Abstract
There is increasing momentum toward the development of gene therapy for heart failure (HF) that is defined by impaired calcium (Ca2+) transport and reduced contractility. We have used FRET (fluorescence resonance energy transfer) between fluorescently-tagged SERCA2a (the cardiac Ca2+ pump) and PLB (phospholamban, ventricular peptide inhibitor of SERCA) to test directly the effectiveness of loss-of-inhibition/gain-of-binding (LOI/GOB) PLB mutants (PLBM) that were engineered to compete with the binding of inhibitory wild-type PLB (PLBWT). Our therapeutic strategy is to relieve PLBWT inhibition of SERCA2a by using the reserve adrenergic capacity mediated by PLB to enhance cardiac contractility. Using a FRET assay, we determined that the combination of a LOI PLB mutation (L31A) and a GOB PLB mutation (I40A) results in a novel engineered LOI/GOB PLBM (L31A/I40A) that effectively competes with PLBWT binding to cardiac SERCA2a in HEK293–6E cells. We demonstrated that co-expression of PLBM enhances SERCA Ca-ATPase activity by increasing enzyme Ca2+ affinity (1/KCa) in PLBWT-inhibited HEK cell homogenates. For an initial assessment of PLBM physiological effectiveness, we used human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CMs) from a healthy individual. In this system, we observed that adeno-associated virus 2 (rAAV2)-driven expression of PLBM enhances the amplitude of SR Ca2+ release and the rate of SR Ca2+ re-uptake. To assess therapeutic potential, we used a hiPSC-CM model of dilated cardiomyopathy (DCM) containing PLB mutation R14del, where we observed that rAAV2-driven expression of PLBM rescues arrhythmic Ca2+ transients and alleviates decreased Ca2+ transport. Thus, we propose that PLBM transgene expression is a promising gene therapy strategy that directly targets the underlying pathophysiology of abnormal Ca2+ transport and thus contractility in underlying systolic heart failure.
Keywords: calcium transport, cardiomyocyte, dilated cardiomyopathy, gene therapy, phospholamban, SERCA
1. Introduction
Heart failure (HF) is characterized by alterations in myocardial contractility resulting in impaired ventricular function, constituting a leading cause of morbidity and mortality worldwide.1 Over the past decades, researchers have made significant progress in identifying intracellular and molecular mechanisms that are altered during HF progression. Defective sarcoplasmic reticulum (SR) Ca2+ transport has been identified as a potential determinant of decreased contractility in the failing hearts.2–4 Ca2+ transport from the cytosol to the SR lumen in human cardiomyocytes (CM) is largely performed (~70% of Ca2+ ions transported) via the SR Ca-ATPase (SERCA2a).5 Despite recent advances in device and pharmacological therapies, the incidence of heart failure continues to rise due to an aging and increasingly obese population, so new therapeutic strategies targeting the underlying physiology are urgently needed. Increasing knowledge of the molecular mechanisms fundamental to cardiac function and disease – including HF – has expanded the therapeutic potential to target key players involved in Ca2+ handling, including SERCA2a.
The activity of SERCA2a is regulated by phospholamban (PLB), a 52-residue single-pass transmembrane protein expressed in the SR of cardiac muscle. PLB binds to SERCA2a and reduces its apparent Ca2+ affinity, as measured from SERCA activity.6 PLB is in equilibrium between homopentamers and monomers, where the oligomeric state is proposed to function as a non-inhibitory reservoir.7–10 Previous studies have identified two functional regions on the transmembrane (TM) helix of PLB.9–11 Single-residue mutations on one side of the TM helix diminish inhibitory function without significantly affecting PLB oligomerization state(s), whereas mutations on the opposite face of the TM helix modulate PLB oligomerization and can lead to enhanced SERCA inhibition.10,12 The regulation of gene expression has been investigated in healthy individuals and HF patients, and decreased SERCA-to-PLB ratio is associated with deteriorated cardiac function, indicating that SERCA/PLB stoichiometry and SERCA2a activity are potential therapeutic targets.13–15 To this end, SERCA2a transgene expression via recombinant adeno-associated virus (rAAV) was achieved in HF animal models to increase the SERCA-to-PLB ratio in the heart. Exogenous SERCA2a expression significantly enhanced cardiac function in disease models by multiple metrics.16,17 Recently, gene therapy designed to increase SERCA2a expression in the human heart underwent clinical trials in patients diagnosed with end-stage HF.18 Despite positive preliminary results in Phase 1/2a,19 the trial did not meet its primary composite end point in phase 2b, and this has been ascribed to dosage constraints and the heterogeneity of heart failure.20 One likely factor is the large size of SERCA2a, which limits steady-state expression levels of the gene therapy construct. Other promising strategies to increase the SERCA-to-PLB ratio include expression of miRNAs that directly target PLB expression21–23 and oligonucleotide-based drugs,24,25 although these strategies have not yet produced an effective therapeutic agent.
In the present study, we have pursued an alternative approach to activate SERCA2a, by expressing a loss-of-inhibition (LOI) PLB mutant (PLBM) that is engineered to compete with the inhibitory wild type PLB (PLBWT) for SERCA2a binding (Fig.A) via viral delivery. In this approach, the ratio between endogenous SERCA and PLB is not targeted; instead, the exogenous PLBM relieves SERCA2a inhibition and enhances Ca2+ transport activity. Thus, this strategy is designed to overcome limitations associated with SERCA2a overexpression-based gene therapy.
