Abstract
Thirty years after initial publications of the concept of a chimeric antigen receptor (CAR), the U.S. Food and Drug Administration (FDA) approved the first anti-CD19 CAR T cell therapy. Unlike other immunotherapies such as immune checkpoint inhibitors and bispecific antibodies, CAR T cells are unique as they are “living drugs”, i.e. gene-edited killer cells that can recognize and kill cancer. During these 30 years of development, the CAR construct, the T cell manufacturing process, and the clinical patient management went through rounds of failures and successes that drove continuous improvement. Tisagenlecleucel was the first gene therapy to receive approval from the FDA for any indication. The initial approval was for relapsed or refractory (r/r) pediatric and young-adult B-cell acute lymphoblastic leukemia in August 2017 and in May 2018 for adult r/r diffuse large B cell lymphoma. Here we review the pre-clinical and clinical development of what began as CART19 at the University of Pennsylvania and later developed into tisagenlecleucel.
Keywords: Chimeric Antigen Receptor T cells, CART, CART19, CTL019, tisagenlecleucel, axicabtagene ciloleucel
Introduction
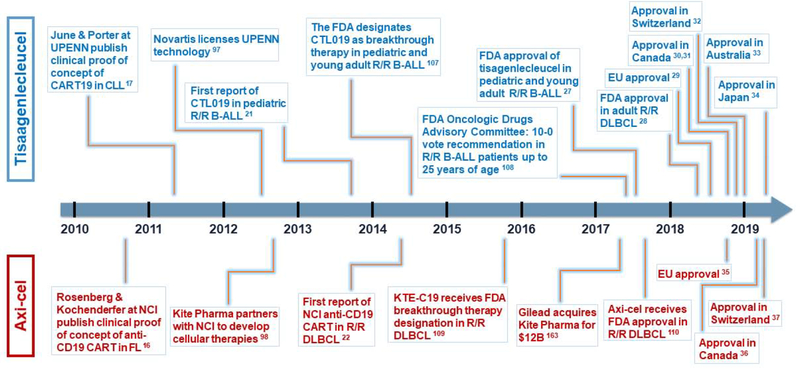
Chimeric antigen receptors (CAR) are proteins generated by the fusion of an antigen-binding domain, typically an antibody-derived single-chain variable fragment (scFv) with the T cell receptor (TCR) signaling domain CD3ζ and improvements have included a selected costimulatory domain. The presence of a tumor-specific CAR makes T cells independent of major histocompatibility complex (MHC)-restriction and virtually any target expressed on the surface of cancer cells can be recognized. After CAR-mediated target recognition and transmission of the signal, the T cell-intrinsic cytotoxic machinery is unleashed. The so-called “first generation” CAR T cells (CART) signal solely by the CD3ζ domain. They were initially used to target HIV [1, 2] and solid tumors [3–7], but resulted in limited or no clinical effect. Costimulatory domains such as CD28 [8–11], 4-1BB [12, 13], and others [12] were added to the CAR construct to enhance anti-tumor efficacy and persistence, leading to “second-generation CARs”. The development of second-generation CART and the choice of CD19 as a tumor antigen significantly increased CART activity in preclinical studies [14, 15] that were eventually translated into unprecedented clinical results in B-cell acute lymphoblastic leukemia (B-ALL) and non-Hodgkin lymphomas (NHL) [16–22]. In particular, CD19-targeted CARTs for B-ALL have since become the prime example of what can be achieved with CART with reported complete remission (CR) rates of 80-90% in r/r pediatric B-ALL, while response rates were 30-50% in chronic lymphocytic leukemia (CLL) and NHL [23–26]. Besides approvals in the US FDA [27, 28], tisagenlecleucel has also been approved in the European Union (EU) [29], Canada [30, 31], Switzerland [32], Australia [33], and Japan [34]. Axicabtagene ciloleucel, the second commercial CAR T cell therapy, is approved for r/r diffuse large B cell lymphoma (DLBCL) by the FDA as well as authorities in the EU [35], Canada [36], and Switzerland [37]. However, despite the clear clinical success, several aspects of CART treatment need to be improved. These include the lower response rates seen in DLBCL and CLL, CD19-negative escape, management of cytokine release syndrome (CRS), neurotoxicity, and the manufacturing cost of goods and services with implications for pricing and reimbursement. This review will describe the basic research, preclinical, and clinical studies that culminated in the FDA approval of tisagenlecleucel in 2017 [27] (key milestone events shown in Figure 1) and discuss current challenges and future perspectives.
Figure 1. Similar trajectories led to FDA approval of first two gene-edited cellular therapies for cancer.
Above timeline (blue): landmarks of tisagenlecleucel road to approval. Below timeline (red): landmarks leading to axicabtagene ciloleucel approval. UPENN, University of Pennsylvania. CART, chimeric antigen receptor T cell. CLL, chronic lymphocytic leukemia. FL, follicular lymphoma. NCI, National Cancer Institute. B-ALL, B-cell acute lymphoblastic leukemia. DLBCL, diffuse large B cell lymphoma. FDA, US Food and Drug Administration. R/R, relapsed-refractory. EU, European Union. Axi-cel, axicabtagene ciloleucel.
Key initial discoveries
CART therapy, like other adoptive cell therapies (ACT), has its roots in allogeneic hematopoietic cell transplantation (allo-HCT) [38]. T cells play an essential role in the success of allo-HCT, as T-cell depletion from the graft, initially pursued to decrease the risk of graft versus host disease (GVHD), increases the risk of relapse [39]. Moreover, malignant cells can be eradicated, even using reduced chemotherapy conditioning regimens, confirming the anti-tumor activity of donor T cells [40]. This notion is confirmed by the fact that post-allo-HCT donor lymphocyte infusions can bring relapsed subjects into a new remission [41]. The significant morbidity and mortality associated with allo-HCT, particularly the associated GVHD, and the risk of relapse gives a rationale for selective use of cancer-specific T cells.
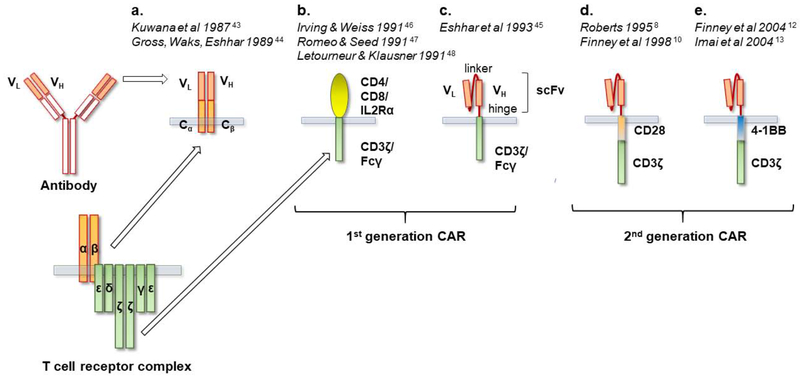
Acknowledging the ability of T cells to reject cancer but also realizing the difficulties in generalizing treatments based on conventional TCR-MHC interaction led researchers to seek other ways of harnessing T cells to target cancer. Concomitantly, advancements in genetic engineering established viral vectors as a tool to manipulate mammalian cells, including T cells [42]. In the late 1980s, two groups were independently working on examining the function and structure of newly elucidated antigen receptors on lymphocytes, both B and T, by creating “chimeric receptors.” From these studies arose the concept of endowing T cells with “at-will” specificities by engineering T cells to recognize antigens in an MHC-independent manner. Kurosawa and coworkers constructed chimeric genes attaching variable light chains (VL) or variable heavy chains (VH) of a monoclonal antibody to the TCR constant α or β chains in 1987 [43] (Figure 2). The VL and VH genes provided specificity for phosphorylcholine, a cell wall component of S. Pneumoniae. Exposure of transfected T cells to heat-killed S. Pneumoniae elicited an intracellular response in the form of calcium influx that was not MHC-restricted.
Figure 2. Evolution of chimeric antigen receptors.
CAR, chimeric antigen receptor. a. First concept of chimeric gene constructs of T cell receptor (TCR) constant regions (Cα and Cβ) fused to immunoglobulin (Ig) variable regions, VH and VL. In the “pre-CAR” concept formation of the antigen recognizing domain VH-VL required pairing of two individual constructs. b. Chimeras of CD4 and other surface molecules are engrafted onto the CD3ζ or Fcγ signaling domains originally with the purpose of elucidating the function of CD3ζ and Fcγ. c. The ”T-body” as proposed by Dr. Eshhar. The variable antibody domains VL and VH are put in serial connection via a linker creating a single chain variable fragment (scFv). The scFv is connected via a hinge to either a CD3ζ or the Fc receptor γ (FcRγ) activating domain. d. and e. Addition of a costimulatory molecule (e.g. CD28 or 4-1BB as shown in figure) established “second-generation CARs”.
In 1989 in Israel, Zelig Eshhar and coworkers generated similar constructs recognizing 2,4,6-trinitrophenyl, a hapten that was historically used to model antibody specificity (Figure 2). Transfected cytotoxic T cell hybridoma cells were able to lyse target-bearing cells and produce IL-2 [44]. Both methods depended on the pairing of the α and β chain in order to obtain the combined specificity of the VL and VH chains. The “T-body” approach would later be refined by using an scFv, containing both VL and VH chains connected via a linker. In “first-generation” CARs, the scFv is connected to either a CD3ζ or the Fc receptor γ (FcRγ) activating domain, via a hinge sequence [45] (Figure 2). The use of an scFv reduces the likelihood of mispairing with the endogenous TCR chains and has remained the most frequently employed extracellular structure used for the design of CAR to this day. Although CARs would be first used experimentally for elucidating the function of the CD3ζ chain [46–48], the potential for cancer treatment was envisioned from the beginning as noted in the discussion of Dr. Eshhar’s 1989 paper: “Construction of cTcRs with anti-tumor specificity will enable testing of the feasibility of this approach in combating human tumors” [44].
Adoptive T Cell Therapy Paving the Way for CARTs
In 1988, Rosenberg and coworkers at the National Cancer Institute (NCI) published an ACT strategy involving isolation of tumor-infiltrating lymphocytes (TIL) from melanoma subjects and in vitro expansion using IL-2, which resulted in the regression of metastatic melanoma in a subset of patients [49]. In a first-in-human clinical study using genetically modified T cells, the Rosenberg group transduced TILs with replication-incompetent murine retrovirus encoding the neomycin resistance gene as a marker for the infused T cells [50]. Five cancer patients received autologous gene-modified TILs, which persisted in circulation for up to two months and could be detected in tumor biopsies. No side effects related to gene transduction were observed and clinical effects were observed in three of five patients. Eshhar and Rosenberg would later collaborate to apply the T-body approach for cancer in research and pre-clinical studies. In 1993, the group transduced TILs with a CAR construct consisting of a folate receptor α (FRα)-specific scFv linked to FcRγ. CAR transduced TILs were able to lyse an ovarian carcinoma cell line (IGROV) in vitro [51], and in vivo [52]. Rosenberg’s group contributed another important principle to ACT by demonstrating that mild lymphodepletion improved the proliferation of adoptively transferred T cells and tumor regression in subjects treated with TILs for melanoma [53]. The group at the University of Pennsylvania showed that adoptive transfer of peripheral blood T cells induced lymphocytosis in the setting of autologous stem cell transplantation [54]. The effect created by lymphodepletion was later coined a “cytokine sink” referring to the increased availability of homeostatic cytokines for the adoptively transferred T cells [55]. Lymphodepletion is now a procedure included in most, though not all, CART therapy protocols [56].
This pioneering work inspired many other groups to study CART with multiple specificities, for example human epidermal growth factor receptor (HER) 2 [57], prostate-specific membrane antigen (PSMA) [58], tumor-associated glycoprotein 72 (TAG-72) [59], carboxy-anhydrase-IX [60], carcinoembryonic antigen (CEA) [61], GD2 [11], CD19 [62, 63], CD20 [64], CD30 [65], and CD171 [7], among others. Some CAR constructs would be a chimera between native molecules in the form of receptors or ligands linked to CD3ζ, for example, heregulin [66], IL13 [67], or CD4 (Figure 2) [47, 68], enabling CART to recognize HER3/4 in breast cancer, IL13Rα2 in glioblastoma, and gp120 on HIV-infected cells, respectively.
