The immune mechanisms that contribute to the efficacy of treatment of cutaneous leishmaniasis (CL) are not fully understood. The aim of this study was to define immune correlates of the outcome of treatment of CL caused by Leishmania (Viannia) species during standard of care treatment with pentavalent antimonials.
KEYWORDS: cutaneous leishmaniasis, antimonials, immune response, inflammation
ABSTRACT
The immune mechanisms that contribute to the efficacy of treatment of cutaneous leishmaniasis (CL) are not fully understood. The aim of this study was to define immune correlates of the outcome of treatment of CL caused by Leishmania (Viannia) species during standard of care treatment with pentavalent antimonials. We conducted a comparative expression profiling of immune response genes in peripheral blood mononuclear cells (PBMCs) and lesion biopsy specimens obtained from CL patients before and at the end of treatment (EoT) with meglumine antimoniate. The ex vivo response of PBMCs to L. (V.) panamensis partially reflected that of lesion microenvironments. Significant downregulation of gene expression profiles consistent with local innate immune responses (monocyte and neutrophil activation and chemoattractant molecules) was observed at EoT in biopsy specimens of patients who cured (n = 8), compared to those from patients with treatment failure (n = 8). Among differentially expressed genes, pretreatment expression of CCL2 was significantly predictive of the therapeutic response (receiver operating characteristic [ROC] curve, area under the curve [AUC] = 0.82, P = 0.02). Polymorphisms in regulatory regions of the CCL2 promoter were analyzed in a pilot cohort of DNA samples from CL patients (cures, n = 20, and treatment failure, n = 20), showing putative association of polymorphisms rs13900(C/T) and rs2857656(G/C) with treatment outcome. Our data indicate that dampening gene expression profiles of monocyte and neutrophil activation characterize clinical cure after treatment of CL, supporting participation of parasite-sustained inflammation or deregulated innate immune responses in treatment failure.
INTRODUCTION
Pathogenesis of dermal leishmaniasis is mediated by the immune and inflammatory responses; thus, resolution of disease and control of infection are intimately linked to the host response. Consequently, antileishmanial drugs alone are often insufficient to clinically resolve disease even in immunocompetent individuals (1–3). Although clinical resolution of cutaneous leishmaniasis (CL) is accompanied by a reduction in parasite burden, Leishmania has been documented to persist following treatment and clinical cure (4–6), indicating that parasite elimination is not required for clinical cure (resolution of pathology).
It is generally accepted that the triggering event of the pathogenesis of CL is the recognition of the invading parasite by innate immune cells, unleashing a cascade of immune-inflammatory responses that lead to the immunopathology of cutaneous lesions. Hence parasite persistence in clinically cured patients represents a risk of disease reactivation if the immune homeostasis is perturbed by either immunosuppression or provocation of an inflammatory response (7).
Treatment failure of first-line antileishmanial drugs (miltefosine and pentavalent antimonial compounds meglumine antimoniate [Glucantime] and sodium stibogluconate [Pentostam]) has been reported to occur in close to 30% of cases in controlled clinical trials in Latin America (1, 8–12). Immunotherapy, in combination with currently available antileishmanials, is recognized as a promising alternative for treatment of visceral and dermal leishmaniasis (8, 13–15). However, harnessing these advancements for effective intervention of CL has been limited despite substantial progress in the development of targeted, less toxic, localized pharmacological interventions of the human immune and inflammatory responses for other diseases (16–19).
Human immunological studies largely rely on assessment of peripheral blood cell responses as readouts of a particular immunological state. This approach is based on the assumption that systemic responses reflect tissue-specific responses. However, evidence suggests that this is not always the case (20, 21). Moreover, changes in ex vivo peripheral blood cell responses can be easily induced by unintended perturbations, such as time-to-blood processing (22), adding to the inter- and intraexperimental variability that could interfere with results and their interpretation (23). Our study aimed to characterize the local and systemic immune profiles of CL patients undergoing treatment with first-line meglumine antimoniate and to compare these profiles between patients who cured or who failed treatment. Understanding the immunological responses associated with a therapeutically achieved cure will inform the development of novel therapeutic approaches, particularly those that are host centered, and the optimization of available treatments.
RESULTS
Patient characteristics.
Sixty patients were enrolled in the study (Table 1, Fig. S1 in the supplemental material) (24). Participants were predominantly young males of Afro-Colombian descent. A median of two cutaneous lesions per patient was reported, with a median lesion duration of 2 months (interquartile range [IQR], 1 to 3). L. (V.) panamensis was the most frequently isolated species (45 of 48 strains; isolation of parasites was not achieved for 12 study participants). After standard-of-care treatment with meglumine antimoniate, 63% of participants were clinically cured (n = 38), while 22 presented treatment failure, which was determined 13 weeks after initiation of treatment. Adherence to treatment, assessed by vial counts and individual patient diaries, was high, with most patients (n = 58) receiving ≥ 85% of the prescribed treatment; the remaining two participants cured despite lower adherence to treatment (69% and 50%; Table 1). Pretreatment peripheral blood mononuclear cells (PBMCs) were obtained from all participants (n = 60). Due to sample volume limitations, gene expression and protein secretion from paired pre- and posttreatment PBMCs were analyzed in samples from 23 patients, while pharmacogenomic profiling was performed in PBMCs from 40 participants (20 who cured and 20 with treatment failure). Paired pre- and posttreatment lesion biopsy specimens were analyzed from 16 CL patients (Fig. S1). The remaining participants did not consent to biopsy sampling or samples were not adequate in terms of RNA quality or concentration.
TABLE 1.
Socio-demographic characteristics of study participantsa
| Variable | Value |
|---|---|
| Median age in years (IQR) | 31.5 (23.5–38.5) |
| No. of males (%) | 51 (85) |
| No. by ethnicity (%) | |
| Afro-Colombian | 40 (66.7) |
| Other | 20 (33.3) |
| No. by study site (%) | |
| Cali | 35 (58.3) |
| Tumaco | 25 (41.7) |
| Clinical characteristics | |
| Median weight in kg (IQR) | 71.4 (62–77.5) |
| Median duration of the oldest lesion in months (IQR) | 2 (1–3) |
| Median no. of lesions (IQR) | 2 (1–2.5) |
| No. by Leishmania strain (%) | |
| L. (V.) panamensis | 45 (75) |
| L. (V.) braziliensis | 2 (3.3) |
| L. (V.) guyanensis | 1 (1.7) |
| Not identified | 12 (20) |
| Median % adherence to treatment (range) | 100 (50–100) |
| No. by treatment response (%) | |
| Cured | 38 (63.3) |
| Failed to cure | 22 (36.7) |
Total participants, n = 60.
Exposure to antimonial drugs modulates local inflammatory responses in CL patients.