2. Materials and methods
2.1. Molecular biology
eGFP and tagRFP were fused to the N-terminus of human SERCA2a and human PLB, respectively. We have PLB demonstrated that attachment of the fluorescent proteins at these sites does not interfere with SERCA activity or inhibition.26,27 PLB cDNA mutations were introduced using the QuikChange mutagenesis kit (Agilent Technologies, Santa Clara, CA), and all expression plasmids were sequenced for confirmation.
2.2. Cell culture
Human embryonic kidney cells 293 (HEK293–6E, NRC, Canada) were cultured in FreeStyle F17 expression medium (Thermo Fisher Scientific, Waltham, MA) supplemented with 2 mM L-glutamine (Invitrogen, Waltham, MA). Cell cultures were maintained in a circular shaker (125 rpm, 37°C, 5% CO2 (Forma Series II Water Jacket CO2 Incubator, Thermo Fisher Scientific, Waltham, MA). For displacement assays, HEK293–6E cells were transiently transfected using 293fectamine with GFP-SERCA2a, RFP-PLBWT, and PLBM or empty vector in a 1:7:7 molar ratio. Cells were then assayed 48 hours post-transfection.
The control hiPSC line (SKiPS-31.3) was cultured as previously described.28 The R14del hiPSC line was derived from the SKiPS-31.3 line using homologous recombination via CRISPR/Cas9. Monolayer cardiac differentiation was performed as described,28 yielding beating cardiomyocytes within 7–10 days.
2.3. Gene expression
RNA was extracted from uninfected and rAAV-infected hiPSC-CMs at day 37, and cDNA was synthesized as previously described.28 Gene expression compared to the housekeeping gene β2-microglobulin (B2M) was determined using qRT-PCR, as assessed by ΔΔCt analysis. See Supplemental Table 1 for the list of primers used for qRT-PCR.
2.4. Immunoblot analysis
Samples were separated on a 4–20% polyacrylamide gradient gel (Bio-Rad, Hercules, CA) and transferred to polyvinylidene difluoride (PVDF) membrane. The PVDF membrane was blocked in Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE) followed by overnight incubation at 4 °C with the primary target antibody: rabbit anti-GFP polyclonal antibody (pAb) (1:1000; ab290, Abcam, Cambridge, United Kingdom), mouse anti-SERCA2 monoclonal antibody (mAb) (1:1000; 2A7-A1, Abcam), rabbit anti-tagRFP pAb (1:1000; ab233, Evrogen), mouse anti-PLB mAb (1:1000, 2D12, Abcam), or rabbit anti-β-actin pAb (1:5000, ab8227, Abcam). Blots were incubated with anti-mouse or anti-rabbit secondary antibodies conjugated to IRDye 680RD or IRDye 800CW, respectively, for 1 h at 23 °C (1:20,000; LI-COR Biosciences). Blots were quantified on the Odyssey scanner (LI-COR Biosciences).
2.5. Fluorescence data acquisition and analysis
Fluorescence lifetime (FLT) measurements were conducted using a top-read FLT plate reader (384-well plates) designed and built by Fluorescence Innovations, Inc. (St. Paul, MN). GFP donor fluorescence was excited with a 473 nm microchip laser from Concepts Research Corporation (Belgium, WI), and emission was acquired with 490 nm long-pass and 520/17 nm band-pass filters (Semrock, Rochester, NY). We previously validated the performance of this FLT plate reader with known fluorescence standards, as well as with a FRET-based high-throughput screening strategy that that targeted 2-color SERCA.29,30 FLT waveforms from donor- and donor/acceptor-labeled samples were analyzed as described in our previous publications and the supplemental methods.29,30
2.6. Ca-ATPase activity assay
An enzyme-coupled, NADH-linked ATPase assay was used to measure Ca2+-activated ATPase activity of SERCA in 96-well microplates. Each well contained HEK293–6E homogenates, 50 mM MOPS (pH 7.0), 100 mM KCl, 1 mM EGTA, 0.2 mM NADH, 1 mM phosphoenol pyruvate, 10 IU/mL of pyruvate kinase, 10 IU/mL of lactate dehydrogenase, 3.5 μg/mL of the Ca2+ ionophore A23187, and CaCl2 added to set [Ca2+]i to the desired values.12 The assay was started by addition of Mg-ATP at a final concentration of 5 mM, and well absorbances were read in a SpectraMax 384 Plus microplate spectrophotometer (Molecular Devices, Sunnyvale, CA). The Ca-ATPase assays were conducted over a range of [Ca2+]i, and the ATPase activities were fitted using the Hill function
| Eq. (1) |
where V is the initial rate of Ca2+-dependent ATPase activity at a specified Ca2+ concentration (pCa), Vmax is the rate of Ca-ATPase activity at saturating [Ca2+]i, n is the Hill coefficient, pKCa is the fitted Ca2+ dissociation constant, and pCa is the concentration of ionized Ca2+ per specific well and V.
2.7. Live-cell Ca2+ transient measurements
hiPSC-CMs (day 35 of differentiation) were enzymatically dissociated using the Detach 2 kit (Promocell, Heidelberg, Germany) and plated on Matrigel-coated German glass coverslips. After 2 days, the plated hiPSC-CMs were loaded with a Ca2+-sensitive fluorescent dye (Fura-2 AM, cell permeant, ThermoFisher, Rockville, MD, USA), and the ratio of fluorescence intensities (excitation ratio of 340/380 nm) were recorded using the IonOptix system (Ionoptix, Milton, MA). The electrically-induced Ca2+ transients were triggered by pulses from a MyoPacer (IonOptix, Milton, MA) at 40 V and 0.5 Hz, with cells at 23 °C. Ca2+ traces were analyzed using IonWizard software (IonOptix) to calculate the release amplitude (peak height relative to baseline) and tau (time of Ca2+ removal). The number of irregular Ca2+ transients was quantified using IonOptix software.
