Abstract
Background:
Peripheral sensory stimulation augments post-stroke upper extremity rehabilitation outcomes. Most sensory stimulations interfere with natural hand tasks and the stimulation duration is limited. We developed TheraBracelet, low-level random-frequency vibration applied via a wristwatch, to enable stimulation during hand tasks and potentially extend stimulation durations.
Objective:
To determine safety of prolonged exposure to TheraBracelet.
Methods:
Single-site double-blind cross-over randomized controlled trial. Chronic stroke survivors were instructed to wear a device on the affected wrist for ≥8hr/day everyday for 2 months while coming to the laboratory weekly for evaluations, with a 2-week break between each month. The device applied vibration at 60% and 1% of the sensory threshold for the real and sham month, respectively. The order of the real and sham months was randomized/balanced. Adverse events (AEs) were assessed weekly, including worsening of hand sensation, dexterity, grip strength, pain, or spasticity and occurrence of skin irritation or swelling. Device-related AE rates were compared between the real and sham month.
Results:
Twenty-five participants completed the study. Six participants (24%) experienced mild AEs involving worsened sensory scores that may be related to the intervention with reasonable possibility. Two experienced them in the real stimulation month only, 3 in the sham month only, and 1 in both months. Therefore, less participants experienced device-related AEs in the real than sham month.
Conclusion:
Daily stimulation using the device for a month is safe for chronic stroke survivors. Future studies examining the efficacy of pairing TheraBracelet with therapy for increasing neurorehabilitation outcomes is a logical next step.
Keywords: Subliminal stimulation, physical stimulation, stroke rehabilitation, upper extremity, paresis, patient safety
1. Introduction
More than two-thirds of nearly 7 million stroke survivors in the U.S.1 have persistent hand impairment, which has a profound negative impact on functional ability and independence.2,3 Recent meta-analysis shows that upper extremity rehabilitation efficacy can improve when therapy is augmented by peripheral sensory stimulation, compared with therapy alone.4 The scientific rationale is that afferent input is a potent driver of change in the motor cortex.5–7
While promising, most sensory stimulation modalities interfere with natural hand tasks: Suprathreshold electrical stimulation causes a tingling sensation8,9 not associated with performing activities of daily living tasks with hand. In addition, wearing a glove8 or a finger cap10 hampers dexterous finger movement and causes a sense of discomfort.11,12 Thus, most sensory stimulations are administered prior to therapy,4 requiring an additional time commitment. These constraints make patient adherence and implementation difficult.13 Furthermore, the effect diminishes 20–30 min after the stimulation,14,15 weakening its effect during the duration of therapy.
To address these practical limitations, TheraBracelet was developed. TheraBracelet is a low-level random-frequency vibratory stimulation applied to the wrist skin via a wristwatch. The theory is that TheraBracelet stimulates mechanoreceptors in wrist skin and afferents16,17 and adds small random currents to sensorimotor cortex neurons to make them readily fire18–20 in coherence21 at the peak of other inputs related to hand tasks. This coherent firing enhances neural communication22,23 for the hand tasks24 and consequently improves hand task performance.25–27 Since TheraBracelet is not readily perceivable and delivered via a wristwatch, it may not interfere with hand movements and hand-object manipulation. In addition, it may potentially be worn all day, allowing the possibility of home use in conjunction with standard rehabilitation therapy for stroke survivors. This home-use paradigm is expected to increase the duration of peripheral sensory stimulation substantially beyond typical clinic visits currently available in the healthcare system.
While the use of TheraBracelet during in-lab therapy sessions was studied in a pilot randomized controlled trial with no safety concerns,27 extended use of TheraBracelet has not been investigated. The purpose of this study was to examine the safety of extended daily use of TheraBracelet at home for a month. We hypothesized that TheraBracelet would have a safety level comparable to wearing essentially a non-vibrating wristwatch.
2. Methods
2.1. Subjects
The study protocol was approved by the Institutional Review Board of the Medical University of South Carolina (Pro00062471). All participants provided written informed consent. Participants were included if they were at least 18 years old, had a stroke at least 3 months prior to enrollment, had at least some movement in the affected hand as seen by the Box and Block Test score greater than 0, and were able to put on the device at home either by themselves or with caregiver assistance. Participants were excluded if they had comorbidities such as peripheral neuropathy and orthopedic conditions in the hand, had compromised skin integrity of the hand or wrist, were participating in an upper limb rehabilitation program, were pregnant, exhibited a language barrier or cognitive impairment that precluded following instructions, exhibited dramatic changes in swelling of the upper extremity during the day, had received botulinum toxin injection in the 3 months prior to the enrollment, or were unable to provide consent. Inclusion/exclusion was determined based on participants’ disclosure except for the Box and Block Test.