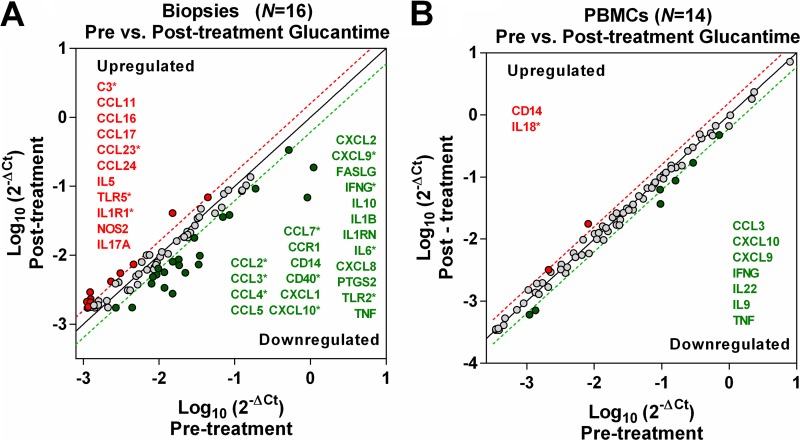
To first explore the relationship between local and systemic immune responses during antileishmanial treatment with meglumine antimoniate, we analyzed and contrasted the gene expression profiles of inflammatory mediators and receptors in lesion biopsy specimens (n = 16) and PBMCs (n = 14) from CL patients obtained before and at the end of treatment (EoT). At EoT, the expression profile of lesion biopsy specimens was characterized by downregulation of gene transcripts involved in homing and activation of monocytes (CCL2, CCL3, CCL4, CCL7, IL6, TNFα, CD14, CD40, TLR2, and CCR1), T cells (FASL, IFNγ, CXCL9, and CXCL10), and neutrophils (CXCL1, CXCL2, and CXCL8), accompanied by induction of transcripts for homing and activation of eosinophils (CCL11, CCL16, CCL23, CCL24, and IL5) and Th17 cells (NOS2 and IL17A), and higher expression of TLR5, IL-1R1 and C3 (Fig. 1 and Table S1).
FIG 1.
Gene expression profiles of lesion biopsy specimens and PBMCs from CL patients after treatment. Lesion biopsy specimens (n = 16) (A) and circulating PBMC samples (n = 14) (B) were obtained from CL patients before and at the end of antimonial treatment. Scatter plots show the average value of gene expression assessed by qRT-PCR of 84 inflammatory genes (chemokines, cytokines, and receptors). Differences in expression are graphically represented as red or green dots (up- or downregulated, respectively) with the corresponding gene names, above or below the 1.5-fold threshold lines. Genes with significantly different expression are shown with an asterisk and represent a fold change difference with a P value of <0.05 based on a Student’s t test.
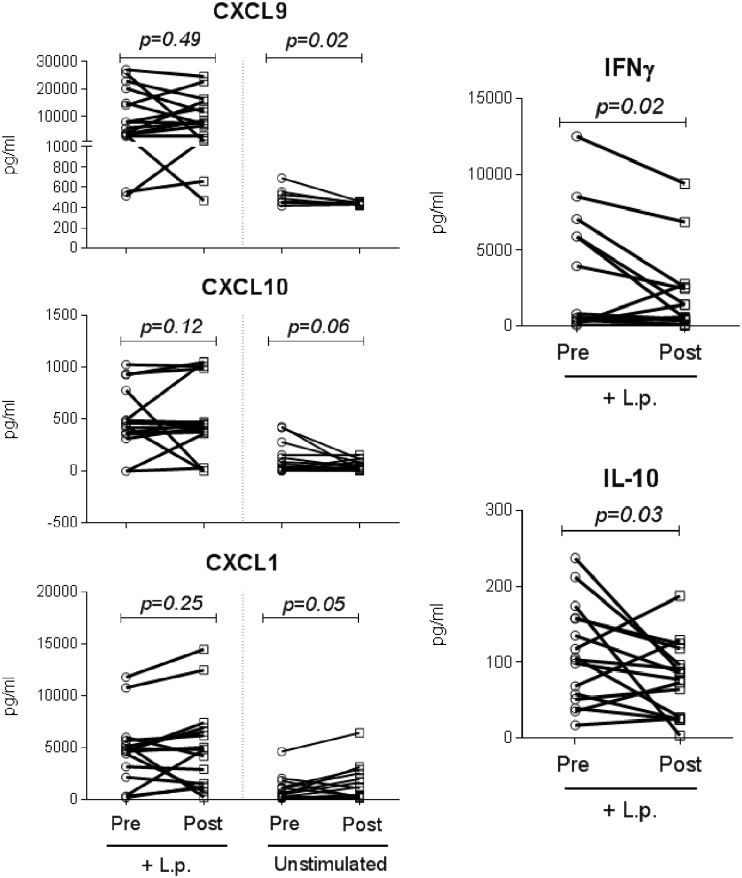
To evaluate the systemic responses, PBMCs from CL patients were collected before treatment and at EoT and infected ex vivo with L. (V.) panamensis (restimulated) to elicit a recall response, and gene expression was measured at 24 h of coculture. Fewer genes were modulated in restimulated PBMCs than in lesion biopsy specimens at EoT. However, consistent with the local response, a trend of downregulation (though not statistically significant) of transcripts mediating a TH1 response (CXCL9, CXCL10, IFNγ, and TNFα) was observed at EoT, along with lower IL22, CCL3, and IL9, while expression of IL18 and CD14 was induced (Fig. 1 and Table S1). Among evaluated genes, protein levels of tumor necrosis factor alpha (TNF-α), CCL7, CCL2, gamma interferon (IFN-γ), CXCL9, CXCL10, interleukin-10 (IL-10), IL-6, IL-17A, CXCL8, CXCL1, and CCL5 were also measured in PBMCs from CL patients (n = 16) collected before initiation of treatment and at EoT, and PBMCs were left unstimulated or were restimulated ex vivo with L. (V.) panamensis (Fig. 2 and Fig. S2). Concordant with the gene expression data, a diminished TH1 profile was also found at the protein level in PBMCs, where significantly lower CXCL9 and a trend of decreased CXCL10 were detected in unstimulated PBMCs at EoT compared to pretreatment samples, and diminished IFN-γ and IL-10 secretion was found in restimulated PBMCs collected at EoT (Fig. 2). Among measured proteins, a trend of increased CXCL1 was observed in unstimulated cells at EoT compared to pretreatment samples (Fig. 2). IFN-γ, TNF-α, IL-10, IL-6, and IL-17A were not detected in unstimulated cells. No differences between pretreatment and EoT protein levels were observed for the other measured proteins in either unstimulated or restimulated cells (Fig. S2). Together, these data show that PBMC responses (either in resting or in Leishmania-restimulated cells) only partially reflect those occurring at the lesion site during exposure to antimonial drugs.
