To understand the role of major histocompatibility complex class I (MHC-I) and MHC-II in vaccine-mediated protection against Coxiella burnetii, we evaluated the protective efficacy of a formalin-inactivated C. burnetii Nine Mile phase I vaccine (PIV) in β2-microglobulin-deficient (B2m KO) and MHC-II-deficient (MHC-II KO) mice. Vaccination reduced disease severity in wild-type (WT) and B2m KO mice but failed to reduce bacterial burden in MHC-II KO mice.
KEYWORDS: CD4+ T cells, Coxiella burnetii, IFN-γ, Tbet-deficient mice, Th1 response, major histocompatibility complex, vaccine-induced immunity
ABSTRACT
To understand the role of major histocompatibility complex class I (MHC-I) and MHC-II in vaccine-mediated protection against Coxiella burnetii, we evaluated the protective efficacy of a formalin-inactivated C. burnetii Nine Mile phase I vaccine (PIV) in β2-microglobulin-deficient (B2m KO) and MHC-II-deficient (MHC-II KO) mice. Vaccination reduced disease severity in wild-type (WT) and B2m KO mice but failed to reduce bacterial burden in MHC-II KO mice. This suggests that the MHC-II antigen presentation pathway is required for PIV-mediated protection against C. burnetii infection. MHC-I and MHC-II affect antibody isotype switching, since both PIV-vaccinated B2m KO and MHC-II KO mice produced less Coxiella-specific IgG than PIV-vaccinated WT mice. Interestingly, MHC-II and CD4 deficiencies were not equivalent in terms of splenomegaly and bacterial clearance. This demonstrates a partial role for CD4+ T cells while revealing MHC-II-restricted, CD4-independent mechanisms. Adoptive transfer of CD4+ T cells from PIV-vaccinated WT mice to naive CD4-deficient (CD4 KO) mice demonstrated that antigen-experienced CD4+ T cells are sufficient to generate protection. Conversely, transfer of naive CD4+ T cells to PIV-vaccinated CD4 KO mice exacerbates disease. Using Tbet-deficient (Tbet KO) mice, we showed a partial role for Th1 subset CD4+ T cells in vaccine protection. Furthermore, Th1-independent roles for Tbet were suggested by significant differences in disease between PIV-vaccinated Tbet KO and CD4 KO mice. Interferon gamma was shown to contribute to the host inflammatory response but not bacterial clearance. Collectively, these findings suggest that vaccine-induced protective immunity against a murine model of experimental Q fever requires MHC-II-restricted, CD4+ T cell-dependent and -independent mechanisms that can be exploited for a new-generation human Q fever vaccine.
INTRODUCTION
Coxiella burnetii is an obligate intracellular Gram-negative bacterium which causes acute and chronic Q fever in humans. Acute Q fever manifests as a flu-like illness with fever, chills, fatigue, headache, and body aches (1). This form of the disease can be asymptomatic and is often self-limiting (2). Consequently, it is believed that disease incidence is significantly underreported (3). Chronic Q fever commonly presents as endocarditis (4–6) and, when left untreated, is fatal in at least 25% of patients (1). Treatment involves dual antibiotic therapy with doxycycline and hydroxychloroquine for at least 18 months (7, 8). However, in one 24-month cohort study (9), more than 30% of Q fever patients retained an impaired health status despite following the prescribed antibiotic regimen. This globally distributed pathogen is transmitted to humans via aerosols from infected ruminants and thus serves as an occupational hazard for individuals working closely with livestock (10–14). Its hardiness in the environment (15), aerosol route of transmission (16, 17), and low infectious dose (18, 19) make C. burnetii an important zoonotic pathogen. Furthermore, C. burnetii has been designated a National Institutes of Health (NIH) category B priority pathogen for its potential threat as a biowarfare agent (20). Considering the incapacitating effects of aerosolized C. burnetii and the shortcomings of current antibiotic therapies, the creation of a safe and effective new-generation Q fever vaccine remains critical.
C. burnetii has two phase variants. Phase I organisms are found in nature and possess full-length lipopolysaccharide (LPS). In contrast, phase II organisms, generated by serial passage in eggs, tissue culture, or synthetic media, have a truncated LPS lacking the O-antigen and outer core regions (21, 22). Virulent C. burnetii phase I is capable of replicating in immunocompetent animals to cause disease, while avirulent C. burnetii phase II is rapidly cleared and does not cause disease (18).
A formalin-inactivated whole-cell vaccine generated from C. burnetii Henzerling phase I (Q-VAX) elicits long-lasting protective immunity in animal models and human vaccinees (10, 23–25); however, it is not approved for use in the United States due to a high incidence of adverse reactions in vaccine recipients (10, 23, 26–29). Multiple screening procedures, including skin tests and serology, are required for safe use of this vaccine (30). Understanding the immunological mechanisms of vaccine protection, as well as the underlying triggers of hypersensitivity, is necessary to develop a vaccine that is both safe and effective.
It has previously been demonstrated that both humoral and cell-mediated immunity contribute to host defense against C. burnetii (25, 31–44). In a murine intraperitoneal (i.p.) infection model, B cells appear to contribute to the host inflammatory response, while T cells and interferon gamma (IFN-γ) are important for bacterial clearance (37). However, only adoptive transfer of immune T cells, not immune B cells, from C. burnetii Nine Mile phase I vaccine (PIV)-vaccinated BALB/c mice to SCID mice reduces disease severity following i.p. challenge (25). These data suggest an important role for T cells in both the primary and the secondary host response against C. burnetii. In a pulmonary infection model, either CD4+ or CD8+ T cells are sufficient to control infection, with CD8+ T cells better controlling inflammation (40). This is in line with our recently published data demonstrating that both major histocompatibility complex class I (MHC-I) and MHC-II are important for primary host defense, with MHC-I playing a more significant role (44). Similarly, either CD4+ or CD8+ T cells are sufficient to generate protection after vaccination with PIV (43); however, the role of MHC-I and MHC-II in vaccine-induced protective immunity is unknown. In addition, it remains unclear how CD4+ and CD8+ T cells contribute to vaccine protection, and which specific T cell subsets respond to vaccination is not clear.
Here, we investigate the role of MHC-I and MHC-II in vaccine protection against C. burnetii and show that MHC-II is important for PIV-mediated protection. The contribution of MHC-II to vaccine-induced protective immunity is only partially dependent on CD4+ T cells, since PIV-vaccinated MHC-II-deficient (MHC-II KO) mice have significantly worse disease than PIV-vaccinated CD4-deficient (CD4 KO) mice. CD4+ T cells are, however, sufficient for protection when they come from an antigen-experienced donor. This is demonstrated by a significant reduction in splenomegaly following adoptive transfer of PIV-vaccinated CD4+ T cells to naive CD4 KO mice. Furthermore, we demonstrate a role for Tbet in PIV protection that is partially dependent on Th1 subset CD4+ T cells. When we evaluated the contribution of IFN-γ, we found that, while IFN-γ does seem to affect inflammation, it does not appear to play a major role in bacterial clearance following secondary challenge. These findings provide novel information about the role of MHC-II, Tbet, CD4+ T cells, and IFN-γ in vaccine-induced protective immunity against a murine model of experimental Q fever. Furthermore, this study highlights key differences in the host response following primary C. burnetii infection and secondary challenge which can inform future Q fever vaccine development.
RESULTS
MHC-II is important for PIV-mediated protection against C. burnetii.
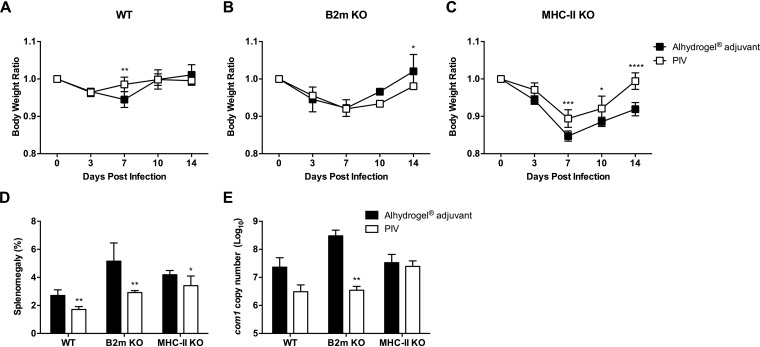
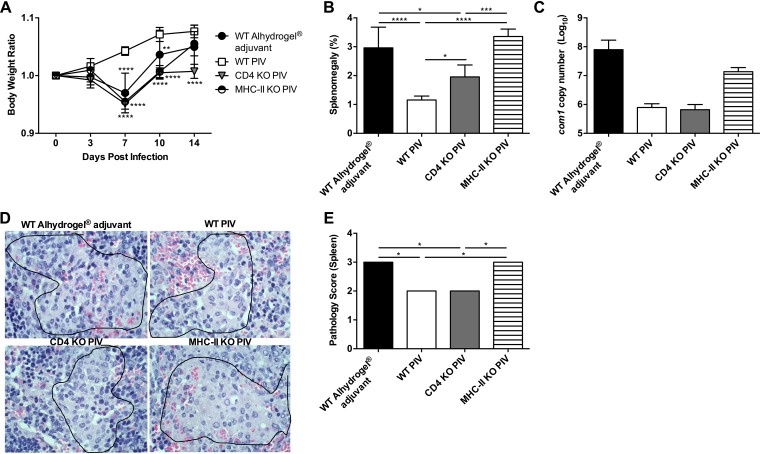
We have previously demonstrated that both MHC-I and MHC-II play important roles in host defense against primary C. burnetii infection, with MHC-I being more critical (44). To determine the role of these complexes in vaccine-mediated protection, we vaccinated MHC-I-deficient (B2m KO) and MHC-II-deficient (MHC-II KO) mice subcutaneously (s.c.) with 10 μg of PIV with Alhydrogel adjuvant followed by intraperitoneal (i.p.) challenge with 1 × 107 genomic copies of C. burnetii Nine Mile phase I (NMI) 28 days postvaccination (dpv). An aluminum hydroxide adjuvant was chosen for these studies based on its widely accepted use in commercially available human vaccines (45). Body weight loss, splenomegaly, and splenic bacterial burden were evaluated to assess the protective efficacy of PIV. PIV-vaccinated wild-type (WT) C57BL/6 mice were protected from body weight loss compared to WT adjuvant control mice, which had a significant drop in body weight 7 days postinfection (dpi; Fig. 1A). This correlated with a significant reduction in splenomegaly (Fig. 1D) and reduced splenic bacterial loads (Fig. 1E) in PIV-vaccinated WT mice compared to adjuvant controls. Vaccination did not change the course of body weight loss in B2m KO mice, which displayed a transient loss in body weight beginning 7 dpi (Fig. 1B). However, vaccination did protect B2m KO mice, as demonstrated by significant reductions in splenomegaly (Fig. 1D) and bacterial burden (Fig. 1E). The most significant drop in body weight occurred in MHC-II KO mice, which lost approximately 15% of their initial body weight by 7 dpi (Fig. 1C). Vaccination of MHC-II KO mice partially abrogated this weight loss, since the PIV-vaccinated cohort had significantly higher body weights 7, 10, and 14 dpi. However, PIV-vaccinated MHC-II KO mice still lost ∼10% of their initial body weight by 7 dpi. In line with these results, PIV-vaccinated MHC-II KO mice only had a small, albeit statistically significant, reduction in splenomegaly (Fig. 1D) and no change in splenic bacterial loads (Fig. 1E). Overall, these data indicate that the MHC-II antigen presentation pathway is important for PIV-mediated protection against C. burnetii.
