Abstract
OBJECTIVE
An imbalance between neuropeptides that promote stress and resilience, such as corticotrophin-releasing factor (CRF) and nociceptin, has been postulated to underlie relapse in addiction. The objective of this study was to develop a paradigm to image the in vivo interaction between stress-promoting neuropeptides and nociceptin receptors (NOP) in humans.
METHODS
[11C]NOP-1A PET was used to measure the binding to NOP receptors at baseline (BASE) and following an intravenous hydrocortisone challenge (POST-CORT) in 19 healthy controls. Hydrocortisone was used as a challenge because in microdialysis studies it has been shown to increase CRF release in extrahypothalamic brain regions such as the amygdala. [11C]NOP-1A total distribution volume (VT) in eleven regions (ROIs) were measured using a two-tissue compartment kinetic analysis. The primary outcome measure was hydrocortisone-induced ΔVT calculated as (VT POST-CORT - VT BASE) / VT BASE.
RESULTS
Hydrocortisone led to an acute increase in plasma cortisol levels. Regional [11C]NOP-1A VT was on average 11 to 16% higher in the POST-CORT compared to BASE condition (linear mixed model, condition, p = 0.005; region, p < 0.001, condition*region, p < 0.001). Independent t-tests in all ROIs were statistically significant and survived multiple comparison correction. Hydrocortisone-induced ΔVT was significantly negatively correlated with baseline VT in several ROIs.
CONCLUSIONS
Hydrocortisone administration increases NOP receptor availability. Increased NOP in response to elevated cortisol might suggest a compensatory mechanism in the brain to counteract CRF/stress. The [11C]NOP-1A and hydrocortisone imaging paradigm should allow for the examination of interactions between stress-promoting neuropeptides and NOP in addictive disorders.
(In Vivo Imaging of Corticotropin-releasing Factor - Nociceptin Receptor Interactions, https://clinicaltrials.gov/ct2/show/NCT03302416 ClinicalTrials.gov Identifier: )
Keywords: [11C]NOP-1A; positron emission tomography, (PET); corticotropin-releasing-factor (CRF); nociceptin/orphanin FQ peptide receptors (NOP); intravenous hydrocortisone
INTRODUCTION
Nociceptin (N/OFQ), also known as orphanin FQ, is an anti-stress/resilience promoting neuropeptide neurotransmitter that binds to its own G-protein coupled receptor, the nociceptin/orphanin FQ peptide (NOP) receptor. A number of rodent studies support a role for this neurotransmitter and its receptor in the regulation of stress. Specifically, an upregulation in NOP receptors in both the central nucleus of the amygdala and the basolateral amygdala has been found in restrained rats compared to controls (1). This finding was also reported in a separate study in which rats exposed to an acute stressor showed NOP receptor upregulation in the amygdala and the paraventricular nucleus of the hypothalamus (2). Rats exposed to a single-prolonged stress in a rodent model for post-traumatic stress disorder (PTSD) also show an increase in NOP receptors in the amygdala and the periaqueductal grey (midbrain) compared to controls (3). Additionally, NOP receptors in socially crowded mice have been shown to be upregulated in the hippocampus (4). In recent human studies, upregulation of NOP receptors in response to stress has also been observed. PET studies conducted with the NOP receptor radiotracer [11C]NOP-1A have demonstrated higher binding to NOP receptors in individuals with cocaine use disorders compared to controls (5). PET studies have also shown that the in vivo binding to NOP receptors is increased in women with more severe PTSD symptoms after sexual violence (6).
In order to understand the upregulation of NOP receptors in conditions of increased stress, it is necessary to have an understanding of the broader stress/anti-stress system. Cortisol levels, which are increased in response to stress, exert a negative feedback effect on the hypothalamic–pituitary–adrenal axis and a positive feedback effect on the extrahypothalamic brain regions such as the amygdala, hippocampus, midbrain and neocortex (7). The release of the stress- and anxiety-promoting neuropeptide neurotransmitter corticotropin releasing factor (CRF) in these extrahypothalamic brain regions is modulated by cortisol (8). The positive feedback relationship between cortisol and CRF release in the extrahypothalamic brain regions has been shown in sheep microdialysis studies in which an intravenous injection of hydrocortisone (cortisol) leads to an ~ 200% increase in CRF in the amygdala (7, 9). CRF is of particular interest because a number of studies have demonstrated a functional antagonism between the stress-promoting neuropeptide CRF and the anti-stress/resilience-promoting neuropeptide N/OFQ. Studies have convincingly demonstrated that N/OFQ, when injected into the brain ventricles, extended amygdala, bed nucleus of the stria terminalis, etc., inhibits CRF-induced anorexia and anxiety-like symptoms in rodents (10–14). Mechanistic studies are consistent with this in showing that N/OFQ’s ability to inhibit the firing of dorsal raphe neurons to regulate serotonin release is potentiated in the presence of CRF (15). Studies that have evaluated restraint-stress in rodents show an ~ 70% increase in NOP receptor protein expression in the extended amygdala to counteract increases in CRF (1). CRF-induced increases in GABAA mediated inhibitory postsynaptic potential are more strongly blocked by N/OFQ in these restraint-stress animals with increased NOP receptors compared to control animals (1). In a more direct documentation of CRF-NOP interactions, an injection of CRF into the lateral cerebroventricle was shown to increase NOP receptor expression by ~ 2-fold in the bed nucleus of the stria terminalis (14). Given the role that CRF and N/OFQ play in the regulation of stress, including in disorders such as addiction and PTSD, a methodology to evaluate their in vivo relationship in humans is of immense interest.
