Abstract
Introduction:
Magnesium sulfate (MgSO4) is utilized for fetal neuroprotection in preterm birth but its mechanism of action is still poorly understood. P2X7 receptor (P2X7R) is required for secretion of IL-1β, and can be blocked by divalent cations such as magnesium (Mg) and its own antagonist, Brilliant Blue G (BBG). We sought to determine, whether during inflammation MgSO4 can block endothelial IL-1β secretion, using an in-vitro model.
Methods:
Human umbilical vein endothelial cell (HUVEC) cultures were treated with varying doses of LPS, 2’(3)-O-(4-Benzoylbenzoyl) adenosine-5’-triphosphate (BzATP), BBG and MgSO4 for 3- or 24-hours. We determined cell cytotoxicity, apoptosis, IL-1β mRNA expression, IL-1β production and secretion and P2X7R expression on HUVECs.
Results:
We demonstrated that MgSO4 is efficacious in blocking IL-1β-mediated-inflammation in HUVECs, at both the initiation and propagation phases of inflammation. MgSO4 exerts these anti-inflammatory effects via downregulation of P2X7Rs on HUVECs.
Conclusion:
LPS-exposure increases IL-1β production and secretion in HUVECs, which is further intensified by P2X7R agonist, BzATP while MgSO4 inhibits IL-1β in both presence and absence of BzATP. This effect is similar to the results of P2X7R antagonist, BBG, suggesting that the anti-inflammatory effects of MgSO4is through P2X7R.
Maternal infection/inflammation in the perinatal period has detrimental consequences on the developing fetus. In addition to being a major risk factor for preterm birth, maternal infection/inflammation, can result in fetal neuro-inflammation, significant perinatal brain injury and life-long disability with motor delays, cognitive, behavioral and psychiatric impairments.(1) Cerebral palsy (CP) is a persistent and non-progressive disorder of posture and movement.(2) Through decades the etiology of CP remained obscure, but emerging data supported the role of neuro-inflammation, excitotoxicity, genetic susceptibility, and immaturity of the central nervous system (CNS).(2-5)
CP is estimated to occur with 1-2.5 in 1000 live births. Although majority of CP occurs in term pregnancies, the incidence of CP increases significantly with decreasing gestational age and birth weight. It is well documented that maternal administration of Magnesium Sulfate (MgSO4) prior to anticipated preterm delivery decreases CP in survivors.(6) Magnesium (Mg) is a divalent cation, which is actively transported across the placenta to the developing fetus.(7) The neuroprotective effect of MgSO4 is believed to be multifactorial although the specific molecular mechanism remains unknown. Mg, reduces excitotoxic injury, can regulate uteroplacental blood flow, decreases cerebral vascular resistance while increasing cerebral blood flow.(8) In contrast, Mg deficiency is associated with inflammation, susceptibility to injury via reactive oxygen species and altered energy metabolism, all of which have been shown to contribute to fetal neuronal damage.(8,9)
Association of intrauterine infection with adverse neurodevelopmental outcomes is well documented in animal models and human neonates.(1, 10) Unfortunately, the neuroprotective effect of MgSO4 in the presence of infection/inflammation is abrogated.(11) Chorioamnionitis is positively associated with CP in both term and preterm infants.(11) In pregnancies complicated with intrauterine infection, inflammatory reaction in the fetus starts with the umbilical vein, and can propagate to a full-blown fetal inflammatory response syndrome (FIRS).(12) Endothelial cells are important mediators of signaling, initiation and propagation of inflammatory and immune responses and are integral to pathogenesis of sepsis.
P2X7 Receptor (P2X7R) belongs to the ligand (ATP) – gated ionotropic nucleotide (P2) receptor family that contains 7 members (P2X1-7).(13) P2X7R’s are abundant in endothelium, immune cells and CNS.(14) Human umbilical vein endothelial cells (HUVECs) only express P2X4R’s and P2X7R’s, of which P2X7R are solely functional.(15)
Lipopolysaccharide (LPS), the outer membrane component of gram negative bacteria, through Toll-like receptor-4 (TLR4), activates a series of intracellular pathways leading to transcription of Pro-IL-1β, while ATP secreted from the damaged cells stimulate P2X7R, activating the NLRP3 inflammasome, and leading to secretion of mature IL-1β. This increases the efflux of intracellular ATP and along with secreted mature IL-1β, augments the inflammation in a vicious circle.(13) IL-1β plays a central role in inflammation-induced fetal brain injury which can be alleviated by IL-1 receptor antagonism.(5) Moreover, P2X7R deficient mice have decreased IL-1β production and attenuation of perinatal brain injury.(13, 16)
In our study, we hypothesized that MgSO4 will inhibit LPS-induced inflammation in HUVECs via P2X7R blockade. We set out to determine whether the protective effects of Mg can be achieved by exposure to MgSO4 at the initiation or propagation phase of inflammation and whether the protective actions of MgSO4 in HUVECs is through P2X7R.
METHODS
Human umbilical vein endothelial cell (HUVEC) cultures:
Commercially available HUVECs were purchased (201p-75n, Cell Applications Inc., San Diego, CA) and grown in Endothelial Cell Growth Medium (ECGM, 211-500, Cell Applications Inc., San Diego, CA) in a 37° C, 5% CO2 humidified incubator. Experiments did not include any human patient samples thus an IRB approval or informed consent was not applicable. ECGM was changed every other day until 60% confluent growth, subcultured at 80% confluence using Subculture reagent kit (Hank’s Balanced Salt Solution (HBSS, 062-100), Trypsin/EDTA solution (070-100), and Trypsin Neutralizing Solution (080-100), Cell Applications Inc., San Diego, CA), counted with a hemocytometer and inoculated to 6-well plates. Experiments were conducted at third to sixth passage in a class II Biological Safety Cabinet, with HEPA filtered laminar airflow.
Reagents:
Lipopolysaccharide from Escherichia coli O55:B5 (LPS, L2880, Sigma), 2’(3)-O-(4-Benzoylbenzoyl) adenosine-5’-triphosphate, triethylammonium salt (BzATP, sc203862, Santa-Cruz), Brilliant Blue G (BBG, B0770, Sigma-Aldrich), MgSO4 (M2643, Sigma) were purchased, stock solutions were prepared for 1 and 10 μl/ml LPS; 10 mM BzATP; 1 mM BBG; 1 M MgSO4; and were kept at −80 °C.
Cell cytotoxicity by LDH assay:
HUVECs were treated with ECGM (control) or LPS (0.1, 1, 5, 10, 100 ng/ml). Cell cytotoxicity was determined by measuring LDH activity in culture supernatants using a commercially available kit (11644793001, Roche, Sigma-Aldrich, St Louis, MO). Briefly, HUVECs were incubated with ECGM or LPS for 3-hours (-hrs) at 37 °C in 96-well plates, then centrifuged at 3000 rpm for 5-minutes. Cell free culture supernatants were incubated with the assay buffer and substrate mix in a new plate at room temperature (RT) and the absorbance at 490nm was measured using a 96-well microplate reader (CLARIOstar BMG LABTECH). The background (spontaneous LDH release) value was measured in non-stimulated cells and subtracted from each measurement. Experiments were performed in triplicates.
