Modeling host-pathogen interactions with human intestinal epithelia using enteroid monolayers on permeable supports (such as Transwells) represents an alternative to animal studies or use of colon cancer-derived cell lines. However, the static monolayer model does not expose epithelial cells to mechanical forces normally present in the intestine, including luminal flow and serosal blood flow (shear force) or peristaltic forces.
KEYWORDS: ETEC, heat-stable enterotoxin, mechanical stress, peristalsis, enteroids, Intestine-Chip
ABSTRACT
Modeling host-pathogen interactions with human intestinal epithelia using enteroid monolayers on permeable supports (such as Transwells) represents an alternative to animal studies or use of colon cancer-derived cell lines. However, the static monolayer model does not expose epithelial cells to mechanical forces normally present in the intestine, including luminal flow and serosal blood flow (shear force) or peristaltic forces. To determine the contribution of mechanical forces in the functional response of human small intestine to a virulence factor of a pathogenic intestinal bacterium, human jejunal enteroids were cultured as monolayers in microengineered fluidic-based Organ-Chips (Intestine-Chips) exposed to enterotoxigenic Escherichia coli heat-stable enterotoxin A (ST) and evaluated under conditions of static fluid, apical and basolateral flow, and flow plus repetitive stretch. Application of flow increased epithelial cell height and apical and basolateral secretion of cyclic GMP (cGMP) under baseline, unstimulated conditions. Addition of ST under flow conditions increased apical and basolateral secretion of cGMP relative to the level under static conditions but did not enhance intracellular cGMP accumulation. Cyclic stretch did not have any significant effect beyond that contributed by flow. This study demonstrates that fluid flow application initiates changes in intestinal epithelial cell characteristics relative to those of static culture conditions under both baseline conditions and with exposure to ST enterotoxin and suggests that further investigations of the application of these mechanical forces will provide insights into physiology and pathophysiology that more closely resemble intact intestine than study under static conditions.
INTRODUCTION
Human adult intestinal stem cell-derived enteroids/organoids retain intestinal segment-specific transcriptional as well as phenotypic characteristics that enable study of untransformed, noncancer epithelia (1–4). Enteroids are generally propagated as three-dimensional (3D) basement membrane matrix-embedded spheroids that are polarized such that the inward-facing apical (AP) cell surface is not directly accessible. This places limitations on host-pathogen interaction studies. Enteroids grown as monolayers on collagen IV-coated permeable supports overcome the challenges of 3D culture by permitting direct application of commensal organisms, pathogens, toxins, nutrients, electrolytes, and drugs to the apical cell surface and enabling subsequent sampling of exported metabolites from both apical and basolateral (BL) surfaces (2, 5–8).
Static enteroid monolayers do not, however, recreate mechanical forces acting on intestinal epithelia in vivo (4) as they lack luminal flow and blood flow (shear stress forces) and the rhythmic contractions that are part of peristalsis. Although multiple efforts have yielded microengineered cell culture systems with flow to recapitulate physical forces of flow and stretch in Organ-on-Chip models (9–12), evidence of discrete advantages associated with inclusion of mechanical forces for modeling host-pathogen interactions within human intestinal epithelia in these systems is not yet clear. In this study, we evaluated the effects of mechanical forces on both baseline characteristics and the responses to heat-stable enterotoxin (ST), a virulence factor of enterotoxigenic Escherichia coli (ETEC) infection that is associated with a large burden of diarrheal illness in low- and middle-income countries (13). The model was a microengineered polydimethylsiloxane (PDMS)-based Intestine-Chip (10, 14, 15). The chip contains two parallel hollow channels separated by a flexible permeable membrane that enables human intestinal epithelial cells to be cultured in the presence of physiologically scaled fluid shear stress forces and repetitive lateral mechanical deformation (see references 14 and 15 for pictures of the experimental model). ETEC infection primarily targets villus enterocytes of the proximal small intestine (16), and thus human jejunal enteroids were used to seed in Intestine-Chips and then allowed to differentiate (17). ST acts by binding to brush border guanylate cyclase C (GCC) and stimulating cyclic GMP (cGMP) synthesis, which ultimately leads to intestinal fluid and electrolyte loss. ST is structurally and functionally related to the endogenously expressed enteric hormones guanylin and uroguanylin, as well as to the synthetic peptide linaclotide, which is used to treat chronic constipation and irritable bowel syndrome with constipation (IBS-C) (9, 18). Interestingly, linaclotide has been previously reported to induce secretion of cGMP that has been suggested as playing a role in symptomatic treatment of IBS-C (19). For this reason, apical (AP), basolateral (BL), and intracellular (IC) pools of cGMP were compared following ST exposure in the Intestine-Chip under static, flow, and flow-plus-rhythmic deformation (termed flow plus stretch) conditions.
RESULTS
Shear stress forces and cyclic strain (mechanical stimuli) induce increased extracellular cGMP secretion under baseline conditions in the Intestine-Chip.
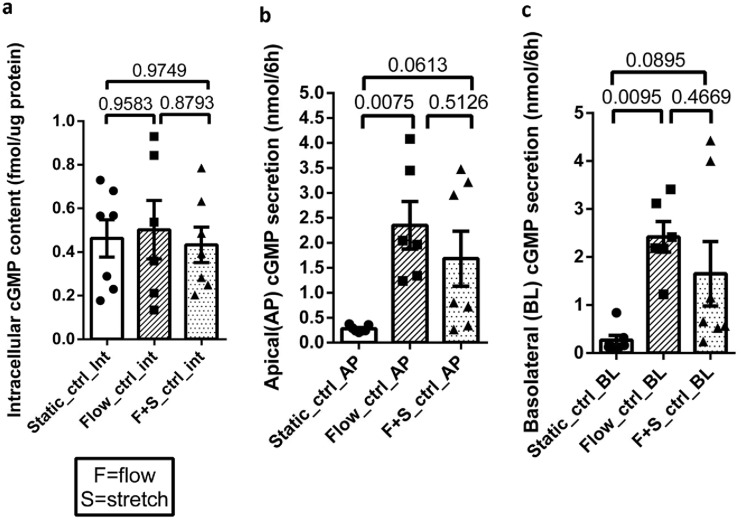
Human jejunal enteroids were seeded into the top channel of the Intestine-Chip on collagen IV-coated membrane and grown in differentiation medium (DM) for 5 days prior to each experiment. Apical and basolateral effluents (∼360 to 500 μl) were separately collected during a 6-h period, and the epithelial cells were lysed for cGMP measurement. Under basal conditions, application of single-pass flow or flow plus stretch for 6 h did not alter intracellular cGMP content relative to that under static conditions (Fig. 1a). However, both apical and basolateral cGMP secretion levels were significantly increased in response to perfusion compared to the level under static conditions (Fig. 1b and c). There was no significant difference in apical and basolateral cGMP secretion levels under otherwise basal conditions between flow alone and flow plus stretch. Of note, addition of stretch to flow was associated with large variations in apical and basolateral cGMP secretion.