Here, we used [11C]NOP-1A and PET to measure the binding to NOP receptors before and after an acute intravenous hydrocortisone challenge (1 mg kg−1 body weight) to image the in vivo interaction between CRF and NOP in humans. Intravenous corticotrophin-releasing hormone was not used as a challenge because it is not transported from the blood to the brain (16). We hypothesized that hydrocortisone-induced increases in CRF will result in an increase in NOP receptors, and this will be detected as higher [11C]NOP-1A receptor binding (VT). Despite the focus of prior basic investigations on limbic-related regions such as the amygdala and hippocampus we had no region-specific hypotheses because both CRF and N/OFQ expressing neurons are widely distributed throughout the brain (17–19).
MATERIALS AND METHODS
Human Subjects
The University of Pittsburgh Human Research Protection Office Institutional Review Board and Radioactive Drug Research Committee approved the study. All subjects provided written informed consent. Nineteen healthy subjects (9 male, 10 female) completed the study. Subjects were recruited via advertisements in newspapers, online ads, and the University of Pittsburgh research registry (Pitt+Me). Participants underwent all clinical and imaging procedures on an outpatient basis.
Study criteria included: (1) males and females between 18–40 years old; (2) no past or current DSM-5 psychiatric or substance use disorders (confirmed via SCID-5, and negative urine drug tests at screening and the day of the PET scan); (3) no history of binge drinking in the past month as defined by NIAAA criteria; (4) not currently on any prescription medical or psychotropic medication; (5) no past or current severe medical, endocrine, cardiovascular, immunological or neurological illnesses as assessed by medical history, physical exam and lab-work; (6) not currently pregnant; (7) no history of radiation exposure in the past twelve months via prior nuclear medicine research studies or occupational exposure such that the total cumulative annual radiation dose exposure would exceed the radiation dose limits specified in the FDA regulations 21 CFR 361.1; (8) no metallic objects in the body that are contraindicated for MRI; (9) and no first-degree relative with psychosis, mood, or anxiety disorders.
PET imaging protocol
Prior to PET imaging, a magnetization prepared rapid gradient echo structural MRI scan was obtained using a Siemens 3T Trio scanner for brain region of interest (ROIs) determination. The synthesis of [11C]NOP-1A and PET imaging protocol (using the Siemens Biograph64 mCT scanner) were conducted using methods that have been described previously (20, 21). Following a low-dose CT scan of the brain for attenuation correction, subjects received an intravenous bolus injection of [11C]NOP-1A and emission data (baseline scan) were collected for 70 minutes (22, 23). Subjects were then administered a 1 mg kg−1 intravenous bolus injection of hydrocortisone over 90 seconds and monitored for two hours for changes in heart rate and blood pressure. These measurements were made every ten minutes for the first hour, and then every fifteen minutes for the next hour. The Perceived Stress Scale (PSS), Hamilton Anxiety Rating Scale (HAM-A) and Center for Epidemiological Depression Scale (CES-D) were used to measure subjective responses before (time, t = 0 minutes) and after the hydrocortisone injection (t = 60 minutes). Plasma cortisol concentrations were also measured at the same time points (i.e., t = 0 and 60 minutes) in the arterial blood. The second [11C]NOP-1A scan was initiated 3.5 hours after the administration hydrocortisone (post-hydrocortisone scan). Since this scan lasted 70 minutes, this allowed for the measurement of [11C]NOP-1A binding to NOP receptors ~ 3.5 – 4.5 hours post-challenge. The timing of the second [11C]NOP-1A scan was based on pre-clinical studies that have documented alterations in NOP ~ 4 to 7 hours after CRF/stress (1, 14). Metabolite-corrected arterial input function measurements were performed and analyzed as previously reported (21, 22). [11C]NOP-1A plasma free fraction (fp) was not pursued in this study because it has poor reproducibility and is highly adherent to the ultracentrifugation filter in a saline buffer solution (5, 6, 21, 22). Heart rate variability (HRV) of subjects during the PET scans were also measured with the Empatica E4 wrist band via a photoplethysmography sensor (Empatica Inc, Cambridge, MA).
