Abstract
Purpose:
Synovial fluid molecular biomarkers help evaluate osteoarthritis (OA) development. Magnetic capture, our new magnetic nanoparticle-based technology, has proven to be an effective tool for determining an extracellular matrix fragment level in two rat OA models. Here, the feasibility of magnetic capture for detecting an OA-related chemokine, monocyte chemoattractant protein-1 (MCP-1 or CCL2), is demonstrated after intra-articular injection of monoiodoacetate (MIA) in the rat knee.
Methods:
Forty-eight male Lewis rats received a right hind limb, intra-articular injection of MIA (1 mg in 25 μl of saline) or 25 μl of saline. Magnetic capture and lavage were performed at 7 days after injection (n=6 per treatment per procedure), with magnetic capture additionally performed at 14 and 28 days post-injection (n=6 per treatment per time point). CCL2 was also assessed in serum samples from each rat.
Results:
Serum CCL2 levels revealed no difference between MIA and saline animals (p=0.0851). In contrast, magnetic capture and lavage detected a significant increase of CCL2 in the MIA-injected knee, with the MIA-injected knee having elevated CCL2 compared to contralateral knees and saline-injected knees (p = 0.00016 (contralateral) and p = 0.00016 (saline) for magnetic capture; p = 0.00023 (contralateral) and p = 0.00049 (saline) for lavage).
Conclusions:
Magnetic capture of CCL2 was successfully developed and applied to determine levels of CCL2 in a rat knee. Magnetic capture detected a statistically significant increase of CCL2 in MIA-injected knees compared to controls, and CCL2 levels stayed relatively stable from week 1 through week 4 post-MIA injection.
Keywords: biomarker, monoiodoacetate, MCP-1 or CCL2, synovial fluid, magnetic particles
Introduction
The translation of osteoarthritis (OA) therapies from the lab to the clinic has been challenging due, in part, to an inability to detect molecular evidence of early-stage OA. At an early stage, the complex disease cascade of OA may be able to be stopped or stalled1–3. As OA is regulated by local increases in catabolic and pro-inflammatory mediators4,5, early molecular evidence for OA is likely contained within the synovial fluid6–9. In addition, molecular evidence for OA may be more stable in synovial fluid than in serum or urine, where protein levels are not joint specific and known to fluctuate throughout the day10,11.
To develop markers of early-stage OA, there is a need to advance parallel measures of OA pathophysiology across species, helping to close the gap between OA animal models and OA patients. However, traditional techniques for the assessment of synovial fluid biomarkers in rodents can have significant technical and quality-related issues12–16. Recently, our group described a new magnetic nanoparticle-based technology, termed magnetic capture. In magnetic capture, superparamagnetic iron oxide nanoparticles are embedded within a polymer (see Figure 1, top left); these particles do not retain stable magnetization in the absence of a magnetic field, but acquire strong magnetization when the field is present. Antibodies specific to the biomarker of interest are conjugated to the polymer surface. When these particles are injected into a joint, they capture a biomarker of interest. After capture, particles are extracted with a small, high gradient permanent magnet inside a catheter. Then, particles are washed off the magnet, and non-covalent bonds between the antibody and biomarker are broken with heat, low pH, or enzymatic cleavage. Biomarker and collected particles are finally quantified and biomarker levels in the joint are determined. Using this approach, we previously detected a cartilage degradation product CTXII (c-terminus telopeptide of type II collagen) in the synovial fluid of two rat OA models17,18. However, magnetic capture has yet to be expanded to protein markers of OA-related inflammation.
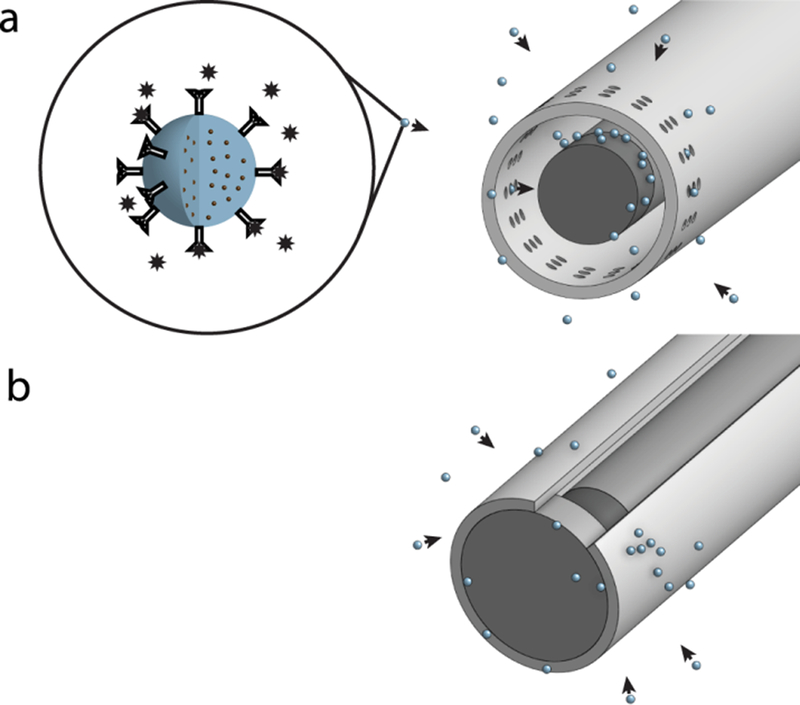
Figure 1. Magnetic capture.
a) Magnetic capture from rat knees.
Biotinylated antibody specific to CCL2 was conjugated to the surface of spherical particles, 1 μm in diameter (zoomed image on the left). The particles contained superparamagnetic nanoparticles embedded within a polymer core. These anti-CCL2 particles were used for all in vitro and in vivo experiments. For collection of anti-CCL2 particles from rat knees, a cylindrically shaped NdFeB magnet (1.0 mm diameter by 1.0 mm length) was attached to a soft magnetic rod and placed inside a 16G catheter, in which small (0.1–0.2 mm) holes were made near the magnet position. The holes in the catheter allowed particles to bind directly to the magnet and protected particles from being wiped from the magnet while the magnetic probe was removed from the knee.
b) In vitro magnetic particle collection.
For in vitro magnetic particle collection, a cylindrically shaped NdFeB magnet (0.75 mm diameter by 1 mm length) on a soft magnetic rod was used. The magnet was placed inside an 18G catheter with no holes. The catheter opening was closed with a rubber cork so particles could not penetrate directly to the magnet. Instead, particles accumulated on the outer surface of the catheter. This allowed particles to be rapidly removed from the catheter via vortexing after the magnet was removed from inside of the catheter.