2.2. Study Design
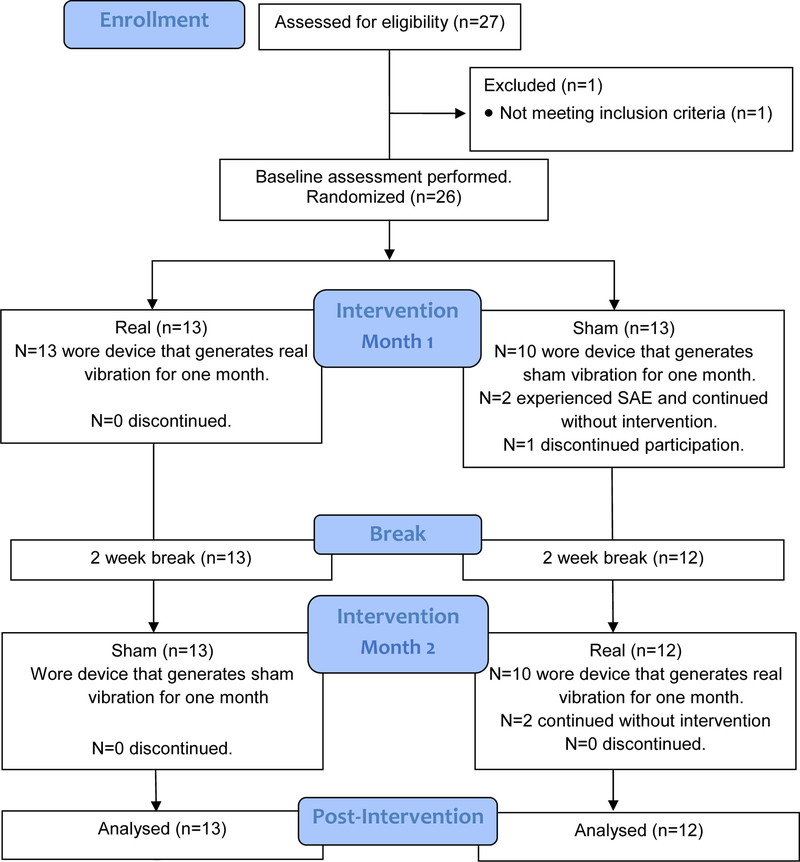
This study was a double-blind, randomized, crossover safety trial. Participants were randomly assigned to either the real or sham stimulation for the first month, followed by a 2-week break, and then crossed over to the other condition for the second month (Figure 1). Block randomization was used to ensure balance in sample size.28 Block sizes were random and blinded to researchers and participants.
Figure 1.
CONSORT Diagram
For both conditions, participants were instructed to wear a wristwatch device (Figure 2) on the affected wrist every day for at least 8 hours/day during daily activity for a month. The device applied random-frequency vibration at 60% of the sensory threshold for the real stimulation condition and 1% of the sensory threshold for the sham condition. This vibration parameter for the real stimulation condition was used becauseit was shown to improve hand dexterity25 and sensation.26 No therapy activities or exercises were prescribed. The rationale was to focus the investigation on the effect of the stimulation without the confounds of a therapy or exercise program.
Figure 2.
Device
The device was composed of an MP3-playing watch (Amazon; 3.7×5.3×1.9cm) with a vibrator (C-3 Tactor, EAI, Casselberry, FL; 18.8mm diameter, 6mm thick) attached underneath the watch surface (Figure 2, total weight=40g). Participants affixed the device to the affected wrist, similar to wearing a watch. The vibrator made direct contact with the wrist skin. Participants were instructed to charge the watch every night during sleep.
Double blinding.
Participant blinding was planned since both real and sham stimulations were set to be imperceptible. Researchers were blinded by use of a custom-developed computer program for randomization and vibration file generation. Specifically, the participant’s sensory threshold was determined at each visit. The custom-developed computer program generated a vibration file. The vibration file contained the real (intensity 40% below the sensory threshold) or sham vibration signal, depending on the randomized condition assignment for the specific participant number and month. The vibration file was uploaded to the watch and used by the participant until the next visit in the following week. The condition assignment sequence was revealed to the biostatistician for final analysis, after all participants’ data were entered into the database.
Evaluation took place at baseline (week 0) and after each week of wearing the device (weeks 1–4) for each month. Evaluation included safety and adherence to the study protocol and safety. Adherence to the study protocol was assessed to gauge the extent that the stimulation was delivered to participants. Each evaluation is detailed below.
Adherence to the study protocol was assessed during weekly evaluations. First, compliance in wearing the device was assessed using participants’ self-reported use logs. Participants brought home a device use log and recorded times at which they put on and took off the device every day. This log was submitted to researchers during their weekly evaluation. Compliance in device wear time was defined a priori as wearing the device for at least half of the prescribed time. The rationale was that 4 hours/day is a substantial exposure to stimulation.
Second, we recorded whether participants were wearing the device on the affected wrist, each time they arrived at the laboratory for weekly evaluations. Third, we assessed whether the device was functioning as intended at weekly evaluations. Specifically, we examined any breakage of the exterior or electrical connection of the cable, and whether the device was turned on and running as intended. We also assessed the vibrator’s frequency and amplitude characteristics using a laser vibrometer (LaserPoint LP01, OMS Corp, Laguna Hills, CA) at week 2 and 4 in each month. We used a template vibration file to verify device function, to not affect blinding of the researchers. All device functions were verified prior to being provided to participants.
Blinding was verified by asking participants at the end of their enrollment whether they felt vibration at any time during the study. If they felt a vibration, then they were asked in which month they felt the vibration (first or second month), and how long and often the perception occurred.
