Abstract
The GABA deficit hypothesis remains one of the most compelling explanations for the information processing impairments in schizophrenia. However, much of the supportive evidence has been derived from post-mortem studies, whereas in vivo studies have largely yielded inconsistent results. We undertook this single voxel proton magnetic resonance (MRS) GABA study to test in a sample of recent onset patients the replicability of our prior finding of reduced early visual cortex GABA in schizophrenia. We also examined the possibility that antipsychotics could represent a significant confound by studying a small subsample of antipsychotic naïve subjects. 23 adults with recent onset schizophrenia and a demographically matched sample of 31 healthy control subjects underwent MRS using a MEGA PRESS sequence on a 3T MR scanner to measure GABA concentration in early visual cortex. To control for in-scanner head movement confounding the results, we quantified the amount of head movement during GABA scans to identify and exclude from analysis scans with excessive movement. Patients demonstrated significantly reduced GABA levels compared to control subjects, p = 0.029. GABA levels did not differ significantly between patients who were antipsychotic naïve (n=7) and patients treated with antipsychotics. This replication a in a recent onset sample suggest that diminished GABA in the visual cortex is a reliable finding, present in early phase of illness and not confounded by illness chronicity.
Keywords: GABA, visual cortex, magnetic resonance spectroscopy
1. Introduction
The GABA deficit hypothesis is one of the leading explanations for the neural mechanisms of schizophrenia (Gonzalez-Burgos, Cho, & Lewis, 2015; Lewis, Curley, Glausier, & Volk, 2012; Lewis & Gonzalez-Burgos, 2006). It proposes that reduced GABA production in cortical interneurons results in local circuit dysfunction and information processing deficits. The strongest evidence for this hypothesis comes from post-mortem studies, which have consistently shown reductions in neocortical mRNA for GAD-67 (Akbarian et al., 1995; Guidotti et al., 2000; Hashimoto, Arion, et al., 2008; Volk, Austin, Pierri, Sampson, & Lewis, 2000), one of two major enzymes synthesizing GABA. GAD-67 is a potent regulator of GABA levels, as demonstrated by studies showing nearly complete absence of GABA in neurons in which the gene coding GAD-67 is deleted (Asada et al., 1997).
Taken together, the GAD-67 regulation of neural GABA content and the post-mortem GAD-67 mRNA abnormalities in schizophrenia predict diminished GABA concentration in schizophrenia that may be detectable using in vivo magnetic resonance spectroscopy (MRS). The ability to reliably measure in vivo GABA deficits in schizophrenia would advance efforts to identify and validate biomarkers of GABA pathology, as well as to determine the functional relevance of GABA deficits in this condition. The advent of editing sequences, such as MEGA PRESS (Mescher, Merkle, Kirsch, Garwood, & Gruetter, 1998), for the reliable quantification of GABA concentration (Bogner et al., 2009) using single voxel proton magnetic resonance spectroscopy (H-MRS) has led to the completion of a growing number of in vivo studies testing the GABA deficit hypothesis in schizophrenia. However, in contrast to the mostly consistent post-mortem findings, results from in vivo MRS studies have been largely inconsistent, as reviewed by Egerton et al. (Egerton, Modinos, Ferrera, & McGuire, 2017). While there have been several reports of diminished GABA in schizophrenia (Kelemen, Kiss, Benedek, & Keri, 2013; Thakkar et al., 2017), including within the PFC (Marenco et al., 2016; Marsman et al., 2014; Rowland et al., 2016), there have also been a number of negative or contradictory studies (Kelemen et al., 2013; Ongur, Prescot, McCarthy, Cohen, & Renshaw, 2010; Tayoshi et al., 2010). A recent meta-analysis of all GABA MRS studies estimated a combined effect size of G =−0.3 of diminished GABA levels in schizophrenia compared to healthy participants (Egerton et al., 2017). These results raise concerns about the reliability and reproducibility of current approaches for measuring in vivo GABA in schizophrenia.
We undertook the present study to test the replicability our prior findings of diminished GABA levels early visual cortex in schizophrenia (Yoon et al., 2010). While much of the interest in GABA deficits in schizophrenia has been focused on higher order cortical regions, such as the prefrontal cortex, the visual cortex is an appealing region of study for in vivo GABA studies in schizophrenia. Post-mortem studies suggest that GABA deficits are pan-cortical, with GAD67 mRNA reduction in interneurons present in all cortices examined, including the visual cortex (Hashimoto, Bazmi, et al., 2008). The visual cortex is a favorable brain region for single voxel MRS studies due to a number of factors enhancing the strength of the spectroscopic signal from this region. Taken together, these considerations suggest that the spectroscopic measurement of GABA concentration in early visual cortex could be a reliable marker for cortical GABA deficits in schizophrenia.
