Abstract
Schizophrenia is a neurocognitive illness characterized by behavioral and neural impairments in both early auditory processing and higher order verbal working memory. Previously we have shown intervention-specific cognitive performance improvements with computerized, targeted training of auditory processing (AT) when compared to a computer games (CG) control intervention that emphasized visual processing. To investigate spatiotemporal changes in patterns of neural activity specific to the AT intervention, the current study used magnetoencephalography (MEG) imaging to derive induced high gamma band oscillations (HGO) during auditory encoding, before and after 50 hours (~10 weeks) of exposure to either the AT or CG intervention. During stimulus encoding, AT intervention-specific changes in high gamma activity occurred in left middle frontal and left middle-superior temporal cortices. In contrast, CG intervention-specific changes were observed in right medial frontal and supramarginal gyri during stimulus encoding, and in bilateral temporal cortices during response preparation. These data reveal that, in schizophrenia, intensive exposure to either training of auditory processing or exposure to visuospatial activities produces significant but complementary patterns of cortical function plasticity within a distributed fronto-temporal network. These results underscore the importance of delineating the specific neuroplastic effects of targeted behavioral interventions to ensure desired neurophysiological changes and avoid unintended consequences on neural system functioning.
Keywords: Cognitive training, neuroplasticity, magnetoencephalography, linguistic processing
1. INTRODUCTION
Behavioral and imaging evidence indicates that schizophrenia is characterized by widespread disturbances in neural systems that subserve auditory processing and verbal memory, at both early and late stages of information processing (Umbricht and Krijes, 2005; Hirano et al, 2008; Kasai et al, 2002; Dale et al, 2010; Force et al, 2008; Leitman et al, 2005; Weiss and Heckers, 2001; Ragland et al, 2004; Kravariti et al, 2009; Mesholam-Gately et al, 2009). Because these impairments likely stem from abnormal neural operations linking early auditory representations with higher-order processes, targeting early auditory processing and higher-order auditory working memory operations with cognitive training should improve verbal memory in schizophrenia (Adcock et al, 2009; Vinogradov et al, 2012). This type of targeted training drives cortical changes and behavioral improvements in verbal learning and memory (VLM) as well as general cognition in people at both early and later stages of illness (Fisher et al, 2009; 2015; Loewy et al, 2016; Popov et al, 2011; Subramaniam et al, 2014).
An unexpected finding of these studies, from three independent samples, is that the “control” group, experiencing visuospatial computer games, shows a temporary decrease in VLM (Fisher et al, 2009; 2015; 2016; Loewy et al, 2016), indicating that this condition has its own cognitive effects. We (Fisher et al, 2015; Loewy et al, 2016) have speculated that poorer VLM performance after the control condition may be the result of “competitive interference”, whereby extended intensive engagement with visuospatial tasks draws away from neural resources that process auditory information, or imposes compensatory consequences. Competitive interference effects may be pronounced in individuals with atypical neural systems, as suggested in both animals (Mao et al, 2011) and humans (Bernstein et al, 2014).
It is critically important to understand neural consequences, or learning-dependent plasticity, of a specific regimen of intensive behavioral exposure to determine the functional implications of different methods of training. This may become especially important as cognitive training becomes utilized as a “circuit activation” strategy, producing neuromodulation to improve a particular cognitive function (Nienow et al, 2016). Understanding intervention-specific neural response patterns may also provide information about underlying pathophysiologic mechanisms that give rise to behavioral patterns occurring before and during different treatment methods.
We used magnetoencephalography (MEG) to examine intervention-related differences in neural oscillatory activity during an untrained auditory working memory task. We focused on high gamma oscillations (HGO) because healthy individuals showed elevated HGO in premotor cortex and along superior temporal gyrus (STG) during successful performance on this task, as well as better coordination of HGO between Broca’s Area and left planum temporale as working memory demands increased (Herman et al, 2013). Further, in this patient population, gamma band abnormalities are related to auditory processing fidelity (Dale et al, 2016), working memory deficits (Haenschel et al 2009; Senkowski and Gallinat, 2015) and symptom severity (Ford et al, 2007; Gallinat et al, 2004; Grutzner et al, 2013; Spencer et al, 2004).
Participants underwent MEG recording while performing the task both before and after 50 hours of targeted training of auditory processing (AT) or 50 hours of visuospatial games (CG). Because these activities show different patterns of improvement on behavioral tasks that index auditory and visual function (Fisher et al, 2009), we expected to observe intervention-specific patterns of HGO plasticity: activation of cortical areas underlying auditory processing and speech encoding should benefit from AT exposure (e.g. Plakke and Romanski, 2014; Binder et al, 1994), but not CG activities that exercise parieto-occipital visual systems (e.g. Kravitz et al, 2011).
2. METHODS
2.1. Recruitment
This report is part of a larger clinical trial investigating efficacy of targeted cognitive training in patients with schizophrenia (ClinicalTrials.gov, ), and includes a subset of participants who were willing and safely able to perform additional tasks during magnetoencephalography (MEG) and magnetic resonance imaging (MRI, see also Adcock et al, 2009; Dale et al, 2010; 2016; Herman et al, 2013; Hinkley et al, 2010; 2011). Chronically ill, clinically stable patients with Axis I diagnosis of schizophrenia (Structured Clinical Interview for DSM-IV, First et al, 2002) were recruited from mental health treatment centers in the San Francisco Bay Area. Participants were in good general physical health, between 18 and 60 years old, had outpatient status for at least 3 months prior to and throughout the study; maintained on stable medication (dosage changes less than 10%); and endorsed English as their first language. Individuals meeting criterion for substance dependence or current substance abuse were excluded from enrollment. Recreational use of marijuana and nicotine was not exclusionary, although participants were advised that intoxication during training or assessments was not allowed. Full recruitment, medication, and clinical assessment details are available in Fisher et al (2009), and summarized for the imaging cohort in section 1 of Supplemental Materials. The trial was carried out in accordance with The Declaration of Helsinki, and reviewed by the Institutional Review Board at the University of California, San Francisco.
2.2. Neuropsychological assessment and group assignment
After study procedures were explained, participants gave written informed consent and underwent initial behavioral and clinical assessments. Measures recommended by the Measurement and Treatment Research to Improve Cognition in Schizophrenia were used to evaluate cognition (MATRICS, Nuechterlein et al, 2008), including the Hopkins Verbal Learning Test-Revised to assess verbal learning and memory (VLM). Symptom severity was assessed via Positive and Negative Syndrome Scales (PANSS, Kay et al, 1987). MATRICS measures, score transformations, and behavioral results from the larger clinical trial are reported elsewhere (Fisher et al, 2009; 2010; 2015).