Safety evaluation took place at each weekly evaluation, without the device on the affected wrist. Worsening in hand sensation, dexterity, grip strength, upper limb pain, or spasticity, occurrence of skin irritation or swelling, and any other adverse events (AEs) were assessed. The following criteria for AEs were defined a priori. Worsening in hand sensation was defined as emergence of numbness based on the participant’s verbal report, or an increase in any of the monofilament or 2-point discrimination scores29 by more than 2 levels and a category (e.g. “normal” to “diminished light touch” for the monofilament test, “fair” to “poor” for the 2-point discrimination test30) for any of the thumb, index, and little fingers. Worsening in hand dexterity was defined as an increase in time to complete the Nine Hole Peg Test (NHPT) by more than 20% which is considered clinically meaningful31 and more than the minimum detectable change of 32.8 sec.32 Worsening in grip strength was defined as a decrease in grip strength by more than 20% which is approximately a standard error of measurement33 and more than the minimum detectable change of 28.4 N.32 Upper limb pain was assessed using the visual analog pain rating scale from 0 (no pain) to 10 (worst possible pain). Worsening in upper limb pain was defined as an increase in the pain level by more than two which is approximately a clinically important difference.34,35 Spasticity was assessed using the Modified Ashworth Scale (MAS) for the thumb, finger, and wrist joints. Worsening in spasticity was defined as an increase in MAS by more than a level, which is approximately the effect expected from botulinum toxin A.36 Emergence of wrist skin irritation based on the participant’s verbal report or researcher’s examination was noted as an adverse event. Occurrence of swelling was defined as emergence of swelling based on the participant’s verbal report or an increase in the wrist circumference by more than 1 cm which is the limit of agreement defined in literature.37 Worsening or occurrence was defined as compared with the baseline of each month. All AEs were categorized for severity38 and relatedness to the intervention39 by the study team in blinding. DSMB reviewed and approved results every 3 months during the study period.
User feedback was obtained at the end of each month. Specifically, participants rated their perceived safety of the device, perceived comfort in wearing the device, and perceived improvement in the movement or use of the affected upper extremity from the device on a 7-point Likert scale (1=strongly agree, 2=agree, 3=more or less agree, 4=neutral, 5=more or less disagree, 6=disagree, 7=strongly disagree), based on the technology acceptance model.40,41
2.3. Sample size and power analysis
Power analysis was performed to obtain the sample size needed to determine safety. We deemed a difference of 20% or more in AE rates between the real and sham stimulation conditions would render the device unsafe due to the following reasons. For a regulatory approval, a safety level similar to a comparable FDA-approved device is desired. The most comparable device with available safety data in literature42 and FDA approval is a Bioness electrical stimulator for foot drop. The Bioness was reported to have a 21% higher likelihood for AEs compared to a standard ankle-foot orthosis (82 out of 99 participants had AEs with the Bioness vs. 61 out of 98 participants had AEs with the standard orthosis).42 In addition, based on informal conversations with healthcare providers, stroke survivors, and clinicians, it was deemed, if 80% of participants gain benefits while at most 20% experience AEs, the device would be viable.
Therefore, 20% tolerance was used to determine the sample size needed for testing the hypothesis of equivalence using a 2×2 crossover43,44 design at 5% significance level (PASS 2008, NCSS Statistical Software). A sample size of 24 (12 in each group at randomization) provides at least 80% power to conclude equivalence within a difference in AE of 20% (of the total group) between the discordant pairs (those who are safe with sham but unsafe with real vs. those with the opposite), assuming a total of 10% discordance rate (proportion of participants with different responses for the two conditions) and a 90% safe rate for the sham condition. Thus, the study is powered to statistically conclude equivalence (hence safety) if 3 or less out of 24 participants had device-related AE in the real vs. sham stimulation month. Statistical equivalence would suggest that TheraBracelet is at least as safe as the Bioness electrical stimulator for foot drop.
2.4. Analysis
The primary analysis involved a comparison of device-related AE rates between the real and sham stimulation conditions using the test of equivalence.43,44 The intent to treat paradigm45 was used. Summative statistics were obtained for all measures, including adherence to the protocol and user feedback.
As a secondary analysis for safety, a repeated-measures generalized linear mixed model was used to detect statistically significant group-level change for each safety measure. The model included week (0, 1, 2, 3, 4), month (1 and 2), condition (real vs. sham), and their interactions. An additional factor of fingers and joints were included for the sensory and MAS scores, respectively. MAS was treated as an ordinal variable, while others were continuous. Transformation (1/x2) was applied for 2-point discrimination scores since they were not normally distributed. Other measures were normally distributed. The factor of interest was the interaction between condition and week.
In addition, reliability of the NHPT was assessed by computing a Pearson correlation between the scores obtained during the visits and scores obtained via review of video recordings of the NHPT. This correlation was examined for randomly selected 25% of the NHPTs at the baseline and week 4 of each month.
3. Results
3.1. Subjects
3.1.1. Recruitment/Participation
Twenty-six chronic stroke survivors were enrolled and randomized (Figure 1). One participant dropped out the next day due to scheduling conflict, resulting in 96% retention rate. Two participants had a serious adverse event (SAE) in the second week of the first month. Both participants were instructed to stop wearing the device but continued with study procedure (i.e., no intervention but weekly safety assessments) per intent-to-treat paradigm. Both participants’ data are included in the safety results.
3.1.2. Baseline characteristics
Participant characteristics are shown in Table 1. Participants were chronic stroke survivors with mild to moderate upper limb impairment. Demographic and clinical characteristics were comparable between the two groups.
Table 1.