PET image analysis
PET data were reconstructed by filtered back projection using the camera’s built-in software. The image analysis software PMOD, version 3.802 (PMOD Technologies LLC, Zurich, Switzerland) was used to conduct frame-to-frame motion correction for head movement. The MR-PET image alignment was performed using a normalized mutual information algorithm. ROIs included the amygdala, hippocampus, midbrain, ventral striatum, caudate, putamen, dorsolateral prefrontal cortex, orbital frontal cortex, medial prefrontal cortex, anterior cingulate cortex and cerebellum (22). ROIs were generated for each subject based on the AAL-VOI atlas using the built-in brain parcellation workflow within PMOD’s Neuro Tool (PNEURO module). Regional volumes and time activity curves were also generated in PMOD. Derivation of [11C]NOP-1A volume of distribution expressed relative to total plasma concentration (VT) in the ROIs was performed using a two-tissue compartment kinetic analysis using the arterial input function implemented in MATLAB (21, 22). VT, which includes both the receptor-bound specific and non-specific binding, was used as the outcome measure because there is no reference region to estimate [11C]NOP-1A non-specific binding (VND) in the brain (24).
Heart rate variability analysis
HRV, which depends on the autonomic nervous system (both the sympathetic and parasympathetic branches) and the respiratory sinus arrhythmia (which is mediated via the baroreceptor reflex and changes in vascular tone) is lowered during stress (25, 26). Studies have also demonstrated an association between low HRV and increased cortisol in plasma and urine (27, 28). Thus, we were interested in HRV response to increased cortisol because it provides an objective measure of the autonomic nervous system’s ability to adapt to the hydrocortisone challenge. HRV measurements were derived from the inter-beat intervals. These inter-beat intervals are the time between heart beat peaks, which are known as the R-R intervals. Raw data for determination of HRV were processed using Kubios HRV ver. 3.0 (29). Medium artefact correction was applied to all samples, correcting on average seven percent of inter-beat intervals for each 70-minute sample. HRV outcome measures examined were the time-domain based index variables: the standard deviation of the normal to normal R-R intervals (SDNN) and the root mean square of successive R-R interval differences (RMSSD). SDNN values increase as heart rate variability increases and becomes more irregular. SDNN is considered a marker of physiological resilience against stress because it is influenced by both the sympathetic and parasympathetic nervous systems (25, 26). RMSSD, which is the beat to beat variance in heart rate, reflects the parasympathetic neural regulation of HRV. The 70-minute inter-beat interval data collected during the baseline and post-hydrocortisone [11C]NOP-1A scans were used to derive the SDNN and RMSSD.
Hydrocortisone effect
Hydrocortisone-induced change in VT (ΔVT) was calculated as the difference between VT measured in the post-hydrocortisone condition (VT CORT) and VT measured in the baseline condition (VT BASE), and expressed as a percentage of VT BASE.
Hydrocortisone-induced changes in cortisol and HRV measures were also calculated using this approach. Hydrocortisone-induced changes in clinical rating scale scores (PSS, HAM-A and CES-D) were calculated as the difference between the score measured in the baseline and post-hydrocortisone condition (this was necessary because several subjects reported relatively low scores including zero in both the baseline and post-hydrocortisone conditions).
Statistical analysis
All statistical analyses were performed with IBM SPSS Statistics v.25. Group demographic and baseline scan parameter, such as injected dose, mass, and plasma clearance, comparisons were performed with paired t-tests. Overall group differences in [11C]NOP-1A VT were analyzed with a linear mixed model (LMM) analysis performed with ROIs as a repeated measure and condition (baseline vs. post-hydrocortisone) as the fixed factor. This test was followed up with post-hoc independent two-tailed paired t-tests in all eleven ROI. The relationships between the PET, clinical and HRV measure were explored by the Pearson product moment correlation coefficients. A two-tailed probability value of p < 0.05 was selected as the significance level for all the analyses. A Benjamini-Hochberg false discovery rate correction (FDR) with α = 0.05 was applied to correct for multiple comparisons (n = 11) in the individual region of interest analyses (30).
RESULTS
Demographics
Nineteen subjects (9 males/10 females; 13 Caucasian, 2 Asian, 1 African American, 1 Hispanic, and 2 more than one race) participated in the study. The mean age of the subjects was 25 ± 4 years (range 21 to 34 years). The mean weight of the subjects was 77 ± 16 kgs (range 53 to 108 kgs). All nineteen subjects who participated in the study were non-smokers.
Scan parameters
[11C]NOP-1A scan parameters, including specific activity, injected dose and mass at the time of injection, and plasma clearance for the baseline and post-hydrocortisone conditions, are listed in Table 1. Despite the between-condition difference in specific activity, no significant differences between the baseline and post-hydrocortisone condition in [11C]NOP-1A injected dose and injected mass were noted. The clearance of [11C]NOP-1A from the plasma was significantly increased post-hydrocortisone compared to baseline (see Table 1).
Table 1.
