Cryptococcosis is an infectious disease caused by two fungal species, Cryptococcus neoformans and Cryptococcus gattii. While C. neoformans affects mainly immunocompromised patients, C. gattii infects both immunocompetent and immunocompromised individuals. Laccase is an important virulence factor that contributes to the virulence of C. neoformans by promoting pulmonary growth and dissemination to the brain. The presence of laccase in C. neoformans can shift the host immune response toward a nonprotective Th2-type response.
KEYWORDS: Cryptococcus neoformans, Cryptococcus gattii, macrophages, laccase, immune responses
ABSTRACT
Cryptococcosis is an infectious disease caused by two fungal species, Cryptococcus neoformans and Cryptococcus gattii. While C. neoformans affects mainly immunocompromised patients, C. gattii infects both immunocompetent and immunocompromised individuals. Laccase is an important virulence factor that contributes to the virulence of C. neoformans by promoting pulmonary growth and dissemination to the brain. The presence of laccase in C. neoformans can shift the host immune response toward a nonprotective Th2-type response. However, the role of laccase in the immune response against C. gattii remains unclear. In this study, we characterized laccase activity in C. neoformans and C. gattii isolates from Thailand and investigated whether C. gattii that is deficient in laccase might modulate immune responses during infection. C. gattii was found to have higher laccase activity than C. neoformans, indicating the importance of laccase in the pathogenesis of C. gattii infection. The expression of laccase promoted intracellular proliferation in macrophages and inhibited in vitro fungal clearance. Mice infected with a lac1Δ mutant strain of C. gattii had reduced lung burdens at the early but not the late stage of infection. Without affecting type-1 and type-2 responses, the deficiency of laccase in C. gattii induced cryptococcus-specific interleukin-17 (IL-17) cytokine, neutrophil accumulation, and expression of the neutrophil-associated cytokine gene Csf3 and chemokine genes Cxcl1, Cxcl2, and Cxcl5 in vivo, as well as enhanced neutrophil-mediated phagocytosis and killing in vitro. Thus, our data suggest that laccase constitutes an important virulence factor of C. gattii that plays roles in attenuating Th17-type immunity, neutrophil recruitment, and function during the early stage of infection.
INTRODUCTION
Cryptococcus neoformans and Cryptococcus gattii are major basidiomycete fungal pathogens that cause cryptococcosis among humans. While C. neoformans infects mainly immunocompromised individuals, C. gattii has been regarded as a pathogen of immunocompetent patients (1–3). The pathophysiologies and clinical manifestations of C. gattii and C. neoformans differ (3, 4): C. neoformans has often been associated with central nervous system involvement and C. gattii with severe lung disease (5). It has been reported that an underlying host antibody defect may be involved in the predisposition to C. gattii infections (6, 7). Moreover, a failure of C. gattii to efficiently induce an effective immune response among healthy individuals has also been proposed (5). Related studies have suggested that C. gattii elicits a less protective immunity than C. neoformans. Infection with C. gattii inhibited neutrophil migration in the lungs during the initial stage of infection (8, 9). Moreover, C. gattii has been found to modulate dendritic cell (DC) function by attenuating dendritic cell maturation (10). Besides affecting innate immune cell function, we recently reported that C. gattii’s dampening of the Th1 and Th17 immune response may contribute to its ability to infect immunocompetent individuals (11).
Cryptococcus produces numerous virulence factors to promote fungal survival and modulate the host immune response. Although the relative importance of each of these characteristics is not fully understood, the presence of capsule and the presence of laccase are considered major virulence factors (5, 12). The expression of laccase has been correlated with the virulence of C. neoformans (13–17). Laccase plays important roles to promote extrapulmonary dissemination of C. neoformans to the brain (16). Although the expression of LAC1, the predominant isoform, was uninvolved with the pulmonary clearance of low-virulence strains, its expression was essential for the progressive growth of a highly virulent strain of C. neoformans in the lungs (16, 17). Laccase enzyme was required for the biosynthesis of not only melanin but also prostaglandin E2 (18); therefore, its activity has been reported to be associated with enhanced fungal survival in macrophages (19). Indeed, laccase expression can promote the pathogenesis of cryptococcal infections by modulating both host innate and adaptive T helper cell responses (14, 17). Mice infected with C. neoformans strain H99 with a LAC1 mutation displayed diminished pulmonary eosinophilia and shifted immune polarization from deleterious M2 to M1 macrophages and Th2 to Th1/Th17 immune responses (17).
Although several lines of evidence have indicated the importance of laccase expression in promoting disease pathogenesis of C. neoformans infection, the immunomodulatory role of laccase in the virulence of C. gattii remains elusive. In this study, we investigated the laccase expression of C. gattii and characterized its effect in modulating fungal uptake, intracellular survival, and killing in macrophages by using a highly virulent C. gattii strain R265 that is deficient in LAC1 (20). The contribution of laccase expression to the disease caused by C. gattii and to the immune response during infection was also evaluated. C. gattii deficient in laccase was found to have less resistance to fungal clearance in macrophages. The lower virulence of the lac1Δ C. gattii mutant during the early stage of infection was found to be associated with a markedly increased cryptococcus-specific Th17 cytokine response, pulmonary neutrophil infiltration, and pulmonary expression of growth factor for neutrophil gene Csf3 and neutrophil-attracting chemokine genes Cxcl1, Cxcl2, and Cxcl5. Furthermore, laccase expression was found to be an important attribute of C. gattii for inhibiting phagocytosis and killing by neutrophils in vitro. Our studies suggest differential roles of laccase in C. gattii and C. neoformans. Laccase expression by C. gattii may play a role in promoting pulmonary fungal growth during initial lung infection by suppressing the Th17 cytokine response and the recruitment and function of neutrophils.
RESULTS
Differences in laccase activity of C. neoformans and C. gattii clinical isolates from Thailand.
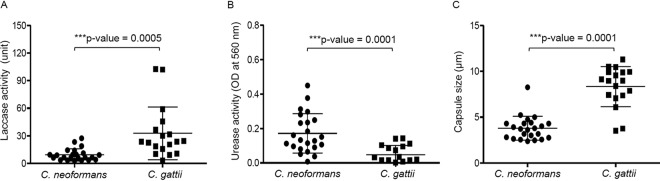
Several reports have shown the enhanced laccase activity of clinical isolates of C. neoformans; however, only a few studies have investigated those of C. gattii. To understand the importance of the laccase activity of C. gattii, we evaluated this enzyme activity together with other key virulence factors, namely, urease activity and capsule size, in comparison with those of C. neoformans. The fungal isolates used in this study are the previously described 23 isolates of C. neoformans and 18 isolates of C. gattii from Thailand (21). Laccase and urease enzyme activities were detected spectrophotometrically for the production of black melanin after exposure to l-DOPA (l-3,4-dihydroxyphenylalanine) medium and for the presence of pH change after urea hydrolysis, respectively. We found increased laccase and urease enzyme activities in both C. neoformans and C. gattii. While C. neoformans appeared to possess higher urease activity (P = 0.0001), the mean laccase activity of C. gattii was significantly higher than that of C. neoformans (P = 0.0005) (Fig. 1A and B). We also measured the capsule sizes of these isolates using India ink counterstaining after capsule induction. The capsule size detected of C. gattii was much greater than that of C. neoformans (P = 0.0001) (Fig. 1C). These data demonstrate significant differences in these virulence factors between C. neoformans and C. gattii clinical isolates. The high laccase enzyme activity in C. gattii suggests the importance of this attribute in disease pathogenesis of not only C. neoformans but also C. gattii infection.
FIG 1.
Activities of laccase and urease and sizes of capsules of C. neoformans and C. gattii clinical isolates. Laccase activity (A), urease activity (B), and capsule size (C) of C. neoformans and C. gattii clinical isolates are shown. Each dot represents one clinical isolate. Results were expressed as the mean of results of three to six experimental repeats. Error bars denote means ± SD. Significance was determined using an unpaired t test (two-tailed).
Effect of LAC1 gene disruption in C. gattii on fungal uptake rate, intracellular fungal load, intracellular proliferation rate, and killing by macrophages.
