Abstract
Background:
Hepatocellular carcinoma (HCC) is the fastest rising cause of cancer-related mortality in the US and a leading indication for liver transplantation (LT). Changes in chronic liver disease burden have led to aging of the chronic liver disease population, but how this affects patients with HCC is unknown. This study aims to characterize trends and transplant-associated outcomes among patients ≥65y listed for LT with HCC.
Methods:
Using the UNOS database, we analyzed all patients ≥18y listed for LT 2003–2017 in the United States; grouped by age at listing (<65y or ≥65y). Nonparametric tests and standardized regression coefficients were used to compare time trends between HCC and non-HCC patients, stratified by disease etiology. Competing risk and Cox regression associated HCC and age with waitlist and post-LT survival. Results: Included were 161,724 liver transplant candidates: 14% were ≥65y at listing and 25% had HCC. The proportion of patients ≥65y rose significantly faster among those with HCC, as compared to those without HCC (Δ=0.80, P<0.001). Age ≥65y was significantly associated with both waitlist mortality (aSHR 1.51, 95CI 1.40–1.64) and post-LT mortality (aHR 1.50, 95CI 1.41–1.60) in multivariable analysis. There were significant interactions between age and HCC on both waitlist (P<0.001) and post-liver transplant mortality (P=0.04), suggesting older age does not impact patients with HCC as much as patients without HCC.
Conclusions:
The proportion of older adults with HCC listed for LT has nearly tripled from 2003–2017, a rate that is over twice as fast as among those without HCC. The rapidly growing population of older adults with HCC may provide an opportunity to expand liver transplant access without compromising outcomes.
Keywords: older adults, mortality, cirrhosis, Hepatitis C
INTRODUCTION
The burden of hepatocellular carcinoma (HCC) is rising rapidly, with an incidence that has nearly tripled in the United States (US) over the past several decades.1–4 As a result, HCC has become the fastest-growing cause of cancer-related mortality in the US. 2,3 These trends have significantly impacted the liver transplant population: not only has the proportion of liver transplant recipients with HCC increased greater than 6-fold over the last two decades5,6, but HCC currently represents the most common indication for liver transplantation in the US.7
The rising incidence of HCC is believed to be due to an increased prevalence of cirrhosis in the United States – the biggest risk factor for HCC – among those with chronic hepatitis C (HCV) as well as the rapid emergence of obesity-related liver disease known as non-alcoholic fatty liver disease (NAFLD). It is well-documented that these changes in liver disease etiology have resulted in an older liver transplant population in the US.8–10 However, it is not known how these evolving demographics have specifically impacted patients with HCC, which may have important implications for HCC-related morbidity and mortality. In the present study, we aimed to characterize aging trends in the HCC population and their impact on transplant-related outcomes among HCC patients.
MATERIALS AND METHODS
All adult (≥ 18 years) patients listed for liver transplantation in the United Network for Organ Sharing/Organ Procurement and Transplantation Network (UNOS/OPTN) registry from January 1, 2003 through December 31, 2017 were evaluated for inclusion in this study.
Recipient and donor characteristics
Data used in this analysis were obtained from the UNOS/OPTN registry as of April 6, 2018. The Model for End-Stage Liver Disease (MELD) score at listing and at transplant or waitlist removal was calculated and capped at 6 and 40.11,12 Because serum sodium was not routinely collected in the UNOS/OPTN database until 2014, we used MELD and not model for end-stage liver disease including serum sodium (MELDNA) in this study looking at long-term trends. Ascites and hepatic encephalopathy were considered present if they were recorded at either listing or waitlist removal/transplant. Liver donor characteristics included those used to calculate the donor risk index (DRI), a summary metric to quantify liver allograft quality.13 Given significant regional variation in transplant MELD scores.
Age groups
Older patients were defined as those ≥65 years at the time of listing, while younger patients were defined as those <65 years at listing. Age 65 is a commonly used cutoff for older adults in the medical and liver transplant literature. 9,14,15
Hepatocellular carcinoma and cirrhosis etiology
Patients were categorized as having HCC if any of the following were true: (1) they were granted HCC exception points; (2) their primary or secondary diagnosis at listing or transplant was HCC; or (3) they were designated as ever having had HCC. Listing diagnoses were grouped into the following common diagnostic categories: hepatitis C virus (HCV), nonalcoholic steatohepatitis (NASH, including cryptogenic cirrhosis), alcohol-related cirrhosis, autoimmune etiologies (including primary biliary cirrhosis, primary sclerosing cholangitis and autoimmune hepatitis), and other etiologies of cirrhosis (any other listing code that met inclusion criteria). For time trend analyses, these diagnoses were further grouped into HCV, NASH, and non-HCV/non-NASH (which included all other categories).