Patients with schizophrenia were stratified by age, education, gender, and symptom severity, then randomized to either AT or CG conditions within the parent trial. AT involved computer-based auditory processing exercises focusing on fundamental aspects of language comprehension (e.g. changes in frequency, phoneme recognition and memory), embedded within a suite of individually-adaptive and increasingly-complex auditory working memory and verbal learning tasks, delivered under a frequent reward schedule. Patients in the CG group played 16 different visually engaging commercial computer games (e.g., visuospatial puzzles, clue-gathering, pinball). Patients completed 50 hours of either AT or CG over an approximately 10-week period and, along with assessment personnel, were blind to group assignment (c.f. Supplemental materials). Compliance, via completion of the exercises, was verified through the software and by study staff who monitored attendance and engagement, answered questions participants had about the exercises, and logged their total intervention time. Interventions were reported as equally enjoyable in the parent trial (Fisher et al, 2009) via the Intrinsic Motivation Inventory (Deci et al, 1994). Effort was equally rewarded via attendance-based payment schedules. Additional detail regarding randomization into, and delivery of, interventions is found in Supplemental Materials and Fisher et al (2009).
Prior to and after the 10-week intervention period, participants underwent MEG and MRI imaging. Of several auditory tasks performed during MEG, we report results from an auditory working memory task. Thirty-nine patients (22 AT, 17 CG) completed two sessions of MEG and one session of MR imaging. The final sample included 16 AT and 14 CG participants, as 9 patients (6 AT, 3 CG) were removed due to poor MEG data quality (c.f. section 2.3, Figure S1). Table 1 lists demographic characteristics, medication status, baseline VLM and symptom scores for each group.
Table 1. Demographic and clinical profiles for each intervention group.
Gender ratio, age, education and pre-intervention clinical measures for each group are presented, with the statistical results of univariate ANOVA testing for group differences on each measure prior to intervention. Baseline VLM and PANSS z-scores were converted using age-stratified normative data published by test authors (c.f. Fisher et al, 2009). The standard error of the mean for each measure, aside from gender ratio, is provided in parentheses.
| AT | CG | t | P | |
|---|---|---|---|---|
| Number | 16 | 14 | ||
| Male:Female | 12:4 | 10:4 | ||
| Age in years | 33.4 (2.7) | 40.5 (2.9) | −1.8 | .08 |
| Education | 14.1 (.5) | 13.6 (.5) | .68 | .50 |
| Age at Onset | 18.7 (1.2)a | 22.2 (1.2) | −2.1 | .05 |
| IQ | 107.3 (3.6) | 107.6 (3.8) | −.05 | .96 |
| Medicationb | 206.5 (57) | 331.9 (62) | −1.5 | .15 |
| Baseline VLM | −2.1 (.3) | −1.7 (.3) | −.75 | .46 |
| PANSS-Pos | 3.1 (.3) | 2.7 (.3) | .69 | .50 |
| PANSS-Neg | 2.7 (.3) | 2.3 (.3) | 1.2 | .24 |
| PANSS-Total | 2.5 (.1) | 2.4 (.2) | .59 | .56 |
Age at Onset unavailable for one participant in AT.
Medication reported as the group mean (se) in chlorpromazine equivalents.
2.3. MEG Data Acquisition and Processing
A 275-channel sensor array in a magnetically shielded room recorded MEG (Omega 2000, CTF Systems Inc./VSM Medtech, Ltd. Port Coquitlam, BC, Canada; c.f. Ahonen et al, 1993; Vrba and Robinson, 2001). To correct for distant magnetic field disturbance, 29 reference sensors were used to calculate a synthetic third-order gradiometer (Vrba and Robinson, 2001; Weinberg et al, 1984). MEG was acquired at a 1200Hz sampling rate and 0.001–300Hz filtering. Radio-emitting coils on nasion, left, and right ears (1 cm anterior to the periauricular point in the plane of the nasion) monitored head position relative to the sensor array during recording, and served as fiducial landmarks for co-registration to structural images. Scans were repeated for any movement greater than 2 cm.
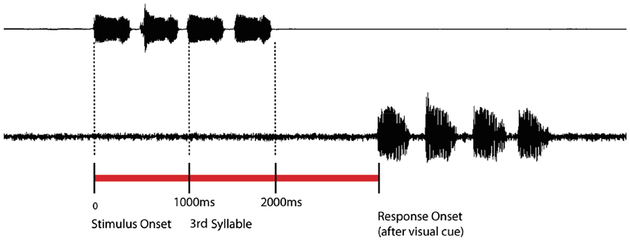
The auditory working memory probe task consisted of two or four syllables involving mixtures of /ba/, /pa/, and /da/, pre-recorded by a female speaker; each lasting 470 msec with 50 msec inter-stimulus intervals (Figure 1). Participants were instructed to listen to, remember, and verbally repeat the syllables after a visual cue (50 to 150 msec variable offset-to-cue delay). A run included 160 trials of 7 s each, with 80 four-phoneme trials randomly interleaved with 80 two-phoneme trials. Two staff members listened to responses to index correct trials.
Figure 1. Schematic of the Auditory Working Memory task.
Either two or four syllables (/ba/, /da/, or /pa/, each syllable pair consisting of different syllables) of roughly 470 msec each, separated by 50 msec were presented on each trial. Figure 1 illustrates stimulus (top) and response (bottom) events using a digitized pattern of stimuli and vocalizations for a 4-syllable trial. Participants were instructed to begin a verbal response after a visual cue appeared on screen at a variable trial-onset-to-cue delay between 2050 and 2150 msec, with up to 3 sec to complete a response after the visual cue. Responses were collected by microphone, digitized within the MEG dataset in real-time, and evaluated by staff for accuracy of syllable repetition.
Epochs from −1s to 7s relative to first syllable onset were submitted to analyses, including markers for stimulus onset, verbal response, and number of phonemes. Epochs were rejected with activity greater than 1.5 pT, an incorrect response, or in which the participant spoke during stimulus presentation. Participants were included in analyses if more than 70 epochs remained after artifact rejection and structural MRI data was available for co-registration.