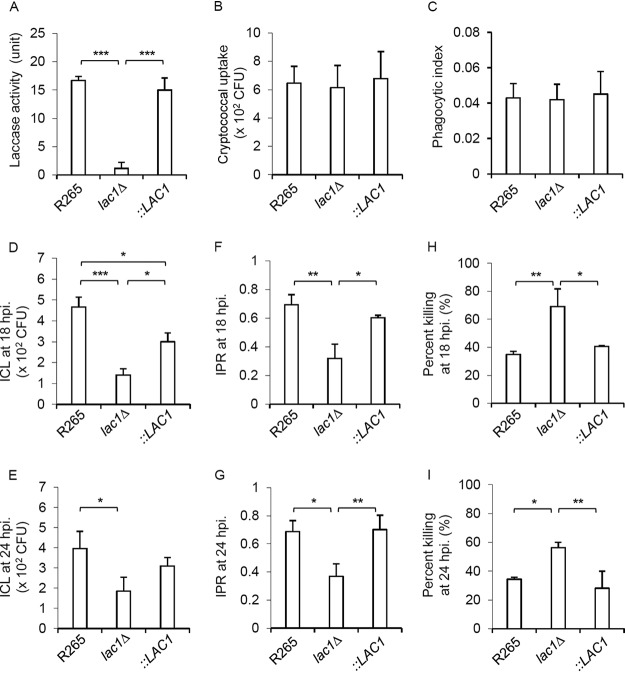
Laccase expression in C. neoformans has been shown to contribute to protection against killing by macrophages (14). Because high laccase activity was observed in C. gattii, we examined whether disruption of the LAC1 gene in C. gattii might enhance macrophage responses, since the LAC1 gene is conserved and considered to be the dominant laccase gene of both C. neoformans and C. gattii (20, 22). We tested the effect of laccase expression in C. gattii on Cryptococcus-macrophage interactions using the previously described melanin-deficient lac1Δ mutant of strain R265 and the reconstituted lac1Δ::LAC1 strain that restored melanin production (20). We confirmed the absence of laccase enzyme activity in the lac1Δ mutant and the restored melanin production in the lac1Δ::LAC1 strain, in comparison to the parental strain R265, by use of the laccase activity assay described in Materials and Methods (Fig. 2A). We determined the effect of laccase expression in C. gattii infection on host macrophage function by assessing phagocytosis rate, phagocytic index, intracellular fungal load (intracellular cryptococcal load [ICL]), intracellular proliferation rate (IPR), and killing by macrophages. While the phagocytosis rates and phagocytic indexes of these strains were similar (Fig. 2B and C), the intracellular fungal load and intracellular proliferation rate of the lac1Δ mutant were significantly lower than those of parental strain R265 and the reconstituted strain (Fig. 2D to G). Moreover, macrophage-mediated killing of the lac1Δ mutant appeared to be greater than killing of the parental and reconstituted strains (Fig. 2H and I). Thus, our data suggest that laccase expression in C. gattii is responsible for the protection against macrophage function in promoting intracellular fungal growth and proliferation.
FIG 2.
Laccase is crucial for intracellular proliferation and survival of C. gattii within macrophages. (A) Laccase activity of LAC1 deletion strains (lac1Δ) was determined and compared with that of the parental (R265) and LAC1 complementary (lac1Δ::LAC1 [::LAC1]) strains. (B to I) The J774 macrophage was infected by C. gattii R265, lac1Δ, and complementary strains at a 1:10 ratio, followed by washing out the extracellular yeasts after 2 h of infection and then lysis at 2, 18, and 24 h postinfection to determine cryptococcal uptake (B) and phagocytic index (C) at 2 h, intracellular loads (ICL) at 18 h (D) and 24 h (E), intracellular proliferation rate (IPR) at 18 h (F) and 24 h (G), and percent killing at 18 h (H) and 24 h (I) using the CFU assay. Graphs depict means ± SD and are representative of three experiments, and significance was determined by one-way ANOVA with Tukey’s post hoc analysis; *, P < 0.05; **, P < 0.01; ***, P < 0.0001.
Laccase expression in C. gattii is required for promoting pulmonary fungal growth at early but not late stages of infection.
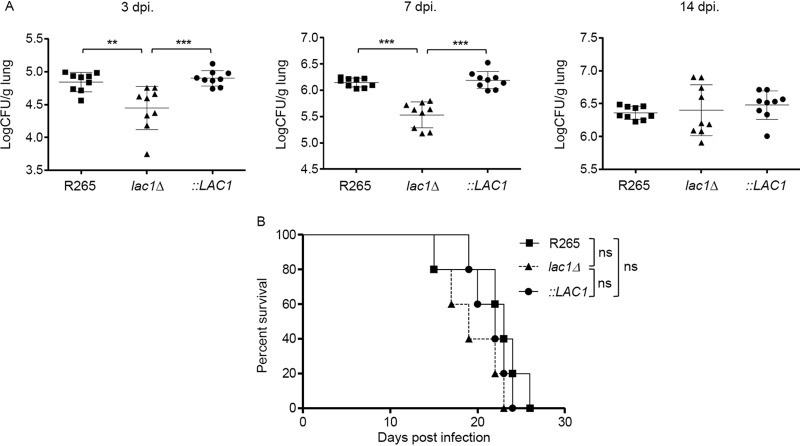
The expression of laccase has been shown to be required for the virulence of C. neoformans by supporting pulmonary persistence and facilitating brain dissemination (16, 17). Because we observed better fungal clearance of the lac1Δ mutant by macrophages, we further determined whether laccase deficiency in C. gattii may decrease virulence in a mouse model of cryptococcosis using BALB/c mice infected with Cryptococcus via intranasal inhalation as previously described (21). We evaluated pulmonary fungal burdens at days 3, 7, and 14 after infection and assessed mouse survival following infection with lac1Δ and lac1Δ::LAC1 strains and the parental C. gattii R265 strain. In comparison with infection with complementary and parental strains, there was significantly less fungal burden in mice infected with the lac1Δ strain at days 3 and 7 but not at day 14 postinfection (Fig. 3A). Like related studies indicating minimal brain involvement of C. gattii strain R265 (11), we indeed did not detect fungal burdens in the brains of mice infected with these strains. Despite the fact that lung fungal burden in mice infected with the lac1Δ strain in the early stage of infection was reduced, the survival rates were comparable between mice infected with C. gattii deficient in laccase and those infected with complementary and parental strains (Fig. 3B). These data suggest that whereas laccase expression in C. gattii is unrequired for supporting fungal growth in the late stage of infection, it acts as an early protective factor from host defense.
FIG 3.
Laccase is required for growth and proliferation of C. gattii during the early stage of infection. BALB/c mice were intranasally infected with C. gattii R265, LAC1 deletion (lac1Δ), and LAC1 complementary (lac1Δ::LAC1 [::LAC1]) strains at 5 × 104 yeast cells/mouse. (A) Lung fungal burdens of infected mice were enumerated at 3, 7, and 14 days postinfection. Graphs show results for individual mice and means ± SD of results from three pooled independent experiments (n = 3 mice per group per experiment). Significance was determined using one-way ANOVA with Tukey’s post hoc analysis; **, P < 0.01; ***, P < 0.0001; ns, not significantly different. (B) For survival analysis, infected mice were monitored for survival daily for 60 days. Survival curves were generated from the results obtained with eight mice per group and evaluated for statistical significance with Kaplan-Meier survival curves, and P values were obtained from a log rank test. ns, not significantly different.
Deficiency of laccase in C. gattii enhanced cryptococcus-specific IL-17 but not type-1/2 immune responses during the early stage of infection.