Pioneering clinical trials with first-generation CART
Romeo and Seed first described specific lysis of HIVgp120/gp41 complex expressing cells by T cells transiently transduced with the CAR CD4-CD3ζ [47]. Margo Roberts and colleagues at Cell Genesys Inc. carried out in vitro studies showing that HIV-infected CD4+ T cells could be specifically lysed by CD8+ T cells stably expressing a CD4-CD3ζ CAR following retroviral transduction [68]. Based upon these observations, the first clinical CAR trials were initiated in the 1990s, in HIV-infected subjects [1, 2]. Kristen Hege, also at Cell Genesys Inc., led a concurrent clinical CAR trial targeting TAG-72 in colorectal cancer, the first initiated for cancer [3]. The CD4-CD3ζ CART demonstrated the overall safety of retroviral transduction of T cells with CAR constructs as well as prolonged in vivo persistence of CART [69]. In the TAG-72 CART trial, one patient showed clinical evidence of CRS and a 50 percent decrease in levels of CEA, but no positive clinical outcomes were obtained [3].
These trials also served as confirmation of the robustness of a new T cell clinical manufacturing protocol using anti-CD3 and anti-CD28 coated magnetic beads [70]. These beads yielded significantly more robust in vivo persistence than T cells expanded using anti-CD3 antibody plus IL-2 [1, 71]. The first clinical use of CD3/CD28 activated T cells occurred in HIV+ subjects. Improvements in CD4 counts, CD4:CD8 ratios, and immune function were observed following dose escalation of their autologous polyclonal CD4+ T cells [72, 73]. The bead-activation method would subsequently go on to be utilized in thousands of subjects enrolled in T cell engineering clinical trials, and for tisagenlecleucel.
The CD4-cD3ζ CART clinical trials, as well as other gene transfer trials up until the early 2000s, had utilized murine gammaretroviral vectors (RV) for gene transfer. The first-in-human use of a lentiviral vector (LV) for gene transfer occurred in a clinical trial of autologous CD4+ T cells carrying an anti-sense to the HIV envelope gene in HIV+ subjects who had developed resistance to antiviral drugs [74]. The advent of third-generation LV further increased the safety profile of this vector [75]. Replication-competent LV and RV have not been detected in vector products and vector-transduced cells from numerous clinical trials [76–79]. Nor have there been any reports of oncogenic insertional mutagenesis from clinical trials involving mature T cells using either LV or RV, although oncogenic transformation of mature T cells has been reported in mice using RV [80]. T cell lymphomas due to insertional oncogenesis occurred in non-human primates when a RV contaminated with replication-competent virus was used in a hematopoietic stem cell transplant experiments [81].
Costimulation takes CART to the next level
The importance of costimulation in T cell activation was unfolding in the wake of the generally disappointing results of phase I trials with first-generation CARTs [3, 4, 82]. The two signal hypothesis proposed in 1970 by Bretscher and Cohn [83] stated that in order to obtain optimal activation a lymphocyte needs an antigen-specific signal delivered through its antigen receptor and an unspecific signal, delivered via costimulatory ligand-receptor interaction. Several early observations highlighted the potential significance of costimulation in the CART context. First, the inability of tumor-reactive T cells to reject malignant cells could be reverted by engineering the malignant cells to express B7/CD80 [84, 85]. Secondly, the function and proliferation of first-generation CARTs were enhanced by stimulation with artificial antigen-presenting cells (aAPC) co-expressing CD80 as well as target antigen [58, 62]. Additionally, EBV-specific T cells transduced with CAR most likely received costimulation from autologous antigen-presenting cells when reintroduced in the host, improving CART function and persistence [6]. The first publications of a second-generation CAR construct can be attributed to two independent groups. Margo Roberts, at Cell Genesys Inc., was the first to patent the concept of integrating a costimulatory domain in the CAR construct, the costimulatory domain being either CD2 or CD28 (patent filed February 1995) (Figure 2) [8]. Finney and colleagues at Celltech Therapeutics Ltd. filed a similar patent December 1996 and published their findings in 1998 [9, 10] describing a construct of an scFv recognizing CD33 and a CD28 costimulatory domain inserted proximally to CD3ζ. Jurkat cells transduced with the novel construct generated a twenty-fold stronger IL-2 response compared to an scFV-CD3ζ construct [10]. Sadelain and coworkers described an scFV-CD28 construct, which did not include a CD3ζ-domain, inducing enhanced anti-apoptotic and proliferation of transduced T cells upon recognition of the cognate antigen of the scFV [11]. Subsequently an scFV-CD28-CD3ζ CAR with the scFV being specific for PSMA was developed [86]. Then followed multiple reports by several groups on in vitro models using CAR against other antigen specificities and costimulatory molecules [12, 13, 86, 87]. Thus, while the first publications on CARs originated in academia (Kurosawa, Eshhar) both the first clinical trials of CARTs (Cell Genesys) and the first costimulatory CARs described and patented (Cell Genesys, Celltech) originated in industry laboratories.
The making of CART19
By the first decade of the 2000s, multiple groups were focusing on CD19 as a target for CART [13–15, 62, 88–90], with early preclinical work showing in vivo activity of a first-generation CAR when facilitated by CD80 stimulation from aAPCs and tumor cells [62]. CD19 is an attractive tumor antigen as it is restricted to malignant B cells as well as B-cell committed progenitors and mature B cells and to date it is the most successful tumor antigen for CART therapy [91]. Importantly, the expected on-target off-tumor toxicity, i.e. B cell aplasia, can be managed with repeated immunoglobulin infusions.
Following the observation that aAPC with 4-1BB ligand were able to augment CD8 T cell growth and function beyond what had been observed for CD80-CD28 interaction [92–94], preclinical studies began using a lentivirally encoded 4-1BB-CD3ζ, CD19-targeted CAR (Figure 2). The 4-1BB-CD3ζ, CD19-targeted CAR construct had been initially developed in a retroviral vector system [13] and subsequently improved by the insertion of a different promoter, elongation factor 1α (EF-1α), and inclusion in a lentiviral vector [15]. In an in vivo model of primary B-ALL, injection of 4-1BB-CD3ζ CD19-targeted CAR T cells resulted in improved survival of T cells compared to CD28-CD3ζ CD19-targeted CAR T cells [15]. Importantly longer leukemia-free survival of animals was observed when using a 4-1 BB second-generation CAR consistent with longer persistence of 4-1BB-stimulated CART. In addition, different promoters in the CAR vector were tested, providing evidence that EF-1α resulted in the highest and most stable CAR expression in both CD4+ and CD8+ T cells. This was the prototype of the CD19-targeted CAR that would start as CART19, later become CTL019 in clinical trials, and finally, tisagenlecleucel, the first FDA approved gene therapy.
Clinical evidence of potent and durable CART anti-tumor activity
Promising activity targeting CD19 was reported by the NCI in one subject with follicular lymphoma (FL) who obtained minimal residual disease (MRD) negativity of bone marrow and PR in lymph nodes [16]. In 2010 the University of Pennsylvania launched a CART19 phase I trial () recruiting adult subjects with r/r CD19+ B cell leukemia and lymphomas (key trials and publications leading to tisagenlecleucel approval are summarized in Table 1). Initially, three CLL subjects were infused. All demonstrated clinical responses to CART19, two obtaining CR, and one obtaining partial remission (PR) [17, 95]. Between 2.9 and 7.8 pounds of leukemia were destroyed in a few weeks by the engineered CAR T cells [95]. Absence of funding to treat more than three patients led to a delay in subsequent enrollments until the end of 2011 [96]. However, the publications attracted interest in licensing CART19 technology. In August 2012, the establishment of a research and development alliance between Novartis and the University of Pennsylvania was announced [97]. Several months later, Kite Pharma partnered with the NCI to develop engineered cellular therapies [98].
Table 1.
Tisagenlecleucel: pivotal clinical trials leading to approval.
| Disease | Primary study population | Reference | Sponsor | Clinical trial identifier | Alias | Design | Location | Phase | No. of patients infused | CR/CRi at 3 months (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| R/R CLL | Adult | [17, 95, 99] | UPENN | Single center | ACC/UPENN | Pilot/I | 14 | 29 | ||
| R/R B-ALL | Children | [100] | UPENN | Single center | CHOP/UPENN | I/IIa | 30 | 90 | ||
| Children | [101] | Novartis | Multicenter | United States | II | 29 | 69 | |||
| Children | [23] | Novartis | ELIANA | Multicenter | Multinational | II | 75 | 81 | ||
| R/R DLBCL | Adult | [104] | UPENN | Single center | ACC/UPENN | IIa | 14 | 43* | ||
| Adult | [105] | Novartis | JULIET | Multicenter | Multinational | II | 93 | 40 |
CR, complete remission. CRi, complete remission with incomplete hematologic recovery. R/R, relapsed/refractory. CLL, chronic lymphocytic leukemia. B-ALL, B-cell acute lymphoblastic leukemia. DLBCL, diffuse large B cell lymphoma. UPENN, University of Pennsylvania. CHOP, Childrens Hospital of Philadelphia. ACC, Abramson Cancer Center.
Percent of patients in CR by month 6.
In total, the first University of Pennsylvania CART19 trial () infused 14 subjects with CLL [99]. Four subjects obtained CR and 4 obtained PR. Hence, following the first three subjects, this first CLL trial cohort clinical response rate was disappointing, though confirmed by observations at Memorial Sloan Kettering Cancer Center and NCI [18, 19]. While bed to bench investigations to improve the consistency and potency of CART products from CLL subjects were initiated, a shift was made to focus clinical efforts in pediatric ALL [21, 23, 100]. Results of the first two subjects treated on the University of Pennsylvania/Children’s Hospital of Philadelphia pediatric trial of CART19 in B-ALL (), were published in 2013 [21]. Both subjects obtained CR, though one subject later relapsed with CD19 negative disease [21]. This was also the first publication on the successful use of the anti-IL-6 receptor blocking antibody tocilizumab to treat CRS. Seventy-two percent of pediatric subjects receiving CART19 had previously been treated with allo-HCT, and 88% had had two or more relapses. Given the extremely poor prognosis in r/r B-ALL, the observation that 90% of CART19-treated subjects (25 pediatric subjects and five adults subjects with r/r B-ALL enrolled in and ) went into CR following CART19 was unexpected and stimulated accelerated development [100]. Of note, the discovery that tocilizumab can successfully treat CRS, drastically changed the feasibility of CART19 leading to even greater interest from both academia and pharma.
From single center trials to global clinical studies
The clinical development that followed for pediatric/young adult B-ALL subjects was the initiation of two multi-center studies. A phase II multi-center trial at sites within the United States () enrolled pediatric and young adults (three to 21 years of age) with r/r B-ALL. Results of interim clinical and pharmacokinetic analyses have been published [101–103]. Twenty-nine of 35 subjects enrolled (83%) were infused with CART19, by then referred to as CTL019. The overall remission rate (ORR) at 6 months, defined as CR or CR with incomplete hematologic recovery, was 69% in infused subjects and relapse-free survival (RFS) was 66%. Ninety percent of subjects experienced CRS and grade 3 or 4 CRS was observed in 38% of the subjects [101]. The second trial, a Novartis global trial with 25 enrollment centers in 11 countries on four continents, called ELIANA () enrolled pediatric and adult subjects up to 30 years of age with r/r B-ALL. Of 92 enrolled subjects, 75 underwent infusion. At an interim analysis, ORR was 81%, and RFS among subjects was 80% at six months and 59% at 12 months. Seventy-seven percent experienced CRS, and 46% had grade 3-4 CRS [23]. Collectively, these data demonstrated the durable induction of clinical responses in the r/r B-ALL cohort.
CTL019 was also evaluated in a phase IIa trial for r/r DLBCL, FL, and mantle cell lymphoma () at the University of Pennsylvania starting 2014. In 28 subjects treated, CR was obtained in 6/14 DLBCL subjects and 10/14 subjects with FL at six months [104]. Importantly, all subjects in CR by six months remained in remission at a median follow-up of 29.3 months. Overall, severe adverse events were lower than what has been observed in B-ALL subjects; 18% developed grade 3 or higher CRS and 11% developed grade 3 or higher neurotoxic events. A Novartis multi-national phase II trial, JULIET (), was initiated and results published [105]. Ninety-three adult subjects with r/r DLBCL received CTL019. Forty percent obtained CR, and 12% obtained PR. The estimated probability of survival at 12 months among subjects in CR was 90%.
