Abstract
We used a screening strategy to test for reprogramming factors for the conversion of human cardiac progenitor cells (CPCs) into Pacemaker-like cells. Human transcription factors SHOX2, TBX3, TBX5, TBX18, and the channel protein HCN2, were transiently induced as single factors and in trio combinations into CPCs, first transduced with the connexin 30.2 (CX30.2) mCherry reporter. Following screens for reporter CX30.2 mCherry gene activation and FACS enrichment, we observed the definitive expression of many pacemaker specific genes; including, CX30.2, KCNN4, HCN4, HCN3, HCN1, and SCN3b. These findings suggest that the SHOX2, HCN2, and TBX5 (SHT5) combination of transcription factors is a much better candidate in driving the CPCs into Pacemaker-like cells than other combinations and single transcription factors. Additionally, single-cell RNA sequencing of SHT5 mCherry+ cells revealed cellular enrichment of pacemaker specific genes including TBX3, KCNN4, CX30.2, and BMP2, as well as pacemaker specific potassium and calcium channels (KCND2, KCNK2, and CACNB1). In addition, similar to human and mouse sinoatrial node (SAN) studies, we also observed the down-regulation of NKX2.5. Patch-clamp recordings of the converted Pacemaker-like cells exhibited HCN currents demonstrated the functional characteristic of pacemaker cells. These studies will facilitate the development of an optimal Pacemaker-like cell-based therapy within failing hearts through the recovery of SAN dysfunction.
Keywords: hADMSCs, CPCs, sinoatrial node, HCN, pacemaker cells
ONE SENTENCE SUMMARY
The SHOX2, HCN2, and TBX5 (SHT5) combination of transcription factors and channel proteins can be used to reprogram CPCs into Pacemaker-like cells as a potential stem cell therapy for sick sinus syndrome (SSS).
INTRODUCTION
The electrical cardiac conduction system (CCS), which includes the sinoatrial node (SAN), atrioventricular node (AVN) and the Purkinje fibers, coordinates the heart’s rate and rhythm (1). The SAN is responsible for initiating electric impulses down through a hierarchical pattern of the CCS to coordinate the asynchronous contractions of the atria and ventricles (2). However, failure of the SAN or a block at any point in the CCS results in arrhythmias. One major conduction disorder that results in cardiac arrhythmias is sick sinus syndrome (SSS); in which the SAN does not function properly (3). Failure of the SAN has been attributed to multiple factors including congenital defects, sarcoidosis (infections), and cardiomyopathies. Further, myocardial ischemia (MI) results in cardiomyocyte loss and scar formation, thereby creating a mechanical barrier and abnormal electrical conduction; eventually contributing to the development of cardiac arrhythmias. Furthermore, heart rhythm abnormalities are often caused or worsened by medications and these abnormalities increase with age (4). In order to circumvent these problems, the focus over the past decade has been on the development of biological pacemakers, as an alternative treatment for conduction system disorders, cardiac repair after an MI, and the limitations of the electronic pacemaker.
The SAN is the primary pacemaker of the heart and is responsible for generating the electric impulse or beat (5). Native cardiac pacemaker cells are anatomically confined within the SAN, a small structure comprised of just a few thousand specialized pacemaker cells (6). During embryonic development, the cardiac pacemaker cells originate from a subset of progenitors distinct from the first cells marked by NKX2.5 (7) and reviewed in Burkhard et al (8). SHOX2, a member of the short stature paired-homeodomain family of transcription factors, is a major genetic determinant of the SAN genetic pathway and is restrictedly expressed in the region of the SAN (9). SHOX2 inhibits NKX2.5 expression and activates a pacemaker genetic pathway that results in up-regulation of the GATA6 and TBX3 transcription factors, and HCN4 channel (10). Along with the T-box transcription factor TBX3, TBX2 maintains SHOX2 marked cells in a state characteristic that of pacemaker-nodal cardiac myocytes (11). Other T-box transcription factors, such as TBX5 and TBX18, may also stimulate pacemaker cell activity through unknown mechanisms (12, 13).
Cell-based cardiac tissue engineering strategies may, therefore, provide regenerative therapeutic options of equivalent function to mechanical and electrical devices. To reinforce this notion, we have reprogrammed human adipogenic mesenchymal stem cells (hADMSCs) into cardiac progenitor cells (CPCs) with ETS2 and MESP1, as co-activators of cardiac differentiation (14). Human ADMSCs are ideal for cell-based therapies since they can be obtained from self-donor patients, treated, and transplanted back to the patient without the burden of immune rejection and or tumor formation (15). Clinical trials worldwide involving hADMSCs in the treatment of human disease (clinicaltrials.gov) have been proven safe and preserved ventricular function in patients (15); however, hADMSCs have failed to be successfully reprogrammed into either cardiac myocytes, vascular cells, pacemaker cell, or Purkinje cells. Although several recent studies have indicated the potential of hADMSCs for cardiac conversion (15–17), no study has convincingly demonstrated their conversions to conduction cells such as pacemaker cells and/or Purkinje cells. Our strategy was to use reprogrammed human CPCs (14) in a novel screening assay using a variety of transcription factors and channel proteins to convert into human cardiac Pacemaker-like cells.
MATERIAL AND METHODS
Reprogramming of hADMSCs into CPCs
Human ADMSCs (hADMSCs) were reprogrammed into CPCs using the human transcription factors ETS2/MESP1 for the differentiation of human cardiac fibroblasts into CPCs (14). Initially, hADMSCs were pre-infected with NKX2.5 td-tomato puromycin reporter, followed by treatment with TAT-fused proteins ETS2 and MESP1 for 3-days, at a concentration of 50 μM each. Subsequently, these cells were forced aggregated (600 cells per aggregate) and kept in hanging drops for 2-days. Afterward, the cells were plated and treated with Activin and BMP (2-days) with normal media change. On activation of NKX2.5 td-tomato reporter, the marker for CPC development, the cells were drug selected via puromycin (2-days drug selection and left for an additional 8-days to grow).
Conversion of CPCs into Pacemaker-like Cells
Reprogramming of CPCs was initially accomplished by infecting 70–80% confluent plates with rtTA2 lentiviral vector and pWPI-CX30.2-puro IRES mCherry reporter lentiviral pacemaker-specific vectors for tracking and flow cytometry sorting of reprogrammed cells. To reprogram CPCs into Pacemaker-like cells, CX30.2 vector infected CPCs were split into eight plates and infected with pDox-SHOX2-eGFP, pDox-HCN2-eGFP, pDox-TBX3-eGFP, pDox-TBX5-eGFP, and pDox-TBX18-eGFP individually and in multiple combinations for transient gene expression. Lentiviral infected cultures were treated with 1 μg/ml doxycycline for 3-days to induce transient transcription factor expression. After 3-days, the doxycycline-induced expressed enhanced Green Fluorescent Protein (eGFP) labeled transcription factors were then observed under the microscope and the expressed transcription factor proteins were confirmed by Western blot of cell lysates. On day-4, all the different combinations were FACS sorted for eGFP+ cells and cultured under similar conditions as CPCs. After a week, the eGFP+ cells were FACS sorted for mCherry+ cells, followed by gene expression analysis by RT-PCR, patch-clamp recording, RNA sequencing, and single-cell RNA sequencing studies.
Cell Culture
CPCs were cultured in alpha-MEM (Life Technologies Corporation) supplemented with 1% (v/v) 1X Glutamax (Life Technologies Corporation), 10% (v/v) FBS (GenDEPOT) and 100 U/ml penicillin. Media for cells was changed every 2-days. Cells were washed twice with Dulbecco’s phosphate-buffered saline (GenDEPOT) prior to trypsin-EDTA (1X) (GenDEPOT) treatment with the purpose of passaging, FACS sorting, other downstream experiments, and freezing. Cell freezing media was composed of alpha-MEM supplemented with 20% (v/v) FBS and 7–8% (v/v) DMSO. Transient gene expression was induced by supplementing the cell culture media with doxycycline (1μg/ml) (Clontech). 293T cells were cultured in DMEM (Life Technologies Corporation) supplemented with 10% (v/v) FBS and 100U/ml penicillin. Control CPCs were cultured for the same amount of time as the lentiviral infected CPC cultures.