For this study, magnetic capture technique was significantly modified to improve quality and extend its applicability to an early marker of OA pathogenesis – CCL2. CCL2 is a member of the C-C chemokine family and is a potent chemotactic factor regulating migration and infiltration of monocytes, memory T lymphocytes, and natural killer (NK) cells. Many cell types produce CCL2, although the major source of CCL2 is monocytes/macrophages. In OA, CCL2 secreted by synovial stromal cells can attract circulating monocytes to the synovium19. Monocytes recruited via CCL2 propagate inflammation and tissue damage in OA, while deficiency of either CCL2 or its receptor (CCR2) protects mice from OA and is associated with fewer synovial macrophages and inflammatory mediators20. Macrophages that infiltrate synovial tissues can further elevate levels of inflammatory cytokines and chemokines in the joint21–23. In addition, OA symptoms and radiographic OA severity correlate with the number of activated macrophages in the synovial tissue24, and synovial fluid levels of CCL2 in humans correlate positively with recent knee injury, OA-related pain, physical disability, and the x-ray based OA diagnosis25–27. Because activated macrophages are well known to facilitate tissue damage in the OA-developing joint28, early detection of CCL2 may be a key factor for understanding OA development.
In rodent models of OA, serum levels of CCL2 show no differences between OA and non-OA animals29; however, synovial fluid levels of CCL2 in rodents have yet to be directly assessed. Thus, the purpose of this study is to expand the use of magnetic capture for the detection of an inflammatory mediator (CCL2) in a rat model of OA knee, thereby extending our ability to assess early signs of OA in the rodent model.
Materials and Methods
Anti-CCL2 magnetic particles
Biotinylated antibody specific to CCL2 (Cat. # 505908, Biolegend, San Diego, CA) was conjugated to the surface of spherical superparamagnetic iron oxide/polymer particles, 1 μm in diameter, with a monolayer of recombinant streptavidin covalently coupled to the particle surface (Dynabeads MyOne™ Streptavidin C1, Cat. # 65001, Life Technologies, Carlsbad, CA). This conjugation was done as follows: Dynabeads were washed 3 times with phosphate buffered saline (PBS), incubated with 20 ng of antibody per 50 μg of particles for 2 h at room temperature, and then overnight at 4°C. Particles were then washed three times with PBS containing 2% BSA and 2 mM EDTA (hereafter referred to as capture buffer). These anti-CCL2 particles were used for all in vitro and in vivo experiments (Figure 1a).
Magnetic collection
For magnetic collection from rat knees, a cylindrical NdFeB (neodymium) magnet (1.0 mm diameter by 1 mm length, Grade N50, Cat. # D0110, Super Magnet Man, Birmingham, AL) was attached to a soft magnetic rod and placed inside a 16G X 1¼ catheter (Exel Safelet, model 26730, State Surgical Supply, Springdale, AR). The catheter protected particles from being wiped away as the magnetic probe was removed from the knee joint. In the catheter, 0.1–0.2 mm diameter holes were made near the magnet position to facilitate particle binding directly to the magnet during the 10 min collection time (Figure 1a). Following collection, particles were washed off the magnet with capture buffer and a rubber scraper.
For in vitro magnetic capture, a 0.75 mm diameter by 1 mm length magnet (Grade N50, Cat. # Cyl0010, Super Magnet Man, Birmingham, AL) was attached to a soft magnetic rod. This magnet was placed inside an 18G X 1¼ catheter (Exel Safelet, model 26735, State Surgical Supply, Springdale, AR) with no holes. Moreover, the catheter opening was closed with a rubber cork; this design prevented particles from directly attaching to the magnet, instead accumulating on the outer surface of the catheter (Figure 1b). In vitro collection was performed for 20 s. After magnetic collection, the magnet was removed from inside of the catheter and particles were washed off the empty catheter by vortexing the catheter in 200 μl of capture buffer.
CCL2 release from particles
After magnetic collection, particles were washed in capture buffer and then incubated for 15 min in the 100 mM Glycine-Tris buffer, pH 3.1, containing 2% BSA and 2 mM EDTA (release buffer). This temporary pH 3.1 treatment broke the non-covalent bond between the particle and CCL2, releasing CCL2 for quantification. Please note, prior magnetic capture approaches for extracellular matrix fragments have used heat; however, preliminary testing of heat effects on CCL2 were found to destroy the target molecule. Following release, magnetic particles were removed from the sample by magnetic separation, leaving collected CCL2 in the supernatant. Then, pH was adjusted to 8.3 for the best enzyme-linked immunosorbent assay (ELISA) detection sensitivity.
ELISA assay
CCL2 was quantified using a rat CCL2 ELISA kit (Cat. # KRC1012 Life Technologies, Carlsbad, CA). The kit was used according to the manufacturer’s instructions with the following modifications: 50 μl of samples, standards, or controls in 100 mM glycine-tris buffer (pH 8.3 containing 2% BSA and 2 mM EDTA) were added to the plate followed by the addition of 50 μl rat CCL2 biotin conjugate solution (detection antibody from the kit). The plate was incubated on a plate shaker at room temperature for 30 min, and then placed at 4°C overnight. On the next day, the plate was incubated on a plate shaker for 30 min at room temperature. The rest of the procedure was performed according to the manufacturer’s instructions: wash, incubation for 30 min with 50 μl of streptavidin-HRP (HRP, horseradish peroxidase) solution, another wash followed by incubation for 30 min with 100 μl of tetramethylbenzidine (TMB) substrate, addition of the 100 μl of stop solution (diluted sulfuric acid), and reading absorbance at 450 and 650 nm. These modifications to the ELISA procedure standardized the pH between the samples and standards, and also significantly increased the CCL2 detection sensitivity (decreased the lower limit of CCL2 detection 8-fold).
Particle count
Particles, magnetically separated after the pH treatment, were washed with capture buffer, re-suspended in 60 μl of capture buffer, and sonicated 3 times for 1 min intervals in an ultrasonic bath (Model M1800H, Branson Ultrasonic Corporation, Danbury, CT, USA) filled with ice water. Then, particle suspensions were read for absorbance at 450 nm using Synergy 2 Multi-Mode Microplate Reader. Suspensions of known particle concentrations were used as standards.
Determining the affinity of anti-CCL2 particles to CCL2
To experimentally determine the affinity of the anti-CCL2 particles to CCL2 (characterized by the equilibrium dissociation constant, KD), increasing amounts of anti-CCL2 particles were added to rat serum samples, each containing 3.2 pg of CCL2 (final volume 20 μl). Samples were incubated on a tube rotator for 2 h to achieve binding equilibrium, and then particles were separated from serum using a magnetic separator. After incubation, the particles were washed with 40 μl of capture buffer, and this wash was added to the 20 μl of serum initially separated from particles (final volume 60 μl). The wash step ensured CCL2 that may have bound to the particles non-specifically would end up in the “not bound” fraction. Then, particles were washed one more time in 60 μl of capture buffer, and were placed in a temporary pH 3.1 treatment (release buffer) to release CCL2. The “released” and “not bound” CCL2 samples were assessed with ELISA. The particles were counted using absorbance, as described above.