Extraction of RNA and quantitative real time Polymerase Chain Reaction (RT-qPCR):
HUVECs were cultured with ECGM, LPS (10, 100 ng/ml), BzATP (10, 100 μM), BBG (100 μM), or MgSO4 (0.1, 1, 10, 100 mM), for 3-hrs. Total RNA was isolated using RNeasy Plus Mini Kit (74136, Qiagen, Valencia, CA). Complementary DNA was reverse transcribed from mRNA using an iScript™ cDNA synthesis kit (1708890, Bio-Rad, Hercules, CA) by priming for 5 mins at 25 °C, reverse transcription (RT) for 20 mins at 46 °C, and RT inactivation for 1 min at 95 °C. qPCR reaction was performed on a CFX384 Touch Real-Time PCR Detection System (Bio Rad, Hercules, CA), with SensiFAST Probe No-ROX (Bioline, Taunton, MA) using iTaq Universal Probes Supermix (1725130, Bio-Rad, Hercules, CA) in 20-μl reactions for 40 cycles, using manufacturer’s protocol for temperature cycling (Bio-Rad, Hercules, CA) and denaturation at 95°C for 5 secs and extension at 60°C for 30 secs. Primers for IL-1β (Primer 1: 5"-GCAGACTCAAATTCCAGCTTG-3', Primer 2: 5'-ATGATAAGCCCACTCTACAGC-3",Probe: 5"-/56-FAM/AGAGTGTAG/ZEN/ATCCCAAAAATTACCCAAAGAAGAA//3IABkFQ/-3",Integrated DNA Technologies, Coralville, IA) and for 18S rRNA (4310893E, probe dye: VIC-TAMRA, Applied Biosystems, Foster City, CA) were used. Data analysis was performed with CFX Manager Software (Bio Rad). The results were subjected to melting curve analysis, and the data were analyzed using the 2-ΔΔCq method. The expression levels of IL-1β mRNA were normalized to 18S and represented as fold change. Experiments were performed in triplicates.
Enzyme linked immunoabsorbent assay (ELISA):
HUVECs were cultured in 6-well plates for 24-hrs with ECGM or LPS (100, 1000 ng/ml), BzATP 100 μM, BBG 100 μM,MgSO4 (1, 10 mM). Supernatants and cell pellets were stored at −80°C until analysis. IL-1β production in HUVECs and secretion into supernatant were measured using IL-1β Human ELISA Kit (ab 100562, Abcam, Cambridge, MA). Total protein amount in cell lysates were measured by Pierce™ BCA Protein Assay Kit (No. 23225, Thermo, Rockford, IL) and IL-1β levels were normalized to total protein. Experiments were performed in triplicates.
Immunohistochemistry:
Following 3-hrs of pre-incubation with LPS and/or MgSO4, HUVECs were fixed in 4% paraformaldehyde and stained with anti-cleaved caspase-3 antibodies at 1:100 dilution (Asp175, 9661L, cell signaling technology Inc., MA) or following 24-hrs incubation with LPS, and/or BzATP, BBG, MgSO4, with P2X7 polyclonal antibody at 1:100 dilution (PA5-25581, Invitrogen, NY) overnight at 4°C, rinsed in PBS, and stained with secondary antibody, donkey, anti-rabbit IgG H&L (Alexa Flour 568) preadsorbed at 1:500 dilution (Ab175692, Abcam, MA) for 3-hrs at RT. DAPI was used to stain cell nuclei. Experiments were conducted in triplicates. Three images (Right, Midline and Left along the midline radius) were obtained per well using an Axioplan 2 imaging system (Carl Zeiss, Thornwood, NY). Mean staining intensity was quantified using Image J (NIH).
Statistical analyses:
Data was tested for normality, one-way and two-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test was used to compare experimental groups. For column statistics 1-way ANOVA, D’Agostino-Pearson omnibus test was performed. Statistical analysis was performed with GraphPad Prism software (version 6.0, San Diego, CA). Data is presented as mean ± standard error of the mean (SEM) and p<0.05 is deemed statistically significant.
RESULTS
MgSO4 reduced LPS-induced cell cytotoxicity in HUVECs:
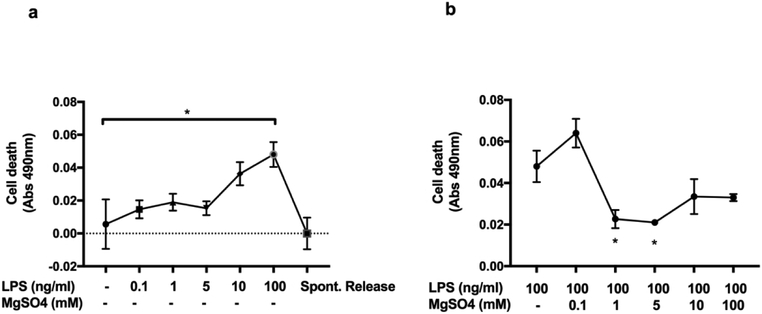
We determined LPS-induced cell cytotoxicity using varying concentrations of LPS on HUVECs. 100 ng/ml of LPS significantly increased cell cytotoxicity compared to control media following 3-hrs of exposure. Whereas, 10 ng/ml of LPS did not affect cell death. It is likely that 10 ng/ml of LPS may not have sufficiently induced the P2X7R-mediated-inflammation at this time-point (Figure 1a). Next, we tested whether MgSO4 could reduce LPS-induced cell cytotoxicity. 1 and 5 mM of MgSO4 were most effective in inhibiting LPS-induced cell cytotoxicity when we co-incubated HUVECs with 100 ng/ml of LPS and MgSO4 (0, 0.1, 1, 5, 10, 100 mM) (Figure 1b).
Figure 1. Cell cytotoxicity was induced by LPS, reduced by MgSO4.
Cell cytotoxicity was determined by LDH assay. LPS stimulation with 100 ng/ml induced cell death (Abs 490nm) in human umbilical vein endothelial cell cultures compared to control media (ECGM) (Fig1a). MgSO4 (1, 5 mM), in the presence of LPS 100 ng/ml, inhibited cell death compared to LPS alone (Fig1b). Values are expressed as mean±SEM, p<0.05, 2-way ANOVA.