FIG 1.
MHC-II is important for PIV-mediated protection against C. burnetii. B2m-deficient (B2m KO), MHC-II-deficient (MHC-II KO), and WT C57BL/6 mice were vaccinated s.c. with 10 μg of formalin-inactivated C. burnetii Nine Mile phase I vaccine (PIV) and challenged i.p. with 1 × 107 genomic copies of C. burnetii NMI 28 days postvaccination (dpv). Mice receiving Alhydrogel adjuvant alone served as unvaccinated controls. (A to C) Relative body weights calculated throughout the infection. (D and E) Splenomegaly (E) and splenic bacterial burden (E) were evaluated at 14 dpi to compare protection. The results are expressed as the percent splenomegaly, i.e., (spleen weight/body weight) × 100. The bacterial burden was determined by real-time quantitative PCR (qPCR) and is expressed as log10 C. burnetii com1 gene copy numbers. Each experimental group includes five mice, with error bars representing the standard deviations from the mean. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (as determined by two-way ANOVA with Sidak’s multiple-comparison test [A to C] or t test [D and E]).
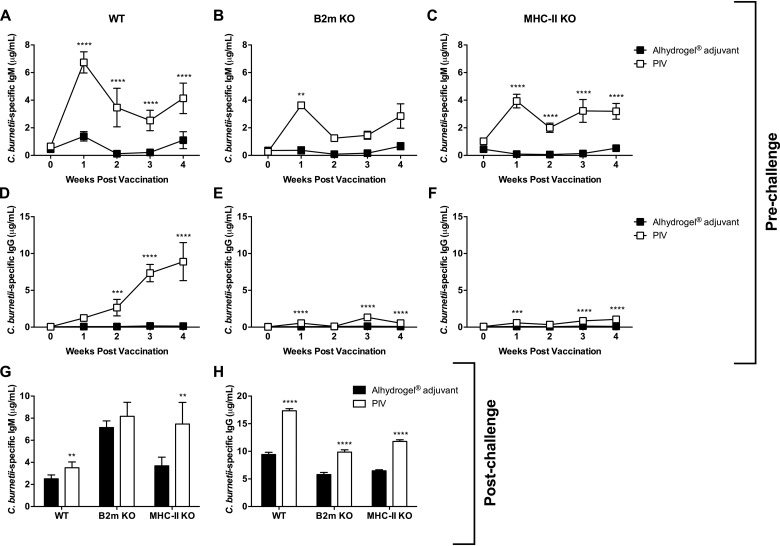
Next, we evaluated the development of specific antibody titers following vaccination and challenge. PIV-vaccinated WT mice produced significant levels of Coxiella-specific IgM, peaking at 7 dpv, compared to WT adjuvant-treated controls (Fig. 2A). Vaccination also elicited specific IgM in B2m KO (Fig. 2B) and MHC-II KO mice (Fig. 2C), which peaked 7 dpv. However, the overall IgM concentrations were markedly reduced compared to those in PIV-vaccinated WT mice. IgM levels were similar between vaccinated groups at the time of challenge. Significant C. burnetii NMI-specific IgG production began 14 dpv in PIV-vaccinated WT mice and continued to rise until the time of challenge (Fig. 2D). In contrast, the concentration of specific IgG produced by PIV-vaccinated B2m KO (Fig. 2E) and MHC-II KO mice (Fig. 2F) was markedly reduced. When evaluated 14 dpi, PIV-vaccinated WT mice had significantly elevated IgM (Fig. 2G) and IgG (Fig. 2H) compared to adjuvant-treated controls. Adjuvant-treated and PIV-vaccinated B2m KO mice produced similar levels of specific IgM (Fig. 2G), indicating that vaccination had no effect on IgM production in the absence of MHC-I. In contrast, PIV-vaccinated MHC-II KO mice had significantly higher levels of IgM than adjuvant-treated controls (Fig. 2G). Similar to PIV-vaccinated WT mice, PIV-vaccinated B2m KO and MHC-II KO mice produced significantly higher levels of specific IgG than adjuvant-treated controls following challenge (Fig. 2H). However, the levels of specific IgG were markedly reduced in the absence of MHC-I or MHC-II. Collectively, these data suggest a role for both MHC-I and MHC-II in antibody isotype switching.
FIG 2.
MHC-I and MHC-II are involved in antibody isotype switching. B2m KO, MHC-II KO, and WT C57BL/6 mice were vaccinated s.c. with 10 μg of PIV and challenged i.p. with 1 × 107 genomic copies of C. burnetii NMI 28 dpv. Mice receiving Alhydrogel adjuvant alone served as unvaccinated controls. C. burnetii NMI-specific serum IgM (A to C) and IgG (D to F) were evaluated weekly following vaccination. Specific IgM (G) and IgG (H) were also evaluated 14 dpi. Each experimental group includes five mice, with error bars representing the standard deviations from the mean. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (as determined by two-way ANOVA with Sidak’s multiple-comparison test [A to F] or t test [G and H]).
MHC-II-dependent vaccine protection is partially dependent on CD4+ T cells.
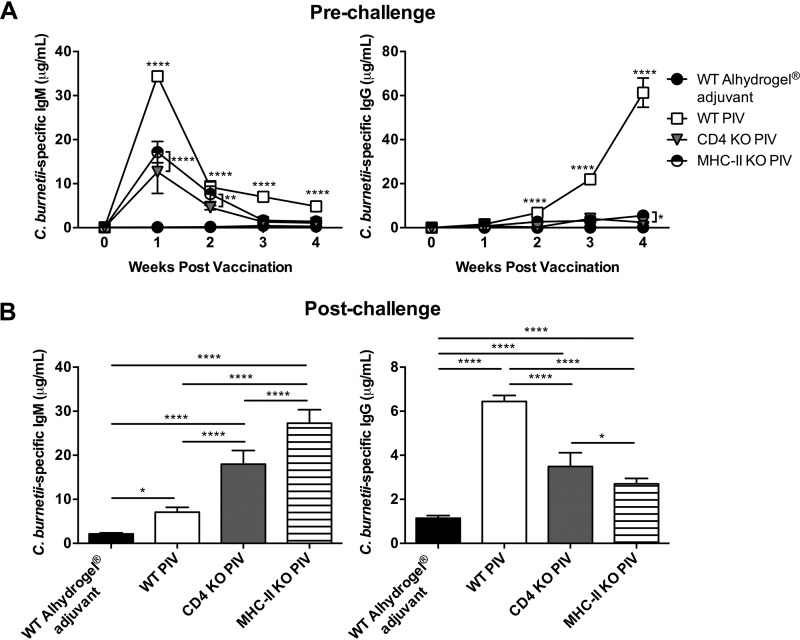
CD4+ T cells are the primary target of MHC-II-mediated antigen presentation. Therefore, to determine the contribution of CD4+ T cells to PIV-mediated protection, we vaccinated and challenged CD4-deficient (CD4 KO) and MHC-II KO mice as described previously. Body weight loss, splenomegaly, and splenic bacterial burden were evaluated to assess vaccine protection. CD4 KO and MHC-II KO mice had similar levels of body weight loss, which were significantly worse than body weight loss of PIV-vaccinated WT mice 7 and 10 dpi (Fig. 3A). CD4 KO mice also had significant body weight loss compared to PIV-vaccinated WT mice 14 dpi. At this time point, CD4 KO and MHC-II KO mice both had significantly worse splenomegaly than PIV-vaccinated WT mice (Fig. 3B). Interestingly, the degree of splenomegaly in MHC-II KO mice was significantly worse than in CD4 KO mice. In line with these results, MHC-II KO mice had higher splenic bacterial burden than PIV-vaccinated WT or CD4 KO mice (Fig. 3C). Bacterial loads were similar between PIV-vaccinated WT and CD4 KO mice, which also had similar degrees of spleen pathology (Fig. 3D and E). In contrast, MHC-II KO mice had significantly worse spleen pathology than both PIV-vaccinated WT and CD4 KO mice. These results suggest that CD4+ T cells may only play a partial role in MHC-II-dependent vaccine protection.