FIG 1.
Basal levels of apical (AP) and basolateral (BL) cGMP secretion were increased with shear force provided by flow. (a) Intracellular cGMP content was similar under all conditions. (b and c) AP and BL cGMP secretion levels were significantly higher with application of sheer force by flow than under static conditions; flow plus stretch caused a similar magnitude of increase, but the increase did not reach statistical significance compared to the level under static conditions. There was no significant difference in the secretion levels caused by flow versus those of flow plus stretch. Results are means ± SEM (static, n = 7; flow, n = 6; flow plus stretch, n = 7). P values were determined by one-way ANOVA with Tukey’s multiple-comparison tests.
ST increases cGMP content and enhances mechanically stimulated extracellular cGMP secretion in the Intestine-Chip.
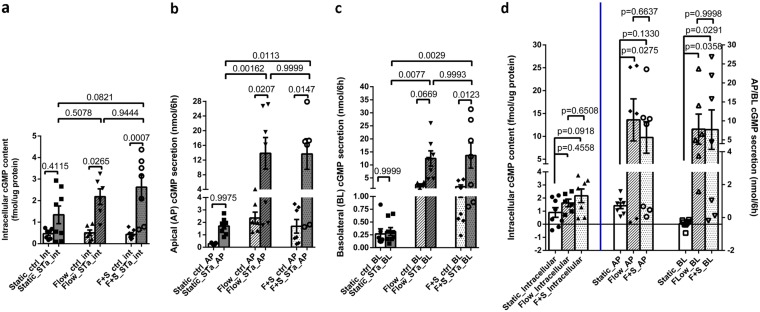
Compartmentalization was evaluated under static and mechanical force-exposed conditions by introducing ST (1 nM) in the static apical medium or the apical perfusate over 6 h. ST significantly increased intracellular cGMP content under flow and flow-plus-stretch conditions, but the increase was not significant under the static condition (Fig. 2a). However, the ST-induced increase in cGMP content was not significantly different when results under the static, flow, and flow-plus-stretch conditions were compared (Fig. 2d). ST significantly increased cGMP secretion apically under flow and flow-plus-stretch conditions but not under static conditions (Fig. 2b). The magnitudes of the increase were not statistically different between the flow and flow-plus-stretch conditions (Fig. 2b and d).
FIG 2.
Mechanical stimuli increased ST-induced AP and BL cGMP secretion levels but did not alter the ST increase in intracellular cGMP content. (a) ST increased the intracellular cGMP content under all conditions although the increase was not significant under static conditions. (b) ST significantly increased AP cGMP secretion with flow and flow plus stretch but not under static conditions. There was no difference in the AP cGMP secretion levels between the flow and flow-plus-stretch conditions. (c) ST significantly increased BL cGMP secretion only under the flow-plus-stretch condition. However, there was no difference in the BL cGMP secretion levels between the flow and flow-plus-stretch conditions. (d) The ST effect on AP cGMP secretion (difference of cGMP secretion in the presence of ST minus that under basal conditions performed on paired samples) under flow and flow plus stretch was significantly greater than the ST effect under static conditions. The ST effect on BL cGMP secretion was significantly higher under flow-plus-stretch conditions than under static conditions and was increased, although not statistically significantly, under flow alone. The ST-induced increases of AP and BL cGMP secretion levels under both flow and flow plus stretch were not different from each other. Results are means ± SEM (static basal, n = 7, and ST, n = 8; flow basal, n = 6, and ST, n = 6; flow-plus-stretch basal, n = 7, and ST, n = 7). P values were determined by one-way ANOVA with Tukey’s multiple-comparison tests. F+S, flow plus stretch.
Results were somewhat different for ST-induced basolateral secretion. ST significantly increased BL cGMP secretion only under flow-plus-stretch conditions (Fig. 2c). The ST-induced increase in basolateral secretion, which was not significant under static conditions, was significantly increased by both flow and flow plus stretch, with the magnitudes of the increase not significantly different between flow and flow-plus-stretch conditions (Fig. 2d).
In conclusion, both apical and basolateral cGMP secretion were stimulated by ST (Fig. 2d) (the ST effect is calculated as the value for the ST minus that of the control). The amounts of secretion were similar under flow and flow-plus-stretch conditions and exceeded the amount under static conditions. In contrast, ST exposure increased intracellular cGMP to surprisingly small but similar extents under static conditions and with application of flow and flow plus stretch. Hence, we hypothesized that both flow and flow plus stretch altered the ability of the enteroid to transport cGMP out of the epithelial cells in such a way that minimized the increase in intracellular cGMP content.
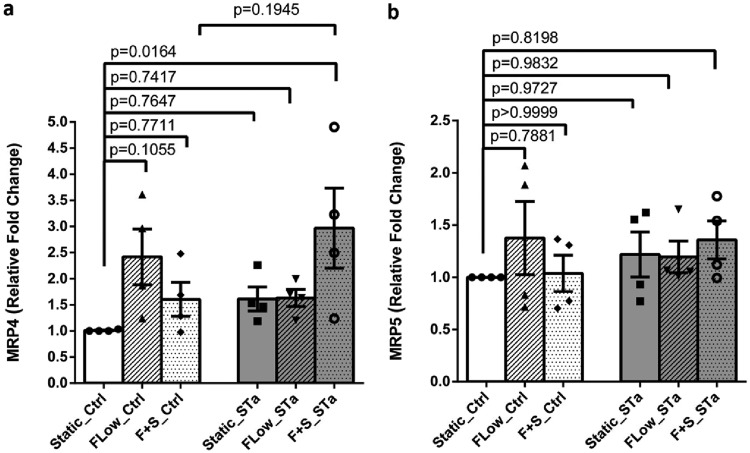
Mechanical stimuli increase jejunal MRP4 mRNA.
Further studies were performed to begin to understand the mechanism of the increased cGMP secretion. We had previously reported that ST-induced cGMP was transported out of the jejunal enteroid by a process inhibited by the multidrug resistance protein (MRP) inhibitor MK571 (3). Thus, the effects of flow and flow plus stretch were determined on jejunal MRP4 and MRP5, which are known to transport cyclic nucleotides. Both flow and flow plus stretch caused small increases in MRP4 mRNA levels under baseline conditions; however, the increases in mRNA failed to reach statistical significance (Fig. 3a). However, in the presence of ST, flow plus stretch significantly increased MRP4 mRNA. mRNA for MRP5 was not altered under any of the conditions tested (Fig. 3b). Of note, MRP5 mRNA expression was lower than that of MRP4.