Plasmid Extraction
All the plasmid DNA which was used for transfection were amplified by transforming into DH5 alpha cells. Plasmid DNA was extracted using a maxi prep kit according to the manufacturer’s instructions (#12162, Qiagen) and quantified using Nanodrop.
Lentiviral Production and Transduction
Lentivirus production for all the plasmids was carried out in 70–80% confluent HEK-293T cells (DNA : transfection reagent; 1:2) using JetPrime transfection reagent (Polyplus-transfection) according to the manufacturer’s instruction. Viral supernatant collected at 48 and 72 hours was filtered through 0.45 μm cellulose filter and then concentrated using Lenti-X concentrator (#631232, Takara Bio USA, Inc.) following the manufacturer’s protocol. For virus transduction, the virus was added to alpha-MEM media containing polybrene at 8 μg/ml concentration. Twenty-four hours after plating the CPCs, the alpha-MEM media was replaced with viral media containing freshly made polybrene. After 24 h, the viral media was replaced with fresh media.
Western Blot Analysis
The cell samples were lysed using radioimmunoprecipitation assay (RIPA) buffer supplemented with protease inhibitor cocktail (P8340, Sigma Aldrich). Lysed samples were denatured by boiling at 100°C for 5 minutes with NuPAGE LDS Sample Buffer (NP0007, Life Technologies) and subjected to electrophoresis with Novex NUPAGE system. This was followed by transferring protein onto polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% milk for an hour. The blots were then treated with primary antibodies overnight, followed by TBS (0.1% tween 20) wash 3 times each for 5 minutes. Then the membranes were probed with horseradish peroxidase (HRP) conjugated secondary antibody for 2 hours at RT. The blots were developed using Enhanced Chemiluminescence Western Lightning Plus (Cat #NEL104001EA, Perkin Elmer).
| Primer | Sequence | Concentration | Primerbank ID |
|---|---|---|---|
| SHOX2 | Mouse Monoclonal | 1:1000 | Sigma Aldrich |
| HCN2 | Rabbit Monoclonal | 1:1000 | Celi Signaling |
| TBX3 | Mouse Monoclonal | 1:1000 | Santa Cruz Biotechnology |
| HA-Tag | Mouse Monoclonal | 1:1000 | GenDEPOT |
| TBX18 | Mouse Monoclonal | 1:1000 | Santa Cruz Biotechnology |
| Anti-mouse | 1:10,000 | Celi Signaling | |
| Anti-rabbit | 1:10,000 | Celi Signaling |
List of antibodies, species, their concentration, and company.
Fluorescence Microscopy
Brightfield and fluorescence images of cell cultures were acquired using a Nikon Ti-E inverted microscope attached with a DS-Fi 1 5-megapixel color camera (Nikon Instruments). Images were captured and analyzed using NIS Elements software v4.13 (Nikon Instruments).
FACS Analysis and Sorting
CPCs at the time-points mentioned were first washed with PBS twice and then dissociated using 0.25% trypsin. Following which the cells were then neutralized using FBS and centrifuged at 1000 rpm for 5 min to get the cell pellet. The cell pellet was then resuspended in alpha-MEM supplemented with 1% FBS, counted using hemocytometer and diluted to obtain 1–1.5 * 106 cells/ml. The cells were then passed through a cell strainer and into a conical FACS tube. A BD LSRII flow cytometer (BD Biosciences) was used to perform FACS analysis and then subsequently followed by sorting on a FACSaria II flow cytometer (BD Biosciences). Sorted cells were collected in alpha-MEM supplemented with 50% FBS and then plated on cell culture plates.
RNA Isolation and Real-time RT-PCR analysis
RNA was isolated from mCherry+ cells after FACS sorting by following the manufacturer’s instructions (R1054, Zymo Research) and quantified using Nanodrop. cDNA synthesis was performed using a high capacity RT-PCR kit (#4368814, Life Technologies) according to the manufacturer’s instructions. cDNA was subjected to qRT-PCR using Power SYBR Green PCR Master Mix (#4367659, Life Technologies) in a StepOnePlus Real-Time PCR System (v. 2.0, Applied Biosystems). Normally, all the real-time PCR reactions were carried out as 15 μL reactions in 96-well plates. Each reaction mixture contained 1 μL diluted cDNA, 2 μl each of forward and reverse primers (10 μM), 7.5 μL 2X SYBR Green PCR Master Mix, and 2.5 μL water. PCR primers were from the PrimerBank database (18). Normalization was performed using GAPDH mRNA levels.
| Primer | Sequence | Primerbank ID |
|---|---|---|
|
HCN1 – forward HCN1 – reverse |
5′ CATGCCACCGCTTTAATCCAG 3′ 5′ ATTGTAGCCACCAGTTTCCGA 3′ |
349501105c2 |
|
HCN3 – forward HCN3 – reverse |
5′ AGCAGTGGAAATCGAGCAGG 3′ 5′ GGTCCCAGTAAAACCGGAAGT 3′ |
38327036c2 |
|
HCN4 – forward HCN4 – reverse |
5′ GAACAGGAGAGGGTCAAGTCG 3′ 5′ CATTGAAGACAATCCAGGGTGT 3′ |
210147528c2 |
|
SCN3b – forward
SCN3b – reverse |
5′ GCCTTCAATAGATTGTTTCCCCT 3′ 5′ CTCGGGCCTGTAGAACCAT 3′ |
93587331c1 |
|
Cx30.2 (GJC3) – forward
Cx30.2 (GJC3) – reverse |
5′ TGGAGTCAGCGGTTTCTGTC 3′ 5′ TTGTGTCTTCTGGTGCTCTCT 3’ |
289177042c3 |
|
KCNN4 – forward
KCNN4 – reverse |
5′ CTGCTGCGTCTCTACCTGG 3′ 5′AGGGTGCGTGTTCATGTAAAG 3′ |
25777651c1 |
|
GAPDH – forward
GAPDH – reverse |
5′ GGAGCGAGATCCCTCCAAAAT 3′ 5′ GGCTGTTGTCATACTTCTCATGG 3′ |
378404907c1 |
Primer sequences with their respective Primerbank ID.
Patch Clamp Electrophysiology
Whole-cell voltage-clamp recordings were carried out on each cell line [CPCs (control) and SHT5 mCherry+ FACS sorted cells (reprogrammed)]. The external solution for HCN currents contained the following (in mM): 140 NaCl, 5.4 KCl, 1 MgCl2, 5 BaCl2, 2 CoCl2, 0.5 4-aminopyridine, 10 glucose, 5 HEPES, 1.8 CaCl2 and with the pH adjusted to 7.4 with NaOH. Cesium chloride (CsCl, 5 mM) was added to the external solution as the Cs+ external solution. The pipette solution was (in mM): 130K-aspartate, 5 Na2-ATP, 5 CaCl2, 2 MgCl2, 11 EGTA, 10 HEPES and with the pH adjusted to 7.35 with KOH. A patch-clamp amplifier (Model 2400. A-M Systems, Carlsborg, WA, USA) was used to record currents at room temperature. This was followed by low pass-filtering of signals at 2 kHz and using the Digidata 1550A interface and pCLAMP 10.5 software (Axon Instruments/Molecular Devices, Union City, CA, USA) for digitizing signals at 5 kHz. Electrode capacitance was compensated after the formation of gigaohm signals.
A 5 mV (100 ms) depolarizing voltage step was applied from −40 mV of holding potential to record series and input resistance as well as membrane capacitance for the recorded cells. Whole-cell capacitance value was determined from the membrane capacitance reading (19). Cells were held at −40 mV, and the HCN currents were recorded immediately after the whole-cell configurations were formed using beta-escin (50 μM, Sigma). The current was elicited from a holding potential at –40 mV and a hyperpolarizing step to −130mV for 1.5 sec. Each cell was first recorded with the regular external solution (without Cs+) followed by recording with the Cs+-external solution. In order to completely exchange the buffer, the recording chamber was perfused with Cs+ external buffer for at least 2 min. The current density (pA/pF) was obtained by dividing the current amplitude (pA) with the membrane capacitance (pF).