Outcomes
To better understand the impact of an aging liver transplant cohort, we analyzed the impact of age in those with and without HCC on two outcomes: 1) waitlist mortality, defined as the combined outcome of death before transplant or removal for being too sick for transplant, and 2) post-liver transplant mortality. For waitlist mortality, patient follow-up began on the date of listing for liver transplant and ended at the time of death, removal from the waitlist, or transplant. Patients removed from the waitlist for recovery of hepatic function, social reasons, or living donor liver transplant were censored at the time of their removal. For post-transplant mortality, follow-up time was defined as the time between the date of transplant and the date of death or last follow-up. Patients remaining alive at last follow-up were censored at that time.
Statistical analysis
Demographics analysis
Categorical variables were compared between age and HCC groups by the chi square test. Continuous variables were compared between groups by the Wilcoxon rank sum test given nonparametric distributions.
Trend analysis
We determined the percentage of patients ≥65 years by listing year from 2003–2017. This was first completed in all patients with and without HCC. We then determined the percentage of patients ≥65 years in those with and without HCC stratified by etiology of cirrhosis (i.e. HCV, NASH, and non-HCV/non-NASH). To test for statistical trends over time, we evaluated the percentage of patients ≥65 stratified by HCC status, treating list year as the continuous variable. We tested for differences in the trends over time using nonparametric tests and standardized regression coefficients (Figure 1).
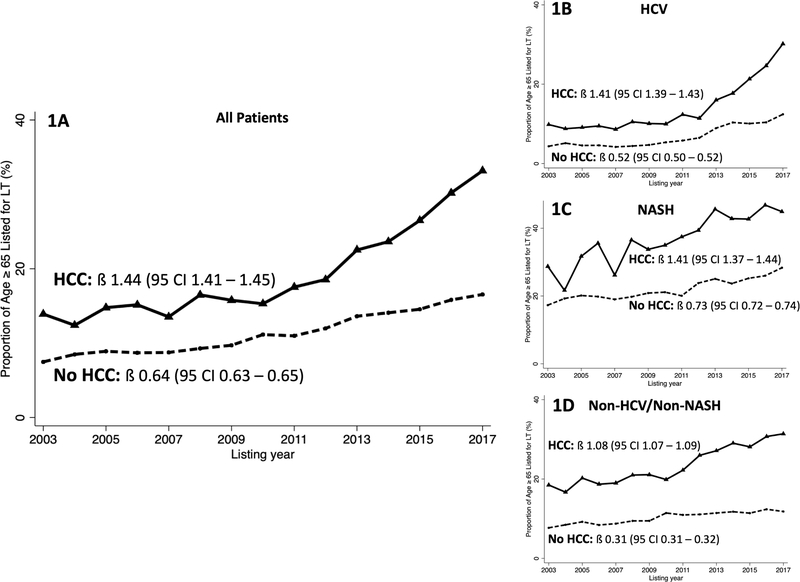
Figure 1. Trends in the proportion of patients listed for liver transplant in the United States between 2003 and 2017 who are ≥65 years at time of listing.
(A) Proportion of all listed patients ≥65 years by HCC status. (B) Proportion of listed patients with HCV cirrhosis ≥65 years by HCC status. (C) Proportion of listed patients with NASH cirrhosis ≥65 years by HCC status. (D) Proportion of listed patients with Non-HCV/non-NASH cirrhosis ≥65 years by HCC status. β represents regression coefficient.
To evaluate the correlation between the changes in screening recommendations for HCV, the introduction of direct acting antivral (DAA) therapy, and the percentage of older patients with HCC, we compared regression coefficients for patients listed before and after January 1, 2014. This date was chosen because during 2013, national guidelines were changed to recommend universal HCV screening for all patients born from 1945–1965, and the Food and Drug Administration approved the first DAA, sofosbuvir. 16–18 Era of transplantation was categorized as: 2003–2005, 2006–2009, 2010–2013, and 2014–2017. Eras were selected a priori based on: (1) significant improvements in both waitlist and post-liver transplant mortality over time; (2) several updates to HCC transplantation policies and management strategies; (3) recommendation changes for HCV screeningin late 2013; and (4) availability of DAA therapy in early 20149,16–22.
Survival analysis
For the outcome of waitlist mortality, competing risk regression was used to associate age ≥65 years with waitlist mortality, accounting for liver transplantation. For the outcome of post-liver transplant mortality, Cox proportional hazard regression models were used to associate age ≥65 years with post-transplant mortality. To account for center variability, all models were adjusted for region and the era of transplantation, and clustered on transplant center. We formally tested the interaction between HCC and age.