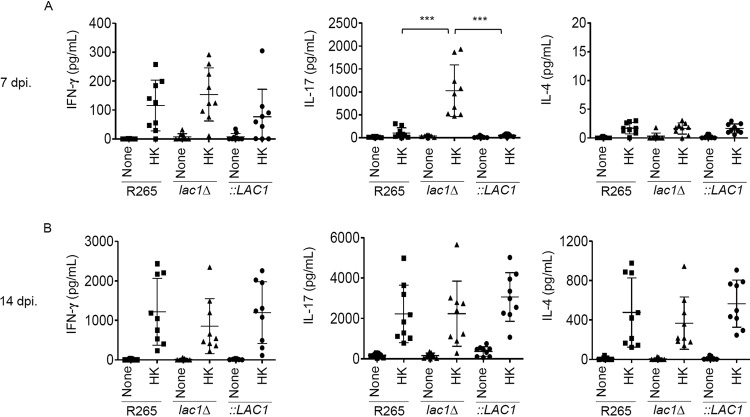
A recent study demonstrated that the deletion of LAC1 in C. neoformans resulted in reduced virulence by shifting Th2 to Th1 and Th17 cytokine responses (17). Therefore, we tested whether reduced fungal growth in the early stage of lac1Δ C. gattii infection might be associated with lower Th2-type cytokine induction during infection. To further understand the role of laccase in modulating the T helper cytokine response against C. gattii, we assessed the effect of the LAC1 deletion in C. gattii on cryptococcus-specific cytokine production in lung-draining lymph nodes at 7 and 14 days postinfection. Lung-draining lymph nodes harvested from mice infected with the parental C. gattii R265 strain and those infected with lac1Δ and lac1 complementary (lac1Δ::LAC1) strains were activated with the same heat-killed cryptococcal strain for 3 days in vitro before the culture supernatants were collected to measure cryptococcus-specific cytokines by ELISA. As previously reported (11), the production of cryptococcus-specific gamma interferon (IFN-γ) and interleukin-17 (IL-17) was low in lung-draining lymph nodes of mice infected with the parental C. gattii R265 strain at 7 and 14 days postinfection (Fig. 4A and B). While IL-4 in the lung-draining lymph nodes of these mice was undetected in the early stage of infection, we found higher induction of cryptococcus-specific IL-4 at 14 days postinfection (Fig. 4B). Unlike C. neoformans with a targeted LAC1 gene deletion (lac1Δ), C. gattii deficient in laccase did not alter the production of IFN-γ and IL-4 compared with that of the parental strain R265 (Fig. 4A and B). Interestingly, we detected significantly higher induction of cryptococcus-specific IL-17 production in lung-draining lymph nodes of mice infected with the lac1Δ mutant at 7 days (Fig. 4A) but not 14 days (Fig. 4B) postinfection. The laccase-reconstituted strain impaired the induction of cryptococcus-specific IL-17 without affecting IFN-γ and IL-4 (Fig. 4A and B), as observed for the parental strain. These data suggest that although no role in switching type-1 to type-2 immune responses was observed, laccase expression in C. gattii is able to suppress the induction of IL-17 cytokine production.
FIG 4.
C. gattii laccase deletion strain potentiates the induction of cryptococcus-specific IL-17 responses during the early phase of infection. BALB/c mice were intranasally infected with C. gattii R265, LAC1 deletion (lac1Δ), and LAC1 complementary (lac1Δ::LAC1 [::LAC1]) strains at 5 × 104 yeast cells/mouse. At the indicated time points, lung-draining lymph nodes were harvested and cell suspensions were prepared to analyze cryptococcus-specific cytokine production. After lymph node cells were stimulated with heat-killed Cryptococcus cells for 72 h, supernatant from lymph node stimulation at 7 (A) and 14 (B) days postinfection (dpi) was collected and subjected to analysis of cytokines IFN-γ, IL-17, and IL-4 by ELISA. Graphs show results for individual mice and means ± SD from three pooled independent experiments (n = 3 mice per group per experiment). Significance was determined by one-way ANOVA with Tukey’s post hoc analysis; ***, P < 0.0001.
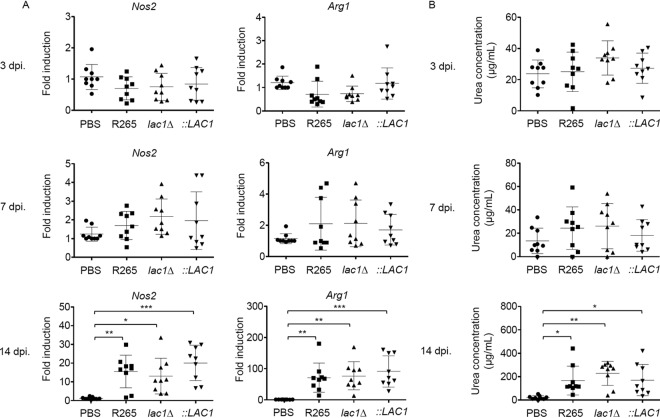
The expression of laccase in C. neoformans has been shown to promote alternative activation of macrophages in infected lungs (17); therefore, we determined whether the phenotypes of macrophages were altered in mice infected with C. gattii deficient in laccase. Mice were infected with lac1Δ mutant, lac1Δ::LAC1, and R265 strains, and the expression of signature genes that specify classically (M1) (Nos2) and alternatively (M2) (Arg1) activated macrophages in the total lungs of infected mice was evaluated using real-time PCR analysis. While the upregulation of Nos2 and Arg1 was detected at 14 days postinfection, no significant differences were observed in the expression of Nos2 and Arg1 among mice infected with lac1Δ mutant, lac1Δ::LAC1, and R265 strains at any stage of infection (Fig. 5A). Likewise, the activity of the arginase enzyme indicated by the concentration of produced urea by bronchoalveolar lavage using a colorimetric method with α-isonitrosopropiophenone was found to be induced after 14 days postinfection with the C. gattii R265 strain but remained unaltered in mice infected with the lac1Δ mutant (Fig. 5B). All together, these data suggest that the expression of laccase in C. gattii may be uninvolved with the induction of type-2 immune responses but plays a role in attenuating Th17-mediated cytokine responses.
FIG 5.
Effect of laccase expression of C. gattii on the polarization of pulmonary M1 and M2 macrophages. BALB/c mice received PBS or were intranasally infected with C. gattii R265 (parental), LAC1 deletion (lac1Δ), and LAC1 complementary (lac1Δ::LAC1 [::LAC1]) strains at 5 × 104 yeast cells/mouse. (A) The expression of genes associated with M1 (Nos2) and M2 (Arg1) macrophages in lungs at 3, 7, and 14 days postinfection. (B) Arginase activity in BAL fluids was determined by photometric measurement of the urea concentration (μg/ml) produced at 3, 7, and 14 days postinfection. Graphs show results for individual mice and means ± SD from three pooled independent experiments (n = 3 mice per group per experiment). Significance was determined by one-way ANOVA with Tukey’s post hoc analysis; *, P < 0.05; **, P < 0.01; ***, P < 0.0001.
Laccase expression in C. gattii is required to inhibit neutrophil recruitment and pulmonary chemokine expression at the early stage of infection.
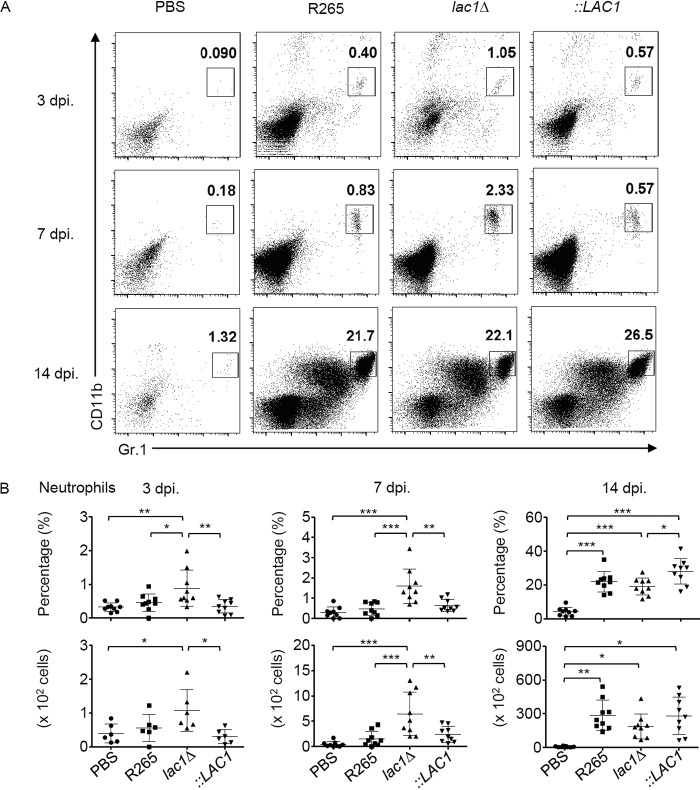
Th17 cells play a role in host defense by mediating the recruitment of neutrophils. Related studies have indicated that highly virulent C. gattii was able to inhibit neutrophil migration in vitro and infiltration in the lungs after in vivo infection (8, 9). Because disruption of LAC1 in C. gattii resulted in an enhanced early control of fungal growth in mouse lungs that was associated with increased IL-17 cytokine responses in the early stage of infection, we further tested whether the expression of laccase in C. gattii might contribute to the inhibition of neutrophil recruitment in the lungs. The frequencies of neutrophils in the lungs of mice infected with lac1Δ, lac1Δ::LAC1, and parental C. gattii R265 strains were enumerated at days 3, 7, and 14 postinfection. While infection with the C. gattii R265 strain induced very low neutrophil infiltration in lungs following infection, we detected a significantly increased number of pulmonary neutrophils in mice infected with the lac1Δ mutant strain at days 3 and 7 but not 14 days postinfection (Fig. 6A and B). Interestingly, the reconstituted strain was able to restore the impaired neutrophil recruitment in infected lungs to a level comparable to that of the parental strain (Fig. 6A and B). Notably, we did not observe any differences in infiltration of other pulmonary leukocytes, including dendritic cells, macrophages, and CD4+ T helper cells, among these mice (see Fig. S1 in the supplemental material). These data indicate that laccase expression by C. gattii contributed to the attenuation of neutrophil infiltration in the lungs during the early infection phase.