A series of hyperpolarizing step commend (from −135 mV to −55 mV with a 10 mV increment, each step for 3 sec) followed by a depolarization step (to +5 mV for 1 sec) was applied to determine the channel activation kinetics. To generate the activation curve, the Cs+ sensitive current at each voltage-step (I) was normalized to the peak current (Imax at −135 mV), and the I/Imax was plotted then fitted with the Boltzmann equation: I/Imax = 1 / [1-exp ((V1/2-V) / k)]. The voltage that elicited half of the maximal current was also determined as V1/2. For isoproterenol (ISO) treatment, the 0.5 mM ISO in DMSO stock solution was prepared freshly and diluted with the culture medium. The cells were incubated for 1 h in the presence of 100 nM ISO or the vesicle DMSO (as the control). Then the cells subjected to patch-clamp recordings. All data are presented as mean ± standard error of mean (s.e.m.). The Student’s t-test was used for the statistical analyses. Throughout, *p<0.05 was regarded as significant.
RNA-Sequencing Library Preparation and Sequencing
RNA was extracted using Mirneasy Mini Kit (Qiagen) with on-column RNase-Free DNase (Qiagen) digestion following the manufacturer’s instructions. Extracted RNA samples underwent quality control assessment using the RNA Nano 6000 chip on Bioanalyzer 2100 (Agilent) and were quantified with Qubit Fluorometer (Thermo Fisher). The RNA libraries were prepared and sequenced at the University of Houston Seq-N-Edit Core per standard protocols. mRNA libraries were prepared with Universal Plus mRNA-Seq kit (NuGen) using 1000 ng input RNA. The size selection for libraries was performed using SPRIselect beads (Beckman Coulter) and purity of the libraries was analyzed using the High Sensitivity DNA chip on Bioanalyzer 2100 (Agilent). The prepared libraries were pooled and sequenced using NextSeq 500 (Illumina); generating ~20 million 2×76 bp paired-end reads per samples.
RNA-Sequencing Transcriptome Analysis
The RNA-seq raw fastq data were processed with RNA-Seq Alignment app within the Illumina BaseSpace app suite (www.basespace.illumina.com): the adaptors were trimmed and reads were mapped to hg19 human reference genome using the STAR aligner (20) to generate BAM files, and FPKM estimation of reference genes and transcripts were performed using Cufflinks 2 (21). Based on this gene count matrix, we used “DESeq2” package (22) to identify differentially expressed genes between SHT5 cells versus CPCs. The significance level of FDR adjusted p-value of 0.05 was used to identify differentially expressed genes.
Single-cell RNA-Sequencing Library Preparation and Sequencing
Cells were re-suspended in PBS with 0.04% BSA (Ambion) to a final concentration of 200 cells per μl on the day of single-cell capture and library preparation. This cell suspension was used as input for automated microfluidic single-cell capture and barcoding using the 10X Genomics Full Chromium platform. Approximately 560 single-cells were captured for each sample using the 10X Genomics Single Cell 3’ Chip (as per manufacturer recommendations Single Cell3’ Re version CG00052) at the University of Houston Seq-N-Edit Core. Single-cell gel beads in emulsion (GEMs) were generated and single cells were uniquely barcoded. cDNA was recovered and selected for using DynaBead MyOne Silane Beads (Thermo Fisher Scientific) and SPRIselect beads (Beckman Coulter). The library was indexed by addition of a 4 random 8 bp indexes “GCATCTCC” “TGTAAGGT” “CTGCGATG” and “CTGCGATG” which are Illumina sequencer compatible i7 indexes. This sequence library then underwent quality control assessment by using a High-sensitivity DNA chip on 2100 BioAnalyzer (Agilent) and then quantified with a Qubit Fluorometer (Thermo Fisher Scientific) and with a Kapa Library Quantification Kit (Kapa Biosystems) by using the AriaMX instrument (Agilent). Libraries were sequenced using NextSeq 500 (Illumina) in stand-alone mode to obtain pair-end sequencing 26 bp (read1) X 98bp (read2) and a single index 8 bp in length obtaining ~40,000 reads per cell.
Single-cell RNA-Sequencing Transcriptome Analysis
The single-cell RNA sequencing data analysis was performed on the Maxwell High-Performance Cluster at the University of Houston. The analytical program used was the Cell Ranger 2.1.1 Single Cell Analysis Pipelines (10X Genomics). Raw base call files generated by Nextseq 500 were demultiplexed using “cellranger mkfastq” pipeline to FASTQ files. FASTQ files were aligned to hg38 human reference genome using “cellranger count” which used STAR aligner (20). Gene expression matrix was reduced using Principal Components Analysis (PCA) and visualized in 2-d space by passing PCA data into t-distributed stochastic neighbor embedding (t-SNE), a nonlinear dimensionality reduction method (23). Graph-based hierarchical clustering algorithm operating in PCA space was used to cluster cells based on the similarity of expression. Differentially expressed genes between clusters were found using sSeq method (24). The top 100 differentially expressed genes from cluster-1 were used for gene ontology. The gene list was analyzed using Gene MANIA package on Cytoscape.
| Gene Ontology | Gene List | Q-value |
|---|---|---|
| Notch Signaling Pathway | Notch4, DLL4, Maml2, Maml3, PSENEN, APH1A, APH1B, NCSTN | 9.50E-07 |
| Wnt Signaling Pathway | Wnt7B, FZD2, FZD10, FZD1, FZD4 | 5.30E-02 |
| Blood Vessel Development | Wnt7b, FZD4, Notch4, DLL4, RPBJ | 3.20E-02 |
Gene ontology of the top 100 differentially expressed genes from cluster-1.
Statistics
Data were processed using GraphPad Prism 5.0 software and Microsoft Excel. The qPCR data and patch-clamp data are presented as the mean ± standard deviation (S.D.) and mean ± standard error of the mean (S.E.M.), respectively. For qPCR, the data were analyzed based on at least two independent experiments in three independent PCR reactions. Analysis of qPCR was performed using one-way ANOVA. Dunnett’s multiple comparison tests were performed on qPCR data for comparison of multiple groups with significant differences. Student’s t-test was used for statistical analysis to compare the control and positive groups for patch-clamp recording. A test was considered significant for p < 0.05 and p < 0.001.