FIG 3.
Flow significantly increased MRP4 but not MRP5 mRNA. MRP4 and MRP5 mRNA levels were determined by qRT-PCR under basal conditions and after 6 h of ST exposure. (a) Message for MRP4 was increased by flow and flow plus stretch under baseline conditions (statistically not significant), while ST significantly increased MRP4 mRNA under conditions of flow plus stretch. (b) In contrast, MRP5 mRNA levels were similar under basal conditions and did not change significantly with ST treatment. The level of MPR5 mRNA was lower than that of MRP4. Results are means ± SEM (n = 4). P values were determined by one-way ANOVA with Tukey’s multiple-comparison tests.
Another possible mechanism contributing to changes in cGMP in the enteroids was that physical forces increased cGMP via increasing GCC activity. As shown in Fig. 4, neither flow nor flow plus stretch altered enteroid GCC activity under baseline conditions or after ST exposure.
FIG 4.
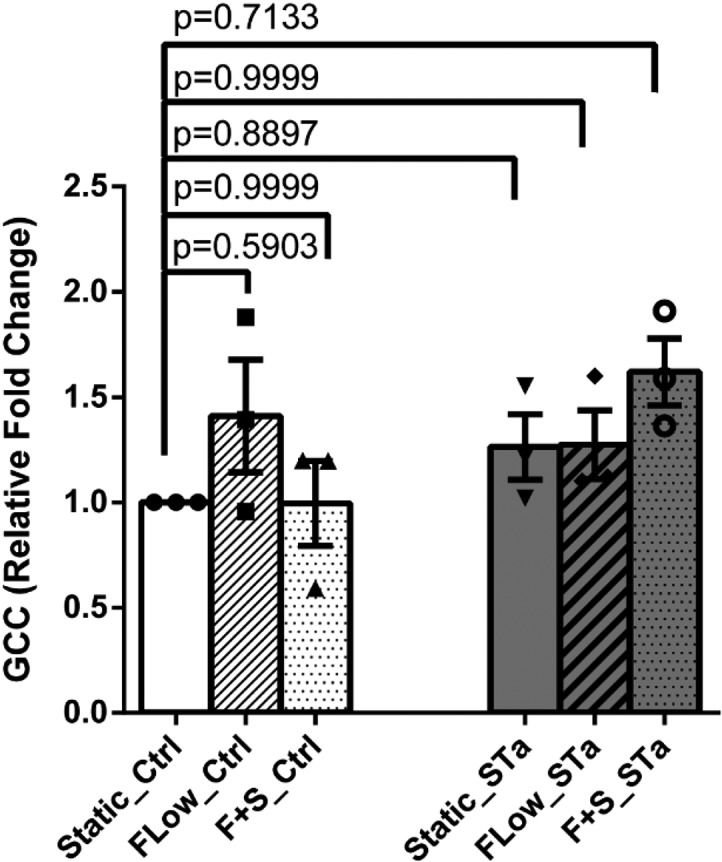
GCC mRNA was not altered by mechanical stimuli. GCC mRNA levels of enteroids under static, flow, and flow-plus-stretch conditions did not show any change either among these three conditions in the basal state or with the addition of ST. Results are means ± SEM (n = 30). P values were determined by one-way ANOVA with Tukey’s multiple-comparison tests.
Epithelia under mechanical stimuli are more columnar than under static conditions.
Intestinal epithelium is exposed to a wide range of mechanical forces in both normal and pathophysiologic states. To determine whether mechanical forces caused structural changes in the enteroids in the Intestine-Chip, heights of the monolayers were determined by confocal microscopy. Both flow and flow plus stretch significantly increased epithelial cell height compared to the height under static conditions (Fig. 5a and b). Morphologically, xz dimensions of cells grown under static condition were 10 to 15 μm tall with a cuboidal appearance. Epithelium under flow and flow plus stretch was 20 to 25 μm in the xz direction and resembled columnar enterocytes.
FIG 5.
Mechanical stimuli enhance epithelial cell height. (a) Representative xz views of confocal microscopic images showing jejunal enteroids immunostained for phalloidin (white), sucrase-isomaltase (green), and nucleus (blue) under static, flow, and flow-plus-stretch conditions, as indicated. Bar, 10 μm. (b) Quantitation of the cell height under static, flow, and flow plus stretch. For all conditions, n = 3 regions of interest from two different sets of experiments (means ± SEM). P values were determined by Student’s paired t tests.
Additional studies were performed to assess the effect of the mechanical forces on the differentiation state of the enteroids. Comparison was made among enteroids grown on Transwell inserts under differentiated conditions (5 days of Wnt removal) and enteroids grown in the Intestine-Chip (static, flow, flow-plus-stretch conditions). The genes studied were LGR5, a stem cell marker, three genes the expression of which increases greatly with enteroid differentiation (MUC2, sucrase-isomaltase, and downregulated-in-adenoma [DRA]) (2, 17), and lysozyme, a gene expressed in Paneth cells (see Fig. S1 in the supplemental material). LGR5 was expressed similarly in the enteroids grown on Transwells and in the Intestine-Chip under all conditions (Fig. S1e). MUC2 was expressed similarly in the Transwell insert-grown enteroids and the enteroids grown under static conditions in the Intestine-Chip, but it was significantly and similarly increased in the enteroids grown in the Intestine-Chip under both flow and flow-plus-stretch conditions. However, compared to expression under the Intestine-Chip static condition, only flow significantly increased MUC2 mRNA expression (Fig. S1c). mRNAs for sucrase-isomaltase and DRA were significantly increased similarly for all conditions of enteroids in the Intestine-Chip (the only exception was for DRA under static conditions) (Fig. S1a and b). The lysozyme levels were similar under all conditions (Fig. S1d). These results demonstrate that the enteroids in the Intestine-Chip under all conditions studied were differentiated and apparently to a greater extent than the enteroids grown in Transwell inserts. The changes in gene expression were related to flow, which caused a significant increase in the mRNA expression for MUC2 compared to that under the static condition in the Intestine-Chip; the major effect in differentiation was caused by the enteroid growth in the Intestine-Chip compared to growth on the Transwell inserts.