Covariables with P < 0.2 were considered for inclusion in multivariable models. Backward elimination was used for final models, with covariables not reaching significance of P < 0.05 being sequentially eliminated. Two-sided P values <0.05 were considered statistically significant. Waitlist and post-transplant patient mortality were estimated by age and HCC status using Kaplan-Meier plots. Plots were compared using a log-rank test.
Software and database
All analyses were performed using Stata 15.0 statistical software (College Station, TX). This study was approved by the institutional review board at the University of California, San Francisco. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
RESULTS
Demographics and clinical characteristics by age and HCC status
There were 161,724 adult patients listed for liver transplant during the study period. Of these, 35.5% were female and 70.8% were non-Hispanic white. The median age at listing was 55 years [interquartile range (IQR) 49–61], with 22,092 (13.7%) patients ≥65 years at listing. Among the 40,293 (24.9%) patients with HCC, the median age was 59 years (IQR 54–64), with 20.6% of these patients ≥65 years at listing. In comparison among patients without HCC, the median age was 54 (IQR 47–60), with 11.4% of patients ≥65 years at listing (Table 1).
Table 1.
Characteristics and outcomes of patients listed for liver transplantation in the United States 2003–2017 by HCC status and age1
| Hepatocellular Carcinoma | No Hepatocellular Carcinoma | |||||
|---|---|---|---|---|---|---|
| Age ≥65 years (n = 8 300) |
Age <65 years (n = 31 993) |
P value | Age ≥65 years (n = 13 792) |
Age <65 years (n = 107 639) |
P value | |
| Female gender | 2 335 (28) | 6 840 (21) | <0.001 | 6 318 (46) | 41 959 (39) | <0.001 |
| Ethnicity | ||||||
| White | 5 388 (65) | 20 621 (65) | 10 741 (78) | 77 797 (72) | ||
| Black | 660 (8) | 3 203 (10) | 733 (5) | 9 724 (9) | ||
| Hispanic | 1 287 (16) | 5 199 (16) | <0.001 | 1 744 (13) | 15 382 (14) | <0.001 |
| Asian | 878 (11) | 2 505 (8) | 1 744 (13) | 15 382 (14) | ||
| Other | 87 (1) | 465 (2) | 110 (1) | 1 438 (1) | ||
| Liver diagnosis | ||||||
| Alcohol | 1 043 (13) | 2 826 (9) | 2 403 (17) | 21 059 (20) | ||
| Hepatitis C | 3 444 (42) | 19 627 (61) | 2 272 (17) | 35 040 (33) | ||
| NASH/Cryptogenic | 1 758 (21) | 2 635 (8) | <0.001 | 5 193 (38) | 17 597 (16) | <0.001 |
| Autoimmune | 326 (4) | 758 (2) | 2 079 (15) | 14 149 (13) | ||
| Other | 1 729 (21) | 6 147 (19) | 1 845 (13) | 19 794 (18) | ||
| Days on waitlist | 188 (71 – 349) | 184 (64 −392) | 0.03 | 132 (26 – 447) | 148 (22 – 588) | <0.001 |
| MELD at listing | 11 (8 – 14) | 11 (8 – 15) | <0.001 | 17 (13 – 24) | 17 (13 – 25) | <0.001 |
| MELD at transplant | 12 (8 – 18) | 13 (9 – 19) | <0.001 | 21 (15 – 30) | 21 (14 – 41) | 0.16 |
| Hepatic encephalopathy | 591 (7) | 2 732 (9) | <0.001 | 2 956 (21) | 24 527 (23) | <0.001 |
| Ascites | 1 427 (17) | 6 412 (20) | <0.001 | 5 920 (43) | 44 386 (41) | <0.001 |
| Karnofsky Performance Status | 70 (60 – 80) | 70 (60 – 80) | 0.60 | 60 (50 – 80) | 70 (40 – 80) | 0.12 |
| Donor risk index | 1.5 (1.2 – 1.8) | 1.5 (1.2 – 1.8) | <0.001 | 1.5 (1.2 – 1.9) | 1.4 (1.2 – 1.7) | <0.001 |
| Private Insurance | 2 369 (29) | 20038 (63) | <0.001 | 3672 (27) | 66970 (62) | <0.001 |
|
Age ≥65 years (n = 8 300) |
Age <65 years (n = 31 993) |
P value |