RESULTS
Conversion of CPCs into Pacemaker-like Cells Expressing CX30.2
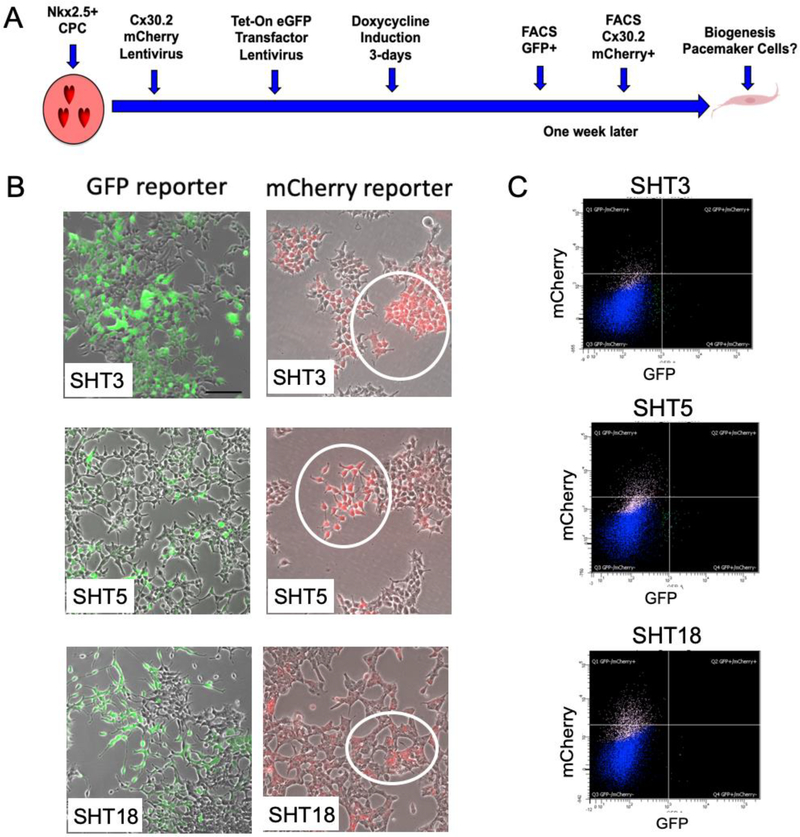
The schematic diagram in Figure-1A shows the screening strategy for converting human CPCs into pacemaker cells (detailedin Figure-S1). Previously we reprogrammed human adipogenic mesenchymal stem cells (hADMSCs) into cardiac progenitor cells (CPCs) that expressed the cardiac mesoderm marker KDR+ and cardiac-specific transfactor, NKX2.5, by using ETS2/MESP1 human transcription factors. We have also shown that ETS2/MESP1 effectively reprogrammed foreskin fibroblasts into CPCs (14). Here, we tested human transcription factors SHOX2, TBX3 channel protein HCN2, as reprogramming factors, to convert CPCs into Pacemaker-like cells. We used tetracycline-controlled transcriptional activation (tet-on) inducible reprogramming factors to regulate their transient expression within the CPCs. First, CPCs were transduced with the connexin 30.2 (CX30.2) mCherry reporter, a conduction cell marker, followed by lentiviral vectors that provided doxycycline-induction of the reporter eGFP gene fused to either SHOX2 (S), HCN2 (H), TBX3 (T3), TBX5 (T5), and TBX18 (T18) factors individually and in combinations (SHT3, SHT5, and SHT18). The CX30.2 reporter gene is a slow conduction junction channel known to render the uncoupling characteristic of pacemaker tissue to the SAN (25) as well as being responsible for integrating all the pacemaker cells with different intrinsic frequency (26). The human orthologue of the mouse CX30.2 gene is CX31.9 and has been found in human ventricular biopsies of healthy individuals (27). Upon 3-days of doxycycline induction to transiently express the reprogramming factors, we observed eGFP+ cells (Figure-1B; left panel) which were FACS sorted to obtain a pure population of eGFP+ cells (Figure-1C, Figure-S2, and Figure-S3A–E). Transient expression of these reprogramming factors was validated by SDS/PAGE and antibodies for Western blot (Figure-S4).
Figure-1: Conversion of human CPCs into Pacemaker-like cells.
(A) Schematic showing a screening strategy by which reprogrammed CPCs are engineered into Pacemaker-like cells. Initially, all the CPCs were infected with CX30.2 mCherry reporter lentivirus, followed by infection with tet-on inducible transcription factors singularly and/or in combination. Transient 3-day induction of doxycycline was carried out to induce the expression of transcription factors, followed by FACS sorting for eGFP+ cells. A week later, when mCherry+ cells were observed under the microscope, reprogrammed cells were FACS sorted for a pure population of mCherry+ cells. These mCherry+ cells were further analyzed by RT-qPCR, RNA sequencing and single-cell RNA sequencing for expression analysis and whole-cell patch-clamp analysis for functional efficiency (i.e. biogenesis of cardiac Pacemaker-like cells). (B) Fluorescence microscopy of the reprogrammed CPCs for GPF+ cells and mCherry+ cells after FACS sorting. Reprogramming was induced by the addition of 1 μg/ml doxycycline to tet-on lentivirus-transduced CPCs for 3-days with the transcription factors, both individually and in combination. Images on the left show eGFP+ cells that were treated with a combination of transcription factors (SHT3, SHT5, and SHT18). Following an additional 7-days, CPCs that were initially transduced with the CX30.2 mCherry reporter genespecific for cardiac pacemaker cells were observed for mCherry+ expression. Images on the right show mCherry+ cells treated with the combination of transcription factors (SHT3, SHT5, and SHT18). SHT5 and SHT18 generated the highest conversion rate of cell positive for mCherry labeled CX30.2 (5.9% and 8.43%, respectively). Note the mCherry+ labeling of the reprogrammed CPCs (circled). (C) FACS plots showing sorting of the transduced CPCs for mCherry+ cells (CX30.2) following induction of the transcription factors in combinations (SHT3, SHT5, and SHT18).
A week later, we observed mCherry+ cells (Figure-1B; right panel), the pacemaker-restricted cell marker, and FACS sorted the cells again to obtain a pure population of CX30.2 reporter mCherry+ cells (Figure-1C, Figure-S2, and Figure-S3F–J). All the reprogramming factors transduced singularly and in combinations generated a small population of cells positive for mCherry (Figure-1B; right panel and Figure-S3F–J). After cell sorting, we analyzed the percentage of mCherry+ cells obtained from individual combinations of transcription factors to determine the percent conversion of CPCs to Pacemaker-like cells (Table-1). Our studies showed that SHT18 and SHT5 combination of transcription factors induced the highest percent conversion of 8.4% and 5.9% CPCs to become positive for CX30.2-mCherry expression. On the contrary, the channel factor HCN2 (< 1%) had the lowest conversion rate for mCherry+ cells.
Table-1: Quantification of mCherry+ cells from FACS analyses to determine the percentage of conversion.
SHT5 and SHT18 cells exhibited a higher conversion rate, while HCN2 showed the lowest conversion rate.
| Transcription Factor Combinations | % mCherry+ Cells |
|---|---|
| SHOX2 | 2.40 |
| HCN2 | 0.22 |
| TBX3 | 4.11 |
| TBX5 | 2.64 |
| TBX18 | 5.45 |
| SHOX2-HCN2-TBX3 (SHT3) | 1.74 |
| SHOX2-HCN2-TBX5 (SHT5) | 5.90 |
| SHOX2-HCN2-TBX18 (SHT18) | 8.43 |
SHOX2, HCN2, and TBX5 (SHT5) Combination Drive Pacemaker Gene Expression
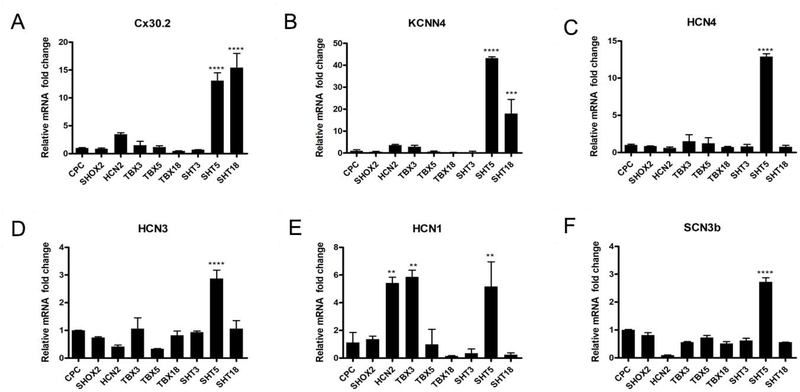
A week after tet-on induced genes were silenced, we evaluated mCherry+ cells for readout of pacemaker-specific target genes (CX30.2, KCNN4, HCN4, HCN3, HCN1, and SCN3b shown in Figure-2). Trio-factor combinations, such as SHT5 and SHT18 resulted in significant accumulation of CX30.2 and KCNN4 gene transcripts (p < 0.001), in comparison to untreated control CPCs (Figure-2A&B). KCNN4, a calcium-activated potassium channel, is critical for functional Pacemaker-like activity (28, 29). Unlike Tbx5 expression in murine ES cells which resulted in increased Cx30.2 expression, here TBX5 by itself did not increase CX30.2 expression but required the combinatorial co-expression with SHOX2 and HCN2 to elevate CX30.2 expression. (12). We observed the other individual reprogramming factors were also insufficient to raise the co-expression of CX30.2 and KCNN4. HCN4, a potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel, is a molecular marker for functional SAN cells activity, which initiates the funny current (If), playing a crucial role in the pacemaker potential (9, 30, 31). Pacemaker ion channels HCN1, HCN3, and HCN4 transcripts appeared in many of the mCherry+ cells, by the transient treatment of SHT5 (Figure-2C–E). Previously, TBX3 was shown to upregulate the HCN1 channels (32), which we confirmed (Figure-2E). Additionally, the HCN2 channel factor influenced HCN1 channel expression (Figure-2E). We also identified significant upregulation of SCN3b by the combination of SHT5 (Figure-2F). Interestingly, the knockout of Scn3b resulted in SAN abnormality in mice (33). Overall, the combination of SHOX2, HCN2, and TBX5 (SHT5) transcription factors resulted in optimization of pacemaker-specific gene activity.