To account for a potential loss of particles during washes, the amount of bound CCL2 was adjusted as follows:
| (Eq. 1) |
where MELISA is the amount of CCL2 determined by ELISA. Note, total CCL2 concentration in this experiment was 160 pg/ml, which is much lower than the total anti-CCL2 antibody concentration for any data point; thus, CCL2 did not saturate anti-CCL2 particles. Moreover, for each data point collected, the concentration of antibody not bound to CCL2 was practically equal to the total antibody concentration. Thus, data can be fit to a simple equation to determine KD:
| (Eq. 2) |
where [Ab] is the concentration of total added antibody.
Determining total CCL2 in the joint
Total amount of CCL2 in a knee (or within a sample tube) is calculated by multiplying the amount of CCL2 per particle by the number of injected particles. This calculation is valid at the condition that all CCL2 present in the synovial fluid (or tube) is bound to particles at the time of magnetic collection17. To satisfy this condition, the particles should carry a molar amount of antibody (Abinjected) exceeding a molar amount of the CCL2 in the sample. Moreover, the antibody concentration in synovial fluid during the particle incubation step should significantly exceed the equilibrium dissociation constant KD:
| (Eq. 3) |
where V is a volume of synovial fluid in the joint. At binding equilibrium, a ratio of concentrations of the CCL2 captured by particles [Ab-CCL2] to the not captured, free CCL2 [CCL2] is equal to the ratio of the concentration of free antibody [Ab] to KD30:
| (Eq. 4) |
Thus, the larger [Ab] is compared to KD, the less unbound target is left over once the antibody and target reach a dynamic binding equilibrium. Therefore Abinjected should be sufficiently large to satisfy Equation 3 (Abinjected / V = [Ab-CCL2] + [Ab]).
Abinjected was estimated from Equation 3 as follows. Using a calculation method for V via urea standardization31 and the results reported in our prior work18, V was estimated for OA and non-OA rat knees (note, V rarely exceeds 80 μl in a rat OA knee). With the determination of the dissociation constant for our particles (experiment described above and results presented later in Figure 2, KD ≈ 29 nM) and with the restriction that less than 3% of CCL2 should remain unbound after the collection phase of magnetic capture, the ratio of [Ab]/KD was estimated to be approximately 30. Using this ratio, one obtains from Equation 3:
| (Eq. 5) |
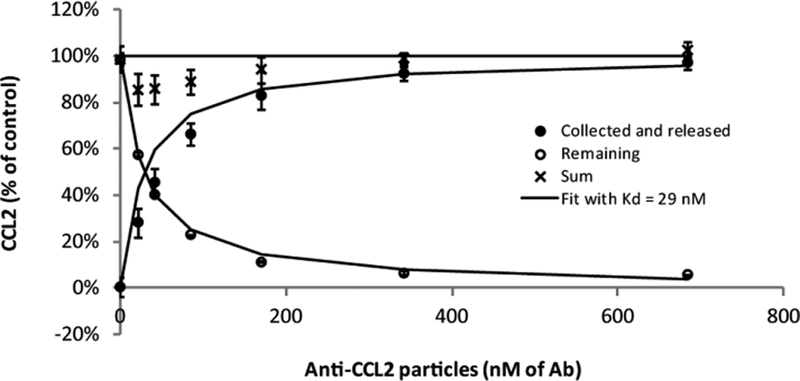
Figure 2. Affinity of anti-CCL2 particles to CCL2.
In samples with 12 pM of CCL2 (160 pg/ml), 10 μl of anti-CCL2 particles of increasing concentration were mixed with 10 μl of normal rat serum and incubated for 2 h on a tube rotator. After incubation, particles were separated from the supernatant, and CCL2 was released from particles with a temporary pH 3.1 treatment (release buffer). CCL2 was assessed with ELISA, and the particles were counted using light absorbance. The amount of released CCL2 was adjusted by the particle count (see Methods). Higher amounts of anti-CCL2 particles, and thus higher concentrations of anti-CCL2 antibody, bound more CCL2 during the 2 h incubation period (closed circles), resulting in less free CCL2 remaining in the serum (open circles). Non-linear fit to the remaining CCL2 (descending curve) using Equation 5, provides KD = 29 nM. Incubation of the particles at pH 3.1 resulted in the breakdown of the non-covalent bond between CCL2 and the antibody and recovery of the CCL2 from the anti-CCL2 particles (black circles, ascending line is a theoretical curve with KD = 29 nM). Moreover, sum of the percentages of CCL2 collected on the magnetic particles and the CCL2 remaining in the fluid (crosses) was close to 100%, indicating limited losses. Error bars correspond to standard deviation of 3 samples at each particle concentration.
An alternate estimate of Abinjected could be based on the requirement that particles carry a molar amount of antibody equal to or exceeding a molar amount of the CCL2 in synovial fluid; this estimate provides a much smaller value of Abinjected (approximately 220 pg, see Figure 4 in Results). This shows CCL2SF did not exceed 20 pg, or approximately 1.5 fmol, in any knee. As such, 10 μg of antibody on the anti-CCL2 particles was determined as a more than sufficient amount of Abinjected for rat knee injections, thereby satisfying the conditions for magnetic capture quantification (see17 for additional details).
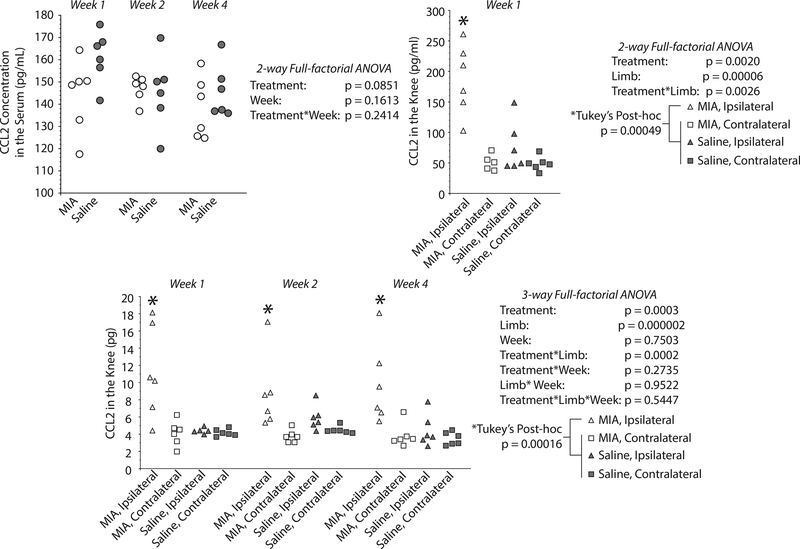
Figure 4. MIA-injected knees show elevated CCL-2 levels compared to controls and contralateral knees.