Effect of MgSO4 on apoptosis in HUVECs:
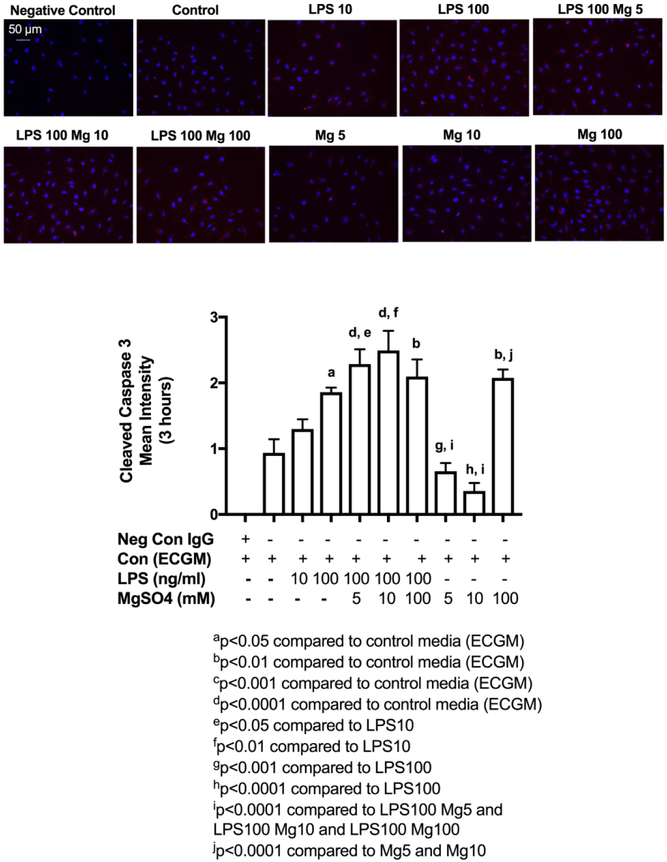
Next, we evaluated HUVEC apoptotic responses to LPS and MgSO4. Following 3-hrs exposure to LPS 100 ng/ml, we showed significant increase in staining intensity of cleaved or activated caspase-3, indicating apoptosis, but not with LPS 10 ng/ml (Figure 2). In the presence of inflammation (LPS 100 ng/ml), varying concentrations of MgSO4 (5, 10, 100 mM), did not decrease or increase cleaved caspase-3 staining in HUVECs when compared to LPS 100 ng/ml (Figure 2). In the absence of inflammation, 100 mM MgSO4 had a direct effect of significantly increasing apoptosis in HUVECs compared to 5 and 10 mM MgSO4.
Figure 2. LPS-induced apoptosis in HUVECs.
HUVECs were stimulated with control media (ECGM), LPS10 or LPS100 (10, 100 ng/ml) or co-stimulated with LPS100 and MgSO4 5, 10 or 100 (5, 10, 100 mM) or MgSO4 5, 10 or 100 alone and were stained for cleaved (activated) caspase-3 (red), and DAPI (blue). IgG staining was used as negative control. LPS100 increased cleaved caspase-3 expression compared to ECGM. In the presence of inflammation MgSO4 did not affect apoptosis at any dose whereas in the absence of inflammation MgSO4 100 resulted in significant increase in apoptosis. Values are expressed as mean±SEM, p<0.05, one-way ANOVA with Tukey’s multiple comparisons test. Scale bar shows 50-μM.
Determining the mechanism of MgSO4-induced suppression of inflammation:
We subsequently set out to determine the mechanism of action behind MgSO4-induced inhibition of inflammation in HUVECs, following exposure to either short term (3-hrs) or long-term (24-hrs) inflammation. We hypothesized that this is through inhibition of P2X7R on HUVECs. We stimulated HUVECs with LPS (100 ng/ml) and BBG (100 μM), a specific P2X7R antagonist or with LPS (100 ng/ml) and BzATP (10, 100 μM), an ammonium salt that is a potent activator of P2X channels or LPS (100 ng/ml) and MgSO4 (1, 10 mM). BzATP is not specific for P2X7R’s however HUVECs only have P2X4R and P2X7R, and of these two receptors P2X4R’s are functionally inactive.(15)
MgSO4 inhibits HUVEC IL-1β mRNA expression following exposure to short-term (3-hrs) inflammation:
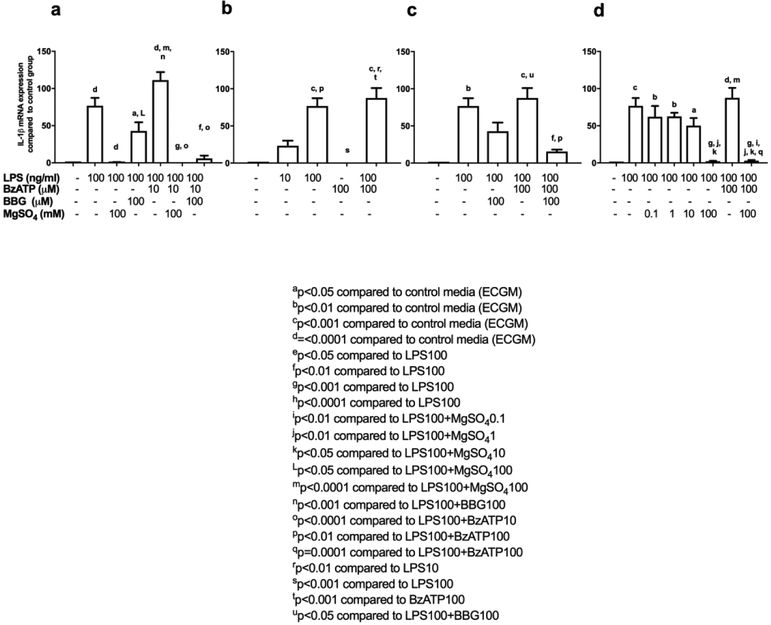
We focused our experiments to our cytokine of interest IL-1β and tested whether MgSO4 inhibited LPS-induced IL-1β mRNA expression in HUVECs following 3-hrs of incubation and whether the observed effects of MgSO4 were through P2X7R. 100 ng/ml of LPS significantly increased IL-1β mRNA expression compared with control media (Figure 3a). IL-1β mRNA expression was almost completely abrogated in HUVECs exposed to 100 ng/ml of LPS and 100 mM of MgSO4 when compared with 100 ng/ml of LPS alone (Figure 3a). We demonstrated that 100 μM BBG significantly inhibited IL-1β mRNA expression under inflammatory-excitotoxic conditions following 3-hr exposure to LPS100 and BzATP10 (Figure 3a). Similarly, 100 mM MgSO4, significantly decreased IL-1β mRNA expression under inflammatory-excitotoxic conditions following 3-hr exposure to LPS100 and BzATP10 (Figure 3a). We did not detect a statistical significant difference between LPS100 and LPS100+BzATP10, thus we chose BzATP100 to generate excitotoxic stimuli in our further experiments (Figure 3a).