Age ≥65 years (n = 13 792) |
Age <65 years (n = 107 639) |
P value | |
| Waitlist Outcomes | ||||||
| Still Waiting | 1 460 (18) | 4 359 (14) | <0.001 | 2 976 (22) | 25 292 (24) | <0.001 |
| Death/Sickness | 1 565 (19) | 5 027 (16) | 4 316 (31) | 24 591 (23) | ||
| Live donor liver transplant | 99 (1) | 433 (1) | 301 (2) | 2 404 (2) | ||
| Deceased donor liver transplant | 5 176 (62) | 22 174 (69) | 6 199 (45) | 55 352 (51) | ||
| 1-year post-transplant mortality | 536 (10) | 1 801 (8) | <0.001 | 846 (14) | 5 522 (10) | <0.001 |
| 5-year post-transplant mortality | 1 108 (21) | 4 433 (20) | 0.02 | 1 396 (23) | 9 989 (18) | <0.001 |
NASH, nonalcoholic steatohepatitis; MELD, model for end stage liver disease
Data presented as number (percent) or median (IQR)
Among the 40,293 patients with HCC, those ≥65 years, as compared to those <65 years, were more likely to be female (28.1% vs 21.4%, P<0.001) and to have NASH cirrhosis (21.2% vs 8.2%, P<0.001). They were also less likely to have decompensated cirrhosis, as measured by ascites (17.2% vs 20.0%, P<0.001) and hepatic encephalopathy (7.1% vs 8.5%, P<0.001). Among the 121,431 non-HCC patients, those ≥65 years were also more likely to be female (45.8% vs 39.0%, P<0.001) and to have NASH cirrhosis (37.7% vs 16.3%, P<0.001). In contrast to those with HCC, in this cohort, rates of ascites and hepatic encephalopathy were clinically similar between age groups.
Trends in percentage of patients ≥65 years
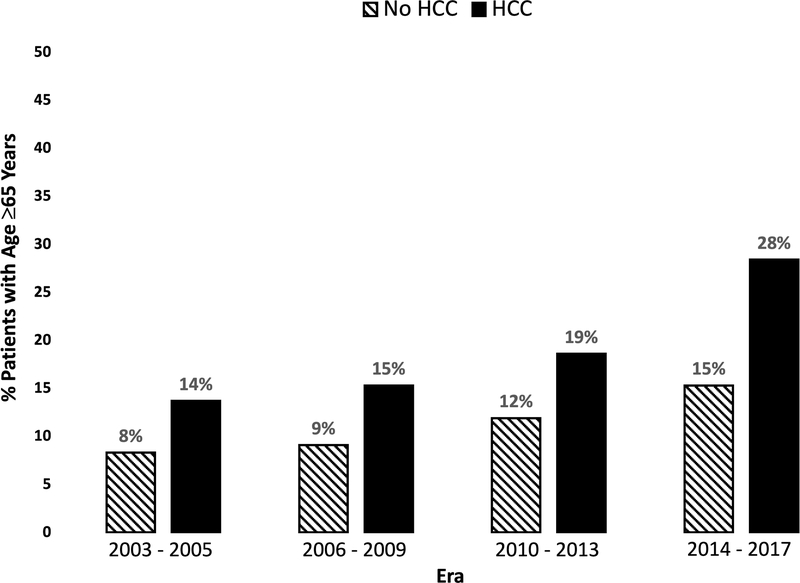
Among all patients with HCC, the median age at listing increased from 54 (49 – 63) years in 2003 to 62 (57 – 66) years; among all patients without HCC, the median age at listing increased, but a slower rate, from 52 (46 – 57) years to 56 (47 – 62) years. Among all patients with HCC, the percentage ≥65 years increased from 13.9% in 2003 to 33.2% in 2017 (ß=1.44, P<0.001). Although the percentage of patients ≥65 years also increased from 2003–2017 among those without HCC (ß=0.64, P<0.001), the rise was significantly faster among those with HCC (Δ=0.80, P<0.001; nonparametric test for trend, P<0.001) (Figure 1A). The proportion of patients ≥65 years also increased by era of transplantation, as shown in Figure 2.
Figure 2. Proportion of patients ≥65 years by era of transplantation.
Hashed bars represent patients without HCC and solid bars represent patients with HCC.
To evaluate the differential increase of the proportion of patients ≥65 years by HCC status in more detail, we performed a trend analysis stratified by primary etiology of cirrhosis. Among all patients listed with HCV cirrhosis, the percentage ≥65 years among those with HCV cirrhosis with HCC rose from 9.9% in 2003 to 30.2% in 2017 (ß=1.41, P<0.001). Simultaneously, among those with HCV cirrhosis without HCC, the percentage ≥65 years rose from 4.5% to 12.4% (ß=0.52, P<0.001). The relative increase between 2003 and 2017 in the percentage of patients ≥65 years was significantly larger among those with HCC compared to those without (Δ=0.89, P<0.001; nonparametric test for trend, P<0.001), as shown in Figure 1B. Trend analysis confirmed that there was a significant increase in the rate of rise of the percentage ≥65 years among those with HCV after the implementation of national screening policies and introduction of DAA therapyHC (Δ=2.43, P<0.001; nonparametric test for trend, P<0.001).