Regulatory Approval for CAR T Cells In the United States and Internationally
Prior to the initiation of the ELIANA trials Novartis had submitted a special protocol assessment (SPA) in March 2014, which was accepted by the FDA [106]. SPA agreements indicate that the FDA accepts the overall trial designs which may support later drug application to the FDA. Shortly after Novartis filed for Breakthrough Therapy Designation of CTL019 in r/r adult and pediatric B-ALL, which was granted by the FDA in July 2014 [107]. This designation is intended to expedite the development and review of new medicines – both drugs and biologic agents – that treat serious or life-threatening conditions if the therapy has demonstrated substantial improvement over available therapies. The FDA had previously granted Breakthrough Therapy Designation to only four other biologic agents, and CTL019 was the first personalized cellular therapy for the treatment of cancer to receive this. With data from the phase II results of the ELIANA trial and supported by the previous University of Pennsylvania/Children’s Hospital of Philadelphia clinical trials, Novartis filed a Biologies License Application with the FDA in early 2017. Priority review designation was granted March 2017. In July 2017, the FDA Oncologic Drugs Advisory Committee, which reviews and recommends investigational human drug products for cancer treatment, gave a unanimous 10-0 vote recommending tisagenlecleucel to treat pediatric and young adult r/r B-ALL [108].
On August 30, 2017, tisagenlecleucel (formerly CTL019), was approved by the FDA for the treatment of subjects up to 25 years of age with B-ALL [27], as the first FDA approved gene therapy and marking a historic date for genetically-engineered cellular therapies for cancer. Kite Pharma’s KTE-C19 received Breakthrough Therapy Designation for r/r aggressive NHL December, 2015 [109], October 2017, the FDA approved axicabtagene ciloleucel (formerly KTE-C19) for adult patients with large B-cell lymphoma failing at least two other kinds of treatment, including DLBCL, primary mediastinal large B-cell lymphoma, high-grade B-cell lymphoma and DLBCL arising from FL [110]. In April 2017 Breakthrough Therapy Designation was granted by the FDA for the use of CTL019 in r/r DLBCL. This was followed by the FDA approval of tisagenlecleucel for DLBCL in May 2018 [28].
Tisagenlecleucel was later authorized for clinical use in the EU by the European Medicines Agency for the treatment of r/r B-ALL and DLBCL in August 2018 [29], and also in Canada [30, 31], Switzerland [32], Australia [33], and Japan [34] for the same indications. As of September 28th, 2019, 101 tisagenlecleucel treatment centers have been established in the US [111]. Axicabtagene ciloleucel is also approved by the European Medicines Agency [35], Health Canada [36], and Switzerland [37], and as of September 28th, 2019, is available at 83 centers in the US [112]. Thus, these two novel CD19-directed CART therapies, out of all cell therapies approved by national health authorities, are available in the largest number of countries [113].
Current concepts of tisagenlecleucel treatment failure
While striking clinical responses following CAR T cell treatment are observed in otherwise untreatable r/r CD19+ B cell malignancies, not all subjects respond to treatment, specifically 10-20% of pediatric and young adult B-ALL and 50-60% of adult DLBCL. Moreover, a significant portion of subjects are either not eligible for the treatment or may not survive during the time needed to schedule, manufacture, and deliver their CAR T cell product. Lastly, a significant subset of subjects (40-50%) relapses within one year after reaching a CR in B-ALL [23, 24, 114].
A deep understanding of the mechanisms leading to relapse is needed to increase response rates and reduce relapses. Two major mechanisms of relapse are observed amongst subjects treated with CART targeting CD19 for CD19+ B-ALL, irrespective of the type of costimulatory domain [115, 116]. One is the relapse of CD19+ B-ALL, typically due to inadequate expansion and persistence of CART [100, 102, 103, 117]. The second major mechanism of relapse involves the emergence of CD19-negative B-ALL [100, 102, 118]. In DLBCL CD19-negative relapses are less frequent although they have been described [119–121]. Therefore, strategies that, from one side, avoid antigen-escape, and on the other increase CART activity and persistence are being pursued. One avenue of preventing CD19-negative relapse is through the use of CAR T cells targeting multiple tumor targets. For example, CD19 and CD123 [122] or more commonly CD19 and CD22 [123, 124] have been proposed as targets for dual-targeted CART therapy. A plethora of multi-targeted clinical CART trials has now been initiated (Table 3). Multiple factors influence the activity and persistence of CART, such as T cell subtypes [125], exhaustion, and interaction with the tumor microenvironment [126]. It is likely the tumor microenvironment of lymphomas and CLL, not unlike that of solid tumors, is challenging for T cells and at least partially explains the lower response rates observed in DLBCL and CLL as compared to B-ALL. In addition, age differences between B-ALL and DLBCL/CLL subjects could play a role as increasing age is known to affect general T cell fitness [127]. Indeed, T cell fitness has been shown to be a determinant of response to CART therapy in CLL [125]. Moreover T cells of CLL subjects may have proliferative defects even when compared to age-matched subjects with other hematological diseases [128]. New strategies being tested to improve CART function include the concomitant treatment with small molecule inhibitors [129]. Ibrutinib is a small molecule inhibitor targeting Bruton’s tyrosine kinase (BTK) that improved CART19 function in a preclinical model [128, 130]. A recent interim analysis of a pilot trial with humanized CART19 (CTL119) and ibrutinib in CLL revealed MRD negativity in the bone marrow at three months in 14 of 18 evaluable subjects, supporting a synergistic effect of dual therapy [131].
Table 3.
Multi-targeted clinical CAR T cell trials.
| Antigens | Sponsor / Collaborator | Location | Disease | Clinical trial reference number |
|---|---|---|---|---|
| CD19 & CD22 | University of Pennsylvania | United States | B-ALL | |
| Crystal Mackall / Lucile Packard Children’s Hospital / Stanford University / National Cancer Institute | United States | B-ALL | ||
| Seattle Children’s Hospital / Children’s National Medical Center Washington | United States | B-ALL | ||
| Crystal Mackall / California Institute for Regenerative Medicine / Stanford University | United States | B-ALL & DLBCL | ||
| National Cancer Institute | United States | B-ALL, CLL, NHL, & lymphosarcoma | ||
| Autolus Limited / Great Ormond Street Hospital / University College London Hospitals / Royal Manchester Children’s Hospital | United Kingdom | B-ALL | ||
| Autolus Limited / University College London Hospitals / Manchester University / Freeman Hospital, The Newcastle upon Tyne Hospitals | United Kingdom | DLBCL | ||
| Xuzhou Medical University | China | B cell malignancies | ||
| CD19 & CD20 | Medical College of Wisconsin / Children’s Hospital and Health System Foundation, Wisconsin | United States | NHL & CLL | |
| Miltenvi Biotec / ICON plc / University Hospital of Cologne | Germany | NHL & CLL | ||
| CD10, CD20, & CD22 | Southern Medical University, Zhujiang Hospital | China | B-ALL | |
| CD22, CD123, CD38, CD10, CD20, TSLPR | Shenzhen Geno-Immune Medical Institute | China | B-ALL | |
| CD19, CD20, CD22, CD30, CD38, CD70, CD123 | Shenzhen Geno-Immune Medical Institute | China | B cell malignancies | |
| BCMA, CD19 | University of Pennsylvania / Novartis | United States | multiple myeloma | |
| The First Affiliated Hospital of Soochow University | China | multiple myeloma | ||
| Peng Liu / Hrain Biotechnology / Shanghai East Hospital | China | multiple myeloma | ||
| Shenzhen Second People’s Hospital | China | multiple myeloma | ||
| BCMA, CD38 | Chinese PLA General Hospital | China | multiple myeloma | |
| BCMA, TACI | Autolus Limited / VU University Medical Centre Amsterdam / University College London Hospitals / The Christie / Freeman Hospital | UK & Netherlands | multiple myeloma | |
| BCMA, CD19, CD38 | The First Affiliated Hospital of Soochow University | China | multiple myeloma | |
| BCMA, CD38, CD56, CD138 | Shenzhen Geno-Immune Medical Institute | China | multiple myeloma | |
| Zhujiang Hospital / Nanfang Hospital of Southern Medical University / The Third Affiliated Hospital of Southern Medical University / Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University | China | multiple myeloma | ||
| Integrin β7, BCMA, CS1, CD38, CD138 | The Sixth Affiliated Hospital of Wenzhou Medical University | China | multiple myeloma | |
| CD33, CD123 or CLL-1 | Shenzhen Geno-Immune Medical Institute | China | AML | |
| MUC1, CLL1, CD33, CD38, CD56, CD123 | Shenzhen Geno-Immune Medical Institute / Zhujiang Hospital, Southern Medical University / The Cancer Hospital of Yunnan | China | AML | |
| CD33, CD38, CD56, CD123, CD117, CD133, CD34, MUC1 | Zhujiang Hospital / Nanfang Hospital of Southern Medical University / The Third Affiliated Hospital of Southern Medical University / Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University | China | AML | |
| EGFRvIII/DR5/NY-ESO-1/Mesothelin | Shenzhen BinDeBio Ltd. / Henan Provincial Hospital | China | Solid Malignancies | |
| HER2, Mesothelin, PSCA, MUC1, Lewis-Y, CD80/86 | Second Affiliated Hospital of Guangzhou Medical University / Hunan Zhaotai Yongren Medical Innovation Co. Ltd. / Guangdong Zhaotai InVivo Biomedicine Co. Ltd. / First Affiliated Hospital, Sun Yat-Sen University | China | Solid Malignancies | |
TSLPR, thymic stromal lymphopoietin receptor. BCMA, B cell maturation antigen. TACI, Transmembrane activator and calcium modulator and cyclophilin ligand interactor. CS1, CD319 or SLAMF7. CLL-1, C-type lectin domain family 12 member A. MUC1, Mucin 1 cell surface associated. EGFRvIII, Epidermal growth factor receptor variant III. DR5, Death receptor 5. NY-ESO-1, Cancer testis antigen 1B. HER2, human epidermal growth factor receptor 2. PSCA, Prostate stem cell antigen. Lewis-Y, Lewis-Y antigen.
Another strategy to increase CART function and reduce immunosuppression is direct checkpoint inhibition of the programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) axis. In a recently published case report the PD-1 blocking antibody pembrolizumab was administered to a subject who had progressive DLBCL despite tisagenlecleucel therapy [132]. Following administration of pembrolizumab, CART19 expanded, and PD-1/Eomes co-expression was decreased. Clinically the subject’s enlarged lymph nodes shrank. A clinical trial () has been initiated testing pembrolizumab administration in subjects with relapsed or progressive disease following tisagenlecleucel or CTL119 therapy and results have recently been published [133]. In children with B-ALL or B lymphoblastic lymphoma with early B cell recovery, residual bulky disease, or unresponsiveness to therapy pembrolizumab or nivolumab was administered early after tisagenlecleucel or CTL119 therapy [134]. In three of six subjects with early B cell recovery, B cell aplasia was reestablished. In four subjects treated for bulky disease, two subjects obtained PR and two obtained CR. PORTIA is an active clinical trial () testing pembrolizumab in combination with tisagenlecleucel in r/r DLBCL subjects. Similarly, atezolizumab, a PD-L1 inhibitor is being studied as combination therapy with axicabtagene ciloleucel for subjects with refractory DLBCL (). Table 2 summarizes past, current, and projected tisagenlecleucel trials. Pivotal trials leading to tisagenlecleucel approval are summarized in Table 1.
Table 2.