Participant Characteristics
| Real then sham (n=13) | Sham then real (n=13) | p-value | |
|---|---|---|---|
| Age, mean (SD) years | 61 (10) | 64 (8) | 0.38 |
| Gender, male/female | 9/4 | 9/4 | 1.00* |
| Affected hand, right/left | 6/7 | 9/5 | 0.70* |
| Stroke type, ischemic/hemorrhagic | 9/4 | 11/3 | 1.00* |
| Time since stroke, mean (SD) months | 36.6 (30.0) | 37.5 (40.0) | 0.97 |
| Fugl-Meyer Assessment - Upper Extremity Score (/66), mean (SD) | 51.5 (5.7) | 49.8 (8.4) | 0.29 |
| Nine Hole Peg Test, mean (SD) | 93.5 (65.8) | 97.8 (61.5) | 0.86 |
| Box and Block Test score, mean (SD) | 29.2 (11.0) | 27.0 (15.5) | 0.24 |
Fisher’s Exact Tests were used. For others, two-sample t-tests were used.
3.2. Adherence to the protocol
Adherence to the protocol is detailed below. For two participants with the SAE, data for adherence to the protocol are included only prior to the SAE.
3.2.1. Compliance in device wear time
The compliance rate in device wear time was 100% (SE=0%) in the real stimulation month, and 99% (SE=1%) in the sham month (Table 2A). The mean compliance rate was computed as the number of participants who were compliant divided by the total number of participants, averaged for 4 weeks. All participants reported mean device wear time of minimum 4 hours/day for all weeks and months, except for one case: One participant in the real then sham group stopped wearing the device in the last week of the sham month. The mean device wear time was 10±0.5 hours/day (Table 2A).
Table 2.
Adherence to the study protocol. (A) Rate of compliance in device wear time and mean±SE wear time from self-reported device use logs. (B) Rate of compliance in wearing the device on the affected wrist observed at the time of arrival at the laboratory for weekly evaluations (mean±SE). (C) Rate of proper device functioning observed during the weekly evaluations (mean ± SE).
| (A) | Month 1 | Month 2 | |
|---|---|---|---|
| Real then sham (n=13) | Compliance rate | 100% ± 0% | 98% ± 1% |
| Wear time | 9.9 ± 0.5 hours/day | 9.4 ± 0.4 hours/day | |
| Sham then real (n=12) | Compliance rate | 100% ± 0% | 100% ± 0% |
| Wear time | 10.9 ± 0.6 hours/day | 10.8 ± 0.6 hours/day |
| (B) | Month 1 | Month 2 |
|---|---|---|
| Real then sham (n=13) | 88% ± 3% | 81% ± 1% |
| Sham then real (n=12) | 90% ± 3% | 96% ± 1% |
| (C) | Month 1 | Month 2 |
|---|---|---|
| Real then sham (n=13) | 88% ± 3% | 96% ± 1% |
| Sham then real (n=12) | 96% ± 1% | 98% ± 1% |
3.2.2. Compliance in device wear at the time of arrival to the laboratory
The compliance rate in wearing the device on the affected wrist when participants arrived at the laboratory for weekly evaluations was 92% (SE=2%) in the real stimulation month and 88% (SE=2%) in the sham month (Table 2B). Fifteen participants out of 25 were compliant for all weekly visits in both months. Seven participants were found not wearing the device at the time of arrival for only 1 out of 4 weekly visits in any month. In these 7 participants, 1 participant was not wearing the device one time in the real month only, 5 participants in the sham month only, and 1 participant in both months. Three participants were found not wearing the device for at least 2 out of 4 weekly visits in any month at the time of their arrival. Specifically, one participant had the device on the unaffected wrist 1 time in the first real stimulation month and was not wearing the device twice in the second sham month. This participant stated that s/he “was in a hurry to get to the laboratory and it was faster to put on the device on the unaffected wrist” and that s/he “wore the device on the affected wrist throughout the week except for that day.” One participant was not wearing the device twice in the first real month and 4 times in the second sham month. This participant typically put on the device in the mid-day and took off the device in the evening according to the use log. Therefore, this participant typically did not start wearing the device at the time of the laboratory visit in the morning. One participant was not wearing the device 3 times in each month, primarily due to repeated device breakage. A new device was provided each time.
3.2.3. Device function
The device was found to be functioning as intended when participants came to the laboratory for weekly evaluations for 93% (SE=2%) of the participants on average in the real stimulation month, and 97% (SE=1%) in the sham month (Table 2C). Eighteen participants out of 25 had no device issues. Five participants had a device issue only one time during their enrollment. One participant had an intermittent electrical connection issue for the cable between the vibrator and watch two times in the first real month. One participant had a device exterior breakage twice in each of the real and sham month. The vibrator’s frequency and amplitude characteristics measured by a laser vibrometer were unaltered at all times. Whenever a device issue was found, a new device verified for its function was provided to the participant.
3.2.4. Blinding
Seventeen participants out of 25 did not feel vibration from the device at any time. Eight participants reported feeling vibration from the device. Among them, 2 participants reported feeling vibration in both months (real and sham stimulation conditions). Six participants reported feeling vibration during the month of the real stimulation condition only. All eight participants reported feeling vibration for a few seconds or a few minutes at a time sporadically.
3.3. Safety
Eighteen out of 25 participants experienced AEs. These 18 participants experienced a total of 76 AEs. The severity and relatedness to intervention for each AE are detailed in Table 3. All AEs were mild, except 3 SAEs.
Table 3.