FIG 2.
Cytokine and chemokine protein measurement in PBMCs of CL patients before and after treatment. Secreted protein levels were measured from culture supernatants of PBMCs from CL patients (n = 16) collected before and at the end of treatment. Unstimulated PBMCs and cells restimulated with L. (V.) panamensis promastigotes (+L.p.) were analyzed. Median fluorescence intensity is shown, with data expressed in pg/ml. P values were calculated using either a Wilcoxon matched-pair signed rank test or a paired t test, depending on the distribution of the data.
The local immune profile of cure is characterized by downregulation of mediators involved in homing and activation of monocytes and neutrophils.
Based on the above-described findings, the immune profiles associated with treatment outcome were characterized in skin lesion biopsy specimens. Gene expression was evaluated in biopsy specimens from patients who cured (n = 8) or who had treatment failure (n = 8). Significant (P < 0.05) and specific downregulation of monocyte and neutrophil activation and chemoattractant molecules (CCL2, CCL7, TLR2, MYD88, IL1β, IL6, CXCL1, and CXCL2) and upregulation of NR3C1 were observed at the end of treatment in patients who cured (Fig. 3). In contrast, in patients who failed treatment, a significant (P < 0.05) induction of C3, CCL23, and CCR4 and downregulation of PTGS2 and CD40 were observed. Common to patients who cured or failed treatment, CXCL10, IFNγ, and MYD88 transcripts were downregulated after treatment, whereas IL-1R1 gene expression was consistently induced (Fig. 3). These findings suggest that clinical cure after treatment may be related to tissue-specific downregulation of mediators of innate immune responses at the lesion site.
FIG 3.
Gene expression profiles of lesion biopsy specimens from CL patients undergoing treatment with antimonials. Quantitative differences in gene expression after antimonial drug treatment in skin lesion biopsy specimens from CL patients who cured (n = 8) or did not respond to treatment (n = 8). Gene expression levels are reported as log2(2−ΔCt). Statistical differences were established comparing pretreatment and posttreatment samples by parametric or nonparametric univariate tests, depending on the distribution of the data. Shown are the genes that, among the 84 analyzed, showed statistically significant differences in pre- versus posttreatment samples or between patient groups. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.0001.
CCL2 expression and gene polymorphisms are discriminatory of the outcome of treatment of CL patients.
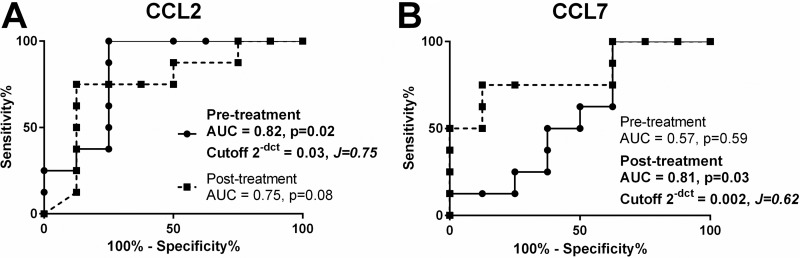
Among differentially expressed genes, the pretreatment levels of CCL2 were found to be significantly predictive of the therapeutic response (receiver operating characteristic [ROC] curve area under the curve [AUC] = 0.82, P = 0.02, 95% confidence interval [95% CI] = 0.6 to 1.0), while posttreatment expression of CCL7 was discriminatory of treatment outcome (ROC curve AUC = 0.81, P = 0.03, 95% CI = 0.3 to 0.8) (Fig. 4). CCL2 is a central node of the monocyte/macrophage chemotaxis and activation network. Based on this, we explored whether single nucleotide polymorphisms (SNPs) in the CCL2 3′ untranslated region (3′ UTR) [rs13900(C/T)] and promoter region [rs2857656(G/C)] could be associated with the therapeutic response. A pilot screening of SNPs in a gender- and ethnicity-matched CL patient subgroup (n = 20 cures and n = 20 failures, Table S2) showed a significant risk of treatment failure among individuals carrying the polymorphic alleles rs13900T and rs2857656C (2.8 and 3.5 odds ratio, respectively [Table 2]).
FIG 4.
Representation of CCL2 and CCL7 gene expression ROC curves as predictors of therapeutic outcome. Receiver operating characteristic (ROC) curves represent the area under the curve (AUC) of the false positive rate versus the true positive rate. Data from all samples for each individual gene were used to construct the ROC curves of gene expression data in pretreatment (continuous line) and posttreatment (dotted line) biopsy specimens. P values, AUC, and cutoff values based on Youden’s J statistic are shown in the graph plots.
TABLE 2.
Genotypes and allele frequencies of SNPs in CCL2 promoter region
| SNP | Genotype or allele | Frequency (%)a
|
P valueb | Odds ratio (95% CI) | |
|---|---|---|---|---|---|
| Cured | Treatment failure | ||||
| CCL2 - rs13900 | |||||
| Genotype | CC | 9 (45) | 3 (15) | 0.082 | 4.6 (1.0–21.0) |
| TT + CT | 11 (55) | 17 (85) | |||
| Alleles | C | 27 (68) | 17 (42) | 0.042 | 2.8 (1.1–7.0) |
| T | 13 (33) | 23 (58) | |||
| CCL2 - rs2857656 | |||||
| Genotype | GG | 9 (45) | 1 (5) | 0.008 | 15.6 (1.7–139.7) |
| CC + GC | 11 (55) | 19 (95) | |||
| Alleles | G | 25 (62) | 13 (32) | 0.013 | 3.5 (1.4–8.7) |
| C | 15 (38) | 27 (68) | |||
Total cured patients, n = 20; total treatment failure patients, n = 20.
Significant associations are shown in bold italic type.
DISCUSSION
Therapeutic response to antimonial drugs is multifactorial, involving the parasite (pathogenicity and drug susceptibility of intracellular Leishmania) (25, 26), pharmacokinetic (PK) and pharmacodynamic (PD) parameters of antileishmanial drugs (27), and the host immune response (28, 29). In this study, we profiled local (skin lesions) and systemic immune responses of cutaneous leishmaniasis (CL) patients undergoing treatment with meglumine antimoniate, substantiating the contribution of monocyte/macrophage and neutrophil functions to the outcome of treatment of CL. Concomitant analysis of skin and peripheral blood responses in naturally infected individuals undergoing supervised antileishmanial treatment provides a unique opportunity to identify host determinants of the therapeutic response in CL. Our study cohort consisted of patients infected with L. (Viannia) species, autochthonous to the Americas. Whether these findings extend to infections with other species and other clinical manifestations such as visceral or mucosal disease remains to be determined.