Data acquired before and after the hydrocortisone challenge
| Scan parameters | Baseline condition | Post-hydrocortisone condition |
|---|---|---|
| Injected dose (mCi) | 12.3 ± 1.3 | 12.7 ± 1.2 |
| Specific Activity (Ci/mmoles) | 2085 ± 708 | 2757 ±1165* |
| Injected mass (pg) | 2.7 ± 0.9 | 2.3 ± 0.8 |
| Clearance (L/h) | 142.3 ± 36.2 | 167.7 ± 49.2* |
|
Clinical parameters‡ | ||
| Perceived Stress Scale (0 to 40) | 4.9 ± 5.3 | 3.8 ± 4.8* |
| Hamilton Anxiety Rating Scale (0 to 56) | 1.5 ± 2.0 | 0.8 ± 1.5* |
| Center for Epidemiological Studies in Depression (0 to 60) | 1.6 ± 2.4 | 1.1 ± 2.3 |
| Cortisol (pg/dL)† | 10.5 ± 3.9 | 79.9 ± 17.1* |
|
HRV parameters
†† | ||
| Heart Rate (beats per minute) | 63 ± 8 | 74 ± 13* |
| Standard deviation of the normal to normal R-R intervals (ms) | 70 ± 18 | 53 ± 24* |
| Root mean square of successive R-R interval differences (ms) | 65 ± 16 | 46 ± 20* |
Values are mean ± standard deviation
p < 0.05
Data only available from n = 17 subjects ft Data only available from n = 16 subjects
Clinical data were acquired at time, t = 0 min and 60 min after the hydrocortisone challenge.
Note: all other scan and HRV data shown correspond to the baseline and post-hydrocortisone PET scans.
Clinical and HRV parameters
Hydrocortisone administration led to a statistically significant increase ~ 7.5-fold in plasma cortisol levels (Table 1). There was a small, but significant decrease in perceived stress and anxiety (but not depressive) symptom scores after the hydrocortisone challenge (see Table 1). Mean HR was significantly higher during the post-hydrocortisone PET scan compared to the baseline PET scan. HRV measures, SDNN and RMSSD were significantly lower in the post-hydrocortisone condition relative to baseline (see Table 1, HR and HRV data was only available from n = 16 subjects due to problems encountered with acquisition).
Hydrocortisone effect on [11C]NOP-1A VT
[11C]NOP-1A VT was significantly higher in the post-hydrocortisone condition compared to baseline (LMM, effect of condition, F (1, 36) = 8.95, p = 0.005; effect of region, F (10, 360) = 708.99, p < 0.001; region * condition interaction, F (10, 360) = 4.09, p < 0.001). Regional values for VT BASE, VT CORT, ΔVT and the uncorrected p-values from the corresponding paired t-tests are shown in Table 2. All p-values in ROIs remained significant after correction for multiple comparisons using FDR.
Table 2.
Effect of intravenous hydrocortisone on [11C]NOP-1A VT
| Regions | [11C]NOP-1A VT | ΔVT (%) | p-values | |
|---|---|---|---|---|
| Baseline condition | Post-hydrocortisone condition | |||
| Amygdala | 16.3 ± 1.9 | 18.7 ± 2.8 | 16.0 ± 21.2 | 0.0046 |
| Hippocampus | 11.6 ± 1.3 | 13.3 ± 2.0 | 15.2 ± 20.2 | 0.0047 |
| Midbrain | 9.4 ± 1.0 | 10.6 ± 1.6 | 13.7 ± 17.5 | 0.0035 |
| Ventral Striatum | 15.1 ± 1.7 | 17.1 ± 2.1 | 13.7 ± 17.6 | 0.0041 |
| Caudate | 12.7 ± 1.4 | 14.0 ± 1.9 | 11.4 ± 18.1 | 0.0162 |
| Putamen | 14.3 ± 1.6 | 16.0 ± 2.0 | 13.3 ± 18.4 | 0.0057 |
| Dorsolateral Prefrontal Cortex | 14.4 ± 1.8 | 16.4 ± 2.4 | 14.3 ± 18.7 | 0.0039 |
| Orbital Frontal Cortex | 14.9 ± 1.8 | 17.1 ± 2.6 | 15.6 ± 18.1 | 0.0016 |
| Medial Prefrontal Cortex | 14.3 ± 1.8 | 16.2 ± 2.4 | 14.3 ± 19.3 | 0.0046 |
| Anterior Cingulate Cortex | 14.6 ± 1.7 | 16.6 ± 2.6 | 14.9 ± 18.6 | 0.0030 |
| Cerebellum | 8.2 ± 0.9 | 9.3 ± 1.3 | 13.9 ± 17.3 | 0.0030 |
p-values shown are from the independent paired t-tests not corrected for multiple comparisons. All regional p-values survived the FDR for multiple comparison correction.