CCL2 levels in rat knee and in serum at 1, 2, and 4 weeks after MIA injection, as assessed by lavage, magnetic capture, or direct ELISA (serum). No statistically significant differences were identified between the CCL2 serum levels of MIA and saline injected animals (2-way ANOVA, left top panel). On the contrast, magnetic capture detected an increase of CCL2 in the MIA-injected knee relative to saline animals and relative to the contralateral knees within the same time point; time point was not a significant factor (3-way ANOVA, bottom panel). Lavage results confirm elevated CCL2 concentration in MIA-injected knee at the one-week time point (right top panel).
In vitro validation of magnetic capture
An experiment was designed to confirm that magnetic capture can accurately determine the total amount of CCL2 in a sample (flow chart of the design is shown in the top of Figure 3 in the results). Sera from three naive rats (# 1, 2, and 3) were used as a source of CCL2; serum from rat # 2 was diluted two times with capture buffer prior to the experiment to provide a varying level of CCL2 concentrations. Either anti-CCL2 particles or unconjugated particles were added to a tube containing 10 μl of capture buffer or serum (n = 3 for each group), and allowed to bind for at least 2 hours. Then, magnetic collection and CCL2 release was performed as described above. CCL2 was assessed via ELISA, and particles were counted using absorbance, as described above.
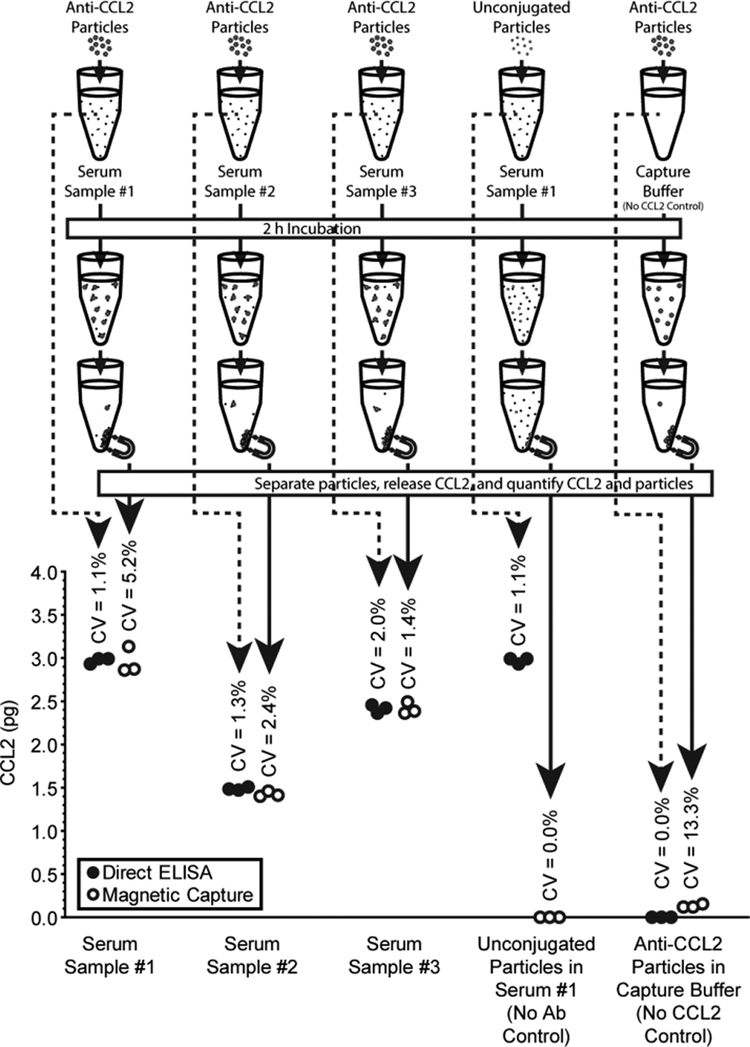
Figure 3. Magnetic capture test in vitro.
Either anti-CCL2 particles or the same amount of unconjugated particles were added to a tube containing 10 μl of rat serum (n = 3 for each group) or capture buffer. Binding was allowed to equilibrate for at least 2 hours. Then, magnetic collection was performed, particles washed in capture buffer, and CCL2 was released using release buffer (as described in Methods). CCL2 was assessed before and after via ELISA (dotted line and solid line, respectively). For magnetic capture, particles were counted using light absorbance. CCL2 levels estimated via magnetic capture were similar to the levels assessed via direct ELISA. Initial CCL2 concentrations were 298±3 pg/ml (Serum 1), 149±2 pg/ml (two times diluted Serum 2), and 242±5 pg/ml (Serum 3).
Animal experiment design
All studies described herein were conducted under IACUC-approved protocols at the University of Florida. Forty-eight male Lewis rats (3 months, ~250 g) were obtained from Charles River Laboratories (Wilmington, MA) and acclimated for 4–8 weeks. Acclimation periods varied to allow for the experiment to take place in eight cohorts of six animals (two cohorts per time point, with MIA and saline injection equally represented in each cohort).
An experimental design flow chart is included as Supplemental Figure 1. For intra-articular injection, anesthesia was induced in rats using a 4% isoflurane sleep box, then maintained with 1–2% isoflurane via mask inhalation. Right knees were aseptically prepared with povidone iodine and 70% alcohol in triplicate, ending with a final application of povidone iodine. Twenty-four rats received an intra-articular injection of MIA (1 mg in 25 μl of saline, MIA – Cat. # AC170970250, ThermoFisher Scientific, Waltham, MA). An additional 24 animals received intra-articular injection of saline (25 μl). Injections were conducted by directing a 29½ gage, U-100 insulin syringe behind the patellar ligament, along the femoral groove, into the joint space. After injection, animals received buprenorphine twice per day for 2 days.
After injection, OA was allowed to develop for 7, 14, or 28 days. At 7 days, either magnetic capture or lavage was performed (n=6 per method per treatment); at 14 and 28 days after injection, magnetic capture was performed (see Supplemental Figure S1). Prior to each magnetic capture or lavage procedure, animals were euthanized via cardiac puncture and exsanguination under deep anesthesia. Blood from each animal was processed to serum samples using blood-separating vacutainers (Part # 367981 BD, Franklin Lakes, NJ), according to the manufacturer’s instructions. Serum was assessed for CCL2 via direct ELISA, as described above.
Lavage
For lavage, a 29G insulin syringe was directed behind the patellar ligament and along the femoral groove. Sterile saline (100 ul) was then injected into the knee. After injection and needle removal, the knee was fully flexed 10 times. A clean 29 gauge insulin syringe was then placed along a similar path, and back pressure was used to collect as much fluid as possible without opening the joint. Lavage and serum samples were then analyzed for CCL2 and urea, and CCL2 concentration in synovial fluid was calculated as follows32:
| (6) |
CCL2 was quantified via ELISA, and urea was quantified using Urea Nitrogen (BUN) Colorimetric Detection Kit (Cat. # EIABUN, ThermoFisher Scientific, Waltham, MA) according to the manufacturer’s instructions.