Figure 3. MgSO4 downregulates IL-1β mRNA expression from HUVECs.
| Fig 3a. | Mean (Fold Change) | SEM | n |
|---|---|---|---|
| Control | 1 | 0 | 3 |
| LPS100 | 76.63316 | 10.7092 | 3 |
| LPS100+MgSO4100 | 0.9004739 | 0.2190636 | 3 |
| LPS100+BBG100 | 42.80225 | 11.72334 | 3 |
| LPS100+BzATP10 | 111.2056 | 10.80206 | 3 |
| LPS100+BzATP10+MgSO4100 | 0.05896 | 0.03743 | 3 |
| LPS100+BzATP10+BBG100 | 5.92331 | 3.761908 | 3 |
| Fig 3b. | |||
| Control | 1 | 0 | 3 |
| LPS10 | 23.28879 | 6.860098 | 3 |
| LPS100 | 76.63316 | 10.7092 | 3 |
| BzATP100 | 0 | 0 | 3 |
| LPS100+BzATP100 | 87.5097 | 13.39503 | 3 |
| Fig 3c. | |||
| Control | 1 | 0 | 3 |
| LPS100 | 76.63316 | 10.7092 | 3 |
| LPS100+BBG100 | 42.80225 | 11.72334 | 3 |
| LPS100+BzATP100 | 87.5097 | 13.39503 | 3 |
| LPS100+BzATP100+BBG100 | 15.52824 | 2.632421 | 3 |
| Fig 3d. | |||
| Control | 1 | 0 | 3 |
| LPS100 | 76.63316 | 10.7092 | 3 |
| LPS100+MgSO40.1 | 62.12084 | 14.59779 | 3 |
| LPS100+MgSO41 | 62.61264 | 4.782178 | 3 |
| LPS100+MgSO410 | 50.23698 | 10.24755 | 3 |
| LPS100+MgSO4100 | 1.958895 | 0.8618461 | 3 |
| LPS100+BzATP100 | 87.5097 | 13.39503 | 3 |
| LPS100+BzATP100+MgSO4100 | 2.927146 | 1.023443 | 3 |
100 μM of BzATP alone did not result in IL-1β mRNA expression (Figure 3b). In addition, 100 μM BBG significantly inhibited IL-1β mRNA expression under inflammatory-excitotoxic conditions following 3-hr exposure to LPS100 and BzATP100 (Figure 3c). 100 mM MgSO4, significantly decreased IL-1β mRNA expression under inflammatory and inflammatory-excitotoxic conditions following 3-hr exposure to LPS100 and BzATP10 (Figure 3d). Lower concentrations of MgSO4 did not alter IL-1β mRNA expression under inflammatory conditions for this time-point.
MgSO4 inhibits HUVEC IL-1β protein production and secretion following exposure to long-term (24-hrs) inflammation:
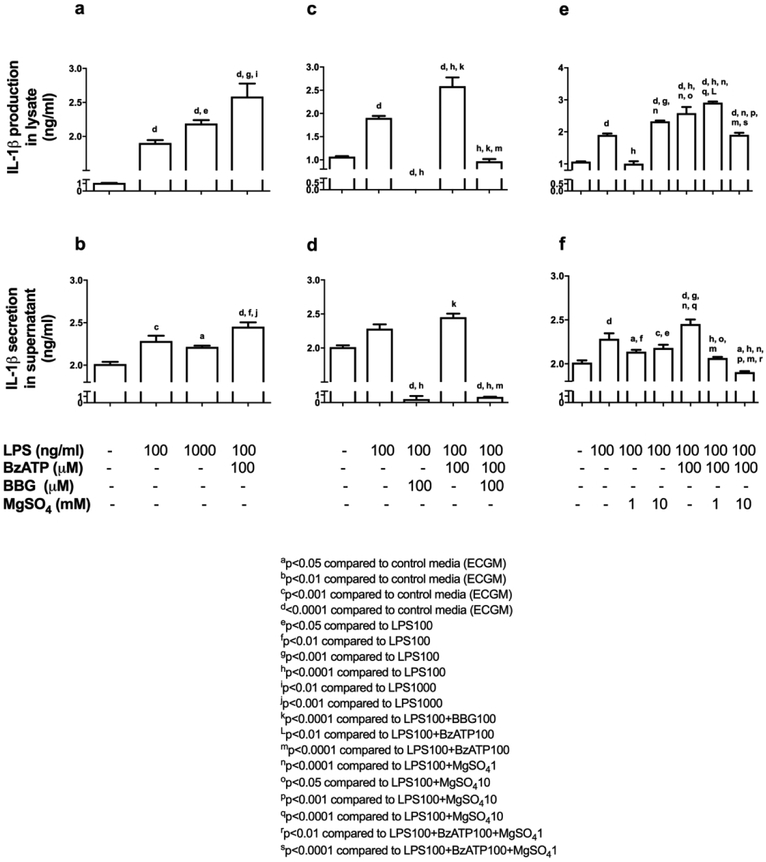
We subsequently measured IL-1β protein production and secretion following 24-hrs of LPS-exposure (100, 1000 ng/ml) in HUVECs (Table 1). We detected a significant increase in IL-1β production and secretion from HUVECs compared to control media, following 24-hrs of LPS-exposure with 100 ng/ml and 1000 ng/ml. IL-1β protein levels from HUVEC lysates exposed to 1000 ng/ml of LPS was significantly increased compared to 100 ng/ml of LPS, however IL-1β protein from the supernatants were similar (Figure 4a-b). Thus, we elected to use 100 ng/ml of LPS for all further experiments.
TABLE 1. Interleukin-1 βeta (IL-1β) protein levels in HUVEC cultures following 24 hour of incubation.
IL-1β protein concentration (ng/ml) was measured by ELISA assay in the HUVEC lysates and supernatants following various culture conditions and compared to control media ECGM following manufacturer’s recommendations. Values are expressed as mean±SEM, p<0.05, one-way ANOVA with Tukey’s multiple comparisons test. Column statistics by one-way ANOVA D’Agostino-Pearson omnibus test.
| Treatment Condition | Lysate (24 hours) ng/ml | Control (ECGM) ng/ml |
Supernatant (24 hours) ng/ml | Control (ECGM) ng/ml |
|---|---|---|---|---|
| LPS100 | 1.905±0.02446 | 1.07±0.006866 | 2.285±0.03598 | 2.013±0.01581 |
| LPS1000 | 2.191±0.02917 | 1.07±0.006866 | 2.215±0.007839 | 2.013±0.01581 |
| LPS100+BzATP100 | 2.587±0.1101 | 1.07±0.006866 | 2.453±0.03007 | 2.013±0.01581 |
| LPS100+BBG100 | 0±0 | 1.07±0.006866 | 0.4667±0.2333 | 2.013±0.01581 |
| LPS100+BzATP100+BBG100 | 0.9675±0.02932 | 1.07±0.006866 | 0.7443±0.01074 | 2.013±0.01581 |
| LPS100+MgSO4 (1) | 1.007±0.04359 | 1.07±0.006866 | 2.138±0.01193 | 2.013±0.01581 |
| LPS100+MgSO4 (10) | 2.33±0.01531 | 1.07±0.006866 | 2.181±0.02088 | 2.013±0.01581 |
| LPS100+BzATP100+MgSO4 (1) | 2.912±0.02101 | 1.07±0.006866 | 2.066±0.007211 | 2.013±0.01581 |
| LPS100+BzATP100+MgSO4 (10) | 1.906±0.03724 | 1.07±0.006866 | 1.906±0.005696 | 2.013±0.01581 |
Figure 4. MgSO4 inhibits IL-1β protein production and secretion from HUVECs.