2.4. Data analysis
2.4.1. MRI acquisition and co-registration with MEG
To enable neural source localization, high-resolution anatomical images were acquired from each individual on a 3 Tesla General Electric Signa LX 15 scanner, utilizing 3D magnetization prepared rapid gradient echo MRI (160 1-mm slices; field of view = 260 mm, matrix = 256 × 256, echo time = 6 msec, repetition time = 35 msec, flip angle = 30°). T1-weighted images were co-registered with MEG data via fiducial landmarks, and spatially normalized to the Montreal Neurological Institute template (MNI, via SPM2: fil.ion.co.uk/spm2, c.f. Friston et al, 1995). A multiple spheres head model was calculated using the position of each sensor relative to the volume within CTF software.
2.4.2. MEG source reconstruction
Sources of neural activity oscillating at the high gamma frequency band (63–117 Hz) were localized to 5 mm voxel regions across the cortex using a time-frequency-optimized spatially adaptive filter implemented in the Neurodynamic Utility Toolbox for MEG (NUTMEG: http://bil.ucsf.edu; Dalal et al, 2008; 2011) via the shared computing cluster at the California Institute for Quantitative Biomedical Research (www.qb3.org). In order to avoid mislocalizations from temporally-correlated auditory sources, data from sensors above each hemisphere were analyzed separately in stimulus-locked analyses.
The contrast utilized to derive voxel-by-voxel neural changes in an individual’s data depended on the hypothesis to be tested. Separate time-frequency analyses were performed to examine: 1) effect of intervention on neural activity associated with accurate stimulus processing and encoding, via stimulus-locked HGO during the first two phonemes, and 2) effects of intervention on neural systems associated with retrieval of auditory representations and response preparation, via response-locked HGO during the 850 msec preceding each correct verbal response. Pseudo F-values reflecting stimulus- or response-locked activity indexed levels of induced HGO within each cortical voxel for each participant. These noise-corrected pseudo-F ratios were assessed in 100 msec windows sliding at 25 msec intervals post-stimulus or pre-response, relative to a static 100 msec control period that started 500 msec prior to first syllable onset.
2.4.3. Group averaging and statistical analysis of MEG data
The cortical map of pseudo-F values was spatially normalized to the MNI template using the same normalization parameters as for structural images. Session-related F-values were calculated and saved for each participant by comparing pseudo-F values from Session Two to those from Session One in within-group analyses. These session-related F-values were then submitted to between-group analyses.
Voxel-by-voxel within-group changes and between-group differences were assessed using statistical non-parametric methods (SnPM; Singh et al, 2003), whereby three-dimensional average and variance maps across subjects were calculated at each time point and smoothed with a 20×20×20mm3 Gaussian kernel (Dalal et al, 2008). From this map, pseudo-t statistics evaluated the magnitude of the contrast at each voxel and time. Voxel labels were permuted to create a T-distribution map for within- and between-group contrasts (2N permutations, N = number of subjects, up to 10,000 permutations). Each voxel’s t-value was evaluated using 2N degrees of freedom to determine the corresponding p-value. To de-emphasize differences arising from minor variations in spatial extent or duration of activation, additional criteria were used to threshold each voxel. First, a spatial requirement of at least 26 contiguous voxels with p<.005 was applied to each voxel. Second, spatially-thresholded clusters of voxels were required to persist for two or more 25 msec time windows.
2.4.4. Cluster Identification and Extraction
Clusters of voxels with significant between-group difference under spatiotemporal thresholding of p<.005 were identified by MNI coordinates and assigned a region label from the SPM template. Within cluster boundaries, the voxel with maximum absolute t-value was obtained over time windows showing significant activity. This coordinate, with its associated time window and p value, was utilized as the cluster focal point in further analyses and reporting of results. Magnitude and direction of within-group changes in Session Two relative to Session One at each cluster was determined by querying the voxel with maximum absolute t-value in the within-group contrast, constrained within 2 cm of the between-group focal point and in its time range.
2.5. Statistical evaluation of behavioral data
Session-related and Group-related changes in task performance and clinical measures were assessed using repeated-measures analysis of variance (ANOVA, IBM SPSS Statistics for OSX, Version 23.0. Armonk, NY: IBM Corp.).
3. RESULTS
3.1. Effects of intervention on task performance and cognitive measures
Accuracy of syllable repetition during the MEG task was similar across groups and sessions (Sessions One and Two, respectively: AT=.76 and .76, n=16; CG=.71 and .76, n=13; standard error=.04 for all values). In the larger study from which participants were recruited, session-related improvement in General Cognition, Non-verbal working memory, Visual Learning and Problem Solving was found regardless of group assignment, with differential improvement observed in AT relative to CG in measures of General Cognition and VLM (Fisher et al, 2009). The subgroup of MEG participants, however, showed no significant group by session effects in neuropsychological measures, although a trend level interaction for VLM mirrored the previously reported effect (Mean change AT=.55, CG=-.29, F[1, 25]=3.457, p=.075).
3.2. Stimulus-locked changes in high gamma during the task
Location and timing of intervention-related HGO differed between groups for stimulus-locked data, shown in Figure 2. Six clusters of significant group difference in HGO change were revealed during presentation of the first two syllables, presumably reflecting differential patterns of cortical plasticity effected by the intervention experience.
Figure 2. Intervention-related differences in HGO change during auditory processing.

The time course of statistically-significant differential activation post-stimulus between the groups is depicted along a timeline representing the first 1 sec in the trial (first syllable pair of 2-syllable and 4-syllable trials). Red clusters depict elevation of HGO obtained in the AT group relative to the CG group, in voxels where positive t-values exceeded the p<.005 threshold over 26 contiguous voxels and 2 consecutive time windows. Blue clusters represent elevation of HGO in the CG relative to AT group using the same significance thresholding as above for negative t-values. Cortical renderings represent the time during which the absolute maximum t-value for the difference between the two interventions was obtained, while duration of statistically-significant differences in HGO is represented via length of corresponding brackets along the timeline. Table 2 provides the maximal or minimal t-value and its location in MNI coordinates, as well as the specific time window designations for each cluster shown.
After 50 hours of AT, HGO increases in left hemisphere during the first syllable presentation and just prior to second syllable offset were observed relative to that of the CG group. In contrast, CG showed enhanced HGO in right hemisphere at first syllable onset and well into second stimulus presentation. Left and right locations exhibiting intervention-related neuroplasticity are largely confined to auditory association cortex within posterior STG, middle temporal gyrus (MTG), supramarginal gyrus (SMG), and regions of frontal cortex associated with cognitive control and executive function. Table 2 details the location and time of each significant cluster displayed in Figure 2.
Table 2. Foci of intervention-related difference in HGO change during auditory processing.