Over decades, investigations have analyzed the immune response of CL patients in peripheral blood mononuclear cells (PBMCs), under the assumption that systemic responses are a surrogate of local immune responses, usually based on quantification of “classical” immune mediators of TH1/TH2 responses (30–33). Illustrating this approach, a significant reduction of IFN-γ, TNF-α, and IL-10 production was shown in ex vivo recall responses of PBMCs from L. major-infected patients after intralesional or systemic treatment with meglumine antimoniate (23). Our parallel expression profiling of PBMCs and lesion biopsy specimens from CL patients supports and extends these observations, demonstrating the concurrence of some immune response parameters in the skin and blood cells during in vivo exposure to antimonials and also underscoring the importance of direct evaluation of lesion samples to describe and identify the determinants of pathogenesis and healing. Nevertheless, the feasibility and potential to elicit and screen cell- or mediator-specific responses in PBMCs support the usefulness of PBMCs as biologically relevant models to explore mechanisms of modulation and/or intervention of immune and inflammatory pathways (34).
Our approach using gene expression profiling of lesion biopsy specimens revealed participation of innate immune signatures in the outcome of CL treatment, with preferential downregulation of neutrophil and monocyte/macrophage recruitment and activation mediators in skin biopsy specimens of CL patients who cured. A study in a cohort of Peruvian CL patients found that after 10 days of treatment with pentavalent antimonials, IFNγ and IL13 mRNA decreased significantly more in lesions from patients who cured than in those who did not cure after treatment (30). In contrast, our results show no difference between groups in the magnitude of downregulation of IFN-γ transcripts in skin samples at the end of treatment (in addition to other classical mediators of TH1 responses, i.e., MYD88, IL1R1, CXCL9, and CXCL10). This disparity could be attributed to the different sampling times between studies, where the Peruvian cohort was sampled at day 10 (halfway through treatment) while the Colombian cohort was sampled at day 20 (end of treatment), possibly indicating that at earlier time points IFN-γ may be discriminatory of the therapeutic outcome, while at the end of standard-of-care treatment it is instead an indicator of in vivo drug exposure.
Despite a consistent downregulation of mediators of TH1 responses in both PBMCs and lesion biopsy specimens, analysis of lesion biopsy specimens was more informative, revealing downregulation of markers of cell activation and recruitment of innate cells, including monocyte/macrophages (CCL2, CCL7, TLR2, MYD88, IL1β, and IL6) and neutrophils (CXCL1 and CXCL2), together with induction of transcripts for homing and activation of eosinophils and TH17 cells after treatment in patients who cured. CCL2 is a central node of the monocyte/macrophage chemotaxis and activation network and is the most potent monocyte chemoattractant. Interestingly, among all evaluated genes, only expression of CCL2 was significantly higher in pretreatment lesion samples from patients who cured than in samples from those who failed treatment. Early activation and recruitment of monocytes to the lesion could promote rapid parasite elimination upon drug exposure. Consistently, Leishmania infections have been shown to induce CCL2 expression, and CCL2 can activate macrophages for parasite killing in murine as well as human macrophages, favoring early control of infection (35–37). The subsequent downregulation of CCL2 during treatment can favor an anti-inflammatory/wound healing response following parasite kill, dampening the proinflammatory and immunopathogenic activity of sustained monocyte recruitment.
Interindividual differences in CCL2 expression have been associated with genetic variants mapping to the cis-regulatory regions of the gene. The rs1024611(A/G) regulatory gene polymorphism has been associated with a variety of diseases, such as HIV-1-associated neurological disorders (38), higher risk of pulmonary tuberculosis (39), and severity of liver disease caused by HCV infection (40), and importantly it has been found associated with higher risk of mucosal leishmaniasis (ML), an immunopathogenically driven chronic manifestation of L. (Viannia) infection, in a Brazilian patient cohort (41). High linkage disequilibrium of rs1024611 and 3′ UTR rs13900 (r2 = 1) and rs2857656 (r2 > 0.9) has been reported (42). A preliminary exploration of CCL2 SNPs in our patient cohort showed a significant risk of treatment failure in individuals carrying the rs13900T and rs2857656C alleles. Presence of these CCL2 polymorphic variants has been primarily associated with higher serum CCL2 levels (42). The higher frequency of these SNPs in treatment-failure patients, together with the lower levels of CCL2 gene expression in the cutaneous lesions, suggests that retention of monocytes in circulation and limited monocyte migration to the skin may occur in treatment-failure patients, restricting early innate mechanisms of parasite killing. In patients with ML, presence of the polymorphic variant rs1024611G is correlated with higher CCL2 plasma levels (41), possibly also reflecting higher systemic retention of monocytes, limiting macrophage-dependent killing in the affected tissues.
Neutrophils participate in the outcome of CL, where beneficial effects of neutrophil engagement (parasite clearance) as well as detrimental effects (permissiveness for infection and parasite survival) have been reported and are likely dependent on the infecting Leishmania species (43). The involvement of neutrophils in the therapeutic outcome of CL remains unknown. However, recent findings suggest that antimony- as well as miltefosine-resistant L. (V.) panamensis elicits significantly higher neutrophil activation, reflected by increased neutrophil extracellular trap (NET) formation, CD66b expression, and reactive oxygen species (ROS) production, yet promotes enhanced parasite survival within neutrophils compared to infections with the drug-susceptible counterpart (44). These results suggest that neutrophils could contribute to sustained inflammation during drug-resistant Leishmania infections, potentially favoring treatment failure. Our results support the hypothesis that inhibition of neutrophil recruitment to cutaneous lesions promotes clinical cure, evidenced by significant downregulation of CXCL1 and CXCL2 after treatment and clinical cure of CL and induction of C3 in patients experiencing treatment failure. CXCL chemokines and activation of C3 favor recruitment of polymorphonuclear cells during acute and chronic inflammatory processes (45–48), respectively. Therefore, these changes in the expression of mediators of neutrophil recruitment and activation support a detrimental role of neutrophils at the lesion site during treatment of CL. Interestingly, it has been shown that neutrophil recruitment favors chronic CL caused by L. mexicana (49). Macrophage infections with L. (V.) panamensis strains isolated from chronic CL patients strongly induce the neutrophil chemokine axis, including CXCL1, CXCL2, CXCL3, CXCL5, and CLXCL8, in contrast to infections with strains causing self-resolving CL (50). Therefore, downregulation of neutrophil chemoattractants during treatment could promote wound healing by limiting immune-mediated pathology, as well as restricting availability of host cells at the lesion site that favor intracellular persistence and parasite survival.
Participation of complement molecules in Leishmania infections has been reported, especially in relation to parasite adherence to host cells and internalization (51–53). C3-deficient mice infected with L. major are resistant to infection and show limited parasite dissemination (54). Interestingly, parasite entry using the C3bi receptor has been shown to result in inhibition of IL-12 (55), favoring parasite survival. Consistently, our results show a significant increase in C3 gene expression in cutaneous lesions at treatment failure, concomitant with higher expression of CCR4 and CCL23, promoting parasite persistence and enhanced local innate cell infiltration and inflammation.