LPS±BzATP induced IL-1β production and secretion from HUVECs (a, b). BBG suppressed IL-1β production and secretion from HUVECs following LPS±BzATP (c, d). MgSO4 similar to BBG inhibited HUVEC IL-1β production and secretion (e, f). Values are expressed as mean±SEM. p<0.0001, one-way ANOVA with Tukey’s multiple comparisons test.
We incubated HUVECs with LPS (100 ng/ml) and 100 μM BzATP with and without BBG (100 μM) for 24-hrs. In a separate subset of experiments, we tested HUVECs with LPS (100 ng/ml) and 100 μM BzATP with and without MgSO4 (1, 10 mM) to determine the efficacy of MgSO4-induced inhibition of IL-1β protein production and secretion at 24-hrs. When HUVECs were incubated with LPS (100 ng/ml) and BBG (100 μM), IL-1β protein in HUVEC lysates was undetectable at 24-hrs compared to LPS. In addition, there was a significant decrease in the detectable IL-1β levels in the supernatant. When BBG (100 μM) was added to LPS (100 ng/ml) and BzATP (100 μM), there was a significant decrease in IL-1β protein in HUVEC lysates in addition to significantly reduced IL-1β secretion from HUVECs into the supernatant, compared to LPS+BzATP (Figure 4c-d).
Low dose (1 mM) MgSO4 in the presence of inflammation (LPS 100 ng/ml) and excitotoxicity (BzATP 100 μM), did not decrease IL-1β protein production in the HUVEC lysates but significantly reduced IL-1β secretion to the supernatant when compared to LPS+BzATP. In contrast, following exposure to high dose (10 mM) MgSO4 under similar inflammatory (LPS 100 ng/ml) and excitatory (BzATP 100 μM) conditions, we detected significantly decreased IL-1β protein in the HUVEC lysates and secretion of IL-1β into the supernatant, compared to LPS+BzATP. Furthermore, MgSO4 10 mM was significantly superior in decreasing IL-1β protein in HUVEC lysates and supernatant compared to 1 mM when cultured with LPS+BzATP (Figure 4e-f).
MgSO4 exerts its anti-inflammatory effects via downregulation of P2X7Rs on HUVECs:
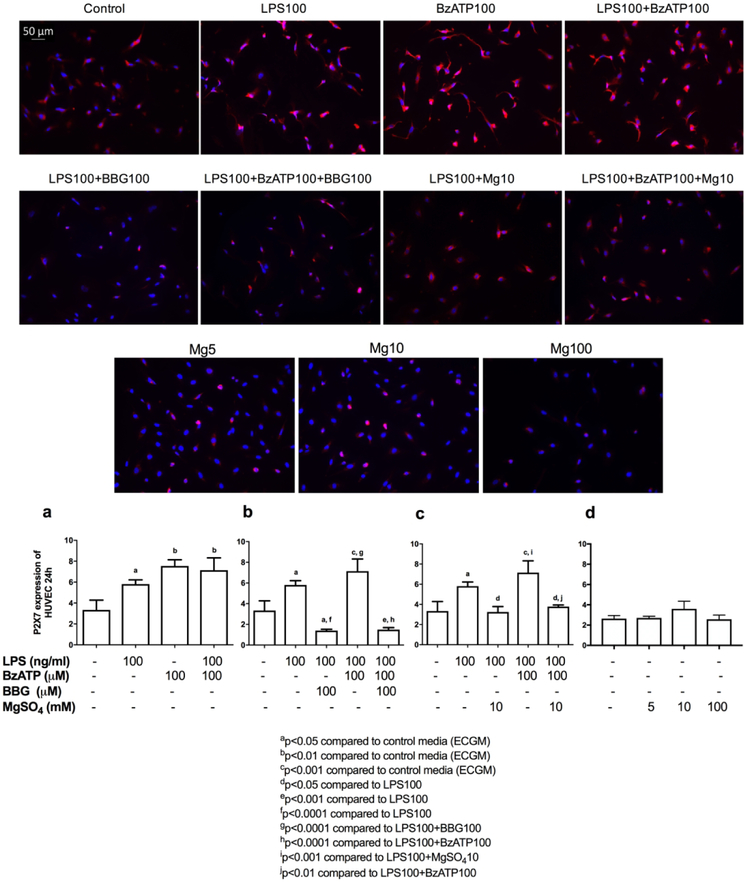
Last, we investigated HUVEC P2X7R expression under various culture conditions. LPS and BzATP individually and together were able to significantly increase HUVEC P2X7Rs following 24-hrs of exposure, compared to control media (Figure 5a). As expected, BBG100 significantly decreased P2X7Rs in HUVECs under inflammatory (LPS100+BBG100) and inflammatory-excitotoxic (LPS100+BzATP100+BBG100) conditions compared to LPS alone(Figure 5b). MgSO4 (MgSO4-10) significantly decreased P2X7Rs, similar to BBG, under inflammatory (LPS100+MgSO4-10) and inflammatory-excitotoxic (LPS100+BzATP100+MgSO4-10) conditions (Figure 5c). Whereas, in the absence of inflammation and/or excitotoxic stimuli, MgSO4 alone, did not alter P2X7R expression on HUVECs (Figure 5d). There was no statistically significant difference in P2X7R expression when incubated for 3- or 6-hrs (data not shown).
Figure 5. P2X7 receptor expression on HUVECs.
LPS±BzATP increased expression of P2X7Rs on HUVECs (a). Similar to BBG (b), MgSO4 (c) inhibited HUVEC P2X7R expression in the presence of inflammation and/or excitotoxicity, MgSO4 alone did not affect P2X7R expression (d). Values are expressed as mean±SEM. (a) p=0.0011, (b) p<0.0001, (c) p=0.0002, (d) Not-significant, one-way ANOVA with Tukey’s multiple comparisons test. Scale bar shows 50-μm.