FIG 3.
MHC-II-dependent vaccine protection is partially dependent on CD4+ T cells. CD4-deficient (CD4 KO), MHC-II KO, and WT C57BL/6 mice were vaccinated s.c. with 10 μg of PIV and challenged i.p. with 1 × 107 genomic copies of C. burnetii NMI 28 dpv. Mice receiving Alhydrogel adjuvant alone served as unvaccinated controls. (A) The relative body weight was determined throughout the infection. Splenomegaly (B) and splenic bacterial burden (C) were assessed 14 dpi to compare protection. (D and E) Spleen sections from WT, CD4 KO, and MHC-II KO mice were evaluated 14 dpi for histiocytic inflammation in red pulp based on the following scale: 0, no accumulations of macrophages; 1, small accumulations of macrophages; 2, small to moderate accumulations of macrophages; and 3, moderate to large accumulations of macrophages. Differences in spleen volume between groups were largely the result of extramedullary hematopoiesis. The results are expressed as the percent splenomegaly, i.e., (spleen weight/body weight) × 100. The bacterial burden was determined by real-time quantitative PCR (qPCR) and is expressed as log10 C. burnetii com1 gene copy numbers. Each experimental group includes five mice, with error bars representing the standard deviations from the mean. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (as determined by two-way ANOVA with Dunnett’s multiple-comparison test [A], one-way ANOVA with Tukey’s multiple-comparison test [B and C], or Kruskal-Wallis with Dunn’s multiple-comparison test [E]).
Upon evaluation of the Coxiella-specific antibody response following vaccination, CD4 KO and MHC-II KO mice had significantly less IgM and undetectable levels of IgG compared to PIV-vaccinated WT mice (Fig. 4A). At 14 dpi, both CD4 KO and MHC-II KO mice had significantly higher IgM than PIV-vaccinated WT mice, as well as significantly less IgG (Fig. 4B). This suggests CD4+ T cells are important for isotype switching and the generation of a protective antibody response against C. burnetii. However, the fact that MHC-II KO mice had significantly lower IgG titers and more severe splenomegaly than CD4 KO mice suggests additional CD4-independent mechanisms may be involved. Collectively, these data indicate that CD4+ T cells partially contribute to the MHC-II-dependent protection generated by PIV.
FIG 4.
CD4+ T cells are important for antibody isotype switching. WT, CD4 KO, and MHC-II KO mice were vaccinated and challenged as previously described. Mice receiving Alhydrogel adjuvant alone served as unvaccinated controls. C. burnetii NMI-specific serum IgM and IgG were evaluated weekly until 28 dpv (A) and 14 dpi (B). Each experimental group includes five mice, with error bars representing the standard deviations from the mean. *, P < 0.05; **, P < 0.01; ****, P < 0.0001 (as determined by two-way ANOVA with Tukey’s multiple-comparison test [A] or one-way ANOVA with Tukey’s multiple-comparison test [B]).
CD4+ T cells from PIV-vaccinated mice are sufficient to generate protection.
To further examine the role of CD4+ T cells in PIV-mediated protection, we obtained purified CD4+ T cells from naive WT mice and WT mice which had been vaccinated with PIV as previously described. Approximately 5 × 106 purified CD4+ T cells were adoptively transferred to naive CD4 KO mice via i.p. injection 24 h before infection with C. burnetii. Body weight loss, splenomegaly, and splenic bacterial burden were evaluated to assess the protective efficacy of CD4+ T cell transfer. PIV-vaccinated CD4 KO mice, as well as all CD4 KO recipient mice, had significant body weight loss throughout infection compared to PIV-vaccinated WT mice (Fig. 5A). However, by 14 dpi, the recipient mice which received PIV-vaccinated CD4+ T cells had increased body weight compared to those recipients which received naive CD4+ T cells. Furthermore, CD4 KO recipient mice receiving PIV-vaccinated CD4+ T cells had a significant reduction in splenomegaly which was similar to PIV-vaccinated WT and CD4 KO mice (Fig. 5B). Similarly, CD4 KO recipient mice receiving PIV-vaccinated CD4+ T cells had splenic bacterial loads comparable to PIV-vaccinated WT and CD4 KO mice (Fig. 5C). This is in contrast to CD4 KO recipient mice receiving naive CD4+ T cells, which had similar splenomegaly and bacterial burden to WT adjuvant control mice. In summary, these data indicate that CD4+ T cells from PIV-vaccinated WT mice are sufficient to generate protection and reduce clinical disease in naive CD4 KO mice.
FIG 5.
CD4+ T cells from PIV-vaccinated mice are sufficient to generate protection. CD4 KO and WT C57BL/6 mice were vaccinated s.c. with 10 μg of PIV and challenged i.p. with 1 × 107 genomic copies of C. burnetii NMI 28 dpv. Naive CD4 KO mice received 5 × 106 purified CD4+ T cells by i.p. injection from either naive or PIV-vaccinated WT mice 24 h before infection. Mice receiving Alhydrogel adjuvant alone served as unvaccinated controls. (A) The relative body weight was measured throughout the infection. Splenomegaly (B) and splenic bacterial burden (C) were evaluated at 14 dpi to compare protection. The results are expressed as the percent splenomegaly, i.e., (spleen weight/body weight) × 100. Bacterial burden was determined by real-time quantitative PCR (qPCR) and is expressed as log10 C. burnetii com1 gene copy numbers. The experiments with results shown in Fig. 5 and 6 were performed concurrently, so the WT Alhydrogel adjuvant, WT PIV, and CD4 KO PIV groups represented in these figures are identical. Each experimental group includes five mice, with error bars representing the standard deviations from the mean. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (as determined by two-way ANOVA with Dunnett’s multiple-comparison test [A] or one-way ANOVA with Tukey’s multiple-comparison test [B and C]).
To determine whether this phenotype was the result of direct antigen stimulation of CD4+ T cells, we next performed an adoptive transfer of naive or PIV-vaccinated CD4+ T cells to PIV-vaccinated CD4 KO mice. We again saw significant body weight loss in PIV-vaccinated CD4 KO mice as well as all CD4 KO recipient mice compared to PIV-vaccinated WT mice (Fig. 6A). The most significant body weight loss was seen in PIV-vaccinated CD4 KO mice which received naive CD4+ T cells. In line with these results, PIV-vaccinated CD4 KO mice which received naive CD4+ T cells had significant splenomegaly (Fig. 6B), equivalent to that of WT adjuvant control mice, as well as higher splenic bacterial loads (Fig. 6C). This suggests that naive CD4+ T cells may play a detrimental role when introduced into a PIV-vaccinated CD4-deficient host. In contrast, PIV-vaccinated CD4 KO recipients which received CD4+ T cells from PIV-vaccinated WT donors had splenomegaly and bacterial burden comparable to PIV-vaccinated WT mice. Overall, these data suggest that CD4+ T cells from PIV-vaccinated WT mice are sufficient to generate protection, while those from naive donors may exacerbate disease.
FIG 6.
CD4+ T cells from naive mice are detrimental to protection. CD4 KO and WT C57BL/6 mice were vaccinated s.c. with 10 μg of PIV and challenged i.p. with 1 × 107 genomic copies of C. burnetii NMI 28 dpv. PIV-vaccinated CD4 KO mice received 5 × 106 purified CD4+ T cells by i.p. injection from either naive or PIV-vaccinated WT mice at 24 h before infection. Mice receiving Alhydrogel adjuvant alone served as unvaccinated controls. (A) The relative body weight was calculated throughout the infection. Splenomegaly (B) and splenic bacterial burden (C) were evaluated at 14 dpi to compare protection. The results are expressed as the percent splenomegaly, i.e., (spleen weight/body weight) × 100. Bacterial burden was determined by real-time quantitative PCR (qPCR) and is expressed as log10 C. burnetii com1 gene copy numbers. The experiments with results shown in Fig. 5 and 6 were performed concurrently, so the WT Alhydrogel adjuvant, WT PIV, and CD4 KO PIV groups represented in these figures are identical. Each experimental group includes five mice, with error bars representing the standard deviations from the mean. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (as determined by two-way ANOVA with Dunnett’s multiple-comparison test [A] or one-way ANOVA with Tukey’s multiple-comparison test [B and C]).
Tbet plays a role in vaccine protection that partially depends on Th1 CD4+ T cells.
