Abstract
Background
Patients with heart failure (HF) with preserved ejection fraction (HFpEF) and obesity display a number of pathophysiologic features that may render them more or less vulnerable to negative effects of decongestion on renal function, including greater right ventricular remodeling, plasma volume expansion, and pericardial restraint. We aimed to contrast the renal response to decongestion in obese compared to non-obese HFpEF
Methods and Results
NIH heart failure network studies that enrolled patients with acute decompensated HFpEF (EF≥50%) were included [DOSE, CARRESS, ROSE and ATHENA]. Obese HFpEF was defined as a body mass index (BMI) ≥30 kg/m2. Compared to non-obese HFpEF (n=118), patients with obese HFpEF (n=214) were an average of 9 years younger (71 vs 80 years,<0.001), with more diabetes (64% vs 31%, p<0.001) but less atrial fibrillation (56% vs 75%, p<0.001). Renal dysfunction (GFR<60 mL/min/1.73m2) was present in 82% of patients, with no difference at baseline in obese and non-obese patients. Despite similar weight loss with decongestive therapies, obese HFpEF patients demonstrated a greater rise in creatinine(Cr) and decline in GFR, with a two-fold higher incidence of mild worsening renal function (rise in Cr≥0.3 mg/dl) (28 vs 14%, p=0.008) and a substantially greater increase in severe worsening of renal function (rise in Cr>0.5mg/dl) (9 vs 0%, p=0.002).
Conclusion
Despite being nearly a decade younger, obese HFpEF patients experience greater deterioration in renal function during decongestion compared to non-obese HFpEF. Further study to elucidate the complex relationships between volume distribution, cardiorenal hemodynamics and adiposity in HFpEF are needed.
Keywords: heart failure, HFpEF, obesity, hospitalization
Introduction
Obesity is associated with inflammation, neurohormonal activation and plasma volume expansion.(1,2) Patients with heart failure (HF) with preserved ejection fraction (HFpEF) and obesity display a number of pathophysiologic features that differentiate them from non-obese patients, including greater plasma volume expansion, right ventricular (RV) dysfunction, cardiomegaly, and pericardial restraint.(3) This mileau sets the stage for enhanced ventricular interaction, whereby RV overload (as with decompensation) interferes with left ventricular (LV) filling to impair cardiac output.
In patients with HF with reduced EF (HFrEF), acute reduction in RV volumes with maneuvers to reduce venous return improves LV filling and cardiac output by decreasing pericardial restraint and ventricular interdependence.(4,5) This would suggest that patients with the obese phenotype of HFpEF, who display this physiology, may respond more favorably to decongestion in the setting of decompensated heart failure. Conversely, patients with obesity often display reduction in systemic vascular resistance,(6) which may lead to arterial underfilling, enhanced sodium retention and greater plasma volume expansion to maintain adequate intravascular volume and cardiac preload.(1,2,7) Aggressive decongestion in such patients might destabilize cardiorenal function if the cardiovascular system is somehow reliant on maintenance of a hypervolemic state in obesity.
In order to determine if there is a differential response to decongestion in patients with the obese phenotype of HFpEF, we examined patients who were hospitalized for acute decompensated HF participating in the prospective inpatient studies that included HFpEF conducted within the NHLBI-sponsored HF clinical research network.(8–11)
Methods
Study subjects
The DOSE, CARRESS, ROSE and ATHENA trials were multicenter, randomized clinical trials conducted conducted by the NHLBI-sponsored Heart Failure Clinical Research Network in hospitalized HF patients testing diuretic infusion strategies, ultrafiltration compared to conventional therapy, addition of dopamine/nesiritide to diuretic therapy, and high dose spironolactone in tandem with diuretic therapy.(8–11) Participants from these studies with unequivocal decompensated HF and an EF≥50% were included in this analysis.
Patient Assessment
All participants underwent history and physical examination by a cardiologist prior to receipt of study intervention, wherein functional class was determined using the NYHA classification. The evaluating cardiologist was blinded to individual treatment assignment. Serum NT-proBNP concentration was measured prospectively in all patients at study entry along with serum creatinine. Estimated glomerular filtration rate was determined by the Modification of Diet in Renal Disease equation. The baseline data reported from these studies was prior to receipt of study medications. Plasma volume was estimated by: (1 − hematocrit) × (a + [b × weight in kg]), where a = 1,530 in men and 864 in women, and b = 41 in men and 47.9 in women.(12) This ancillary study was designed and approved by the Heart Failure Network (HFN) ancillary studies committee and all analyses were completed by the HFN Coordinating Center.