The transduction efficiency and transduction protocol using rAAV2 vectors to transduce hiPSC-CMs was previously documented.31 Briefly, rAAV2.L31A, rAAV2.I40A, and rAAV2.L31A/I40A PLB viruses were used to transuce hiPSC-CMs at day 30 of differentiation (1e4 MOI), enzymatically dissociated on day 35, and plated on Matri-gel coated German glass coverslips, as described above. Fura-2 fluorescence measurements were recorded in AAV-infected hiPSC-CMs on day 37 and compared to non-infected control hiPSC-CMs. AAV viruses were purified according to the two-plasmid method with iodixanol gradient as described.32
2.8. Statistical analyses
All statistics were performed using Prism (GraphPad, La Jolla, CA), and analysis was done by one-way ANOVA followed by the Bonferroni post hoc test; analysis of two group comparisons was done by Student’s t-test (*P < 0.05 and ‘ns’ is not significant). Data are presented as mean ± standard error of the mean (SEM), and all statistical values were calculated from a minimum of three separate experiments.
3. Results
3.1. FRET assay demonstrates SERCA-binding competition between PLBWT and loss-of-function PLBM
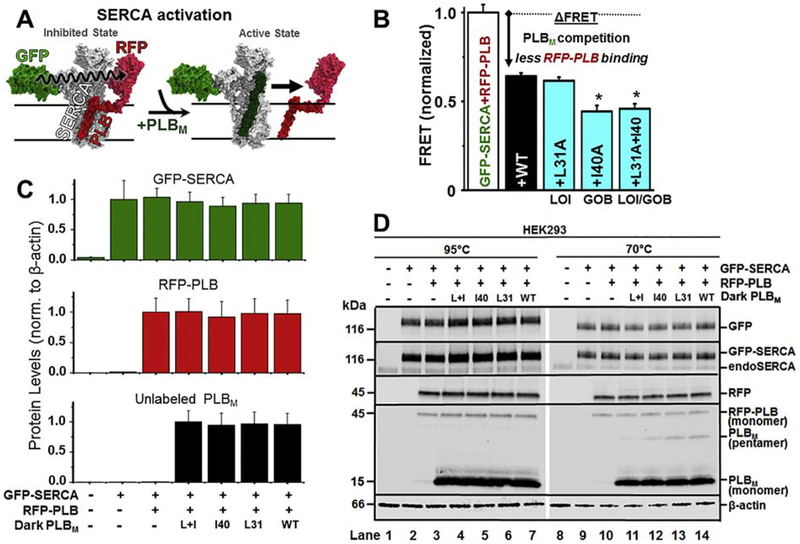
Previously, two classes of point mutations within the PLB TM domain had been identified via alanine-scanning mutagenesis, with disparate outcomes relative to SERCA: loss-of-inhibitory function (LOI) and gain-of-binding function (GOB).10,33 We hypothesized that combining LOI and GOB mutations in a single PLB would result in a LOI-PLB that can effectively compete with the inhibitory PLBWT. To test this, we used a SERCA2a-PLB biosensor system that consists of (a) co-expressed GFP-tagged SERCA2a and RFP-tagged PLBWT in HEK293–6E cells and (b) a fluorescence lifetime (FLT) readout of FRET. FLT-FRET is used to resolve changes in SERCA2a-PLB complex structure and binding (Fig.1A).29 We varied the ratio between the donor molecule (GFP-SERCA2a) and acceptor molecule (RFP-PLBWT), and found that the maximal energy transfer efficiency E (fractional decrease of the fluorescence lifetime) in this live-cell based system saturates at 0.10, as previously reported.29 We transfected cells expressing GFP-SERCA2a and RFP-PLBWT with either untagged PLBWT or PLBM containing TM mutations (L31A, I40A, or L31A/I40A). Displacement of the RFP-PLBWT from its interaction with GFP-SERCA2a was observed as a decrease in the FRET efficiency relative to that measured in control cells expressing only the GFP-SERCA/RFP-PLBWT donor-acceptor pair (Fig.1B). FRET between GFP-SERCA2a and RFP-PLBWT decreased significantly upon co-expression of unlabeled PLBWT, indicating that the RFP-PLBWT and unlabeled PLBWT are in equilibrium for hetero-dimeric binding to GFP-SERCA2a. An alanine substitution at L31A (LOI mutation) did not significantly alter the FRET value relative to the wild-type control. In contrast, an alanine substitution at I40A (associated with increased SERCA inhibition via decreased Ca2+ sensitivity) further decreased FRET, indicating that I40A-PLBM competes effectively with RFP-PLBWT with a potency (affinity) comparable to or greater than that of unlabeled PLBWT. The combination of L31A and I40A mutations resulted in a PLBM with binding similar to that of I40A-PLBM, consistent with our hypothesis that PLBM with both mutation types can retain high affinity toward SERCA2a.
Fig. 1. Co-expression of SERCA, PLB, and PLBM.