The location, time window, duration, and voxel-wise extent of each cluster of intervention-related difference in stimulus-locked HGO are shown in Table 2. Voxels surviving the spatiotemporal thresholding at p<.005, corresponding to those shown in Figure 2, were tabulated and their associated anatomic labels listed. The voxel location and single time window where the absolute maximal t-value was identified in between-group analyses are listed in the MNI column, with associated t- and p-values to the right. The two right-most columns display the maximum or minimum t-value, as appropriate, obtained from the within-group contrasts in a 1.5 cm3 region around the between-group voxel at either a significance level of p<.005 or p<.05.
| Regions with Between-group clusters | Time Range, mseca | MNIb (mseca) | Max t/p | # voxels | AT t-value | CG t-value |
|---|---|---|---|---|---|---|
| Left Middle and Superior Frontal Gyri (BA9, BA10) | 125 to 175 | −35, 60, 15 (150) | 4.25/.0002 | 52 | 4.26c | −2.51d |
| Left Insula/Putamen | 200 to 225 | −30, 15, 0 (225) | 3.03/.0017 | 22 | 2.35d | −2.71d |
| Right Medial Frontal (BA10, BA11) | 775 to 950 | 20, 50, −30 (800) | −4.75/.0018 | 158 | −3.38d | 4.13d |
| Right Supramarginal Gyrus | ||||||
| 925 to end | 60 −45 55 (975) | −3.93/.0009 | 43 | ns | 3.57d | |
| Middle Temporal | 950 to end | −60, −55, 15 (975) | 3.35/.0009 | 9 | 2.99d | −3.28d |
Time reported as the start of the relevant 25 msec window.
MNI coordinate reported where the absolute maximal t-value occurred in the cluster of active voxels.
Within-group t-value is significant at p < .005.
Within-group t-value is significant at p < .05.
Within-group t values, reported in the right two columns of Table 2, reveal significant between-group clusters arise via asymmetric, and in some cases opposing, patterns of HGO change for AT and CG groups. That is, left hemisphere clusters within frontal and temporal cortex show within-group elevations of HGO for AT, producing positive t-values, while negative t-values were obtained within the CG group, reflecting reductions in HGO post-intervention. In contrast, right medial frontal cortex decreases in HGO are observed within the AT group, while increases appear for CG. In right SMG, at both early and late time periods, HGO increases are less robust for AT than for CG groups, though both show neuroplasticity between Session One and Session Two. Direct comparison of HGO between groups during Session One did not reveal any statistically-significant differences, thus the two patient groups had relatively equivalent activity prior to intervention. Regions with significant session-related changes in HGO obtained in within-group contrasts, independent of focal points obtained from between-group analyses, are listed in Supplementary Tables 1 (AT) and 2 (CG).
3.3. Response-locked changes in high gamma during the syllable identification task
Figure 3 illustrates intervention-related HGO differences between groups in Session Two relative to Session One, for the period prior to the verbal response. Relative to AT, the CG group showed greater HGO change between Sessions in locations within both left and right hemispheres.
Figure 3. Intervention-related differences in HGO change during verbal response preparation.
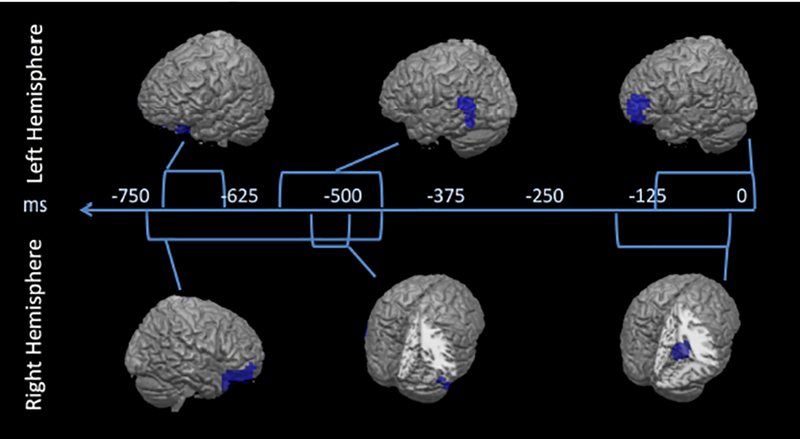
The time course of significant differential activation between the two intervention groups prior to response production is depicted along a timeline representing the 850 msec prior to vocalization. Between-group clusters of significant HGO change were found as elevation of HGO in the CG relative to AT group (negative t-values), at a spatiotemporal threshold of p<.005. Each cortical rendering represents the time during which the absolute maximum t-value for the difference between the two interventions was obtained, while the duration of the statistically-significant HGO difference is represented in length of corresponding brackets along the timeline. Table 3 provides the t-values and MNI coordinates for each cluster depicted in Figure 3.
Compared to that of AT, HGO changes in the CG group occurred early in both left and right PFC, then left temporal auditory association regions (posterior STG and inferior temporal gyrus, ITG), followed by left MFG and right parahippocampus, extending superiorly into posterior cingulate. Table 3 provides peak locations and times of these changes in HGO, as well as within- group t-values for each cluster.
Table 3. Foci of intervention-related difference in HGO change during response preparation.
The location, time window, duration, and voxel-wise extent of each cluster of intervention-related difference in response-locked HGO are shown in Table 3. Voxels surviving the spatiotemporal thresholding at p<.005, corresponding to those shown in Figure 3, were tabulated and their associated anatomic labels listed. The voxel location and single time window where the absolute maximal t-value was identified in between-group analyses are listed in the MNI column, with associated t- and p-values to the right. The two right-most columns display the maximum or minimum t-value, as appropriate, obtained from the within-group contrasts in a 1.5 cm3 region around the between-group voxel at either a significance level of p<.005 or p<.05.
| Regions with Between-group clusters | Time Range, mseca | MNIb (mseca) | Max t/p | # voxel | AT t-value | CG t-value |
|---|---|---|---|---|---|---|
| Right and Left Anterior Middle Frontal Gyrus (BA46, 11, 10) | ||||||
| −125 to end | −60, 55, 5 (0) | −5.33/.0001 | 113 | −2.25d | 3.39d | |
| Right Parahippocampus, Cingulate (BA30) | −175 to −50 | 15, −45, 0 (−75) | −3.16/.0005 | 52 | ns | 2.11d |
| Left Posterior Temporal (BA37, 39) | −575 to −475 | −70, −75, 20 (-500) | −5.89/.0004 | 76 | ns | 2.76d |
| Right Inferior Temporal (BA37) | −525 to −500 | 70, −60, −25 (−525) | −3.97/.0004 | 18 | 1.68d | 4.01c |
Time reported as the start of the relevant 25 msec window.