In testing the hypothesis of the replicability of reduced visual cortex GABA in schizophrenia, we attempted to account for a number of potentially important confounds. Since the prior finding of diminished GABA in early visual cortex could have been confounded by illness chronicity, in the present study we tested the GABA deficit hypothesis in a sample of recent onset (within 5 years of testing) patients. We examined the possibility of antipsychotic medications confounding results by studying a subsample of patients who were antipsychotic naïve at time of study. Since head movement during scanning could be a significant confound, particularly in clinical studies in which one group may exhibit differential levels of movement compared to another, we measured the magnitude of head movement during scanning, allowing us to detect and exclude scans with excessive movement from analysis.
2. Experimental Materials and Methods
2.1. Subjects
A total of 54 subjects participated in this study; 23 subjects with schizophrenia, in whom onset of illness occurred within 5 years of study participation, and 31 demographically matched sample of healthy subjects with no history of major psychiatric illness and without first degree relatives with psychotic disorders. As described below, one control and one patient were excluded due to excessive head movement during scanning, bringing the total sample to 30 healthy subjects and 22 patients. The demographic and clinical characteristics of participants are displayed in Table 1.
Table 1.
Sample demographics and clinical profile
| Patient (N=23) | Control (N=31) | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-value | |
| Age (years) | 21.8 | 2.9 | 22.9 | 4.8 | 0.33 |
| Gender (%male) | 87% | 74% | 0.25 | ||
| Education (years) | 13.0 | 2.7 | 15.0 | 2.0 | 0.02 |
| Parental Education (years) | 14.0 | 3.2 | 14.2 | 3.0 | 0.82 |
| Handedness (% R) | 96% | 87% | 0.28 | ||
| GAS | 58.4 | 12.7 | |||
| SANS Total | 5.1 | 4.0 | |||
| SAPS Total | 4.2 | 4.3 | |||
| BPRS Total | 35.3 | 9.6 | |||
| Anti-Psychotics | |||||
| Typical | 0% | ||||
| Atypical | 70% | ||||
| None | 30% | ||||
| CPZ Equivalents | 196.6 | 216.5 | |||
| Duration of Illness (weeks) | 16.1 | 9.1 | |||
Brief Psychiatric Rating Scale (BPRS); Scale for the Assessment of Negative Symptoms (SANS); Scale for the Assessment of Positive Symptoms (SAPS), and Global Assessment of Symptoms (GAS).
Patients were recruited primarily from the Early Diagnosis and Preventive Treatment (EDAPT) Clinic at UC Davis and healthy controls were recruited from the surrounding Sacramento community. Diagnostic evaluations with the Structured Clinical Interview for DSM-IV-TR (First et al., SCID ref) were conducted by either Masters or Doctoral level clinicians to confirm the diagnosis of schizophrenia or schizoaffective disorder (hereby collectively referred to as schizophrenia) in patients and to exclude major psychiatric illness in controls. Controls with a first-degree relative with a psychotic disorder were also excluded. Diagnoses were confirmed by consensus conference. Symptoms were quantified with the Brief Psychiatric Rating Scale (BPRS) (Overall, 1974), Scales for the Assessment of Negative Symptoms (SANS) (Andreasen, 1982) and Scales for the Assessment of Positive Symptoms (SAPS) (Andreasen, 1984). Sub-scores from the BPRS, SANS, and SAPS will be used to derive an index of disorganization (Barch, Carter, MacDonald, Braver, & Cohen, 2003). Exclusion criteria for all subjects were: IQ < 70, drug/alcohol dependence history or abuse in the previous three months or a positive urine drug screen on the day of testing, significant head trauma, or any known contraindication to MR scanning. After complete description of the study, written informed consent was obtained. This study was approved by the Institutional Review Board of the University of California Davis School of Medicine.
This study utilized essentially a frequency matching approach to achieve similar demographic characteristics between patient and control at the group level. In the first stage of study enrollment, subject selection proceeded in parallel across the two groups and without regard to demographic variables. Once one-half of the target sample size for the patient group was achieved, the investigators began comparing the distribution of the two samples for gender and age using a chi-square and t-test, respectively. If the p-value of the group difference in the distribution of a demographic variable were < 0.2, then only control subjects whose enrollment would reduce group differences in the demographic variable of interest would be enrolled. For example, if group means for patient and controls were 20 and 25 years of age respectively, with group difference of p < 0.2, then the next control subject to be enrolled would have to be younger than 25.