Our findings suggest that a therapeutic cure will be associated with inhibition of the inflammatory response mediated primarily by downregulation of proinflammatory cytokines and chemokines involved in monocyte and neutrophil recruitment and activation. A signature of chronic inflammation portrayed by neutrophil recruitment and activation, together with diminished control of classical proinflammatory mechanisms (monocyte and TH1 cell functions) and effector molecules in patients with nonresolving therapeutic outcomes, strongly supports the participation of heightened innate inflammatory responses in treatment failure, either sustained by parasite persistence or mediated by deregulated immune pathways.
MATERIALS AND METHODS
Ethics statement.
This study was approved and monitored by the institutional review board for ethical conduct of research involving human subjects of the Centro Internacional de Entrenamiento e Investigaciones Médicas (CIDEIM) in accordance with national (Colombian resolutions 8430 of 1993 and 2378 of 2008) and international (Declaration of Helsinki and amendments of 2013 and International Conference of Harmonization, ICH) guidelines. All individuals voluntarily participated in the study and provided written informed consent.
Study subjects and clinical samples.
Adult patients between 18 and 65 years of age with parasitological confirmation of cutaneous leishmaniasis (CL) who consulted the clinical facilities of Centro Internacional de Entrenamiento e Investigaciones Médicas (CIDEIM) in the cities of Cali and Tumaco, Colombia, were included in this study. Exclusion criteria were as follows: pregnancy, mucocutaneous disease, medical history of cardiac, renal, or hepatic disease, use of any antileishmanial drug during the 3 months prior to enrollment, HIV-positive test, and contraindications for antimonial treatment (i.e., amylase, aspartate aminotransferase [AST], alanine aminotransferase [ALT], creatinine, or serum urea nitrogen [BUN] levels outside the normal ranges).
Participants received standard-of-care treatment for CL, which was intramuscular injections of meglumine antimoniate at a dose of 20 mg/kg body weight/day for 20 days (56). Clinical evaluation was conducted at enrollment, at the end of treatment, and at 13 weeks after initiation of treatment (±7 days). Lesion aspirates for parasite culture and identification (57) were performed at enrollment. For analysis of immune responses, a 100-ml peripheral blood sample and a 4-mm biopsy specimen of the border of one skin lesion were obtained at the beginning and the end of treatment. Pre- and posttreatment skin biopsy specimens were obtained from the same lesion and cryopreserved with optimal cutting temperature (OCT) compound for further analysis.
Clinical cure was defined as complete reepithelialization, absence of inflammatory signs for all cutaneous leishmaniasis lesions, and absence of new leishmaniasis lesions at 13 weeks of follow up (58). For patients presenting with therapeutic failure, rescue treatment was prescribed according to Colombian national guidelines (56) and was monitored by personnel of the Clinical Research Unit of CIDEIM.
Leishmania strain, peripheral blood cell isolation, and infection.
Promastigotes of the Sb-susceptible Leishmania (V.) panamensis MHOM/CO/2002/3594 strain, stably transfected with the luciferase reporter gene (L.p.-LUC001) (59), were cultured at 25°C in RPMI medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco), 5 mg/ml hemin and 120 mg/ml Geneticin. Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation over a Ficoll-Hypaque 1077 gradient (Sigma). Cells were cryopreserved in 10% dimethyl sulfoxide (DMSO) and 90% FBS. For experiments, cells were gently thawed and resuspended in complete RPMI medium (10% FBS, 100 μg/ml streptomycin, and 100 U/ml penicillin). Cell viability was determined with trypan blue (Sigma) and defined acceptable when above 80%. PBMCs were infected with stationary-phase L.p.-LUC001 promastigotes at a 1:10 parasite-to-monocyte ratio for 24 h at 34°C and 5% CO2 prior to evaluation of gene expression and 72 h for protein secretion experiments.
Gene expression.
Expression of 84 inflammatory mediators and receptors, including chemokines, cytokines, and associated receptors, was measured by real-time quantitative PCR (RT-qPCR) (PCR Arrays, catalog number PAHS-077ZD, Qiagen). Total RNA was extracted from PBMCs using TRIzol reagent (Invitrogen, USA) followed by RNA cleanup with RNeasy minikit columns (Qiagen, USA). RNA from skin biopsy specimens was extracted as previously described (29). cDNA was synthesized using the RT first strand synthesis kit (Qiagen). RT-qPCRs were run on a CFX96 Real Time System (Bio-Rad). Data were normalized using five housekeeping genes: β-actin (ACTB), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), hypoxanthine guanine phosphoribosyl transferase (HPRT1), β-2-microglobulin (B2M), and ribosomal protein large P0 (RPLP0). Fold change gene expression was calculated by the ΔΔCt method.
Cytokine/chemokine multiplex assay.
Protein secretion was evaluated in culture supernatants of PBMCs obtained before and at the end of treatment, using a custom-made Luminex assay (R&D Systems, Minneapolis, MN), which included detection of TNF-α, CCL7, CCL2, IFN-γ, CXCL9, CXCL10, IL-10, IL-6, IL-17A, CXCL8, CXCL1, and CCL5, according to the manufacturer’s instructions. Samples were acquired on a Luminex 200 instrument using default settings. Median fluorescence intensity data were analyzed using the five-parameter logistic curve fitting method for calculating cytokine and chemokine concentrations. Protein secretion was measured in ex vivo-infected PBMCs and uninfected cells and expressed as pg/ml.
CCL2 genotyping.
DNA was isolated from PBMCs. Based on the CCL2 reference gene sequence (NG_012123.1), two primer sets were designed to amplify the 3′UTR and the promoter regions, containing rs13900(C/T) and rs2857656(G/C) SNPs, respectively. SetA primers were 5′–TCC ACT GCT TAC TCA TGT CCC–3′ and 5′–GTG GGC TGT TAA ATC TGC TGA G–3′. SetB primers were 5′–CAG CTT CAA GAC CAT TGT GGC–3′ and 5′–AAA CAT CCC AGG GGT AGA ACT–3′. PCR products were separated in 1.3% agarose gels and products of approximately 980 bp were extracted and purified for bi-directional Sanger sequencing. Sequences were edited and analyzed using BioEdit v7.2.5.
Statistical analysis.