Histology
After magnetic capture, knees were dissected, fixed in 10% neutral buffered formalin for 48 h, decalcified in Cal-Ex for 3 weeks, then embedded in paraffin via vacuum infiltration. Knees were sectioned frontally at 10 μm, with at least one section acquired every 100 μm between the anterior and posterior horn of the medial meniscus. Slides from the central aspect of the joint were stained with toluidine blue and then graded according to OARSI recommendations33 and our prior work grading synovial changes34.
Immunohistochemistry
Immunostaining of the rat knee sections was carried out with the N-Histofine Simple Stain Rat MAX PO (R) stain (Cat. # 414181F, AS ONE International, Inc., Santa Clara, CA) according to the manufacturer’s instructions. Rabbit polyclonal anti-CCL2 antibody (Cat. # PA5–34505, ThermoFisher Scientific, Waltham, MA) was used as the primary antibody. PBS with no primary antibody was used as a negative control.
Statistical analysis
For statistical analysis of CCL2 in the knee, differences between treatment group, limb, and time point were assessed using a 3-way ANOVA (Statistica, Tulsa, OK). Serum CCL2 levels were assessed using a 2-way ANOVA (treatment, time). Lavage samples were assessed using a 2-way ANOVA (treatment, limb). OARSI scores were analysed using a 2-way ANOVA (treatment, time). Post-hoc Tukey’s HSD tests were conducted when indicated by the ANOVA.
Results
Affinity of anti-CCL2 particles to CCL2
As discussed in the methods, total CCL2 in a knee (or a sample tube) following magnetic capture is calculated by multiplying the amount of CCL2 per collected particle by the number of injected particles. This calculation is valid for the condition that nearly all CCL2 present in the joint synovial fluid (or in the tube) is bound to particles at the time of magnetic collection17. Again, to satisfy this condition, the particles should carry a molar amount of antibody (Abinjected) exceeding a molar amount of the CCL2 in the sample, and the antibody concentration in synovial fluid during the particle incubation step should significantly exceed an equilibrium dissociation constant KD characterizing the affinity of antibody to its target (see methods for details). To verify this condition, the constant KD needs to be determined for each particular target-antibody pair.
Figure 2 shows determination of the affinity of our anti-CCL2 particles to CCL2, post-conjugation. Increasing amounts of the anti-CCL2 particles were added to rat serum samples, each containing 3.2 pg of CCL2. Note, total CCL2 concentration in this experiment was 160 pg/ml = 12 pM, which is much lower than the total anti-CCL2 antibody concentration for any data point on Figure 2. Therefore, CCL2 did not saturate anti-CCL2 particles. Moreover, for each data point, the concentration of antibody not bound to CCL2 was practically equal to the total antibody concentration. Thus, the data could be fit to Equation 2. Non-linear fit to the remaining CCL2 (descending line, open circles) provided KD ≈ 29 nM. These data also demonstrate CCL2 can be released for quantification via pH treatment with acceptable losses (Figure 2, sum).
Based on this result, 10 μg of antibody on the anti-CCL2 particles was determined as an amount sufficient for the rat knee injection, thereby satisfying the conditions for magnetic capture quantification.
In vitro validation of magnetic capture
CCL2 amounts assessed via magnetic capture compared favourably to the CCL2 amounts assessed via direct ELISA (Figure 3). As expected, the variability of magnetic capture is slightly larger than that for a direct ELISA (Figure 3), as magnetic capture includes more processing than direct ELISA. The test experiment includes two negative controls: 1) magnetic capture performed with unconjugated particles (no antibody control); 2) magnetic capture performed in capture buffer containing no CCL2 (no CCL2 control). As expected, unconjugated particles did not collect any CCL2, and conjugated particles in capture buffer produced only a small background signal (Figure 3).
Magnetic Capture in the Rat Knee
While CCL2 levels in serum revealed no statistically significant difference between MIA and saline animals (p = 0.0851), magnetic capture and lavage detected a significant increase of CCL2 in the MIA-injected knee at 1 week post-injection (Figure 4). Magnetic capture also detected elevated levels of CCL2 in the OA-affected knee at 2 weeks and 4 weeks. The MIA-injected knee had elevated CCL2 compared to the contralateral knees and saline-injected knees for magnetic capture (p = 0.00016 for contralateral, and p = 0.00016 for saline) and lavage (p = 0.00023 for contralateral, and p = 0.00049 for saline). Note that while both lavage and magnetic capture were able to detect elevated levels of CCL2, magnetic capture detects picograms of CCL2 in the knee, while lavage detects concentration.
Histology
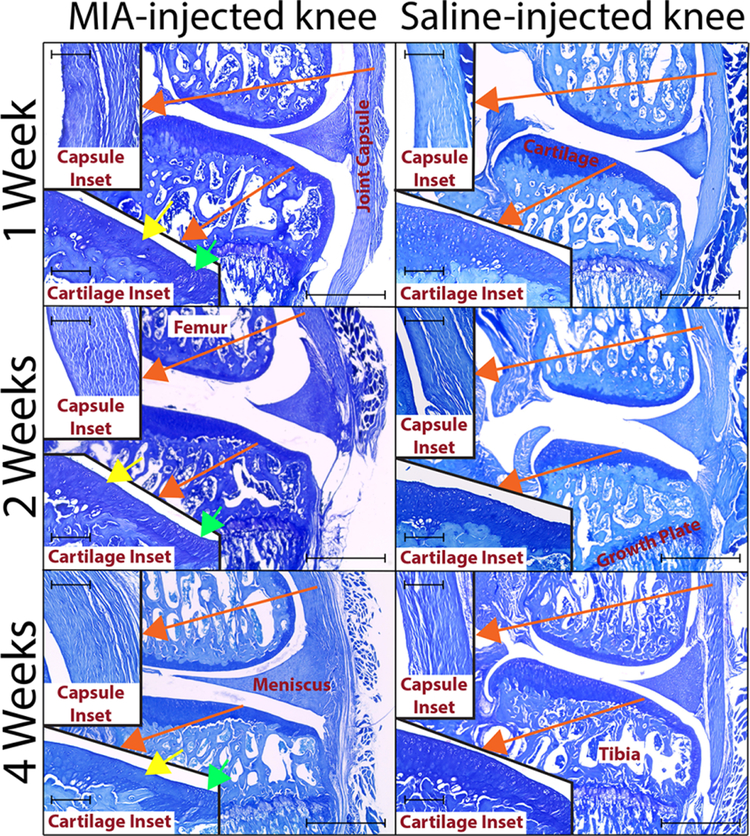
Cartilage matrix loss was not observed in any animal; however, mild cartilage degeneration was observed in MIA injected animals, including areas of chondrocyte death (yellow arrows, Figure 5) and chondrocyte clustering (green arrows, Figure 5). Histological scoring confirmed significant differences in the cartilage health of MIA and saline-injected knees (Supplemental Table 1).