Among patients with NASH and HCC, the percentage ≥65 years rose from 28.6% in 2003 to 45.6% in 2017 (ß= 1.41, P<0.001), compared with a rise from 13.8% to 29.4% among those without HCC (ß= 0.73, P<0.001). Similar to patients with HCV, the rise in the proportion of patients ≥65 years was significantly faster among those with HCC compared to those without HCC (Δ=0.68, P<0.001; nonparametric test for trend, P<0.001) (Figure 1C).
Similarly, among those with non-HCV/non-NASH and HCC, the percentage ≥65 years rose from 19.7% in 2003 to 32.1% in 2017 (ß= 1.08, P<0.001), compared with a smaller rise from 9.3% to 12.7% among those with non-HCV/non-NASH cirrhosis without HCC (ß= 0.31, P<0.001) (Δ = 0.77, P<0.001; nonparametric test for trend, P<0.001)(Figure 1D).
Waitlist outcomes by age and HCC status
Among all patients, 22.0% experienced waitlist mortality. By HCC status, waitlist mortality was 23.8% among those without HCC and 16.4% among those with HCC (P<0.001). By age, waitlist mortality was 26.6% among those ≥65 years, compared with 21.1% among those <65 years (P<0.001) (Table 1).
In univariable competing risk regression, age ≥65 years was significantly associated with waitlist mortality [subhazard ratio (SHR) 1.43, 95% confidence interval (CI) 1.36–1.50, p<0.001]. Other factors associated with waitlist mortality included female gender (SHR 1.19, 95% CI 1.10–1.28), NASH compared with alcohol-related cirrhosis (SHR 1.15, 95% CI 1.02–1.31), final MELD (SHR 1.05, 95% CI 1.02–1.08), hepatic encephalopathy (SHR 2.84, 95% CI 2.09–3.87), and private insurance (SHR 0.76, 95% CI 0.71–0.81). HCC was not significantly associated with waitlist mortality on univariable analysis (SHR 0.83, 95% CI 0.66–1.05, P=0.12). In multivariable regression, age ≥65 years remained independently associated with waitlist mortality (aSHR 1.51, 95% CI 1.40–1.64) (Table 2).
Table 2.
Competing risk analysis for waitlist mortality among all patients listed for liver transplant
| Univariable | Multivariable* | |||||
|---|---|---|---|---|---|---|
| SHR | 95% CI | P value | SHR | 95% CI | P value | |
| Age ≥65 (at listing) | 1.43 | 1.36–1.50 | <0.001 | 1.51 | 1.40 – 1.64 | <0.001 |
| HCC | 0.83 | 0.66–1.05 | 0.12 | 0.87 | 0.55 – 1.37 | 0.55 |
| Female gender | 1.19 | 1.10–1.28 | <0.001 | |||
| Liver diagnosis | ||||||
| Alcohol | Ref | Ref | ||||
| Hepatitis C | 1.09 | 0.95–1.24 | 0.22 | 1.16 | 1.11 – 1.23 | <0.001 |
| NASH | 1.15 | 1.02–1.31 | 0.03 | 1.08 | 1.03 – 1.14 | 0.002 |
| Autoimmune/cholestatic | 0.97 | 0.86–1.09 | 0.62 | 0.93 | 0.85 – 1.02 | 0.12 |
| Other | 1.11 | 0.90–1.36 | 0.33 | 1.08 | 0.94 – 1.24 | 0.30 |
| MELD at transplant per point | 1.05 | 1.02–1.08 | <0.001 | 1.04 | 1.01–1.07 | 0.01 |
| Hepatic encephalopathy | 2.84 | 2.09–3.87 | <0.001 | 1.75 | 1.55–1.97 | <0.001 |
| Private insurance | 0.76 | 0.71–0.81 | <0.001 | 0.86 | 0.79–0.93 | <0.001 |
SHR, subhazard ratio; CI, confidence interval; HCC, hepatocellular carcinoma; NASH, nonalcoholic steatohepatitis; MELD, model for end stage liver disease
Multivariable model also adjusted for blood type, region, era, and interaction term between age and HCC
There was a significant interaction between age ≥65 years and HCC status on waitlist mortality (P<0.001), with age ≥65 years being associated with a significantly lower risk of waitlist mortality in those with HCC compared to those without HCC.