DISCUSSION
In our current study, we show for the first time, MgSO4-induced inhibition of inflammation in HUVECs is through the downregulation of P2X7R expression. Furthermore, we demonstrate that MgSO4 is efficacious in blocking IL-1β-mediated-inflammation in HUVECs at the initiation of inflammation and more specifically during the propagation phase of excitotoxic-inflammatory injury and this effect is via P2X7Rs. In addition, we characterize that MgSO4-induced attenuation of excitotoxic-inflammatory injury is dose dependent, such that higher doses (10 mM) of MgSO4 is significantly superior in inhibiting IL-1β production and secretion from HUVECs during the excitotoxic phase of inflammation-induced cellular damage. Moreover, in the absence of inflammation, higher doses (100 mM) of MgSO4 increases apoptosis, however 1 or 10 mM of MgSO4 do not cause apoptosis in HUVECs.
We hypothesized that inflammatory signaling in HUVECs is through P2X7R and MgSO4 dependent downregulation of P2X7R will dampen initiation and propagation of inflammatory signaling and tested our hypothesis utilizing HUVECs in-vitro. We showed that inflammatory signaling in the human endothelial vein can be inhibited by MgSO4 through P2X7R in-vitro. Further in-vivo studies would be necessary to determine whether P2X7R dependent inhibition of endothelial inflammatory signaling is involved in MgSO4 associated neuroprotection from CP and whether the decreased efficacy of MgSO4 for CP prevention, in the presence of chorioamnionitis, may be related to the timing or dose of MgSO4 administration after the acute inflammatory insult. Despite two thirds of CP cases occur in term infants, preterm infants remain at increased risk of CP, the risk increasing with decreasing gestational age. Survival among periviable (22-24 weeks) infants are increasing in US academic centers which is accompanied by a similar rate of rise in survival with and without neurodevelopmental impairment (NDI).(17) Overall, NDI remains high (43%) and moderate or severe CP rates are not declining among periviable infants (5-15%).(17) Among low birth weight infants, the prevalence of CP was 3.5 (95% CI 3.2-3.9) and 2.9 (95% CI 2.3-3.2) per 1000 for year 2006 and 2010, respectively.(18)
In the simulated pharmacokinetic analysis of the original “Beneficial Effects of Antenatal MgSO4 (BEAM)” trial, prevention of CP correlated to maternal serum Mg levels at delivery, specifically 3.7-4.4 mg/dl, minimized the risk of CP in premature infants.(19) In this first pharmacokinetics/pharmacodynamics study, authors did not observe a difference between MgSO4 dose and severity of CP and concluded that more studies are needed to optimize the dose and the duration of this common treatment strategy for prevention of CP. In secondary analysis of MFMU BEAM trial, when neonates were stratified based on their cord blood Mg levels (≥ 2.9 or ≤ 1.5 mg/ml), higher levels (≥ 2.9) correlated with a slight but not significant decrease in the odds of MDI<70.(20) We calculated in-vitro equivalence of 1.5 mg/ml Mg as 8.3 mM and 2.9 mg/ml as 16 mM. Thus, 10 mM of MgSO4 used in our study, is comparable to observed neonatal levels. Furthermore, fetal neuroprotection from MgSO4 disappeared in women with chorioamnionitis compared to women without chorioamnionitis.(11)
Endothelium plays a central role in vascular function, normal hemostasis versus thrombosis, inflammation and immune function. Endothelial cell dysfunction contributes to pregnancy complications such as preterm labor, preeclampsia and FIRS and neonatal diseases such as sepsis.(21, 22) The results of our study support that the timing, dose and duration of MgSO4-exposure, is critical for MgSO4-induced suppression of excitotoxic-inflammation in human umbilical vein endothelium.
We detected an increase in caspase-3 in HUVECs, after 3-hrs of in-vitro LPS-exposure. Other researchers also showed that LPS-induced apoptosis in HUVECs is caspase-3 dependent, in agreement with our findings.(23, 24) However, despite attenuating inflammation and excitotoxicity, MgSO4 did not decrease apoptosis in HUVECs during inflammation. Interestingly, in the absence of inflammation, we showed that 100 mM MgSO4 significantly increased apoptosis in HUVECs, while 5 or 10 mM MgSO4 did not have this adverse effect. We opted to use 1 and 10 mM of MgSO4 for our subsequent experiments at 24-hrs of exposure given the recent FDA relabeling of MgSO4 from category A to D due to increased risk of bone fractures in fetus and neonate following longer exposures to this drug with cumulatively higher doses (25) and our current data showing increased endothelial cell apoptosis at 100 mM. However, by using varying concentrations of MgSO4 (i.e., 10-100mM), we may have changed the osmolarity of the culture media. A past study has shown that hyperosmolar stress can induce ATP release via cell lysis/death and these extracellular ATP can then activate P2X7R.(26) Therefore, it is plausible that the increased apoptosis noted at 100mM of MgSO4 may be an effect of hyperosmolarity rather than due to the cytotoxicity of MgSO4 at that level.
In-vitro and in-vivo studies showed that reduced levels of Mg is associated with inflammation.(9, 27, 28) For instance, HUVECs cultured in low Mg (0.1-1 mM) concentrations, in the absence of LPS, synthesize greater levels of IL-1.(27) Moreover, MgSO4 administration, either prior to or at the initiation of inflammation, but not after inflammation was established, decreased HUVEC inflammatory responses via blocking nuclear translocation of NFKB.(29) Almousa et. al. preincubated HUVECs with low (0.1 mM) or high (10 mM) concentrations of MgSO4 for 72-hrs and then exposed HUVECs to 4-hrs of LPS and showed that while pre-incubation with low (0.1 mM) MgSO4 exacerbated LPS responses, high (10 mM) MgSO4 dampened LPS responses.(9) We detected significantly decreased expressions of IL-1β, in HUVECs co-stimulated with LPS and MgSO4 compared to LPS stimulation without MgSO4, concurrent with the existing literature. Furthermore, MgSO4 was sufficient to completely abrogate HUVEC IL-1β secretion, which has been implicated in the pathogenesis of chorioamnionitis, FIRS and neonatal brain injury.(3, 4, 22) Burd et al., demonstrated attenuation of inflammation-induced brain injury following MgSO4, in a well-established model of intrauterine inflammation in mice.(30) In that study, administration of MgSO4 following maternal inflammation, restored fetal neuronal dendritic process counts, however failed to dampen IL-1β expression in fetal brains. Humans and mice have comparable inhibition of P2X7R by Mg.(31) However, species differences exist, for instance IC50 value of Mg on P2X7R is significantly higher in rats compared to humans.(32) Therefore, mice models may be superior to rat, to study the in-vivo effects of Mg. Our current and previous findings further signify that preventative effects of MgSO4 on endothelial injury may be related to timing of treatment in relation to the excitotoxic-inflammatory insult, severity of infection/inflammation and the dose of MgSO4.
