which are defined by the loss of transcription factor PLZF and Fc receptor γ-chain. Adaptive NK cells dominated ADCC responses, and their frequency within total NK cells correlated with lower parasitemia and resistance to malaria (43). This is reminiscent of the enhanced ADCC responses reported for a subset of memory-like NK cells that expand in CMV-infected individuals (65–67).
To investigate whether PD-1 expression modulates NK cell function, or correlates with NK functional properties, we performed functional assays that trigger two different mechanisms of NK cell activation. In a natural cytotoxicity assay, in which NK-sensitive target cells lacking ligands of inhibitory receptors trigger NK cells, we found that PD-1-expressing NK cells showed diminished degranulation relative to PD-1-negative NK cells, although some individuals had higher basal levels of CD107a+ NK cells in the PD-1+ subset; the origin and significance of this observation requires further investigation. Conversely, in an ADCC assay, in which NK cells are stimulated by antibody-coated target cells through the Fc receptor CD16, we demonstrated enhanced degranulation of PD-1-expressing NK cells compared to that of PD-1-negative NK cells. In addition to the expression of PD-1, these NK cells also displayed elevated levels of CD16, which might explain the increased activity detected in the ADCC functional assay. In T cells, engagement of the PD-1 receptor with one of its two ligands, PD-L1 or PD-L2, delivers a coinhibitory signal to proximal TCR signaling complexes (19–21, 68), leading to attenuated T cell receptor signaling, thereby limiting or preventing cytokine production and proliferation of these cells (reviewed in reference 18). However, the diminished degranulation by PD-1+ NK cells observed here might not be due to inhibitory signaling by PD-1, since the target cell K562 does not express PD-L1 or PD-L2.
It is possible that PD-1+ NK cells are “educated” (or “tuned”) to become more responsive by hematopoietic cells that express PD-L1 or PD-L2. NK cell inhibitory receptors, in particular those that bind to MHC class I, have a dual function: they inhibit NK cell reactivity toward cells expressing MHC class I but also maintain NK cells in a state of responsiveness to subsequent activation events, a process referred to as “education.” Similarly, PD-1+ NK cells may acquire stronger responsiveness toward PD-L-negative cells. The enhanced response of PD-1+ NK cells that we observed here was limited to their ADCC activity. In contrast, natural cytotoxicity by PD-1+ NK cells toward K562 target cells was reduced. This could be due to the fundamental differences in the signaling pathways that lead to NK cell activation through CD16 and through synergistic combination of coactivation receptors (54, 69).
Previous studies have described enhanced NK cell cytotoxicity against PD-L1-expressing multiple myeloma tumor cells after blocking PD-1 in vitro (34). Similarly, NK cells from Mycobacterium tuberculosis-infected subjects show elevated IFN-γ production and degranulation when stimulated with M. tuberculosis antigen while blocking PD-1 in vitro (37). In addition, PD-1-expressing NK cells from individuals with renal cell carcinoma or hepatitis C virus infection downregulate PD-1 expression after tumor removal (31) or successful antiviral treatment (36), respectively. This suggests that PD-1 expression on NK cells may be a consequence of continuous stimulation with a specific antigen, and although it is not known which combination of activating and/or inhibiting receptors on NK cells are recognizing the specific antigen, in general, PD-1 expression appears to be associated with less-responsive NK cells in the context of cancer and other infectious diseases.
During Plasmodium infections, NK cells are a major source of proinflammatory cytokines (47, 51, 70, 71) and eliminate iRBCs via ADCC in vitro (42). Whether iRBCs express activating ligands that can induce natural cytotoxicity in NK cells is still under active investigation. However, it is well known that individuals acquire antibodies against blood-stage antigens after repeated malaria infections (72), which is consistent with enhanced elimination of iRBCs by ADCC. If repeated Plasmodium infections lead to the development of an NK cell population that expresses the inhibitory receptor PD-1 and at the same time an elevated level of the activating receptor CD16, these NK cells might display reduced activity toward potential target cells expressing one of the PD-1 ligands but enhanced ADCC activity against iRBCs that do not express PD-L1 or PD-L2. That disease severity declines and PD-1 expression on NK cells increases with cumulative malaria exposure supports this hypothesis. However, it remains to be determined if PD-1+ and PD-1− NK cells differ in their ability to destroy iRBCs.