2.2. Spectroscopy methods
All scans were conducted at the UC Davis Imaging Research Center. GABA was measured with proton MRS using a Siemens 3 Tesla TIM Trio MRI System with a 32-channel phased-array head coil (Siemens HealthCare, Erlangan, Germany). We sampled early visual cortex with a 35 × 30 × 25mm voxel centered on the calcarine sulci bilaterally, with its posterior border approximately 5 mm anterior to the dura. A single voxel MEGA-PRESS J-difference spectral editing sequence measured total GABA (Mescher et al., 1998). Alternating subspectra were obtained with and without a frequency-selective inversion pulse applied to the GABA C3 resonance. The subspectra were subtracted to generate a difference spectrum containing an upright total GABA signal at about 3.0ppm. The following scanning parameters were used: TR=1500 msec; TE=68 msec; edit frequency 1.9ppm; delta frequency=−1.7; edit pulse bandwidth 45 Hz; NEX = 256 for each of three subscans (total NEX = 768). Each subscan was of approximately 6.5 min duration.
Using jMRUI software (Stefan et al., 2009), all spectra were phase-aligned with reference to water, zero-filled from 1024 to 4096, apodized with a 4Hz Gaussian filter, and frequency aligned to creatine at 3.02ppm. Peak integration, using a custom-made, user-independent, peak integration algorithm quantified total GABA (2.99 ± 0.12ppm) and Glx (glutamine + glutamate) (3.76 ± 0.11ppm) in the difference spectra and creatine (3.02 ± 0.09ppm) in the summed spectra. The ratio of total GABA/total creatine signal was used for hypothesis testing. Normalizing to creatine reduces inter-subject variance due to differences in global signal strength and CSF fraction within the voxel and has been shown to yield reliable GABA concentration estimates (Bogner et al., 2009).
2.3. In-Scanner Head Movement
Although the effects of head movement on MRS measurements have yet to be precisely clarified, studies of other neuroimaging modalities, such as resting state fMRI (Power et al. 2012), suggest that head movement likely represents an important source of variance for GABA measurements. Mitigating the effects of movement may be particularly important in clinical studies that rely on group comparisons for hypothesis testing (Weinberger and Radulescu 2015) because head movement may lead to either false negative or false positive results, depending on the effect of movement on GABA estimates and how well groups are matched on head movement. Our solution to this problem was to measure the magnitude of head movement during scanning so that we could detect and exclude scans with excessive head movement.
This was accomplished by videotaping a visual marker affixed to the subject’s head during scanning and quantifying the movement of this marker using an automated algorithm. Details of this method can be found in a preprint by Cui et al. (Cui et al. 2018). We derived two metrics of movement: 1) root mean square (RMS) of the magnitude of displacement (the linear movement of the head away from its original position when the MRS voxel was first placed at the beginning of the scan) and 2) speed, (moment-by-moment head movement). Subscans with movement greater than two standard deviations from the mean of all subjects for either metric were excluded. We obtained three sequential GABA subscans for each subject rather than one long scan. This allowed for the possibility of identifying and excluding individual scans with excessive movement. For example, in cases where excessive movement occurred in only one of the three subscans, then only the excessive movement subscan would be excluded, and the subscans with acceptable levels of movement from the subject would be retained in the analysis. A total of six control and nine patient GABA subscans exhibited excessive movement and thus were excluded. GABA estimates for a subject was obtained averaging across the values from the retained subscans. One control and one patient exhibited excessive movement in all of their GABA subscans, excluding these participants from the analyses.
2.4. Creatine Linewidth
Creatine linewidths from the off editing spectra were obtained using Gannet 3.1 (Edden, Puts, Harris, Barker, & Evans, 2014) (https://github.com/richardedden/Gannet3.1/archive/master.zip).
2.5. Statistical analysis
Testing of the primary hypothesis was accomplished by comparing group distributions of GABA/Cr values with independent samples t-test. A one-tailed test of significance was utilized due to the strong directional hypothesis of lower GABA in SZ compared to control sample in the visual cortex. This prediction was based on the consistency of MRS findings of a deficit in visual cortex GABA concentration in schizophrenia (Kelemen et al., 2013; Thakkar et al., 2017; Yoon et al., 2010). For all other inferential tests, a two-tailed test of significance was utilized. Associations with potential confounds, such as head movement, antipsychotic dose, and duration of illness, was tested, in part, with bivariate Pearson correlation.