Baseline VT predicts the magnitude of the hydrocortisone-induced ΔVt
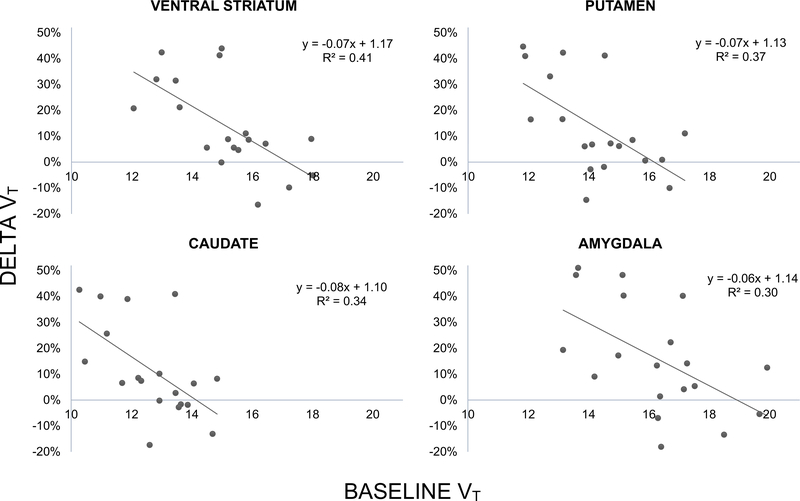
Significant negative relationships were observed between baseline VT and ΔVT in the ventral striatum (r = ‒0.64, p = 0.003), putamen (r = ‒0.60, p = 0.006), caudate (r = ‒0.58, p = 0.009), amygdala (r = ‒0.55, p = 0.016), medial prefrontal cortex (r = ‒0.50, p = 0.031), hippocampus (r = ‒ 0.49, p = 0.034), dorsolateral prefrontal cortex (r = ‒0.49, p = 0.035), and cerebellum (r = ‒0.48, p = 0.038). The same relationship was at trend level in the remaining regions of interest (p < 0.1, data not shown). The negative relationships in the amygdala and striatal regions (ventral striatum, caudate and putamen) survived the FDR correction for multiple comparisons (shown in Figure 1).
Figure 1.
shows the negative relationship between VT BASE and ΔVT in the four ROIs that were significant after correction for multiple hypothesis testing. Smaller VT values at baseline are predictive of a larger increase in VT after the hydrocortisone challenge.
Relationship between hydrocortisone-induced changes in clinical / HRV parameters and ΔVT
No significant relationships were noted between the hydrocortisone-induced change in scores in perceived stress, anxiety, depression, etc. and ΔVT in any of the ROIs. There were also no relationships between the hydrocortisone-induced change in plasma cortisol levels and ΔVT in any of the ROIs.
Hydrocortisone-induced ΔHR was not correlated with ΔVT in the ROIs. There were significant negative relationships between the hydrocortisone-induced ΔSDNN and ΔVT in the caudate (r = −0.56, p = 0.025), putamen (r = −0.53, p = 0.035) and hippocampus (r = −0.50, p = 0.049). In other words, hydrocortisone-induced decreases in SDNN were associated with increases in NOP-1A VT after the challenge (Supplement Figure S1). This relationship was also at trend level in the cerebellum, midbrain, amygdala, and anterior cingulate, dorsolateral prefrontal and medial prefrontal cortices (p < 0.1, data not shown). None of these relationships survived the FDR correction for multiple comparisons. No significant relationships were observed between ΔRMSSD and ΔVT in any of the ROIs.
Exploratory analyses examining the relationships between baseline measures such as PSS, HAM-A, HAM-D, cortisol levels, SDNN, RMSSD, etc., and ΔVT in ROIs were all not significant.
Effect of sex and age on baseline VT and ΔVT
A LMM analyses to examine the effect of sex on regional VT was not significant (effect of sex, F (1, 17) = 0.00, p = 0.98; effect of region, F (10, 170) = 413.92, p < 0.001; region * sex interaction, F (10, 170) = 1.21, p = 0.29). Sex also had no effect on ΔVT (LMM, effect of sex, F (1, 17) = 0.61, p = 0.45; effect of region, F (10, 170) = 3.09, p = 0.001; region * sex interaction, F (10, 170) = 0.53, p = 0.87).
Bivariate correlations showed no significant relationships between age and VT, or ΔVT in the ROIs.
DISCUSSION
The main findings of this study are: (1) hydrocortisone-induced elevations in plasma cortisol increased [11C]NOP-1A VT by ~ 10 to 15% in the ROIs; and (2) hydrocortisone-induced increases in VT were negatively correlated with the VT measured in the baseline condition, i.e., a smaller baseline VT was predictive of a large ΔVT and vice versa. The hydrocortisone-induced ΔVT in subjects was highly variable (Supplemental Figure S2). For example, in the orbital frontal cortex ΔVT ranged from −17% to +47%. Nevertheless, in 15 out of 19 subjects the increase in VT after hydrocortisone exceeded the 4% increase in VT reported in the retest scans acquired 3-hours after the test scans in the study by Lohith et al. (22). The mean test-retest variability for [11C]NOP-1A VT derived using compartmental modeling in relatively large regions of interest is 12% (22). This suggests an effect size (calculated as the hydrocortisone-induced ΔVT / test-retest variability) for the hydrocortisone-induced Δ[11C]NOP-1A VT in the range of 0.9 to 1.3 in the regions of interest. This effect size is comparable to other imaging paradigms used in the field such as the [11C]FLB 457-amphetamine ( ~ 1) and the [11C]flumazenil-tiagabine (~ 0.6) paradigms, which measure dopamine-release and GABA-release in the cortex (31, 32). However, it is smaller than observed for D2/3 radiotracers such as [11C]raclopride and [11C]NPA (~ 2 to 4), which measure dopamine release in the striatum (33). A result that fell short of significance was the relationship between hydrocortisone-induced increases in VT and decreases in SDNN – the HRV measure related to stress-resilience. An important negative finding in this study was the failure to replicate our previous report of lower regional baseline VT in females compared to males (5). The hydrocortisone-induced increases in VT in this study cannot be explained by the between-condition differences in [11C]NOP-1A specific activity and plasma clearance (shown in Table 1). This is supported by the lack of differences in [11C]NOP-1A injected mass (Table 1) and the use of VT as the PET outcome measure (34). Because VT is the ratio of tissue to free plasma tracer concentration at equilibrium it is not vulnerable to changes plasma clearance. A key limitation is the inability to exclude hydrocortisone-induced changes in plasma free fraction (fp) and non-specific binding (VND) as factors that influenced ΔVT because they could not be quantified. Other limitations include the lack of a placebo-control and the failure to account for the influence of the circadian variations in cortisol that may have altered NOP receptors binding parameters in the study design. The examination of [11C]NOP-1A VT before and after a hydrocortisone challenge at the same time of the day is necessary to exclude the influence of circadian variations in a future study.