Post-liver transplant outcomes by age and HCC status
Among the 161,724 listed for deceased donor liver transplant, 88,901 patients underwent deceased donor liver transplant (55.0%). Among these patients, 1- and 5-year post-liver transplant mortality rates were 9.8% and 19.0% respectively (Table 1). The rates of 1-year and 5-year mortality differed less between patients ≥65 years and <65 years among those with HCC (1-year: 10.4% v. 8.1%, P<0.001; 5-year: 21.4% v. 20.0%, P = 0.02), than it did between patients ≥65 years and <65 years without HCC (1-year: 13.7% v. 10.0%, P<0.001; 5-year: 22.5% v. 18.1%, P<0.001).
Among all patients who underwent deceased donor liver transplant, age ≥65 years (HR 1.39, 95% CI 1.33 – 1.45) was associated with increased post-liver transplant mortality as was presence of HCC (HR 1.13, 95% CI 1.09 – 1.18). Other factors associated with post-liver transplant mortality were: HCV compared with alcohol-related cirrhosis (HR 1.26, 95% CI 1.21 – 1.32), NASH compared with alcohol-related cirrhosis (HR 1.07, 95% CI 1.01–1.12), baseline Karnofsky Performance Status (HR 0.96 per 10 points, 95% CI 0.95 – 0.97), MELD at transplant (HR 1.01, 95% CI 1.01–1.01), waiting list time (HR 0.99 per 90 days, 95% CI 0.98 – 0.99), hepatic encephalopathy (HR 1.21, 95% CI 1.16–1.26), and DRI (HR 1.03 per 0.1 points, 95% CI 1.03–1.04). In multivariable analysis, age ≥65 years remained independently associated with post-liver transplant mortality (aHR 1.50, 95% CI 1.41–1.60, P<0.001) (Table 3).
Table 3.
Cox regression analysis for post-liver transplantation mortality among all patients listed for liver transplant
| Univariable | Multivariable* | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age ≥65 (at listing) | 1.39 | 1.33–1.45 | <0.001 | 1.50 | 1.41 – 1.60 | <0.001 |
| HCC | 1.13 | 1.09–1.18 | <0.001 | 1.26 | 1.18 – 1.33 | <0.001 |
| Female gender | 0.93 | 0.90–0.96 | <0.001 | |||
| Liver diagnosis | ||||||
| Alcohol | Ref | Ref | ||||
| Hepatitis C | 1.26 | 1.21–1.32 | <0.001 | 1.34 | 1.28 – 1.40 | <0.001 |
| NASH | 1.06 | 1.01–1.12 | 0.02 | 1.07 | 1.01 – 1.12 | 0.02 |
| Autoimmune | 0.81 | 0.77–0.86 | <0.001 | 0.87 | 0.81 – 0.93 | <0.001 |
| Other | 1.10 | 1.02–1.17 | 0.007 | 1.11 | 1.04 – 1.18 | 0.001 |
| MELD (final) per point | 1.01 | 1.01–1.01 | <0.001 | 1.01 | 1.01 – 1.02 | <0.001 |
| Waiting List Time per 90 days | 0.99 | 0.98 – 0.99 | <0.001 | 0.99 | 0.98 – 0.997 | 0.03 |
| KPS per 10 points | 1.00 | 0.00–1.00 | <0.001 | 0.96 | 0.95 – 0.97 | <0.001 |
| Hepatic encephalopathy | 1.21 | 1.16–1.26 | <0.001 | 1.11 | 1.05 – 1.18 | <0.001 |
| Donor risk index per 0.1 points | 1.38 | 1.30–1.47 | <0.001 | 1.03 | 1.03 – 1.04 | <0.001 |
HR, hazard ratio; CI, confidence interval; HCC, hepatocellular carcinoma; NASH, nonalcoholic steatohepatitis; MELD, model for end stage liver disease; KPS, Karnofsky Performance Status
Multivariable model also adjusted for interaction term between age and HCC
Similar to waitlist mortality, there was a significant interaction between age and HCC on post-liver transplant mortality (P=0.04). Among patients <65 years, HCC was associated with an average increased risk of post-liver transplant mortality by 37% (95% CI 23%−52%, P<0.001), but among those ≥65 years, the risk associated with HCC was significantly lower (30% [95% CI 12% – 48%, P<0.001]).
DISCUSSION
In this national study of over 160,000 patients listed for liver transplantation in the US, we observed that the proportion of older adults with HCC listed for transplant has nearly tripled between 2003 to 2017, with over one third of patients listed for liver transplant with HCC aged 65 or older by 2017. While older age was a risk factor for both waitlist and post-liver transplant mortality, it was more strongly associated with mortality among non-HCC patients compared to those with HCC. While prior studies have reported on the aging US liver transplant population,8–10,23 our study is the first to explore how the rising age among patients with chronic liver disease specifically impacts patients listed for liver transplant with HCC.