Group assignment
Obese HFpEF was defined as a body mass index (BMI) ≥30 kg/m2 and non-obese HFpEF as a BMI<30 kg/m2. To mirror previously-utilized definitions,(3) sensitivity analyses defining obese HFpEF with grade 2 obesity as a BMI ≥35 kg/m2, and stratified by World Health Organization BMI categories were also performed.
Outcome assessment
Worsening Renal Function (WRF) was defined as a 0.3mg/dl or greater increase in serum creatinine during hospitalization. In addition, severe WRF was defined as a 0.5 mg/dl or greater increase in serum creatinine. Clinical characteristics, response to decongestion therapies, and death and rehospitalization rates at 30 days were compared.
Statistical Analysis
Categorical variables were summarized by frequencies with percentages, and compared using Chi square or Fisher’s exact test. Continuous variables were summarized by the median, 25th and 75th percentiles, and compared using Kruskal-Wallis test. Thirty day outcomes were compared using Cox proportional Hazard models adjusted for trial, age, sex, heart rate and blood pressure. All statistical tests were two-sided, and a p value of <0.05 was considered statistically significant. Given the exploratory nature of the analyses, adjustment for multiple comparisons was not performed. All the statistical analyses were performed using SAS statistical software (version 9.4, SAS Institute, Cary, NC).
Results
Study sample
A total of 332 patients hospitalized with decompensated HFpEF across the 4 trials (ROSE 107, DOSE 79, CARRESS 58, ATHENA 88) were included. The overall prevalence of obesity, defined by BMI≥30 kg/m2, was 64%. The proportion of obese HFpEF patients was similar across all 4 trials (ROSE 62%, DOSE 66%, CARRESS 69%, ATHENA 64%). There was no significant difference in prevalence of obesity between the placebo and treatment groups across each of the 4 study samples (p>0.1 for all, data not shown).
Baseline demographics
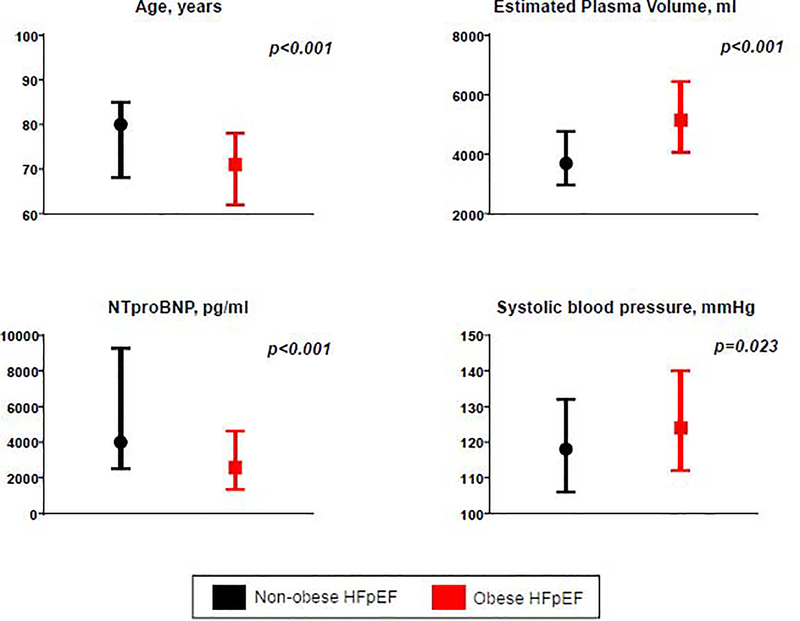
Compared to non-obese HFpEF (n=118), patients with obese HFpEF (n=214) were an average of 9 years younger at the time of HF hospitalization and tended to be more likely to be female and of non-white enthnicity (Table 1, Figure 1). Obese HFpEF patients were more likely to have had a prior HF hospitalization in the past year (67 vs 56%, p=0.043) and had a two-fold greater prevalence of diabetes mellitus (64 vs 31%, p<0.001). In contrast, atrial fibrillation was less common in obese HFpEF as compared to non-obese HFpEF (56 vs 75%, p<0.001). Blood pressure was higher in obese HFpEF patients and prevalence of hypertension tended to be higher (Figure 1, Table 1).