The Kolmogorov-Smirnov and Shapiro-Wilk tests were used to determine data distribution. Thereafter, comparisons of gene expression and cytokine secretion profiles of CL patients who cured or failed to respond to treatment were analyzed using Student’s t test for parametric data, and the Mann-Whitney test was applied for nonparametric data. For comparisons of paired data obtained from samples before and after treatment, parametric and nonparametric data were analyzed using the paired t test and the Wilcoxon signed-rank test, respectively. For gene expression data, the significance of the fold change is shown as a P value based on Student’s t test 2(−ΔCt) values for each gene using the GeneGlobe Data Analysis Center (Qiagen). Receiver operating characteristic (ROC) curves were used to explore the predictive/prognostic potential of gene expression levels to discriminate therapeutic cure and failure. Sensitivity and specificity parameters were calculated for differentially expressed genes using each value as the cutoff value. Youden’s J statistic was used to define cutoff values. Area under the curve (AUC), 95% confidence intervals (CI), and two-tailed z ratios were also calculated to estimate statistical significance. Odds ratios and 95% confidence intervals were calculated for SNP data using Fisher’s exact test. Statistical significance was established at P < 0.05. Data were analyzed using Prism 6 software (GraphPad Software, Inc., La Jolla, CA) and STATA 14.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the patients who participated in this study.
Special thanks to Carolina Pustrovrh and Liliana Salazar at Universidad del Valle for their support in processing tissue biopsy samples, and John Rioux at Universite de Montreal for discussions on SNP profiling.
This research received support from NIAID/NIH RO1AI093775, Fogarty/NIH D43 TW006589, Wellcome Trust 107595/Z/15/Z, Colciencias Project number 222956933302, and National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number U19AI129910.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other agencies.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Palacios R, Osorio LE, Grajalew LF, Ochoa MT. 2001. Treatment failure in children in a randomized clinical trial with 10 and 20 days of meglumine antimonate for cutaneous leishmaniasis due to Leishmania viannia species. Am J Trop Med Hyg 64:187–193. doi: 10.4269/ajtmh.2001.64.187. [DOI] [PubMed] [Google Scholar]
- 2.Soto J, Toledo J, Vega J, Berman J. 2005. Short report: efficacy of pentavalent antimony for treatment of colombian cutaneous leishmaniasis. Am J Trop Med Hyg 72:421–422. doi: 10.4269/ajtmh.2005.72.421. [DOI] [PubMed] [Google Scholar]
- 3.Vélez I, López L, Sánchez X, Mestra L, Rojas C, Rodríguez E. 2010. Efficacy of miltefosine for the treatment of American cutaneous leishmaniasis. Am J Trop Med Hyg 83:351–356. doi: 10.4269/ajtmh.2010.10-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camera P, de O, Junger J, Pires F, do ESS, Mattos M, Oliveira-Neto MP, Fernandes O, Pirmez C. 2006. Haematogenous dissemination of Leishmania (Viannia) braziliensis in human American tegumentary leishmaniasis. Trans R Soc Trop Med Hyg 100:1112–1117. doi: 10.1016/j.trstmh.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Rosales-Chilama M, Gongora RE, Valderrama L, Jojoa J, Alexander N, Rubiano LC, Cossio A, Adams ER, Saravia NG, Gomez MA. 2015. Parasitological confirmation and analysis of Leishmania diversity in asymptomatic and subclinical infection following resolution of cutaneous leishmaniasis. PLoS Negl Trop Dis 9:e0004273. doi: 10.1371/journal.pntd.0004273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vergel C, Palacios R, Cadena H, Posso CJ, Valderrama L, Perez M, Walker J, Travi BL, Saravia NG. 2006. Evidence for Leishmania (Viannia) parasites in the skin and blood of patients before and after treatment. J Infect Dis 194:503–511. doi: 10.1086/505583. [DOI] [PubMed] [Google Scholar]
- 7.Wortmann GW, Aronson NE, Miller RS, Blazes D, Oster CN. 2000. Cutaneous leishmaniasis following local trauma: a clinical pearl. Clin Infect Dis 31:199–201. doi: 10.1086/313924. [DOI] [PubMed] [Google Scholar]
- 8.Machado PRL, Lessa H, Lessa M, Guimaraes LH, Bang H, Ho JL, Carvalho EM. 2007. Oral pentoxifylline combined with pentavalent antimony: a randomized trial for mucosal leishmaniasis. Clin Infect Dis 44:788–793. doi: 10.1086/511643. [DOI] [PubMed] [Google Scholar]
- 9.Navin TR, Arana BA, Arana FE, Berman JD, Chajón JF. 1992. Placebo-controlled clinical trial of sodium stibogluconate (Pentostam) versus ketoconazole for treating cutaneous leishmaniasis in Guatemala. J Infect Dis 165:528–534. doi: 10.1093/infdis/165.3.528. [DOI] [PubMed] [Google Scholar]
- 10.Romero GA, Guerra MV, Paes MG, Macêdo VO. 2001. Comparison of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis and L. (V.) guyanensis in Brazil: therapeutic response to meglumine antimoniate. Am J Trop Med Hyg 65:456–465. doi: 10.4269/ajtmh.2001.65.456. [DOI] [PubMed] [Google Scholar]
- 11.Soto J, Rea J, Balderrama M, Toledo J, Soto P, Valda L, Berman JD. 2008. Short report: efficacy of miltefosine for Bolivian cutaneous leishmaniasis. Am J Trop Med Hyg 78:210–211. doi: 10.4269/ajtmh.2008.78.210. [DOI] [PubMed] [Google Scholar]
- 12.Tuon FF, Amato VS, Graf ME, Siqueira AM, Nicodemo AC, Amato Neto V. 2008. Treatment of New World cutaneous leishmaniasis–a systematic review with a meta-analysis. Int J Dermatol 47:109–124. doi: 10.1111/j.1365-4632.2008.03417.x. [DOI] [PubMed] [Google Scholar]
- 13.Arevalo I, Ward B, Miller R, Meng T, Najar E, Alvarez E, Matlashewski G, Llanos‐Cuentas A. 2001. Successful treatment of drug‐resistant cutaneous Leishmaniasis in humans by use of imiquimod, an immunomodulator. Clin Infect Dis 33:1847–1851. doi: 10.1086/324161. [DOI] [PubMed] [Google Scholar]
- 14.Musa AM, Khalil EAG, Mahgoub FAE, Elgawi SHH, Modabber F, Elkadaru A, Aboud MH, Noazin S, Ghalib HW, El-Hassan AM. 2008. Immunochemotherapy of persistent post-kala-azar dermal leishmaniasis: a novel approach to treatment. Trans R Soc Trop Med Hyg 102:58–63. doi: 10.1016/j.trstmh.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. 2010. Control of the leishmaniases: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, 22 to 26 March 2010. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 16.Fecci PE, Sampson JH. 2019. The current state of immunotherapy for gliomas: an eye toward the future. J Neurosurg 131:657–666. doi: 10.3171/2019.5.JNS181762. [DOI] [PubMed] [Google Scholar]