Tisagenlecleucel: past, current, and projected trials (pivotal trials leading to approval are shown in Table 1).
| Primary disease | Primary study population | Description | Publication | Main sponsor | Clinical trial identifier | Alias | Design | Location | Phase | Actual or estimated enrollment | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| B-ALL | |||||||||||
| R/R B-ALL | Adult | Randomized comparison blinatumomab and inotuzumab | Novartis | OBERON | Multicenter | Multinational | III | 220 | NYR | ||
| B-ALL | Pediatric | tisagenlecleu cel if MRD+ following first line treatment | Novartis | CASSI OPEIA | Single center | UPENN/CHOP | II | 140 | R | ||
| R/R B-ALL | Pediatric | Optimization study of tocilizumab for CRS | UPENN | Single center | UPENN/CHOP | Pilot | 80 | ANR | |||
| R/R B-ALL | Pediatric | Expanded access/compassionate use | Novartis | Multicenter | Multinational | II | |||||
| R/R B-ALL & DLBCL | Adult & pediatric | tisagenlecleu cel-axicabtagene ciloleucel head-to-head in DLBCL | MCC | Single center | MCC | II | 120 | R | |||
| R/R B-ALL & DLBCL | Pediatric | CTL119 | UPENN | Single center | ACC/UPENN | ||||||
| B-ALL & DLBCL | Adult & pediatric | managed access program | Novartis | Multicenter | United States | NA | NA | NA | |||
| R/R B-ALL | Adult | Allogeneic CART19 | UPENN | Single center | ACC/UPENN | I | 2 | Completed | |||
| R/R B-ALL | Adult | UPENN | Single center | ACC/UPENN | II | 30 | Completed | ||||
| R/R B-ALL | Pediatric | CTL119 for very high risk subsets of pediatric B-ALL | UPENN | Single center | UPENN/CHOP | II | 85 | R | |||
| R/R B-ALL | Adult | CART22 alone or in combination with CTL119 | UPENN | Single center | UPENN | I | 18 | R | |||
| Lymphoma | |||||||||||
| R/R NHL | Pediatric | Novartis | BIANCA | Multicenter | United States & Spain | II | 35 | R | |||
| R/R NHL | Adult | Novartis | BELINDA | NA | NA | III | 318 | NYR | |||
| R/R DLBCL | Adult | tisagenlecleu cel-ibrutinib combination therapy | Novartis | Single center | UPENN | Ib | 40 | NYR | |||
| R/R NHL | Adult | Pembrolizum ab to subjects failing or relapsing post-tisagenlecleu cel | [133] | UPENN | Single center | ACC/UP | I/II | 12 | NA | ||
| R/R NHL | Adult | FDG-PET response post-CART19 | UPENN | Single center | ACC/UP ENN | I | 9 | Completed | |||
| R/R DLBCL | Adult | tisagenlecleu cel-pembrolizum ab combination therapy | Novartis | PORTIA | Multicenter | United States & Austria | Ib | 32 | R | ||
| R/R FL | Adult | Novartis | ELARA | Multicenter | United States & Australia | II | 113 | R | |||
| CLL | |||||||||||
| R/R CLL | Adult | CTL119 + ibrutinib | [131] | UPENN | Single center | ACC/UPENN | Pilot | 20 | ANR | ||
| R/R CLL/SLL | Adult | Randomization between two different doses | [128, 171] | UPENN | Single center | ACC/UPENN | II | Completed | |||
| B cell malignancies (all kinds) | |||||||||||
| R/R | All ages | Determine safety and maximum tolerated dose of tisagenlecleu cel | MDA/NCI | Single center | MDA | I | 26 | ANR | |||
| All ages | Long term follow up All ages CD19-directed CART | Novartis | Multicenter | Global | NA | 620 | R | ||||
| Multiple myeloma | |||||||||||
| Adult | CART19 day 2 post-ASCT following early relapse | [172, 173] | UPENN | Single center | ACC/UPENN | I | 13 | Completed | |||
| Adult | CART19 day 60 post-ASCT | UPENN | Single center | UPENN/ACC | II | 5 | Terminated | ||||
| Relapsed | Adult | Up-front BCMA-CART alone or with CTL119 | UPENN | Single center | UPENN | I | 39 | R | |||
| Solid cancer | |||||||||||
| Pancreatic cancer | Adult | tisagenlecleu cel to prolong meso-specific CART response | UPENN/UCSF | Single center | UCSF | I | 4 | Completed | |||
R/R, relapsed/refractory. CLL, chronic lymphocytic leukemia. B-ALL, B-cell acute lymphoblastic leukemia. DLBCL, diffuse large B cell lymphoma. FL, follicular lymphoma. MM, multiple myeloma. NHL, non-Hodgkin lymphoma. MRD, minimal residual disease. UPENN, University of Pennsylvania. CHOP, Childrens Hospital of Philadelphia. ACC, Abramson Cancer Center. ASCT, autologous stem cell transplant. MCC, Masonic Cancer Center, University of Minnesota. MDA, MD Anderson Cancer center. NCI, National Cancer Institute. UCSF, University of California - San Francisco. NA, not available/not applicable. NYR, not yet recruiting. R, recruiting, ANR, active, not recruiting. CART, chimeric antigen receptor T cell.
Novel information added
Future perspectives
Propelled by the approvals of the first CART therapies, there has been an exponential growth of clinical trials involving CART and other cellular therapies for cancer [135]. More than 1,000 cell therapies are currently in the pipeline, and CAR T cell products make up more than half of these [135].
While tisagenlecleucel is available in ~150 clinical centers worldwide, production of CART is extraordinarily complex and takes place in a few specialized GMP facilities in the US and EU. Collection, manufacturing, logistics, and transportation of CART are critical factors that are essential considerations in the continuum of this therapy [136]. Thus, improvements to manufacturing protocols, to analytics methods, and more seamless logistics, will allow more potent products to reach patients in need more quickly and potentially also reduce patient-to-patient variation [137–139]. Off-the-shelf, allogeneic CART products from several companies and academic centers are in early phase clinical trials and have the obvious benefit of eliminating time-delay for manufacturing as well as being a source when sufficient CAR T cell numbers cannot be generated [140–146]. The limitations of allogeneic CART are the risk of GVHD as well as host versus graft elimination of CART. Current trials ( and ) of a CD19-directed allogeneic CART (UCART19) in B-ALL use CART as a bridge to subsequent allo-HCT [140].
Several strategies are addressing the challenges of increasing persistence and potency or fine-tuning CAR trafficking. Optimizing spacer length between the CAR domains [147], incorporating additional costimulatory domains (“third-generation” CAR) [148], and the inclusion of a cytokine expression cassette (so-called TRUCKS) [149] have been explored as means to improve CART potency. Gene editing by CRISPR-Cas9 or other modalities is being used increasingly to fashion CARTs for specific purposes (allogeneic) or augment potency. In a murine model, T cell persistence improved following knockout of PD-1 [145]. CRISPR-Cas9 has also been used in a preclinical model to knock out CD33 on hematopoietic stem cells, imparting resistance to CD33-targeted CART [150]. This strategy allows CD33-targeted CART therapy of AML without killing myeloid progenitor cells.
Several other methods are being explored to reduce the short-term toxicity of CART therapy. Examples are options that permit the elimination of CART after infusion (e.g., inducible apoptosis systems [151] or co-expression of depletion markers [152]). Conditional CAR systems explore strategies for controlling CAR-mediated activation. Wu and coauthors developed “remote-control CARs” which are split CAR designs that require a small molecule in order for the extracellular antigen-binding domain to associate with the intracellular signaling domain, an “On-switch” [153]. Small molecule-dependent systems can also be used as an “Off-switch” of CAR transgene expression [154]. Combinatorial antigen-sensing circuits or “switchable CARs” are elegant solutions that allow for both safety in terms of controlled CART activation and versatility in terms of broad applicability against multiple antigens [155]. In the model explored by Rodgers and coauthors, T cells were transduced with a CAR recognizing a non-human neoepitope, in turn, the neo-epitope is engrafted on an antigen-binding fragment (Fab) recognizing a tumor antigen. Addition of Fab can thus redirect CART to tumor cells [155].
In extending CART therapy to other malignancies, alternative approaches to circumvent the absence of truly tumor-specific antigens have been proposed [116]. Among others, these involve scFv affinity and CAR density modulation [156], a combination of VL and VH chains from different antibodies recognizing the same antigen [157, 158], and establishing micro-circuitry systems to enable CART activation only when the right combination of antigens are present [159–161]. Targeting supportive cells in the tumor microenvironment, as shown by targeting CD123 on tumor-associated macrophages in Hodgkin lymphoma, may be a solution for improved disease control [162]. Unlike the clinical responses seen in hematological malignancies, attempts at treating solid tumors with CART have so far achieved limited results. The ability of CART therapy to overcome the tumor microenvironment of solid tumors and with acceptable on-target off-tumor toxicities will require more sophisticated potency-enhancing strategies.
Tisagenlecleucel was the most expensive cancer therapy to have been approved in the US, which rightfully raises questions of the cost effectiveness of therapy. Recent studies suggest that comparable healthcare costs of allo-HCT for relapsed pediatric ALL and DLBCL remain high in the years following allo-HCT in large part due to complications and relapse [163, 164]. Conventional treatment for childhood cancers carries significant long-term toxicities [165]. In comparison, CART can induce durable responses in subjects with CD19+ malignancies that have no other treatment options with only short-term manageable toxicities. Justification and value of tisagenlecleucel will ultimately depend on the fraction of patients that achieve long-term remission as well as the frequency of morbidity related to treatment [166].
The growing number of trials now registered for tisagenlecleucel (Table 2) and for CART therapy, in general, attests to the investment both from industry and academia in this novel therapy [135, 167]. Interestingly, CTL119 is now being tested in a clinical trial in the first line setting in pediatric B-ALL () and axicabtagene ciloleucel is being tested against standard of care second-line therapy in r/r DLBCL (); the results of these trials could drastically change the treatment algorithm for r/r B-ALL and lymphoma. Moreover, the role of allogeneic transplant, especially in B-ALL, will be redefined potentially allowing for the optimization of its safety and efficacy profile [168]. Many other industry players have ventured into CART development since Novartis and Kite Pharma/Gilead’s original partnerships [97, 98, 169, 170]. The clinical approval of tisagenlecleucel in several countries all around the world is a landmark in cellular immunotherapy and genetic engineering for cancer. There are multiple avenues to pursue in order to increase efficacy and safety of CAR T cell therapy, spawning hope of further improvements in a near future that will enable more patients to be successfully treated with these new medicines.
Acknowledgments
The authors would like to thank Carl June, Michael Milone, David Porter, Stephen Schuster, Stephan Grupp, Shannon Maude, as well as past and current colleagues in the Center for Cellular Immunotherapies, Abramson Cancer Center and Children’s Hospital of Philadelphia. Special thanks to the patients and families who participated in the clinical trials of CART19/CTL019/tisagenlecleucel. We would like to thank and acknowledge all of the investigators, centers, and now companies, engaged in CAR T cell research, translation and clinical trials. This review focused on the development of CART19 to tisagenlecleucel, and we regret that due to space limitations, the contributions of all could not be included.
This work was supported by grants from the ITMAT (PI: M.R.), the ASH-Scholar Award (P.I.: M.R.), the NIH NCI 1K99CA212302-01A1 and R00CA212302-03 (PI: M.R.), and the Mark Foundation (P.I.: M.R.). P.B. received grants from the Danish Cancer Society, “Dagmar Marshalls fond”, “Fabrikant Einar Willumsens Mindelegat”, “Københavns Universitets kræftforsknings fond”, “Fonden til fremme af klinisk cancer forskning”, and “Anders Hasselbalchs fond til leukaemiens bekaempelse”. The providers of funding were not involved in the writing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
P.B.: Unrestricted research grant from Novartis Healthcare Denmark contributed to his institution. M.R.: research funding from Novartis; inventor in patents involving the use of CART immunotherapy for cancer. Consultant/advisor Nanostring, Abclon B.L.L.: Scientific Advisory Board of Avectas, ThermoFisher Viral Vector Systems (formerly Brammer Bio), Cure Genetics, Immuneel, Incysus, Ori Biotech, Vycellix, consultancy fees CRC Oncology, licensed intellectual property to Novartis Pharmaceuticals Corporation and Tmunity Therapeutics, and co-Founder, equity holder of Tmunity Therapeutics.