Table 1:
Baseline Characteristics
| Non-Obese HFpEF (BMI<30) (N=118) | Obese HFpEF (BMI≥30) (N=214) | p-value | |
|---|---|---|---|
| Age, years | 80 (68 – 85) | 71 (62 – 78) | <0.001 |
| Female, n (%) | 41 (35) | 97 (45) | 0.061 |
| White race, n (%) | 100 (85) | 163 (76) | 0.065 |
| Anthropometries and Vital Signs | |||
| Weight, lbs | 167 (149–185) | 243 (215–286) | <0.001 |
| Body Mass Index, kg/m2 | 26.8 (24.4 –28.0) | 38.0 (34.1–45.4) | <0.001 |
| Heart rate, bpm | 75 (66 –81) | 72 (64–82) | 0.44 |
| Systolic Blood Pressure, mmHg | 118 (106 –132) | 124 (112–140) | 0.023 |
| Disease Severity and Ventricular Function | |||
| Hospitalization for HF in past year, n (%) | 65 (56) | 141 (67) | 0.043 |
| Ejection Fraction, % | 58 (55–63) | 55 (55–63) | 0.68 |
| Comorbidites | |||
| Hypertension, n (%) | 100 (85) | 194 (91) | 0.11 |
| Atrial fibrillation/flutter, n (%) | 88 (75) | 119 (56) | <.001 |
| Diabetes, n (%) | 37 (31) | 137 (64) | <.001 |
| Estimated Glomerular Filtration Rate | 0.81 | ||
| <30 mL/min/1.73 m2 | 22 (19) | 46 (22) | |
| 30–59 mL/min/1.73m2 | 74 (63) | 128 (60) | |
| >60 mL/min/1.73m2 | 22 (19) | 39 (18) | |
| Medications on admission | |||
| ACE inhibitor/ARB, n (%) | 45 (28) | 100 (47) | 0.13 |
| Beta blocker, n (%) | 91 (77) | 154 (72) | 0.31 |
| Aldosterone antagonist, n (%) | 20 (17) | 35 (17) | 0.92 |
| Diuretic, n (%) | 113 (96) | 206 (96) | 0.78 |
| Calcium channel blocker, n (%) | 37 (31) | 67 (32) | 0.99 |
Values represent Median (25th–75th) or % unless otherwise specified
Figure 1: Clinical characteristics of obese decompensated HFpEF.
Obese HFpEF patients were younger with higher systolic blood pressure which would be expected to protect against renal hypoperfusion. In addition, they had greater estimated plasma volume despite lower NT-proBNP levels.
Renal function at the time of enrollment was similar in obese and non-obese patients, with 82% demonstrating an impaired glomerular filtration rate (GFR) <60 mL/min/1.73m2 consistent with stage 3 chronic kidney disease or worse (Tables 1 and 2). Renal function was similar between groups even after adjusting for age, race, diabetes and hypertension (p=0.45). Estimated plasma volume was 40% higher in the obese HFpEF group as compared to non-obese HFpEF (Figure 1), with greater severity of peripheral edema, but similar prevalence of jugular vein distention and orthopnea, exceeding 90% in both groups. Most patients were functional class III or IV, with no difference between the obese and non-obese HFpEF groups. Despite greater plasma volume expansion and peripheral edema in the obese HFpEF group, NT proBNP levels were lower compared to non-obese patients with HFpEF (2577[1328–4613] vs 4006[2519–9271] pg/ml, p<0.001) (Table 2).