3. Results
3.1. Sample Characteristics
The demographics and clinical characteristics of the study sample are summarized in Table 1. The groups were well-matched for age, gender, handedness and parental education. Patients had significantly less education compared to healthy subjects and on average were diagnosed with schizophrenia 16.1 weeks prior to study participation, with a range of 7 – 40 weeks. 30% of patients were antipsychotic naïve at time of study participation. Patients who were taking antipsychotic during testing were all on atypical antipsychotics.
3.2. GABA
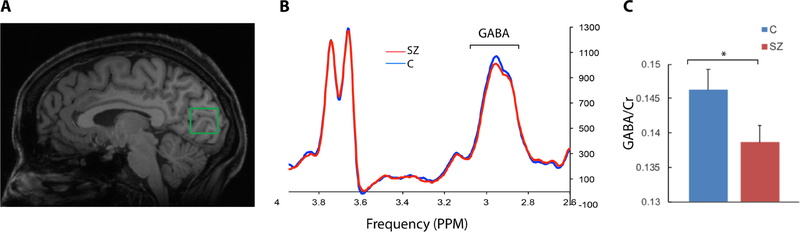
Creatine linewidth, a proxy for spectral data quality, did not significantly differ between groups: Cmean = 8.567 +/−0.571Hz; SZmean = 8.685 +/− 0.643Hz (t = 0.678, df = 50, p = 0.501. Patients exhibited significantly lower GABA/Cr concentration compared to healthy subjects (Figure 1); Cmean = 0.146 +/−0.014; SZmean = 0.139 +/− 0.013, (t = 1.945, df = 50, p = 0.029). The effect size of this group difference (Cohen’s D) was 0.55. The creatine peak did not differ significantly between groups, Cmean = 257262 +/−25956; SZmean = 261197 +/−36497 (t = 0.455, df = 50, p = 0.651). In this clinical study, our acquisition of 768 averages is more than is often used for measuring GABA. We tested if diminished GABA in the patient sample could be detected with fewer MRS averages for each subject. We did so by using subsets of the acquired averages to generate the GABA spectra. When we included the first 256 averages, the GABA concentration was non-significantly lower in patients, p = 0.277. When we included the first 512 averages, the GABA concentration was again non-significantly lower in patients, p = 0.059. One control subject and three patients were smokers. The exclusion of these subjects did not alter our findings. The samples did not exhibit significant difference in Glx/Cr levels: Cmean = 0.128 +/− 0.012; SZmean = 0.127 +/− 0.019, (t = 0.328, df = 50, p = 0.744).
Figure 1. GABA Spectroscopy Results.
A) An example of the voxel position within the early visual cortex in a representative subject. The voxel was centered over the calcarine fissure and positioned such that there remained, at minimum, 2 mm distance between the edge of the voxel and cortical surface of the brain. B) Group averaged edited spectra shows the characteristic pseudo-doublet peak of the GABA line graph at ~3.0 ppm and the doublet peak of Glx at ~3.7 ppm and higher GABA peak in the control compared to the patient group. B). Group average of the integrals of the creatine-normalized GABA peaks. * p < 0.05.
3.3. Effect of Antipsychotic Exposure
To examine the possibility that antipsychotics could affect GABA levels, we compared GABA levels between 7 patients who were antipsychotic naïve with 15 patients taking antipsychotics at time of testing. These groups’ GABA levels did not differ significantly: antipsychotic naïve sample mean = 0.138 +/− 0.014; medicated sample mean = 0.139 +/− 0.013. The difference in GABA levels was non-significant (df = 20, t=.066, p = .940) and the effect size of group difference was 0.034. In addition, the correlation between GABA concentration and D2 receptor load as indexed by CPZ equivalent dosage (Davis, 1974; Woods, 2003) was low, R = 0.013, p = 0.954.
3.4. Duration of Illness
We tested whether duration of illness (DOI), the interval between onset of psychosis and time of scanning, is associated with GABA levels in two ways. We first compared GABA levels in patients with the highest 50th percentile with those from the lowest 50th percentile for DOI. GABA levels did not differ between the two groups: low DOI GABA mean = 0.141, high DOI GABA mean = 0.136, p = 0.344. Second, we tested the correlation between illness duration and GABA levels and the correlation was nonsignificant (R = 0.021, p = 0.928).
3.5. Associations with Symptoms
Since associations with symptoms was not predicted or hypothesized a priori, we conducted an exploratory analysis examining the association between level of psychopathology and GABA levels. No significant correlations were observed with GAS and total scores on BPRS, SAN, SAPS or disorganization, absolute value of R < 0.294 for all comparisons.