References
- [1].Walker RE, Bechtel CM, Natarajan V, Baseler M, Hege KM, Metcalf JA, Stevens R, Hazen A, Blaese RM, Chen CC, Leitman SF, Palensky J, Wittes J, Davey RT Jr., Falloon J, Polis MA, Kovacs JA, Broad DF, Levine BL, Roberts MR, Masur H, Lane HC, Long-term in vivo survival of receptor-modified syngeneic T cells in patients with human immunodeficiency virus infection, Blood 96(2) (2000) 467–74. [PubMed] [Google Scholar]
- [2].Mitsuyasu RT, Anton PA, Deeks SG, Scadden DT, Connick E, Downs MT, Bakker A, Roberts MR, June CH, Jalali S, Lin AA, Pennathur-Das R, Hege KM, Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects, Blood 96(3) (2000) 785–93. [PubMed] [Google Scholar]
- [3].Hege KM, Bergsland EK, Fisher GA, Nemunaitis JJ, Warren RS, McArthur JG, Lin AA, Schlom J, June CH, Sherwin SA, Safety, tumor trafficking and immunogenicity of chimeric antigen receptor (CAR)-T cells specific for TAG-72 in colorectal cancer, Journal for immunotherapy of cancer 5 (2017) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, Chen CC, Yang JC, Rosenberg SA, Hwu P, A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer, Clinical cancer research : an official journal of the American Association for Cancer Research 12(20 Pt 1) (2006) 6106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, Gratama JW, Stoter G, Oosterwijk E, Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 24(13) (2006) e20–2. [DOI] [PubMed] [Google Scholar]
- [6].Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, Yvon E, Weiss HL, Liu H, Rooney CM, Heslop HE, Brenner MK, Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma, Nature medicine 14(11) (2008) 1264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, Meechoovet HB, Bautista C, Chang WC, Ostberg JR, Jensen MC, Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma, Molecular therapy : the journal of the American Society of Gene Therapy 15(4) (2007) 825–33. [DOI] [PubMed] [Google Scholar]
- [8].Roberts MR, Chimeric receptor molecules for delivery of co-stimulatory signals, 1995. http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&u=%2Fnetahtml%2FPTO%2Fsearch-adv.htm&r=55&f=G&l=50&d=PTXT&s1=5,712,149&p=2&OS=5,712,149&RS=5,712,149. (Accessed July 24th 2019).
- [9].Bebbington CR, Lawson AD, Weir N, Finney HM, CELL ACTIVATION PROCESS AND REAGENTS THEREFOR, 1996. https://patentscope.wipo.int/search/en/detail.isf?docId=WO1997023613&tab=PCTBIBLIO. (Accessed July 24th 2019).
- [10].Finney HM, Lawson AD, Bebbington CR, Weir AN, Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product, J Immunol 161(6) (1998) 2791–7. [PubMed] [Google Scholar]
- [11].Krause A, Guo HF, Latouche JB, Tan C, Cheung NK, Sadelain M, Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes, The Journal of experimental medicine 188(4) (1998) 619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Finney HM, Akbar AN, Lawson AD, Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain, Journal of immunology (Baltimore, Md.: 1950) 172(1) (2004) 104–13. [DOI] [PubMed] [Google Scholar]
- [13].Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, Campana D, Chimeric receptors with 4–1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia, Leukemia 18(4) (2004) 676–84. [DOI] [PubMed] [Google Scholar]
- [14].Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La Perle K, Quintas-Cardama A, Larson SM, Sadelain M, Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts, Clinical cancer research : an official journal of the American Association for Cancer Research 13(18 Pt 1) (2007) 5426–35. [DOI] [PubMed] [Google Scholar]
- [15].Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, Samanta M, Lakhal M, Gloss B, Danet-Desnoyers G, Campana D, Riley JL, Grupp SA, June CH, Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo, Molecular therapy: the journal of the American Society of Gene Therapy 17(8) (2009) 1453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, Maric I, Raffeld M, Nathan DA, Lanier BJ, Morgan RA, Rosenberg SA, Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19, Blood 116(20) (2010) 4099–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Porter DL, Levine BL, Kalos M, Bagg A, June CH, Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia, The New England journal of medicine 365(8) (2011) 725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda O, Olszewska M, Bernal Y, Pegram H, Przybylowski M, Hollyman D, Usachenko Y, Pirraglia D, Hosey J, Santos E, Halton E, Maslak P, Scheinberg D, Jurcic J, Heaney M, Heller G, Frattini M, Sadelain M, Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias, Blood 118(18) (2011) 4817–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, Yang JC, Kammula US, Devillier L, Carpenter R, Nathan DA, Morgan RA, Laurencot C, Rosenberg SA, B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells, Blood 119(12) (2012) 2709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, Borquez-Ojeda O, Qu J, Wasielewska T, He Q, Bernal Y, Rijo IV, Hedvat C, Kobos R, Curran K, Steinherz P, Jurcic J, Rosenblat T, Maslak P, Frattini M, Sadelain M, CD19-Targeted T Cells Rapidly Induce Molecular Remissions in Adults with Chemotherapy-Refractory Acute Lymphoblastic Leukemia, Science translational medicine 5(177) (2013) 177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Grupp SA, Kalos M, Barrett D, Aplenc R, Porter D, Rheingold S, Teachey D, Chew A, Hauck B, Wright J, Milone M, Levine B, June C, Chimeric antigen receptor-modified T cells for acute lymphoid leukemia, New England Journal of Medicine 368(16) (2013) 1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM, Raffeld M, Feldman S, Lu L, Li YF, Ngo LT, Goy A, Feldman T, Spaner DE, Wang ML, Chen CC, Kranick SM, Nath A, Nathan DA, Morton KE, Toomey MA, Rosenberg SA, Chemotherapy-Refractory Diffuse Large B-Cell Lymphoma and Indolent B-Cell Malignancies Can Be Effectively Treated With Autologous T Cells Expressing an Anti-CD19 Chimeric Antigen Receptor, Journal of Clinical Oncology (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, Schlis K, Davis KL, Martin PL, Nemecek ER, Yanik GA, Peters C, Baruchel A, Boissel N, Mechinaud F, Balduzzi A, Krueger J, June CH, Levine BL, Wood P, Taran T, Leung M, Mueller KT, Zhang Y, Sen K, Lebwohl D, Pulsipher MA, Grupp SA, Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia, The New England journal of medicine 378(5) (2018) 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Park JH, Riviere I, Gonen M, Wang X, Senechal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, Roshal M, Maslak P, Davila M, Brentjens RJ, Sadelain M, Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia, The New England journal of medicine 378(5) (2018) 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, Robinson E, Steevens NN, Chaney C, Soma L, Chen X, Yeung C, Wood B, Li D, Cao J, Heimfeld S, Jensen MC, Riddell SR, Maloney DG, CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients, The Journal of clinical investigation (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, Yuan C, Zhang H, Zhang L, Rosenberg SA, Wayne AS, Mackall CL, T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial, Lancet 385(9967) (2015) 517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].US Food and Drug Administration, FDA approves tisagenlecleucel for B-cell ALL and tocilizumab for cytokine release syndrome, 2017. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm574154.htm. (Accessed July 27th 2019).
- [28].US Food and Drug Administration, FDA approves tisagenlecleucel for adults with relapsed or refractory large B-cell lymphoma, 2018. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm606540.htm. (Accessed July 27th 2019).
- [29].European Medicines Agency, Kymriah, 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/kymriah. (Accessed July 27th 2019).
- [30].Health Canada, Regulatory Decision Summary for Kymriah (Control no. 213547), 2018. https://hpr-rps.hres.ca/reg-content/regulatory-decision-summary-detail.php?linkID=RDS00423. (Accessed 2019.
- [31].Health Canada, Regulatory Decision Summary for Kymriah (Control no. 213698), 2018. https://hpr-rps.hres.ca/reg-content/regulatory-decision-summary-detail.php?linkID=RDS00422. (Accessed July 27th 2019).
- [32].Swissmedic, Kymriah TM, Zellsuspension zur Infusion (Tisagenlecleucelum), 2018. https://www.swissmedic.ch/swissmedic/en/home/humanarzneimittel/authorisations/authorised-medicinal-products-with-new-active-substances/kymriahtm_zellsuspensionzurinfusiontisagenlecleucelum.html. (Accessed July 27th 2019).
- [33].Therapeutic Goods Administration, Inclusions of new biologicaIs, 2018. https://www.tga.gov.au/inclusions-new-biologicals. (Accessed July 27th 2019).
- [34].Oxford Biomedica, Oxford Biomedica notes the Japanese approval of Kymriah® (tisagenlecleucel), the first CAR-T cell therapy authorised in Asia, 2019. https://www.oxfordbiomedica.co.uk/news-media/press-release/oxford-biomedica-notes-iapanese-approval-kymriah%C2%AE-tisagenlecleucel-first. (Accessed July 27th 2019).
- [35].European Medicines Agency, Yescarta, 2019. https://www.ema.europa.eu/en/medicines/human/EPAR/yescarta. (Accessed July 27th 2019).
- [36].Health Canada, Regulatory Decision Summary - Yescarta - Health Canada, 2019. https://hpr-rps.hres.ca/reg-content/regulatory-decision-summary-detail.php?lang=en&linkID=RDS00486. (Accessed July 27th 2019).
- [37].Swissmedic, Yescarta®, 0,4 – 2 x 108 Zellen Infusionsdispersion (axicabtagene ciloleucel), 2019. https://www.swissmedic.ch/swissmedic/en/home/humanarzneimittel/authorisations/authorised-medicinal-products-with-new-active-substances/yescarta_zellen_infusionsdispersion_axicabtagene_ciloleucel.html. (Accessed September 28th 2019).
- [38].Gyurkocza B, Rezvani A, Storb RF, Allogeneic hematopoietic cell transplantation: the state of the art, Expert review of hematology 3(3) (2010) 285–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Marmont AM, Horowitz MM, Gale RP, Sobocinski K, Ash RC, van Bekkum DW, Champlin RE, Dicke KA, Goldman JM, Good RA, et al. , T-cell depletion of HLA-identical transplants in leukemia, Blood 78(8) (1991) 2120–30. [PubMed] [Google Scholar]
- [40].Baron F, Storb R, Allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning as treatment for hematologic malignancies and inherited blood disorders, Mol Ther 13(1) (2006) 26–41. [DOI] [PubMed] [Google Scholar]
- [41].Chang X, Zang X, Xia CQ, New strategies of DLI in the management of relapse of hematological malignancies after allogeneic hematopoietic SCT, Bone Marrow Transplant 51(3) (2016) 324–32. [DOI] [PubMed] [Google Scholar]
- [42].Friedmann T, A brief history of gene therapy, Nature genetics 2(2) (1992) 93–8. [DOI] [PubMed] [Google Scholar]
- [43].Kuwana Y, Asakura Y, Utsunomiya N, Nakanishi M, Arata Y, Itoh S, Nagase F, Kurosawa Y, Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions, Biochem Biophys Res Commun 149(3) (1987) 960–8. [DOI] [PubMed] [Google Scholar]
- [44].Gross G, Waks T, Eshhar Z, Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity, Proceedings of the National Academy of Sciences of the United States of America 86(24) (1989) 10024–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Eshhar Z, Waks T, Gross G, Schindler DG, Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors, Proceedings of the National Academy of Sciences of the United States of America 90(2) (1993) 720–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Irving BA, Weiss A, The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways, Cell 64(5) (1991) 891–901. [DOI] [PubMed] [Google Scholar]
- [47].Romeo C, Seed B, Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides, Cell 64(5) (1991) 1037–46. [DOI] [PubMed] [Google Scholar]
- [48].Letourneur F, Klausner RD, T-cell and basophil activation through the cytoplasmic tail of T-cell-receptor zeta family proteins, Proceedings of the National Academy of Sciences of the United States of America 88(20) (1991) 8905–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, et al. , Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report, The New England journal of medicine 319(25) (1988) 1676–80. [DOI] [PubMed] [Google Scholar]
- [50].Rosenberg SA, Aebersold P, Cornetta K, Kasid A, Morgan RA, Moen R, Karson EM, Lotze MT, Yang JC, Topalian SL, et al. , Gene transfer into humans--immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction, N Engl J Med 323(9) (1990) 570–8. [DOI] [PubMed] [Google Scholar]
