Abstract
Purpose:
To examine the prevalence of, and factors associated with, atypical antipsychotic use among U.S. pregnant women, and potential associations between early pregnancy atypical antipsychotic use and risk for 14 birth defects.
Methods:
We analyzed data from the National Birth Defects Prevention Study (1997–2011), a U.S. population-based case-control study examining risk factors for major structural birth defects.
Results:
Atypical antipsychotic use during pregnancy was more common among women with pre-pregnancy obesity, and women who reported illicit drug use before and during pregnancy, smoking during pregnancy, alcohol use during pregnancy, or use of other psychiatric medications during pregnancy. We observed elevated associations (defined as a crude odds ratio [cOR] ≥2.0) between early pregnancy atypical antipsychotic use and conotruncal heart defects (6 exposed cases; cOR: 2.3, 95% confidence interval [CI]: 0.9–6.1), and more specifically Tetralogy of Fallot (3 exposed cases; cOR: 2.5, 95% CI: 0.7–8.8), cleft palate (4 exposed cases; cOR: 2.5, 95% CI: 0.8–7.6), anorectal atresia/stenosis (3 exposed cases; cOR: 2.8, 95% CI: 0.8–9.9), and gastroschisis (3 exposed cases; cOR: 2.1, 95% CI: 0.6–7.3).
Conclusions:
Our findings support the close clinical monitoring of pregnant women using atypical antipsychotics. Women treated with atypical antipsychotics generally access healthcare services before pregnancy; efforts to reduce correlates of atypical antipsychotic use might improve maternal and infant health in this population.
Keywords: antipsychotics, birth defects, mental health, pharmacoepidemiology
1. Introduction
Atypical or second-generation antipsychotic medications are used as first-line treatment to manage primary psychotic disorders and phases of bipolar disorders (ACOG, 2008; Poo and Agius, 2015). These medications are also used as pharmacological adjuncts to manage the symptoms of treatment-resistant unipolar major depression and anxiety disorders (Demler, 2011; Park et al., 2017; Toh et al., 2013). Atypical antipsychotics have fewer neuromuscular side effects than typical first-generation antipsychotics (Leucht et al., 1999), and for this reason, are now more commonly prescribed for mental disorders than typical antipsychotics (Fisher et al., 2014; Park et al., 2017). Onset of these mental health disorders often begins in adolescence or early adulthood, and many of the disorders that can be treated with antipsychotics are more common in women than men (NIMH, 2018). However, the prevalence of atypical antipsychotic use among the population cumulatively affected by these mental health disorders in the United States is largely unknown.
Previous studies suggest a growing proportion of pregnant women are prescribed atypical antipsychotics. In Denmark, filled prescriptions for antipsychotic medications during pregnancy have increased from 1.5 per 1,000 pregnancies to 3.8 per 1,000 pregnancies with a delivery from 2000–2016 (Damkier et al., 2018). In the United States, Park and colleagues (2017) showed an increase in atypical antipsychotic prescription claims among Medicaid-insured U.S. pregnant women between 2001 (0.4%) and 2010 (1.3%). The prevalence of atypical antipsychotic medication use among more representative populations of U.S. pregnant women is unclear, as most available U.S. estimates were drawn from Medicaid-insured populations and based on prescription claims data (Epstein et al., 2013; Park et al., 2017; Toh et al., 2013). The increase in potential use suggests it is important to understand these mothers’ characteristics. Women who use atypical antipsychotics during pregnancy and have mental disorders may have other factors (e.g., unintended pregnancies, pre-pregnancy obesity, diabetes, substance use; Habermann et al., 2013; Park et al., 2017; Petersen et al., 2016; Reis and Kallen, 2008) associated with poor pregnancy and infant outcomes. Pregnant women who use atypical antipsychotics may also more commonly have concomitantly-prescribed psychotropic medications (e.g., anxiolytics, anticonvulsants, antidepressants) (Park et al., 2017; Petersen et al., 2016; Sadowski et al., 2013). Notably, most estimates of maternal factors associated with atypical antipsychotic use are drawn from non-U.S. populations, where prenatal and mental health care differ from the United States.