MNI coordinate reported where the absolute maximal t-value occurred in the cluster of active voxels.
Within-group t-value is significant at p < .005.
Within-group t-value is significant at p < .05.
Within the AT group, t-values reflecting HGO change in left MFG indicated reductions in, or suppression of, HGO in post- relative to pre-training, perhaps because verbal response initiation requires less effortful cognitive control or has more efficient spatiotemporal activation after experiencing training of auditory processing. Curiously, the AT group showed no significant intervention-related changes in left posterior temporal regions during response preparation, while CG appears to show elevated HGO in temporal cortex, roughly 500 msec prior to verbalization, at a lower statistical threshold.
4. DISCUSSION
4.1. Summary of findings
In participants with schizophrenia, we compared intervention-specific patterns of brain plasticity via changes in induced neural oscillations within auditory encoding and working memory networks. We found that 50 hours of auditory training enhanced HGO during auditory stimulus encoding, relative to pre-intervention levels, within a left hemisphere-dominant, fronto-temporal network known to play a role in auditory processing and speech encoding (c.f. Hickock and Poeppel, 2007; Friederici, 2012; Price, 2012); this enhancement was not associated with a significant change in task performance and thus not due to changes in overall ability to perform the task. In contrast, the equivalent duration of visually-intensive computer games enhanced HGO during stimulus encoding within regions of right hemisphere that are not traditionally involved in language processing, also not due to changes in ability to perform the task. Below, we discuss the broader implications of our findings on intervention-specific neuroplasticity and the potential for competitive interference among auditory and visual networks.
4.2. Intervention-specific cortical neuroplasticity during auditory working memory
Both AT and CG regimens provide engagement with, and emphasis on, different perceptual and higher order cognitive processes that promote changes in different cortical systems when probed during the auditory working memory task. During stimulus encoding, the AT group revealed enhanced HGO in left PFC at 150 msec post-syllable-onset in regions encompassing Brodmann’s Areas 9 and 10, suggesting more efficient fronto-temporal perceptual control (Miller and Cohen, 2001). Additionally, AT exhibited enhanced HGO in left insula during first syllable presentation and, at second syllable termination, within the left STG and MTG auditory regions associated with phonological processing (Hickok and Poeppel, 2007; Oh et al, 2014; Vigneau et al, 2006). In contrast, the CG group enhanced HGO within right hemisphere during auditory stimulus encoding; areas associated with visual processing, spatial skills, and cognitive control (Aminoff et al, 2013; Vigneau et al, 2011). Toward the end of the stimulus encoding period, the CG group revealed relative HGO increases in right medial frontal and temporal-parietal regions associated with cognitive and/or inhibitory control of perceptual processing (Garavan et al, 1999; Munakata et al, 2011), with HGO suppression in left hemisphere regions associated with verbal auditory representations (Hickok and Poeppel, 2007).
The CG group also showed significant HGO enhancement during response preparation relative to the AT group, although with similar task performance, observed across regions associated with both cognitive control (bilateral MFG, Miller and Cohen, 2001) and auditory representations (left STG and right ITG: Vigneau et al, 2006; 2011). Additionally, CG showed elevated HGO within right parahippocampus and posterior cingulate cortex: areas that aid in establishing contextual associations between multimodal stimuli (Aminoff et al, 2013). Within-group analysis for the AT group during the response period showed HGO suppression in left PFC relative to baseline in areas thought to exert control over linguistic responses, perhaps due to increased efficiency in earlier stimulus processing.
In sum, comparison of patterns of HGO change suggests that AT participants show early differential increases in left STG and PFC activity, consistent with enhanced auditory processing and encoding, as well as early cognitive control. In contrast, those experiencing the CG condition exhibit right hemisphere activation consistent with relatively later engagement of cognitive control, but also later left linguistic enhancement that may indicate decreased efficiency in early stimulus processing and later retrieval of auditory representations. This decreased efficiency post-intervention potentially explains VLM decrements observed in the larger cohort (c.f. Fisher et al, 2009).
4.3. Competitive Interference between verbal and visual systems: Developing informed training regimens for schizophrenia
The current study examines neuroplasticity in patients with schizophrenia after experience that emphasizes either auditory or visual processing, using a probe task that relies on auditory working memory. We found that both “intervention” groups were capable of performing the auditory working memory task at similar accuracy levels, showing consistent levels of task performance over time, but relied on different cortical mechanisms. The CG condition promoted a reliance on bilateral frontal activity related to high-level cognitive properties of the task, with later-onset modulation of sensory regions, while the AT condition effected a rapid and enhanced sensory response favoring left hemisphere language and auditory processing areas.
In AT, enhanced HGO within a left fronto-temporal cortical network subserving auditory working memory may be supporting the improvement in verbal memory seen with this type of training (ie: Fisher et al, 2009; 2015; 2016; Loewy et al, 2016; Popov et al, 2011; 2012; 2015). In contrast, those experiencing CG showed plasticity in cortical regions not known for verbal processing, thus their successful performance may be due to increased reliance on executive and non-sensory processes. Essentially, extensive practice with visuospatial games and puzzles produced plasticity during auditory working memory, but in cortical networks not typically utilized for verbal processing. These regions are, however, associated with spatial and executive functioning, presumably exercised by the CG activities; this is generally consistent with increased prefrontal activation observed in fMRI studies of people with chronic schizophrenia after a range of learning experiences (Bor et al, 2011; Penades et al, 2013). This “compensatory” neural mechanism ultimately produced a similar outcome with respect to task performance during auditory working memory.
A large perceptual learning literature indicates that repeated exposure to perceptual information carries a high degree of specificity for trained features (Ahissar and Hochstein, 1997; Fiorentini and Bernardi, 1980; Irvine et al, 2000; c.f. Seitz and Watanabe, 2005 for review), may not transfer across modality (Alais and Cass, 2010), and can alter sensory cortex in a feature-specific manner (Schoups et al, 2001; Furmanski et al, 2004; Bao et al, 2004). However, this specificity depends on the type of training task used (Green et al, 2015), as cross-modal effects of perceptual training have also been reported: auditory information training can improve subsequent visual discrimination (Beer and Watanabe, 2009) and exposure to visually-presented verbal information alters auditory representations within superior temporal gyrus (Bonte et al, 2017). Thus, in some cases, training within one sensory modality can drive changes in neural processing within another; changes that are sometimes beneficial, and at other times deleterious, for tasks performed in that modality.