Figure-2: Expression of Pacemaker Specific Genes.
RT-PCR of the reprogrammed CPCs for expression of pacemaker specific genes: (A) CX30.2, (B) KCNN4, (C) HCN4, (D) HCN3, (E) HCN1, and (F) SCN3b. CX30.2 and KCNN4 were significantly expressed in cells treated with the SHT5 and SHT18 combination of transcription factors. Similarly, expression of the HCN4 and HCN3 ion channels were significantly increased in cells treated with the SHT5 combination. However, while HCN1 expression was significantly increased in cells active by HCN2, TBX3, and SHT5 transcription factors, SCN3b expression was only increased in SHT5 activated cells. One-way ANOVA followed by Dunnett’s multiple comparison tests was applied for RT-PCR analysis. All data are expressed as mean ± SD. A significance level of **p < 0.05, ***p < 0.001, ****p < 0.0001 versus CPCs is indicated by an asterisk(s).
Transient SHOX2, HCN2, and TBX5 (SHT5) expression induced a Pacemaker Transcriptome Profile
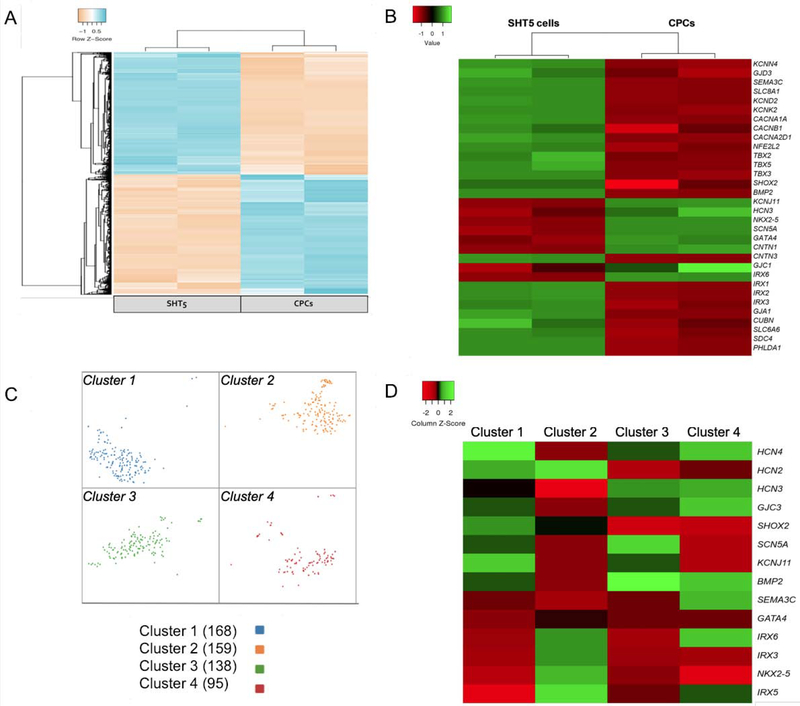
To determine the transcriptome profiling of the reprogrammed cells, we performed RNA sequencing of SHT5 cells and CPCs. The principal component analysis (PCA) plot revealed that both the SHT5 and CPC samples within the plot grouped, as defined clusters and that the biological samples of each of these two cell types were clustered tightly (Figure-S5). The global heat map revealed that thousands of genes were differentially expressed between the SHT5 mCherry+ cells and the CPCs (Figure3A). Furthermore, we identified that the SHT5 reprogrammed cells were enriched in pacemaker-specific markers including SHOX2, GJD3 (CX30.2), TBX5, TBX3, BMP2, and KCNN4 (Figure-3B), as reported by others (6, 34). In addition, the SHT5 cells also exhibited enrichment of pacemaker-specific transcripts for calcium and potassium channels including KCND2, KCNK2, CACNB1, and CACNA1A (32) (Figure-3B).
Figure-3: Transcriptome analysis of SHT5 reprogrammed cells.
Transcriptome analysis of SHT5 reprogrammed human CPCs into Pacemaker-like cells were characterized by both RNA sequencing and single-cell RNA sequencing. (A) Heat map displaying thousands of genes that are differentially expressed (adjusted p-value < 0.05) between SHT5 and CPCs indicating a change in transcriptome during reprogramming. (B) Heat map of SHT5 cells and CPCs showing differentially expressed (adjusted p-value < 0.05) transcriptome of genes marking the pacemaker phenotype. (C) A t-distributed stochastic neighbor embedding (t-SNE) plot from the single-cell RNA sequencing of 560 SHT5 cells showing the distribution in four distinctive clusters. (D) Heat map displaying differentially expressed genes between four clusters of SHT5 cells (adjusted p-value < 0.05); cluster 1 (blue), cluster 2 (orange), cluster 3 (green), and cluster 4 (red).
Previous studies showed that NKX2.5 is not expressed in human and mouse SANs (35); whereas, NKX2.5 is a specific ventricular conduction marker and highly enriched in Purkinje fibers (36). In addition to NKX2.5, CNTN2 is also a specific ventricular conduction marker that is highly enriched in Purkinje fibers (36). In agreement with these studies, our data showed that Purkinje-restricted NKX2.5 and CNTN2 gene transcripts along with the cardiomyocyte-specific GATA4 mRNA were not detectable in the SHT5 cells (Figure-3B). Similarly, Purkinje-specific SCN5a and IRX6 gene transcript expression were not enriched in the SHT5 cells (Figure-3B) (36). However, our data did reveal expression of IRX1, IRX2, and CNTN3, markers of Purkinje cells, in SHT5 cells (Figure-3B) (36, 37).
These findings, therefore, raised a question of whether we have heterogeneity in the SHT5 population; hence, we decided to perform single-cell RNA sequencing on the SHT5 activated cells to better understand the transcriptome of the individual cells within the total cell population. In such, we captured 560 SHT5 cells and assessed the individual transcriptomes of each cell. We found four transcriptionally distinct clusters by graph-based hierarchical clustering, as represented in the t-distributed stochastic neighbor embedding (t-SNE) plot (Figure-3C). We observed that the cells in cluster-1 were enriched in the pacemaker-specific genes including HCN2, HCN3, HCN4, SHOX2, GJC3, and BMP2 and thus consistent with the pacemaker phenotype (Figure-3D). We also found that while both cluster-3 and cluster-4 exhibited enrichment of few pacemaker genes, they also exhibited low levels of SHOX2 and HCN2 (Figure-3D). Interestingly, we observed that clusters-1, cluster-3, and cluster-4 exhibited similarity in their downregulation of NKX2.5, GATA4, and IRX3. Thus, while NKX2.5 downregulation drives the progenitor cells towards the pacemaker phenotype, its upregulation is essential for the Purkinje fiber upregulation (38–40). Consistent with our RNA sequencing data, we also did not observe an enrichment of CNTN2, a specific molecular marker for the Purkinje fibers, in the four clusters (Figure-3D). In contrast, cluster-2 exhibited upregulation of HCN2 and IRX3 as well as downregulation of SHOX2, HCN3, HCN4, among other genes thus possibly representing the heterogeneity of a non-pacemaker cell. Therefore, both RNA sequencing data and single-cell RNA sequencing data suggests that while having a population of cells which have all the three factors SHOX2, HCN2, and TBX5 (SHT5) exhibiting the pacemaker phenotype, some cells were not converted into pacemaker cells. Non-converted cells may have occurred due to not receiving either one or two of the combination of transcription factors during lentiviral infection.