Naive CD4+ T cells can differentiate into different T helper subsets (e.g., Th1, Th2, Th17, T follicular helper, T regulatory, etc.) based on transcriptional regulation and the local cytokine environment (46). To determine which CD4+ T helper subsets are involved in vaccine protection, we vaccinated and challenged transcription factor deficient mice as previously described. Body weight loss, splenomegaly, and splenic bacterial burden were evaluated to assess vaccine protection. Tbet KO mice, which are deficient in the Th1 subset and display markedly reduced interferon gamma (IFN-γ) (47), showed significant body weight loss compared to PIV-vaccinated WT mice 3, 7, and 10 dpi (Fig. 7A). No significant body weight loss occurred in Stat6 KO mice, which are nonresponsive to interleukin 4 (IL-4)/IL-13 signaling and lack the Th2 subset (48). Th17-deficient RORγt KO mice (49) did not lose significant body weight until 14 dpi. At this time point, Tbet KO mice had significantly worse splenomegaly than PIV-vaccinated WT mice (Fig. 7B). In contrast, both Stat6 KO and RORγt KO mice had similar splenomegaly to PIV-vaccinated WT mice. Considering Tbet KO mice had worse splenomegaly than CD4 KO mice, there may be a role for Tbet in vaccine protection that is independent of its role in Th1 differentiation. In terms of splenic bacterial burden, all deficient strains had a statistically significant reduction compared to WT adjuvant controls and were similar to PIV-vaccinated WT mice (Fig. 7C). This suggests that Th1 cells serve a role in inflammation modulation, though they may not contribute to bacterial clearance. Taken together, these results indicate that Tbet plays a role in vaccine protection which partially depends on Th1 CD4+ T cells.
FIG 7.
Tbet plays a role in vaccine protection that partially depends on Th1 CD4+ T cells. Tbet-deficient (Tbet KO), Stat6-deficient (Stat6 KO), RORγt-deficient (RORγt KO), CD4 KO, and WT C57BL/6 mice were vaccinated s.c. with 10 μg of PIV and challenged i.p. with 1 × 107 genomic copies of C. burnetii NMI 28 dpv. Mice receiving Alhydrogel adjuvant alone served as unvaccinated controls. (A) The relative body weight was determined throughout the infection. Splenomegaly (B) and splenic bacterial burden (C) were evaluated at 14 dpi to compare protection. The results are expressed as the percent splenomegaly, i.e., (spleen weight/body weight) × 100. The bacterial burden was determined by real-time quantitative PCR (qPCR) and is expressed as log10 C. burnetii com1 gene copy numbers. Each experimental group includes five mice, with error bars representing the standard deviations from the mean. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (as determined by two-way ANOVA with Dunnett’s multiple-comparison test [A] or one-way ANOVA with Tukey’s multiple-comparison test [B and C]).
IFN-γ modulates inflammation but may not be involved in bacterial clearance.
IFN-γ, in conjunction with Tbet, influences the differentiation of naive T cells into the Th1 subset. Furthermore, IFN-γ has been previously reported to play an important role in the clearance of C. burnetii following primary challenge (37). To evaluate the role of IFN-γ in PIV-mediated protection, we vaccinated and challenged IFN-γ-deficient (IFN-γ KO) mice and measured body weight loss, splenomegaly, and splenic bacterial burden. IFN-γ KO mice had significant body weight loss compared to PIV-vaccinated WT mice beginning 7 dpi (Fig. 8A). Furthermore, IFN-γ KO mice had significantly worse splenomegaly than PIV-vaccinated WT mice at 14 dpi (Fig. 8B). Interestingly, we saw no significant difference in splenic bacterial burden between PIV-vaccinated WT and IFN-γ KO mice (Fig. 8C). Collectively, these results suggest that IFN-γ may not be required for bacterial clearance during secondary challenge and may only serve to modulate the host inflammatory response.
FIG 8.
IFN-γ modulates inflammation but may not be involved in bacterial clearance. IFN-γ-deficient (IFN-γ KO) and WT C57BL/6 mice were vaccinated s.c. with 10 μg of PIV and challenged i.p. with 1 × 107 genomic copies of C. burnetii NMI 28 dpv. Mice receiving Alhydrogel adjuvant alone served as unvaccinated controls. (A) Relative body weight was measured throughout infection. (B) Splenomegaly and (C) splenic bacterial burden were evaluated at 14 dpi to compare protection. The results are expressed as the percent splenomegaly, i.e., (spleen weight/body weight) × 100. The bacterial burden was determined by real-time quantitative PCR (qPCR) and is expressed as log10 C. burnetii com1 gene copy numbers. Each experimental group includes five mice, with error bars representing the standard deviations from the mean. *, P < 0.05; **, P < 0.01; ****, P < 0.0001 (as determined by two-way ANOVA with Dunnett’s multiple-comparison test [A] or one-way ANOVA with Tukey’s multiple-comparison test [B and C]).
DISCUSSION
Q-VAX has successfully reduced the spread of Q fever among occupational risk groups in Australia since the implementation of a National Q Fever Management Program in 2001 (50); however, it has failed to gain approval by the U.S. Food and Drug Administration due to the high incidence of adverse reactions in vaccinated populations. Understanding which vaccine components elicit these hypersensitivities, as well as the mechanisms of vaccine-induced protective immunity, is necessary to rationally design a safe and effective vaccine.
Whole-cell inactivated C. burnetii vaccines, including Q-VAX, are highly efficacious in healthy volunteers without adjuvant (10, 23, 24, 51, 52). Still, vaccines which are successfully tested in healthy adults are often nonimmunogenic in young children and the elderly (53). Indeed, it is reported that children’s risk of exposure to C. burnetii in South West Queensland is similar to the high risk of abattoir workers (54), and yet Q-VAX is not currently recommended for children (55). As such, immunopotentiation in these risk groups should be the focus of a new generation Q fever vaccine. Adjuvantation is one approach to this aim. Furthermore, the addition of adjuvant to enhance immunogenicity may allow for reduction in the amount of C. burnetii antigen required to generate protection. This “dose sparing” approach may, in turn, reduce reactogenicity since it appears to occur in a dose-dependent manner (52).
MHC molecules guide T cell development and shape adaptive immunity. In the thymus, MHC-I and MHC-II select for CD8+ and CD4+ T cells, respectively. As such, mice deficient in β2-microglobulin (B2m), a component of MHC-I, do not express MHC-I on their cell surface and are virtually devoid of CD8+ T cells (56). Similarly, MHC-II KO mice lack CD4+ T cells (57). Using these deficient mouse strains, we have previously demonstrated that both MHC-I and MHC-II are required for host defense against primary C. burnetii infection, with MHC-I deficiency having a greater impact on disease severity (44). In this study, we sought to determine the role of MHC-I and MHC-II in PIV-mediated immunity and found that, in contrast to primary infection, MHC-II appears to play an important role during secondary responses. PIV-vaccinated MHC-II KO mice had significant body weight loss, only a mild reduction in splenomegaly, and no change in com1 gene copy numbers compared to adjuvant controls. MHC-II is important for vaccine-mediated immunity against other intracellular pathogens, including herpes simplex virus (58, 59), influenza virus (60), and Mycobacterium bovis bacillus Calmette-Guerin (61). In these models, the importance of MHC-II is often associated with CD4+ T cell help for the generation of protective antibody responses. When we evaluated the Coxiella-specific antibody response following vaccination, we found that PIV-vaccinated B2m KO and MHC-II KO mice had early defects in IgM which were restored to WT levels at the time of challenge. In addition, PIV-vaccinated B2m KO and MHC-II KO mice produced markedly reduced IgG over the course of vaccination. The lack of Coxiella-specific IgG in PIV-vaccinated B2m KO mice was unexpected; however, there are at least two possible explanations for this phenomenon. B2m is not only involved in the assembly of MHC-I at the cell surface but is also a component of the neonatal Fc receptor (FcRn) (62). As such, B2m KO mice do not express FcRn on the cell surface. FcRn has been shown to protect IgG from catabolism; therefore, the lack of IgG could be due to rapid turnover in this mouse strain (63–65). Indeed, it has been previously demonstrated that B2m KO mice produce significantly less virus-specific IgG in response to vaccinia virus infection than their WT counterparts (66). Alternatively, a follicular CD8+ T cell subset has recently been described which promotes antibody isotype switching through the secretion of IL-21 (67). The latter may be more likely considering both PIV-vaccinated B2m KO and MHC-II KO mice produced IgG following challenge, albeit less than did WT mice. Further investigation is required to understand the role MHC-I and MHC-II play in generating Coxiella-specific protective antibodies.
Following the observation that MHC-II KO mice were more severely affected than B2m KO mice, we wanted to evaluate the role of CD4+ T cells in PIV protection. CD4+ T cells receive signals from professional antigen-presenting cells through T cell receptor recognition of peptide-loaded MHC-II. Interestingly, we found that the disease burden in CD4 KO mice was not equivalent to that in MHC-II KO mice. PIV-vaccinated CD4 KO mice had significantly elevated splenomegaly compared to PIV-vaccinated WT mice; however, their splenomegaly and splenic bacterial burden were still significantly less than those in MHC-II KO mice. This suggests that CD4-independent mechanisms may exist which contribute to vaccine protection in the absence of CD4. It has previously been reported that MHC-II-restricted CD4– CD8+ and CD4– CD8− αβ+ T cells expand to fill the T cell compartment in the absence of CD4 (68–73). These noncanonical T cell subsets are capable of protecting mice from bacterial and viral infections (69, 70, 72). Indeed, protection from lethal Leishmania major in CD4 KO mice was found to depend on IFN-γ-secreting, MHC-II-restricted CD4– CD8− double-negative αβ+ T cells (69). Furthermore, similar to classical CD4+ T cells, these cells are capable of B cell helper functions. Zheng et al. (74) found that germinal center formation, Ig somatic hypermutation, affinity maturation, and B cell memory generation were all able to progress normally in CD4 KO mice, albeit at a lower level. When we examined the Coxiella-specific antibody response in CD4 KO mice following vaccination, we found that they did not make significant specific IgG compared to PIV-vaccinated WT mice. However, they did produce significantly higher levels of specific IgG than MHC-II KO mice and WT adjuvant controls following challenge. This suggests that the contribution of one of these noncanonical MHC-II-restricted T cell subsets is possible; however, they are not as efficient as CD4+ T cells considering that CD4 KO mice had significantly less IgG than WT mice. γδ+ T cells are another population which could be mediating protection in the absence of CD4. Schneider et al. (75) reported elevated CD45RO+ HLA-DR+ γδ+ T cells in patients with acute Q fever compared to healthy controls. These γδ+ T cells were more activated than αβ+ T cells, which suggests an important role for them in host defense against C. burnetii. The role these unique T cell populations play in vaccine-mediated immunity is an interesting area for future research.