- 17.Garofano F, Gonzalez-Carmona MA, Skowasch D, Schmidt-Wolf R, Abramian A, Hauser S, Strassburg CP, Schmidt-Wolf I. 2019. Clinical trials with combination of cytokine-induced killer cells and dendritic cells for cancer therapy. Int J Mol Sci 20:4307. doi: 10.3390/ijms20174307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo J, Nishikawa G, Prasad V. 2019. A systematic review of head-to-head trials of approved monoclonal antibodies used in cancer: an overview of the clinical trials agenda. J Cancer Res Clin Oncol 145:2303–2311. doi: 10.1007/s00432-019-02984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q, Guldner IH, Golomb SM, Sun L, Harris JA, Lu X, Zhang S. 2019. Single-cell profiling guided combinatorial immunotherapy for fast-evolving CDK4/6 inhibitor-resistant HER2-positive breast cancer. Nat Commun 10:3817. doi: 10.1038/s41467-019-11729-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acosta Davila JA, Hernandez De Los Rios A. 2019. An overview of peripheral blood mononuclear cells as a model for immunological research of Toxoplasma gondii and other apicomplexan parasites. Front Cell Infect Microbiol 9:24. doi: 10.3389/fcimb.2019.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhavanam S, Rayat GR, Keelan M, Kunimoto D, Drews SJ. 2018. Characterization of immune responses of human PBMCs infected with Mycobacterium tuberculosis H37Ra: impact of donor declared BCG vaccination history on immune responses and M. tuberculosis growth. PLoS One 13:e0203822. doi: 10.1371/journal.pone.0203822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navas A, Giraldo-Parra L, Prieto MD, Cabrera J, Gómez MA. 2019. Phenotypic and functional stability of leukocytes from human peripheral blood samples: considerations for the design of immunological studies. BMC Immunol 20:5. doi: 10.1186/s12865-019-0286-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lakhal-Naouar I, Slike BM, Aronson NE, Marovich MA. 2015. The immunology of a healing response in cutaneous leishmaniasis treated with localized heat or systemic antimonial therapy. PLoS Negl Trop Dis 9:e0004178. doi: 10.1371/journal.pntd.0004178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bardou P, Mariette J, Escudié F, Djemiel C, Klopp C. 2014. jvenn: an interactive Venn diagram viewer. BMC Bioinformatics 15:293. doi: 10.1186/1471-2105-15-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rojas R, Valderrama L, Valderrama M, Varona MX, Ouellette M, Saravia NG. 2006. Resistance to antimony and treatment failure in human Leishmania (Viannia) infection. J Infect Dis 193:1375–1383. doi: 10.1086/503371. [DOI] [PubMed] [Google Scholar]
- 26.Obonaga R, Fernández OL, Valderrama L, Rubiano LC, Castro MDM, Barrera MC, Gomez MA, Gore Saravia N. 2014. Treatment failure and miltefosine susceptibility in dermal leishmaniasis caused by Leishmania subgenus Viannia species. Antimicrob Agents Chemother 58:144–152. doi: 10.1128/AAC.01023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruz A, Rainey PM, Herwaldt BL, Stagni G, Palacios R, Trujillo R, Saravia NG. 2007. Pharmacokinetics of antimony in children treated for leishmaniasis with meglumine antimoniate. J Infect Dis 195:602–608. doi: 10.1086/510860. [DOI] [PubMed] [Google Scholar]
- 28.Castro M del M, Cossio A, Velasco C, Osorio L. 2017. Risk factors for therapeutic failure to meglumine antimoniate and miltefosine in adults and children with cutaneous leishmaniasis in Colombia: a cohort study. PLoS Negl Trop Dis 11:e0005515. doi: 10.1371/journal.pntd.0005515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez-Pinto D, Navas A, Blanco VM, Ramírez L, Garcerant D, Cruz A, Craft N, Saravia NG. 2012. Regulatory T cells in the pathogenesis and healing of chronic human dermal leishmaniasis caused by Leishmania (Viannia) species. PLoS Negl Trop Dis 6:e1627. doi: 10.1371/journal.pntd.0001627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurer-Cecchini A, Decuypere S, Chappuis F, Alexandrenne C, De Doncker S, Boelaert M, Dujardin JC, Loutan L, Dayer JM, Tulliano G, Arevalo J, Llanos-Cuentas A, Chizzolini C. 2009. Immunological determinants of clinical outcome in Peruvian patients with tegumentary leishmaniasis treated with pentavalent antimonials. Infect Immun 77:2022–2029. doi: 10.1128/IAI.01513-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nogueira MF, Goto H, Sotto MN, Cuce LC. 2008. Cytokine profile in Montenegro skin test of patients with localized cutaneous and mucocutaneous leishmaniasis. Rev Inst Med Trop Sao Paulo 50:333–337. doi: 10.1590/s0036-46652008000600004. [DOI] [PubMed] [Google Scholar]
- 32.Saravia NG, Valderrama L, Labrada M, Holguin AF, Navas C, Palma G, Weigle KA. 1989. The relationship of Leishmania braziliensis subspecies and immune response to disease expression in New World leishmaniasis. J Infect Dis 159:725–735. doi: 10.1093/infdis/159.4.725. [DOI] [PubMed] [Google Scholar]
- 33.Diaz YR, Rojas R, Valderrama L, Saravia NG. 2010. T-bet, GATA-3, and Foxp3 expression and Th1/Th2 cytokine production in the clinical outcome of human infection with Leishmania (Viannia) species. J Infect Dis 202:406–415. doi: 10.1086/653829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez-Fajardo L, Fernández OL, McMahon-Pratt D, Saravia NG. 2015. Ex vivo host and parasite response to antileishmanial drugs and immunomodulators. PLoS Negl Trop Dis 9:e0003820. doi: 10.1371/journal.pntd.0003820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritter U, Moll H. 2000. Monocyte chemotactic protein-1 stimulates the killing of leishmania major by human monocytes, acts synergistically with IFN-gamma and is antagonized by IL-4. Eur J Immunol 30:3111–3120. doi:. [DOI] [PubMed] [Google Scholar]
- 36.Ritter U, Körner H. 2002. Divergent expression of inflammatory dermal chemokines in cutaneous leishmaniasis. Parasite Immunol 24:295–301. doi: 10.1046/j.1365-3024.2002.00467.x. [DOI] [PubMed] [Google Scholar]