AEs. The number of AEs is shown in each severity and relatedness category. The number of AEs in each cell is further divided into the number of AEs that occurred in the real and sham months (in parenthesis). In addition, the number of participants out of a total of 25 who experienced AEs is shown in each severity and relatedness category. The number of participants is further divided into the number of participants that experienced AEs in the real month only, sham month only, and both (in parenthesis).
| #=Number of AEs (in real
month/sham month) n=Number of participants with AE (in real month only/sham month only/both) |
|||
|---|---|---|---|
| Relatedness╲Severity | |||
| Not Related | #=12 (8/4) n=8 (6/1/1) |
0 | #=1 (1*/0) n=1 (1/0/0) |
| Unlikely | #=32 (18/14) n=14 (4/3/7) |
0 | #=2 (0/2†) n=2 (0/2/0) |
| Reasonable possibility | #=29 (9/20) n=6 (2/3/1) |
0 | 0 |
| Definitely | 0 | 0 | 0 |
| Total | #=73 (35/38) n=18 (6/3/9) |
0 | #=3 (1/2) n=3 (1/2/0) |
The AE severity and relatedness categorization was reviewed and approved by the DSMB in blinding.
Facial skin surgery which has been routine for this participant for past 30 years.
Transient ischemic attack-like symptoms were experienced. Both participants were medically examined and no diagnosis was given.
The 3 SAEs were deemed either unlikely or not related to intervention. One SAE was determined to be not related to intervention, because this was a facial skin surgery that this participant had been having routinely for the past 30 years. The other 2 SAEs involved two participants experiencing transient ischemic attack-like symptoms. The participants were evaluated by the study physician and their medical providers. No diagnosis was made. The study team determined that these SAEs are unlikely related to the intervention, which was agreed upon by the DSMB. Double blinding was preserved during this investigation. After all of the data were collected, group assignment information was revealed. These two SAEs were found to have occurred during the sham stimulation in the first month for both participants and thus could not be related to the real stimulation.
Among 73 mild AEs, 12 AEs were determined to be not related to the intervention, because they involved known causes unrelated to the intervention, such as bug bites, cat scratches, sunburn, cold symptoms, and a bruise on the unaffected upper limb. The 32 mild AEs were determined to be unlikely related to intervention because they were sporadic and receded in the following assessments within the same month.
The 29 mild AEs were determined to have reasonable possibility to be related to intervention because they sustained until the end of the month or occurred at the end of the month. Out of 29, 9 AEs occurred in the real stimulation month, and 20 AEs occurred in the sham month. These 29 AEs occurred in 6 participants. They occurred in the real stimulation month for 2 participants, in the sham month for 3 participants, and in both months for 1 participant. These AEs were worsening in sensation (in 2-point discrimination or monofilament score in one or two fingers) and emergence of skin irritation (in the sham month only).
The primary analysis for safety involved comparison of rates of device-related AE occurrence between the real and sham conditions. Since the number of participants experiencing device-related AEs (with reasonable possibility or definitely) was less in the real month vs. sham month (2 vs. 3), it is concluded that the real stimulation did not increase the number of participants experiencing AEs compared with the sham stimulation.
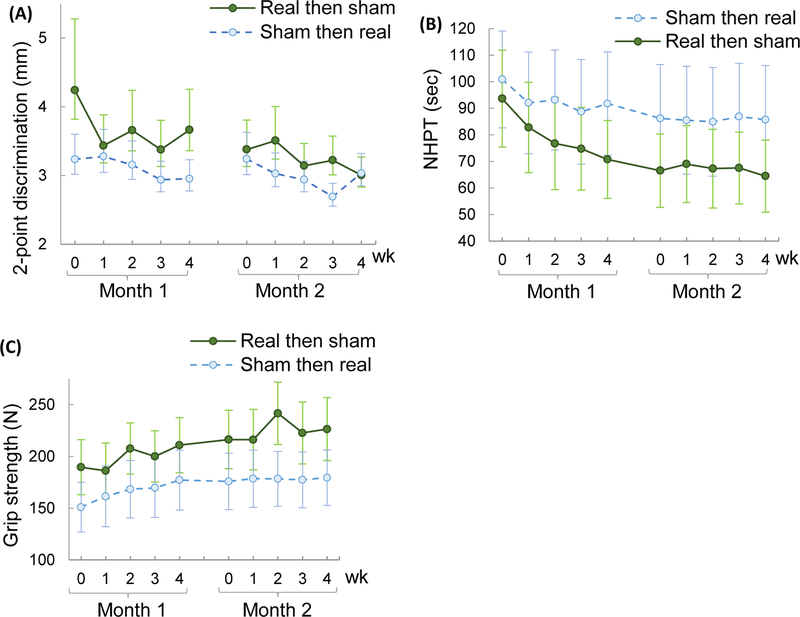
As a secondary analysis, we examined if there is a statistically significant deterioration for any of the non-dichotomous safety measures as a group. The main factor of interest was the condition × week interaction. This interaction was significant for 2-point discrimination only. Specifically, the 2-point discrimination score significantly varied by week (p=0.0219), condition × week (p=0.0431), and finger (p=0.0054). Other factors were not significant (p>0.1). The mean change was 0.4 mm (SE=1.0) in the real month and 0.3 mm (SE=0.9) in the sham month (Figure 3A). The mean change over the enrollment period (10 weeks) was 1.2 mm (SE=1.1) for the real then sham group, and 0.2 mm (SE=1.4) for the sham then real group. These changes are considered to be very small since the test resolution is 1 mm.
Figure 3.
Mean±SE 2-point discrimination test (A), Nine Hole Peg Test (NHPT) time (B), and grip strength (C) during the enrollment period for the two groups (one group had the sham month followed by the real stimulation month vs. another group had the real stimulation month followed by sham month). Back-transformed data are shown for the 2-point discrimination test (A).