Increased binding of [11C]NOP-1A to NOP in response to an acute elevation in cortisol is consistent with numerous rodent investigations that have demonstrated an upregulation of NOP receptors in response to stress (reviewed in introduction). This finding also suggests that the increased NOP receptors previously reported in patients with cocaine use disorders compared to controls is related to the increased cortisol and CRF levels associated with stress (5). Interpreting the hydrocortisone-induced upregulation of NOP receptors observed in this study using a simple receptor occupancy model would suggest that the hydrocortisone challenge depletes N/OFQ levels. However, such a decrease in endogenous N/OFQ levels following a CRF or behavioral stress challenge is not supported by basic investigations (1, 14). For example, N/OFQ levels were unchanged in the study by Rodi et al., that reported NOP receptor upregulation in response to a CRF challenge (14). Also consistent with this are unpublished rodent data in which N/OFQ (and NOP receptor) levels were found to be unchanged 20-minutes after a CRF challenge (Ciccocioppo lab). Based on this it is tempting to speculate that NOP receptor upregulation, as opposed to N/OFQ release, is the primary mechanism by which N/OFQ signaling is enhanced to counter increased CRF/stress. The fact that increased binding to NOP receptors is not detected 20 minutes after the hydrocortisone/CRF challenge but is detected 4–6 hours after the challenge also suggests that it takes significant time to either synthesize new NOP receptor proteins or recycle the previously inaccessible intracellular NOP receptors to the cell surface. The latter is supported by numerous in vitro cell studies that have shown NOP receptors to internalize within the cell via endocytosis in the presence of the endogenous agonist N/OFQ (35–39). Recycling of these internalized NOP receptors in an active form back to the plasma membrane is a critical mechanism by which N/OFQ signaling is restored (for example, after desensitization) or enhanced (35, 36). Assuming that [11C]NOP-1A detects only receptors on the cell surface (or has lower affinity for receptors that are internalized compared to those on the cell surface) the internalization model would suggest that hydrocortisone-induced ΔVT reflects the number of internalized NOP receptors that have recycled back to the cell surface in an active form. This model also has the potential to explain a possible ceiling effect that is apparent in individuals with higher baseline NOP VT. Here, we postulate that subjects with less N/OFQ levels at baseline have a higher VT because they have less internalized receptors and more surface receptors. In contrast, subjects with greater N/OFQ have a smaller baseline VT because they have more internalized and less surface NOP receptors. It is possible that the total number of NOP receptors in both these subject groups (less N/OFQ, more N/OFQ) is the same and sets the ceiling. However, the fraction of NOP receptors located in the internalized and surface pools are different in the two subject groups. Under such a scenario, a subject who is exposed to an acute stress/hydrocortisone challenge with more internalized receptors would have more reserve capacity to upregulate NOP, whereas the one with less internalized receptors would have less reserve to upregulate NOP. In theory, CUD patients who exhibit a higher NOP VT at baseline would not be able to upregulate NOP in response to a hydrocortisone challenge. Linking hydrocortisone-induced NOP upregulation with clinically relevant measures such as relapse to cocaine use in future studies has the potential inform us as to whether this phenomenon represents an adaptive or maladaptive response to stress. We also cannot exclude the possibility that a higher dose of hydrocortisone would elicit a more robust response in individuals with a higher baseline NOP VT. Future studies with higher hydrocortisone doses are necessary to clarify this issue. In vitro studies in cells are also warranted to confirm whether [11C]NOP-1A binds with preference to cell surface versus internalized NOP receptors—a key assumption in this model.
In summary, we used [11C]NOP-1A PET and hydrocortisone to demonstrate increased binding to NOP receptors following an acute elevation in plasma cortisol levels, and by extension brain CRF and N/OFQ. Lower baseline VT values predicted larger increase in VT after the hydrocortisone challenge. Combining this imaging paradigm with HRV measures and physiological stress tasks (such as the cold pressor task, or Trier Social Stress Task) have the potential to further our understanding of its clinical relevance in stress-resilience. The use of the hydrocortisone-[11C]NOP-1A PET imaging paradigm should allow for the examination of the interactions between stress and anti-stress/resilience promoting neuropeptides in addictive and stress disorders.