In summary, these data suggest that PD-1 may contribute to the regulation of NK cell effector functions during infections such as malaria, findings that may have implications for PD-1-targeted therapies for infectious diseases, cancer, and autoimmunity.
MATERIALS AND METHODS
Ethics statement.
The study in Mali was approved by the Ethics Committee of the Faculty of Medicine, Pharmacy and Odonto-Stomatology at the University of Bamako, Mali, and the Institutional Review Board at the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIAID protocol numbers 06-I-N147 and 11-I-N126). PBMCs from healthy U.S. adults were obtained from the NIH blood bank (NIH protocol number 99-CC-0168). Written informed consent was obtained from adult study participants and the parents or legal guardians of participating children.
Study site and participants.
This study was conducted in the rural villages of Kambila and Kalifabougou, Mali, where P. falciparum transmission is seasonal and intense from July through December. Detailed descriptions of both study sites and study designs have been reported previously (73, 74). The present study analyzed peripheral blood mononuclear cells (PBMCs) collected at four time points: at the end of the 6-month dry season (a period of negligible malaria transmission), just before treatment of the first acute malaria episode of the ensuing 6-month malaria season and 10 days later, and at the end of the subsequent 6-month dry season. Acute malaria was defined as an axillary temperature ≥37.5°C, asexual parasitemia of ≥5,000 parasites/μl of blood, and no other cause of fever discernible on physical examination. PBMCs from healthy U.S. adults were obtained from the NIH blood bank; previous P. falciparum exposure history is unknown in these anonymous U.S. donors but is highly unlikely.
PBMC isolation.
Malian blood samples were drawn by venipuncture into sodium citrate-containing cell preparation tubes (BD, Franklin Lakes, NJ) and transported to the laboratory, and PBMCs were isolated within 2 h according to the manufacturer’s instructions. The cell preparation tubes contain an inert gel that forms a physical barrier during centrifugation to separate PBMCs from whole blood. PBMCs were washed twice with phosphate-buffered saline (PBS; KD Medical, Columbia, MD) and frozen in heat-inactivated fetal bovine serum (FBS; Gibco, Grand Island, NY) and 7.5% dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO). PBMCs were kept in freezing containers (VWR International) at −80°C for 24 h and then transferred to liquid nitrogen. PBMCs from U.S. donors were isolated by Ficoll-Hypaque (Amersham Biosciences) density gradient centrifugation of leukapheresis samples (in vitro stimulation assays) or whole blood collected into sodium citrate-containing tubes (ex vivo phenotyping by flow cytometry) and then frozen as described for Mali samples. For analysis, PBMCs were rapidly thawed in a 37°C water bath and washed first in PBS with 10% heat-inactivated FBS and then in complete RPMI medium (RPMI 1640 with l-glutamine, supplemented with 10% heat-inactivated FBS, penicillin/streptomycin at 10,000 μg/ml, and 50 μM β-mercaptoethanol [all from Gibco, Invitrogen, Grand Island, NY]). PBMC viability determined by trypan blue exclusion was between 85% and 95%.
Cell lines.
The human erythroblast cell line K562 and the murine mastocytoma cell line P815 (both obtained from the American Type Culture Collection [ATCC]) were maintained in RPMI medium (Gibco) supplemented with 10% fetal bovine serum (FBS).
Flow cytometry.
Thawed PBMCs (1 × 106 to 2 × 106) were transferred to 96-well U-bottom plates, washed with PBS, and incubated for 20 min with a live/dead stain (Molecular Probes, Invitrogen, Eugene, OR). Surface antigens were stained for 30 min in fluorescence-activated cell sorter (FACS) buffer (PBS, 3% heat-inactivated FBS, 0.03% sodium azide) using the following antibodies: anti-human CD3 BD Horizon V500 (UCHT1), anti-human PD-1 phycoerythrin (PE) (MIH4) obtained from BD Biosciences, anti-human CD56 Alexa Fluor 488 (NCAM) obtained from eBioscience, and anti-human CD16 allophycocyanin (APC)-Cy7 (3G8) from Biolegend. Isotype-matched control antibodies and fluorescence minus one (FMO) controls were used to determine background staining. PBMCs were analyzed with a BD LSRII flow cytometer (BD Biosciences), and data analysis was performed using FlowJo software (Tree Star, Inc.; version 6.0).