- [51].Hwu P, Shafer GE, Treisman J, Schindler DG, Gross G, Cowherd R, Rosenberg SA, Eshhar Z, Lysis of ovarian cancer cells by human lymphocytes redirected with a chimeric gene composed of an antibody variable region and the Fc receptor gamma chain, The Journal of experimental medicine 178(1) (1993) 361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hwu P, Yang JC, Cowherd R, Treisman J, Shafer GE, Eshhar Z, Rosenberg SA, In vivo antitumor activity of T cells redirected with chimeric antibody/T-cell receptor genes, Cancer research 55(15) (1995) 3369–73. [PubMed] [Google Scholar]
- [53].Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA, Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes, Science (New York, N.Y.) 298(5594) (2002) 850–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Laport GG, Levine BL, Stadtmauer EA, Schuster SJ, Luger SM, Grupp S, Bunin N, Strobl FJ, Cotte J, Zheng Z, Gregson B, Rivers P, Vonderheide RH, Liebowitz DN, Porter DL, June CH, Adoptive transfer of costimulated T cells induces lymphocytosis in patients with relapsed/refractory non-Hodgkin lymphoma following CD34+-selected hematopoietic cell transplantation, Blood 102(6) (2003) 2004–13. [DOI] [PubMed] [Google Scholar]
- [55].Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP, Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy, Trends Immunol 26(2) (2005) 111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Maus MV, June CH, Making Better Chimeric Antigen Receptors for Adoptive T-cell Therapy, Clinical cancer research : an official journal of the American Association for Cancer Research 22(8) (2016) 1875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Moritz D, Wels W, Mattern J, Groner B, Cytotoxic T lymphocytes with a grafted recognition specificity for ERBB2-expressing tumor cells, Proceedings of the National Academy of Sciences of the United States of America 91(10) (1994) 4318–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gong MC, Latouche JB, Krause A, Heston WD, Bander NH, Sadelain M, Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen, Neoplasia 1(2) (1999) 123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].McGuinness RP, Ge Y, Patel SD, Kashmiri SV, Lee HS, Hand PH, Schlom J, Finer MH, McArthur JG, Anti-tumor activity of human T cells expressing the CC49-zeta chimeric immune receptor, Human gene therapy 10(2) (1999) 165–73. [DOI] [PubMed] [Google Scholar]
- [60].Weijtens ME, Willemsen RA, Valerio D, Stam K, Bolhuis RL, Single chain Ig/gamma gene-redirected human T lymphocytes produce cytokines, specifically lyse tumor cells, and recycle lytic capacity, Journal of immunology (Baltimore, Md. : 1950) 157(2) (1996) 836–43. [PubMed] [Google Scholar]
- [61].Nolan KF, Yun CO, Akamatsu Y, Murphy JC, Leung SO, Beecham EJ, Junghans RP, Bypassing immunization: optimized design of “designer T cells” against carcinoembryonic antigen (CEA)-expressing tumors, and lack of suppression by soluble CEA, Clinical cancer research : an official journal of the American Association for Cancer Research 5(12) (1999) 3928–41. [PubMed] [Google Scholar]
- [62].Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, King PD, Larson S, Weiss M, Riviere I, Sadelain M, Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15, Nature medicine 9(3) (2003) 279–86. [DOI] [PubMed] [Google Scholar]
- [63].Cooper LJ, Topp MS, Serrano LM, Gonzalez S, Chang WC, Naranjo A, Wright C, Popplewell L, Raubitschek A, Forman SJ, Jensen MC, T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect, Blood 101(4) (2003) 1637–44. [DOI] [PubMed] [Google Scholar]
- [64].Wang J, Press OW, Lindgren CG, Greenberg P, Riddell S, Qian X, Laugen C, Raubitschek A, Forman SJ, Jensen MC, Cellular immunotherapy for follicular lymphoma using genetically modified CD20-specific CD8+ cytotoxic T lymphocytes, Molecular therapy : the journal of the American Society of Gene Therapy 9(4) (2004) 577–86. [DOI] [PubMed] [Google Scholar]
- [65].Hombach A, Muche JM, Gerken M, Gellrich S, Heuser C, Pohl C, Sterry W, Abken H, T cells engrafted with a recombinant anti-CD30 receptor target autologous CD30(+) cutaneous lymphoma cells, Gene therapy 8(11) (2001) 891–5. [DOI] [PubMed] [Google Scholar]
- [66].Altenschmidt U, Kahl R, Moritz D, Schnierle BS, Gerstmayer B, Wels W, Groner B, Cytolysis of tumor cells expressing the Neu/erbB-2, erbB-3, and erbB-4 receptors by genetically targeted naive T lymphocytes, Clinical cancer research : an official journal of the American Association for Cancer Research 2(6) (1996) 1001–8. [PubMed] [Google Scholar]
- [67].Kahlon KS, Brown C, Cooper LJ, Raubitschek A, Forman SJ, Jensen MC, Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells, Cancer research 64(24) (2004) 9160–6. [DOI] [PubMed] [Google Scholar]
- [68].Roberts MR, Qin L, Zhang D, Smith DH, Tran AC, Dull TJ, Groopman JE, Capon DJ, Byrn RA, Finer MH, Targeting of human immunodeficiency virus-infected cells by CD8+ T lymphocytes armed with universal T-cell receptors, Blood 84(9) (1994) 2878–89. [PubMed] [Google Scholar]
- [69].Scholler J, Brady TL, Binder-Scholl G, Hwang WT, Plesa G, Hege KM, Vogel AN, Kalos M, Riley JL, Deeks SG, Mitsuyasu RT, Bernstein WB, Aronson NE, Levine BL, Bushman FD, June CH, Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells, Science translational medicine 4(132) (2012) 132ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Levine BL, Bernstein WB, Connors M, Craighead N, Lindsten T, Thompson CB, June CH, Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells, Journal of immunology (Baltimore, Md.: 1950) 159(12) (1997) 5921–30. [PubMed] [Google Scholar]
- [71].Levine BL, Cotte J, Small CC, Carroll RG, Riley JL, Bernstein WB, Van Epps DE, Hardwick RA, June CH, Large-scale production of CD4+ T cells from HIV-1-infected donors after CD3/CD28 costimulation, Journal of hematotherapy 7(5) (1998) 437–48. [DOI] [PubMed] [Google Scholar]
- [72].Levine BL, Bernstein WB, Aronson NE, Schlienger K, Cotte J, Perfetto S, Humphries MJ, Ratto-Kim S, Birx DL, Steffens C, Landay A, Carroll RG, June CH, Adoptive transfer of costimulated CD4+ T cells induces expansion of peripheral T cells and decreased CCR5 expression in HIV infection, Nature medicine 8(1) (2002) 47–53. [DOI] [PubMed] [Google Scholar]
- [73].Bernstein WB, Cox JH, Aronson NE, Tracy L, Schlienger K, Ratto-Kim S, Garner R, Cotte J, Zheng Z, Winestone L, Liebig C, Galley LM, Connors M, Birx DL, Carroll RG, Levine BL, Immune reconstitution following autologous transfers of CD3/CD28 stimulated CD4(+) T cells to HIV-infected persons, Clinical immunology (Orlando, Fla.) 111(3) (2004) 262–74. [DOI] [PubMed] [Google Scholar]
- [74].Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X, Binder GK, Slepushkin V, Lemiale F, Mascola JR, Bushman FD, Dropulic B, June CH, Gene transfer in humans using a conditionally replicating lentiviral vector, Proceedings of the National Academy of Sciences of the United States of America 103(46) (2006) 17372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L, A third-generation lentivirus vector with a conditional packaging system, Journal of virology 72(11) (1998) 8463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].McGarrity GJ, Hoyah G, Winemiller A, Andre K, Stein D, Blick G, Greenberg RN, Kinder C, Zolopa A, Binder-Scholl G, Tebas P, June CH, Humeau LM, Rebello T, Patient monitoring and follow-up in lentiviral clinical trials, The journal of gene medicine 15(2) (2013) 78–82. [DOI] [PubMed] [Google Scholar]
- [77].Cornetta K, Duffy L, Turtle CJ, Jensen M, Forman S, Binder-Scholl G, Fry T, Chew A, Maloney DG, June CH, Absence of Replication-Competent Lentivirus in the Clinic: Analysis of Infused T Cell Products, Molecular therapy: the journal of the American Society of Gene Therapy 26(1) (2018) 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Marcucci KT, Jadlowsky JK, Hwang WT, Suhoski-Davis M, Gonzalez VE, Kulikovskaya I, Gupta M, Lacey SF, Plesa G, Chew A, Melenhorst JJ, Levine BL, June CH, Retroviral and Lentiviral Safety Analysis of Gene-Modified T Cell Products and Infused HIV and Oncology Patients, Molecular therapy: the journal of the American Society of Gene Therapy 26(1) (2018) 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lyon D, Lapteva N, Gee AP, Absence of Replication-Competent Retrovirus in Vectors, T Cell Products, and Patient Follow-Up Samples, Molecular therapy: the journal of the American Society of Gene Therapy 26(1) (2018) 6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Heinrich T, Rengstl B, Muik A, Petkova M, Schmid F, Wistinghausen R, Warner K, Crispatzu G, Hansmann ML, Herling M, von Laer D, Newrzela S, Mature T-cell lymphomagenesis induced by retroviral insertional activation of Janus kinase 1, Molecular therapy: the journal of the American Society of Gene Therapy 21(6) (2013) 1160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Donahue RE, Kessler SW, Bodine D, McDonagh K, Dunbar C, Goodman S, Agricola B, Byrne E, Raffeld M, Moen R, et al. , Helper virus induced T cell lymphoma in nonhuman primates after retroviral mediated gene transfer, The Journal of experimental medicine 176(4) (1992) 1125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Sadelain M, Brentjens R, Riviere I, The promise and potential pitfalls of chimeric antigen receptors, Current opinion in immunology 21(2) (2009) 215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Bretscher P, Cohn M, A theory of self-nonself discrimination, Science (New York, N.Y.) 169(3950) (1970) 1042–9. [DOI] [PubMed] [Google Scholar]
- [84].Chen L, Ashe S, Brady WA, Hellstrom I, Hellstrom KE, Ledbetter JA, McGowan P, Linsley PS, Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4, Cell 71(7) (1992) 1093–102. [DOI] [PubMed] [Google Scholar]
- [85].Baskar S, Ostrand-Rosenberg S, Nabavi N, Nadler LM, Freeman GJ, Glimcher LH, Constitutive expression of B7 restores immunogenicity of tumor cells expressing truncated major histocompatibility complex class II molecules, Proc Natl Acad Sci U S A 90(12) (1993) 5687–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M, Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor, Nature biotechnology 20(1) (2002) 70–5. [DOI] [PubMed] [Google Scholar]
- [87].Friedmann-Morvinski D, Bendavid A, Waks T, Schindler D, Eshhar Z, Redirected primary T cells harboring a chimeric receptor require costimulation for their antigen-specific activation, Blood 105(8) (2005) 3087–93. [DOI] [PubMed] [Google Scholar]
- [88].Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N, Smith DD, Forman SJ, Jensen MC, Cooper LJ, CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells, Cancer research 66(22) (2006) 10995–1004. [DOI] [PubMed] [Google Scholar]
- [89].Roessig C, Scherer SP, Baer A, Vormoor J, Rooney CM, Brenner MK, Juergens H, Targeting CD19 with genetically modified EBV-specific human T lymphocytes, Annals of hematology 81 Suppl 2 (2002) S42–3. [PubMed] [Google Scholar]
- [90].Huang X, Guo H, Kang J, Choi S, Zhou TC, Tammana S, Lees CJ, Li ZZ, Milone M, Levine BL, Tolar J, June CH, Scott McIvor R, Wagner JE, Blazar BR, Zhou X, Sleeping Beauty transposon-mediated engineering of human primary T cells for therapy of CD19+ lymphoid malignancies, Molecular therapy: the journal of the American Society of Gene Therapy 16(3) (2008) 580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Scheuermann RH, Racila E, CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy, Leukemia & lymphoma 18(5–6) (1995) 385–97. [DOI] [PubMed] [Google Scholar]
- [92].Suhoski MM, Golovina TN, Aqui NA, Tai VC, Varela-Rohena A, Milone MC, Carroll RG, Riley JL, June CH, Engineering artificial antigen-presenting cells to express a diverse array of costimulatory molecules, Molecular therapy: the journal of the American Society of Gene Therapy 15(5) (2007) 981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Zhang H, Snyder KM, Suhoski MM, Maus MV, Kapoor V, June CH, Mackall CL, 4–1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy, Journal of immunology (Baltimore, Md.: 1950) 179(7) (2007) 4910–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Maus MV, Thomas AK, Leonard DG, Allman D, Addya K, Schlienger K, Riley JL, June CH, Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4–1BB, Nature biotechnology 20(2) (2002) 143–8. [DOI] [PubMed] [Google Scholar]
- [95].Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH, T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia, Science translational medicine 3(95) (2011) 95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Rosenbaum L, Tragedy, Perseverance, and Chance - The Story of CAR-T Therapy, The New England journal of medicine 377(14) (2017) 1313–1315. [DOI] [PubMed] [Google Scholar]
- [97].Penn Medicine News, University of Pennsylvania and Novartis Form Alliance to Expand Use of Personalized T Cell Therapy for Cancer Patients, 2012. https://www.pennmedicine.org/news/news-releases/2012/august/university-of-pennsylvania-and. (Accessed July 27th 2019).