The NHPT time varied significantly by week, month, week × month interaction (p<0.0001 for all) and condition (p=0.0276). Other factors were not significant (p>0.1). Specifically, NHPT time decreased over weeks during the first month more so than during the second month (Figure 3A). The mean change in NHPT was −16 sec (SE=5) in the first month (−23 in the real month and −9 in the sham month), and −1 sec (SE=2) in the second month (−2 in the sham month and 0 in the real month). The mean change throughout the enrollment duration was −26 sec (SE=6) (−29 for the real then sham group, and −15 for the sham then real group). These changes are less than the minimum detectable change of 32.8 sec.32 The condition effect was associated with the starting NHPT time between the conditions. The reliability of the NHPT measurement was excellent: the Pearson correlation was 0.999 between the scores obtained during the visits and the scores obtained via review of video recordings (p<0.001).
Grip strength varied significantly by week, month (p<.0001 for both) and condition (p=0.0015). Other factors were not significant (p>0.05). The mean grip strength increased by 24 N (SE=7) in the first month and 7 N (SE=9) in the second month (Figure 3C). The mean change over the 10-week enrollment duration was 33 N (SE=9), which is greater than the minimum detectable change of 28.4 N.32 The condition effect was associated with the different starting grip strength between the conditions.
No statistical significance was found for the monofilament test, pain, spasticity, and wrist circumference. In summary, the secondary analysis showed that the stimulation did not deteriorate hand sensation, dexterity, strength, pain, spasticity, and swelling at the group level.
3.4. User Feedback
The mean perceived safety of the device was 1 (1st quartile = 1, 3rd quartile = 1) for the real stimulation month and 1 (1, 1) for the sham month. The mean perceived comfort in wearing the device was 1 (1, 2) for the real month and 2 (1, 3) for the sham month. The mean perceived improvement in the movement or use of the affected upper extremity from the device was 3 (3, 3) for the real month and 3 (2, 4) for the sham month. The scale was 7-point Likert scale (1=strongly agree, 2=agree, 3=more or less agree, 4=neutral, 5=more or less disagree, 6=disagree, 7=strongly disagree).
4. Discussion
This study systematically investigated the safety of daily use of TheraBracelet at home over a month in chronic stroke survivors. The extended daily use of TheraBracelet was likely achieved as suggested by the high rates of adherence to the study protocol, except for the two participants with SAE who were instructed to stop wearing the device. TheraBracelet stimulation did not increase the number of participants experiencing device-related AEs compared with the sham stimulation. Relatively high occurrence of mild AEs regardless of the conditions could be due to extensive tests performed weekly and measurement reliability issues for the 2-point discrimination and monofilament tests that have been shown to have little to moderate reliabilities.29 Participants perceived that the device was safe, as seen by the user feedback. Therefore, it is concluded that long-term use of TheraBracelet stimulation at home (i.e., >4hr/day daily over a month) is safe for chronic stroke survivors.
This study was not designed to test efficacy. Participants were not instructed to perform any hand task practices, exercises, or therapeutic activities during enrollment. Rather, participants were instructed to continue with their routine lifestyle. The reason was to focus the investigation on the safety of the stimulation, in the absence of the confounding effects of upper extremity therapy. Chronic stroke survivors generally have a reduced amount of affected upper limb movement (mean 5 hours/day of movement46 compared to 9 hours/day for age-matched controls47 detected based on accelerometry). Their daily routine may not involve much movement of the affected upper extremity. Because no upper extremity therapeutic activity was encouraged, the anticipated effects of the TheraBracelet stimulation on hand function was minimal. Despite having no intervention other than wearing the stimulation device, secondary safety analysis showed that there was a general trend of improvements in the 2-point discrimination test score, NHPT time, and grip strength over weeks. However, changes in the 2-point discrimination and NHPT were small or less than the minimum detectable change. Change in grip strength was not specific to the real stimulation month. Thus, it may represent a placebo or practice effect.
Limitations of this study are as follows. A relatively small size was used (n=25). Perfect covariate balance does not manifest in small trials. However, the differences observed in our study are insignificant and therefore simply due to chance. This study focused on examination of the safety of TheraBracelet in the absence of any therapeutic activities. Therefore, this study lacks information on the efficacy of using TheraBracelet in conjunction with a standard therapy program with home exercises. This study did not systematically examine whether the device interferes with any therapeutic activities. A cross-over design was used because it was initially anticipated that changes in measures that occurred during the first month would subside during 2 weeks of not wearing the device. However, changes were sustained, resulting in different baselines for the two months (Figure 3). This trend informs that future trials may use a parallel design rather than crossover. We chose the cross-over design and powered it sufficiently to account for the known variability in the stroke population. However, larger stratified studies considering subpopulations may be warranted to further explore the heterogeneity of the stroke population. Although the vibration intensity was adjusted to be 40% below their sensory threshold at the weekly visit, some participants were able to feel vibration for a brief period during the following week, suggesting changes in the sensory threshold over time. This study did not use any wear sensor to objectively monitor wear time. We cannot rule out the possibility that participants may inflate their self-reported wear time. Some device function issues such as breakage were observed, suggesting the need to improve the device exterior design.
5. Conclusion
Daily long-term use of TheraBracelet stimulation at home (i.e., >4hr/day over a month) is safe in chronic stroke survivors. It is a logical step to plan a phase II study investigating the efficacy of pairing TheraBracelet with a standard hand rehabilitation therapy in increasing hand motor outcomes and functional independence while continuing to monitor safety.