These mechanisms may be especially critical to consider when designing training regimens for illnesses characterized by inefficient sensory processing. For example, if some forms of schizophrenia are characterized by inefficiencies in auditory representational processes, then training heavily on visual perceptual and visuospatial processes may: 1) Prove beneficial for some by strengthening underlying adaptive visual cognitive processes to help develop “work-around” cognitive operations that compensate for impaired auditory and verbal processing; or 2) Prove deleterious for others by driving changes in visual systems that compete or interfere with already inefficient auditory systems. In the larger cohort from which the current participants were drawn, cross-modal interference appears to have been unidirectional, as visually-oriented behavioral measures were not significantly reduced in the AT group after training, while verbal learning was impaired after the intervention in the CG group (Fisher et al, 2009).
4.4. Limitations
Schizophrenia is a heterogeneous illness and although subject groups were matched on education, symptom severity, and medication regimens, other between-group differences may contribute to variance in cortical processing. While a no-contact chronic schizophrenia group could provide a neutral control to inform results specific to intervention versus treatment-as-usual, additional confounds related to differential study contact might be introduced. Ultimately, the intervention activities were designed for different purposes: AT to target and improve cortical underpinnings of auditory processing abilities, and CG to minimize reliance on auditory and linguistic processing while maintaining similar levels of computer activity and staff contact -- without explicitly targeting visuospatial competencies or cortical pathways. It is likely that the primary limitation of this study lies in the (unimodal) selection of MEG probe task: the auditory working memory probe task is expected to rely on processing pathways supported by AT, yet a similar visuospatial probe task was not run to examine the converse situation.
The complex longitudinal study design contributed to significant subject attrition, resulting in small sample sizes that limit generalizability of conclusions. Far fewer participants consented to additional sessions of MEG and MRI imaging than were in the cohort from which original cognitive results were obtained, so current data may be underpowered to reveal relationships between intervention-related neural plasticity and change in cognitive measures. Prior work has shown that delivery of AT in 18 and 19 patients can promote improvement of VLM and/or other cognitive domains examined via standardized instruments (Popov et al, 2011; Popov, et al, 2015), thus the current sample of 17 AT-assigned patients is likely at the lower end of appropriate sample size for this purpose.
While MEG is ideally suited to examine neural oscillations non-invasively, this modality is primarily sensitive to neocortical sources, especially in gyri, with reduced sensitivity to deeper midbrain structures. Furthermore, although reconstruction of time-frequency event related brain activity from MEG using time-frequency optimized adaptive spatial filtering algorithms have high-fidelity in comparison to other non-adaptive reconstruction algorithms, they are sensitive to impact from highly correlated brain source activity, noise and forward modeling errors.
Finally, the degree that illness duration and medications affect HGO and/or interact with exposure to the intervention activities (see Vinogradov et al, 2009 for effects of anticholinergic burden and response to cognitive training) are open questions. Chronic SZ patients with cumulative illness burden and exposure to antipsychotic treatments show different patterns of neural activity/connectivity, and neurotransmitter metabolites than drug-naïve patients experiencing a first-episode of psychosis (Griffa et al, 2019; Marsman et al, 2013). Our results do not address neurotransmitter/neuromodulator effects on HGO for AT and CG groups after intervention, and it is probable that they do not generalize to young patients in the earliest stage of schizophrenia. Multimodal imaging studies that relate measures of neural metabolites and HGO across the progression of disease would be an informative future direction.
4.5. Broader implications
While many questions remain, the current study shows that intensive engagement with computerized activities targeting different functional competencies can drive different patterns of cortical neuroplasticity during auditory working memory. This has implications for the design of cognitive training interventions; for understanding potential neurocognitive effects of recreational activities such as video game playing; and, perhaps, for a deeper understanding of complex pathophysiologic processes that contribute to schizophrenia.
Although the current data cannot elaborate on behavioral significance of different training regimens for people with schizophrenia at different stages of illness, or the neurotransmitter alterations resulting from experience with different regimens of training, it is clear that the visuospatial computer games experience produced a distinct pattern of neural change during auditory working memory from that seen in auditory training participants: one suggestive of less efficient auditory perceptual processing and a higher reliance on cognitive control mechanisms. This decreased efficiency may be a mechanism contributing to the poorer VLM performance we have previously reported in the computer games condition across three different study samples (Fisher et al, 2009; 2015; Loewy et al, 2016).
Supplementary Material
Acknowledgements
We thank Dr. Tracy Luks for technical assistance with structural imaging, and Mary Vertinski and Alex Genevsky for assistance with data collection.
Funding Body Disclosure
The work described in this manuscript was supported by National Institutes of Health R01DC004855 (SSN), R01DC010145 (SSN), R21NS076171 (SV) R01MH068725 (SV), the Howard Hughes Medical Institute Medical Fellows program (EGB), and by the San Francisco Department of Veterans Affairs Medical Center (SV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The cognitive training software used in this study was supplied to the senior author free of charge by Posit Science. Dr. Vinogradov is a paid consultant to Brain Plasticity Inc., a company with a commercial interest in cognitive training software. None of the other authors have any financial interest in Brain Plasticity Inc. or Posit Science. Dr. Vinogradov serves on advisory boards for Genentech and Hoffman-LaRoche, and is a consultant to Amgen, Genentech, and Hoffman-LaRoche. Drs. Brown, Herman, Dale, Hinkley, Fisher, Subramaniam, and Nagarajan report no potential conflicts of interest.