Figure 5. Histology.
Representative images are shown for saline and MIA animals at each week (toluidine blue). Medial compartment of each section (10 μm sections) was imaged at 4-x magnification on an EVOS XL Core (scale bars indicates 1 mm). Inserts indicated by red arrows show images of the corresponding areas taken at a 20-x magnification (scale bars indicate 0.2 mm). At all weeks, cartilage shows areas of chondrocyte death (yellow arrows) and clustering (green arrows) for MIA animals (20× cartilage inset). Synovium also shows differences between MIA and saline animals: synovium is significantly thicker in MIA animals, and its structure is altered compared to the saline animal (20-x synovial inset). Moreover, synovial subintimal fibroblasts in MIA animals are spherical and lack directionality, unlike cells in saline animals that appear as spindles with strong population directionality.
In addition, the synovium was significantly thicker in MIA animals, and its structure was altered compared to the saline animal (20× capsule inset). Synovial subintimal fibroblasts in MIA animals were also spherical and lack directionality, while cells in saline animals appear as spindles with strong population directionality. Again, histological scoring confirmed significant differences in the synovial lining of MIA and saline-injected knees (Supplemental Table 1).
Immunohistochemistry
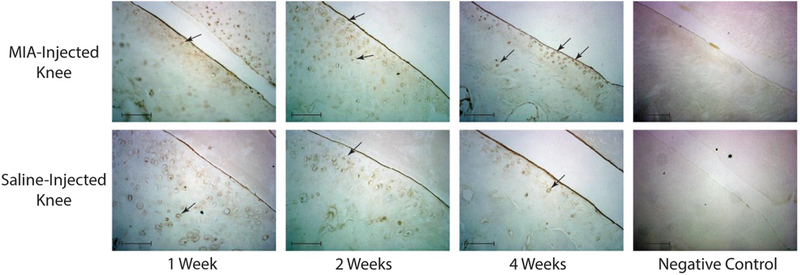
Immunohistochemistry found increased number of CCL2 positive chondrocytes in the MIA injected knees (Figure 6), consistent with elevated levels of CCL2 found by magnetic capture and lavage results.
Figure 6. Immunohistochemistry.
Immunohistochemistry was used to stain the medial part of articular cartilage in the saline and MIA animals (representative images). Immunostaining was carried out with the N-Histofine Simple Stain Rat MAX PO (R) stain and rabbit polyclonal anti-CCL2 antibody as described in methods. Images were obtained with 20-x objective of the Wirth Zeiss axioskop microscope (Camera: 3.3 MPX; Software: ImagingPlanet, scale bars indicate 0.1 mm). Black arrows indicate CCL2-expressing chondrocytes. CCL2 staining was increased in the MIA injected knees, consistent with elevated levels of CCL2 found by magnetic capture and lavage.
Discussion
This work demonstrates the ability to detect CCL2 in rat knees using magnetic capture. Importantly, elevated CCL2 was detected despite limited cartilage matrix loss. However, regions of cell death, chondrocyte cloning, and synovial thickening were identified, suggesting CCL2 may be indicative of either early matrix or synovial changes in the OA knee. Moreover, changes in CCL2, as detected by magnetic capture, were confirmed by an alternate technique (lavage) at the 1 week time point. It is also worth noting that our prior work with magnetic capture focused on large extracellular matrix fragments, which can be released from magnetic particles using heat. These data demonstrate magnetic capture can also assess the levels of a small chemokine in a rat OA model using a pH release.
Compared to the original magnetic capture procedure17, this study introduced several important modifications. The modifications included: 1) use of the streptavidin-coated particles and biotinylated antibody instead of conjugating the antibody via COOH groups; 2) quantifying particles via light absorbance instead of fluorescence; and importantly, 3) the use of a pH treatment for the protein target release instead of heat. These modifications improved efficiency of the procedure. Use of the biotin-streptavidin pair simplified conjugation and increased the antibody binding capacity of the particles. Counting particles via light absorbance allowed for the use of the non-fluorescent particles, creating additional options for the particle choice. Finally, the use of pH treatment instead of heat represents a more general approach for the protein target release, which magnetic capture can use with other targets.
In the magnetic capture procedure from this study, all target biomarkers within a joint are driven into a bound state on the particles, then released via pH for quantification. An alternative quantification approach would be the development of on-particle ELISAs, which could be quantified via flow cytometry and further reduce processing. In addition, another variation of magnetic capture (applicable to large joints) involves collection of only small percentage of biomarker from the joint, then relying on the law of mass action to approximate concentration. In this case, magnetic capture can detect biomarker concentration in synovial fluid rather than its total amount, without the need to aspirate synovial fluid. While concentration could also be obtained with lavage (shown in this study), magnetic capture has a final potential not available with lavage – ability to assess biomarker production rates. When applied in vivo, small samples could be acquired longitudinally, allowing change in biomarker attached to the particle surface to be assessed. This advance would allow for the in vivo assessment of biomarker production rates. To be clear, this application of magnetic capture would also require assessment of particle clearance and the immune response to the particle alone, which is the subject of on-going work in our lab.
In addition to these scientific advances, magnetic capture could improve the diagnosis or understanding of OA pathogenesis in human joints in the future. Specifically, magnetic capture has advantages in smaller joints affected by OA, including the metacarpophalangeal and interphalangeal joints of the hand and the facet joints of the spine, amongst others. Magnetic capture may also reduce the incidence of dry taps (inability to collect synovial fluid), as smaller needles and probes may be used than those needed to remove viscous synovial fluid. Finally, magnetic capture can facilitate the collection of joint-level biomarkers in patients independent of joint swelling, providing a measure of OA pathogenesis that is independent from joint effusion. However, to be clear, this translational potential for magnetic capture requires significant work beyond this proof-of-concept study.
In addition to validation relative to lavage at week 1, our in vitro assays demonstrate CCL2 amounts assessed via magnetic capture are comparable to CCL2 amounts assessed via direct ELISA (Figure 3). While the variability of magnetic capture is slightly larger than that for a direct ELISA (1.1–2.0% CV for direct ELISA vs. 1.4–5.2% CV for magnetic capture), this is an expected outcome – an ELISA occurs in both procedures and magnetic capture requires additional processing. However, a direct ELISA is not viable for rodent knees, as synovial fluid cannot be directly aspirated. Instead, synovial fluid is typically recovered with a lavage procedure. Magnetic capture provides an alternative whereby a protein target could be magnetically isolated from the synovial fluid, thereby concentrating (rather than diluting) the target. In addition, magnetic capture quantifies total protein amount, rather than concentration. Hence, magnetic capture may be beneficial for proteins that are markedly affected by joint effusion.