(A) Schematic of gene therapy strategy: a tight-binding, non-inhibitory PLB mutant (PLBM, dark green) displaces inhibitory RFP-PLB (PLBWT, dark red), resulting in a FRET decrease. (B) FRET assay of PLBM competition in HEK293–6E. GFP-SERCA-RFP-PLBWT FRET (binding, white bar) is decreased (arrow) by PLB variants. LOI = Loss-of-inhibitory function mutant. GOB = Gain-of-inhibition mutant. (C/D) Immunoblots of HEK homogenate samples incubated at 95°C (disrupting PLB pentamer, lanes 1–7) or at 70°C (lanes 8–14) from untransfected HEK293–6E cells (lane 1&8), cells expressing GFP-SERCA2a (lanes 2&9), or cells expressing GFP-SERCA2a+RFP-PLB (lanes 3–7&11–14) +L31A/I40A-PLB (lanes 4&11), +I40A-PLB (lanes 5&12), +L31A-PLB (lanes 6&13), and +WT-PLB (lanes 7&14). Primary antibodies used (from top to bottom of 1D panels) are anti-GFP, anti-SERCA2, anti-RFP, anti-PLB, and anti-β-actin. Expression levels were quantitated by densitometry in lanes 1–7. Error bars indicate SEM (n=3). *P < 0.05.
Expression of the constructs was confirmed by SDS-PAGE and immunoblot (Fig.1C/D). GFP-tagged SERCA2a was resolved from endogenous SERCA2b, as verified by binding of a GFP-specific antibody. For GFP-SERCA, there were no bands of lower mobility (apparent proteolysis) and no bands of higher mobility (apparent aggregation) in intact cells. Expression of RFP-PLB produced a single band recognized by RFP- and PLB-specific antibodies in samples heated to 95°C prior to electrophoresis. The I40A mutation disrupts pentamer formation, and we observed the disappearance of the band corresponding to PLB pentamer when the I40A mutation is present (Fig.1D; lanes 11 and 12). PLB pentamer was detected for PLBWT and L31A-PLB (Fig.1D; lanes 13 and14). As the expression level of GFP-SERCA2a, RFP-PLBWT, and PLBM is not significantly different between respective samples, we conclude that the observed changes in FRET are due to displacement of RFP-tagged PLBWT via competition (to GFP-SERCA2a) with untagged PLBM. The Ca-ATPase activity of GFP-SERCA is similar to that of untagged SERCA, so the GFP tag does not perturb endogenous function.26
3.2. Effects of loss-of-function and gain-of-binding PLB mutants on SERCA2a regulation
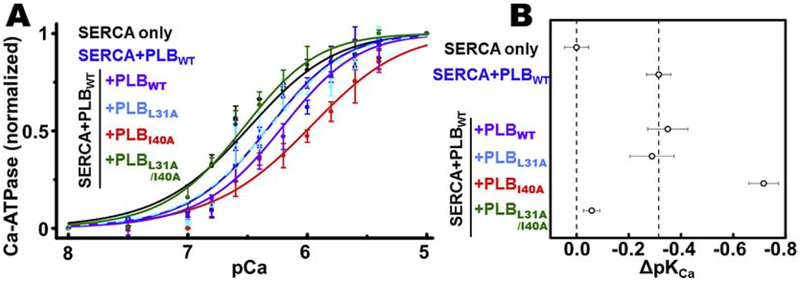
The effects of PLBM overexpression on the Ca2+-dependence of SERCA2a activity (measured by pKCa) were determined in HEK293–6E homogenate samples, enabling co-expression of PLBWT and PLBM in a cell-based system similar to the FRET assays. Ca-ATPase activity in HEK293–6E cells expressing SERCA2a alone (Fig.2A, black) increases with Ca2+ at physiological Ca2+ concentrations (e.g., between pCa 7 and pCa 6) and saturates at higher Ca2+ concentrations (pCa 5), consistent with previous measurements.29 Concomitant expression of PLBWT (Fig.2A/B, blue) decreases Ca2+ affinity (increases pKCa) of SERCA2a, and the additional expression of PLBWT (purple) or L31A-PLBM (cyan dashes) does not significantly decrease the apparent Ca2+ affinity; indicating that SERCA2a is fully inhibited and that L31A-PLBM is not sufficient to relieve PLBWT inhibition under these experimental conditions. We observed a further decrease in the apparent Ca2+ affinity upon co-expression of I40A-PLBM (red) with SERCA2a and PLBWT, suggesting that I40A-PLBM shows competitive binding to SERCA2a in the presence of PLBWT (consistent with FRET measurements) and acts as a super-shifter/inhibitor.10,12,34 Co-expression with L31A/I40A-PLBM (green) increased Ca2+ affinity to values similar to the SERCA-only sample, indicating that the PLB inhibition was relieved. Taken together, the structural (FRET) and functional (Ca-ATPase) assay results demonstrate that L31A/I40A-PLBM is non-inhibitory and binds to SERCA2a in HEK293–6E cells.
Fig. 2. SERCA activation by PLBM competition.
(A) Normalized Ca-ATPase activity of HEK293–6E cell homogenates was measured 48 hours after transfection using +empty vector (negative control, not shown), +WT-SERCA2a (black), +WT-SERCA2a/WT-PLB (blue), +WT-SERCA2a/WT-PLB +WT-PLB (purple), +WT-SERCA2a/WT-PLB +L31A-PLB (cyan, dashed line), WT-SERCA2a/WT-PLB +I40A-PLB (red), or WT-SERCA2a/WT-PLB +L31A/I40A-PLB (green). (B) Quantification of apparent Ca2+ affinity (1/pKCa) and PLBM-induced change (Δ) from (A). Error bars indicate SEM (n = 3).