DISCUSSION
The mechanical forces exerted on the intestine by luminal flow and blood flow and the cyclic strain of peristalsis have been established as playing an important role in normal physiologic and pathological states (20–23, 46). These forces affect all of the multiple cell types in the intestine, and thus the effects directly on the epithelial cells have been difficult to describe in isolation. The study of human stem cell-derived enteroid monolayers has allowed characterization of normal human epithelial cells. In addition, Ingber et al. developed a gut-on-a-chip platform they called the “Intestine-Chip” which has allowed application of apical flow that recreates intestinal luminal fluid flow and basolateral flow in the area where blood flow normally occurs (10, 14, 15, 24–26). Administering repetitive vacuum pressure to mechanically stretch and relax the flexible PDMS membrane and adherent tissues in the central channel has been used to mimic peristaltic motions of the living human small intestine. The Intestine-Chip has been shown to alter some structural and functional aspects of human intestinal cells (15, 24, 25); however, most of these studies were performed with the human colon cancer cell line, Caco-2, cocultured with endothelial cells (15). Here, we extended these studies using the Intestine-Chip and normal human jejunal enteroid-derived chips to examine the pathophysiologic effects of a virulence factor (ST) from a human enteric pathogen, ETEC, that affects the proximal small intestine in order to begin evaluation of the contributions of these mechanical forces on normal human intestine and in intestinal host-pathogen interactions. In addition, to understand the contribution of the mechanical forces to the structure and function of enteroids, we compared the effects of these forces to characteristics of the same enteroids which were derived from the same donor and were studied in the absence of the physical forces but in the same platform, referred to as the “static” condition, acknowledging that the Intestine-Chip was designed to include the mechanical forces.
The enteroids were studied in the differentiated state due to removal of Wnt in the conditioned medium in which the enteroids were grown, which we had shown previously to represent the small intestinal villus compartment (2, 17). The concentration of ST studied corresponded to the level of this toxin that was produced when these jejunal enteroid monolayers were exposed to 109 CFU ETEC for 6 to 8 h in a Transwell filter (3). The flow rate in the current study was chosen to recapitulate the postprandial cardiac output to the intestinal mucosa (see Data Set S1 in the supplemental material) (27–29).
Compared to cGMP secretions of the static jejunal enteroid monolayers in the Intestine-Chip, flow alone significantly increased the apical and basolateral cGMP secretion levels under baseline conditions. Morphologically, both flow and flow plus stretch equally increased the epithelial cell height. Flow and flow plus stretch also equivalently increased the ST-induced increases in intracellular cGMP content and apical and basolateral cGMP secretion. However, the ST-induced basolateral cGMP secretion under flow conditions failed to reach statistical significance. Of note, the magnitude of the ST-induced increase in basolateral cGMP secretion was not different when data from flow and flow plus stretch were compared, supporting equivalent effects of the mechanical forces (Fig. 2d). Consideration of what explained the increased transport of cGMP out of the enteroids centered on MRP4 and MRP5 since a general MRP inhibitor, MK571, prevented the increase (3). In the current studies, MRP4 message expression was slightly increased under basal conditions of flow and flow plus stretch, while ST under flow-plus-stretch conditions significantly increased MRP4 but not MRP5 expression. This increase in MRP4 potentially contributes to the increased cGMP secretion, but measurement of MRP4 and MRP5 protein expression is needed to define the role of MRPs in enteroid cGMP secretion. Knockdown studies are being undertaken in a separate study to examine if altering MRP expression alters ST-induced cGMP secretion (unpublished data). We conclude that the major effect of regulation of cGMP secretion is driven by flow since addition of stretch did not significantly affect either the intracellular cGMP content or extracellular cGMP secretion beyond that caused by flow alone.
An aim of this study was to characterize the contribution of mechanical forces created by flow and stretch to the effect of a single pathophysiologic enterotoxin (ST) on human enteroids. The data indicate an effect of flow but no additional effect of stretch on ST effects on jejunal cGMP handling. We also point out that multiple factors must be considered in evaluating the relevant normal physiology being modeled in the Intestine-Chip, including the following. (i) The flow in the Intestine-Chip is laminar, while in vivo the intestine experiences turbulent flow. This could create some differences in the effects. (ii) The system of repetitive stretch used was designed to recreate the repetitive distention that occurs with respiration (30), while peristalsis consists of more complex repetitive, sweeping, cephalad to caudal contractions that are not entirely regular. Thus, the muscle contractions that occur with peristalsis provide a more complex force than occurs with the Intestine-Chip. (iii) Other studies with the Intestine-Chip used one of several endothelial cell types on the enteroid basolateral surface, including intestinal microvascular cells, the effects of which were not considered here (15, 25). This difference may have contributed to the lack of effect of the stretch on some of the functions studied here.
Under the static conditions in the Intestine-Chip, the epithelial cells were shorter and more cuboidal in appearance than the same cells grown as differentiated monolayers on Transwell filters, while the epithelial cell height of differentiated enteroids grown on Transwell filters (8, 31) was similar to the height of those exposed to flow and flow plus stretch in the Intestine-Chip. However, the extent of differentiation of these enteroids in the Intestine-Chip under all conditions compared to differentiation of the same differentiated jejunal enteroids on Transwell inserts indicates an increased state of differentiation, as judged by mRNA levels of MUC2, sucrase-isomaltase, and SLC26A3 (downregulated-in-adenoma [DRA]) and the equally low level of LGR5 mRNA (Fig. S1). We present this to indicate the need for further studies to understand how the epithelial height is determined and point out that there are multiple differences in the conditions the enteroids are exposed to in the two platforms, including the materials the cells were grown on, level of oxygenation, hydrostatic pressure from the enclosed Intestine-Chip versus the open state of the Transwell, and the fact that the cells on Transwell filters were grown on expansion medium before they were switched to differentiation medium 5 days before the experiment (2) whereas cells in the current study were exposed to differentiation medium from the time of seeding.
Because of the failure of ST to significantly increase the cGMP content in jejunal enteroids studied in Transwell filters without the presence of phosphodiesterase inhibitors (3), 3-isobutyl-1-methylxanthine (IBMX) and the cGMP-specific phosphodiesterase-5 inhibitor vardenafil (32–34) were included under all conditions. In spite of their inclusion, ST failed to alter intracellular cGMP content under static conditions, and the significant increase in cGMP content with flow was surprisingly small, which we attribute, in part, to the intracellular removal. Flow and flow plus stretch were important for both apical and basolateral cGMP secretion, with either small or no increases occurring under static conditions. We can only speculate why the epithelial cells control intracellular cGMP content so carefully, but the well-described role of intracellular cGMP as an inhibitor of proliferation would predict that it is likely to be stringently regulated (35).