Supplementary Material
ACKNOWLEDGEMENTS
The project described above was funded by R21DA042633 and R01DA026472 from the National Institute on Drug Abuse (NIDA). Recruitment of subjects were also supported by a research registry (Pitt+Me), which is funded by the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, Award Number UL1 TR001857.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDA or the National Institutes of Health.
Footnotes
FINANCIAL DISCLOSURE
The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Ciccocioppo R, de Guglielmo G, Hansson AC, Ubaldi M, Kallupi M, Cruz MT, et al. (2014): Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: significance for anxiety-like behaviors. J Neurosci. 34:363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green MK, Devine DP (2009): Nociceptin/orphanin FQ and NOP receptor gene regulation after acute or repeated social defeat stress. Neuropeptides. 43:507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Simpson-Durand CD, Standifer KM (2015): Nociceptin/orphanin FQ peptide receptor antagonist JTC-801 reverses pain and anxiety symptoms in a rat model of post-traumatic stress disorder. Br J Pharmacol. 172:571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiss D, Wolter-Sutter A, Krezel W, Ouagazzal AM (2007): Effects of social crowding on emotionality and expression of hippocampal nociceptin/orphanin FQ system transcripts in mice. Behav Brain Res. 184:167–173. [DOI] [PubMed] [Google Scholar]
- 5.Narendran R, Tollefson S, Himes ML, Paris J, Lopresti B, Ciccocioppo R, et al. (2019): Nociceptin Receptors Upregulated in Cocaine Use Disorder: A Positron Emission Tomography Imaging Study Using [(11)C]NOP-1A. Am J Psychiatry.appiajp201918081007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narendran R, Tollefson S, Fasenmyer K, Paris J, Himes ML, Lopresti B, et al. (2019): Decreased Nociceptin Receptors Are Related to Resilience and Recovery in College Women Who Have Experienced Sexual Violence: Therapeutic Implications for Posttraumatic Stress Disorder. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook CJ (2002): Glucocorticoid feedback increases the sensitivity of the limbic system to stress. Physiol Behav. 75:455–464. [DOI] [PubMed] [Google Scholar]
- 8.Koob GF (1999): Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 46:1167–1180. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz ML, Tator CH, Hoffman HJ (1972): The uptake of hydrocortisone in mouse brain and ependymoblastoma. J Neurosurg. 36:178–183. [DOI] [PubMed] [Google Scholar]
- 10.Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, Massi M (2003): The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. J Neurosci. 23:9445–9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciccocioppo R, Biondini M, Antonelli L, Wichmann J, Jenck F, Massi M (2002): Reversal of stress- and CRF-induced anorexia in rats by the synthetic nociceptin/orphanin FQ receptor agonist, Ro 64–6198. Psychopharmacology (Berl). 161:113–119. [DOI] [PubMed] [Google Scholar]
- 12.Ciccocioppo R, Martin-Fardon R, Weiss F, Massi M (2001): Nociceptin/orphanin FQ inhibits stress- and CRF-induced anorexia in rats. Neuroreport. 12:1145–1149. [DOI] [PubMed] [Google Scholar]
- 13.Ciccocioppo R, Cippitelli A, Economidou D, Fedeli A, Massi M (2004): Nociceptin/orphanin FQ acts as a functional antagonist of corticotropin-releasing factor to inhibit its anorectic effect. Physiol Behav. 82:63–68. [DOI] [PubMed] [Google Scholar]
- 14.Rodi D, Zucchini S, Simonato M, Cifani C, Massi M, Polidori C (2008): Functional antagonism between nociceptin/orphanin FQ (N/OFQ) and corticotropin-releasing factor (CRF) in the rat brain: evidence for involvement of the bed nucleus of the stria terminalis. Psychopharmacology (Berl). 196:523–531. [DOI] [PubMed] [Google Scholar]
- 15.Nazzaro C, Barbieri M, Varani K, Beani L, Valentino RJ, Siniscalchi A (2010): Swim stress enhances nociceptin/orphanin FQ-induced inhibition of rat dorsal raphe nucleus activity in vivo and in vitro: role of corticotropin releasing factor. Neuropharmacology. 58:457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martins JM, Kastin AJ, Banks WA (1996): Unidirectional specific and modulated brain to blood transport of corticotropin-releasing hormone. Neuroendocrinology. 63:338–348. [DOI] [PubMed] [Google Scholar]
- 17.Merchenthaler I (1984): Corticotropin releasing factor (CRF)-like immunoreactivity in the rat central nervous system. Extrahypothalamic distribution. Peptides. 5 Suppl 1:53–69. [DOI] [PubMed] [Google Scholar]
- 18.Potter E, Behan DP, Linton EA, Lowry PJ, Sawchenko PE, Vale WW (1992): The central distribution of a corticotropin-releasing factor (CRF)-binding protein predicts multiple sites and modes of interaction with CRF. Proc Natl Acad Sci U S A. 89:4192–4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witta J, Palkovits M, Rosenberger J, Cox BM (2004): Distribution of nociceptin/orphanin FQ in adult human brain. Brain Res. 997:24–29. [DOI] [PubMed] [Google Scholar]