There is a critical need to understand these medications’ safety during pregnancy given their potentially increased use among pregnant women. Studies have suggested that there may be an increased risk for any major structural birth defect (Bellet et al., 2015; Coughlin et al., 2015; Habermann et al., 2013; Huybrechts et al., 2016; Kulkarni et al., 2014; Petersen et al., 2016; Reis and Kallen, 2008; Sadowski et al., 2013; Terrana et al., 2015) or any structural congenital heart defect (Coughlin et al., 2015; Habermann et al., 2013; Huybrechts et al., 2015) associated with use of antipsychotic medications generally during pregnancy. However, few data are available about atypical antipsychotic medication use specifically during early pregnancy (the period of organogenesis) and the risk for specific birth defects. Examining associations between maternal early pregnancy atypical antipsychotic use and specific birth defects is critical because potential teratogens rarely result in increased risks for all defects; instead, teratogens alter specific developmental processes that result in specific birth defects (Khoury et al., 1992).
We used data from the U.S. population-based National Birth Defects Prevention Study to examine the prevalence of, and maternal factors associated with, atypical antipsychotic medication use among pregnant women. Given limited research on atypical antipsychotic use and risk for specific birth defects, we conducted an exploratory analysis that examined possible associations between early pregnancy atypical antipsychotic use and risk for specific defects.
2. Materials and methods
2.1. Participants
We analyzed data from the National Birth Defects Prevention Study (NBDPS) on pregnancies ending on or after October 1, 1997, through those with an estimated date of delivery (EDD) on or before December 31, 2011. The NBDPS was a population-based, multi-site case-control study that examined risk factors for more than 30 major structural birth defects with unknown etiologies; infants with recognized single-gene disorders or chromosomal abnormalities were excluded (Reefhuis et al., 2015). Ten sites located in Arkansas, California, Georgia, Iowa, Massachusetts, New Jersey, New York, North Carolina, Texas, and Utah collaborated on the NBDPS. Each site ascertained data on pregnancies affected by selected birth defects from active, population-based birth defects surveillance systems using standard, detailed case definitions. Birth defect cases included live births (all sites), stillbirths (all sites except NY before 2000 and NJ), and terminations (all sites except GA before 1999, MA before 2011, NY before 2000, and NJ). Clinical data were abstracted from medical records to confirm that birth defect(s) met eligibility criteria, and clinical geneticists and other expert clinicians then classified cases into homogeneous defect categories using procedures described elsewhere (Rasmussen et al., 2003; Reefhuis et al., 2015). Controls were liveborn infants without major birth defects who were randomly sampled from the same geographic location and study years as birth defect cases using data from hospital birth logs or vital records. More detailed information about the design of NBDPS has been published previously (Rasmussen et al., 2003; Reefhuis et al., 2015). All participating institutions received IRB approval, and consent was obtained.
Mothers were invited to participate in an English or Spanish computer-assisted telephone interview 6 weeks to 24 months after their EDD (participation rate: 67% for case and 65% for control mothers) (Reefhuis et al., 2015). The interview included questions to assess maternal sociodemographic factors, health and pregnancy history, behavioral exposures, and over-the-counter and prescription medication, supplement, and vitamin use. The median time to interview was 11 months for case and 9 months for control mothers. Mothers reported information about medication use (start/stop dates, duration, and frequency) before and during pregnancy using calendar dates or pregnancy months (consecutive 30-day intervals during the time before and during conception). Pregnancy exposure timing was based on the estimated date of conception.
Atypical antipsychotic exposure was defined as maternal report of any use of ≥1 of the following medications: aripiprazole, asenapine, clozapine, iloperidone, lurasidone, olanzapine, paliperidone, quetiapine, risperidone, or ziprasidone. Case and control mothers may have reported medications when asked about any: (a) medications taken before and during pregnancy or (b) diseases or illnesses occurring before and during pregnancy, with subsequent probing for which medications were used to treat the reported condition. Other medication data were collected using the same procedures. Anxiolytic, anticonvulsant, and antidepressant exposure were each defined using the National Institute of Mental Health medication list (NIMH, 2010). The Slone Drug Dictionary (licensed from Boston University) was used to link medication components to corresponding drug products.