To further investigate the function of CD4+ T cells in PIV-mediated protection, we next performed adoptive-transfer experiments to CD4 KO mice. CD4+ T cells from PIV-vaccinated WT donor mice resulted in similar splenomegaly and splenic bacterial burden to PIV-vaccinated WT mice when administered to naive CD4 KO mice, which suggests that antigen-experienced CD4+ T cells are sufficient to generate protection. We use the term “antigen experienced” to describe T cells derived from an animal which has previously been vaccinated, realizing that the isolated cells are a mixed population of naive and antigen-specific memory T cells. The fact that PIV is a whole-cell vaccine with multiple antigens precludes the use of MHC tetramers for isolation of antigen-experienced CD4+ T cells. While CD4+ T cells from PIV-vaccinated WT donor mice are sufficient to protect naive CD4 KO recipients, naive CD4+ T cells appear to exacerbate disease in PIV-vaccinated CD4 KO recipients. This suggests that vaccination reprograms CD4+ T cells. Indeed, Zhang et al. (25) reported decreased serum IL-12p40, tumor necrosis factor alpha (TNF-α), IFN-γ, IL-17, and granulocyte colony-stimulating factor in PIV-vaccinated mice following i.p. challenge with C. burnetii NMI compared to a nonprotective phase II vaccine (PIIV) and adjuvant controls. With this in mind, we hypothesize that transfer of naive CD4+ T cells to PIV-vaccinated CD4 KO mice results in elevated proinflammatory cytokines. We did measure serum IFN-γ at 14 dpi and found elevated levels in the naive WT donor/PIV-vaccinated CD4 KO recipient group; however, the difference was not statistically significant (data not shown). The time point examined was likely a factor in this result. It would be interesting to evaluate the differences in cytokine responses between PIV-vaccinated CD4 KO mice and PIV-vaccinated CD4 KO mice which received naive CD4+ T cells following in vitro restimulation with C. burnetii NMI.
Naive CD4+ T cells can differentiate into different effector subsets (Th1, Th2, Th17, Treg, and Tfh) following activation. Th1 subset CD4+ T cells are a proinflammatory population which is transcriptionally regulated by Tbet (76). Their development is further driven by IL-12 and IFN-γ. Th2 cells are canonically involved in antibody-mediated immunity, and their development is guided by GATA-3 and IL-4/Stat6 (48, 77, 78). Th17 subset CD4+ T cells develop through signals from RORγt and IL-23 (79). To understand the role of these subsets in PIV-mediated immunity, we utilized Tbet-deficient, Stat6-deficient, and RORγt-deficient mice. Stat6 and RORγt KO mice had similar splenomegaly and bacterial burden to PIV-vaccinated WT mice, which suggests that Th2 and Th17 subset CD4+ T cells do not contribute significantly to protection. Conversely, Tbet KO mice had significantly worse splenomegaly than PIV-vaccinated WT mice. This suggests that Th1 cells are important for PIV-mediated protection. Interestingly, Tbet KO mice had worse disease than CD4 KO mice, indicating that there are likely Th1-independent roles for Tbet in vaccine immunity. Indeed, Tbet is known to be involved in the development and effector function of many other cell subsets, including dendritic cells (DCs), B cells, CD8+ T cells, and NK cells. DCs have been shown to require Tbet for the production of IFN-γ and activation of antigen-specific T cell responses (80). As such, the phenotype seen in Tbet KO mice may be the result of a loss of DC function. DCs are critical to vaccine protection and DCs pulsed with protein antigens from C. burnetii NMI have been shown to elicit a protective CD4+ T cell response (81). Alternatively, Tbet is involved in isotype switching in B cells. Tbet-deficient B cells fail to undergo class-switch recombination to the IgG2a isotype (82), especially in response to T-independent antigens like LPS (83). Antibody-mediated immunity, and more specifically IgG2a, is known to be required for PIV protection. Zhang et al. (25) reported a 4:1 ratio of IgG2a to IgG1 following vaccination with 0.2 μg of PIV compared to a 1:2 IgG2a/IgG1 ratio following vaccination with 0.2 μg of PIIV. Therefore, the increased disease burden observed in Tbet KO mice could be due to a lack of Coxiella-specific antibody production. Considering Tbet is involved in T-independent isotype switching, this would be a plausible CD4-independent mechanism. Indeed, it has previously been demonstrated that passive transfer of antibody produced following PIV vaccination of CD4 KO mice is sufficient for protection against C. burnetii infection (43). Otherwise, CD8+ T cell and NK cell dysfunction could also be playing a role. Effector and memory CD8+ T cell fates are regulated by Tbet and are impaired in Tbet KO mice (84–86). In the context of Tbet deficiency, the lack of a Th1 CD4+ T cell response coupled with dysfunctional CD8+ T cells likely has an additive effect on disease severity and the lack of vaccine protection. Indeed, nude mice lacking both subsets of T cells have worse disease than either CD4 or CD8 KO mice following vaccination and challenge with C. burnetii NMI (43). NK cells have not been studied in the context of Q fever vaccination; however, they do not appear to play a significant role following primary challenge (37). Overall, the role of Tbet in PIV-mediated protection is likely multifaceted and will be better elucidated with adoptive transfer studies using specific Tbet deficient cell subsets.
Regardless of the cell population studied, the consensus is that defects resulting from Tbet deficiency are due to dysregulated IFN-γ. Tbet transactivates the IFN-γ gene and has been shown to control IFN-γ production in CD4+ T cells, CD8+ T cells, and NK cells (47). Furthermore, the susceptibility of Tbet KO mice to multiple other intracellular pathogens, including Mycobacterium tuberculosis (87), Salmonella Typhimurium (88), and Leishmania major (47), has been attributed to insufficient IFN-γ. To determine the role of IFN-γ in vaccine-mediated protection against C. burnetii, we vaccinated and challenged IFN-γ KO mice. Interestingly, while IFN-γ KO mice had more severe splenomegaly than PIV-vaccinated WT mice, there was no difference in splenic bacterial burden. This corresponds with the phenotype observed in Tbet KO mice in which there was significant splenomegaly compared to PIV-vaccinated WT mice with no difference in splenic bacterial burden. Collectively, these data suggest that Tbet and IFN-γ modulate the host inflammatory response but do not appear to play a critical role in bacterial clearance following secondary challenge. This is in contrast to the critical role of IFN-γ after primary challenge (37). However, we did observe differences in the kinetics of body weight loss between Tbet KO and IFN-γ KO mice, which suggests that their roles in vaccine protection are not indistinguishable. Tbet KO mice displayed significant body weight loss beginning 3 dpi that recovered to the level of PIV-vaccinated WT mice by 14 dpi. However, IFN-γ KO mice exhibited significant body weight loss beginning 7 dpi and failed to recover by 14 dpi. While Tbet is required for optimal IFN-γ production, IFN-γ can be secreted in the absence of Tbet (89) and could explain this difference in body weight loss.
In summary, this study provides novel information about mechanisms of cell-mediated immunity in vaccine protection against a murine model of experimental Q fever. We report a previously uncharacterized MHC-II-dependent mechanism of protection against C. burnetii that is partially dependent on Tbet, CD4+ T cells, and IFN-γ. Furthermore, this study highlights differences in the primary and secondary immune response which should be considered when designing future vaccines against Q fever.
MATERIALS AND METHODS
Animals.
Four- to eight-week-old B2m KO (stock no. 002087), MHC-II KO (stock no. 003584), CD4 KO (stock no. 002663), Tbet KO (stock no. 004648), Stat6 KO (stock no. 005977), RORγt KO (stock no. 007571), and WT C57BL/6 (stock no. 000664) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). IFN-γ KO mice were kindly provided by Robyn Klein at Washington University (St. Louis, MO). Animals were housed in sterile microisolator cages in a conventional animal facility or an animal biosafety level 3 (ABSL3) facility at the University of Missouri Laboratory for Infectious Disease Research (MU-LIDR). Animals were provided food and water ad libitum. All research involving animals was conducted per animal care and use guidelines and all animal use protocols were approved by the Animal Care and Use Committee at the University of Missouri and following the Guide for the Care and Use of Laboratory Animals (92) and other federal statutes and regulations relating to the use of animals (Animal Welfare Assurance Number A38903). All infections were conducted in an ABSL3 facility at the MU-LIDR.
Bacterial strains.
C. burnetii Nine Mile phase I (NMI) clone 7 (RSA 493) was propagated in acidified citrate cysteine medium-2 (ACCM-2) as previously described (90). Bacteria were purified by centrifugation at 15,000 × g for 30 min, followed by two washes with sterile 1× phosphate-buffered saline (PBS). NMI was handled under biosafety level 3 (BSL3) conditions at the MU-LIDR.
Vaccination.