REFERENCES:
- Adcock RA, Dale C, Fisher M, Aldebot S, Genevesky A, Simpson GV, et al. (2009): When top-down meets bottom-up: auditory training enhances verbal memory in schizophrenia. Schizophr Bull 35: 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahissar M, Hochstein S (1997): Task difficulty and the specificity of perceptual learning. Nature 387: 401–406. [DOI] [PubMed] [Google Scholar]
- Ahonen AI, Hamalainen MS, Ilmoniemi RJ, Kajola MJ, Knuutila J, Simola JT, Vilkman VA (1993): Sampling theory for neuromagnetic detector arrays. IEEE Trans Biomed Eng 40(9):859–69. [DOI] [PubMed] [Google Scholar]
- Alais D, Cass J (2010): Multisensory perceptual learning of temporal order: audiovisual learning transfers to vision but not audition. PLoS One 5(6):e11283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff EM, Kveraga K, Bar M (2013): The role of the parahippocampal cortex in cognition. Trends Cogn Sci 17(8):379–390. doi: 10.1016/j.tics.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chang EF, Woods J, Merzenich MM (2004): Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nat. Neurosci 7(9): 974–981. [DOI] [PubMed] [Google Scholar]
- Beer AL, Watanabe T (2009): Specificity of auditory-guided visual perceptual learning suggests crossmodal plasticity in early visual cortex. Exp Brain Res 198(2–3):353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein LE, Eberhardt SP, Auer ET Jr (2014): Audiovisual spoken word training can promote or impede auditory-only perceptual learning: prelingually deafened adults with late-acquired cochlear implants versus normal hearing adults. Front Psychol 5(934) 1–20. doi: 10.3389/fpsyg.2014.00934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Rao SM, Hammeke TA, Yetkin FZ, Jesmanowicz A, Bandettini PA, Wong EC, Estkowski LD, Goldstein MD, et al. (1994). Functional magnetic resonance imaging of human auditory cortex, Annals of Neurology 35(6). [DOI] [PubMed] [Google Scholar]
- Bonte M, Correia JM, Keetels M, Vroomen J, Formisano E (2017): Reading-induced shifts of perceptual speech representations in auditory cortex. Sci Rep 7(1):5143. doi: 10.1038/s41598-017-05356-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor J, Brunelin J, d’Amato T, Costes N, Suaud-Chagny MF, Saoud M, Poulet E (2011): How can cognitive remediation therapy modulate brain activations in schizophrenia? An fMRI study. Psychiatry Res 192(3):160–6. doi: 10.1016/j.pscychresns.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Dalal SS, Guggisberg AG, Edwards E, Sekihara K, Findlay AM, Canolty RT, et al. (2008): Five-dimensional neuroimaging: localization of the time-frequency dynamics of cortical activity. Neuroimage 40: 1686–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal SS, Zumer JM, Guggisberg AG, Trumpis M, Wong DDE, Sekihara K, Nagarajan SS (2011): MEG/EEG Source Reconstruction, Statistical Evaluation, and Visualization with NUTMEG. Comput Intell Neurosci 2011:758973. doi: 10.1155/2011/758973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale CL, Brown EG, Fisher M, Herman AB, Dowling AF, Hinkley LB, et al. (2016): Auditory cortical plasticity drives training-induced cognitive changes in schizophrenia. Schizophr Bull 42(1): 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale CL, Findlay AM, Adcock RA, Vertinski M, Fisher M, Genevsky A, et al. (2010): Timing is everything: neural response dynamics during syllable processing and its relation to higher order cognition in schizophrenia and healthy comparison subjects. Int J Psychophysiol 75(2):183–193. doi: 10.1016/j.ijpsycho.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini A, Berardi N (1980): Perceptual learning specific for orientation and spatial frequency. Nature 287: 43–44. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (2002): Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fisher M, Holland C, Merzenich MM, Vinogradov S (2009): Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry 166: 805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Holland C, Subramaniam K, Vinogradov S, (2010): Neuroplasticity-based cognitive training in schizophrenia an interim report on the effects 6 months later. Schizophr Bull 36(4):869–79. doi: 10.1093/schbul/sbn170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Loewy R, Carter C, Lee A, Ragland JD, Niendam T et al. (2015): Neuroplasticity-based auditory training via laptop computer improves cognition in young individuals with recent onset schizophrenia. Schizophr Bull 41(1):250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Mellon SH, Wolkowitz O, Vinogradov S (2016): Neuroscience-informed auditory training in schizophrenia: a final report of the effects on cognition on serum brain-derived neurotrophic factor. Schizophr Res Cogn 3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force RB, Venables NC, Sponheim SR, (2008): An auditory processing abnormality specific to liability for schizophrenia. Schizophr Res 103: 298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Faustman WO, Mathalon DH (2007): Synch before you speak: auditory hallucinations in schizophrenia. Am J Psychiatry 164: 458–466. [DOI] [PubMed] [Google Scholar]
- Friederici AD (2012): The cortical language circuit: from auditory perception to sentence comprehension. Trends Cogn Sci 16(5):262–268. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Poline J, Frith C, Heather J, Frackowiak RSJ (1995): Spatial registration and normalization of images. Hum Brain Mapp 2:165–189 [Google Scholar]
- Furmanski CS, Schluppeck D, Engel SA (2004): Learning strengthens the response of primary visual cortex to simple patterns. Curr Biol 14(7): 573–578. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Winterer G, Herrmann CS, Senkowski D (2004): Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin Neurophysiol 115:1863–1874. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA (1999): Right Hhemispheric dominance of inhibitory control: An event-related functional MRI study. Proc Natl Acad Sci USA 96: 8301–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CS, Kattner F, Siegel MH, Kersten D, Schrater PR (2015): Differences in perceptual learning transfer as a function of training task. J Vis 15(10):5. [DOI] [PubMed] [Google Scholar]
- Griffa A, Baumann PS, Klauser P, Mullier E, Cleusix M, Jenni R, van den Heuvel MP, Do KQ, Conus P, Hagmann P (2019): Brain connectivity alterations in early psychosis: from clinical to neuroimaging staging. Transl Psychiatry 9(1):62. doi: 10.1038/s41398-019-0392-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grützner C, Wibral M, Sun L, Rivolta D, Singer W, Maurer K, Uhlhaas PJ (2013): Deficits in high- (>60 Hz) gamma-band oscillations during visual processing in schizophrenia. Front Hum Neurosci 7:88–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenschel C, Bittner RA, Waltz J, Haertling F, Wibral M, Singer W, et al. (2009): Cortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia. J Neurosci 29, 9481–9489. doi: 10.1523/JNEUROSCI.1428-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman AB, Houde JF, Vinogradov S, Nagarajan SS (2013): Parsing the phonological loop: activation timing in the dorsal speech stream determines accuracy in speech reproduction. J Neurosci 33: 5439–5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickock G, Peoppel D (2007): The cortical organization of speech processing. Nat Rev Neurosci 8: 393–402. [DOI] [PubMed] [Google Scholar]