NK cell functional assays.
PBMCs were thawed and rested overnight at 37°C in flat-bottom 96-well plates at 5 × 105 PBMCs/100 μl complete RPMI medium. The percentage of NK cells expressing PD-1 was determined by flow cytometry after the overnight resting period by using the following monoclonal antibodies: anti-human CD3 BV510 (OKT3; Biolegend), anti-human CD56 Alexa Fluor 488 (NCAM; Affymetrix eBioscience), and anti-human PD-1 PE (MIH4; BD Biosciences). NK cell activity was determined by IFN-γ production and by CD107a expression as a measure of NK cell degranulation (75). Briefly, 5 × 105 PBMCs were incubated with or without target cells in the presence of anti-human CD107a PE-Cy7 (H4A3, BD Biosciences) and BD GolgiStop (1:100 diluted; BD Biosciences) for 5 h at 37°C. The target cells were either K562 cells at a 1:1 ratio (PBMCs to K562 cells) or P815 cells at a 2:1 ratio (PBMCs to P815 cells). P815 cells were pretreated with or without 2 μg/ml purified anti-mouse CD16/CD32 antibody (2.4G2; BD Pharmingen) for 30 min on ice and washed thoroughly before they were added to the PBMCs. Following the incubation period, the cell mixture was stained with the above-mentioned monoclonal antibodies against CD3, CD56, and PD-1. After the cells were fixed and lysed (eBioscience FoxP3/transcription factor staining buffer set), intracellular IFN-γ was stained with anti-human IFN-γ PE-CF594 (B27; BD Biosciences). The cells were acquired with a BD LSRII flow cytometer (BD Biosciences). NK cells were identified as CD3− CD56+ cells within the FSC-SSC gated lymphocyte population after gating on singlet cells. The same experiments were repeated independently with similar results after substituting the following monoclonal antibodies: anti-human CD3 fluorescein isothiocyanate (FITC) (HIT3a; Biolegend), Brilliant Violet 421 anti-human CD279 (PD-1), Brilliant Violet 785 anti-human CD107a (Biolegend; clone H4A3c), and anti-human IFN-γ PE/Dazzle 594. Cells were then acquired with a BD X20 (BD Biosciences) flow cytometer. In one set of experiments, after the PBMCs were incubated with GoInVivo purified anti-human PDL-1 blocking antibody (10 μg/ml) or control IgG for 1 h, K562 target cells were added to the culture at a 1:1 ratio (PBMCs to K562 cells) and stained with the above-described monoclonal antibodies.
Preparation of iRBCs for in vitro stimulation of PBMCs.
The 3D7 strain of P. falciparum was maintained in fresh human ORh+ erythrocytes at 3 to 5% hematocrit in RPMI 1640 medium (KD Medical) supplemented with 10% heat-inactivated ORh+ human serum (Interstate Blood Bank, Memphis, TN), 7.4% sodium bicarbonate (Life Technologies), and 25 mg/ml of gentamicin (Life Technologies) at 37°C in the presence of a gas mixture containing 5% O2, 5%CO2, and 90% N2. Parasite cultures were shown to be free of Mycoplasma. P. falciparum schizont-infected RBCs (iRBCs) were isolated in RPMI 1640 medium supplemented with 0.25% Albumax (GIBCO, Invitrogen) and 7.4% sodium bicarbonate (Life Technologies) using magnetic columns (LD MACS separation columns; Miltenyi Biotec). Control preparations of uninfected RBCs from the same blood donor were obtained and tested in all experiments. Lysates of P. falciparum-infected and uninfected RBCs were obtained by three freeze-thaw cycles in liquid nitrogen and a 37°C water bath.
In vitro stimulation of PBMCs with P. falciparum lysate.
PBMCs were thawed and rested for 4 h at 37°C in flat-bottom 6-well plates at 3 × 106 PBMCs/ml in complete RPMI medium. After the initial resting period, either iRBCs or uninfected control RBCs were added to the culture at a ratio of 1:30 (PBMCs to iRBCs or RBCs) and kept at 37°C for 3 days. After the incubation period, cells were harvested and stained with the following monoclonal antibodies: anti-human CD3 FITC (Biolegend; cloneHIT3a), Brilliant Violet 421anti-human PD1 (Biolegend; clone NAT105), and BUV 737 anti-human CD56 (BD Bioscience; clone NCAM16.2). The cells were acquired with a BD X20 (BD Biosciences) flow cytometer. NK cells were identified as CD3− CD56+ cells within the FSC-SSC lymphocyte population after gating on singlet cells.