3.3. rAAV-driven expression of L31A/I40A-PLBM enhances Ca2+ transients in hiPSC-CMs
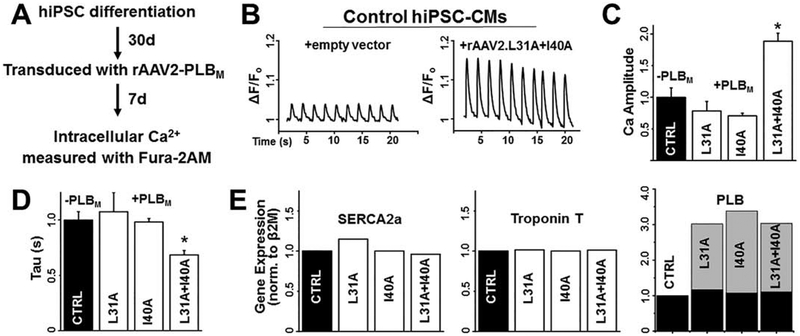
We differentiated hiPSCs derived from a healthy individual (SKiPS-31.3 line) into cardiomyocytes using established protocols that yields a predominant ventricular-like population.28 To assess physiological relevance in this native SERCA-PLB cardiac system, we tested the effects of rAAV-delivered PLBM by infecting hiPSC-CMs with empty virus, rAAV2.L31A-, rAAV2.I40A-, or rAAV2.L31A/I40A-PLBM at day 30 of differentiation and recording Ca2+ transients via fura-2AM upon electrical stimulation at day 37 (Fig.3A). Ca2+ transients were regular in appearance (Fig.3B). hiPSC-CMs expressing rAAV2.L31A-PLBM or rAAV2.I40A-PLBM did not show significant changes in peak amplitude or the rate of Ca2+ removal relative to control cells expressing empty vector (Fig.3C). However, there were striking improvements in Ca2+ transient parameters (increased peak amplitude, increased Ca2+ removal rate) in hiPSC-CMs infected with rAAV2.L31A/I40A-PLBM (Fig.3B/C). Expression of the exogenous PLBM was confirmed by qRT-PCR where virally-infected hiPSC-CMs had approximately two PLBM per one PLBWT (Fig.3D). We observed no differences in SERCA2a levels indicating that enhanced Ca2+ transport is due to altered PLB regulation and not a compensatory change in SERCA2a expression (Fig.3D). Cardiac troponin T expression levels were also unchanged, suggesting that PLBM does not significantly alter hiPSC-CM differentiation.
Fig. 3. In normal, healthy hiPSC-CMs, rAAV2-driven expression of L31A/I40A-PLB enhances Ca2+ release amplitude and Ca2+ removal rate.
(A) Schematic of experimental design for hiPSC differentiation, transduction, and cytosolic Ca2+ assay. (B) Representative Ca2+ transients in control hiPSC-CMs (left) compared to rAAV2-L31A/I40A PLB transduced hiPSC-CMs (right). (C) Quantification of Ca2+ release amplitude (peak height) normalized to uninfected control (CTRL) and (D) Ca2+ removal time constant (Tau) of hiPSC-CMs normalized to uninfected control (CTRL). Error bars indicate SEM (n = 3). *P < 0.05. (E) qRT-PCR analysis of gene expression of Ca2+ transport and contractility proteins: SERCA2a (left), cardiac troponin T (center), and PLB (right; black = endogenous PLBWT, gray = exogenous PLBM) of uninfected (CTRL) or infected (rAAV2-L31A/I40A PLB) hiPSC-CMs. Gene expression values are normalized to uninfected control.
3.4. Relief of SERCA2a inhibition by PLBM rescues irregular Ca2+ transients in cardiomyopathic R14del-PLB hiPSC-CMs
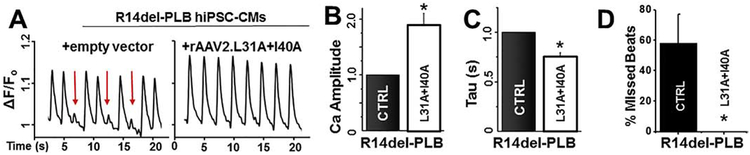
Autosomal dominant mutations in PLB have been linked to DCM and these mutations include R9C35, R14del36–38, R25C39, and L39stop40. Recent work using hiPSC-CMs derived from patients heterozygous for the R14del-PLB mutation show mislocalization of R14del-PLB into aggregates leading to higher levels of autophagy and accompanying irregular Ca2+ transients.41,42 Mice heterozygous for the R14del-PLB mutation have severe defects in SERCA2a activity and Ca2+ transport. We sought to characterize the effects of viral expression of PLBM in this dilated cardiomyopathic model to evaluate therapeutic potential. To accomplish this, we generated a R14del-PLB knock-in hiPSC line using homologous recombination via CRISPR/Cas9 to insert the R14del-PLB mutation into the control SKiPS-31.3 line (Fig.3), producing an isogenic control to test our therapies.28 While the R14del-PLB hiPSC-CMs display no differences in Ca2+ transients parameters measured at day 21 (compared to wild type cells), we observed an arrhythmic (irregular) Ca2+ transient profile in R14del-PLB hiPSC-CMs after day 37 of differentiation. This irregularity occurred at a frequency of 58 ± 19% of Ca2+ transient recorded under paced conditions. (n = 85 cells) (Fig.4A/D). This phenotype switch was also observed in patient-derived R14del hiPSC-CMs.41 Upon infection with rAAV2.L31A/I40A-PLB, we observed an improvement in Ca2+ handling properties of the R14del-PLB hiPSC-CMs. Representative Ca2+ tracings show a significant reduction in irregular Ca2+ transients and improvements in Ca2+ amplitude and tau upon rAAV2.L31A/I40A-PLB infection (Fig.4B/C). Finally, no irregular Ca2+ transients (n = 20 cells) were observed after infection with rAAV2.L31A/I40A-PLB (Fig.4D). Altogether, these data indicate an impairment in Ca2+ cycling in R14del-PLB hiPSC-CMs that is corrected by exogenous expression of the SERCA activating L31A/I40A-PLBM.