To be clear, elevated levels of CCL2 in the synovial fluid of MIA rats at 1 week were observed with both magnetic capture and lavage in this study, indicating CCL2 may be detected with either method (at least within 1 week). This elevation of CCL2 is consistent with results from the synovial fluid of patients with OA or knee injury26,27. While others have detected elevation of CCL2 protein histologically or via digestion of tissue explants25, to our knowledge, this is the first study to detect elevated CCL2 levels in the synovial fluid of the rat knee. Also consistent with prior studies in mice20, serum levels of CCL2 following MIA injection revealed no statistically significant difference compared to saline controls.
Recent studies indicate CCL2/CCR2 signalling can contribute directly to both inflammation and pain development35–38. CCL2 plays a major role in attracting monocytes to the synovium, and monocytes recruited via CCL2/CCR2 propagate inflammation and tissue damage in OA. Peripheral neuron endings in the synovium are likely a source of the nociceptive stimuli in OA38–40. Infiltrating macrophages secrete pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6, facilitating the development of inflammation and triggering sensitization of the peripheral nerve endings in the synovium. Moreover, CCL2 likely participates directly in the nociceptive sensitization through its own mechanism35–38. While the 1 mg of MIA used in this study only led to a mild amount joint damage, gait studies in our group using this same 1 mg MIA dose have shown shuffle-step compensations that are similar to gait compensations observed in surgical OA models41. Hence, while the damage was limited in this model, the detection of CCL2 may still relate to OA-related pain and disability in the rat.
CCL2 or CCR2 deficiency in mice also reduces OA-related inflammation and pain development after the destabilization of the medial meniscus (DMM) injury42,43. In both CCL2 and CCR2 deficient mice, post DMM pain-related behaviour was delayed, and a lower average chondropathy score was observed, compared with wild type mice42. Furthermore, 8 weeks after DMM surgery, mRNA levels for both CCL2 and CCR2 in the dorsal root ganglia (DRG) were greatly increased in wild type mice, and cultured neurons from DMM wild type mice produced increased levels of CCL2 protein compared to naïve and sham controls43. This indicates the involvement of the CCL2/CCR2 in OA pain development is not limited to processes occurring within an OA joint. Our data indicate these processes may start with early upregulation of CCL2 in the joint space, potentially driving some pathophysiologic characteristics of chronic OA.
There remain significant limitations to the study. First, while a theoretical advantage of magnetic capture over lavage is the concentration of the biomarker on the particle surface, CCL2 can be detected via lavage at least 1 week post-MIA injection. While these data validate magnetic capture as accurate, this study does not demonstrate an advantage for magnetic capture over lavage for CCL2 detection. Second, only male rats were used in this study. While our data demonstrate CCL2 detection is possible with magnetic capture, the role of CCL2 in OA pathogenesis is not fully defined as female rats were not explored. Along with this point, it is notable that cartilage matrix loss was not observed at 4 weeks after intra-articular injection of 1 mg of MIA; thus, it is not known if joint damage would progress to full-thickness cartilage damage, despite the detection of CCL2 after this injection. Finally, magnetic capture at this stage has only be validated for use in the rat. Adapting this protocol to other species would require new characterization of particle-target binding kinetics and additional optimization of release protocols.
In summary, magnetic capture of CCL2 was successfully developed, tested in vitro, and applied to determine levels of CCL2 in a rat knee. Magnetic capture detected a statistically significant increase of CCL2 in MIA-injected knees compared to controls, and CCL2 levels stayed relatively stable from week 1 through week 4 post-MIA injection.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health under grants R01AR068424 and R21AR064402; and by the J. Crayton Pruitt Family Department of Biomedical Engineering at the University of Florida. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure of interest:
The authors report no conflict of interest.
References
- 1.Le Graverand-Gastineau MP (2010). “Disease modifying osteoarthritis drugs: facing development challenges and choosing molecular targets.” Curr Drug Targets 11(5): 528–535. [DOI] [PubMed] [Google Scholar]
- 2.Hunter DJ (2011). “Pharmacologic therapy for osteoarthritis--the era of disease modification.” Nat Rev Rheumatol 7(1): 13–22. [DOI] [PubMed] [Google Scholar]
- 3.Kraus VB (2005). “Biomarkers in osteoarthritis.” Curr Opin Rheumatol 17(5): 641–646. [DOI] [PubMed] [Google Scholar]
- 4.Lotz MK and Kraus VB (2010). “New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options.” Arthritis Res Ther 12(3): 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldring MB and Otero M (2011). “Inflammation in osteoarthritis.” Curr Opin Rheumatol 23(5): 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ratcliffe A, Flatow EL, Roth N, Saed-Nejad F and Bigliani LU (1996). “Biochemical markers in synovial fluid identify early osteoarthritis of the glenohumeral joint.” Clin Orthop Relat Res(330): 45–53. [DOI] [PubMed] [Google Scholar]
- 7.Naito K, Takahashi M, Kushida K, Suzuki M, Ohishi T, Miura M, Inoue T and Nagano A (1999). “Measurement of matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases-1 (TIMP-1) in patients with knee osteoarthritis: comparison with generalized osteoarthritis.” Rheumatology (Oxford) 38(6): 510–515. [DOI] [PubMed] [Google Scholar]
- 8.Catterall JB, Stabler TV, Flannery CR and Kraus VB (2010). “Changes in serum and synovial fluid biomarkers after acute injury (NCT00332254).” Arthritis Res Ther 12(6): R229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Arman MM, El-Fayoumi G, El-Shal E, El-Boghdady I and El-Ghaweet A (2010). “Aggrecan and cartilage oligomeric matrix protein in serum and synovial fluid of patients with knee osteoarthritis.” HSS J 6(2): 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong SY, Stabler TV, Criscione LG, Elliott AL, Jordan JM and Kraus VB (2006). “Diurnal variation of serum and urine biomarkers in patients with radiographic knee osteoarthritis.” Arthritis Rheum 54(8): 2496–2504. [DOI] [PubMed] [Google Scholar]