Purified C. burnetii NMI was inactivated for 48 h in 10% formalin, followed by dialysis in deionized water. Antigen concentration was then measured using a Micro BCA protein assay kit (Pierce, Rockford, IL). Mice were vaccinated s.c. with 10 μg of formalin-inactivated C. burnetii Nine Mile phase I vaccine (PIV) in 50 μl of sterile 1× PBS with 50 μl of aluminum hydroxide adjuvant (Alhydrogel adjuvant 2%; InvivoGen, San Diego, CA) for a final delivered volume of 100 μl as previously described (91). Unvaccinated control mice were given 50 μl of sterile 1× PBS with 50 μl of adjuvant for a final delivered volume of 100 μl. Vaccines were prepared with a 1:1 ratio of antigen/adjuvant per manufacturer instructions. Vaccinated and unvaccinated control mice were then challenged to assess protectivity.
Infection.
Vaccinated and unvaccinated control mice were challenged 28 days postvaccination by intraperitoneal (i.p.) injection with 1 × 107 genomic copies of C. burnetii NMI in 400 μl sterile 1× PBS. Mice were weighed throughout the course of infection and relative body weight (current body weight/day 0 body weight) was calculated to monitor disease progression. The protective efficacy of PIV was evaluated 14 dpi by comparing the percent splenomegaly [i.e., (spleen weight/body weight) × 100] against unvaccinated controls. The bacterial burden in the spleen was also measured using real-time quantitative PCR (qPCR).
qPCR.
Spleen pieces were weighed, diluted 20× in lysis buffer (1 M Tris, 0.5 M EDTA, 7 mg/ml glucose, 28 mg/ml lysozyme), homogenized, and filtered through a 100-μm nylon mesh to remove any connective tissue. Then, 10 μl of proteinase K (20 mg/ml) was added to 200 μl of filtered spleen homogenate, followed by incubation at 60°C overnight. Next, 21 μl 10% sodium dodecyl sulfate was added to samples, followed by incubation at room temperature for 1 h. Finally, DNA was extracted using a High Pure PCR template preparation kit (Roche, Indianapolis, IN). The bacterial burden was determined by quantifying com1 gene copy numbers using a standard curve with SYBR green (Applied Biosystems, Foster City, CA) on an Applied Biosystems StepOnePlus real-time PCR system. The standard curve was generated using recombinant plasmid DNA (com1 gene ligated into pBluescript vector). Splenic bacterial burden was calculated using the following equation: {[(genomic equivalents × lysis buffer)/2] × spleen weight}/spleen piece weight.
Coxiella-specific enzyme-linked immunosorbent assay.
Sera from vaccinated and unvaccinated control mice were used for quantification of total C. burnetii-specific IgM and IgG. Ninety-six-well microtiter plates were coated overnight at 4°C with 100 μl of inactivated NMI antigen (0.5 μg/ml) or unlabeled anti-IgM or -IgG antibody (0.5 μg/ml, for the standard curve; Southern Biotech, Birmingham, AL) in 0.05 M carbonate/bicarbonate coating buffer (pH 9.6). Plates were then blocked for 1 h with 1% bovine serum albumin in PBS-T buffer (0.05% Tween 20 in 1× PBS), followed by incubation with 200 μl of diluted sample serum (1:200 to 1:1,200) or serially diluted pure IgM or IgG (Southern Biotech) for 2 h at room temperature. Plates were washed with PBS-T buffer and then incubated with 100 μl of diluted horseradish peroxidase-conjugated goat anti-mouse IgM or IgG (1:4,000 to 1:8,000) at room temperature for 1 h. The plates were again washed with PBS-T, followed by the addition of 100 μl of TMB substrate (Thermo Fisher Scientific, Waltham, MA). Reactions were stopped using 1 M H3PO4, and the absorbance was measured at 450 nm using SpectraMax (Molecular Devices, San Jose, CA) or Infinite F50 (Tecan, Switzerland) microplate readers.
Histopathology.
Spleens were fixed in 10% formalin for 48 h before processing by the University of Missouri Veterinary Medicine Diagnostic Laboratory. Briefly, tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Spleen sections were then scored for histiocytic inflammation in red pulp after blinded review by a trained pathologist. Pathology scores were assigned based on the following scale: 0, no accumulations of macrophages; 1, small accumulations of macrophages; 2, small to moderate accumulations of macrophages; and 3, moderate to large accumulations of macrophages.
Adoptive cell transfer.
Spleen cells were harvested from naive and PIV-vaccinated WT C57BL/6 mice for adoptive cell transfer at 28 dpv. Briefly, spleens were homogenized, and the cell suspension was filtered through a 100-μm nylon mesh to remove any connective tissue. The spleen cells were pelleted by centrifugation at 500 × g for 8 min and resuspended in 5 ml of ACK lysis buffer for 5 min at room temperature to lyse the red blood cells. The remaining cells were then pelleted by centrifugation at 500 × g for 8 min and resuspended in sterile 1× PBS for counting. To obtain purified CD4+ T cells, magnetic cell separation was performed using a negative isolation kit (Miltenyi Biotec, Germany). Purity was assessed by flow cytometry to be >85% CD4+ T cells. Naive and PIV-vaccinated CD4 KO mice received 5 × 106 purified CD4+ T cells via i.p. injection 24 h before infection.
Statistical analysis.
Prism 5.0 (GraphPad Software, Inc., San Diego, CA) was used for all statistical analyses. Results represent means ± the standard deviations. Parametric data were compared using t tests, one- or two-way analysis of variance (ANOVA) with Sidak’s, Tukey’s, or Dunnett’s multiple-comparison test. Nonparametric data were compared using Kruskal-Wallis with Dunn’s multiple-comparison test. Differences were considered significant at P < 0.05.
ACKNOWLEDGMENTS
This study was supported by NIH/NIAID grants R01AI134681, R21AI130347, and R21AI137504 to G.Z.
We thank the staff at the University of Missouri Laboratory for Infectious Disease Research (MU-LIDR) for their assistance with these experiments. We also thank Charles Brown for critical reading and editing of the manuscript.
REFERENCES
- 1.CDC. 2019. Q fever. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/qfever/. [Google Scholar]
- 2.Raoult D, Marrie T, Mege J. 2005. Natural history and pathophysiology of Q fever. Lancet Infect Dis 5:219–226. doi: 10.1016/S1473-3099(05)70052-9. [DOI] [PubMed] [Google Scholar]
- 3.Akamine CM, Perez ML, Lee JH, Ing MB. 2019. Q fever in southern California: a case series of 20 patients from a VA medical center. Am J Trop Med Hyg 101:33–39. doi: 10.4269/ajtmh.18-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baca OG, Paretsky D. 1983. Q fever and Coxiella burnetii: a model for host-parasite interactions. Microbiol Rev 47:127–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maurin M, Raoult D. 1999. Q fever. Clin Microbiol Rev 12:518–553. doi: 10.1128/CMR.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker NR, Barralet JH, Bell AM. 2006. Q fever. Lancet 367:679–688. doi: 10.1016/S0140-6736(06)68266-4. [DOI] [PubMed] [Google Scholar]
- 7.Raoult D, Houpikian P, Tissot Dupont H, Riss JM, Arditi-Djiane J, Brouqui P. 1999. Treatment of Q fever endocarditis: comparison of two regimens containing doxycycline and ofloxacin or hydroxychloroquine. Arch Intern Med 159:167–173. doi: 10.1001/archinte.159.2.167. [DOI] [PubMed] [Google Scholar]
- 8.Brennan RE, Samuel JE. 2003. Evaluation of Coxiella burnetii antibiotic susceptibilities by real-time PCR assay. J Clin Microbiol 41:1869–1874. doi: 10.1128/jcm.41.5.1869-1874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Loenhout JA, Hautvast JL, Vercoulen JH, Akkermans RP, Wijkmans CJ, van der Velden K, Paget WJ. 2015. Q-fever patients suffer from impaired health status long after the acute phase of the illness: results from a 24-month cohort study. J Infect 70:237–246. doi: 10.1016/j.jinf.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Marmion BP, Ormsbee RA, Kyrkou M, Wright J, Worswick D, Cameron S, Esterman A, Feery B, Collins W. 1984. Vaccine prophylaxis of abattoir-associated Q fever. Lancet 2:1411–1414. doi: 10.1016/s0140-6736(84)91617-9. [DOI] [PubMed] [Google Scholar]
- 11.McQuiston JH, Holman RC, McCall CL, Childs JE, Swerdlow DL, Thompson HA. 2006. National surveillance and the epidemiology of human Q fever in the United States, 1978–2004. Am J Trop Med Hyg 75:36–40. doi: 10.4269/ajtmh.2006.75.1.0750036. [DOI] [PubMed] [Google Scholar]
- 12.Whitney EA, Massung RF, Candee AJ, Ailes EC, Myers LM, Patterson NE, Berkelman RL. 2009. Seroepidemiologic and occupational risk survey for Coxiella burnetii antibodies among US veterinarians. Clin Infect Dis 48:550–557. doi: 10.1086/596705. [DOI] [PubMed] [Google Scholar]
- 13.Walsh MG. 2012. Assessing Q fever in a representative sample from the United States population: identification of a potential occupational hazard. Epidemiol Infect 140:42–46. doi: 10.1017/S0950268811000227. [DOI] [PubMed] [Google Scholar]