The apical and basolateral secretion levels of cGMP were similar in magnitude in the Intestine-Chip, and while it is the control of intracellular cGMP that appears to drive the regulation of cGMP secretion, there is growing evidence of extracellular cGMP having effects on cell physiology and pathophysiology by an auto- or paracrine mechanism. Luminal cGMP inhibits chloride reabsorption in the medullary thick ascending limb of rabbit tubules, and extracellular cGMP inhibits transepithelial sodium transport in porcine renal tubular cells (36). Potential intestinal effects of extracellular cGMP include regulation by apical cGMP of Escherichia coli proliferation, release of ST or other ETEC virulence factors and other effects on the intestinal microbiome, and regulation by basolateral cGMP of immune cells in the lamina propria, which is known to affect ETEC (8, 31), as well as effects on the enteric nervous system (ENS). We have performed preliminary studies evaluating the effect of apical cGMP on ETEC proliferation and ST release in jejunal enteroids with no effect on either parameter found (data not shown). While there is no defined pathophysiologic role as yet for apical cGMP secretion, multiple studies have suggested that cGMP secreted at the basolateral site of enterocytes may affect the enteric nervous system, particularly by inhibiting pain sensation, such as the increased visceral sensitivity that is present in IBS-C. Studies of the effects of cGMP on visceral pain sensation have shown that uroguanylin, a naturally occurring intestinal hormone, activates GCC, leading to apical and basolateral secretion of cGMP in Caco-2 cells and in rat colon. In the latter, the cGMP secreted on the basolateral surface inhibits firing of afferent nerves, and cGMP added directly to rat colon inhibited the neural stretch response as well. In addition, cGMP inhibited murine colon nociception with an effect much greater in mice with chronically induced visceral hypersensitivity (37–39). Most of these studies were undertaken to support the concept that linaclotide, which clinically is an effective laxative, reduces abdominal discomfort associated with IBS-C, and an important preliminary observation indicates that this effect occurs even without the laxative effect occurring (19, 40).
The mechanism leading to secretion of cGMP is speculated to involve the ABC transporter family members, multidrug resistance proteins (MRPs). Using MDCK cells, Wijnholds et al. showed that basolaterally localized MRP5 transports nucleotides. They suggested that since MRP4 is closely related to MRP5, it also was likely to transport nucleotides (41). Moreover, Wielinga and his group showed that MRP4- and MRP5-overexpressing cells, when stimulated with the nitric oxide-releasing compound sodium nitroprusside and the adenylate cyclase stimulator forskolin, effluxed more cGMP and cAMP, respectively, than under basal conditions and did so in an ATP-dependent manner (42, 47). They suggested that MRP4 and MRP5 might function as overflow pumps, decreasing steep increases in intracellular cGMP levels. In the current study, under basal conditions without ST exposure, MRP4 mRNA was increased under flow and flow-plus-stretch (statistically significant) conditions compared to levels under static conditions, in agreement with increased extracellular cGMP accumulation. Of note, MRP4 has some increased specificity for cGMP over cAMP (43). Hoque et al. demonstrated NHERF1 as a major determinant of MRP4 trafficking to apical membranes of mammalian kidney cells. Moreover, they suggested that MRP4 may be localized to either apical or basolateral membranes in polarized cells, depending on the cell type (43). However, lack of specificity of the MRP4 and MRP5 antibodies available has prevented localization of these MRPs as well as quantitation by immunoblotting. Further studies, including knockout or knockdown studies, are needed to define the role of MRP4 and MRP5 in human jejunal enteroids and to determine whether other cyclic nucleotide transporters might be affected by flow and/or stretch to affect cGMP secretion.
This study demonstrates that the mechanical shear stress forces provided by luminal and/or basolateral flow, compared to results of studies using the same platform under static conditions, affect multiple aspects of normal human jejunal enteroid structure and function and also multiple aspects relating to the effects of E. coli heat-stable enterotoxin on enteroids. The shear force from apical-plus-basolateral flow induced an increase in cell height, apical and basolateral cGMP secretion under basal conditions (without ST), and altered host-pathogen interactions including the magnitude of the ST-induced apical and basolateral cGMP secretion. Repetitive stretch did not add to these effects. In this study, only the effects of a single virulence factor of one human diarrheal disease were studied. Thus, we suggest that further studies of host-pathogen interactions are necessary to determine the contribution of these physical forces to normal intestinal physiology and pathophysiology, including host-pathogen interactions, as well as potential contributions to studies of drug pharmacokinetics and personalized drug therapy.
MATERIALS AND METHODS
Human jejunal enteroid culture and propagation in vitro.
Enteroids were established from crypts containing stem cells isolated from jejunal biopsy specimens from healthy adult subjects using the protocol of Sato et al. (44, 45), with minor modifications as previously described (2). All studies were approved by the Johns Hopkins University School of Medicine institutional review board. Enteroids were maintained in Matrigel (Corning, Tewksbury, MA) in expansion medium composed of advanced Dulbecco’s modified Eagle medium/F12 (Life Technologies, Carlsbad, CA) containing 100 U/ml penicillin-streptomycin (Quality Biological, Gaithersburg, MD), 10 mmol/liter HEPES (Life Technologies), and 1× GlutaMAX (Life Technologies), with 50% Wnt3A-conditioned medium (produced by L-Wnt3A cell line, ATCC CRL-2647), 15% R-spondin 1-conditioned medium (produced by HEK293T cell line stably expressing mouse R-spondin 1; kindly provided by Calvin Kuo, Stanford University, Stanford, CA), 10% Noggin-conditioned medium (produced by HEK293T cell line stably expressing mouse Noggin), 1× B-27 supplement (Life Technologies), 1 mmol/liter N-acetylcysteine (Sigma-Aldrich), 50 ng/ml human epidermal growth factor (Life Technologies), 1 μg/ml (Leu-15) gastrin (AnaSpec, Fremont, CA), 500 nmol/liter A83-01 (Tocris, Bristol, United Kingdom), 10 μmol/liter SB202190 (Sigma-Aldrich), and 100 μg/ml Primocin (InvivoGen, San Diego, CA). Enteroids were cultured in a 5% CO2 atmosphere at 37°C and passaged every 7 to 12 days. Expansion medium was supplemented with 10 μmol/liter Y-27632 (Tocris) and 10 μmol/liter CHIR99021 (Tocris) during the first 2 days after passaging. The effect of ST was initially evaluated in jejunal enteroids made from three normal human subjects and studied as monolayers grown on Transwell inserts, as described previously (2). In all cases, ST rapidly increased intracellular, apical, and basolateral cGMP levels, with the biggest changes occurring in basolateral cGMP secretion (data not shown). One of the jejunal enteroid lines was selected for the studies using the Intestine-Chip (passage numbers 30 to 45).