Table 2:
Severity of Congestion and Laboratories
| Non-Obese HFpEF (BMI<30) (N=118) | Obese HFpEF (BMI≥30) (N=214) | p-value | |
|---|---|---|---|
| Indicators of Congestion | |||
| Estimated plasma volume, ml | 3685 (2954–4765) | 5147 (4053–6450) | <0.001 |
| Edema ≥2+, n (%) | 86 (73) | 177 (84) | 0.022 |
| Jugular vein distention ≥8 cm, n (%) | 110 (93) | 198 (93) | 0.95 |
| Orthopnea, n (%) | 107 (92) | 196 (93) | 0.64 |
| NYHA Functional Class at Assessment | 0.54 | ||
| II, n (%) | 9 (8) | 11 (6) | |
| III, n (%) | 76 (67) | 124 (65) | |
| IV, n (%) | 28 (25) | 57 (30) | |
| Laboratory values | |||
| Sodium, mEq/L | 139 (136 – 141) | 140 (137 – 142) | 0.009 |
| Hemoglobin, g/dl | 10.8 (9.9 – 11.9) | 10.6 (9.7 –12.2) | 0.73 |
| Blood urea nitrogen, mg/dl | 38 (25 – 50) | 33 (23 – 53) | 0.39 |
| GFR,mL/min/1.73m2 | 44 (33 – 54) | 42 (32 – 56) | 0.62 |
| Serum Creatinine, mg/dl | 1.5 (1.2 – 1.9) | 1.6 (1.2 – 2.1) | 0.70 |
| NT-pro BNP, pg/ml | 4006 (2519 – 9271) | 2577 (1328 – 4613) | <.001 |
Values represent Median (25th–75th) or % unless otherwise specified
Response to decongestion
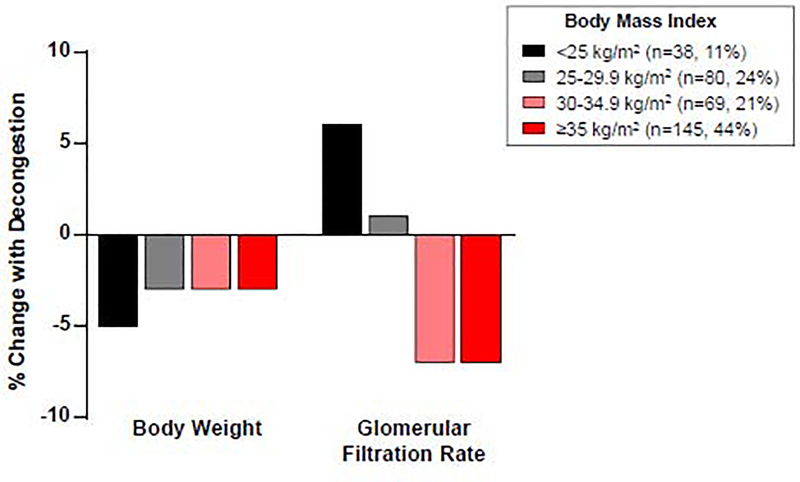
Median length of stay was 6 (4, 8) days, with no difference between groups (p=0.37, Table 3). Despite similar degrees of absolute weight loss, and less proportional weight loss, obese HFpEF patients demonstrated a greater rise in creatinine and greater decline in estimated GFR (Figure 2, Table 3). There was a two-fold higher incidence of worsening renal function during decongestion (28 vs 14%, p=0.008) and a substantially greater incidence of severe worsening renal function (rise in creatinine≥0.5 mg/dl) in obese HFpEF (9 vs 0%, p=0.002). Although baseline blood pressure was higher in the obese HFpEF patients, the blood pressure decline during diuresis was similar in both groups.
Table 3:
Renal Response to Decongestion
| Non-Obese HFpEF (BMI<30) (N=118) | Obese HFpEF (BMI≥30) (N=214) | p-value | |
|---|---|---|---|
| Diuretic dose at Randomization, mg/daya | 80 (40, 160) | 80 (40, 160) | 0.39 |
| Length of Stay, Days | 5 (3, 8) | 6 (4, 8) | 0.37 |
| Changes in Renal Function | |||
| Change in Creatinine, mg/dl | −0.02 (−0.20, 0.12) | +0.10 (−0.10, 0.30) | <0.001 |
| % Change in Creatinine | −2 (−12, 11) | +6 (−6, 20) | <0.001 |
| Worsening renal function (rise in Cr≥0.3 mg/dl), n (%) | 16 (14) | 56 (28) | 0.008 |
| Worsening renal function (rise in Cr≥0.5 mg/dl), n (%) | 0 (0) | 17 (9) | 0.002 |
| Change in eGFR, mL/min/1.73m2 | 0.7 (−5.1, 5.4) | −2.6 (−8.6, 2.8) | 0.002 |
| % Change in eGFR | 2 (−11, 16) | −7 (−19, 7) | <0.001 |
| Estimates of Decongestion | |||
| Change in Weight, lb | −6 (−11, −3) | −7 (−14, −2) | 0.23 |
| % Change in Weight | −4 (−7, −2) | −3 (−5, −1) | 0.04 |
| Change in NT proBNP, pg/ml | −1046 (−2153, −83) | −583 (−1561, −106) | 0.16 |
| % Change in NT proBNP | −24 (−43, −6) | −35 (−57, −6) | 0.058 |
| Hemodynamic Changes | |||
| Change in systolic blood pressure, mmHg | −6 (−20, 6) | −2 (−18, 7) | 0.39 |
| Change in mean blood pressure, mmHg | −5 (−15, 3) | −3 (−13, 8) | 0.29 |
Values represent Median (25th–75th) or % unless otherwise specified
Dose in intravenous furosemide equivalents
Figure 2: Renal response to decongestion in obese HFpEF.