FIG 6.
Effect of laccase expression in C. gattii on pulmonary neutrophil infiltration. BALB/c mice received PBS or were intranasally infected with C. gattii R265, LAC1 deletion (Δlac1), or LAC1 complementary (lac1Δ::LAC1 [::LAC1]) strains at 5 × 104 yeast cells/mouse. At the indicated time points, mice were sacrificed and bronchoalveolar lavage (BAL) fluid and cells were collected to analyze neutrophils by a flow cytometry assay. (A) Representative dot plot showing strategy for characterization of neutrophils (CD11b+ Gr.1hi) from BAL fluid cells of infected mice at 3, 7, and 14 days postinfection. (B) Percentage and absolute number of BAL fluid neutrophils at 3, 7, and 14 days postinfection. Graphs show results for individual mice and means ± SD of results from three pooled independent experiments (n = 3 mice per group per experiment). Significance was determined using one-way ANOVA with Tukey’s post hoc analysis; *, P < 0.05; **, P < 0.01; ***, P < 0.0001.
To understand the involvement of laccase expression in C. gattii in potentiating neutrophil inhibition, we analyzed the pulmonary gene expression of key cytokines and chemokines associated with the survival and recruitment of neutrophils as well as a family of extracellular proteases and matrix metalloproteinases-12 (MMP-12) that were previously shown to contribute to the regulation of pulmonary inflammation in Cryptococcus infection (23). At day 7 postinfection, infection with C. gattii strain R265 was unable to elicit the pulmonary gene expression of key neutrophil-attracting chemokines, cytokines, and MMP-12 (Fig. 7A). While the lac1Δ::LAC1 complementary strain and the R265 strain induced similar expression patterns, we found a significant upregulation of Mmp12, Csf2, Csf3, Cxcl1, Cxcl2, and Cxcl5 at day 7 postinfection with the lac1Δ mutant (Fig. 7A). Consistent with the comparable neutrophil accumulation in the lungs at day 14 postinfection, we did not detect different expression patterns of MMP-12, neutrophil-attracting chemokines, and growth factors in mouse lungs infected with R265 and the lac1Δ mutant strain (Fig. 7B). Collectively, our data suggest that the expression of laccase in C. gattii contributed to the inefficient induction of pulmonary neutrophil infiltration and inflammatory chemokine expression that may support C. gattii survival during the early infection phase.
FIG 7.
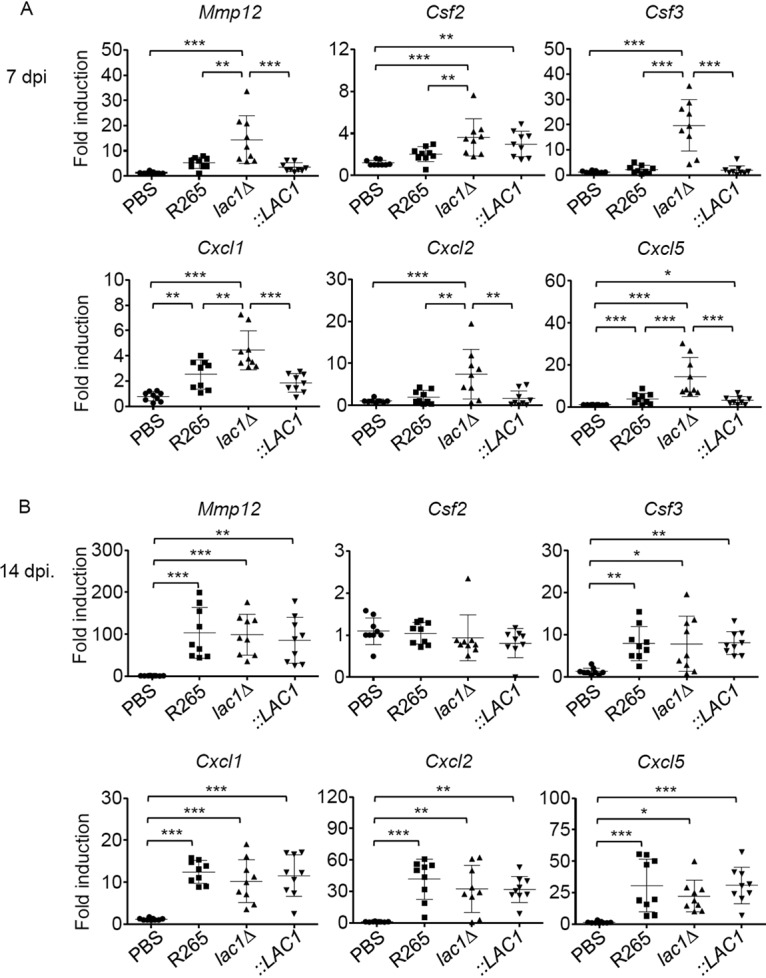
Mice infected with a C. gattii laccase deletion strain induced expression of neutrophil growth factor and neutrophil-attracting chemokines in the lung. BALB/c mice received PBS or were intranasally infected with C. gattii R265, LAC1 deletion (lac1Δ), or LAC1 complementary (lac1Δ::LAC1 [::LAC1]) strains at 5 × 104 yeast cells/mouse. At the indicated time points, mice were sacrificed and lungs were collected. Total lung RNA was isolated and subjected to cDNA synthesis, followed by real-time PCR analysis of genes associated with the growth and recruitment of neutrophils, including Mmp12, Csf2, Csf3, Cxcl1, Cxcl2, and Cxcl5 at 7 (A) and 14 (B) days postinfection. Expression levels of target genes were normalized to endogenous actin (Actb) transcript levels, and relative quantification of samples was compared with the PBS control serving as the baseline. Graphs show results for individual mice and means ± SD from three pooled independent experiments (n = 3 mice per group per experiment). Significance was determined using one-way ANOVA with Tukey’s post hoc analysis; *, P < 0.05; **, P < 0.01; ***, P < 0.0001.
Laccase expression in C. gattii is required to inhibit neutrophil-mediated antifungal activity in vitro.
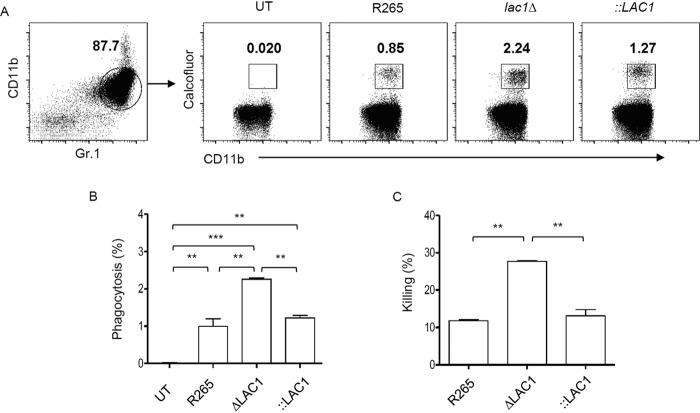
One recent study indicated that neutrophils can mediate antifungal activity against the highly virulent C. gattii strain R265 in vitro (24). Because the laccase enzyme contributes to the inhibition of neutrophil recruitment in the lungs at the early stage of C. gattii infection, we further investigated whether the expression of laccase in C. gattii might inhibit phagocytosis and neutrophil fungicidal activity in vitro. We evaluated the percentage of phagocytosis of lac1Δ, lac1Δ::LAC1, and parental C. gattii R265 strains stained with calcofluor white by neutrophils by use of flow cytometry analysis. As described in a related study (24), we found that C. gattii R265 could be engulfed by neutrophils (Fig. 8A and B). Interestingly, the percentage of yeast cells phagocytosed by neutrophils was much higher with infection with the lac1Δ mutant strain than with infection with R265 and complementary strains (Fig. 8A and B). We next assessed the fungicidal effect of neutrophils against C. gattii R265 and laccase-deficient strains. We found that neutrophils were able to kill C. gattii R265 (Fig. 8C). However, the killing ability of the neutrophils was much greater with infection with the laccase-deficient C. gattii strain (Fig. 8C). All together, our data suggest that laccase expression in C. gattii plays an important role in suppressing neutrophil function in vitro.