- 11.Gordon CD, Stabler TV and Kraus VB (2008). “Variation in osteoarthritis biomarkers from activity not food consumption.” Clin Chim Acta 398(1–2): 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barton NJ, Stevens DA, Hughes JP, Rossi AG, Chessell IP, Reeve AJ, McQueen DS. Demonstration of a novel technique to quantitatively assess inflammatory mediators and cells in rat knee joints. J Inflamm (Lond). 2007. June 13;4:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel L, Sun W, Glasson SS, Morris EA, Flannery CR, Chockalingam PS. Tenascin-C induces inflammatory mediators and matrix degradation in osteoarthritic cartilage. BMC Musculoskelet Disord. 2011. July 15;12:164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swearingen CA, Chambers MG, Lin C, Marimuthu J, Rito CJ, Carter QL, Dotzlaf J, Liu C, Chandrasekhar S, Duffin KL, Mitchell PG, Durham TB, Wiley MR, Thirunavukkarasu K. A short-term pharmacodynamic model for monitoring aggrecanase activity: injection of monosodium iodoacetate (MIA) in rats and assessment of aggrecan neoepitope release in synovial fluid using novel ELISAs. Osteoarthritis Cartilage. 2010. September;18(9):1159–1166. [DOI] [PubMed] [Google Scholar]
- 15.Allen KD, Mata BA, Gabr MA, Huebner JL, Adams SB Jr, Kraus VB, Schmitt DO, Setton LA. Kinematic and dynamic gait compensations resulting from knee instability in a rat model of osteoarthritis. Arthritis Res Ther. 2012. Apr;14(2):R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seifer DR, Furman BD, Guilak F, Olson SA, Brooks SC 3rd, Kraus VB. Novel synovial fluid recovery method allows for quantification of a marker of arthritis in mice. Osteoarthritis Cartilage. 2008. December;16(12):1532–1538. Erratum in: Osteoarthritis Cartilage. 2012 Jan;20(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yarmola EG, Shah Y, Arnold DP, Dobson J, Allen KD. Magnetic Capture of a Molecular Biomarker from Synovial Fluid in a Rat Model of Knee Osteoarthritis. Ann Biomed Eng. 2016. April;44(4):1159–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yarmola EG, Shah YY, Kloefkorn HE, Dobson J, Allen KD. Comparing intra-articular CTXII levels assessed via magnetic capture or lavage in a rat knee osteoarthritis model. Osteoarthritis Cartilage. 2017. July;25(7):1189–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashida K, Nanki T, Girschick H, Yavuz S, Ochi T, Lipsky PE. Synovial stromal cells from rheumatoid arthritis patients attract monocytes by producing MCP-1 and IL-8. Arthritis Res. 2001;3(2):118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raghu H, Lepus CM, Wang Q, Wong HH, Lingampalli N, Oliviero F, Punzi L, Giori NJ, Goodman SB, Chu CR, Sokolove JB, Robinson WH. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann Rheum Dis. 2017. May;76(5):914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011. Jan;7(1):33–42. [DOI] [PubMed] [Google Scholar]
- 22.Rahmati M, Mobasheri A, Mozafari M. Inflammatory mediators in osteoarthritis: A critical review of the state-of-the-art, current prospects, and future challenges. Bone. 2016. April;85:81–90. [DOI] [PubMed] [Google Scholar]
- 23.Berenbaum F Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013. January;21(1):16–21. [DOI] [PubMed] [Google Scholar]
- 24.Kraus VB, McDaniel G, Huebner JL, Stabler TV, Pieper CF, Shipes SW, Petry NA, Low PS, Shen J, McNearney TA, Mitchell P. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthritis Cartilage. 2016. September;24(9):1613–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris Q, Seto J, O’Brien K, Lee PS, Kondo C, Heard BJ, Hart DA, Krawetz RJ. Monocyte chemotactic protein-1 inhibits chondrogenesis of synovial mesenchymal progenitor cells: an in vitro study. Stem Cells. 2013. October;31(10):2253–2265. [DOI] [PubMed] [Google Scholar]
- 26.Watt FE, Paterson E, Freidin A, Kenny M, Judge A, Saklatvala J, Williams A, Vincent TL. Acute Molecular Changes in Synovial Fluid Following Human Knee Injury: Association With Early Clinical Outcomes. Arthritis Rheumatol. 2016. Sep;68(9):2129–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, Jiang BE. Serum and synovial fluid chemokine ligand 2/monocyte chemoattractant protein 1 concentrations correlates with symptomatic severity in patients with knee osteoarthritis. Ann Clin Biochem. 2015. March;52(Pt 2):276–282. [DOI] [PubMed] [Google Scholar]
- 28.Rahmati M, Nalesso G, Mobasheri A, Mozafari M. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Res Rev. 2017. November;40:20–30. [DOI] [PubMed] [Google Scholar]
- 29.Bowles RD, Mata BA, Bell RD, Mwangi TK, Huebner JL, Kraus VB, Setton LA. In vivo luminescence imaging of NF-κB activity and serum cytokine levels predict pain sensitivities in a rodent model of osteoarthritis. Arthritis Rheumatol. 2014. March;66(3):637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehninger AL, Nelson DL, Cox MM. Lehninger Principles of Biochemistry, 3rd ed. New York (NY): Worth Publishers; 2003. p 96. [Google Scholar]
- 31.Kraus VB, Stabler TV, Kong SY, Varju G, McDaniel G. Measurement of synovial fluid volume using urea. Osteoarthritis Cartilage. 2007. October;15(10):1217–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kraus VB, Huebner JL, Fink C, King JB, Brown S, Vail TP, Guilak F. Urea as a passive transport marker for arthritis biomarker studies. Arthritis Rheum. 2002. February;46(2):420–7. [DOI] [PubMed] [Google Scholar]
- 33.Gerwin N, Bendele AM, Glasson S, Carlson CS. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage. 2010. October;18 Suppl 3:S24–34. [DOI] [PubMed] [Google Scholar]
- 34.Kloefkorn HE, Allen KD. Quantitative histological grading methods to assess subchondral bone and synovium changes subsequent to medial meniscus transection in the rat. Connect Tissue Res. 2017. May-Jul;58(3–4):373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White FA, Bhangoo SK, Miller RJ. Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov. 2005. October;4(10):834–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White FA, Feldman P, Miller RJ. Chemokine signaling and the management of neuropathic pain. Mol Interv. 2009. August;9(4):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009;(194):417–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009. June;29(6):313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005. March 12–18;365(9463):965–973. [DOI] [PubMed] [Google Scholar]
- 40.Kidd BL, Photiou A, Inglis JJ. The role of inflammatory mediators on nociception and pain in arthritis. Novartis Found Symp. 2004;260:122–133; discussion 133–138, 277–279. [PubMed] [Google Scholar]
- 41.Lakes EH, Allen KD. Quadrupedal rodent gait compensations in a low dose monoiodoacetate model of osteoarthritis. Gait Posture. 2018. Jun; 63: 73–79. [DOI] [PubMed] [Google Scholar]
- 42.Miotla Zarebska J, Chanalaris A, Driscoll C, Burleigh A, Miller RE, Malfait AM, Stott B, Vincent TL. CCL2 and CCR2 regulate pain-related behaviour and early gene expression in post-traumatic murine osteoarthritis but contribute little to chondropathy. Osteoarthritis Cartilage. 2017. March;25(3):406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller RE, Tran PB, Das R, Ghoreishi-Haack N, Ren D, Miller RJ, Malfait AM. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc Natl Acad Sci U S A. 2012. December 11;109(50):20602–20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.