Despite similar weight loss with diuresis in HFpEF patients with increasing body mass index, there was greater reduction in glomerular filtration rate with decongestion as compared to non-obese patients.
Clinical Outcomes
At 30 days, the overall mortality rate was 5.2% and rehospitalization rate was 22% (Table 4). Despite the differences in worsening renal function, there was no difference in survival or rehospitalization in obese as compared to non-obese HFpEF patients.
Table 4:
Clinical Outcomes at 30 Days
| Non-Obese HFpEF (BMI<30) (N=118) | Obese HFpEF (BMI≥30) (N=214) | p-value | |
|---|---|---|---|
| Mortality event rate, % (95% CI)a | 6.5 (3.2, 13.2) | 4.5 (2.4, 8.6) | 0.49 |
| Rehospitalization event rate, %(95% CI)a | 25 (17, 34) | 21 (16, 28) | 0.54 |
| Death or rehospitalization event rate, % (95% CI)a | 27 (19, 36) | 24 (18, 31) | 0.62 |
Values represent Median (25th–75th) or % unless otherwise specified
Comparisons adjusted for trial, age, sex, heart rate and blood pressure
Sensitivity analysis
In a sensitivity analysis evaluating patients with Grade 2 or higher obesity compared to non-obese HFpEF, results were similar, with a 2-fold greater incindence of worsening renal function during decongestion in obese HFpEF (Supplemental table 1). Stratification of results by diabetes showed results directionally similar to the primary analysis, with a greater decline in estimated GFR with diuresis in obesity, which tended to be somewhat greater in obese patients with diabetes (Supplemental table 2). Additional sensitivity analyses stratified by World Health Organization BMI categorization (Supplemental table 3) and comparing normal weight HFpEF patients with severe (≥grade II) obesity (Supplemental table 4) produced similar results.
Discussion
The major findings of our study can be summarized as follows: 1) among decompensated patients with HFpEF admitted for volume overload, patients with the obese phenotype are, on average, nearly a decade younger than non-obese HFpEF patients, 2) despite younger age, nearly 80% of obese HFpEF patients have a glomerular filtration rate consistent with at least stage 3 chronic kidney disease, and most significantly 3) decongestion was less well tolerated from a renal filtration standpoint in obese HFpEF, with a greater proportion experiencing worsening renal function, despite similar to lesser magnitude of net decongestion reflected by weight loss.
This study demonstrates a potentially important difference in the response to decongestion in patients with HFpEF based upon the presence or absence of obesity. Because patients with the obese phenotype of HFpEF display greater plasma volume expansion, more profound cardiomegaly, and heightended extrinsic pericardial restraint on the heart,(3) one could posit that decongestion would enhance renal function by reducing RV volumes to mitigate external restraint more in this cohort, improving LV filling, cardiac performance and by extension, end-organ perfusion. This phenomenon is well-described in patients with advanced HFrEF, where reduction in cardiac venous return improves LV preload and stroke volume because of enhanced diastolic ventricular interaction.(4,5) Reduction in pericardial restraint with decongestion would be expected to lower central venous pressure and reduce renal congestion,(5) with secondary improvements in renal function, as has been observed in experimental preparations(13) and from clinical studies of HF with reduced ejection fraction.(14,15)
Instead, we observed that obese HFpEF patients were two-fold more likely to develop worsening renal function with decongestion and an even more substantially greater likelihood of developing more substantial worsening renal function, despite similar absolute weight loss and lower relative weight loss. This is particularly notable since obese HFpEF patients had greater estimated plasma volume expansion and peripheral edema. The mechanism for the differential response cannot be discerned from these data, but a number of possibilities merit consideration.