Why has the population of HCC patients seeking liver transplantation aged faster than the population of non-HCC patients? Our analyses suggest that this is driven in large part by the aging of patients with HCV-related liver disease. In the US, the peak HCV antibody prevalence occurred in individuals born between 1940 and 1965 – the Baby Boomers – with most having been infected between the ages of 20–35.24,25 The majority of these individuals are now ≥65 years and have had HCV infection for >30 years, both of which have been shown to be to be independent risk factors for HCC.26 In addition, US guidelines changed in 2013 to recommend universal HCC screening for all Baby Boomers, increasing diagnosis of HCV, many of whom had cirrhosis at diagnosis, and therefore qualifyied for HCC screening. 27–29 Although the widespread treatment of HCV-infected individuals with DAA therapy significantly reduces lifetime risk of HCC, those who have advanced fibrosis at the time of DAA therapy remain at risk for HCC at older ages, rather than dying of hepatic decompensation.30
Another major explanation for the rapid rise in older liver transplant patients with HCC is the obesity epidemic, which has led to a burgeoning population of patients with NAFLD. Given the relatively slow progression of obesity-related liver disease, cirrhosis related to NAFLD often does not develop until more advanced ages, in comparison to to other liver diseases. Therefore, as the underlying etiology driving the development of HCC more frequently becomes NAFLD, the age of patients listed for transplant with HCC is expected to increase.
The temporal trends in advancing age that we observed among patients seeking liver transplantation for HCC are particularly relevant in the context of their transplant-related outcomes. In contrast to previous studies which have suggested that older patients universally have worse liver transplant outcomes, 8,23 our findings demonstrate that the relationship between age and post-transplant outcomes is more nuanced. With regard to waitlist mortality, older HCC patients had half the risk of death of older non-HCC patients. Similarly, among patients <65 years, HCC was associated with substantially increased risk of post-liver transplant mortality, whereas HCC was associated with a smaller though still increased risk of post-transplant mortality among patients ≥65 years. This may be explained by increased clinical reserve among the cohort of patients ≥65 years selected to undergo liver transplant, compared to those ≥65 years with decompensated cirrhosis.
We acknowledge the following limitations to our study. First, given that we analyzed data from a national administrative database, we were unable to fully account for center-specific policies that might influence the age of HCC patients being considered for liver transplantation. However, given that center-specific policies, when present, typically impose age limits on liver transplantation rather than liberalize age cut-offs, we would anticipate that such policies would lead to underestimation of age trends among patients with HCC nationwide. Further, our survival analyses were clustered on center, and therefore any center based variation in outcomes should have been accounted for. Second, as national HCV screening guidelines changed around 2013, it is possible that our findings reflected increased screening practices rather than true increased incidence or earlier HCC diagnosis resulting in lead time bias. Finally, we acknowledge that by using the UNOS/OPTN database there is the possibility of a selection bias of only including older patients deemed to have the functional reserve to undergo transplant. This limits the conclusions that can be made regarding all older patients with HCC, but we believe our finding that age impacts those with HCC less than those without HCC, highlights the need to reassess how we are capturing age-associated risk among liver transplant candidates.
Our findings have significant implications for the management of older patients with chronic liver disease and HCC. We demonstrated that the median age of the HCC population is rising rapidly in the US, significantly faster than those without HCC. Given the increased HCV screening among the Baby Boomers, increasing availability of DAA therapy, and the ongoing epidemic of obesity-related liver disease – all of which may be contributing to the aging of the HCC population – our observations may represent merely the tip of the iceberg. In light of the favorable interaction that we observed between HCC and older age on both waitlist and post-transplant mortality, our findings suggest that older HCC patients may present an opportunity to expand liver transplant access to adults at older ages without significantly compromising outcomes. However, we also confirmed that age is a risk factor for waitlist and post-liver transplant mortality in all populations, including HCC. Thus, patient selection for liver transplantation remains essential in this population; future research should focus on developing better metrics—perhaps based on comorbidities and frailty—to more accurately risk-stratify older HCC patients and evaluate their candidacy for liver transplantation.
Acknowledgments
Grant Support:
This study was funded by NIA Research Project Grant (R01AG059183, Lai), NIA Paul B. Beeson Career Development Award in Aging (K23AG048337, Lai), and NIDDK National Research Service Award Hepatology Training Grant (T32DK060414, Cullaro, Rubin).