FIG 8.
Laccase expression of C. gattii suppresses phagocytosis and killing by neutrophils in vitro. Freshly isolated murine neutrophils were cultured with live C. gattii R265, LAC1 deletion (lac1Δ), and LAC1 complementary (lac1Δ::LAC1 [::LAC1]) strains. For neutrophil phagocytosis, cryptococci were opsonized with fresh mouse serum and labeled with calcofluor white (CFW; 1:100), followed by incubation with neutrophils for 3 h. The percent phagocytosis was evaluated by flow cytometry. (A) Representative dot plot showing strategy for characterization of neutrophils associated with Cryptococcus (CD11b+ Gr.1hi CFW+ neutrophils). (B) Graph showing percent phagocytosis of Cryptococcus by neutrophils. For neutrophil killing, cryptococci were opsonized with fresh mouse serum and incubated with neutrophils for 4 h. After neutrophil lysis, the suspension was plated on an SDA plate to measure the number of viable fungi (CFU/ml) and the percent killing was calculated by comparison with a Cryptococcus culture without neutrophils. (C) Graph showing percent killing of Cryptococcus by neutrophils. Graphs depict means ± SD and are representative of results of three independent experiments. Significance was determined using one-way ANOVA with Tukey’s post hoc analysis; **, P < 0.01; ***, P < 0.0001.
DISCUSSION
The complex interactions between Cryptococcus and the immune system shape the consequence of disease pathogenesis. Laccase activity and melanin synthesis have been implicated as major factors responsible for virulence in C. neoformans. The higher laccase activity of a C. neoformans strain of HIV-positive patients appeared to heighten fungal survival in cerebrospinal fluid (CSF) (19). Moreover, laccase has been shown to be an important immune modulator of host-C. neoformans interaction (17, 25). The protective function of laccase in C. neoformans against antimicrobial activities and its role in modulating macrophage and T helper cell phenotypes promoted the progressive pulmonary fungal growth and dissemination to the brain. Unlike C. neoformans, C. gattii appears to cause infection in immunocompetent hosts. The influences of laccase expression on the virulence of C. gattii and the ability to modulate the host immune response have not been clearly addressed. In the present study, we examined the effect of laccase expression in C. gattii on the host immune response to better understand the divergence of C. neoformans and C. gattii in modulating the immune response and disease pathogenesis. We found that laccase expression of C. gattii contributed to the inhibition of macrophage defense, IL-17 cytokine, and neutrophil responses in the early stage of infection, as well as the suppression of neutrophil fungicidal activity in vitro.
Based on our results of virulence factor characterization of C. neoformans and C. gattii isolates from Thailand, C. neoformans appears to be a higher urease producer than C. gattii, as has been suggested by other studies (26). One recent study showed an important role of urease in C. gattii infection, because the urease mutation caused reduced blood burdens and a delayed time of death (27). It has been demonstrated that capsule size can differ dramatically between C. neoformans and C. gattii (5). We found a greater capsule size for C. gattii than for C. neoformans, suggesting the involvement of capsule size in disease pathogenesis of C. gattii infection. Indeed, a recent study suggested that whereas the capsular strain impaired the induction of DC and T cells, the acapsular mutant of C. gattii induced human monocyte-derived DC maturation and T cell proliferation, suggesting its contribution to the mechanisms of immune evasion (28). While laccase activity of C. neoformans has been recognized as an important virulence factor that plays multiple roles to protect against the host immune defense, the role of laccase expression in C. gattii infections is less clear. Related studies have found variable degrees of laccase enzyme activity in C. gattii isolated from immunocompetent goats (29). A higher level of enzyme activity has been associated with a highly virulent strain of C. gattii (20, 29). In accordance with these related studies, we investigated the laccase enzyme activity in Cryptococcus isolates from Thailand and found an expressed laccase enzyme activity not only in C. neoformans but also in C. gattii. The average laccase enzyme activity of the investigated C. gattii isolates was even higher than those of the C. neoformans isolates. Our data thus emphasize that laccase activity is an important virulence factor for not only C. neoformans but also C. gattii.
As with C. neoformans, a related study using the lac1Δ mutant strain of C. gattii R265 (the C. gattii Vancouver Island outbreak strain) indicated that LAC1 was the main gene responsible for melanin production (20). Using l-DOPA as the substrate, we did not detect laccase activity in the lac1Δ mutant C. gattii R265 strain. A related study suggested that there were no other phenotypic defects, including capsule formation and growth at 37°C in the lac1Δ mutant C. gattii R265 strain (20). Moreover, the enzyme activity could be restored in the lac1Δ::LAC1 complementary strain, suggesting that this strain could be used to further address the function of laccase expression in C. gattii. By evaluating the interaction with macrophages in vitro, we found laccase expression in C. gattii to be involved in fungal growth, intracellular proliferation, and resistance to killing in macrophages without affecting the phagocytosis rate. Similar to related findings regarding C. neoformans, laccase expression has been associated with ex vivo CSF survival and the in vivo rate of fungal clearance (19) and a laccase-negative strain was more susceptible to the antifungal activity of alveolar macrophages (14). Our data suggest that laccase expression in C. gattii may have a function similar to that in C. neoformans, to protect the fungus from the antifungal activity of macrophages.
By investigating the contribution of laccase expression in C. gattii to fungal virulence in mice, we found that laccase expression is important for control of the early growth of the fungus in the lungs without affecting fungal burdens during the chronic stage or the mouse survival rate. The expression of laccase in C. neoformans has been shown to mainly promote fungal dissemination to the brain (16, 17). Moreover, the effect of laccase on pulmonary persistence has been detected in a high-virulence C. neoformans strain but not a low-virulence C. neoformans strain deficient in laccase. Because the Vancouver Island outbreak strain R265 exhibited a high level of laccase activity, it is likely that the expression level of laccase in high-virulence strains is correlated with their ability to suppress the protective pulmonary host response. A related study quantifying laccase expression in lung tissue during the course of infection showed that laccase is highly expressed in early infection but decreases at later stages of infection (30). Indeed, we observed increased laccase activity in C. gattii in pulmonary cells during the course of infection (data not shown), suggesting the role of laccase during infection. Moreover, the functional compensation of LAC2 in the absence of LAC1, as has been described in C. neoformans (31), in long-term virulence of C. gattii cannot be excluded in this study.
Related research has suggested that the mechanism by which laccase contributes to cryptococcal virulence may be due to augmentation of the development of nonprotective type-2 immune responses and inhibition of the polarization of Th1 and Th17 (17, 32). Unlike for C. neoformans, the presence of laccase in C. gattii did not affect the production of cryptococcus-specific Th1/Th2 cytokines or the development of M1/M2 macrophages. However, consistent with previous studies (17, 32), we found that laccase expression in C. gattii attenuated the induction of cryptococcus-specific IL-17 cytokine responses. Comparable to observations in C. neoformans (18, 32), laccase in C. gattii may be required for prostaglandin E2 (PGE2) synthesis, exhibiting an inhibitory effect on IL-17 production, but this requires further investigation.
IL-17 has been found to promote neutrophil chemotaxis (33), and correspondingly, we observed that laccase of C. gattii inhibited pulmonary neutrophil accumulation and neutrophil-attracting chemokine expression during the early infection phase, in which a reduced IL-17 cytokine level was measured. Furthermore, it has been demonstrated that C. gattii suppresses the recruitment of neutrophils in vitro and in vivo at the early stage of infection (8, 9). Therefore, laccase expression in C. gattii may be central to neutrophil inhibition. Moreover, we observed that the percentage of phagocytosis and killing by neutrophils in vitro was much more pronounced in the laccase-deficient strain, indicating that laccase expression allows C. gattii R265 to resist neutrophil-mediated killing to some extent. Although a recent study indicated that neutrophils can recognize, phagocytose, and kill a highly virulent C. gattii R265 strain (24), it has been suggested that the killing of C. gattii R265 by neutrophils may not be efficient for fungal sterilization. Likewise, related research suggested that IL-17 enhances the host defense against pulmonary infection with C. neoformans by affecting inflammatory cell recruitment but without affecting mouse survival (34). Quite possibly, the expression of laccase in C. gattii is required to support fungal growth in the early lung infection phase by suppressing the production of IL-17 and the recruitment and function of neutrophils. The possible mechanisms by which laccase inhibits antifungal activities might be due to the suppression of respiratory burst or oxidative stress in phagocytes, as previously described in a model of C. neoformans (14, 35). It has been suggested that C. gattii is more resistant to oxidative stress than C. neoformans and that oxidative killing is not effective for sterilization of C. gattii R265 (24). It is likely that different mechanisms may be required for the neutrophil-mediated antifungal effects on C. gattii and C. neoformans. Although neutrophils seem to be detrimental in some mouse strains and in HIV-infected patients with cryptococcal meningitis (36, 37), there have been several reports in mouse and human studies indicating the importance of neutrophils in Cryptococcus clearance (38–40). In mouse strains with more resistance to progressive pulmonary infection, the early neutrophilia was found to promote protective immune responses against C. neoformans (39). Considering the ability of C. gattii to cause disease in immunocompetent hosts, neutrophils may be important in the early protection of hosts against progressive pulmonary cryptococcosis. The definitive roles of neutrophils in mediating protective effects against cryptococcosis caused by C. gattii should be investigated further in both mice and humans.