3.6. Head Movement
The mean RMS of distance for the control group was 1.120 mm +/− 0.460 mm while for the patient group it was 1.044 mm +/− 0.384 mm. The group means did not differ significantly, P = 0.775. The mean RMS of speed for the control group was 0.0152 mm/sec +/− 0.007 mm/sec while for the patient group it was 0.014 mm/sec +/− 0.0117 mm/sec. The group means were not significantly different, P = 0.284. The creatine linewidths of the subscans with excessive movement were significantly higher than those associated with the subscans with non-excessive movement, p = 0.003.
4. Discussion
This study found lower GABA concentration in early visual cortex in a sample of subjects with recent onset schizophrenia compared to a demographically matched healthy sample. This finding replicates prior results from our group (Yoon et al., 2010) and others (Kelemen et al., 2013; Thakkar et al., 2017). To our knowledge, there are no published studies of early visual cortex GABA levels in schizophrenia that have failed to observe lower GABA in the patient group. The consistency of these findings from early visual cortex suggests that reduced GABA levels in schizophrenia can be reliably measured in vivo using single voxel H MRS in this region. The finding of lower GABA concentration in a recent onset sample suggest that lower visual cortex GABA in schizophrenia is not confounded by factors associated with illness chronicity. This study also found nearly equivalent GABA levels between unmedicated and medicated patients.
An important determinant of the reliability and reproducibility of MRS findings in schizophrenia could be the brain region being sampled. The consistency of findings from early visual cortex contrasts with the variability of results from studies that have measured GABA in other brain regions such as the medial PFC and the parietal-occipital cortex as reviewed by Egerton et al. (Egerton et al., 2017). Studies of the PFC in particular have been inconsistent with some studies showing elevated (de la Fuente-Sandoval et al., 2018; Kegeles et al., 2012), others showing diminished (Marenco et al., 2016; Marsman et al., 2014; Rowland et al., 2016) (Chiu et al. 2018) or unchanged (Reid et al. 2019) GABA in schizophrenia compared to a healthy comparison sample. It is notable that all early visual cortex studies utilized the same method for positioning the spectroscopic voxel - centering the voxel around the calcarine - enhancing the likelihood that the same brain region was sampled across studies. Variance in voxel placement, and therefore, the possible heterogeneity of brain region sampled across studies, could be one reason for the variability of results of studies that have measured GABA in other brain regions.
One potential explanation for the apparent discrepancy between some in vivo and post-mortem GABA findings is to posit a model of GABA pathology in schizophrenia in which GABA levels varies across stages of illness, with elevated and reduced levels occurring in early and late phases of illness, respectively. This model would account for the increased GABA observed in some (de la Fuente-Sandoval et al., 2018; Kegeles et al., 2012), though not all (Chiu et al. 2018), studies of first episode patients and clinical high-risk individuals (de la Fuente-Sandoval et al., 2015) and the diminished GABA levels observed in chronic samples by post-mortem GAD67 (Akbarian et al., 1995; Guidotti et al., 2000; Hashimoto, Arion, et al., 2008; Volk et al., 2000) and MRS studies (Kelemen et al., 2013; Thakkar et al., 2017; Yoon et al., 2010), (Marenco et al., 2016; Marsman et al., 2014; Rowland et al., 2016). Results from the present study are mostly inconsistent with this model. The present study is the second to have found reduced GABA in early visual cortex in a recent onset sample (Kelemen et al., 2013). In addition, the low correlation between DOI and GABA levels and absence of a difference in GABA levels between low compared to high DOI subsamples in the present study provide additional data inconsistent with the model of high GABA levels in early stage of illness. However, a comparison of the effect sizes of group differences in GABA levels in early visual cortex in the present with the prior study in chronic patients suggests that the GABA deficit may become more prominent with illness chronicity. The effect size was modest for the recent onset sample in this study, 0.55, while it was much larger in the chronic sample, 2.60. Future, longitudinal studies that sample GABA across the full range of illness stages and multiple brain regions will be required to fully test the possibility of changes in GABA across illness stage.
Other plausible explanations for the inconsistency between the results of the present study with the stage of illness model of GABA abnormalities in schizophrenia include the possibility that elevated GABA occurs only in the very earliest stages of illness, as suggested by one study in a high risk sample (de la Fuente-Sandoval et al., 2015). If this is the case, the limited sampling of patients in the earliest phase of illness by the present study may have led to missing the elevation in GABA levels that may occur. Another possibility is that elevated GABA in early illness is region specific, such that it does not occur in the visual cortex and may be limited to or most apparent in other cortical regions such as the PFC.