- 14.de Rooij MM, Schimmer B, Versteeg B, Schneeberger P, Berends BR, Heederik D, van der Hoek W, Wouters IM. 2012. Risk factors of Coxiella burnetii (Q fever) seropositivity in veterinary medicine students. PLoS One 7:e32108. doi: 10.1371/journal.pone.0032108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams JC, Thompson HA (ed). 1991. Q fever: the biology of Coxiella burnetii, vol 2, p 21–71. CRC Press, Boca Raton, FL. [Google Scholar]
- 16.Hawker JI, Ayres JG, Blair I, Evans MR, Smith DL, Smith EG, Burge PS, Carpenter MJ, Caul EO, Coupland B, Desselberger U, Farrell ID, Saunders PJ, Wood MJ. 1998. A large outbreak of Q fever in the West Midlands: windborne spread into a metropolitan area? Commun Dis Public Health 1:180–187. [PubMed] [Google Scholar]
- 17.Tissot-Dupont H, Torres S, Nezri M, Raoult D. 1999. Hyperendemic focus of Q fever related to sheep and wind. Am J Epidemiol 150:67–74. doi: 10.1093/oxfordjournals.aje.a009920. [DOI] [PubMed] [Google Scholar]
- 18.Moos A, Hackstadt T. 1987. Comparative virulence of intra- and interstrain lipopolysaccharide variants of Coxiella burnetii in the guinea pig model. Infect Immun 55:1144–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brooke RJ, Kretzschmar ME, Mutters NT, Teunis PF. 2013. Human dose response relation for airborne exposure to Coxiella burnetii. BMC Infect Dis 13:488. doi: 10.1186/1471-2334-13-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oyston PC, Davies C. 2011. Q fever: the neglected biothreat agent. J Med Microbiol 60:9–21. doi: 10.1099/jmm.0.024778-0. [DOI] [PubMed] [Google Scholar]
- 21.Hackstadt T, Peacock MG, Hitchcock PJ, Cole RL. 1985. Lipopolysaccharide variation in Coxiella burnetii: intrastrain heterogeneity in structure and antigenicity. Infect Immun 48:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kersh GJ, Oliver LD, Self JS, Fitzpatrick KA, Massung RF. 2011. Virulence of pathogenic Coxiella burnetii strains after growth in the absence of host cells. Vector Borne Zoonotic Dis 11:1433–1438. doi: 10.1089/vbz.2011.0670. [DOI] [PubMed] [Google Scholar]
- 23.Marmion BP, Ormsbee RA, Kyrkou M, Wright J, Worswick DA, Izzo AA, Esterman A, Feery B, Shapiro RA. 1990. Vaccine prophylaxis of abattoir-associated Q fever: eight years’ experience in Australian abattoirs. Epidemiol Infect 104:275–287. doi: 10.1017/s0950268800059458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ackland JR, Worswick DA, Marmion BP. 1994. Vaccine prophylaxis of Q fever. A follow-up study of the efficacy of Q-Vax (CSL) 1985–1990. Med J Aust 160:704–708. doi: 10.5694/j.1326-5377.1994.tb125909.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang G, Russell-Lodrigue KE, Andoh M, Zhang Y, Hendrix LR, Samuel JE. 2007. Mechanisms of vaccine-induced protective immunity against Coxiella burnetii infection in BALB/c mice. J Immunol 179:8372–8380. doi: 10.4049/jimmunol.179.12.8372. [DOI] [PubMed] [Google Scholar]
- 26.Smadel JE, Snyder MJ, Robbins FC. 1948. Vaccination against Q fever. Am J Hyg 47:71–81. doi: 10.1093/oxfordjournals.aje.a119187. [DOI] [PubMed] [Google Scholar]
- 27.Meiklejohn G, Lennette EH. 1950. Q fever in California. I. Observations on vaccination of human beings. Am J Hyg 52:54–64. [PubMed] [Google Scholar]
- 28.Stoker MG. 1957. Q fever down the drain. Br Med J 1:425–427. doi: 10.1136/bmj.1.5016.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell JF, Lackman DB, Meis A, Hadlow WJ. 1964. Recurrent reaction of site of Q fever vaccination in a sensitized person. Mil Med 129:591–595. doi: 10.1093/milmed/129.7.591. [DOI] [PubMed] [Google Scholar]
- 30.Seqirus. 2017. Q-VAX Q fever vaccine and Q-VAX skin test (AUST R 100517 & 100518). Sequirus, Maidenhead, United Kingdom: https://labeling.seqirus.com/PI/AU/Q-Vax/EN/Q-Vax-Product-Information.pdf. [Google Scholar]
- 31.Lennette EH, Clark WH, Jensen FW, Toomb CJ. 1952. Q fever studies. XV. Development and persistence in man of complement-fixing and agglutinating antibodies to Coxiella burnetii. J Immunol 68:591–598. [PubMed] [Google Scholar]
- 32.Kishimoto RA, Burger GT. 1977. Appearance of cellular and humoral immunity in guinea pigs after infection with Coxiella burnetii administered in small-particle aerosols. Infect Immun 16:518–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peacock MG, Fiset P, Ormsbee RA, Wisseman CL Jr. 1979. Antibody response in man following a small intradermal inoculation with Coxiella burnetii phase I vaccine. Acta Virol 23:73–81. [PubMed] [Google Scholar]
- 34.Humphres RC, Hinrichs DJ. 1981. Role of antibody in Coxiella burnetii infection. Infect Immun 31:641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Izzo AA, Marmion BP, Worswick DA. 1988. Markers of cell-mediated immunity after vaccination with an inactivated, whole-cell Q fever vaccine. J Infect Dis 157:781–789. doi: 10.1093/infdis/157.4.781. [DOI] [PubMed] [Google Scholar]
- 36.Andoh M, Russell-Lodrigue KE, Zhang G, Samuel JE. 2005. Comparative virulence of phase I and II Coxiella burnetii in immunodeficient mice. Ann N Y Acad Sci 1063:167–170. doi: 10.1196/annals.1355.026. [DOI] [PubMed] [Google Scholar]
- 37.Andoh M, Zhang G, Russell-Lodrigue KE, Shive HR, Weeks BR, Samuel JE. 2007. T cells are essential for bacterial clearance, and gamma interferon, tumor necrosis factor alpha, and B cells are crucial for disease development in Coxiella burnetii infection in mice. Infect Immun 75:3245–3255. doi: 10.1128/IAI.01767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shannon JG, Cockrell DC, Takahashi K, Stahl GL, Heinzen RA. 2009. Antibody-mediated immunity to the obligate intracellular bacterial pathogen Coxiella burnetii is Fc receptor and complement independent. BMC Immunol 10:26. doi: 10.1186/1471-2172-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shannon JG, Heinzen RA. 2009. Adaptive immunity to the obligate intracellular pathogen Coxiella burnetii. Immunol Res 43:138–148. doi: 10.1007/s12026-008-8059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Read AJ, Erickson S, Harmsen AG. 2010. Role of CD4+ and CD8+ T cells in clearance of primary pulmonary infection with Coxiella burnetii. Infect Immun 78:3019–3026. doi: 10.1128/IAI.00101-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capo C, Mege JL. 2012. Role of innate and adaptive immunity in the control of Q fever. Adv Exp Med Biol 984:273–286. doi: 10.1007/978-94-007-4315-1_14. [DOI] [PubMed] [Google Scholar]
- 42.Zhang G, Zhang Y, Samuel JE. 2012. Components of protective immunity. Adv Exp Med Biol 984:91–104. doi: 10.1007/978-94-007-4315-1_5. [DOI] [PubMed] [Google Scholar]
- 43.Zhang G, Peng Y, Schoenlaub L, Elliott A, Mitchell W, Zhang Y. 2013. Formalin-inactivated Coxiella burnetii phase I vaccine-induced protection depends on B cells to produce protective IgM and IgG. Infect Immun 81:2112–2122. doi: 10.1128/IAI.00297-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buttrum L, Ledbetter L, Cherla R, Zhang Y, Mitchell WJ, Zhang G. 2018. Both major histocompatibility complex class I (MHC-I) and MHC-II molecules are required, while MHC-I appears to play a critical role in host defense against primary Coxiella burnetii infection. Infect Immun 86:e00602-17. doi: 10.1128/IAI.00602-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanicas M, Rossi S, Giudice GD, Rambe DS. 2015. Safety and mechanism of action of licensed vaccine adjuvants. Int Curr Pharm J 4:420–431. doi: 10.3329/icpj.v4i8.24024. [DOI] [Google Scholar]
- 46.Crotty S, Johnston RJ, Schoenberger SP. 2010. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol 11:114–120. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. 2002. Distinct effects of T-bet in Th1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science 295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 48.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. 1996. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity 4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 49.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. 2006. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 50.Gidding HF, Wallace C, Lawrence GL, McIntyre PB. 2009. Australia’s national Q fever vaccination program. Vaccine 27:2037–2041. doi: 10.1016/j.vaccine.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Shapiro RA, Siskind V, Schofield FD, Stallman N, Worswick DA, Marmion BP. 1990. A randomized, controlled, double-blind, cross-over, clinical trial of Q fever vaccine in selected Queensland abattoirs. Epidemiol Infect 104:267–273. doi: 10.1017/s0950268800059446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fries LF, Waag DM, Williams JC. 1993. Safety and immunogenicity in human volunteers of a chloroform-methanol residue vaccine for Q fever. Infect Immun 61:1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiedermann U, Garner-Spitzer E, Wagner A. 2016. Primary vaccine failure to routine vaccines: why and what to do? Hum Vaccin Immunother 12:239–243. doi: 10.1080/21645515.2015.1093263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parker N, Robson J, Bell M. 2010. A serosurvey of Coxiella burnetii infection in children and young adults in South West Queensland. Aust N Z J Public Health 34:79–82. doi: 10.1111/j.1753-6405.2010.00478.x. [DOI] [PubMed] [Google Scholar]