In summary, our present data demonstrate that laccase is required for the early growth of C. gattii strain R265 in the lungs and provide evidence that laccase significantly contributes to the virulence of not only C. neoformans but also C. gattii in pulmonary infection. The expression of laccase in C. gattii has been associated with the inhibition of the Th17-type cytokine response and neutrophil recruitment and function without affecting type-1 and type-2 immune responses. While C. neoformans has been associated with dissemination to the brain, C. gattii has been known to cause fatal lung infection with minimal brain involvement. Because a type-2 immune response was stimulated by C. neoformans but not C. gattii, the differences in the mammalian immune responses to these fungi likely contribute to differing disease outcomes. The function of laccase in C. gattii may help us to gain clearer insights into the involvement of fungal virulence in evading host immune responses and promoting cryptococcal diseases.
MATERIALS AND METHODS
Mice.
Female BALB/c mice, 6 to 8 weeks old, were obtained from Nomura Siam International Co., Ltd., Thailand, and housed under specific-pathogen-free conditions in the animal facility of Thammasat University. Mice were euthanized with CO2 by controlled gradual displacement using a flow meter in accordance with IACUC and the American Veterinary Medicine Association (AMVA) guidelines on euthanasia. All animal studies were approved by the Thammasat University Animal Care and Use Committee (008/2557).
Cryptococcus strains.
All clinical isolates of the Cryptococcus neoformans-Cryptococcus gattii species complex and reference strains of C. neoformans (H99) and C. gattii (R265) (21) were retrieved from a culture collection of the Department of Medical Technology, Faculty of Allied Health Sciences, Thammasat University. Genetically engineered strains of C. gattii (R265), LAC1-deleted (lac1Δ), and LAC1-complemented (lac1Δ::LAC1) strains generated from a related study (20) were retrieved from a culture collection of the Department of Microbiology, Faculty of Medicine, Siriraj Hospital, Mahidol University. Cryptococcus strains were thawed and maintained in Sabouraud dextrose agar (SDA) (Thermo Fisher Scientific) at room temperature. A single colony of yeast cells was cultured in Sabouraud dextrose broth (SDB) at 37°C with shaking at 200 rpm for 24 h before infection. This study’s ethics were approved by the Thammasat Institutional Review Board under certificate number 041/2558 (exempt).
Laccase activity.
The activity of laccase was determined as previously described (19). Briefly, a single isolated colony from SDA was resuspended in l-DOPA medium (0.1% glucose anhydrous, 0.1% l-asparagine, 0.3% KH2PO4, 0.025% MgSO4·7H2O, and 0.01% l-DOPA, pH 5.56) and adjusted to achieve a concentration of 4 × 106 cells/ml. The inoculum was incubated for 16 h at 37°C and then for 24 h at 25°C, with shaking at 250 rpm to induce melanin production. The supernatant was collected after centrifugation at 4,000 × g for 5 min, and the concentration of melanin was determined spectrophotometrically at a 475-nm wavelength and converted to laccase units (U) by a factor of 0.001 optical density (OD) equal to 1 U (19). The reference strains of C. neoformans (H99) and C. gattii (R265) were used as control strains, and the assays were repeated two or three times.
Urease activity.
The urease activity of Cryptococcus was determined as previously described (41). Briefly, 24-h cryptococcal cultures were washed and resuspended in 2 ml of sterile phosphate-buffered saline (PBS) at pH 7.2. The cell suspensions were adjusted to an OD of 0.7 at 570 nm and mixed with an equal volume of 2× RUH broth and cultured at 37°C with shaking at 200 rpm for 8 h. Urease activity in the culture supernatants was determined at 570 nm using PBS as a blank control. A magenta red color was considered a positive reaction, and an orangish yellow color was considered a negative reaction.
Capsule induction assay.
To induce capsule production in vitro, 24-h cryptococcal cultures were washed and adjusted to 2 × 106 cells/ml in capsule-inducing medium (Dulbecco’s modified Eagle medium [DMEM] with 1% NCTC-109 medium and 10% heat-inactivated fetal bovine serum [FBS]) and incubated for 48 h at 37°C with 10% CO2. Cells were subsequently harvested, counterstained with India ink, and observed under a 40× bright-field objective using an Olympus DP21-SAL light microscope (Olympus Canada). The capsule diameter was calculated as the average of (total cell diameter – cell body diameter)/2 for 50 to 100 cells (19).
In vitro phagocytosis, intracellular proliferation, and killing assays.
The murine macrophage cell line J774 (ATCC TIB-67) was used for in vitro phagocytosis, intracellular proliferation, and killing assays as previously described (21). Briefly, macrophages were cultured in complete Dulbecco’s modified Eagle medium (cDMEM), i.e., DMEM medium supplemented with 10% FBS, 1% l-glutamine, and 1% penicillin and streptomycin, in a 5% CO2 humidified atmosphere at 37°C. Before infection, 1.5 × 105 cells of J774 macrophages were cultured in a 24-well culture plate (Corning) containing cDMEM for 24 h at 37°C with 5% CO2 and in serum-free medium for 2 h, followed by activation with 1 μg/ml phorbol myristate acetate (PMA) for 30 min at 37°C with 5% CO2. Macrophages were then infected with cryptococci that were opsonized with anti-capsule monoclonal antibody (MAb) 18B7 (kindly provided by Arturo Casadevall, Albert Einstein College of Medicine, New York, USA) at a ratio of 1:10 and incubated at 37°C with 5% CO2. Cryptococcal uptake was determined by the number of intracellular cryptococci within the macrophages 2 h after infection. After 2 h of infection, extracellular cryptococci were removed, and macrophage cultures were lysed with sterile distilled water and plated on Sabouraud dextrose agar to analyze CFU. The phagocytic index (PI) was calculated using the following formula: PI = (CFU at 2 hpi/total number of macrophages) × 100 (where hpi is hours postinfection) (42). To determine the intracellular cryptococci (intracellular cryptococcal load [ICL]), the remaining wells were maintained and lysed at 18 and 24 h as described above. The IPR of Cryptococcus at 18 and 24 h postinfection was calculated from the number of intracellular cryptococci at 18 or 24 h postinfection divided by the number of intracellular cryptococci at 2 h postinfection. For intracellular killing, the percent killing of Cryptococcus within macrophages at 18 and 24 h postinfection was calculated by the following formula: % killing at 18 or 24 hpi = [100 − (CFU at 18 or 24 hpi × 100)/CFU at 2 hpi].
Murine model of Cryptococcus infection.
The 24-h cryptococcal cultures were washed, counted, and resuspended in PBS at a concentration of 1 × 106 yeast cells/ml as previously described (21). After anesthetization with isoflurane, the mice were treated with PBS or infected with Cryptococcus by intranasal inoculation of 50 μl of the yeast cell suspension (5 × 104 yeast cells/mouse) (43). For survival analysis, BALB/c mice were infected with Cryptococcus (8 mice/group) and monitored for their survival by inspection twice daily and euthanized when they appeared to be in pain or moribund (11). For organ isolation and the CFU assay, the lungs of Cryptococcus-infected mice were excised, weighed, and homogenized in sterile PBS with 1% penicillin and streptomycin and plated at various dilutions on SDA. The number of CFU was calculated following incubation at 30°C for 48 h.
BAL fluid collection and inflammatory cell analysis.
Bronchoalveolar lavage (BAL) fluid was collected at the time points indicated in the figures and stored at −80°C for arginase activity. BAL cells were analyzed for inflammatory cells by staining them with the following antibodies: fluorescein isothiocyanate (FITC)-conjugated anti-CD11b (M1/70), phycoerythrin (PE)-conjugated anti-CD11c (HL3), and allophycocyanin (APC)-conjugated-anti-Gr.1 (RB6-8C5) antibodies. Neutrophils were analyzed based on the expression of CD11b and Gr.1 (CD11b+ Gr.1hi) as previously described (11). Fc receptors were blocked by the addition of anti-CD16/32 antibody. Flow cytometric analysis was performed using a BD FACSVerse cytometer (BD Biosciences, San Jose, CA, USA).