Intestine-Chip.
Organ-Chips were fabricated from PDMS and assembled as described previously (14, 20, 24) (Emulate, Inc., Boston, MA). The top (1 mm high by 1 mm wide) and bottom (0.2 mm high by 1 mm wide) channels were separated by a thin (50-μm), flexible, PDMS membrane containing 7-μm-diameter pores with 40-μm spacing and surrounded on either side by two vacuum chambers (1 mm high by 300 μm wide). Chips were sterilized with ethyl alcohol (70%) and distilled deionized water. This step was followed by activation of the chip according to the manufacturer’s protocol (Emulate, Inc.) (15). The chips were then coated with collagen IV solution (Sigma-Aldrich) (200 μg/ml in phosphate-buffered saline [PBS]) and incubated in a humidified 37°C incubator for 2 h. Enteroids were isolated from Matrigel by treatment with cell recovery solution (Cultrex) at 4°C for 30 min and dissociated with recombinant enzymes (TrypLE; Gibco) to obtain enteroid fragments.
Fragmented enteroids were then suspended in differentiation medium prepared by removing Wnt3A, R- spondin 1, and SB202190 in the expansion medium supplemented with Y-27632 and CHIR99021 for the first 2 days. Approximately 200 fragments/chip were seeded on the upper channel of the Intestine-Chip and incubated overnight without any flow at 37°C. The following day (day 1 postseeding) the enteroids were perfused at a flow rate of 60 μl/h modeled to mimic the postprandial cardiac output directed to the small intestine (Data Set S1) until day 5. For the flow-plus-stretch condition, cyclic membrane deformation (10% strain; 0.15 Hz) was applied. Both of the dynamic components of Intestine-Chip culture, flow and cyclic stretch, plus control of O2/CO2, were performed using Human Emulation System instrumentation (Emulate, Inc.) (15).
The enteroid monolayers were confluent on day 5, at which time they were incubated with differentiation medium containing IMBX (1 mM) (MP, Biomedicals) and vardenafil (1 μM) (Sigma-Aldrich) without (control) or with synthetic STp (1 nM) (Bachem/ThermoFisher) for 6 h. ST was exposed to the apical channel only. Apical and basolateral samples were collected for 6 h and analyzed for cGMP content by enzyme-linked immunosorbent assay (ELISA; Enzo Biochem, Inc.). For intracellular cGMP determination, cells were lysed with 0.5 M HCl (according to the ELISA protocol). Protein concentration was measured using a Pierce bicinchoninic acid (BCA) protein assay (ThermoFisher).
Morphological analysis.
Enteroids in Intestine-Chips on day 5 postplating were fixed in paraformaldehyde (4%) and permeabilized in 5% (wt/vol) bovine serum albumin (BSA)–0.1% (vol/vol) Triton X-100 before being incubated at 4°C overnight with primary antibodies directed against sucrase-isomaltase (1:100) (Developmental Studies Hybridoma Bank, University of Iowa). The next day, cells were washed with PBS and incubated with Alexa Fluor-488 and -568 secondary antibodies (1:100), Hoechst 33342 (10 μg/ml), and Alexa Fluor-647–phalloidin (1:200) at room temperature for 1 h. Images were acquired with an upright scanning confocal microscope (Leica SP5 X MP DMI-6000, 25× water objective).
Quantitative real-time PCR.
Total RNA was extracted from monolayers in the Intestine-Chip using a PureLink RNA Mini kit (Life Technologies) according to the manufacturer’s protocol. cDNA was synthesized from 1 to 2 μg of RNA using SuperScript VILO Master Mix (Life Technologies). Quantitative real-time PCR (qRT-PCR) was performed using Power SYBR green Master Mix (Life Technologies) on a QuantStudio 12K Flex real-time PCR system (Applied Biosystems, Foster City, CA). Each sample was studied in triplicate, and 5 ng of RNA-equivalent cDNA was used for each reaction. The sequences of gene-specific primers were as follows: MRP4, GAAGCGCCTGGAATCTACAA (forward) and AGAGCCCCTGGAGAGAAGAT (reverse); MRP5, CACCATCCACGCCTACAATAAA (forward) and CACCGCATCGCACACGTA (reverse); RN18S, GCAATTATTCCCCATGAACG (forward) and GGGACTTAATCAACGCAAGC (reverse); SI, TTTTGGCATCCAGATTCGAC (forward) and ATCCAGGCAGCCAAGAATC (reverse); DRA, CCATCATCGTGCTGATTGTC (forward) and AGCTGCCAGGACGGACTT (reverse); MUC2, ACTCCAACATCTCCGTGTCC (forward) and AGCCACACTTGTCTGCAGTG (reverse); lysozyme, GGCCAAATGGGAGAGTGGTTA (forward) and CCAGTAGCGGCTATTGATCTGAA (reverse); LGR5, ACCAGACTATGCCTTTGGAAAC (forward) and TTCCCAGGGAGTGGATTCTAT (reverse).
The relative fold changes in mRNA levels of MRP4 and MRP5 between static, flow, and flow-plus-stretch conditions were determined using the 2−ΔΔCT (where CT is threshold cycle) method with human 18S rRNA as an internal control for normalization (2).
Statistical analysis.
Data are presented as means ± standard errors of the means (SEM). Statistical analyses were conducted using Student’s t test for comparisons between two conditions, and one-way analysis of variance (ANOVA) with Tukey’s multiple-comparison test was used when more than two conditions were compared. A P value of ≤0.05 was considered statistically significant. Studies were performed using one jejunal enteroid line derived from a normal human subject. Experiments were repeated at least three times.
Supplementary Material
ACKNOWLEDGMENTS
This study was partly supported by NIH NIAID PO1AI125181 and NIH NIDDK P30DK089502 and by a grant-in-aid from Emulate, Inc.