P2X7R, is an ATP-gated ion channel, present on many cell types, immune cells, endothelium including HUVECs, and CNS, and has major roles in activation of NLRP3 inflammasome and IL-1β secretion.(14) P2X7R’s can be activated by BzATP, a potent excitatory stimulant (33) and can be blocked by BBG, a specific, non-competitive antagonist of P2X7R.(34) We detected an upregulation of P2X7R on HUVECs in inflammation and inflammatory-excitotoxic conditions. As anticipated, HUVECs co-cultured with BBG showed decreased P2X7Rs. More importantly, MgSO4 similar to BBG, resulted in a significant downregulation of P2X7Rs under inflammatory and inflammatory-excitotoxic culture conditions, suggesting that the role of Mg in decreasing IL-1β-mediated-inflammation via P2X7R blockade.
HUVECs were once believed to be devoid of P2X7R expression.(35) Valdecantos et al., identified the presence of functional P2X7R in the smooth muscle layer of placental blood vessels both at the maternal chorion and fetal umbilical vein and artery.(36) Wilson et al., subsequently demonstrated the presence of functional P2X7R’s on HUVECs at baseline and with exposure to inflammation.(15) Furthermore, they confirmed that HUVECs were capable of secreting IL-1β under inflammatory conditions via P2X7R dependent mechanism, however in their experiments the net effect of such stimulation was anti-inflammatory, likely due to simultaneous IL-1Ra secretion.(15) Moreover, alterations in blood flow, simulated with exposing HUVECs to different shear stresses, also induced endothelial P2X7-mediated-inflammation.(37) Thus, it is plausible that an unbalanced P2X7R-mediated-endothelial-inflammation may play an integral role in pathological pregnancies and adverse fetal and neonatal outcomes, and specifically in adverse neurodevelopmental outcomes.
Our 3-hr data matches well with the activation patterns of molecular pathways at the initiation of inflammation. LPS, initially stimulates TLR4 receptors and after activation MyD88, cellular release of ATP results in activation of P2X7R.(13) This may explain why we did not observe inhibition of IL-1β after LPS+BBG compared to LPS at 3-hrs due to the lack of excitotoxic stimulus, however when we cultured HUVECs with LPS+BzATP+BBG there was a significant decrease in IL-1β. When we tested HUVECs at 24-hrs however, BBG attenuated inflammation in both LPS exposed and LPS+BzATP exposed samples. This may suggest that 3-hrs may be too early for P2X7R related excitotoxicity to occur and hence BBG is not effective at this time-point on HUVECs. In addition, at 24-hrs, we showed that higher concentration of MgSO4 (10 mM) was significantly superior to lower (1 mM) in suppressing IL-1β under inflammatory (LPS) and excitotoxic (BzATP) conditions compared to just inflammation in HUVECs. This suggests that MgSO4 can attenuate the propagation of inflammation by downregulation of P2X7R’s during the excitotoxic phase of inflammation. However, a couple limitations to this study must be addressed. Our study did not control for the places downstream of P2X7R activation where MgSO4 could have possibly been having its effect. Furthermore, higher concentrations of MgSO4 may interfere with either the potassium efflux or calcium influx in the HUVECs, which are both indicated in the activation of P2X7R to induce the activation of the inflammasome.(38) Despite these limitations, our study establishes the importance of MgSO4 in the overall inflammatory process involving HUVECs.
Using 100 μM BBG, a specific P2X7R blocker for HUVECs, we showed complete abrogation of LPS-induced inflammatory responses, specifically expression of IL-1β in HUVEC lysates with a significant decrease in secreted IL-1β at 24-hrs. We also determined that this dose of BBG was sufficient to inhibit HUVEC inflammatory responses even in the presence of other excitatory stimuli such as BzATP. When we repeated our experiments with MgSO4, we demonstrated decreased HUVEC IL-1β production and secretion when cultured with and without BzATP. Our data shows similar reductions in HUVEC IL-1β production and secretion in culture conditions supplemented with MgSO4 in comparison to BBG. This supports our hypothesis that MgSO4 acts through P2X7R to inhibit HUVEC inflammatory responses. As expected, MgSO4 in the absence of inflammation did not alter P2X7R expression on HUVECs. Other researchers have shown that Mg-induced inhibition of P2X7Rs is “voltage-independent”, in other words, Mg acts allosterically by changing the binding affinity of ATP to P2X7R and not by direct blockade of the P2X7R, which is in support of our findings.(32, 39)
In sum, we demonstrate that MgSO4 is effective in blocking the initiation and propagation of inflammation in human endothelial vein in-vitro. Furthermore, MgSO4 dampens the excitotoxic-inflammation in a dose dependent manner and through downregulation of endothelial P2X7Rs. Our data shows that the efficacy of MgSO4 in dampening human endothelial vein inflammation may be related to the timing, dose and duration of MgSO4 administration. Further in-vivo studies are necessary to determine whether MgSO4 prevents CP by dampening the excitatory-inflammation through inhibition of P2X7R in the fetus.
Acknowledgments and Statement of Financial Support:
Integrated Research Center for Fetal Medicine Fund
Footnotes
Conflict of Interest: All authors have no conflicts of interests to disclose.
Presented orally at the 65th Annual Scientific Meeting of the Society of Reproductive Immunology, San Diego, CA, March 6-10, 2018.