Acknowledgments
The authors would like to thank the Data and Safety Monitoring Board members, Drs. Robert Adams, Craig Velozo, and Caitlyn Meinzer.
Funding: This study was possible through funding from the NIH/NICHD Small Business Technology Transfer (STTR) R41HD090792 awarded to TheraBracelet Inc. with a subcontract to the Medical University of South Carolina (PI: Seo), and NIH/NIGMS P20GM109040 COBRE for Stroke Recovery (PI: Kautz).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Clinical Trial Registration
Clinical trial registration number: NCT03318341
Ethical Approval: All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Conflict of Interest: N.J. Seo and L.R. Enders are inventors of a patent regarding the investigated sensory stimulation. Seo has no part in the company that sponsored this trial. Enders is a scientist in the company that sponsored this trial. The other authors report no conflicts of interest.
Contributor Information
Na Jin Seo, Division of Occupational Therapy, Department of Health Professions, Department of Health Sciences and Research, Medical University of South Carolina (MUSC), Charleston, SC
Leah R. Enders, TheraBracelet Inc., Louisville, KY
Andrew Fortune, Department of Health Sciences and Research, MUSC
Shannon Cain, Division of Occupational Therapy, Department of Health Professions, MUSC
Amanda A. Vatinno, Department of Health Sciences and Research, MUSC
Eli Schuster, Department of Health Professions, MUSC.
Viswanathan Ramakrishnan, Department of Public Health Sciences, MUSC
Wuwei Feng, Department of Neurology, Duke University Medical Center
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2015 Update: A Report From the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2.Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75(4):394–398. [DOI] [PubMed] [Google Scholar]
- 3.Stewart JC, Cramer SC. Patient-reported measures provide unique insights into motor function after stroke. Stroke. 2013;44(4):1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conforto AB, Dos Anjos SM, Bernardo WM, et al. Repetitive Peripheral Sensory Stimulation and Upper Limb Performance in Stroke: A Systematic Review and Meta-analysis. Neurorehabil Neural Repair. 2018;32(10):863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaneko T, Caria MA, Asanuma H. Information processing within the motor cortex. II. Intracortical connections between neurons receiving somatosensory cortical input and motor output neurons of the cortex. J Comp Neurol. 1994;345(2):172–184. [DOI] [PubMed] [Google Scholar]
- 6.Kaneko T, Caria MA, Asanuma H. Information processing within the motor cortex. I. Responses of morphologically identified motor cortical cells to stimulation of the somatosensory cortex. J Comp Neurol. 1994;345(2):161–171. [DOI] [PubMed] [Google Scholar]
- 7.Matyas F, Sreenivasan V, Marbach F, et al. Motor control by sensory cortex. Science. 2010;330(6008):1240–1243. [DOI] [PubMed] [Google Scholar]
- 8.Golaszewski SM, Siedentopf CM, Koppelstaetter F, et al. Modulatory effects on human sensorimotor cortex by whole-hand afferent electrical stimulation. Neurology. 2004;62(12):2262–2269. [DOI] [PubMed] [Google Scholar]
- 9.Meesen RL, Cuypers K, Rothwell JC, Swinnen SP, Levin O. The effect of long-term TENS on persistent neuroplastic changes in the human cerebral cortex. Hum Brain Mapp. 2011;32(6):872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurita Y, Shinohara M, Ueda J. Wearable Sensorimotor Enhancer for Fingertip Based on Stochastic Resonance Effect. IEEE Transactions on Human-Machine Systems. 2013;43(3):333–337. [Google Scholar]
- 11.Yu A, Yick KL, Ng SP, Yip J. Case study on the effects of fit and material of sports gloves on hand performance. Appl Ergon. 2019;75:17–26. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita H Effect of gloves on prehensile forces during lifting and holding tasks. Ergonomics. 1999;42(10):1372–1385. [DOI] [PubMed] [Google Scholar]
- 13.Jin J, Sklar GE, Min Sen Oh V, Chuen Li S. Factors affecting therapeutic compliance: A review from the patient’s perspective. Ther Clin Risk Manag. 2008;4(1):269–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaelin-Lang A, Luft AR, Sawaki L, Burstein AH, Sohn YH, Cohen LG. Modulation of human corticomotor excitability by somatosensory input. J Physiol. 2002;540(Pt 2):623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith L, Brouwer B. Effectiveness of muscle vibration in modulating corticospinal excitability. J Rehabil Res Dev. 2005;42(6):787–794. [DOI] [PubMed] [Google Scholar]
- 16.Vallbo AB, Johansson RS. Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Hum Neurobiol. 1984;3(1):3–14. [PubMed] [Google Scholar]
- 17.Vallbo AB. Microneurography: how it started and how it works. J Neurophysiol. 2018;120(3):1415–1427. [DOI] [PubMed] [Google Scholar]
- 18.Moss F, Ward LM, Sannita WG. Stochastic resonance and sensory information processing: a tutorial and review of application. Clin Neurophysiol. 2004;115(2):267–281. [DOI] [PubMed] [Google Scholar]
- 19.Seo NJ, Lakshminarayanan K, Bonilha L, Lauer AW, Schmit BD. Effect of imperceptible vibratory noise applied to wrist skin on fingertip touch evoked potentials - an EEG study. Physiol Rep. 2015;3(11):e12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seo NJ, Lakshminarayanan K, Lauer AW, et al. Use of imperceptible wrist vibration to modulate sensorimotor cortical activity. Exp Brain Res. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward LM. Physics of neural synchronisation mediated by stochastic resonance. Contemporary Physics. 2009;50(5):563–574. [Google Scholar]