Fig. 4. In cardiomyopathic R14del hiPSC-CMs, viral delivery of L31A/I40A-PLB rescues arrhythmogenic Ca2+ transients and enhances cellular Ca2+ cycling.
(A) Representative Ca2+ transients in R14del PLB hiPSC-CMs (left) andR14del hiPSC-CMs infected with rAAV2.L31A/I40A-PLB (right). Arrhythmogenic Ca2+ transients are identified by red arrow. (B) Ca2+ release peak amplitude (fluorescence intensity) normalized to uninfected R14del hiPSC-CMs. (C) Ca2+ re-uptake time constant (Tau) normalized to uninfected R14del hiPSC-CMs. (D) Irregular Ca2+ transients determined by quantifying the number of individual cells exhibiting irregular Ca2+ transients. Error bars indicate SEM (n = 3). *P < 0.05.
4. Discussion
Hallmarks of heart failure include decreased contractile velocity, decreased relaxation rates, and pathological remodeling.1 Although the critical events that lead to impaired cardiac performance are still being determined, it is clear that pathways controlling intracellular Ca2+ homeostasis significantly contribute to decreased cardiomyocyte and contractile function.2–4 SERCA2a and PLB are major determinants of SR Ca2+ transport in the heart, and alterations to their function has profound effects on intracellular Ca2+ cycling.5 We demonstrated previously that the interaction between the cardiac Ca2+ pump, SERCA2a, and its principal inhibitor, PLB, can be measured via FRET in HEK293 cells.29 In the present study, we applied this FRET assay to measure the competition of non-inhibitory PLBM and RFP-labeled PLBWT for binding to GFP-SERCA2a. We used this assay to measure the relative affinity of non-inhibitory PLBM and identified a double mutant (L31A/I40A) that binds with affinity greater than PLBWT. Expression of L31A/I40A-PLBM increased Ca-ATPase activity in PLBWT-expressing HEK293 cells. Mechanistically, these results strongly suggest that L31A/I40A-PLBM enhances SERCA activity by competitively displacing the inhibitory form of PLB. These results demonstrate that it is possible to separate the inhibitory potency of PLB from its binding affinity. Specifically, the properties of the PLB double mutant (L31A = loss-of-inhibition = LOI; I40A = gain-of-binding = GOB) show the dominant effect of the LOI mutation (without inhibition by the GOB mutation), while preserving binding affinity for SERCA2a. Ryanodine receptor (RyR), the SR Ca2+-release channel, is hyperphosphorylated by PKA in chronic cardiac diseases, causing dissociation of FKBP12.6 from RyR2 and increased RyR2 open probability (SR Ca2+ leak), resulting in reduced SR Ca2+ content.43 We observed that the basal (unstimulated) cytosolic Ca2+ concentration was similar in the control (empty vector) transduced hiPSC-CMs, as compared to PLB or PLBM transduced cells, indicating that there is not a significant change in RyR function or RyR leak (Fig.3B; Fig.4A).
The rate and amplitude of Ca2+ transients in cardiac cells provides two key paraments that correlate with effects on the excitation-contraction coupling in the heart.44 We found that rAAV2-driven expression of L31A/I40A-PLBM significantly improved Ca2+ release amplitude and Ca2+ removal in both healthy and pathogenic hiPSC-CM lines. Using CRISPR/Cas9 we created a knock-in hiPSC cell line that is heterozygous for the DCM-causing mutation R14del-PLB. We differentiated this cell line into cardiomyocytes to study potential defects in Ca transport and homeostasis. Similar to hiPSC-CMs derived from R14del-PLB human patients,41 our cell line developed an arrhythmia-like Ca2+ transient phenotype by day 37 of differentiation. We demonstrated that expression of L31A/I40A-PLBM reversed Ca transport dysfunction and irregular Ca2+ transients in R14del-PLB hiPSC-CMs.
In this study, we have combined FRET-based SERCA and PLB biosensors in HEK293 cells with the use of control and cardiomyopathic hiPSC-CMs to correlate effects on SERCA2a structure-function. We acknowledge the limitations of the HEK293 and hiPSC cell models. HEK293 cells expressing human SERCA and PLB have been a standard in biophysical approaches.10,26,29,30,45,46 However, these cells do not express other components that compose the cardiomyocyte proteosome, including key Ca2+ handling components and contractile elements. hiPSC-CM is a renewable human cardiac cell line that retains the disease mutation and can be gene-edited, avoids invasive approaches to obtain human cardiac tissue, and can be utilized for drug discovery. However, hiPSC-CMs are immature compared to adult myocytes and are a heterogeneous population.47 These results may justify future studies to test potential therapeutic effects in vivo and in human heart tissues. Altogether, this work paves the way for potential novel therapies for heart diseases associated with impaired Ca2+ transport and decreased contractility.
Highlights.
SERCA activation improves contractility in cardiac diseases with impaired Ca2+ transport.
Engineered PLB mutant (PLBM) binds SERCA to relieve inhibition by PLBWT.
PLBM increased the Ca2+ affinity and Ca2+-activated ATPase activity of SERCA.
Viral expression of PLBM in hiPSC-CMs enhanced the Ca2+ uptake rate and release.
PLBM rescued irregular Ca2+ transients in dilated cardiomyopathic hiPSC-CMs.
Acknowledgments
This study was supported by N.I.H. grants to D.D.T. (GM27906, HL129814, and AG26160). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Bengt Svensson for help with figures. Spectrophotometric assays were performed in the Biophysical Technology Center at the University of Minnesota Department of Biochemistry, Molecular Biology, and Biophysics.