SHOX2, HCN2, and TBX5 (SHT5) Combination Impose Functional Pacemaker Properties
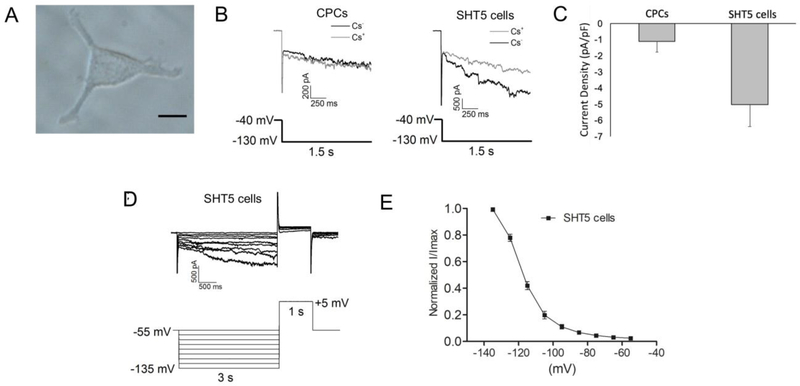
Based on the pacemaker gene expression profile with different transcription factors, we chose SHT5 mCherry+ cells (Figure-4A) for the functional analysis. We observed that SHT5 mCherry+ cells exhibited the HCN4 current, and this 4 current was absent in the control CPCs (Figure-4B). Specifically, cells were recorded with and without cesium (Cs+), where the Cs+ sensitive fraction of the hyperpolarization-activated current was defined as the pacemaker current (i.e. funny current or the HCN current). The SHT5 cells exhibited a robust HCN4 inward current, characteristic of pacemaker cells. As expected, the addition of Cs+ resulted in a complete blockade of HCN currents in the SHT5 cells. We observed a significantly higher current density (pA/pF) of approximately 3-fold in SHT5 cells (−5.03±1.36 pA/pF, n=17) when compared to the CPCs (−1.12±0.64 pA/pF, n=21) (Figure-4C). To further analyze the HCN channel activation kinetics, SHT5 cells were given a series of hyperpolarization command followed by a depolarization step (Figure-4D), and the voltage that elicited 50% of the maximal response (V1/2) when SHT5 cells were hyperpolarized from −55 mV to −135 mV was −125.28±3.83 mV (n=14) (Figure-4E).
Figure-4: Whole Cell electrophysiological recordings of functional SHT5 reprogrammed cells.
(A) A representative image of an SHT5 mCherry+ cell used for the electrophysiological recording. (B) Whole-cell current traces were recorded from the CPCs (left panel) or the SHT5 mCherry+ (HCN positive) cells (right panel) in the absence (gray) and presence (black) of 5 mM Cs+. The voltage protocol is indicated below the traces. The HCN current was defined as the Cs+-sensitive fraction of the hyperpolarization-activated current. (C) The current density (pA/pF) from SHT5 cells (−5.03±1.36, n=17) is significantly higher than the current density recorded from CPCs (−1.12±0.64, n=21). (D) A series hyperpolarizing step command followed by a depolarization step was applied to determine the channel activation kinetics. Traces recorded from a representative SHT5 cell is shown. (E) The Cs+ sensitive current at each voltage-step (I) was normalized to the peak current (Imax at −135 mV), and the I/Imax was plotted then fitted with the Boltzmann equation: I/Imax = 1 / [1-exp ((V1/2-V) / k)]. The voltage eliciting half of the maximal current (I/Imax at 0.5) was calculated as −125.28±3.83 mV for the SHT5 (HCN4 positive) cells (n=14).
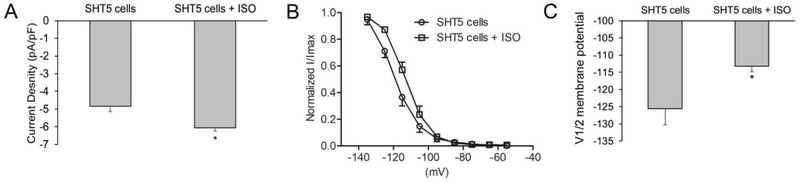
Biological pacemaker cells have the advantage of hormone regulation. The β-adrenergic receptor β-AR) stimulated cAMP binds to the HCN channel and thereby contributes to the HCN pacemaker currents (41). To characterize the effect of β-AR stimulation on HCNs, we treated the SHT5 cells with isoproterenol (ISO). The current density in SHT5 cells increased significantly upon stimulation with ISO (−6.06±0.17 pA/pF, n=9), as compared to the cells without ISO (−4.84±0.30 pA/pF, n=8) (Figure-5A). Furthermore, treatment with ISO resulted in a significant shift of the HCN activation curve towards more positive voltages in the SHT5 cells (Figure-5B) and the V1/2 was shifted from −125.6±4.66 (n=8) in SHT5 cells without ISO to −113.2±1.66 (n=9) with ISO stimulation (Figure-5C). This result indicated that the SHT5 cells exhibit functional HCN Pacemaker currents that are sensitive to β-AR stimulation.
Figure-5: Electrophysiological recordings in the presence of beta-adrenergic receptor stimulation with Isoproterenol (ISO).
(A) Treatment with 100 nM isoproterenol (ISO) significantly increased the HCN4 current density (pA/pF) in SHT5 mCherry+ cells (SHT5: −4.84±0.30 pA/pF, n=8; SHT5+ISO: −6.06±0.17 pA/pF, n=9) (B) Treatment with ISO also shifted the activation curve toward more positive voltages in SHT5 mCherry+ cells. (C) ISO shifted the activation midpoint V1/2 significantly to a more positive voltage in SHT5 mCherry+ cells (SHT5: V1/2 = −125.6 ± 4.66 mV, n=8; SHT5+ISO: −113.2 ± 1.66, n=9). All data are presented as a mean ± SEM. The Student’s t-test was used for statistical analysis, * p < 0.05.
DISCUSSION
Over the last decade, a variety of transcription factors have been used to generate Pacemaker-like cells. In a recent study, Sun, Qiao (42) differentiated adipose-derived stem cells into Pacemaker-like cells through TBX18, while another study reported the conversion of the myocardial cells into the Pacemaker-like cell phenotype by ectopic expression of TBX3 (32). In addition, TBX5 overexpression in the Xenopus laevis model was shown to upregulate conduction system markers (12). During embryonic development of the heart, transcription factors SHOX2, TBX3, and TBX5 regulated the SAN gene program (43). While other core factors, such as TBX18 within the SAN have been shown to suppress cardiomyocyte formation (43). In another study, human mesenchymal stem cell (hMSCs) transduced with the mouse Hcn2 gene generated funny currents, which was suggestive of the role of Hcn2 in yielding functional biological pacemakers (44). Thus, to enhance our chance of reprogramming human CPCs into pacemaker cells, we decided to use the five factors singularly as well as in combination for reprogramming and the CX30.2 mCherry reporter, as a conversion endpoint.
Interestingly, our FACS sorting data yielded mCherry+ cells with all eight different combinations; by either employing SHOX2, HCN2, TBX3, TBX5, and TBX18 transcription factors in single or SHT3, SHT5, and SHT18 transcription factors in combination. These results are consistent with previous studies where these transcription factors individually have resulted in driving the fate of substrate cells, both in vitro and in vivo, into Pacemaker-like cells. However converted mCherry+ cells from the SHT5 cocktail of transcription factors demonstrated the penultimate expression of many pacemaker specific genes; including, CX30.2, KCNN4, HCN4, HCN3, HCN1, and SCN3b. These findings suggest that the SHT5 combination of transcription factors is a much better candidate in driving the CPCs into Pacemaker-like cells than other combinations and single transcription factors. Further, we observed that the transcriptome of SHT5 cells exhibited enrichment of pacemaker specific genes including TBX3, KCNN4, CX30.2, and BMP2, as well as pacemaker specific calcium and potassium channels (KCND2, KCNK2, and CACNB1). In support of our findings, the transcriptome profile of our Pacemaker-like cells closely resembled the differentially expressed genes identified within the developing mouse SAN (45). In addition, similar to human and mouse SAN studies, we observed the down-regulation of NKX2.5. Thus, the transcriptome data and gene assay data revealed that the SHT5 activated cells that share a pacemaker cell phenotype.