In vitro stimulation of purified NK cells with P. falciparum lysate.
NK cells were purified from PBMCs with magnetic separation kits according to the manufacturer’s instructions. First, CD3-positive cells were removed from PBMCs using the Stem Cell Technologies EasySep human CD3-positive selection kit. NK cells were then enriched through negative selection with the EasySep human NK cell enrichment kit, resulting in an NK cell purity of approximately 98%. Isolated NK cells were incubated for 3 days at 37°C with either iRBCs or uninfected control RBCs at a ratio of 1:30 (PBMCs to iRBCs or RBCs). After 3 days, cells were harvested, stained with monoclonal antibodies, and analyzed by flow cytometry as described above. Cytokines from the culture supernatants were measured using the Bio-plex human cytokine kit (Bio-Rad) and a Luminex 200 device (Bio-Rad) per the manufacturer’s protocol.
Statistical analysis.
Statistical tests are indicated in the figure legends. All analyses were performed using GraphPad Prism (GraphPad Software, version 8.0). Two-tailed P values of ≤0.05 were considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank the residents of Kalifabougou and Kambila, Mali, for participating in this study and members of the E.O.L. and P.D.C. laboratories for helpful discussions. J.M., R.G., M.P., K.A., S.L., G.A., B.T., S.R., E.O.L., and P.D.C. designed the study. J.M. and R.G. performed the experiments. J.M., R.G., J.S., and P.D.C. analyzed the data. J.M., R.G., E.O.L., and P.D.C. wrote the manuscript. This study was funded by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.French AR, Yokoyama WM. 2003. Natural killer cells and viral infections. Curr Opin Immunol 15:45–51. doi: 10.1016/s095279150200002x. [DOI] [PubMed] [Google Scholar]
- 2.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol 17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 3.Chen Q, Amaladoss A, Ye W, Liu M, Dummler S, Kong F, Wong LH, Loo HL, Loh E, Tan SQ, Tan TC, Chang KT, Dao M, Suresh S, Preiser PR, Chen J. 2014. Human natural killer cells control Plasmodium falciparum infection by eliminating infected red blood cells. Proc Natl Acad Sci U S A 111:1479–1484. doi: 10.1073/pnas.1323318111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roland J, Soulard V, Sellier C, Drapier AM, Di Santo JP, Cazenave PA, Pied S. 2006. NK cell responses to Plasmodium infection and control of intrahepatic parasite development. J Immunol 177:1229–1239. doi: 10.4049/jimmunol.177.2.1229. [DOI] [PubMed] [Google Scholar]
- 5.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. 2002. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer 2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 6.Waldhauer I, Steinle A. 2008. NK cells and cancer immunosurveillance. Oncogene 27:5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 7.Orange JS. 2008. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol 8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang W, Chai NR, Maric D, Bielekova B. 2011. Unexpected role for granzyme K in CD56bright NK cell-mediated immunoregulation of multiple sclerosis. J Immunol 187:781–790. doi: 10.4049/jimmunol.1100789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flodström-Tullberg M, Bryceson YT, Shi F-D, Höglund P, Ljunggren H-G. 2009. Natural killer cells in human autoimmunity. Curr Opin Immunol 21:634–640. doi: 10.1016/j.coi.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Crouse J, Xu HC, Lang PA, Oxenius A. 2015. NK cells regulating T cell responses: mechanisms and outcome. Trends Immunol 36:49–58. doi: 10.1016/j.it.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. 2007. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity 26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fehniger TA, Cai SF, Cao X, Bredemeyer AJ, Presti RM, French AR, Ley TJ. 2007. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity 26:798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Gidlund M, Orn A, Wigzell H, Senik A, Gresser I. 1978. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature 273:759–761. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- 14.Lanier LL. 1998. NK cell receptors. Annu Rev Immunol 16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama WM. 1998. Natural killer cell receptors. Curr Opin Immunol 10:298–305. doi: 10.1016/s0952-7915(98)80168-4. [DOI] [PubMed] [Google Scholar]