- 55.Barralet JH, Parker NR. 2004. Q fever in children: an emerging public health issue in Queensland. Med J Aust 180:596–597. doi: 10.5694/j.1326-5377.2004.tb06106.x. [DOI] [PubMed] [Google Scholar]
- 56.Koller BH, Marrack P, Kappler JW, Smithies O. 1990. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science 248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 57.Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. 1999. Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci U S A 96:10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghiasi H, Cai S, Nesburn AB, Wechsler SL. 1997. MHC-II but not MHC-I responses are required for vaccine-induced protection against ocular challenge with HSV-1. Curr Eye Res 16:1152–1158. doi: 10.1076/ceyr.16.11.1152.5104. [DOI] [PubMed] [Google Scholar]
- 59.Ghiasi H, Roopenian DC, Slanina S, Cai S, Nesburn AB, Wechsler SL. 1997. The importance of MHC-I and MHC-II responses in vaccine efficacy against lethal herpes simplex virus type 1 challenge. Immunology 91:430–435. doi: 10.1046/j.1365-2567.1997.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O E, Lee Y-T, Ko E-J, Kim K-H, Lee Y-N, Song J-M, Kwon Y-M, Kim M-C, Perez DR, Kang S-M. 2014. Roles of major histocompatibility complex class II in inducing protective immune responses to influenza vaccination. J Virol 88:7764–7775. doi: 10.1128/JVI.00748-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ladel CH, Daugelat S, Kaufmann S. 1995. Immune response to Mycobacterium bovis bacillus Calmette-Guérin infection in major histocompatibility complex class I- and II-deficient knock-out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur J Immunol 25:377–384. doi: 10.1002/eji.1830250211. [DOI] [PubMed] [Google Scholar]
- 62.Praetor A, Hunziker W. 2002. β2-Microglobulin is important for cell surface expression and pH-dependent IgG binding of human FcRn. J Cell Sci 115:2389–2397. [DOI] [PubMed] [Google Scholar]
- 63.Ghetie V, Hubbard JG, Kim J-K, Tsen M-F, Lee Y, Ward ES. 1996. Abnormally short serum half-lives of IgG in β2-microglobulin-deficient mice. Eur J Immunol 26:690–696. doi: 10.1002/eji.1830260327. [DOI] [PubMed] [Google Scholar]
- 64.Israel EJ, Wilsker DF, Hayes KC, Schoenfeld D, Simister NE. 1996. Increased clearance of IgG in mice that lack β2-microglobulin: possible protective role of FcRn. Immunology 89:573–578. doi: 10.1046/j.1365-2567.1996.d01-775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Christianson GJ, Brooks W, Vekasi S, Manolfi EA, Niles J, Roopenian SL, Roths JB, Rothlein R, Roopenian DC. 1997. β2-Microglobulin-deficient mice are protected from hypergammaglobulinemia and have defective antibody responses because of increased IgG catabolism. J Immunol 159:4781–4792. [PubMed] [Google Scholar]
- 66.Spriggs MK, Koller BH, Sato T, Morrissey PJ, Fanslow WC, Smithies O, Voice RF, Widmer MB, Maliszewski CR. 1992. β2-microglobulin-, CD8+ T-cell-deficient mice survive inoculation with high doses of vaccinia virus and exhibit altered IgG responses. Proc Natl Acad Sci U S A 89:6070–6074. doi: 10.1073/pnas.89.13.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang R, Masters AR, Fortner KA, Champagne DP, Yanguas-Casás N, Silberger DJ, Weaver CT, Haynes L, Rincon M. 2016. IL-6 promotes the differentiation of a subset of naive CD8+ T cells into IL-21–producing B helper CD8+ T cells. J Exp Med 213:2281–2291. doi: 10.1084/jem.20160417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rahemtulla A, Fung-Leung WP, Schilham MW, Kundig TM, Sambhara SR, Narendran A, Arabian A, Wakeham A, Paige CJ, Zinkernagel RM. 1991. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature 353:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 69.Locksley RM, Reiner SL, Hatam F, Littman DR, Killeen N. 1993. Helper T cells without CD4: control of leishmaniasis in CD4-deficient mice. Science 261:1448–1451. doi: 10.1126/science.8367726. [DOI] [PubMed] [Google Scholar]
- 70.Rahemtulla A, Kundig TM, Narendran A, Bachmann MF, Julius M, Paige CJ, Ohashi PS, Zinkernagel RM, Mak TW. 1994. Class II major histocompatibility complex-restricted T cell function in CD4-deficient mice. Eur J Immunol 24:2213–2218. doi: 10.1002/eji.1830240942. [DOI] [PubMed] [Google Scholar]
- 71.Matechak EO, Killeen N, Hedrick SM, Fowlkes BJ. 1996. MHC class II-specific T cells can develop in the CD8 lineage when CD4 is absent. Immunity 4:337–347. doi: 10.1016/s1074-7613(00)80247-2. [DOI] [PubMed] [Google Scholar]
- 72.Pearce EL, Shedlock DJ, Shen H. 2004. Functional characterization of MHC class II-restricted CD8+ CD4– and CD8– CD4– T cell responses to infection in CD4–/– mice. J Immunol 173:2494–2499. doi: 10.4049/jimmunol.173.4.2494. [DOI] [PubMed] [Google Scholar]
- 73.Tyznik AJ, Sun JC, Bevan MJ. 2004. The CD8 population in CD4-deficient mice is heavily contaminated with MHC class II-restricted T cells. J Exp Med 199:559–565. doi: 10.1084/jem.20031961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng B, Ozen ZZ, Cao S, Zhang Y, Han S. 2002. CD4-deficient T helper cells are capable of supporting somatic hypermutation and affinity maturation of germinal center B cells. Eur J Immunol 32:3315–3325. doi:. [DOI] [PubMed] [Google Scholar]
- 75.Schneider T, Jahn HU, Liesenfeld O, Steinhoff D, Riecken EO, Zeitz M, Ullrich R. 1997. The number and proportion of Vγ9 Vδ2 T cells rise significantly in the peripheral blood of patients after the onset of acute Coxiella burnetii infection. Clin Infect Dis 24:261–264. doi: 10.1093/clinids/24.2.261. [DOI] [PubMed] [Google Scholar]
- 76.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100:655–669. doi: 10.1016/S0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 77.Zheng W, Flavell RA. 1997. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89:587–596. doi: 10.1016/S0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 78.Zhu J, Guo L, Watson CJ, Hu-Li J, Paul WE. 2001. Stat6 is necessary and sufficient for IL-4’s role in Th2 differentiation and cell expansion. J Immunol 166:7276–7281. doi: 10.4049/jimmunol.166.12.7276. [DOI] [PubMed] [Google Scholar]
- 79.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 80.Lugo-Villarino G, Maldonado-Lopez R, Possemato R, Penaranda C, Glimcher LH. 2003. T-bet is required for optimal production of IFN-gamma and antigen-specific T cell activation by dendritic cells. Proc Natl Acad Sci U S A 100:7749–7754. doi: 10.1073/pnas.1332767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wei Y, Wang X, Xiong X, Wen B. 2011. Coxiella burnetii antigen-stimulated dendritic cells mediated protection against Coxiella burnetii in BALB/c mice. J Infect Dis 203:283–291. doi: 10.1093/infdis/jiq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peng SL, Szabo SJ, Glimcher LH. 2002. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc Natl Acad Sci U S A 99:5545–5550. doi: 10.1073/pnas.082114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gerth AJ, Lin L, Peng SL. 2003. T-bet regulates T-independent IgG2a class switching. Int Immunol 15:937–944. doi: 10.1093/intimm/dxg093. [DOI] [PubMed] [Google Scholar]
- 84.Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. 2003. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci U S A 100:15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. 2005. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol 6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 86.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. 2007. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity 27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sullivan BM, Jobe O, Lazarevic V, Vasquez K, Bronson R, Glimcher LH, Kramnik I. 2005. Increased susceptibility of mice lacking T-bet to infection with Mycobacterium tuberculosis correlates with increased IL-10 and decreased IFN-gamma production. J Immunol 175:4593–4602. doi: 10.4049/jimmunol.175.7.4593. [DOI] [PubMed] [Google Scholar]
- 88.Ravindran R, Foley J, Stoklasek T, Glimcher LH, McSorley SJ. 2005. Expression of T-bet by CD4 T cells is essential for resistance to Salmonella infection. J Immunol 175:4603–4610. doi: 10.4049/jimmunol.175.7.4603. [DOI] [PubMed] [Google Scholar]
- 89.Usui T, Preiss JC, Kanno Y, Yao ZJ, Bream JH, O’Shea JJ, Strober W. 2006. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J Exp Med 203:755–766. doi: 10.1084/jem.20052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Omsland A, Beare PA, Hill J, Cockrell DC, Howe D, Hansen B, Samuel JE, Heinzen RA. 2011. Isolation from animal tissue and genetic transformation of Coxiella burnetii are facilitated by an improved axenic growth medium. Appl Environ Microbiol 77:3720–3725. doi: 10.1128/AEM.02826-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ledbetter L, Cherla R, Chambers C, Zhang Y, Zhang G. 2019. Eosinophils affect antibody isotype switching and may partially contribute to early vaccine-induced immunity against Coxiella burnetii. Infect Immun 87:e00376-19. doi: 10.1128/IAI.00376-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]