We acknowledge scientific discussions and input from Jennifer Foulke-Abel.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Middendorp S, Schneeberger K, Wiegerinck CL, Mokry M, Akkerman RD, van Wijngaarden S, Clevers H, Nieuwenhuis EE. 2014. Adult stem cells in the small intestine are intrinsically programmed with their location-specific function. Stem Cells 32:1083–1091. doi: 10.1002/stem.1655. [DOI] [PubMed] [Google Scholar]
- 2.Yin J, Tse CM, Avula LR, Singh V, Foulke-Abel J, de Jonge HR, Donowitz M. 2018. Molecular basis and differentiation-associated alterations of anion secretion in human duodenal enteroid monolayers. Cell Mol Gastroenterol Hepatol 5:591–609. doi: 10.1016/j.jcmgh.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu H, Sunuwar L, Fleckenstein JM, Donowitz M, Foulke-Abel JD. 2017. Enterotoxigenic E. coli (ETEC) pathogenesis modeled in human enteroid monolayers demonstrates MRP-related cyclic nucleotide secretion. Gastroenterology 152:S57. doi: 10.1016/S0016-5085(17)30548-6. [DOI] [Google Scholar]
- 4.Yu H, Hasan NM, In JG, Estes MK, Kovbasnjuk O, Zachos NC, Donowitz M. 2017. The contributions of human mini-intestines to the study of intestinal physiology and pathophysiology. Annu Rev Physiol 79:291–312. doi: 10.1146/annurev-physiol-021115-105211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kozuka K, He Y, Koo-McCoy S, Kumaraswamy P, Nie B, Shaw K, Chan P, Leadbetter M, He L, Lewis JG, Zhong Z, Charmot D, Balaa M, King AJ, Caldwell JS, Siegel M. 2017. Development and characterization of a human and mouse intestinal epithelial cell monolayer platform. Stem Cell Rep 9:1976–1990. doi: 10.1016/j.stemcr.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott A, Rouch JD, Jabaji Z, Khalil HA, Solorzano S, Lewis M, Martin MG, Stelzner MG, Dunn JC. 2016. Long-term renewable human intestinal epithelial stem cells as monolayers: a potential for clinical use. J Pediatr Surg 51:995–1000. doi: 10.1016/j.jpedsurg.2016.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.In JG, Foulke-Abel J, Estes MK, Zachos NC, Kovbasnjuk O, Donowitz M. 2016. Human mini-guts: new insights into intestinal physiology and host-pathogen interactions. Nat Rev Gastroenterol Hepatol 13:633–642. doi: 10.1038/nrgastro.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noel G, Baetz NW, Staab JF, Donowitz M, Kovbasnjuk O, Pasetti MF, Zachos NC. 2017. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci Rep 7:45270. doi: 10.1038/srep45270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokuyama E, Nagai Y, Takahashi K, Kimata Y, Naruse K. 2015. Mechanical stretch on human skin equivalents increases the epidermal thickness and develops the basement membrane. PLoS One 10:e0141989. doi: 10.1371/journal.pone.0141989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HJ, Ingber DE. 2013. Gut-on-a-chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr Biol (Camb) 5:1130–1140. doi: 10.1039/c3ib40126j. [DOI] [PubMed] [Google Scholar]
- 11.Poling HM, Wu D, Brown N, Baker M, Hausfeld TA, Huynh N, Chaffron S, Dunn JCY, Hogan SP, Wells JM, Helmrath MA, Mahe MM. 2018. Mechanically induced development and maturation of human intestinal organoids in vivo. Nat Biomed Eng 2:429–442. doi: 10.1038/s41551-018-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Workman MJ, Gleeson JP, Troisi EJ, Estrada HQ, Kerns SJ, Hinojosa CD, Hamilton GA, Targan SR, Svendsen CN, Barrett RJ. 2018. Enhanced utilization of induced pluripotent stem cell-derived human intestinal organoids using microengineered chips. Cell Mol Gastroenterol Hepatol 5:669–677.e662. doi: 10.1016/j.jcmgh.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acácio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Li H, Collins JJ, Ingber DE. 2016. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci U S A 113:E7–E15. doi: 10.1073/pnas.1522193112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasendra M, Tovaglieri A, Sontheimer-Phelps A, Jalili-Firoozinezhad S, Bein A, Chalkiadaki A, Scholl W, Zhang C, Rickner H, Richmond CA, Li H, Breault DT, Ingber DE. 2018. Development of a primary human small intestine-on-a-chip using biopsy-derived organoids. Sci Rep 8:2871. doi: 10.1038/s41598-018-21201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleckenstein JM, Munson GM, Rasko DA. 2013. Enterotoxigenic Escherichia coli: orchestrated host engagement. Gut Microbes 4:392–396. doi: 10.4161/gmic.25861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foulke-Abel J, In J, Yin J, Zachos NC, Kovbasnjuk O, Estes MK, de Jonge H, Donowitz M. 2016. Human enteroids as a model of upper small intestinal ion transport physiology and pathophysiology. Gastroenterology 150:638–649.e638. doi: 10.1053/j.gastro.2015.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandar AK. 2017. Diagnosis and treatment of irritable bowel syndrome with predominant constipation in the primary-care setting: focus on linaclotide. Int J Gen Med 10:385–393. doi: 10.2147/IJGM.S126581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black CJ, Burr NE, Quigley EMM, Moayyedi P, Houghton LA, Ford AC. 2018. Efficacy of secretagogues in patients with irritable bowel syndrome with constipation: systematic review and network meta-analysis. Gastroenterology 155:1753–1763. doi: 10.1053/j.gastro.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Huh D, Kim HJ, Fraser JP, Shea DE, Khan M, Bahinski A, Hamilton GA, Ingber DE. 2013. Microfabrication of human organs-on-chips. Nat Protoc 8:2135–2157. doi: 10.1038/nprot.2013.137. [DOI] [PubMed] [Google Scholar]
- 21.Gayer CP, Basson MD. 2009. The effects of mechanical forces on intestinal physiology and pathology. Cell Signal 21:1237–1244. doi: 10.1016/j.cellsig.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinheiro D, Bellaiche Y. 2018. Mechanical force-driven adherens junction remodeling and epithelial dynamics. Dev Cell 47:3–19. doi: 10.1016/j.devcel.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Monier B, Gettings M, Gay G, Mangeat T, Schott S, Guarner A, Suzanne M. 2015. Apico-basal forces exerted by apoptotic cells drive epithelium folding. Nature 518:245–248. doi: 10.1038/nature14152. [DOI] [PubMed] [Google Scholar]