- [98].Gilead, Kite Pharma Partners with the National Cancer Institute to Develop Novel Cellular Immunotherapy Clinical Products, 2012. https://www.gilead.com/news-and-press/press-room/press-releases/2012/10/kite-pharma-partners-with-the-national-cancer-institute-to-develop-novel-cellular-immunotherapy-clinical-products. (Accessed July 27th 2019).
- [99].Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, Ambrose D, Grupp SA, Chew A, Zheng Z, Milone MC, Levine BL, Melenhorst JJ, June CH, Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia, Science translational medicine 7(303) (2015) 303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA, Chimeric antigen receptor T cells for sustained remissions in leukemia, The New England journal of medicine 371(16) (2014) 1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Maude SL, Pulsipher MA, Boyer MW, Grupp SA, Davies SM, Phillips CL, Verneris MR, August KJ, Schlis K, Driscoll TA, Mody R, Capitini CM, June CH, Levine BL, Wood PA, Yi L, Levine JE, Efficacy and Safety of CTL019 in the First US Phase II Multicenter Trial in Pediatric Relapsed/Refractory Acute Lymphoblastic Leukemia: Results of an Interim Analysis, 128(22) (2016) 2801–2801. [Google Scholar]
- [102].Mueller KT, Waldron E, Grupp SA, Levine JE, Laetsch TW, Pulsipher MA, Boyer MW, August KJ, Hamilton J, Awasthi R, Stein AM, Sickert D, Chakraborty A, Levine BL, June CH, Tomassian L, Shah SS, Leung M, Taran T, Wood PA, Maude SL, Clinical Pharmacology of Tisagenlecleucel in B-cell Acute Lymphoblastic Leukemia, Clinical cancer research: an official journal of the American Association for Cancer Research 24(24) (2018) 6175–6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Stein AM, Grupp SA, Levine JE, Laetsch TW, Pulsipher MA, Boyer MW, August KJ, Levine BL, Tomassian L, Shah S, Leung M, Huang PH, Awasthi R, Mueller KT, Wood PA, June CH, Tisagenlecleucel Model-Based Cellular Kinetic Analysis of Chimeric Antigen Receptor-T Cells, CPT: pharmacometrics & systems pharmacology 8(5) (2019) 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak O, Brogdon JL, Pruteanu-Malinici I, Bhoj V, Landsburg D, Wasik M, Levine BL, Lacey SF, Melenhorst JJ, Porter DL, June CH, Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas, The New England journal of medicine (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jager U, Jaglowski S, Andreadis C, Westin JR, Fleury I, Bachanova V, Foley SR, Ho PJ, Mielke S, Magenau JM, Holte H, Pantano S, Pacaud LB, Awasthi R, Chu J, Anak O, Salles G, Maziarz RT, Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma, The New England journal of medicine 380(1) (2019) 45–56. [DOI] [PubMed] [Google Scholar]
- [106].US Food and Drug Administration, Clinical Review - KYMRIAH, 2017. https://www.fda.gov/media/107973/download. (Accessed October 13th 2019).
- [107].Penn Medicine News, University of Pennsylvania’s Personalized Cellular Therapy for Leukemia Receives FDA’s Breakthrough Therapy Designation, 2014. https://www.pennmedicine.org/news/news-releases/2014/yuly/university-of-pennsylvanias-pe. (Accessed October 13th 2019).
- [108].US Food and Drug Administration Center for Drug Evaluation and Research, Summary Minutes of the Oncologic Drugs Advisory Committee, July 12th, 2017. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM573719.pdf (Accessed July 27th 2019).
- [109].Gilead, Kite Pharma Receives FDA Breakthrough Therapy Designation for KTE-C19 for the Treatment of Refractory, Aggressive Non Hodgkin Lymphoma (NHL), 2015. https://www.gilead.com/news-and-press/press-room/press-releases/2015/12/kite-pharma-receives-fda-breakthrough-therapy-designation-for-ktec19-for-the-treatment-of-refractory-aggressive-non-hodgkin-lymphoma-nhl. (Accessed October 13th 2019).
- [110].US Food and Drug Administration, FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma, 2017. https://www.fda.gov/news-events/press-announcements/fda-approves-car-t-cell-therapy-treat-adults-certain-types-large-b-cell-lymphoma. (Accessed July 27th 2019).
- [111].Novartis, Find a Kymriah treatment center, 2019. https://www.us.kymriah.com/treatment-center-locator/. (Accessed September 28th 2019).
- [112].Kite Pharma, WHERE CAN YESCARTA BE RECEIVED?, 2019. https://www.yescarta.com/treatment-centers. (Accessed September 28th 2019).
- [113].Cuende N, Rasko JEJ, Koh MBC, Dominici M, Ikonomou L, Cell, tissue and gene products with marketing authorization in 2018 worldwide, Cytotherapy 20(11) (2018) 1401–1413. [DOI] [PubMed] [Google Scholar]
- [114].Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, Bleakley M, Brown C, Mgebroff S, Kelly-Spratt KS, Hoglund V, Lindgren C, Oron AP, Li D, Riddell SR, Park JR, Jensen MC, Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults, Blood 129(25) (2017) 3322–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Ruella M, Maus MV, Catch me if you can: Leukemia Escape after CD19-Directed T Cell Immunotherapies, Comput Struct Biotechnol J 14 (2016) 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Shah NN, Fry TJ, Mechanisms of resistance to CAR T cell therapy, Nature reviews. Clinical oncology (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Mueller KT, Maude SL, Porter DL, Frey N, Wood P, Han X, Waldron E, Chakraborty A, Awasthi R, Levine BL, Melenhorst JJ, Grupp SA, June CH, Lacey SF, Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia, Blood 130(21) (2017) 2317–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Ruella M, Xu J, Barrett DM, Fraietta JA, Reich TJ, Ambrose DE, Klichinsky M, Shestova O, Patel PR, Kulikovskaya I, Nazimuddin F, Bhoj VG, Orlando EJ, Fry TJ, Bitter H, Maude SL, Levine BL, Nobles CL, Bushman FD, Young RM, Scholler J, Gill SI, June CH, Grupp SA, Lacey SF, Melenhorst JJ, Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell, Nature medicine 24(10) (2018) 1499–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Evans AG, Rothberg PG, Burack WR, Huntington SF, Porter DL, Friedberg JW, Liesveld JL, Evolution to plasmablastic lymphoma evades CD19-directed chimeric antigen receptor T cells, Br J Haematol (2015). [DOI] [PubMed] [Google Scholar]
- [120].Yu H, Sotillo E, Harrington C, Wertheim G, Paessler M, Maude SL, Rheingold SR, Grupp SA, Thomas-Tikhonenko A, Pillai V, Repeated loss of target surface antigen after immunotherapy in primary mediastinal large B cell lymphoma, American journal of hematology 92(1) (2017) E11–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Shalabi H, Kraft IL, Wang HW, Yuan CM, Yates B, Delbrook C, Zimbelman JD, Giller R, Stetler-Stevenson M, Jaffe ES, Lee DW, Shern JF, Fry TJ, Shah NN, Sequential loss of tumor surface antigens following chimeric antigen receptor T-cell therapies in diffuse large B-cell lymphoma, Haematologica 103(5) (2018) e215–e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Ruella M, Barrett DM, Kenderian SS, Shestova O, Hofmann TJ, Perazzelli J, Klichinsky M, Aikawa V, Nazimuddin F, Kozlowski M, Scholler J, Lacey SF, Melenhorst JJ, Morrissette JJ, Christian DA, Hunter CA, Kalos M, Porter DL, June CH, Grupp SA, Gill S, Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies, The Journal of clinical investigation (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S, Delbrook C, Yates B, Shalabi H, Fountaine TJ, Shern JF, Majzner RG, Stroncek DF, Sabatino M, Feng Y, Dimitrov DS, Zhang L, Nguyen S, Qin H, Dropulic B, Lee DW, Mackall CL, CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy, Nature medicine 24(1) (2018) 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Qin H, Ramakrishna S, Nguyen S, Fountaine TJ, Ponduri A, Stetler-Stevenson M, Yuan CM, Haso W, Shern JF, Shah NN, Fry TJ, Preclinical Development of Bivalent Chimeric Antigen Receptors Targeting Both CD19 and CD22, Molecular therapy oncolytics 11 (2018) 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, Boesteanu AC, Wang Y, O’Connor RS, Hwang WT, Pequignot E, Ambrose DE, Zhang C, Wilcox N, Bedoya F, Dorfmeier C, Chen F, Tian L, Parakandi H, Gupta M, Young RM, Johnson FB, Kulikovskaya I, Liu L, Xu J, Kassim SH, Davis MM, Levine BL, Frey NV, Siegel DL, Huang AC, Wherry EJ, Bitter H, Brogdon JL, Porter DL, June CH, Melenhorst JJ, Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia, Nature medicine 24(5) (2018) 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS, Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition, The Journal of clinical investigation 126(8) (2016) 3130–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Goronzy JJ, Weyand CM, Successful and Maladaptive T Cell Aging, Immunity 46(3) (2017) 364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Fraietta JA, Beckwith KA, Patel PR, Ruella M, Zheng Z, Barrett DM, Lacey SF, Melenhorst JJ, McGettigan SE, Cook DR, Zhang C, Xu J, Do P, Hulitt J, Kudchodkar SB, Cogdill AP, Gill S, Porter DL, Woyach JA, Long M, Johnson AJ, Maddocks K, Muthusamy N, Levine BL, June CH, Byrd JC, Maus MV, Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia, Blood 127(9) (2016) 1117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Sadelain M, Riviere I, Riddell S, Therapeutic T cell engineering, Nature 545(7655) (2017) 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Ruella M, Kenderian SS, Shestova O, Klichinsky M, Melenhorst JJ, Wasik MA, Lacey SF, June CH, Gill S, Kinase inhibitor ibrutinib to prevent cytokine-release syndrome after anti-CD19 chimeric antigen receptor T cells for B-cell neoplasms, Leukemia 31(1) (2017) 246–248. [DOI] [PubMed] [Google Scholar]
- [131].Gill S, Vides V, Frey NV, Metzger S, O’Brien M, Hexner E, Mato AR, Lacey SF, Melenhorst J, Pequignot E, Gladney WL, Hwang W-T, Lamontagne A, Davis M, Byrd JC, Schuster SJ, Siegel DL, Isaacs RE, June CH, Porter DL, Prospective Clinical Trial of Anti-CD19 CAR T Cells in Combination with Ibrutinib for the Treatment of Chronic Lymphocytic Leukemia Shows a High Response Rate, Blood vol. 132 no. Suppl 1 298 (2018). [Google Scholar]
- [132].Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine BL, June CH, Schuster SJ, PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR, Blood 129(8) (2017) 1039–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Chong EA, Svoboda J, Nasta SD, Landsburg DJ, Winchell N, Napier E, Mato AR, Melenhorst JJ, Ruella M, Lacey SF, June CH, Schuster SJ, Sequential Anti-CD19 Directed Chimeric Antigen Receptor Modified T-Cell Therapy (CART19) and PD-1 Blockade with Pembrolizumab in Patients with Relapsed or Refractory B-Cell Non-Hodgkin Lymphomas, Blood vol. 132 no. Suppl 1 4198 (2018). [Google Scholar]
- [134].Li AM, Hucks GE, Dinofia AM, Seif AE, Teachey DT, Baniewicz D, Callahan C, Fasano C, McBride B, Gonzalez V, Nazimuddin F, Porter DL, Lacey SF, June CH, Grupp SA, Maude SL, Checkpoint Inhibitors Augment CD19-Directed Chimeric Antigen Receptor (CAR) T Cell Therapy in Relapsed B-Cell Acute Lymphoblastic Leukemia, Blood vol. 132 no. Suppl 1 556 (2018). [Google Scholar]
- [135].Yu JX, Hubbard-Lucey VM, Tang J, The global pipeline of cell therapies for cancer, 2019. https://www.nature.com/articles/d41573-019-00090-z. (Accessed July 31th 2019). [DOI] [PubMed]