Abbreviations:
- CI
confidence interval
- DAA
direct acting antiviral
- DRI
donor risk index
- HCC
hepatocellular carcinoma
- HCV
hepatitis C
- HR
hazard ratio
- IQR
interquartile range
- MELD
Model for End-Stage Liver Disease
- MELDNa
Model for End-Stage Liver Disease including serum sodium
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- SHR
subhazard ratio
- UNOS/OPTN
United Network for Organ Sharing/Organ Procurement and Transplantation Network
- US
United States
Footnotes
Disclosures:
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61(1):191–199. doi: 10.1002/hep.27388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3(4):524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122(9):1312–1337. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control. 2017;24(3):1073274817729245. doi: 10.1177/1073274817729245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ioannou GN, Perkins JD, Carithers RL. Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134(5):1342–1351. doi: 10.1053/j.gastro.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59(6):2188–2195. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 7.Yang JD, Larson JJ, Watt KD, et al. Hepatocellular Carcinoma Is the Most Common Indication for Liver Transplantation and Placement on the Waitlist in the United States. Clin Gastroenterol Hepatol 2017;15(5):767–775.e3. doi: 10.1016/j.cgh.2016.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su F, Yu L, Berry K, et al. Aging of Liver Transplant Registrants and Recipients: Trends and Impact on Waitlist Outcomes, Post-Transplantation Outcomes, and Transplant-Related Survival Benefit. Gastroenterology. 2016;150(2):441–53.e6–quize16. doi: 10.1053/j.gastro.2015.10.043. [DOI] [PubMed] [Google Scholar]
- 9.Haugen CE, Holscher CM, Garonzik Wang J, et al. National Trends in Liver Transplantation in Older Adults. J Am Geriatr Soc 2018;66(12):2321–2326. doi: 10.1111/jgs.15583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durand F, Levitsky J, Cauchy F, Gilgenkrantz H, Soubrane O, Francoz C. Age and liver transplantation. J Hepatol. December 2018. doi: 10.1016/j.jhep.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 12.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 2008;359(10):1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6(4):783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 14.Malinis MF, Chen S, Allore HG, Quagliarello VJ. Outcomes among older adult liver transplantation recipients in the model of end stage liver disease (MELD) era. Ann Transplant. 2014;19:478–487. doi: 10.12659/AOT.890934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haugen CE, McAdams DeMarco M, Holscher CM, et al. Multicenter Study of Age, Frailty, and Waitlist Mortality Among Liver Transplant Candidates. Ann Surg January 2019. doi: 10.1097/SLA.0000000000003207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moyer VA U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2013;159(5):349–357. doi: 10.7326/0003-4819-159-5-201309030-00672. [DOI] [PubMed] [Google Scholar]
- 17.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep 2012;61(RR-4):1–32. [PubMed] [Google Scholar]
- 18.AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932–954. doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 19.Stepanova M, Wai H, Saab S, Mishra A, Venkatesan C, Younossi ZM. The outcomes of adult liver transplants in the United States from 1987 to 2013. Liver Int 2015;35(8):2036–2041. doi: 10.1111/liv.12779. [DOI] [PubMed] [Google Scholar]
- 20.Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109(4):542–553. doi: 10.1038/ajg.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156(2):477–491.e1. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flemming JA, Kim WR, Brosgart CL, Terrault NA. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology. 2017;65(3):804–812. doi: 10.1002/hep.28923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrero JI, Lucena JF, Quiroga J, et al. Liver transplant recipients older than 60 years have lower survival and higher incidence of malignancy. Am J Transplant. 2003;3(11):1407–1412. doi: 10.1046/j.1600-6143.2003.00227.x. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006;144(10):705–714. [DOI] [PubMed] [Google Scholar]
- 25.Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med 2014;160(5):293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asahina Y, Tsuchiya K, Tamaki N, et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology. 2010;52(2):518–527. doi: 10.1002/hep.23691. [DOI] [PubMed] [Google Scholar]
- 27.Moorman AC, Xing J, Ko S, et al. Late diagnosis of hepatitis C virus infection in the Chronic Hepatitis Cohort Study (CHeCS): Missed opportunities for intervention. Hepatology. 2015;61(5):1479–1484. doi: 10.1002/hep.27365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Udompap P, Mannalithara A, Heo N-Y, Kim D, Kim WR. Increasing prevalence of cirrhosis among U.S. adults aware or unaware of their chronic hepatitis C virus infection. J Hepatol 2016;64(5):1027–1032. doi: 10.1016/j.jhep.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barocas JA, Wang J, White LF, et al. Hepatitis C Testing Increased Among Baby Boomers Following The 2012 Change To CDC Testing Recommendations. Health Aff (Millwood). 2017;36(12):2142–2150. doi: 10.1377/hlthaff.2017.0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li DK, Chung RT. Impact of hepatitis C virus eradication on hepatocellular carcinogenesis. Cancer. 2015;121(17):2874–2882. doi: 10.1002/cncr.29528. [DOI] [PMC free article] [PubMed] [Google Scholar]