Arginase activity assay.
Arginase enzyme activity was determined as previously described (21). Briefly, 100-μg/ml total protein concentrations of BAL fluids were added to the activation solution (50 mmol Tris-HCl and 10 mmol MnCl2, pH 7.5) and then incubated at 56°C for 10 min. After the addition of 0.5 M l-arginine (pH 9.7) to the activated mixture, the solution was incubated at 37°C for 2 h, followed by the addition of acid stop solution. For the colorimetric determination of urea, 9% α-isonitrosopropiophenone (ISPF) (Sigma-Aldrich) was added, and the mixture was heated at 100°C for 45 min. The urea concentration was determined spectrophotometrically by measuring the absorbance at 490 nm. The activity of arginase enzyme was determined as a concentration of produced urea (μg/ml).
Quantitative RT-PCR.
Total lung RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. The RNA was used as the template for unspecific first-strand cDNA synthesis by reverse transcription using oligo(dT), random hexamers, and Moloney murine leukemia virus (MMLV) reverse transcriptase (Invitrogen). Real-time PCR (RT-PCR) was performed using iTaq universal SYBR green supermix (Bio-Rad Laboratories) and the cytokine and chemokine primer pairs as previously described (21). Expression levels of target genes were normalized to actin (Actb) transcript levels, and relative quantification of samples was compared with the PBS control serving as the baseline.
Cryptococcus-specific cytokine production.
Single-cell suspensions of lung-draining lymph nodes were plated in a 24-well plate and stimulated with heat-killed C. gattii at a ratio of 1:2. Following 72 h of stimulation at 37°C with 5% CO2, the culture supernatant was collected and kept at −80°C for cytokine analysis. The concentrations of cytokines IL-4, IFN-γ, and IL-17 were measured using the antibody pairs from BD Pharmingen according to the manufacturer’s instructions.
Neutrophil phagocytosis and killing assays.
The neutrophil phagocytosis and killing assays were performed as previously described (24, 44). Briefly, 1.6 × 106 cells of freshly isolated murine neutrophils were incubated in RPMI medium supplemented with 40% fresh mouse serum in the presence of live cryptococci (3.2 × 106 cells) that were opsonized with fresh mouse serum and labeled with calcofluor white (CFW; 1:100 dilution) (Sigma) for 3 h. After incubation, cells suspensions were stained with fluorochrome-conjugated antibodies against CD11b and Gr.1 to determine neutrophils associated with fungal cells (CD11b+ Gr.1hi CFW+ neutrophils) using a BD FACSVerse cytometer (BD Biosciences), and the acquired data were analyzed with FlowJo software (Treestar). For neutrophil killing analysis, freshly isolated neutrophils (3.2 × 106 cells) and live opsonized cryptococcal cells (4 × 104 cells) were incubated in RPMI medium supplemented with 40% fresh mouse serum for 4 h. To assess the killing of Cryptococcus by neutrophils, 1 μl of 10% (vol/vol) Triton X-100 was added to the suspension to lyse the neutrophils, suspensions were diluted and plated on SDA plates to count the number of viable fungi expressed as CFU, and killing percentage were calculated by comparison with CFU of fungus in the absence of neutrophils.
Statistical analysis.
Each experiment was performed two or three times. Data are presented as mean values ± standard deviation (SD). Data were analyzed using an unpaired t test (two-tailed) as well as one-way analysis of variance (ANOVA) with Turkey’s post hoc analysis. Survival curves were evaluated for statistical significance with Kaplan-Meier survival curves, and P values were obtained from a log rank test. Statistical analysis was performed using GraphPad Prism 5 Software. A P value of <0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank the faculty of Allied Health Sciences, Center of Scientific Equipment for Advanced Research, Thammasat University, for their support. We also thank Wieland Meyer, The Westmead Institute for Medical Research, the University of Sydney, Westmead, Australia, for kindly providing the lac1 mutants.
This work was supported by the fiscal year budget of the office of the National Research Council of Thailand to Thammasat University. A.H. was supported by a Royal Golden Jubilee Ph.D. (RGJ-PHD) program scholarship (grant no. PHD/0016/2558).
We declare no conflicting financial interests.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Maziarz EK, Perfect JR. 2016. Cryptococcosis. Infect Dis Clin North Am 30:179–206. doi: 10.1016/j.idc.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.May RC, Stone NR, Wiesner DL, Bicanic T, Nielsen K. 2016. Cryptococcus: from environmental saprophyte to global pathogen. Nat Rev Microbiol 14:106–117. doi: 10.1038/nrmicro.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ngamskulrungroj P, Chang Y, Sionov E, Kwon-Chung KJ. 2012. The primary target organ of Cryptococcus gattii is different from that of Cryptococcus neoformans in a murine model. mBio 3:e00103-12. doi: 10.1128/mBio.00103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrnes EJ III, Bartlett KH, Perfect JR, Heitman J. 2011. Cryptococcus gattii: an emerging fungal pathogen infecting humans and animals. Microbes Infect 13:895–907. doi: 10.1016/j.micinf.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bielska E, May RC. 2016. What makes Cryptococcus gattii a pathogen? FEMS Yeast Res 16:fov106. doi: 10.1093/femsyr/fov106. [DOI] [PubMed] [Google Scholar]
- 6.Rosen LB, Freeman AF, Yang LM, Jutivorakool K, Olivier KN, Angkasekwinai N, Suputtamongkol Y, Bennett JE, Pyrgos V, Williamson PR, Ding L, Holland SM, Browne SK. 2013. Anti-GM-CSF autoantibodies in patients with cryptococcal meningitis. J Immunol 190:3959–3966. doi: 10.4049/jimmunol.1202526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saijo T, Chen J, Chen SC, Rosen LB, Yi J, Sorrell TC, Bennett JE, Holland SM, Browne SK, Kwon-Chung KJ. 2014. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. mBio 5:e00912-14. doi: 10.1128/mBio.00912-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong ZM, Murphy JW. 1995. Effects of the two varieties of Cryptococcus neoformans cells and culture filtrate antigens on neutrophil locomotion. Infect Immun 63:2632–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng PY, Sham A, Kronstad JW. 2009. Cryptococcus gattii isolates from the British Columbia cryptococcosis outbreak induce less protective inflammation in a murine model of infection than Cryptococcus neoformans. Infect Immun 77:4284–4294. doi: 10.1128/IAI.00628-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huston SM, Li SS, Stack D, Timm-McCann M, Jones GJ, Islam A, Berenger BM, Xiang RF, Colarusso P, Mody CH. 2013. Cryptococcus gattii is killed by dendritic cells, but evades adaptive immunity by failing to induce dendritic cell maturation. J Immunol 191:249–261. doi: 10.4049/jimmunol.1202707. [DOI] [PubMed] [Google Scholar]
- 11.Angkasekwinai P, Sringkarin N, Supasorn O, Fungkrajai M, Wang YH, Chayakulkeeree M, Ngamskulrungroj P, Angkasekwinai N, Pattanapanyasat K. 2014. Cryptococcus gattii infection dampens Th1 and Th17 responses by attenuating dendritic cell function and pulmonary chemokine expression in the immunocompetent hosts. Infect Immun 82:3880–3890. doi: 10.1128/IAI.01773-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma H, May RC. 2009. Virulence in Cryptococcus species. Adv Appl Microbiol 67:131–190. doi: 10.1016/S0065-2164(08)01005-8. [DOI] [PubMed] [Google Scholar]