2.2. Statistical Analysis
After excluding mothers who did not complete the interview’s medication section (Figure 1), there were 31 651 case mothers and 11 615 control mothers included in the analysis to assess the prevalence of atypical antipsychotic use during pregnancy and factors associated with use. We estimated the prevalence of any atypical antipsychotic use during pregnancy (including the month prior to conception due to imprecision of pregnancy and exposure timing) for case and control mothers separately. We estimated the prevalence of atypical antipsychotic use during pregnancy for two time-periods (1997–2004; 2005–2011) and across each month of pregnancy. To examine associations between maternal factors and atypical antipsychotic use during pregnancy, we calculated crude odds ratios (cOR) and 95% confidence intervals (CIs) using logistic regression. Associations were assessed separately for case and control mothers. We examined maternal age, race/ethnicity, education, previous births, pregnancy intention, pre-pregnancy obesity, folic acid use in the month before conception through the first month of pregnancy, illicit drug use in the three months before conception to pregnancy end, smoking or alcohol use during pregnancy, and anxiolytic, anticonvulsant, or antidepressant use, regardless of indication, during pregnancy.
Figure 1.

Participant selection for the estimation of prevalence and maternal factors associated with atypical antipsychotic use during pregnancy, and associations between early pregnancy atypical antipsychotic use and specific birth defects, National Birth Defects Prevention Study, 1997–2011
aSample used in the calculation of prevalence estimates for atypical antipsychotic medication use during pregnancy and maternal factors associated with use. Pregnancy was defined as the month prior to conception through the end of pregnancy. bAnticonvulsant medications included in this exclusion can be found in the National Institute of Mental Health anticonvulsant medication list (NIMH, 2010). cEarly pregnancy was defined as the month prior to conception through the third month of pregnancy. dTotal sample of case and control mothers included in the analysis of early pregnancy atypical antipsychotic use and its association with birth defects; includes cases and controls exposed and unexposed to atypical antipsychotic medications.
We used logistic regression to calculate cOR and 95% CIs for the association between any early pregnancy (month prior to conception through the third pregnancy month) atypical antipsychotic use and specific selected birth defects. For the birth defect risk analysis, we excluded mothers who reported pre-pregnancy type 1 or 2 diabetes or anticonvulsant medication exposure in early pregnancy, due to their strong association with several birth defects (Correa et al., 2008; Werler et al., 2011). We also excluded mothers who reported atypical antipsychotic use only outside of the exposure window and birth defects with <3 exposed cases. There were 22 387 case and 11 470 control mothers eligible for the birth defect risk analysis (Figure 1). A priori, based on α = 0.05, β = 0.2 (i.e., power=0.80), and prevalence of atypical antipsychotic use during early pregnancy among controls of 0.1%, we estimated our smallest detectable odds ratios to range from 2.51 for any heart defect to 4.17 for anorectal atresia/stenosis (Schlesselman, 1982). Given this, and to better align our analysis with recent American Statistical Association guidelines (Wasserstein et al., 2019) to evaluate findings in light of effect sizes with clinical or scientific relevance based on knowledge of the content area, regardless of a findings’ statistical significance, we considered associations to be elevated if there was a cOR ≥2.0. This threshold for an elevated association was set for all analyses. Analyses were conducted using SPSS 24.0.
3. Results
3.1. Prevalence of Atypical Antipsychotic Medication Use during Pregnancy
The use of atypical antipsychotics during pregnancy was rare; only 0.2% (67/31 651) of case mothers and 0.2% (17/11 615) of control mothers reported atypical antipsychotic medication use at any point during pregnancy. Among exposed case and control mothers, the most commonly reported atypical antipsychotics were similar and included quetiapine (case: 52.2%; control: 52.9%), aripiprazole (case: 23.9%; control: 23.5%), olanzapine (case: 11.9%; control: 17.6%), and risperidone (case: 13.4%; control: 11.8%). Among exposed mothers, less than 3% of case and no control mothers reported exposure to asenapine or ziprasidone. No case or control mothers reported exposure to other atypical antipsychotic medications. Among all exposed mothers, 6.0% (5/84 exposed mothers, including four case mothers and one control mother) took more than one atypical antipsychotic medication during pregnancy.