Obese HFpEF patients displayed a 2-fold greater burden of diabetes, and it is notable that despite being a decade younger, their baseline GFR was impaired to a similar degree as older non-obese HFpEF patients Since GFR is known to decrease with aging, this implies an earlier onset of kidney disease in obese HFpEF. There is increasing recognition that obesity causes clinical HFpEF at a younger age, where a greater burden of chronic kidney disease has been described.(16,17) The current data further validates the potential impact of obesity on presentation with decompensated volume overload HFpEF at a younger age, in contrast to non-obese HFpEF patients where aging and atrial fibrillation may be important contributors to decompensation.
Glomerular filtration in HF is determined by the combination of intrinsic renal dysfunction, as well as reversible renal hypoperfusion caused by the combination of poor renal arterial perfusion and renal venous congestion.(18) In addition to potential intrinsic diabetic nephropathy, obesity has itself been directly implicated in causing renal dysfunction and glomerular pathology which may occur through a number of mechanisms including glomerular hyperfiltration, adipose mediated inflammation, oxidative stress and adipokine release, renin-angiotensin system activation and ectopic adipose accumulation.(19,20) Increased perinephric fat(21) in addition to heightened abdominal pressure related to increased visceral fat(22) could have a negative effect on renal function in obesity, and children and adults with obesity demonstrate improvements in renal function with weight loss.(23,24) While the blood pressure decline with diuresis was similar in obese and non-obese HFpEF, the former group displayed higher baseline pressure, and it is possible that obese patients may be more reliant on maintaining a higher renal perfusion pressure, and that this could contribute to the observed differences in renal response to decongestion.
In addition to potential differences in intrinsic renal reserve, obese HFpEF patients may be more reliant on volume expansion than non-obese patients. Total blood volume increases monotonically with body weight, but the ratio of volume to body weight decreases with obesity.(7) A recent study including a number of patients with obese HFpEF sugggested that the vast majority of volume lost during diuresis originated from the interstitium rather than the vascular space.(25) If there is insufficient capillary refill to reconstitute vascular volume from the interstitium, this could destabilize cardiorenal homeostasis. This is supported by a recent trial in hospitalized patients with HFpEF (mean BMI 40.8), where continuous furosemide infusion caused a greater rise in creatinine when compared to intermittent bolus diuretic.(26) In HFrEF, diuresis and vasodilation do not necessarily compromise cardiac output,(27) but this may not hold in patients with HFpEF, who display marked diastolic LV chamber stiffening and are more likely to display reduction in stroke volume as LV preload is reduced. This preload sensitivity in combination with frequently observed decreased systemic vascular resistance in obesity(6) may predispose to renal hemodynamic compromise during diuresis. (28)
In this study as well as others, the creatinine elevation was prognostically benign, with no difference in 30 day event rates in the obese and non-obese HFpEF patients. Emerging evidence suggests that such transient elevations in creatinine during diuresis reflect changes in intraglomerular hemodynamics rather than true tubular injury, and the benefits of greater decongestion may outweigh any potentially negative effect on the kidneys.(29,30) While this small elevation in creatinine should not necessarily deter further diuresis for patients that remain clinically congested, these data suggest that closer monitoring may be warranted among patients with obese HFpEF. This may include use of invasive hemodynamic assessment when volume status is uncertain with rising creatinine, or use of implantable pressure monitoring,(31) particularly given the difficulties of physical examination estimates of hemodynamics in obesity. It also is reflective of the worse renal hemodynamic tolerance to diuresis in obese HFpEF which is particularly notable given their younger age, and emphasize our incomplete understanding of the complexity of cardiorenal interactions in obesity-related HFpEF. Further study of the role of novel renal protective agents such as sodium glucose cotransporter 2 inhibitors(32) require investigation and clinical trials in HFpEF are ongoing () ()
Limitations
This was a secondary analysis of prior randomized trials and therefore the results are prone to type 1 error. More sophisticated measures of adiposity were not collected, and how these findings relate to the relatively crude estimate of adiposity using BMI in terms of renal response to diuresis requires additional investigation. Plasma volume was not directly measured but rather estimated using a previously validated equation, though this is less accurate compared to direct assessments.(12) Assessment of proteinuria or changes in hemoglobin during therapy were not available. Mineralocorticoid antagonist use was rather low (17%) and the current data cannot address how decongestion might relate to obesity in patients with more aggressive use of these drugs. Given the exploratory nature of analyses performed, correction for multiple hypothesis testing was not performed.