Some may question the utility of examining GABA in the visual cortex in schizophrenia when the visual system is considered by some as being relatively intact compared to other systems. This view, however, is challenged by the considerable literature documenting abnormalities at multiple levels of the visual system in schizophrenia, including alterations in perception (Butler, Silverstein, & Dakin, 2008) motion processing (Chen, 2011) and eye movement related phenomena (Bolding et al., 2014). Post-mortem studies have shown reductions in GAD-67 (Hashimoto, Bazmi, et al., 2008) as well as other neuropathologic abnormalities (Selemon, Rajkowska, & Goldman-Rakic, 1995) in the early visual cortex resembling those found in higher order cortical regions. These finding suggests that GABA deficits in schizophrenia may be pan cortical and that the early visual cortex could serve as a model experimental system to further study GABAergic and related abnormalities in schizophrenia. The early visual cortex is an appealing region for single voxel MRS studies due to technical factors optimizing the spectroscopic signal. The signal measured from a brain region of interest decreases exponentially with increasing distance from the MR receiver element within the head coil. Thus, maximal GABA signal is detected from regions in close proximity to the receiver elements, such as early visual cortex. Another factor favoring the early visual cortex is that it is relatively devoid of nearby brain tissue/air interfaces, which enhances magnetic field homogeneity and signal recovery. Thus, MRS measures of early visual cortex GABA deficits may be a reliable marker of pan-cortical GABA synthesis abnormalities in schizophrenia. However, it is also possible that MRS-visible, reduced in vivo GABA is not a pan-cortical finding in schizophrenia, but is only manifest in a subset of cortical regions, including the early visual cortex. A recent study found only modest correlations between resting GABA levels across four functionally distinct cortical regions (Greenhouse et al. 2016). For example, their observed correlation between GABA values in right lateral prefrontal cortex and bilateral early visual cortex was r =−0.26. Future studies will be needed to determine if regional differences in the regulation of GABA levels makes specific cortical regions, like early visual cortex, more likely to exhibit decreased in vivo GABA in the context of the GAD67 abnormalities observed post-mortem in schizophrenia patients.
A novel method employed by the present study was the means by which we quantitatively assessed the amount of head movement during MRS scans. Head movement represents a potentially significant source of noise in GABA measurement with MRS, and could limit the ability to detect true differences in GABA levels between two groups of subjects. Given this concern, we quantified the magnitude of head movement so that we may identify scans with excessive head movement and exclude them from analyses.
Limitations of the study include the small size of the antipsychotic naïve sample, which renders as preliminary any conclusions that could be made on antipsychotic effects on GABA levels. However, our finding of an absence of an antipsychotic effect is consistent with some but not all prior MRS studies. Three longitudinal studies that have measured GABA levels in the same individual before and after antipsychotic initiation (Goto, Yoshimura, Kakeda, & Moriya…, 2010; Kelemen et al., 2013) showed an absence of an effect of medications on GABA levels whereas one longitudinal study showed treatment with antipsychotic lowered GABA levels (de la Fuente-Sandoval et al., 2018). Another limitation was the relatively low level of symptomology of our sample. The patient sample falls into the mild to moderately ill level based on their BPRS scores (Leucht et al., 2005). This could, in part, explain the lack of association between GABA levels and symptom severity due to a floor effect.
There is on-going debate regarding methods for quantifying GABA concentration. The two most common are referencing to internal water or creatine. Each approach offers a unique set of advantages and limitations (for a review of these issues see Mikkelson et al. 2019). However, a recent, large, multi-centered study (Mikkelsen et al., 2019) concluded that both quantification methods afforded similar reliability. We have chosen to reference to creatine in order to facilitate comparisons with our prior study (Yoon et al. 2010), which utilized creatine referencing. Another reason for referencing to creatine is to avoid the possibility of error propagation associated with water referencing, which requires additional processing steps to properly estimate water signal from the three major tissue compartments within the spectroscopy voxel. The major argument against referencing to creatine is the possibility of abnormal creatine levels in schizophrenia, rendering referencing to this metabolite problematic for group comparisons. However, meta-analyses have shown little evidence for abnormalities in creatine levels in schizophrenia compared to healthy control subjects (Iwata et al., 2018; Kraguljac et al., 2012). The consistency among studies that have found lower GABA in the visual cortex cannot be attributable to GABA referencing methods. Two utilized creatine (Yoon et al., 2010; Kelemen et al., 2013) while one (Kelemen et al., 2013; Thakkar et al., 2017) utilized water as the reference molecule.