Abbreviations:
- Ca2+
calcium
- B2M
β2-microglobulin
- DCM
dilated cardiomyopathy
- ER
endoplasmic reticulum
- FLT
fluorescence lifetime
- FRET
fluorescence resonance energy transfer
- GOB
gain of binding function
- GFP
green fluorescent protein
- hiPSC-CM
human induced pluripotent stem cell-derived cardiomyocyte
- HEK
human embryonic kidney
- HF
heart failure
- LOI
loss of inhibitory function
- mAb
monoclonal antibody
- miRNA
microRNA
- pAb
polyclonal antibody
- PLB
phospholamban
- PLBM
phospholamban mutant
- PLBWT
wild type phospholamban
- rAAV
recombinant adeno-associated virus
- RFP
red fluorescent protein
- SERCA2a
sarcoendoplasmic reticulum calcium ATPase 2a
- SR
sarcoplasmic reticulum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
None
References
- 1.Writing Group M et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 133, e38–360, doi: 10.1161/CIR.0000000000000350 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Ablorh ND & Thomas DD Phospholamban phosphorylation, mutation, and structural dynamics: a biophysical approach to understanding and treating cardiomyopathy. Biophys Rev 7, 63–76, doi: 10.1007/s12551-014-0157-z (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorski PA, Ceholski DK & Hajjar RJ Altered myocardial calcium cycling and energetics in heart failure--a rational approach for disease treatment. Cell Metab 21, 183–194, doi: 10.1016/j.cmet.2015.01.005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marks AR Calcium cycling proteins and heart failure: mechanisms and therapeutics. J Clin Invest 123, 46–52, doi: 10.1172/JCI62834 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bers DM Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70, 23–49, doi: 10.1146/annurev.physiol.70.113006.100455 (2008). [DOI] [PubMed] [Google Scholar]
- 6.MacLennan DH & Kranias EG Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol 4, 566–577, doi: 10.1038/nrm1151 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Robia SL et al. Forster transfer recovery reveals that phospholamban exchanges slowly from pentamers but rapidly from the SERCA regulatory complex. Circ Res 101, 1123–1129 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Autry JM & Jones LR Functional co-expression of the canine cardiac Ca2+ pump and phospholamban in Spodoptera frugiperda (Sf21) cells reveals new insights on ATPase regulation. J Biol Chem 272, 15872–15880, doi: 10.1074/jbc.272.25.15872 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Cornea RL, Jones LR, Autry JM & Thomas DD Mutation and phosphorylation change the oligomeric structure of phospholamban in lipid bilayers. Biochemistry 36, 2960–2967 (1997). [DOI] [PubMed] [Google Scholar]
- 10.Kimura Y, Kurzydlowski K, Tada M & MacLennan DH Phospholamban inhibitory function is activated by depolymerization. J Biol Chem 272, 15061–15064, doi: 10.1074/jbc.272.24.15061 (1997). [DOI] [PubMed] [Google Scholar]
- 11.Simmerman HK, Kobayashi YM, Autry JM & Jones LR A leucine zipper stabilizes the pentameric membrane domain of phospholamban and forms a coiled-coil pore structure. J Biol Chem 271, 5941–5946, doi: 10.1074/jbc.271.10.5941 (1996). [DOI] [PubMed] [Google Scholar]
- 12.Cornea RL, Autry JM, Chen Z & Jones LR Reexamination of the role of the leucine/isoleucine zipper residues of phospholamban in inhibition of the Ca2+ pump of cardiac sarcoplasmic reticulum. J Biol Chem 275, 41487–41494, doi: 10.1074/jbc.M008195200 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Hasenfuss G et al. Relation between myocardial function and expression of sarcoplasmic reticulum Ca(2+)-ATPase in failing and nonfailing human myocardium. Circ Res 75, 434–442 (1994). [DOI] [PubMed] [Google Scholar]
- 14.Mercadier JJ et al. Altered sarcoplasmic reticulum Ca2(+)-ATPase gene expression in the human ventricle during end-stage heart failure. J Clin Invest 85, 305–309, doi: 10.1172/JCI114429 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagai R et al. Regulation of myocardial Ca2+-ATPase and phospholamban mRNA expression in response to pressure overload and thyroid hormone. Proc Natl Acad Sci U S A 86, 2966–2970, doi: 10.1073/pnas.86.8.2966 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt U et al. Restoration of diastolic function in senescent rat hearts through adenoviral gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase. Circulation 101, 790–796 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Byrne MJ et al. Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther 15, 1550–1557, doi: 10.1038/gt.2008.120 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Jessup M et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation 124, 304–313, doi: 10.1161/CIRCULATIONAHA.111.022889 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zsebo K et al. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circulation research 114, 101–108, doi: 10.1161/CIRCRESAHA.113.302421 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Greenberg B et al. Prevalence of AAV1 neutralizing antibodies and consequences for a clinical trial of gene transfer for advanced heart failure. Gene Ther 23, 313–319, doi: 10.1038/gt.2015.109 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andino LM et al. AAV-mediated knockdown of phospholamban leads to improved contractility and calcium handling in cardiomyocytes. J Gene Med 10, 132–142, doi: 10.1002/jgm.1131 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Grobetal T et al. A novel artificial microRNA expressing AAV vector for phospholamban silencing in cardiomyocytes improves Ca2+ uptake into the sarcoplasmic reticulum. PLoS One 9, e92188, doi: 10.1371/journal.pone.0092188 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bish LT et al. Cardiac gene transfer of short hairpin RNA directed against phospholamban effectively knocks down gene expression but causes cellular toxicity in canines. Hum Gene Ther 22, 969–977, doi: 10.1089/hum.2011.035 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soller