Conclusions
Hospitalized patients with the obese phenotype of HFpEF experience greater acute decline in renal function with decongestion, despite similar weight loss and greater degree of volume overload as compared to non-obese HFpEF patients. These data suggests that closer monitoring may be warranted in this population during treatment for volume overload, and call for further study to elucidate the complex relationships between volume distribution, cardiorenal hemodynamics and adiposity in HFpEF.
Supplementary Material
Highlights.
In this pooled analysis from 4 clinical trials, patients with obese HFpEF were nearly a decade younger than non-obese HFpEF when presenting with decompensated HF requiring hospitalization.
Despite the younger age, obese HFpEF patients experienced greater deterioration in renal function during decongestion.
Given the high prevalence of impaired renal function in hospitalized HFpEF patients, future research into the complex relationships between adiposity, hemodynamics and renal hemodynamics in obese HFpEF is urgently needed.
Acknowledgements
Dr. Borlaug is supported by RO1 HL128526 and U10 HL110262. Dr. Reddy was supported by T32 HL007111. Drs. Reddy, Obokata, Stevenson, Redfield and Borlaug were supported by training grant U10HL110337 from the National Heart, Lung, and Blood Institute.
Abbreviations list
- ATHENA-HF
Aldosterone Targeted NeuroHormonal CombinEd with Natriuresis TherApy in Heart Failure
- DOSE
Diuretic Optimization Strategies Evaluation
- ROSEL
Renal Optimization Strategies Evaluation
- CARRESS-HF
Cardiorenal Rescue Study in Acute Decompensated Heart Failure
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- RV
right ventricular
- LV
left ventricular
- WRF
Worsening renal function
- GFR
Glomerular filtration rate
Footnotes
Disclosures: None
TRIAL REGISTRATIONS
Aldosterone Targeted NeuroHormonal CombinEd with Natriuresis TherApy in Heart Failure (ATHENA-HF) clinicaltrials.gov Identifier: ; Diuretic Optimization Strategies Evaluation (DOSE), ClinicalTrials.gov number, ; Renal Optimization Strategies Evaluation (ROSE), Clinicaltrials.gov Identifier: ; Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF), ClinicalTrials.gov Identifier, .
Relationship with industry: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Packer M Leptin-Aldosterone-Neprilysin Axis: Identification of Its Distinctive Role in the Pathogenesis of the Three Phenotypes of Heart Failure in People With Obesity. Circulation 2018;137:1614–1631. [DOI] [PubMed] [Google Scholar]
- 2.Alexander JK, Dennis EW, Smith WG, Amad KH, Duncan WC, Austin RC. Blood volume, cardiac output, and distribution of systemic blood flow in extreme obesity. Cardiovasc Res Cent Bull 1962;1:39–44. [PubMed] [Google Scholar]
- 3.Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atherton JJ, Moore TD, Lele SS et al. Diastolic ventricular interaction in chronic heart failure. Lancet 1997;349:1720–4. [DOI] [PubMed] [Google Scholar]
- 5.Borlaug BA, Reddy YNV. The Role of the Pericardium in Heart Failure: Implications for Pathophysiology and Treatment. JACC Heart Fail 2019;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy YN, Melenovsky V, Redfield MM, Nishimura RA, Borlaug BA. High-Output Heart Failure: A 15-Year Experience. J Am Coll Cardiol 2016;68:473–82. [DOI] [PubMed] [Google Scholar]
- 7.Feldschuh J, Enson Y. Prediction of the normal blood volume. Relation of blood volume to body habitus. Circulation 1977;56:605–12. [DOI] [PubMed] [Google Scholar]
- 8.Felker GM, Lee KL, Bull DA et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011;364:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bart BA, Goldsmith SR, Lee KL et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen HH, Anstrom KJ, Givertz MM et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013;310:2533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler J, Anstrom KJ, Felker GM et al. Efficacy and Safety of Spironolactone in Acute Heart Failure: The ATHENA-HF Randomized Clinical Trial. JAMA Cardiol 2017;2:950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling HZ, Flint J, Damgaard M et al. Calculated plasma volume status and prognosis in chronic heart failure. Eur J Heart Fail 2015;17:35–43. [DOI] [PubMed] [Google Scholar]