Although this study extends the literature on GABA abnormalities in schizophrenia, it does not directly address the question of the primacy of GABA deficits relative to deficits in other systems in schizophrenia. One of the most well-known alternatives is the NMDA receptor hypofunction hypothesis (Krystal et al., 1994; Luby, Cohen, Rosenbaum, Gottlieb, & Kelley, 1959). Some investigators have proposed the intriguing possibility that the key site of NMDA receptor hypofunction is on GABAergic interneurons (Cohen, Tsien, Goff, & Halassa, 2015; Olney, Newcomer, & Farber, 1999). Thus, GABAergic deficits could be a downstream manifestation of NMDA receptor hypofunction.
In summary, this study found evidence of reduced GABA levels as measured by MRS in early visual cortex of subjects with recent onset schizophrenia. This finding adds to the growing and consistent spectroscopic evidence of reduced in vivo GABA levels in this brain region in schizophrenia. These findings suggest that the early visual cortex may be a reliable experimental system in which to further study the neurobiological mechanisms and the consequences of GABAergic deficits in schizophrenia.
Role of the Funding Source
This research was funded by grants from the National Institute of Mental Health to Jong Yoon, R21 MH090475.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
All authors reports absence of conflict of interest.
5. References
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE Jr., et al. (1995). Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry, 52(4), 258–266. [DOI] [PubMed] [Google Scholar]
- Andreasen NC (1982). Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry, 39(7), 784–788. [DOI] [PubMed] [Google Scholar]
- Andreasen NC (1984). The Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, Iowa: University of Iowa. [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, et al. (1997). Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A, 94(12), 6496–6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Carter CS, MacDonald AW 3rd, Braver TS, & Cohen JD (2003). Context-processing deficits in schizophrenia: diagnostic specificity, 4-week course, and relationships to clinical symptoms. J Abnorm Psychol, 112(1), 132–143. [PubMed] [Google Scholar]
- Bogner W, Gruber S, Doelken M, Stadlbauer A, Ganslandt O, Boettcher U, et al. (2009). In vivo quantification of intracerebral GABA by single-voxel (1)H-MRS-How reproducible are the results? Eur J Radiol. [DOI] [PubMed] [Google Scholar]
- Bolding MS, Lahti AC, White D, Moore C, Gurler D, Gawne TJ, et al. (2014). Vergence eye movements in patients with schizophrenia. Vision Res, 102, 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Silverstein SM, & Dakin SC (2008). Visual perception and its impairment in schizophrenia. Biol Psychiatry, 64(1), 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y (2011). Abnormal visual motion processing in schizophrenia: a review of research progress. Schizophr Bull, 37(4), 709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Tsien RW, Goff DC, & Halassa MM (2015). The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr Res, 167(1–3), 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM (1974). Dose equivalence of the antipsychotic drugs. J Psychiatr Res, 11, 65–69. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Reyes-Madrigal F, Mao X, Leon-Ortiz P, Rodriguez-Mayoral O, Jung-Cook H, et al. (2018). Prefrontal and Striatal Gamma-Aminobutyric Acid Levels and the Effect of Antipsychotic Treatment in First-Episode Psychosis Patients. Biol Psychiatry, 83(6), 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Reyes-Madrigal F, Mao X, Leon-Ortiz P, Rodriguez-Mayoral O, Solis-Vivanco R, et al. (2015). Cortico-Striatal GABAergic and Glutamatergic Dysregulations in Subjects at Ultra-High Risk for Psychosis Investigated with Proton Magnetic Resonance Spectroscopy. Int J Neuropsychopharmacol, 19(3), pyv105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RA, Puts NA, Harris AD, Barker PB, & Evans CJ (2014). Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging, 40(6), 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Modinos G, Ferrera D, & McGuire P (2017). Neuroimaging studies of GABA in schizophrenia: a systematic review with meta-analysis. Transl Psychiatry, 7(6), e1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Cho RY, & Lewis DA (2015). Alterations in Cortical Network Oscillations and Parvalbumin Neurons in Schizophrenia. Biol Psychiatry, 77(12), 1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto N, Yoshimura R, Kakeda S, & Moriya J (2010). No alterations of brain GABA after 6 months treatment with atypical antipsychotic drugs in early-stage first-episode schizophrenia. Progress in Neuro- …. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, et al. (2000). Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry, 57(11), 1061–1069. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, et al. (2008). Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry, 13(2), 147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, & Lewis DA (2008). Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry, 165(4), 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata Y, Nakajima S, Plitman E, Mihashi Y, Caravaggio F, Chung JK, et al. (2018). Neurometabolite levels in antipsychotic-naive/free patients with schizophrenia: A systematic review and meta-analysis of (1)H-MRS studies. Prog Neuropsychopharmacol Biol Psychiatry, 86, 340–352. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, et al. (2012). Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry, 69(5), 449–459. [DOI] [PubMed] [Google Scholar]