- 20.Pike VW, Rash KS, Chen Z, Pedregal C, Statnick MA, Kimura Y, et al. (2011): Synthesis and evaluation of radioligands for imaging brain nociceptin/orphanin FQ peptide (nOp) receptors with positron emission tomography. J Med Chem. 54:2687–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narendran R, Ciccocioppo R, Lopresti B, Paris J, Himes ML, Mason NS (2018): Nociceptin Receptors in Alcohol Use Disorders: A Positron Emission Tomography Study Using [(11)C]NOP-1A. Biol Psychiatry. 84:708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lohith TG, Zoghbi SS, Morse CL, Araneta MD, Barth VN, Goebl NA, et al. (2014): Retest imaging of [11C]NOP-1A binding to nociceptin/orphanin FQ peptide (NOP) receptors in the brain of healthy humans. Neuroimage. 87:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lohith TG, Zoghbi SS, Morse CL, Araneta MF, Barth VN, Goebl NA, et al. (2012): Brain and whole-body imaging of nociceptin/orphanin FQ peptide receptor in humans using the pEt ligand 11C-NOP-1A. J Nucl Med. 53:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura Y, Fujita M, Hong J, Lohith TG, Gladding RL, Zoghbi SS, et al. (2011): Brain and whole-body imaging in rhesus monkeys of 11C-NOP-1A, a promising PET radioligand for nociceptin/orphanin FQ peptide receptors. J Nucl Med. 52:1638–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HG, Cheon EJ, Bai DS, Lee YH, Koo BH (2018): Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 15:235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaffer F, Ginsberg JP (2017): An Overview of Heart Rate Variability Metrics and Norms. Front Public Health. 5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pulopulos MM, Vanderhasselt MA, De Raedt R (2018): Association between changes in heart rate variability during the anticipation of a stressful situation and the stress-induced cortisol response. Psychoneuroendocrinology. 94:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thayer JF, Hall M, Sollers JJ 3rd, Fischer JE (2006): Alcohol use, urinary cortisol, and heart rate variability in apparently healthy men: Evidence for impaired inhibitory control of the HPA axis in heavy drinkers. Int J Psychophysiol. 59:244–250. [DOI] [PubMed] [Google Scholar]
- 29.Tarvainen MP, Niskanen J-P, Lipponen JA, Ranta-aho PO, Karjalainen PA (2014): Kubios HRV - Heart rate variability analysis software. Computer Methods and Programs in Biomedicine. 113:210–220. [DOI] [PubMed] [Google Scholar]
- 30.Benjamini Y, Hochberg Y (1995): Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B.289–300. [Google Scholar]
- 31.Narendran R, Mason NS, May MA, Chen CM, Kendro S, Ridler K, et al. (2011): Positron emission tomography imaging of dopamine D(2)/(3) receptors in the human cortex with [(1)(1)C]FLB 457: reproducibility studies. Synapse. 65:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narendran R, Frankle WG, Mason NS, Rabiner EA, Gunn R, Searle GE, et al. (2009): Positron Emission Tomography Imaging of Amphetamine-Induced Dopamine Release in the Human Cortex: A comparative evaluation of the high affinity dopamine D2/3 radiotracers [11C]FLB 457 and [11C]fallypride. Synapse. 63:447–461. [DOI] [PubMed] [Google Scholar]
- 33.Narendran R, Mason NS, Laymon C, Lopresti B, Velasquez N, May M, et al. (2010): A comparative evaluation of the dopamine D2/3 agonist radiotracer [11C]NPA and antagonist [11C]raclopride to measure amphetamine-induced dopamine release in the human striatum. Journal of Pharmacology and Experimental Therapeutics. 63:574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slifstein M, Laruelle M (2001): Models and methods for derivation of in vivo neuroreceptor parameters with PET and SPECT reversible radiotracers. Nucl Med Biol. 28:595–608. [DOI] [PubMed] [Google Scholar]
- 35.Spampinato S, Baiula M (2006): Agonist-regulated endocytosis and desensitization of the human nociceptin receptor. Neuroreport. 17:173–177. [DOI] [PubMed] [Google Scholar]
- 36.Spampinato S, Baiula M, Calienni M (2007): Agonist-regulated internalization and desensitization of the human nociceptin receptor expressed in CHO cells. Curr Drug Targets. 8:137–146. [DOI] [PubMed] [Google Scholar]
- 37.Toll L, Bruchas MR, Calo G, Cox BM, Zaveri NT (2016): Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems. Pharmacol Rev. 68:419–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corbani M, Gonindard C, Meunier JC (2004): Ligand-regulated internalization of the opioid receptor-like 1: a confocal study. Endocrinology. 145:2876–2885. [DOI] [PubMed] [Google Scholar]
- 39.Mann A, Mouledous L, Froment C, O’Neill PR, Dasgupta P, Gunther T, et al. (2019): Agonist-selective NOP receptor phosphorylation correlates in vitro and in vivo and reveals differential post-activation signaling by chemically diverse agonists. Sci Signal. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.