- 13.Salas SD, Bennett JE, Kwon-Chung KJ, Perfect JR, Williamson PR. 1996. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med 184:377–386. doi: 10.1084/jem.184.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, Tewari RP, Williamson PR. 1999. Laccase protects Cryptococcus neoformans from antifungal activity of alveolar macrophages. Infect Immun 67:6034–6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu X, Williamson PR. 2004. Role of laccase in the biology and virulence of Cryptococcus neoformans. FEMS Yeast Res 5:1–10. doi: 10.1016/j.femsyr.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Noverr MC, Williamson PR, Fajardo RS, Huffnagle GB. 2004. CNLAC1 is required for extrapulmonary dissemination of Cryptococcus neoformans but not pulmonary persistence. Infect Immun 72:1693–1699. doi: 10.1128/iai.72.3.1693-1699.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu Y, Davis MJ, Dayrit JK, Hadd Z, Meister DL, Osterholzer JJ, Williamson PR, Olszewski MA. 2012. Immune modulation mediated by cryptococcal laccase promotes pulmonary growth and brain dissemination of virulent Cryptococcus neoformans in mice. PLoS One 7:e47853. doi: 10.1371/journal.pone.0047853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erb-Downward JR, Noggle RM, Williamson PR, Huffnagle GB. 2008. The role of laccase in prostaglandin production by Cryptococcus neoformans. Mol Microbiol 68:1428–1437. doi: 10.1111/j.1365-2958.2008.06245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabiiti W, Robertson E, Beale MA, Johnston SA, Brouwer AE, Loyse A, Jarvis JN, Gilbert AS, Fisher MC, Harrison TS, May RC, Bicanic T. 2014. Efficient phagocytosis and laccase activity affect the outcome of HIV-associated cryptococcosis. J Clin Invest 124:2000–2008. doi: 10.1172/JCI72950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ngamskulrungroj P, Price J, Sorrell T, Perfect JR, Meyer W. 2011. Cryptococcus gattii virulence composite: candidate genes revealed by microarray analysis of high and less virulent Vancouver Island outbreak strains. PLoS One 6:e16076. doi: 10.1371/journal.pone.0016076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansakon A, Mutthakalin P, Ngamskulrungroj P, Chayakulkeeree M, Angkasekwinai P. 2019. Cryptococcus neoformans and Cryptococcus gattii clinical isolates from Thailand display diverse phenotypic interactions with macrophages. Virulence 10:26–36. doi: 10.1080/21505594.2018.1556150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito-Kuwa S, Nakamura K, Valderrama B, Aoki S, Vidotto V, Osafune T. 2008. Diversity of laccase among Cryptococcus neoformans serotypes. Microbiol Immunol 52:492–498. doi: 10.1111/j.1348-0421.2008.00063.x. [DOI] [PubMed] [Google Scholar]
- 23.Supasorn O, Sringkarin N, Srimanote P, Angkasekwinai P. 2016. Matrix metalloproteinases contribute to the regulation of chemokine expression and pulmonary inflammation in Cryptococcus infection. Clin Exp Immunol 183:431–440. doi: 10.1111/cei.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ueno K, Yanagihara N, Otani Y, Shimizu K, Kinjo Y, Miyazaki Y. 2019. Neutrophil-mediated antifungal activity against highly virulent Cryptococcus gattii strain R265. Med Mycol 57:1046–1054. doi: 10.1093/mmy/myy153. [DOI] [PubMed] [Google Scholar]
- 25.Waterman SR, Hacham M, Panepinto J, Hu G, Shin S, Williamson PR. 2007. Cell wall targeting of laccase of Cryptococcus neoformans during infection of mice. Infect Immun 75:714–722. doi: 10.1128/IAI.01351-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres-Rodriguez JM, Alvarado-Ramirez E, Gutierrez-Gallego R. 2008. Urease activity in Cryptococcus neoformans and Cryptococcus gattii. Rev Iberoam Micol 25:27–31. doi: 10.1016/S1130-1406(08)70007-X. [DOI] [PubMed] [Google Scholar]
- 27.Feder V, Kmetzsch L, Staats CC, Vidal-Figueiredo N, Ligabue-Braun R, Carlini CR, Vainstein MH. 2015. Cryptococcus gattii urease as a virulence factor and the relevance of enzymatic activity in cryptococcosis pathogenesis. FEBS J 282:1406–1418. doi: 10.1111/febs.13229. [DOI] [PubMed] [Google Scholar]
- 28.Huston SM, Ngamskulrungroj P, Xiang RF, Ogbomo H, Stack D, Li SS, Timm-McCann M, Kyei SK, Oykhman P, Kwon-Chung KJ, Mody CH. 2016. Cryptococcus gattii capsule blocks surface recognition required for dendritic cell maturation independent of internalization and antigen processing. J Immunol 196:1259–1271. doi: 10.4049/jimmunol.1501089. [DOI] [PubMed] [Google Scholar]
- 29.Alvarado-Ramírez E, Torres-Rodríguez JM, Sellart M, Vidotto V. 2008. Laccase activity in Cryptococcus gattii strains isolated from goats. Rev Iberoam Micol 25:150–153. doi: 10.1016/s1130-1406(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Rivera J, Tucker SC, Feldmesser M, Williamson PR, Casadevall A. 2005. Laccase expression in murine pulmonary Cryptococcus neoformans infection. Infect Immun 73:3124–3127. doi: 10.1128/IAI.73.5.3124-3127.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pukkila-Worley R, Gerrald QD, Kraus PR, Boily MJ, Davis MJ, Giles SS, Cox GM, Heitman J, Alspaugh JA. 2005. Transcriptional network of multiple capsule and melanin genes governed by the Cryptococcus neoformans cyclic AMP cascade. Eukaryot Cell 4:190–201. doi: 10.1128/EC.4.1.190-201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valdez PA, Vithayathil PJ, Janelsins BM, Shaffer AL, Williamson PR, Datta SK. 2012. Prostaglandin E2 suppresses antifungal immunity by inhibiting interferon regulatory factor 4 function and interleukin-17 expression in T cells. Immunity 36:668–679. doi: 10.1016/j.immuni.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds JM, Angkasekwinai P, Dong C. 2010. IL-17 family member cytokines: regulation and function in innate immunity. Cytokine Growth Factor Rev 21:413–423. doi: 10.1016/j.cytogfr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murdock BJ, Huffnagle GB, Olszewski MA, Osterholzer JJ. 2014. Interleukin-17A enhances host defense against cryptococcal lung infection through effects mediated by leukocyte recruitment, activation, and gamma interferon production. Infect Immun 82:937–948. doi: 10.1128/IAI.01477-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Missall TA, Moran JM, Corbett JA, Lodge JK. 2005. Distinct stress responses of two functional laccases in Cryptococcus neoformans are revealed in the absence of the thiol-specific antioxidant Tsa1. Eukaryot Cell 4:202–208. doi: 10.1128/EC.4.1.202-208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mednick AJ, Feldmesser M, Rivera J, Casadevall A. 2003. Neutropenia alters lung cytokine production in mice and reduces their susceptibility to pulmonary cryptococcosis. Eur J Immunol 33:1744–1753. doi: 10.1002/eji.200323626. [DOI] [PubMed] [Google Scholar]
- 37.Musubire AK, Meya DB, Rhein J, Meintjes G, Bohjanen PR, Nuwagira E, Muzoora C, Boulware DR, Hullsiek KH, COAT and ASTRO Trial Teams. 2018. Blood neutrophil counts in HIV-infected patients with cryptococcal meningitis: association with mortality. PLoS One 13:e0209337. doi: 10.1371/journal.pone.0209337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coffey MJ, Phare SM, George S, Peters-Golden M, Kazanjian PH. 1998. Granulocyte colony-stimulating factor administration to HIV-infected subjects augments reduced leukotriene synthesis and anticryptococcal activity in neutrophils. J Clin Invest 102:663–670. doi: 10.1172/JCI2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guillot L, Carroll SF, Homer R, Qureshi ST. 2008. Enhanced innate immune responsiveness to pulmonary Cryptococcus neoformans infection is associated with resistance to progressive infection. Infect Immun 76:4745–4756. doi: 10.1128/IAI.00341-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun D, Zhang M, Liu G, Wu H, Li C, Zhou H, Zhang X, Shi M. 2016. Intravascular clearance of disseminating Cryptococcus neoformans in the brain can be improved by enhancing neutrophil recruitment in mice. Eur J Immunol 46:1704–1714. doi: 10.1002/eji.201546239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwon-Chung KJ, Wickes BL, Booth JL, Vishniac HS, Bennett JE. 1987. Urease inhibition by EDTA in the two varieties of Cryptococcus neoformans. Infect Immun 55:1751–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang AL, Hole CR, Doering TL. 2019. An automated assay to measure phagocytosis of Cryptococcus neoformans. Curr Protoc Microbiol 53:e79. doi: 10.1002/cpmc.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campuzano A, Castro-Lopez N, Wozniak KL, Leopold Wager CM, Wormley FL Jr. 2017. Dectin-3 is not required for protection against Cryptococcus neoformans infection. PLoS One 12:e0169347. doi: 10.1371/journal.pone.0169347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qureshi A, Grey A, Rose KL, Schey KL, Del Poeta M. 2011. Cryptococcus neoformans modulates extracellular killing by neutrophils. Front Microbiol 2:193. doi: 10.3389/fmicb.2011.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.