- 37.Racoosin EL, Beverley SM. 1997. Leishmania major: promastigotes induce expression of a subset of chemokine genes in murine macrophages. Exp Parasitol 85:283–295. doi: 10.1006/expr.1996.4139. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez E, Rovin BH, Sen L, Cooke G, Dhanda R, Mummidi S, Kulkarni H, Bamshad MJ, Telles V, Anderson SA, Walter EA, Stephan KT, Deucher M, Mangano A, Bologna R, Ahuja SS, Dolan MJ, Ahuja SK. 2002. HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc Natl Acad Sci U S A 99:13795–13800. doi: 10.1073/pnas.202357499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flores-Villanueva PO, Ruiz-Morales JA, Song C-H, Flores LM, Jo E-K, Montaño M, Barnes PF, Selman M, Granados J. 2005. A functional promoter polymorphism in monocyte chemoattractant protein-1 is associated with increased susceptibility to pulmonary tuberculosis. J Exp Med 202:1649–1658. doi: 10.1084/jem.20050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mühlbauer M, Bosserhoff AK, Hartmann A, Thasler WE, Weiss TS, Herfarth H, Lock G, Schölmerich J, Hellerbrand C. 2003. A novel MCP-1 gene polymorphism is associated with hepatic MCP-1 expression and severity of HCV-related liver disease. Gastroenterology 125:1085–1093. doi: 10.1016/s0016-5085(03)01213-7. [DOI] [PubMed] [Google Scholar]
- 41.Ramasawmy R, Menezes E, Magalhães A, Oliveira J, Castellucci L, Almeida R, Rosa MEA, Guimarães LH, Lessa M, Noronha E, Wilson ME, Jamieson SE, Kalil J, Blackwell JM, Carvalho EM, de Jesus AR. 2010. The -2518bp promoter polymorphism at CCL2/MCP1 influences susceptibility to mucosal but not localized cutaneous leishmaniasis in Brazil. Infect Genet Evol 10:607–613. doi: 10.1016/j.meegid.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pham M-H, Bonello GB, Castiblanco J, Le T, Sigala J, He W, Mummidi S. 2012. The rs1024611 regulatory region polymorphism is associated with CCL2 allelic expression imbalance. PLoS One 7:e49498. doi: 10.1371/journal.pone.0049498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hurrell BP, Regli IB, Tacchini-Cottier F. 2016. Different Leishmania species drive distinct neutrophil functions. Trends Parasitol 32:392–401. doi: 10.1016/j.pt.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Regli IB, Fernández OL, Martínez-Salazar B, Gómez MA, Saravia NG, Tacchini-Cottier F. 2018. Resistance of Leishmania (Viannia) panamensis to meglumine antimoniate or miltefosine modulates neutrophil effector functions. Front Immunol 9:3040. doi: 10.3389/fimmu.2018.03040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Futosi K, Fodor S, Mócsai A. 2013. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol 17:638–650. doi: 10.1016/j.intimp.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu F, Zou Q, Ding X, Shi D, Zhu X, Hu W, Liu L, Zhou H. 2016. Complement component C3a plays a critical role in endothelial activation and leukocyte recruitment into the brain. J Neuroinflammation 13:23. doi: 10.1186/s12974-016-0485-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watanabe K, Gilchrist CA, Uddin MJ, Burgess SL, Abhyankar MM, Moonah SN, Noor Z, Donowitz JR, Schneider BN, Arju T, Ahmed E, Kabir M, Alam M, Haque R, Pramoonjago P, Mehrad B, Petri WA. 2017. Microbiome-mediated neutrophil recruitment via CXCR2 and protection from amebic colitis. PLoS Pathog 13:e1006513. doi: 10.1371/journal.ppat.1006513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, Gunzer M, Roers A, Hogg N. 2013. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 121:4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 49.Hurrell BP, Schuster S, Grün E, Coutaz M, Williams RA, Held W, Malissen B, Malissen M, Yousefi S, Simon H-U, Müller AJ, Tacchini-Cottier F. 2015. Rapid sequestration of Leishmania mexicana by neutrophils contributes to the development of chronic lesion. PLoS Pathog 11:e1004929. doi: 10.1371/journal.ppat.1004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Navas A, Vargas DA, Freudzon M, McMahon-Pratt D, Saravia NG, Gómez MA. 2014. Chronicity of dermal leishmaniasis caused by Leishmania panamensis is associated with parasite-mediated induction of chemokine gene expression. Infect Immun 82:2872–2880. doi: 10.1128/IAI.01133-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mosser DM. 1994. Receptors on phagocytic cells involved in microbial recognition. Immunol Ser 60:99–114. [PubMed] [Google Scholar]
- 52.Robledo S, Wozencraft A, Valencia AZ, Saravia N. 1994. Human monocyte infection by Leishmania (Viannia) panamensis. Role of complement receptors and correlation of susceptibility in vitro with clinical phenotype. J Immunol 152:1265–1276. [PubMed] [Google Scholar]
- 53.Mosser DM, Brittingham A. 1997. Leishmania, macrophages and complement: a tale of subversion and exploitation. Parasitology 115 Suppl:S9–S23. doi: 10.1017/s0031182097001789. [DOI] [PubMed] [Google Scholar]
- 54.Jacobs T, Andrä J, Gaworski I, Graefe S, Mellenthin K, Krömer M, Halter R, Borlak J, Clos J. 2005. Complement C3 is required for the progression of cutaneous lesions and neutrophil attraction in Leishmania major infection. Med Microbiol Immunol 194:143–149. doi: 10.1007/s00430-004-0229-y. [DOI] [PubMed] [Google Scholar]
- 55.Ricardo-Carter C, Favila M, Polando RE, Cotton RN, Bogard Horner K, Condon D, Ballhorn W, Whitcomb JP, Yadav M, Geister RL, Schorey JS, Mcdowell MA. 2013. Leishmania major inhibits IL-12 in macrophages by signalling through CR3 (CD11b/CD18) and down-regulation of ETS-mediated transcription. Parasite Immunol 35:409–420. doi: 10.1111/pim.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ministerio de la Protección Social. 2010. Guía para la atención clínica integral del paciente con leishmaniasis. Bogotá, Colombia. [Google Scholar]
- 57.Saravia NG, Weigle K, Navas C, Segura I, Valderrama L, Valencia AZ, Escorcia B, McMahon-Pratt D. 2002. Heterogeneity, geographic distribution, and pathogenicity of serodemes of Leishmania Viannia in Colombia. Am J Trop Med Hyg 66:738–744. doi: 10.4269/ajtmh.2002.66.738. [DOI] [PubMed] [Google Scholar]
- 58.Rubiano LC, Miranda MC, Muvdi Arenas S, Montero LM, Rodríguez-Barraquer I, Garcerant D, Prager M, Osorio L, Rojas MX, Pérez M, Nicholls RS, Saravia NG. 2012. Noninferiority of miltefosine versus meglumine antimoniate for cutaneous leishmaniasis in children. J Infect Dis 205:684–692. doi: 10.1093/infdis/jir816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roy G, Dumas C, Sereno D, Wu Y, Singh AK, Tremblay MJ, Ouellette M, Olivier M, Papadopoulou B. 2000. Episomal and stable expression of the luciferase reporter gene for quantifying Leishmania spp. infections in macrophages and in animal models. Mol Biochem Parasitol 110:195–206. doi: 10.1016/s0166-6851(00)00270-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.