- 16.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. 2013. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol 31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin HT, Ahmed R, Okazaki T. 2011. Role of PD-1 in regulating T-cell immunity. Curr Top Microbiol Immunol 350:17–37. doi: 10.1007/82_2010_116. [DOI] [PubMed] [Google Scholar]
- 18.Kamphorst AO, Ahmed R. 2013. Manipulating the PD-1 pathway to improve immunity. Curr Opin Immunol 25:381–388. doi: 10.1016/j.coi.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, Qiu Y, Jussif JM, Carter LL, Wood CR, Chaudhary D. 2004. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett 574:37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 20.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. 2004. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 21.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. 2005. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. 2010. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, Koren-Michowitz M, Shimoni A, Nagler A. 2008. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res 14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 24.Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. 2015. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med 21:24–33. doi: 10.1016/j.molmed.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 26.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med 203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 28.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. 2011. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol 13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirata S, Senju S, Matsuyoshi H, Fukuma D, Uemura Y, Nishimura Y. 2005. Prevention of experimental autoimmune encephalomyelitis by transfer of embryonic stem cell-derived dendritic cells expressing myelin oligodendrocyte glycoprotein peptide along with TRAIL or programmed death-1 ligand. J Immunol 174:1888–1897. doi: 10.4049/jimmunol.174.4.1888. [DOI] [PubMed] [Google Scholar]
- 30.Ding H, Wu X, Wu J, Yagita H, He Y, Zhang J, Ren J, Gao W. 2006. Delivering PD-1 inhibitory signal concomitant with blocking ICOS co-stimulation suppresses lupus-like syndrome in autoimmune BXSB mice. Clin Immunol 118:258–267. doi: 10.1016/j.clim.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Macfarlane AWt, Jillab M, Plimack ER, Hudes GR, Uzzo RG, Litwin S, Dulaimi E, Al-Saleem T, Campbell KS. 2014. PD-1 expression on peripheral blood cells increases with stage in renal cell carcinoma patients and is rapidly reduced after surgical tumor resection. Cancer Immunol Res 2:320–331. doi: 10.1158/2326-6066.CIR-13-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pesce S, Greppi M, Tabellini G, Rampinelli F, Parolini S, Olive D, Moretta L, Moretta A, Marcenaro E. 2017. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: a phenotypic and functional characterization. J Allergy Clin Immunol 139:335–346.e3. doi: 10.1016/j.jaci.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 33.Beldi-Ferchiou A, Lambert M, Dogniaux S, Vely F, Vivier E, Olive D, Dupuy S, Levasseur F, Zucman D, Lebbe C, Sene D, Hivroz C, Caillat-Zucman S. 2016. PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget 7:72961–72977. doi: 10.18632/oncotarget.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benson DM Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, Baiocchi RA, Zhang J, Yu J, Smith MK, Greenfield CN, Porcu P, Devine SM, Rotem-Yehudar R, Lozanski G, Byrd JC, Caligiuri MA. 2010. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 116:2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norris S, Coleman A, Kuri-Cervantes L, Bower M, Nelson M, Goodier MR. 2012. PD-1 expression on natural killer cells and CD8(+) T cells during chronic HIV-1 infection. Viral Immunol 25:329–332. doi: 10.1089/vim.2011.0096. [DOI] [PubMed] [Google Scholar]
- 36.Golden-Mason L, Klarquist J, Wahed AS, Rosen HR. 2008. Cutting edge: programmed death-1 expression is increased on immunocytes in chronic hepatitis C virus and predicts failure of response to antiviral therapy: race-dependent differences. J Immunol 180:3637–3641. doi: 10.4049/jimmunol.180.6.3637. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez IB, Pasquinelli V, Jurado JO, Abbate E, Musella RM, de la Barrera SS, García VE. 2010. Role played by the programmed death-1-programmed death ligand pathway during innate immunity against Mycobacterium tuberculosis. J Infect Dis 202:524–532. doi: 10.1086/654932. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Correa B, Lopez-Sejas N, Duran E, Labella F, Alonso C, Solana R, Tarazona R. 2019. Modulation of NK cells with checkpoint inhibitors in the context of cancer immunotherapy. Cancer Immunol Immunother 68:861–870. doi: 10.1007/s00262-019-02336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muntasell A, Ochoa MC, Cordeiro L, Berraondo P, López-Díaz de Cerio A, Cabo M, López-Botet M, Melero I. 2017. Targeting NK-cell checkpoints for cancer immunotherapy. Curr Opin Immunol 45:73–81. doi: 10.1016/j.coi.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Guillerey C, Huntington ND, Smyth MJ. 2016. Targeting natural killer cells in cancer immunotherapy. Nat Immunol 17:1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 41.Wiesmayr S, Webber SA, Macedo C, Popescu I, Smith L, Luce J, Metes D. 2012. Decreased NKp46 and NKG2D and elevated PD-1 are associated with altered NK-cell function in pediatric transplant patients with PTLD. Eur J Immunol 42:541–550. doi: 10.1002/eji.201141832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arora G, Hart GT, Manzella-Lapeira J, Doritchamou JY, Narum DL, Thomas LM, Brzostowski J, Rajagopalan S, Doumbo OK, Traore B, Miller LH, Pierce SK, Duffy PE, Crompton PD, Desai SA, Long EO. 2018. NK cells inhibit Plasmodium falciparum growth in red blood cells via antibody-dependent cellular cytotoxicity. Elife 7:e36806. doi: 10.7554/eLife.36806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hart GT, Tran TM, Theorell J, Schlums H, Arora G, Rajagopalan S, Sangala ADJ, Welsh KJ, Traore B, Pierce SK, Crompton PD, Bryceson YT, Long EO. 2019. Adaptive NK cells in people exposed to Plasmodium falciparum correlate with protection from malaria. J Exp Med 216:1280–1290. doi: 10.1084/jem.20181681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mavoungou E, Held J, Mewono L, Kremsner PG. 2007. A Duffy binding-like domain is involved in the NKp30-mediated recognition of Plasmodium falciparum-parasitized erythrocytes by natural killer cells. J Infect Dis 195:1521–1531. doi: 10.1086/515579. [DOI] [PubMed] [Google Scholar]
- 45.Wolf AS, Sherratt S, Riley EM. 2017. NK cells: uncertain allies against malaria. Front Immunol 8:212. doi: 10.3389/fimmu.2017.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doolan DL, Hoffman SL. 1999. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J Immunol 163:884–892. [PubMed] [Google Scholar]
- 47.Mohan K, Moulin P, Stevenson MM. 1997. Natural killer cell cytokine production, not cytotoxicity, contributes to resistance against blood-stage Plasmodium chabaudi AS infection. J Immunol 159:4990–4998. [PubMed] [Google Scholar]
- 48.Orago AS, Facer CA. 1991. Cytotoxicity of human natural killer (NK) cell subsets for Plasmodium falciparum erythrocytic schizonts: stimulation by cytokines and inhibition by neomycin. Clin Exp Immunol 86:22–29. doi: 10.1111/j.1365-2249.1991.tb05768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mavoungou E, Luty AJ, Kremsner PG. 2003. Natural killer (NK) cell-mediated cytolysis of Plasmodium falciparum-infected human red blood cells in vitro. Eur Cytokine Netw 14:134–142. [PubMed] [Google Scholar]
- 50.Hansen DS, Bernard NJ, Nie CQ, Schofield L. 2007. NK cells stimulate recruitment of CXCR3+ T cells to the brain during Plasmodium berghei-mediated cerebral malaria. J Immunol 178:5779–5788. doi: 10.4049/jimmunol.178.9.5779. [DOI] [PubMed] [Google Scholar]
- 51.Agudelo O, Bueno J, Villa A, Maestre A. 2012. High IFN-gamma and TNF production by peripheral NK cells of Colombian patients with different clinical presentation of Plasmodium falciparum. Malar J 11:38. doi: 10.1186/1475-2875-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. 2000. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. 2001. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 54.Bryceson YT, March ME, Ljunggren HG, Long EO. 2006. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang SM, Norris PJ, Nixon DF, Lanier LL. 2011. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A 108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, Lopez-Verges S, Lanier LL, Weisdorf D, Miller JS. 2012. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood 119:2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muntasell A, Vilches C, Angulo A, Lopez-Botet M. 2013. Adaptive reconfiguration of the human NK-cell compartment in response to cytomegalovirus: a different perspective of the host-pathogen interaction. Eur J Immunol 43:1133–1141. doi: 10.1002/eji.201243117. [DOI] [PubMed] [Google Scholar]