To determine the functional efficiency of the reprogrammed cells using the SHT5 combination of transcription factors, we measured the HCN current using patch-clamp recordings, which is characteristic in pacemaker cells. We recorded the hyperpolarization-elicited inward currents with the current density at −5.03±1.36 pA/pF when the SHT5 cells were hyperpolarized from −40 to −130 mV (n=17). Further, the voltage to elicit half of the maximal HCN current (V1/2) was around −125 mV when SHT5 cells were recorded with a series of hyperpolarization steps (from −55 mV to −135 mV; the maximal current was elicited at −135mV). In a recent study, Kapoor, Liang (46) transduced Tbx18 in ventricular myocytes of rats to generate biological pacemaker. They reported that the transduced cells recorded If with a current density of −1.9±0.8 pA/pF at −50mV (n=3). In another study, Yang, Zhang (13) induced differentiation of ADSC using Tbx18 to generate Pacemaker-like cells. They reported that the differentiated Pacemaker-like cells exhibited a current density of −5.43±1.36 pA/pF (n=4). In addition, previous studies have shown that cyclic AMP (cAMP) modulates cardiac pacemaker HCN currents and shifts the activation curve to more positive voltages (47). Our studies also showed that the HCN currents recorded in the SHT5 mCherry+ cells were sensitive to β-AR stimulation by ISO, increasing the current density and shifting the V1/2 to a more positive voltage (−113 mV). Thus, our SHT5 converted Pacemaker-like cells exhibited HCN currents demonstrated functional characteristics of pacemaker cells.
CONCLUSIONS
In our study, we observed that the SHOX2, HCN2, and TBX5 (SHT5) cocktail of transcription factors and channel protein reprogrammed the CPCs into Pacemaker-like cells. The SHT5 factors resulted in upregulation of pacemaker specific gene expression and transcriptome expression, attributing the pacemaker phenotype to these cells. In addition, the SHT5 cells also exhibited the functional characteristic of pacemaker cells (i.e. If recording). Thus, the SHT5 combination of transcription factors and channel protein are able to reprogram human CPCs into human cardiac Pacemaker-like cells and will facilitate the development of cell-based therapies for various cardiac conduction diseases.
Supplementary Material
Figure-S1: Schematic illustration showing the conversion of human CPCs into Pacemaker-like cells
Figure-S2: FACS analysis and sorting of the reprogrammed CPCs into Pacemaker-like cells.
Figure-S3: Fluorescence microscopy of the reprogrammed CPCs into Pacemaker-like cells.
Figure-S4: Western blot analysis for protein expression of the inducible vectors of the reprogrammed CPCs into Pacemaker-like cells.
Figure-S5: Transcriptome analysis by RNA sequencing.
HIGHLIGHTS.
SHOX2, HCN2, and TBX5 (SHT5) cocktail of transcription factors and channel protein reprogrammed CPCs into Pacemaker-like cells. The SHT5 factors resulted in upregulation of pacemaker specific gene expression and transcriptome expression, attributing the pacemaker phenotype to the cells. The SHT5 mCherry+ cells also exhibited the funny current via HCN4 channels, attributing the functional characteristic of pacemaker cells. Thus, the findings of this study show that the SHT5 combination of transcription factors can be used to reprogram CPCs into Pacemaker-like cells as a potential stem cell therapy for sick sinus syndrome (SSS) as well as for other cardiac conduction diseases.
ACKNOWLEDGMENTS
The data in this paper are based on the dissertation thesis submitted in partial fulfillment of the requirements for a Ph.D. (Pharmacology) in the Department of Pharmacological and Pharmaceutical Sciences in the College of Pharmacy at the University of Houston (S.R.).
FUNDING:
Research reported in this paper was supported in part by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH) [R15HL141963 (B.K.M.) and R15HL124458 (B.K.M.)], the American Heart Association (AHA) [18AIREA33960175 (to B.K.M.)], and a grant from Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation (to B.K.M.). The Center for Advanced Science in Space supported research on the conversion of human adipogenic mesenchymal stem cells into cardiac progenitors (R.J.S.). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
ABBREVIATIONS
- AVN
atrioventricular node
- cAMP
cyclic AMP
- β-AR
β-adrenergic receptor
- CCS
cardiac conduction system
- CNTN
contactin
- CPCs
cardiac progenitor cells
- Cs+
cesium
- CsCl
cesium chloride
- CVD
cardiovascualar disease
- Cx
connexins
- ESC
embryonic stem cells
- ETS
E26 transformation-specific
- eGFP
enhanced green fluorescent protein
- HCN
potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel
- HF
heart failure
- hADMSCs
human adipogenic mesenchymal stem cells
- hMSCs
human mesenchymal stem cells
- If
funny current
- IRX
iroquois
- ISO
isoproterenol
- KCNN4
potassium calcium-activated channel subfamily N member 4
- MSC
mesenchymal stem cells
- MESP
mesoderm posterior protein
- MI
myocardial infarction
- NKX2.5
NK2 transcription factor related, locus 5
- pA/pF
current density
- PCA
principal component analysis
- PCR
polymerase chain reaction
- SAN
sinoatrial node
- SCN
sodium channel
- SCN3b
sodium voltage-gated channel beta subunit-3
- SHOX2
short stature homeobox 2
- SSS
sick sinus syndrome
- t-SNE
t-distributed stochastic neighbor embedding plot
- TBX3
T-box transcription factor 3
- TBX5
T-box transcription factor 5
- TBX18
T-box transcription factor 18
Footnotes
DISCLOSURES:
Authors declare no conflicts of interests.