Despite rare overall use during pregnancy, the proportion of case mothers exposed to atypical antipsychotics increased from 0.1% (13/16 192) during 1997–2004 to 0.4% (54/15 458) during 2005–2011. A more modest increase was observed for control mothers (0.1% [5/5,879] during 1997–2004 to 0.2% [12/5,736] during 2005–2011). The prevalence of atypical antipsychotic use decreased as the pregnancy progressed (Figure 2). This decrease was most pronounced from the month before conception through the third month of pregnancy, although the highest prevalence of atypical antipsychotic use was also observed during this period.
Figure 2.
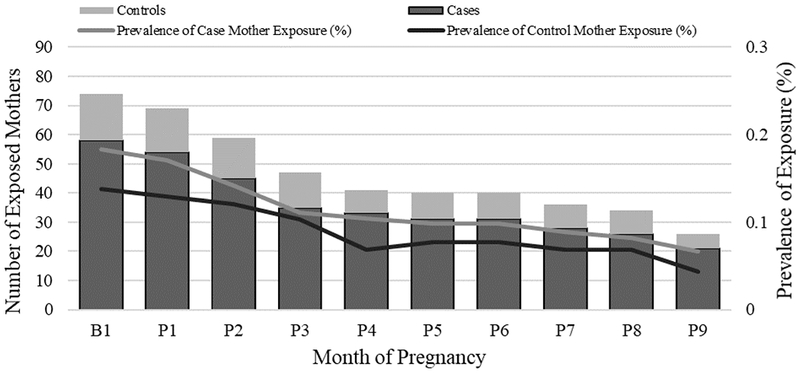
Distribution of any atypical antipsychotic medication use across pregnancy months,a National Birth Defects Prevention Study, 1997–2011
aB1 corresponds to the month before conception, P1–P9 correspond to the 1st–9th month of pregnancy.
3.2. Factors Associated with Atypical Antipsychotic Medication Use during Pregnancy
Among control mothers, we observed elevated associations between atypical antipsychotic medication use during pregnancy and pre-pregnancy obesity (cOR: 4.0, 95% CI: 1.5–10.3), any illicit drug use in the three months before through the end of pregnancy (cOR: 9.6, 95% CI: 3.5–25.9), smoking during pregnancy (cOR: 3.2, 95% CI: 1.2–8.3), and alcohol use during pregnancy (cOR: 2.2, 95% CI: 0.8–5.7) (Table 1). Strong, elevated associations were also observed between atypical antipsychotic use during pregnancy and concurrent use of anxiolytics (cOR: 36.5, 95% CI: 11.7–113.9), anticonvulsants (cOR: 92.5, 95% CI: 31.5–271.9), and antidepressants (cOR: 36.6, 95% CI: 13.5–99.3) (Table 1). Similar patterns were observed for these variables among case mothers (Table 2), although associations with pre-pregnancy obesity and alcohol use during pregnancy did not meet our threshold for elevated associations.
TABLE 1.
Maternal sociodemographic and health factors and any atypical antipsychotic medication usea during pregnancy among control mothers, National Birth Defects Prevention Study, 1997–2011
| Maternal Factors | Exposed to atypical antipsychotic medications during pregnancyb,c (N = 17) | Unexposed to atypical antipsychotic medications during pregnancyb,c (N = 11 598) | cORd | 95% CI |
|---|---|---|---|---|
| Age (years) | ||||
| <35 | 13 (76.5%) | 9,962 (85.9%) | 0.5 | [0.2, 1.6] |
| ≥35 [ref] | 4 (23.5%) | 1,636 (14.1%) | ||
| Non-Hispanic white race/ethnicity | ||||
| Yes | 12 (70.6%) | 6,742 (58.2%) | 1.7 | [0.6, 4.9] |
| No [ref] | 5 (29.4%) | 4,850 (41.8%) | ||
| Education (years) | ||||
| >12 | 9 (52.9%) | 6,845 (59.7%) | 0.8 | [0.3, 2.0] |
| ≤12 [ref] | 8 (47.1%) | 4,621 (40.3%) | ||
| Number of previous birthse | ||||
| One or more | 11 (64.7%) | 7,042 (60.7%) | 1.2 | [0.4, 3.2] |
| None [ref] | 6 (35.3%) | 4,552 (39.3%) | ||
| Pregnancy intentionf | ||||
| Wanted to be pregnant then | 8 (57.1%) | 5,601 (59.3%) | 0.9 | [0.3, 2.6] |
| Other intention [ref] | 6 (42.9%) | 3,847 (40.7%) | ||