Category of Study: Basic science
References
- 1.Burd I, Balakrishnan B, Kannan S 2012. Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am J Reprod Immunol 67:287–294. [DOI] [PubMed] [Google Scholar]
- 2.Zarrei M, Fehlings DL, Mawjee K, et al. 2017. De novo and rare inherited copy-number variations in the hemiplegic form of cerebral palsy. Genet Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuban KC, O'Shea TM, Allred EN, et al. Investigators ES 2014. Systemic inflammation and cerebral palsy risk in extremely preterm infants. J Child Neurol 29:1692–1698.24646503 [Google Scholar]
- 4.Cordeiro CN, Tsimis M, Burd I 2015. Infections and Brain Development. Obstet Gynecol Surv 70:644–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leitner K, Al Shammary M, McLane M, Johnston MV, Elovitz MA, Burd I 2014. IL-1 receptor blockade prevents fetal cortical brain injury but not preterm birth in a mouse model of inflammation-induced preterm birth and perinatal brain injury. Am J Reprod Immunol 71:418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rouse DJ, Hirtz DG, Thom E, et al. Eunice Kennedy Shriver NM-FMUN 2008. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med 359:895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stigson L, Kjellmer I 1997. Serum levels of magnesium at birth related to complications of immaturity. Acta Paediatr 86:991–994. [DOI] [PubMed] [Google Scholar]
- 8.Hirtz DG, Nelson K 1998. Magnesium sulfate and cerebral palsy in premature infants. Curr Opin Pediatr 10:131–137. [DOI] [PubMed] [Google Scholar]
- 9.Almousa LA, Salter AM, Langley-Evans SC 2018. Varying magnesium concentration elicits changes in inflammatory response in human umbilical vein endothelial cells (HUVECs). Magnes Res 31:99–109. [DOI] [PubMed] [Google Scholar]
- 10.Pappas A, Kendrick DE, Shankaran S, et al. Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N 2014. Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates. JAMA Pediatr 168:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards JM, Edwards LE, Swamy GK, Grotegut CA 2017. Magnesium sulfate for neuroprotection in the setting of chorioamnionitis. J Matern Fetal Neonatal Med:1–8. [DOI] [PubMed] [Google Scholar]
- 12.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM 2015. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol 213:S29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S 2017. The P2X7 Receptor in Infection and Inflammation. Immunity 47:15–31. [DOI] [PubMed] [Google Scholar]
- 14.Bartlett R, Stokes L, Sluyter R 2014. The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol Rev 66:638–675. [DOI] [PubMed] [Google Scholar]
- 15.Wilson HL, Varcoe RW, Stokes L, et al. 2007. P2X receptor characterization and IL-1/IL-1Ra release from human endothelial cells. Br J Pharmacol 151:115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsimis ME, Lei J, Rosenzweig JM, et al. 2017. P2X7 receptor blockade prevents preterm birth and perinatal brain injury in a mouse model of intrauterine inflammation. Biol Reprod 97:230–239. [DOI] [PubMed] [Google Scholar]
- 17.Younge N, Goldstein RF, Bann CM, et al. Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N 2017. Survival and Neurodevelopmental Outcomes among Periviable Infants. N Engl J Med 376:617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durkin MS, Benedict RE, Christensen D, et al. 2016. Prevalence of Cerebral Palsy among 8-Year-Old Children in 2010 and Preliminary Evidence of Trends in Its Relationship to Low Birthweight. Paediatr Perinat Epidemiol 30:496–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brookfield KF, Elkomy M, Su F, Drover DR, Carvalho B 2017. Optimization of Maternal Magnesium Sulfate Administration for Fetal Neuroprotection: Application of a Prospectively Constructed Pharmacokinetic Model to the BEAM Cohort. J Clin Pharmacol 57:1419–1424. [DOI] [PubMed] [Google Scholar]
- 20.Edwards JM, Edwards LE, Swamy GK, Grotegut CA 2018. Effect of Cord Blood Magnesium Level at Birth on Non-neurologic Neonatal Outcomes. Am J Perinatol 36: 3–7 [DOI] [PubMed] [Google Scholar]
- 21.Park JS, Park CW, Lockwood CJ, Norwitz ER 2005. Role of cytokines in preterm labor and birth. Minerva Ginecol 57:349–366. [PubMed] [Google Scholar]
- 22.D'Alquen D, Kramer BW, Seidenspinner S, et al. 2005. Activation of umbilical cord endothelial cells and fetal inflammatory response in preterm infants with chorioamnionitis and funisitis. Pediatr Res 57:263–269. [DOI] [PubMed] [Google Scholar]
- 23.Xing YL, Zhou Z, Agula, et al. 2012. Protocatechuic aldehyde inhibits lipopolysaccharide-induced human umbilical vein endothelial cell apoptosis via regulation of caspase-3. Phytother Res 26:1334–1341. [DOI] [PubMed] [Google Scholar]
- 24.Lee TH, Chang J, Kim BM 2014. Saikosaponin C inhibits lipopolysaccharide-induced apoptosis by suppressing caspase-3 activation and subsequent degradation of focal adhesion kinase in human umbilical vein endothelial cells. Biochem Biophys Res Commun 445:615–621. [DOI] [PubMed] [Google Scholar]
- 25.2016. Committee Opinion No 652: Magnesium Sulfate Use in Obstetrics. Obstet Gynecol 127:e52–53. [DOI] [PubMed] [Google Scholar]
- 26.Guzman-Aranguez A, Perez de Lara MJ, Pintor J 2017. Hyperosmotic stress induces ATP release and changes in P2X7 receptor levels in human corneal and conjunctival epithelial cells. Purinergic Signal 13:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maier JA, Malpuech-Brugere C, Zimowska W, Rayssiguier Y, Mazur A 2004. Low magnesium promotes endothelial cell dysfunction: implications for atherosclerosis, inflammation and thrombosis. Biochim Biophys Acta 1689:13–21. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa M, Oono H, Nishio A 2001. Enhanced production of IL-1beta and IL-6 following endotoxin challenge in rats with dietary magnesium deficiency. J Vet Med Sci 63:467–469. [DOI] [PubMed] [Google Scholar]
- 29.Rochelson B, Dowling O, Schwartz N, Metz CN 2007. Magnesium sulfate suppresses inflammatory responses by human umbilical vein endothelial cells (HuVECs) through the NFkappaB pathway. J Reprod Immunol 73:101–107. [DOI] [PubMed] [Google Scholar]
- 30.Burd I, Breen K, Friedman A, Chai J, Elovitz MA 2010. Magnesium sulfate reduces inflammation-associated brain injury in fetal mice. Am J Obstet Gynecol 202:292 e291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujiwara M, Ohbori K, Ohishi A, Nishida K, Uozumi Y, Nagasawa K 2017. Species Difference in Sensitivity of Human and Mouse P2X7 Receptors to Inhibitory Effects of Divalent Metal Cations. Biol Pharm Bull 40:375–380. [DOI] [PubMed] [Google Scholar]
- 32.Virginio C, Church D, North RA, Surprenant A 1997. Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology 36:1285–1294. [DOI] [PubMed] [Google Scholar]
- 33.Torigoe G, Nagao M, Tanabe N, et al. 2017. PYK2 mediates BzATP-induced extracellular matrix proteins synthesis. Biochem Biophys Res Commun 494:663–667. [DOI] [PubMed] [Google Scholar]
- 34.Jiang LH, Mackenzie AB, North RA, Surprenant A 2000. Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Mol Pharmacol 58:82–88. [PubMed] [Google Scholar]
- 35.Beigi RD, Kertesy SB, Aquilina G, Dubyak GR 2003. Oxidized ATP (oATP) attenuates proinflammatory signaling via P2 receptor-independent mechanisms. Br J Pharmacol 140:507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valdecantos P, Briones R, Moya P, Germain A, Huidobro-Toro JP 2003. Pharmacological identification of P2X1, P2X4 and P2X7 nucleotide receptors in the smooth muscles of human umbilical cord and chorionic blood vessels. Placenta 24:17–26. [DOI] [PubMed] [Google Scholar]
- 37.Green JP, Souilhol C, Xanthis I, et al. 2017. Atheroprone flow activates inflammation via endothelial ATP-dependent P2X7-p38 signalling. Cardiovasc Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albalawi F, Lu W, Beckel JM, Lim JC, McCaughey SA, Mitchell CH 2017. The P2X7 Receptor Primes IL-1beta and the NLRP3 Inflammasome in Astrocytes Exposed to Mechanical Strain. Front Cell Neurosci 11:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang LH 2009. Inhibition of P2X(7) receptors by divalent cations: old action and new insight. Eur Biophys J 38:339–346. [DOI] [PubMed] [Google Scholar]