DATA AND MATERIALS AVAILABILITY STATEMENT:
The data will be made available according to the policies of Science Translational Medicine and of the National Academy of Sciences (NAS) for the open sharing of publication-rated data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mangoni ME, Nargeot J. Genesis and regulation of the heart automaticity. Physiol Rev. 2008;88(3):919–82. [DOI] [PubMed] [Google Scholar]
- 2.John RM, Kumar S. Sinus Node and Atrial Arrhythmias. Circulation. 2016;133(19):1892–900. [DOI] [PubMed] [Google Scholar]
- 3.Alonso A, Jensen PN, Lopez FL, Chen LY, Psaty BM, Folsom AR, et al. Association of sick sinus syndrome with incident cardiovascular disease and mortality: the Atherosclerosis Risk in Communities study and Cardiovascular Health Study. PLoS One. 2014;9(10):e109662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324(12):781–8. [DOI] [PubMed] [Google Scholar]
- 5.Boyett MR, Honjo H, Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res. 2000;47(4):658–87. [DOI] [PubMed] [Google Scholar]
- 6.Vedantham V New Approaches to Biological Pacemakers: Links to Sinoatrial Node Development. Trends Mol Med. 2015;21(12):749–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christoffels VM, Mommersteeg MT, Trowe MO, Prall OW, de Gier-de Vries C, Soufan AT, et al. Formation of the venous pole of the heart from an Nkx2–5-negative precursor population requires Tbx18. Circ Res. 2006;98(12):1555–63. [DOI] [PubMed] [Google Scholar]
- 8.Burkhard S, van Eif V, Garric L, Christoffels VM, Bakkers J. On the Evolution of the Cardiac Pacemaker. J Cardiovasc Dev Dis. 2017;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espinoza-Lewis RA, Yu L, He F, Liu H, Tang R, Shi J, et al. Shox2 is essential for the differentiation of cardiac pacemaker cells by repressing Nkx2–5. Dev Biol. 2009;327(2):376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mommersteeg MT, Hoogaars WM, Prall OW, de Gier-de Vries C, Wiese C, Clout DE, et al. Molecular pathway for the localized formation of the sinoatrial node. Circ Res. 2007;100(3):354–62. [DOI] [PubMed] [Google Scholar]
- 11.Singh R, Hoogaars WM, Barnett P, Grieskamp T, Rana MS, Buermans H, et al. Tbx2 and Tbx3 induce atrioventricular myocardial development and endocardial cushion formation. Cell Mol Life Sci. 2012;69(8):1377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrmann F, Bundschu K, Kuhl SJ, Kuhl M. Tbx5 overexpression favors a first heart field lineage in murine embryonic stem cells and in Xenopus laevis embryos. Dev Dyn. 2011;240(12):2634–45. [DOI] [PubMed] [Google Scholar]
- 13.Yang M, Zhang GG, Wang T, Wang X, Tang YH, Huang H, et al. TBX18 gene induces adipose-derived stem cells to differentiate into pacemaker-like cells in the myocardial microenvironment. Int J Mol Med. 2016;38(5):1403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Islas JF, Liu Y, Weng KC, Robertson MJ, Zhang S, Prejusa A, et al. Transcription factors ETS2 and MESP1 transdifferentiate human dermal fibroblasts into cardiac progenitors. Proc Natl Acad Sci U S A. 2012;109(32):13016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100(9):1249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rangappa S, Entwistle JW, Wechsler AS, Kresh JY. Cardiomyocyte-mediated contact programs human mesenchymal stem cells to express cardiogenic phenotype. J Thorac Cardiovasc Surg. 2003;126(1):124–32. [DOI] [PubMed] [Google Scholar]
- 17.Yamada Y, Wang XD, Yokoyama S, Fukuda N, Takakura N. Cardiac progenitor cells in brown adipose tissue repaired damaged myocardium. Biochem Biophys Res Commun. 2006;342(2):662–70. [DOI] [PubMed] [Google Scholar]
- 18.Spandidos A, Wang X, Wang H, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2010;38(Database issue):D792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi L, Chang JY, Yu F, Ko ML, Ko GY. The Contribution of L-Type Cav1.3 Channels to Retinal Light Responses. Front Mol Neurosci. 2017;10:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotechnol. 2013;31(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lvd Maaten. Accelerating t-SNE using Tree-Based Algorithms. Journal of Machine Learning Research. 2018;15:3221–45. [Google Scholar]
- 24.Yu D, Huber W, Vitek O. Shrinkage estimation of dispersion in Negative Binomial models for RNA-seq experiments with small sample size. Bioinformatics (Oxford, England). 292013 p. 1275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreuzberg MM, Sohl G, Kim JS, Verselis VK, Willecke K, Bukauskas FF. Functional properties of mouse connexin30.2 expressed in the conduction system of the heart. Circ Res. 2005;96(11):1169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verheijck EE, van Kempen MJ, Veereschild M, Lurvink J, Jongsma HJ, Bouman LN. Electrophysiological features of the mouse sinoatrial node in relation to connexin distribution. Cardiovasc Res. 2001;52(1):40–50. [DOI] [PubMed] [Google Scholar]
- 27.Munshi NV, McAnally J, Bezprozvannaya S, Berry JM, Richardson JA, Hill JA, et al. Cx30.2 enhancer analysis identifies Gata4 as a novel regulator of atrioventricular delay. Development. 2009;136(15):2665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleger A, Seufferlein T, Malan D, Tischendorf M, Storch A, Wolheim A, et al. Modulation of calcium-activated potassium channels induces cardiogenesis of pluripotent stem cells and enrichment of pacemaker-like cells. Circulation. 2010;122(18):1823–36. [DOI] [PubMed] [Google Scholar]
- 29.Kleger A, Liebau S. Calcium-activated potassium channels, cardiogenesis of pluripotent stem cells, and enrichment of pacemaker-like cells. Trends Cardiovasc Med. 2011;21(3):74–83. [DOI] [PubMed] [Google Scholar]
- 30.Christoffels VM, Smits GJ, Kispert A, Moorman AF. Development of the pacemaker tissues of the heart. Circ Res. 2010;106(2):240–54. [DOI] [PubMed] [Google Scholar]
- 31.Stieber J, Herrmann S, Feil S, Loster J, Feil R, Biel M, et al. The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci U S A. 2003;100(25):15235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakker ML, Boink GJ, Boukens BJ, Verkerk AO, van den Boogaard M, den Haan AD, et al. T-box transcription factor TBX3 reprogrammes mature cardiac myocytes into pacemaker-like cells. Cardiovasc Res. 2012;94(3):439–49. [DOI] [PubMed] [Google Scholar]
- 33.Hakim P, Brice N, Thresher R, Lawrence J, Zhang Y, Jackson AP, et al. Scn3b knockout mice exhibit abnormal sino-atrial and cardiac conduction properties. Acta Physiol (Oxf). 2010;198(1):47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puskaric S, Schmitteckert S, Mori AD, Glaser A, Schneider KU, Bruneau BG, et al. Shox2 mediates Tbx5 activity by regulating Bmp4 in the pacemaker region of the developing heart. Hum Mol Genet. 2010;19(23):4625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Espinoza-Lewis RA, Liu H, Sun C, Chen C, Jiao K, Chen Y. Ectopic expression of Nkx2.5 suppresses the formation of the sinoatrial node in mice. Dev Biol. 2011;356(2):359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maass K, Shekhar A, Lu J, Kang G, See F, Kim EE, et al. Isolation and characterization of embryonic stem cell-derived cardiac Purkinje cells. Stem Cells. 2015;33(4):1102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pallante BA, Giovannone S, Fang-Yu L, Zhang J, Liu N, Kang G, et al. Contactin-2 expression in the cardiac Purkinje fiber network. Circ Arrhythm Electrophysiol. 2010;3(2):186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris BS, Spruill L, Edmonson AM, Rackley MS, Benson DW, O’Brien TX, et al. Differentiation of cardiac Purkinje fibers requires precise spatiotemporal regulation of Nkx2–5 expression. Dev Dyn. 2006;235(1):38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jay PY, Harris BS, Maguire CT, Buerger A, Wakimoto H, Tanaka M, et al. Nkx2–5 mutation causes anatomic hypoplasia of the cardiac conduction system. J Clin Invest. 2004;113(8):1130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schott JJ, Benson DW, Basson CT, Pease W, Silberbach GM, Moak JP, et al. Congenital heart disease caused by mutations in the transcription factor NKX2–5. Science. 1998;281(5373):108–11. [DOI] [PubMed] [Google Scholar]
- 41.Biel M, Michalakis S. Cyclic nucleotide-gated channels. Handb Exp Pharmacol. 2009(191):111–36. [DOI] [PubMed] [Google Scholar]
- 42.Sun AJ, Qiao L, Huang C, Zhang X, Li YQ, Yang XQ. Comparison of mouse brown and white adiposederived stem cell differentiation into pacemakerlike cells induced by TBX18 transduction. Mol Med Rep. 2018;17(5):7055–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Weerd JH, Christoffels VM. The formation and function of the cardiac conduction system. Development. 2016;143(2):197–210. [DOI] [PubMed] [Google Scholar]
- 44.Bruzauskaite I, Bironaite D, Bagdonas E, Skeberdis VA, Denkovskij J, Tamulevicius T, et al. Relevance of HCN2-expressing human mesenchymal stem cells for the generation of biological pacemakers. Stem Cell Res Ther. 2016;7(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodyer WR, Beyersdorf BM, Paik DT, Tian L, Li G, Buikema JW, et al. Transcriptomic Profiling of the Developing Cardiac Conduction System at Single-Cell Resolution. Circ Res. 2019;125(4):379–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kapoor N, Liang W, Marban E, Cho HC. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nat Biotechnol. 2013;31(1):54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351(6322):145–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure-S1: Schematic illustration showing the conversion of human CPCs into Pacemaker-like cells
Figure-S2: FACS analysis and sorting of the reprogrammed CPCs into Pacemaker-like cells.
Figure-S3: Fluorescence microscopy of the reprogrammed CPCs into Pacemaker-like cells.
Figure-S4: Western blot analysis for protein expression of the inducible vectors of the reprogrammed CPCs into Pacemaker-like cells.
Figure-S5: Transcriptome analysis by RNA sequencing.