- [136].Levine BL, Miskin J, Wonnacott K, Keir C, Global Manufacturing of CAR T Cell Therapy, Molecular therapy. Methods & clinical development 4 (2017) 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Zhang W, Jordan KR, Schulte B, Purev E, Characterization of clinical grade CD19 chimeric antigen receptor T cells produced using automated CliniMACS Prodigy system, Drug design, development and therapy 12 (2018) 3343–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Lu TL, Pugach O, Somerville R, Rosenberg SA, Kochenderfer JN, Better M, Feldman SA, A Rapid Cell Expansion Process for Production of Engineered Autologous CAR-T Cell Therapies, Human gene therapy methods 27(6) (2016) 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Levine BL, Fesnak AD, Riviere I, Showcasing Clinical Development and Production of Cellular Therapies, Molecular therapy: the journal of the American Society of Gene Therapy 25(4) (2017) 827–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Qasim W, Zhan H, Samarasinghe S, Adams S, Amrolia P, Stafford S, Butler K, Rivat C, Wright G, Somana K, Ghorashian S, Pinner D, Ahsan G, Gilmour K, Lucchini G, Inglott S, Mifsud W, Chiesa R, Peggs KS, Chan L, Farzeneh F, Thrasher AJ, Vora A, Pule M, Veys P, Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells, Science translational medicine 9(374) (2017). [DOI] [PubMed] [Google Scholar]
- [141].Torikai H, Reik A, Liu PQ, Zhou Y, Zhang L, Maiti S, Huls H, Miller JC, Kebriaei P, Rabinovitch B, Lee DA, Champlin RE, Bonini C, Naldini L, Rebar EJ, Gregory PD, Holmes MC, Cooper LJ, A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR, Blood 119(24) (2012) 5697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y, Multiplex Genome Editing to Generate Universal CAR T Cells Resistant to PD1 Inhibition, Clinical cancer research: an official journal of the American Association for Cancer Research 23(9) (2017) 2255–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Eyquem J, Mansilla-Soto J, Giavridis T, van der Stegen SJ, Hamieh M, Cunanan KM, Odak A, Gonen M, Sadelain M, Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection, Nature 543(7643) (2017) 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Torikai H, Reik A, Soldner F, Warren EH, Yuen C, Zhou Y, Crossland DL, Huls H, Littman N, Zhang Z, Tykodi SS, Kebriaei P, Lee DA, Miller JC, Rebar EJ, Holmes MC, Jaenisch R, Champlin RE, Gregory PD, Cooper LJ, Toward eliminating HLA class I expression to generate universal cells from allogeneic donors, Blood 122(8) (2013) 1341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Ren J, Zhang X, Liu X, Fang C, Jiang S, June CH, Zhao Y, A versatile system for rapid multiplex genome-edited CAR T cell generation, Oncotarget (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Werner Sunderland M, Peggs KS, Successful translation and future prospects of TALEN editing for leukemia patients, Expert opinion on biological therapy 18(7) (2018) 725–726. [DOI] [PubMed] [Google Scholar]
- [147].Watanabe N, Bajgain P, Sukumaran S, Ansari S, Heslop HE, Rooney CM, Brenner MK, Leen AM, Vera JF, Fine-tuning the CAR spacer improves T-cell potency, Oncoimmunology 5(12) (2016) e1253656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, Varela-Rohena A, Haines KM, Heitjan DF, Albelda SM, Carroll RG, Riley JL, Pastan I, June CH, Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains, Proceedings of the National Academy of Sciences of the United States of America 106(9) (2009) 3360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Chmielewski M, Abken H, CAR T cells transform to trucks: chimeric antigen receptor-redirected T cells engineered to deliver inducible IL-12 modulate the tumour stroma to combat cancer, Cancer Immunol Immunother 61(8) (2012) 1269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Kim MY, Yu KR, Kenderian SS, Ruella M, Chen S, Shin TH, Aljanahi AA, Schreeder D, Klichinsky M, Shestova O, Kozlowski MS, Cummins KD, Shan X, Shestov M, Bagg A, Morrissette JJD, Sekhri P, Lazzarotto CR, Calvo KR, Kuhns DB, Donahue RE, Behbehani GK, Tsai SQ, Dunbar CE, Gill S, Genetic Inactivation of CD33 in Hematopoietic Stem Cells to Enable CAR T Cell Immunotherapy for Acute Myeloid Leukemia, Cell 173(6) (2018) 1439–1453.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Minagawa K, Jamil MO, Al-Obaidi M, Pereboeva L, Salzman D, Erba HP, Lamb LS, Bhatia R, Mineishi S, Di Stasi A, In Vitro Pre-Clinical Validation of Suicide Gene Modified Anti-CD33 Redirected Chimeric Antigen Receptor T-Cells for Acute Myeloid Leukemia, PloS one 11(12) (2016) e0166891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Tasian SK, Kenderian SS, Shen F, Ruella M, Shestova O, Kozlowski M, Li Y, Schrank-Hacker A, Morrissette JJ, Carroll M, June CH, Grupp SA, Gill S, Optimized Depletion of Chimeric Antigen Receptor T-Cells in Murine Xenograft Models of Human Acute Myeloid Leukemia, Blood (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Wu CY, Roybal KT, Puchner EM, Onuffer J, Lim WA, Remote control of therapeutic T cells through a small molecule-gated chimeric receptor, Science (New York, N.Y.) 350(6258) (2015) aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Mamonkin M, Mukherjee M, Srinivasan M, Sharma S, Gomes-Silva D, Mo F, Krenciute G, Orange JS, Brenner MK, Reversible Transgene Expression Reduces Fratricide and Permits 4–1BB Costimulation of CAR T Cells Directed to T-cell Malignancies, Cancer immunology research 6(1) (2018) 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Rodgers DT, Mazagova M, Hampton EN, Cao Y, Ramadoss NS, Hardy IR, Schulman A, Du J, Wang F, Singer O, Ma J, Nunez V, Shen J, Woods AK, Wright TM, Schultz PG, Kim CH, Young TS, Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies, Proceedings of the National Academy of Sciences of the United States of America 113(4) (2016) E459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Arcangeli S, Rotiroti MC, Bardelli M, Simonelli L, Magnani CF, Biondi A, Biagi E, Tettamanti S, Varani L, Balance of Anti-CD123 Chimeric Antigen Receptor Binding Affinity and Density for the Targeting of Acute Myeloid Leukemia, Molecular therapy: the journal of the American Society of Gene Therapy 25(8) (2017) 1933–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Thokala R, Olivares S, Mi T, Maiti S, Deniger D, Huls H, Torikai H, Singh H, Champlin RE, Laskowski T, McNamara G, Cooper LJ, Redirecting Specificity of T cells Using the Sleeping Beauty System to Express Chimeric Antigen Receptors by Mix-and-Matching of VL and VH Domains Targeting CD123+ Tumors, PloS one 11(8) (2016) e0159477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Drent E, Themeli M, Poels R, de Jong-Korlaar R, Yuan H, de Bruijn J, Martens ACM, Zweegman S, van de Donk N, Groen RWJ, Lokhorst HM, Mutis T, A Rational Strategy for Reducing On-Target Off-Tumor Effects of CD38-Chimeric Antigen Receptors by Affinity Optimization, Molecular therapy: the journal of the American Society of Gene Therapy 25(8) (2017) 1946–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M, Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells, Nature biotechnology 31(1) (2013) 71–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Lanitis E, Poussin M, Klattenhoff AW, Song D, Sandaltzopoulos R, June CH, Powell DJ Jr., Chimeric antigen receptor T Cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo, Cancer immunology research 1(1) (2013) 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Wilkie S, van Schalkwyk MC, Hobbs S, Davies DM, van der Stegen SJ, Pereira AC, Burbridge SE, Box C, Eccles SA, Maher J, Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling, Journal of clinical immunology 32(5) (2012) 1059–70. [DOI] [PubMed] [Google Scholar]
- [162].Ruella M, Klichinsky M, Kenderian SS, Shestova O, Ziober A, Kraft DO, Feldman M, Wasik MA, June CH, Gill S, Overcoming the Immunosuppressive Tumor Microenvironment of Hodgkin Lymphoma Using Chimeric Antigen Receptor T Cells, Cancer Discov 7(10) (2017) 1154–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Maziarz RT, Guerin A, Gauthier G, Heroux J, Zhdanava M, Wu EQ, Thomas SK, Chen L, Five-year direct costs of acute lymphoblastic leukemia pediatric patients undergoing allogeneic stem cell transplant, International journal of hematologic oncology 5(2) (2016) 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Maziarz RT, Hao Y, Guerin A, Gauthier G, Gauthier-Loiselle M, Thomas SK, Eldjerou L, Economic burden following allogeneic hematopoietic stem cell transplant in patients with diffuse large B-cell lymphoma, Leukemia & lymphoma 59(5) (2018) 1133–1142. [DOI] [PubMed] [Google Scholar]
- [165].Couzin-Frankel J, Beyond survival, Science (New York, N.Y.) 363(6432) (2019) 1166–1169. [DOI] [PubMed] [Google Scholar]
- [166].Lin JK, Lerman BJ, Barnes JI, Boursiquot BC, Tan YJ, Robinson AQL, Davis KL, Owens DK, Goldhaber-Fiebert JD, Cost Effectiveness of Chimeric Antigen Receptor T-Cell Therapy in Relapsed or Refractory Pediatric B-Cell Acute Lymphoblastic Leukemia, Journal of clinical oncology: official journal of the American Society of Clinical Oncology (2018) Jco2018790642. [DOI] [PubMed] [Google Scholar]
- [167].Tang J, Hubbard-Lucey VM, Pearce L, O’Donnell-Tormey J, Shalabi A, The global landscape of cancer cell therapy, Nature reviews. Drug discovery 17(7) (2018) 465–466. [DOI] [PubMed] [Google Scholar]
- [168].Kansagra AJ, Frey NV, Bar M, Laetsch TW, Carpenter PA, Savani BN, Heslop HE, Bollard CM, Komanduri KV, Gastineau DA, Chabannon C, Perales MA, Hudecek M, Aljurf M, Andritsos L, Barrett JA, Bachanova V, Bonini C, Ghobadi A, Gill SI, Hill JA, Kenderian S, Kebriaei P, Nagler A, Maloney D, Liu HD, Shah NN, Kharfan-Dabaja MA, Shpall EJ, Mufti GJ, Johnston L, Jacoby E, Bazarbachi A, DiPersio JF, Pavletic SZ, Porter DL, Grupp SA, Sadelain M, Litzow MR, Mohty M, Hashmi SK, Clinical utilization of Chimeric Antigen Receptor T-cells (CAR-T) in B-cell acute lymphoblastic leukemia (ALL)-an expert opinion from the European Society for Blood and Marrow Transplantation (EBMT) and the American Society for Blood and Marrow Transplantation (ASBMT), Bone marrow transplantation (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Gilead, Gilead Sciences to Acquire Kite Pharma for $11.9 Billion, 2017. https://www.gilead.com/news-and-press/press-room/press-releases/2017/8/gilead-sciences-to-acquire-kite-pharma-for-119-billion. (Accessed October 13th 2019).
- [170].The Economist Intelligence Unit, Bristol-Myers drives into CAR-T therapies, 2019. https://www.eiu.com/industry/article/817665265/bristol-myers-drives-into-car-t-therapies/2019-02-18. (Accessed August 3rd 2019).
- [171].van Bruggen JAC, Martens AWJ, Fraietta JA, Hofland T, Tonino SH, Eldering E, Levin MD, Siska PJ, Endstra S, Rathmell JC, June CH, Porter DL, Melenhorst JJ, Kater AP, van der Windt GJW, Chronic lymphocytic leukemia cells impair mitochondrial fitness in CD8(+) T cells and impede CAR T-cell efficacy, Blood 134(1) (2019) 44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Garfall AL, Maus MV, Hwang WT, Lacey SF, Mahnke YD, Melenhorst JJ, Zheng Z, Vogl DT, Cohen AD, Weiss BM, Dengel K, Kerr ND, Bagg A, Levine BL, June CH, Stadtmauer EA, Chimeric Antigen Receptor T Cells against CD19 for Multiple Myeloma, The New England journal of medicine 373(11) (2015) 1040–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Garfall AL, Stadtmauer EA, Hwang WT, Lacey SF, Melenhorst JJ, Krevvata M, Carroll MP, Matsui WH, Wang Q, Dhodapkar MV, Dhodapkar K, Das R, Vogl DT, Weiss BM, Cohen AD, Mangan PA, Ayers EC, Nunez-Cruz S, Kulikovskaya I, Davis MM, Lamontagne A, Dengel K, Kerr ND, Young RM, Siegel DL, Levine BL, Milone MC, Maus MV, June CH, Anti-CD19 CAR T cells with high-dose melphalan and autologous stem cell transplantation for refractory multiple myeloma, JCI insight 3(8) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]