- Kelemen O, Kiss I, Benedek G, & Keri S (2013). Perceptual and cognitive effects of antipsychotics in first-episode schizophrenia: the potential impact of GABA concentration in the visual cortex. Prog Neuropsychopharmacol Biol Psychiatry, 47, 13–19. [DOI] [PubMed] [Google Scholar]
- Kraguljac NV, Reid M, White D, Jones R, den Hollander J, Lowman D, et al. (2012). Neurometabolites in schizophrenia and bipolar disorder - a systematic review and meta-analysis. Psychiatry Res, 203(2–3), 111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. (1994). Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry, 51(3), 199–214. [DOI] [PubMed] [Google Scholar]
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, & Engel R (2005). Clinical implications of Brief Psychiatric Rating Scale scores. Br J Psychiatry, 187, 366–371. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, & Volk DW (2012). Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci, 35(1), 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, & Gonzalez-Burgos G (2006). Pathophysiologically based treatment interventions in schizophrenia. Nat Med, 12(9), 1016–1022. [DOI] [PubMed] [Google Scholar]
- Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, & Kelley R (1959). Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry, 81(3), 363–369. [DOI] [PubMed] [Google Scholar]
- Marenco S, Meyer C, Kuo S, van der Veen JW, Shen J, DeJong K, et al. (2016). Prefrontal GABA Levels Measured With Magnetic Resonance Spectroscopy in Patients With Psychosis and Unaffected Siblings. Am J Psychiatry, 173(5), 527–534. [DOI] [PubMed] [Google Scholar]
- Marsman A, Mandl RC, Klomp DW, Bohlken MM, Boer VO, Andreychenko A, et al. (2014). GABA and glutamate in schizophrenia: a 7 T (1)H-MRS study. Neuroimage Clin, 6, 398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, & Gruetter R (1998). Simultaneous in vivo spectral editing and water suppression. NMR Biomed, 11(6), 266–272. [DOI] [PubMed] [Google Scholar]
- Mikkelsen M, Rimbault DL, Barker PB, Bhattacharyya PK, Brix MK, Buur PF, et al. (2019). Big GABA II: Water-referenced edited MR spectroscopy at 25 research sites. Neuroimage, 191, 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, & Farber NB (1999). NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res, 33(6), 523–533. [DOI] [PubMed] [Google Scholar]
- Ongur D, Prescot AP, McCarthy J, Cohen BM, & Renshaw PF (2010). Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biol Psychiatry, 68(7), 667–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall J (1974). Psychological measurements in psychopharmacology. Volume editor: Pichot P; co-editor: Olivier-Martin R. Basel, New York,: Karger. [Google Scholar]
- Rowland LM, Krause BW, Wijtenburg SA, McMahon RP, Chiappelli J, Nugent KL, et al. (2016). Medial frontal GABA is lower in older schizophrenia: a MEGA-PRESS with macromolecule suppression study. Mol Psychiatry, 21(2), 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, & Goldman-Rakic PS (1995). Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry, 52(10), 805–818; discussion 819–820. [DOI] [PubMed] [Google Scholar]
- Stefan D, Di Cesare F, Andrasescu A, Popa E, Lazariev A, Vescovo E, et al. (2009). Quantitation of magnetic resonance spectroscopy signals: the mJRUI software package. Meas Sci Technol, 20. [Google Scholar]
- Tayoshi S, Nakataki M, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S, et al. (2010). GABA concentration in schizophrenia patients and the effects of antipsychotic medication: a proton magnetic resonance spectroscopy study. Schizophr Res, 117(1), 83–91. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Rosler L, Wijnen JP, Boer VO, Klomp DW, Cahn W, et al. (2017). 7T Proton Magnetic Resonance Spectroscopy of Gamma-Aminobutyric Acid, Glutamate, and Glutamine Reveals Altered Concentrations in Patients With Schizophrenia and Healthy Siblings. Biol Psychiatry, 81(6), 525–535. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, & Lewis DA (2000). Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry, 57(3), 237–245. [DOI] [PubMed] [Google Scholar]
- Woods SW (2003). Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry, 64(6), 663–667. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, et al. (2010). GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci, 30(10), 3777–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]