KJ et al. Rheostatic regulation of the SERCA/phospholamban membrane protein complex using non-coding RNA and single-stranded DNA oligonucleotides. Sci Rep 5, 13000, doi: 10.1038/srep13000 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soller KJ, Yang J, Veglia G & Bowser MT Reversal of phospholamban inhibition of the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) using short, protein-interacting RNAs and oligonucleotide analogs. J Biol Chem 291, 21510–21518, doi: 10.1074/jbc.M116.738807 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bidwell P, Blackwell DJ, Hou Z, Zima AV & Robia SL Phospholamban binds with differential affinity to calcium pump conformers. J Biol Chem 286, 35044–35050, doi: 10.1074/jbc.M111.266759 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gruber SJ et al. Discovery of enzyme modulators via high-throughput time-resolved FRET in living cells. J Biomol Screen 19, 215–222, doi: 10.1177/1087057113510740 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ceholski DK et al. Functional and transcriptomic insights into pathogenesis of R9C phospholamban mutation using human induced pluripotent stem cell-derived cardiomyocytes. J Mol Cell Cardiol 119, 147–154, doi: 10.1016/j.yjmcc.2018.05.007 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stroik DR et al. Targeting protein-protein interactions for therapeutic discovery via FRET-based high-throughput screening in living cells. Sci Rep 8, 12560, doi: 10.1038/s41598-018-29685-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaaf TM et al. High-throughput spectral and lifetime-based FRET screening in living cells to identify small-molecule effectors of SERCA. SLAS Discov 22, 262–273, doi: 10.1177/1087057116680151 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rapti K et al. Effectiveness of gene delivery systems for pluripotent and differentiated cells. Mol Ther Methods Clin Dev 2, 14067, doi: 10.1038/mtm.2014.67 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Gordo E, Kohlbrenner E, Katz MG & Weber T AAV Vectors for Efficient Gene Delivery to Rodent Hearts. Methods Mol Biol 1950, 311–332, doi: 10.1007/978-1-4939-9139-6_19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asahi M, McKenna E, Kurzydlowski K, Tada M & MacLennan DH Physical interactions between phospholamban and sarco(endo)plasmic reticulum Ca2+-ATPases are dissociated by elevated Ca2+, but not by phospholamban phosphorylation, vanadate, or thapsigargin, and are enhanced by ATP. J Biol Chem 275, 15034–15038, doi: 10.1074/jbc.275.20.15034 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Autry JM & Jones LR High-level coexpression of the canine cardiac calcium pump and phospholamban in Sf21 insect cells. Ann N Y Acad Sci 853, 92–102, doi: 10.1111/j.1749-6632.1998.tb08259.x (1998). [DOI] [PubMed] [Google Scholar]
- 35.Schmitt JP et al. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science 299, 1410–1413, doi: 10.1126/science.1081578299/5611/1410[pii] (2003). [DOI] [PubMed] [Google Scholar]
- 36.Haghighi K et al. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc Natl Acad Sci U S A 103, 1388–1393, doi:0510519103 [pii] 10.1073/pnas.0510519103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karakikes I et al. Correction of human phospholamban R14del mutation associated with cardiomyopathy using targeted nucleases and combination therapy. Nat Commun 6, 6955, doi: 10.1038/ncomms7955 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Heijden JF & Hassink RJ The phospholamban p.Arg14del founder mutation in Dutch patients with arrhythmogenic cardiomyopathy. Neth Heart J 21, 284–285, doi: 10.1007/s12471-013-0413-z (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu GS et al. A novel human R25C-phospholamban mutation is associated with super-inhibition of calcium cycling and ventricular arrhythmia. Cardiovasc Res 107, 164–174, doi: 10.1093/cvr/cvv127 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haghighi K et al. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest 111, 869–876, doi: 10.1172/JCI17892 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karakikes I et al. Correction of human phospholamban R14del mutation associated with cardiomyopathy using targeted nucleases and combination therapy. Nat Commun 6, 6955, doi: 10.1038/ncomms7955 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Te Rijdt WP et al. Phospholamban p.Arg14del cardiomyopathy is characterized by phospholamban aggregates, aggresomes, and autophagic degradation. Histopathology 69, 542–550, doi: 10.1111/his.12963 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Bers DM, Eisner DA & Valdivia HH Sarcoplasmic reticulum Ca2+ and heart failure: roles of diastolic leak and Ca2+ transport. Circ Res 93, 487–490, doi: 10.1161/01.RES.0000091871.54907.6B (2003). [DOI] [PubMed] [Google Scholar]
- 44.Ahola A, Polonen RP, Aalto-Setala K & Hyttinen J Simultaneous Measurement of Contraction and Calcium Transients in Stem Cell Derived Cardiomyocytes. Ann Biomed Eng 46, 148–158, doi: 10.1007/s10439-017-1933-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelly EM, Hou Z, Bossuyt J, Bers DM & Robia SL Phospholamban oligomerization, quaternary structure, and sarco(endo)plasmic reticulum calcium ATPase binding measured by fluorescence resonance energy transfer in living cells. J Biol Chem 283, 12202–12211, doi: 10.1074/jbc.M707590200 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pallikkuth S et al. Phosphorylated phospholamban stabilizes a compact conformation of the cardiac calcium-ATPase. Biophys J 105, 1812–1821, doi: 10.1016/j.bpj.2013.08.045 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamdar F, Klaassen Kamdar A, Koyano-Nakagawa N, Garry MG & Garry DJ Cardiomyopathy in a dish: using human inducible pluripotent stem cells to model inherited cardiomyopathies. J Card Fail 21, 761–770, doi: 10.1016/j.cardfail.2015.04.010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]