- 58.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. 2011. Innate or adaptive immunity? The example of natural killer cells. Science 331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quintin J, Cheng SC, van der Meer JW, Netea MG. 2014. Innate immune memory: towards a better understanding of host defense mechanisms. Curr Opin Immunol 29:1–7. doi: 10.1016/j.coi.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, Wallace DL, de Lara CM, Ghattas H, Asquith B, Worth A, Griffin GE, Taylor GP, Tough DF, Beverley PC, Macallan DC. 2007. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology 121:258–265. doi: 10.1111/j.1365-2567.2007.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nikzad R, Angelo LS, Aviles-Padilla K, Le DT, Singh VK, Bimler L, Vukmanovic-Stejic M, Vendrame E, Ranganath T, Simpson L, Haigwood NL, Blish CA, Akbar AN, Paust S. 2019. Human natural killer cells mediate adaptive immunity to viral antigens. Sci Immunol 4:eaat8116. doi: 10.1126/sciimmunol.aat8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. 2006. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol 7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 63.Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, von Andrian UH. 2010. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol 11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun JC, Beilke JN, Lanier LL. 2009. Adaptive immune features of natural killer cells. Nature 457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Costa-Garcia M, Vera A, Moraru M, Vilches C, López-Botet M, Muntasell A. 2015. Antibody-mediated response of NKG2Cbright NK cells against human cytomegalovirus. J Immunol 194:2715–2724. doi: 10.4049/jimmunol.1402281. [DOI] [PubMed] [Google Scholar]
- 66.Zhang T, Scott JM, Hwang I, Kim S. 2013. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. J Immunol 190:1402–1406. doi: 10.4049/jimmunol.1203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD, Han H, Chiang SC, Foley B, Mattsson K, Larsson S, Schaffer M, Malmberg KJ, Ljunggren HG, Miller JS, Bryceson YT. 2015. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 42:443–456. doi: 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Freeman GJ. 2008. Structures of PD-1 with its ligands: sideways and dancing cheek to cheek. Proc Natl Acad Sci U S A 105:10275–10276. doi: 10.1073/pnas.0805459105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim HS, Long EO. 2012. Complementary phosphorylation sites in the adaptor protein SLP-76 promote synergistic activation of natural killer cells. Sci Signal 5:ra49. doi: 10.1126/scisignal.2002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Artavanis-Tsakonas K, Riley EM. 2002. Innate immune response to malaria: rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J Immunol 169:2956–2963. doi: 10.4049/jimmunol.169.6.2956. [DOI] [PubMed] [Google Scholar]
- 71.McCall MB, Roestenberg M, Ploemen I, Teirlinck A, Hopman J, de Mast Q, Dolo A, Doumbo OK, Luty A, van der Ven AJ, Hermsen CC, Sauerwein RW. 2010. Memory-like IFN-gamma response by NK cells following malaria infection reveals the crucial role of T cells in NK cell activation by P falciparum. Eur J Immunol 40:3472–3477. doi: 10.1002/eji.201040587. [DOI] [PubMed] [Google Scholar]
- 72.Portugal S, Pierce SK, Crompton PD. 2013. Young lives lost as B cells falter: what we are learning about antibody responses in malaria. J Immunol 190:3039–3046. doi: 10.4049/jimmunol.1203067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tran TM, Li S, Doumbo S, Doumtabe D, Huang CY, Dia S, Bathily A, Sangala J, Kone Y, Traore A, Niangaly M, Dara C, Kayentao K, Ongoiba A, Doumbo OK, Traore B, Crompton PD. 2013. An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin Infect Dis doi: 10.1093/cid/cit174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Crompton PD, Traore B, Kayentao K, Doumbo S, Ongoiba A, Diakite SA, Krause MA, Doumtabe D, Kone Y, Weiss G, Huang CY, Doumbia S, Guindo A, Fairhurst RM, Miller LH, Pierce SK, Doumbo OK. 2008. Sickle cell trait is associated with a delayed onset of malaria: implications for time-to-event analysis in clinical studies of malaria. J Infect Dis 198:1265–1275. doi: 10.1086/592224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aktas E, Kucuksezer UC, Bilgic S, Erten G, Deniz G. 2009. Relationship between CD107a expression and cytotoxic activity. Cell Immunol 254:149–154. doi: 10.1016/j.cellimm.2008.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.