- 24.Kim HJ, Huh D, Hamilton G, Ingber DE. 2012. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 12:2165–2174. doi: 10.1039/c2lc40074j. [DOI] [PubMed] [Google Scholar]
- 25.Bein A, Shin W, Jalili-Firoozinezhad S, Park MH, Sontheimer-Phelps A, Tovaglieri A, Chalkiadaki A, Kim HJ, Ingber DE. 2018. Microfluidic organ-on-a-chip models of human intestine. Cell Mol Gastroenterol Hepatol 5:659–668. doi: 10.1016/j.jcmgh.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villenave R, Wales SQ, Hamkins-Indik T, Papafragkou E, Weaver JC, Ferrante TC, Bahinski A, Elkins CA, Kulka M, Ingber DE. 2017. Human gut-on-a-chip supports polarized infection of coxsackie B1 virus in vitro. PLoS One 12:e0169412. doi: 10.1371/journal.pone.0169412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Texter EC., Jr 1963. Small intestinal blood flow. Am J Dig Dis 8:587–613. doi: 10.1007/bf02239457. [DOI] [PubMed] [Google Scholar]
- 28.Hulten L, Jodal M, Lindhagen J, Lundgren O. 1976. Blood flow in the small intestine of cat and man as analyzed by an inert gas washout technique. Gastroenterology 70:45–51. doi: 10.1016/S0016-5085(76)80401-5. [DOI] [PubMed] [Google Scholar]
- 29.Granger DN, Richardson PD, Kvietys PR, Mortillaro NA. 1980. Intestinal blood flow. Gastroenterology 78:837–863. doi: 10.1016/0016-5085(80)90692-7. [DOI] [PubMed] [Google Scholar]
- 30.Benam KH, Mazur M, Choe Y, Ferrante TC, Novak R, Ingber DE. 2017. Human lung small airway-on-a-chip protocol. Methods Mol Biol 1612:345–365. doi: 10.1007/978-1-4939-7021-6_25. [DOI] [PubMed] [Google Scholar]
- 31.Noel G, Doucet M, Nataro JP, Kaper JB, Zachos NC, Pasetti MF. 2017. Enterotoxigenic Escherichia coli is phagocytosed by macrophages underlying villus-like intestinal epithelial cells: modeling ex vivo innate immune defenses of the human gut. Gut Microbes 9:382–389. doi: 10.1080/19490976.2017.1398871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giovannoni MP, Vergelli C, Graziano A, Dal Piaz V. 2010. PDE5 inhibitors and their applications. Curr Med Chem 17:2564–2587. doi: 10.2174/092986710791859360. [DOI] [PubMed] [Google Scholar]
- 33.Ried M, Neu R, Lehle K, Großer C, Szöke T, Lang G, Hofmann H-S, Hoenicka M. 2017. Superior vasodilation of human pulmonary vessels by vardenafil compared with tadalafil and sildenafil: additive effects of bosentan. Interact Cardiovasc Thorac Surg 25:254–259. doi: 10.1093/icvts/ivx108. [DOI] [PubMed] [Google Scholar]
- 34.Ashour AE, Rahman AF, Kassem MG. 2014. Vardenafil dihydrochloride. Profiles Drug Subst Excip Relat Methodol 39:515–544. doi: 10.1016/B978-0-12-800173-8.00009-X. [DOI] [PubMed] [Google Scholar]
- 35.Rappaport JA, Waldman SA. 2018. The guanylate cyclase C-cGMP signaling axis opposes intestinal epithelial injury and neoplasia. Front Oncol 8:299. doi: 10.3389/fonc.2018.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sager G. 2004. Cyclic GMP transporters. Neurochem Int 45:865–873. doi: 10.1016/j.neuint.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Silos-Santiago I, Hannig G, Eutamene H, Ustinova EE, Bernier SG, Ge P, Graul C, Jacobson S, Jin H, Liong E, Kessler MM, Reza T, Rivers S, Shea C, Tchernychev B, Bryant AP, Kurtz CB, Bueno L, Pezzone MA, Currie MG. 2013. Gastrointestinal pain: unraveling a novel endogenous pathway through uroguanylin/guanylate cyclase-C/cGMP activation. Pain 154:1820–1830. doi: 10.1016/j.pain.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 38.Feng B, Kiyatkin ME, La JH, Ge P, Solinga R, Silos-Santiago I, Gebhart GF. 2013. Activation of guanylate cyclase-C attenuates stretch responses and sensitization of mouse colorectal afferents. J Neurosci 33:9831–9839. doi: 10.1523/JNEUROSCI.5114-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castro J, Harrington AM, Hughes PA, Martin CM, Ge P, Shea CM, Jin H, Jacobson S, Hannig G, Mann E, Cohen MB, MacDougall JE, Lavins BJ, Kurtz CB, Silos-Santiago I, Johnston JM, Currie MG, Blackshaw LA, Brierley SM. 2013. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3′,5′-monophosphate. Gastroenterology 145:1334–1346.e11. doi: 10.1053/j.gastro.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 40.Chey WD, Chamberlin P, Bochenek W, Tripp K, Higgins CS, Omniewski N, Fox SM, Hall M, Hashash A, Miller M, O’Dea CR, Currie M. 2017. Targeted delivery of linaclotide to specific areas of the intestine affects clinical efficacy in patients with irritable bowel syndrome with constipation (IBS-C). Gastroenterology 152(Suppl 1):S1314–S1315. doi: 10.1016/S0016-5085(17)34374-3. [DOI] [Google Scholar]
- 41.Wijnholds J, Mol CA, van Deemter L, de Haas M, Scheffer GL, Baas F, Beijnen JH, Scheper RJ, Hatse S, De Clercq E, Balzarini J, Borst P. 2000. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci U S A 97:7476–7481. doi: 10.1073/pnas.120159197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wielinga PR, van der Heijden I, Reid G, Beijnen JH, Wijnholds J, Borst P. 2003. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J Biol Chem 278:17664–17671. doi: 10.1074/jbc.M212723200. [DOI] [PubMed] [Google Scholar]
- 43.Hoque MT, Conseil G, Cole SP. 2009. Involvement of NHERF1 in apical membrane localization of MRP4 in polarized kidney cells. Biochem Biophys Res Commun 379:60–64. doi: 10.1016/j.bbrc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 44.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. 2011. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 45.Sato T, Clevers H. 2013. Primary mouse small intestinal epithelial cell cultures. Methods Mol Biol 945:319–328. doi: 10.1007/978-1-62703-125-7_19. [DOI] [PubMed] [Google Scholar]
- 46.Fernández-Sánchez ME, Barbier S, Whitehead J, Béalle G, Michel A, Latorre-Ossa H, Rey C, Fouassier L, Claperon A, Brullé L, Girard E, Servant N, Rio-Frio T, Marie H, Lesieur S, Housset C, Gennisson J-L, Tanter M, Ménager C, Fre S, Robine S, Farge E. 2015. Mechanical induction of the tumorigenic beta-catenin pathway by tumour growth pressure. Nature 523:92–95. doi: 10.1038/nature14329. [DOI] [PubMed] [Google Scholar]
- 47.Reid G, Wielinga P, Zelcer N, De Haas M, Van Deemter L, Wijnholds J, Balzarini J, Borst P. 2003. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol Pharmacol 63:1094–1103. doi: 10.1124/mol.63.5.1094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.