- 13.Doty JM, Saggi BH, Sugerman HJ et al. Effect of increased renal venous pressure on renal function. J Trauma 1999;47:1000–3. [DOI] [PubMed] [Google Scholar]
- 14.Mullens W, Abrahams Z, Francis GS et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009;53:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Testani JM, Khera AV, St John Sutton MG et al. Effect of right ventricular function and venous congestion on cardiorenal interactions during the treatment of decompensated heart failure. Am J Cardiol 2010;105:511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tromp J, MacDonald M, Tay WT et al. Heart Failure with Preserved Ejection Fraction in the Young. Circulation 2018;138:2763–2773. [DOI] [PubMed] [Google Scholar]
- 17.Zacharias M, Joffe S, Konadu E et al. Clinical epidemiology of heart failure with preserved ejection fraction (HFpEF) in comparatively young hospitalized patients. Int J Cardiol 2016;202:918–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zannad F, Rossignol P. Cardiorenal Syndrome Revisited. Circulation 2018;138:929–944. [DOI] [PubMed] [Google Scholar]
- 19.D’Agati VD, Chagnac A, de Vries AP et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol 2016;12:453–71. [DOI] [PubMed] [Google Scholar]
- 20.Tsuboi N, Okabayashi Y, Shimizu A, Yokoo T. The Renal Pathology of Obesity. Kidney Int Rep 2017;2:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamacchia O, Nicastro V, Camarchio D et al. Para- and perirenal fat thickness is an independent predictor of chronic kidney disease, increased renal resistance index and hyperuricaemia in type-2 diabetic patients. Nephrol Dial Transplant 2011;26:892–8. [DOI] [PubMed] [Google Scholar]
- 22.Lambert DM, Marceau S, Forse RA. Intra-abdominal pressure in the morbidly obese. Obes Surg 2005;15:1225–32. [DOI] [PubMed] [Google Scholar]
- 23.Inge TH, Laffel LM, Jenkins TM et al. Comparison of Surgical and Medical Therapy for Type 2 Diabetes in Severely Obese Adolescents. JAMA Pediatr 2018;172:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bilha SC, Nistor I, Nedelcu A et al. The Effects of Bariatric Surgery on Renal Outcomes: a Systematic Review and Meta-analysis. Obes Surg 2018;28:3815–3833. [DOI] [PubMed] [Google Scholar]
- 25.Miller WL, Mullan BP. Understanding the heterogeneity in volume overload and fluid distribution in decompensated heart failure is key to optimal volume management: role for blood volume quantitation. JACC Heart Fail 2014;2:298–305. [DOI] [PubMed] [Google Scholar]
- 26.Sharma K, Vaishnav J, Kalathiya R et al. Randomized Evaluation of Heart Failure With Preserved Ejection Fraction Patients With Acute Heart Failure and Dopamine: The ROPA-DOP Trial. JACC Heart Fail 2018;6:859–870. [DOI] [PubMed] [Google Scholar]
- 27.Stevenson LW, Tillisch JH. Maintenance of cardiac output with normal filling pressures in patients with dilated heart failure. Circulation 1986;74:1303–8. [DOI] [PubMed] [Google Scholar]
- 28.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol 2012;59:442–51. [DOI] [PubMed] [Google Scholar]
- 29.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 2010;122:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmad T, Jackson K, Rao VS et al. Worsening Renal Function in Patients With Acute Heart Failure Undergoing Aggressive Diuresis Is Not Associated With Tubular Injury. Circulation 2018;137:2016–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adamson PB, Abraham WT, Bourge RC et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail 2014;7:935–44. [DOI] [PubMed] [Google Scholar]
- 32.Wanner C, Inzucchi SE, Lachin JM et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med 2016;375:323–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.