- Hinkley LBN Owen JP, Fisher M, Findlay AM, Vinogradov S, Nagarajan SS (2010): Cognitive impairments in schizophrenia as assessed through activation and connectivity measures of magnetoencephalography. Front Hum Neurosci 3: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkley LBN, Vinogradov S, Guggisberg AG, Fisher M, Findlay AM, Nagarajan SS (2011): Clinical symptoms and alpha band resting-state functional connectivity iaging in patients with schizophrenia: implications for novel approaches to treatment. Biol Psychiatry 70(12): 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Hirano Y, Maekawa T, Obayashi C, Oribe N, Kuroki T, et al. (2008): Abnormal neural oscillatory activity to speech sounds in schizophrenia: a magnetoencephalography study. J Neurosci 28(19):4897–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine DR, Martin RL, Klimkeit E, Smith R (2000): Specificity of perceptual learning in a frequency discrimination task. J Acoust Soc Am 108(6):2964–8. [DOI] [PubMed] [Google Scholar]
- Kasai K, Nakagome K, Itoh K, Koshida I, Hata A, Iwanami A, Fukuda M, et al. (2002): Impaired cortical network for preattentive detection of change in speech sounds in schizophrenia: a high-resolution event-related potential study. Am J Psychiatry 159: 546–553. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA, (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13(2): 261–76. [DOI] [PubMed] [Google Scholar]
- Kravariti E, Morgan K, Fearon P, Zanelli JW, Lappin JM, Dazzan P, et al. (2009): Neuropsychological functioning in first-episode schizophrenia. Br J Psychiatry 195(4): 336–345. [DOI] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Mishkin M (2011). A new neural framework for visuospatial processing, Nat Rev Neurosci. 12(4): 217–230. doi: 10.1038/nrn3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman DI, Foxe JJ, Butler PD, Saperstein A, Revheim N, Javitt DC (2005): Sensory contributions to impaired prosodic processing in Schizophrenia. Biol Psychiatry 58: 56–61. doi: 10.1016/j.biopsych.2005.02.034 [DOI] [PubMed] [Google Scholar]
- Loewy R, Fisher M, Schlosser DA, Biagianti B, Stuart B, Mathalon DH, Vinogradov S (2016): Intensive auditory cognitive training improves verbal memory in adolescents and young adults at clinical high risk for psychosis. Schizophr Bull 42 Suppl. 1: S118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao YT, Hua TM, Pallas SL (2011): Competition and convergence between auditory and crossmodal visual inputs to primary auditory cortical areas. J Neurophysiol 105: 1558–1573. doi: 10.1152/jn.00407.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE (2013): Glutamate in schizophrenia: a focused review and meta-analysis of ¹H-MRS studies. Schizophr Bull 39(1):120–9. doi: 10.1093/schbul/sbr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ (2009): Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology 23: 315–36. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001): An integrative theory of prefrontal cortex function. Ann Rev Neurosci 24: 176–202. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O’Reilly RC (2011): A unified framework for inhibitory control. Trends Cog Sci 15(10): 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienow TM, Lim KO, MacDonald A (2016): TDCS produces incremental gain when combined with working memory training in patients with schizophrenia: A proof of concept pilot study. Schizophrenia Research 172: 2218–219. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. (2008): The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry 165:203–213. [DOI] [PubMed] [Google Scholar]
- Oh A, Duerden EG, Pang EW (2014): The role of the insula in speech and language processing. Brain Lang 135: 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penadés R, Pujol N, Catalán R, Massana G, Rametti G, García-Rizo C, Bargalló N, Gastó C, Bernardo M, Junqué C (2013): Brain effects of cognitive remediation therapy in schizophrenia: a structural and functional neuroimaging study. Biol Psychiatry 73(10):1015–23. doi: 10.1016/j.biopsych.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Plakke B, Romanski LM (2014): Auditory connections and functions of the prefrontal cortex. Front Neurosci 8:199. doi: 10.3389/fnins.2014.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov T, Jordanov T, Rockstroh B, Elbert T, Merzenich MM, Miller GA (2011): Specific cognitive training normalizes auditory sensory gating in schizophrenia: a randomized trial. Biol Psychiatry 69(5):465–471. [DOI] [PubMed] [Google Scholar]
- Popov T, Rockstroh B, Weisz N, Elbert T, Miller GA (2012): Adjusting Brain Dynamics in Schizophrenia by Means of Perceptual and Cognitive Training. PLoS ONE 7(7): e39051. doi: 10.1371/journal.pone.0039051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov TG, Carolus A, Schubring D, Popova P, Miller GA, Rockstroh BS (2015): Targeted training modifies oscillatory brain activity in schizophrenia patients. Neuroimage Clin 7: 807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ (2012): A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage 62: 816–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Valdez J, Turetsky BI, Elliott M, Kohler C, et al. (2004): Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. Am J Psychiatry 161: 1004–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoups A, Vogels R, Qian N, Orban G (2001): Practising orientation identification improves orientation coding in V1 neurons. Nature 412(6846): 549–553. [DOI] [PubMed] [Google Scholar]
- Seitz A, Watanabe T (2005): A unified model for perceptual learning. Trends Cogn Sci 9(7): 203–216. [DOI] [PubMed] [Google Scholar]
- Senkowski D, Gallinat J (2015): Dysfunctional prefrontal gamma-band oscillations reflect working memory and other cognitive deficits in schizophrenia. Biol Psychiatry 77: 1010–1019. [DOI] [PubMed] [Google Scholar]
- Singh KD, Barnes GR, Hillebrand A (2003): Group imaging of task-related changes in cortical synchronisation using nonparametric permutation testing. Neuroimage 19: 1589–1601. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, et al. (2004): Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci USA 101: 17288–17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam K, Luks TL, Garrett C, Chung C, Fisher M, Nagarajan S, Vinogradov S (2014): Intensive cognitive training in schizophrenia enhances working memory and associated prefrontal cortical efficiency in a manner that drives long-term functional gains. Neuroimage 99: 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbricht D, Krljes S (2005): Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res 76(1):1–23. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, et al. (2006): Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage 30, 1414–1432. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Jobard G, Petit L, Crivello F, et al. (2011): What is right-hemisphere contribution to phonological, lexico-semantic, and sentence processing? Insights from a meta-analysis. Neuroimage 54(1): 577–593. doi: 10.1016/j.neuroimage.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, de Villers-Sidani E (2012): Cognitive training for impaired neural systems in neuropsychiatric illness. Neuropsychopharmacology 37(1):43–76. doi: 10.1038/npp.2011.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, Warm H, Holland C, Kirshner MA, Pollock BG (2009): The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry 166(9): 1055–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrba J, Robinson SE (2001): Signal processing in magnetoencephalography. Methods 25: 249–271. [DOI] [PubMed] [Google Scholar]
- Weinberg H, Brickett PA, Vrba J, Fife AA, Burbank MB (1984): The use of a squid third order spatial gradiometer to measure magnetic fields of the brain. Ann N Y Acad Sci 425: 743–752. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Heckers S (2001): Neuroimaging of declarative memory in schizophrenia. Scand J Psychol 42: 239–250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.