- 22.Ward LM, MacLean SE, Kirschner A. Stochastic resonance modulates neural synchronization within and between cortical sources. PLoS One. 2010;5(12):e14371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fries P Rhythms for Cognition: Communication through Coherence. Neuron. 2015;88(1):220–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins JJ, Imhoff TT, Grigg P. Noise-enhanced tactile sensation. Nature. 1996;383(6603):770. [DOI] [PubMed] [Google Scholar]
- 25.Seo NJ, Kosmopoulos ML, Enders LR, Hur P. Effect of remote sensory noise on hand function post stroke. Front Hum Neurosci. 2014;8:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enders LR, Hur P, Johnson MJ, Seo NJ. Remote vibrotactile noise improves light touch sensation in stroke survivors’ fingertips via stochastic resonance. J Neuroeng Rehabil. 2013;10(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seo NJ, Woodbury ML, Bonilha L, et al. TheraBracelet stimulation during task-practice therapy to improve post-stroke upper extremity function – A pilot randomized controlled study. Physical Therapy Journal. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suresh K An overview of randomization techniques: An unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4(1):8–11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Bulut T, Tahta M, Sener U, Sener M. Inter- and intra-tester reliability of sensibility testing in healthy individuals. J Plast Surg Hand Surg. 2018;52(3):189–192. [DOI] [PubMed] [Google Scholar]
- 30.Pendleton HM. Chapter 22 Evaluation of Sensation and Intervention for Sensory Dysfunction In: Pendleton HM, Schultz-Krohn W, eds. Pedretti’s Occupational Therapy: Practice Skills for Physical Dysfunction. 6th ed: Elsevier Health Sciences; 2006. [Google Scholar]
- 31.Feys P, Lamers I, Francis G, et al. The Nine-Hole Peg Test as a manual dexterity performance measure for multiple sclerosis. Mult Scler. 2017;23(5):711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen HM, Chen CC, Hsueh IP, Huang SL, Hsieh CL. Test-retest reproducibility and smallest real difference of 5 hand function tests in patients with stroke. Neurorehabil Neural Repair. 2009;23(5):435–440. [DOI] [PubMed] [Google Scholar]
- 33.Bertrand AM, Mercier C, Bourbonnais D, Desrosiers J, Gravel D. Reliability of maximal static strength measurements of the arms in subjects with hemiparesis. Clin Rehabil. 2007;21(3):248–257. [DOI] [PubMed] [Google Scholar]
- 34.Farrar JT, Young JP Jr., LaMoreaux L, Werth JL, Poole RM Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. [DOI] [PubMed] [Google Scholar]
- 35.Emshoff R, Bertram S, Emshoff I. Clinically important difference thresholds of the visual analog scale: a conceptual model for identifying meaningful intraindividual changes for pain intensity. Pain. 2011;152(10):2277–2282. [DOI] [PubMed] [Google Scholar]
- 36.Shaw LC, Price CI, van Wijck FM, et al. Botulinum Toxin for the Upper Limb after Stroke (BoTULS) Trial: effect on impairment, activity limitation, and pain. Stroke. 2011;42(5):1371–1379. [DOI] [PubMed] [Google Scholar]
- 37.Campagna G, Zampetti S, Gallozzi A, et al. Excellent Intra and Inter-Observer Reproducibility of Wrist Circumference Measurements in Obese Children and Adolescents. PLoS One. 2016;11(6):e0156646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.US Department of Health and Human Services NIoH, National Cancer Institute. Common terminology criteria for adverse events (CTCAE) version 5.0. 2017; https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50.
- 39.NINDS. Adverse event relatedness scale. 2017; https://cde.nlm.nih.gov/deView?tinyId=XrsKeH6EJCC.
- 40.Bertrand M, Bouchard S. Applying the technology acceptance model to VR with people who are favorable to its use. Journal of Cyber Therapy & Rehabilitation. 2008;1(2):200–210. [Google Scholar]
- 41.Cranen K, Veld RHit, Ijzerman M, Vollenbroek-Hutten M Change of patients’ perceptions of telemedicine after brief use. Telemedicine and e-Health. 2011;17(7):530–535. [DOI] [PubMed] [Google Scholar]
- 42.Kluding PM, Dunning K, O’Dell MW, et al. Foot drop stimulation versus ankle foot orthosis after stroke: 30-week outcomes. Stroke. 2013;44(6):1660–1669. [DOI] [PubMed] [Google Scholar]
- 43.Liu JP, Hsueh HM, Hsieh E, Chen JJ. Tests for equivalence or non-inferiority for paired binary data. Statistics in medicine. 2002;21(2):231–245. [DOI] [PubMed] [Google Scholar]
- 44.Nam JM, Blackwelder WC. Analysis of the ratio of marginal probabilities in a matched-pair setting. Statistics in medicine. 2002;21(5):689–699. [DOI] [PubMed] [Google Scholar]
- 45.Gupta SK. Intention-to-treat concept: A review. Perspect Clin Res. 2011;2(3):109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bailey RR, Birkenmeier RL, Lang CE. Real-world affected upper limb activity in chronic stroke: an examination of potential modifying factors. Top Stroke Rehabil. 2015;22(1):26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bailey RR, Lang CE. Upper-limb activity in adults: referent values using accelerometry. J Rehabil Res Dev. 2013;50(9):1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]