| Pre-pregnancy obesity (BMI kg/m2) | ||||
| ≥30 | 8 (47.1%) | 2,035 (18.3%) | 4.0 | [1.5, 10.3] |
| <30 [ref] | 9 (52.9%) | 9,080 (81.7%) | ||
| Any early pregnancy folic acid useg | ||||
| Yes | 5 (29.4%) | 6,135 (52.9%) | 0.4 | [0.1, 1.1] |
| No [ref] | 12 (70.6%) | 5,462 (47.1%) | ||
| Any illicit drug useh | ||||
| Yes | 6 (35.3%) | 626 (5.4%) | 9.6 | [3.5, 25.9] |
| No/Not Known [ref] | 11 (64.7%) | 10 972 (94.6%) | ||
| Any smokingb | ||||
| Yes | 7 (41.2%) | 2,087 (18.1%) | 3.2 | [1.2, 8.3] |
| No [ref] | 10 (58.8%) | 9,420 (81.9%) | ||
| Any alcohol useb | ||||
| Yes | 10 (58.8%) | 4,544 (39.6%) | 2.2 | [0.8, 5.7] |
| No [ref] | 7 (41.2%) | 6,932 (60.4%) | ||
| Other Psychiatric Medication Use | ||||
| Anxiolytic medicationsb,i | ||||
| Yes | 4 (23.5%) | 97 (0.8%) | 36.5 | [11.7, 113.9] |
| No [ref] | 13 (76.5%) | 11 500 (99.2%) | ||
| Anticonvulsant medicationsb,i | ||||
| Yes | 5 (29.4%) | 52 (0.4%) | 92.5 | [31.5, 271.9] |
| No [ref] | 12 (70.6%) | 11 545 (99.6%) | ||
| Antidepressant medicationsb,i | ||||
| Yes | 11 (64.7%) | 553 (4.8%) | 36.6 | [13.5, 99.3] |
| No [ref] | 6 (35.3%) | 11 037(95.2%) |
Mothers of control infants reported use ot the following atypical antipsychotic medications: aripiprazole, olanzapine, quetiapine, and risperidone;
From the month before conception to pregnancy end;
Column counts and percentages may not equal the expected N due to missing data on maternal factors;
Bolded results in the table reflect elevated associations with cOR ≥2.0;
Includes previous live births and stillbirths;
Other intention includes women who wanted to wait until later to become pregnant, those who did not want to be pregnant at all, and those who did not care about becoming pregnant;
From the month before conception through the first month of pregnancy;
From three months before conception to pregnancy end;
Medications included in the anxiolytic, anticonvulsant, and antidepressant medication categories can be found in the National Institute of Mental Health mental health medication list;
Abbreviations: 95% CI, 95% confidence interval; cOR, crude (unadjusted) odds ratio; Ref, reference category.
TABLE 2.
Maternal sociodemographic and health factors and any atypical antipsychotic medication usea during pregnancy among case mothers, National Birth Defects Prevention Study, 1997–2011
| Maternal Factors | Exposed to atypical antipsychotic medications during pregnancyb,c(N = 67) | Unexposed to atypical antipsychotic medications during pregnancyb,c (N = 31 584) | cORd | 95% CI |
|---|---|---|---|---|
| Age (years) | ||||
| <35 | 51 (76.1%) | 26 809 (84.9%) | 0.6 | [0.3, 1.0] |
| ≥35 [ref] | 16 (23.9%) | 4775 (15.1%) | ||
| Non-Hispanic white race/ethnicity | ||||
| Yes | 41 (61.2%) | 18 569 (58.8%) | 1.1 | [0.7, 1.8] |
| No [ref] | 26 (38.8%) | 13 008 (41.2%) | ||
| Education (years) | ||||
| >12 | 28 (42.4%) | 17 733 (56.8%) | 0.6 | [0.3, 0.9] |
| ≤12 [ref] | 38 (57.6%) | 13 510 (43.2%) | ||
| Number of previous birthse | ||||
| One or more | 36 (53.7%) | 18 259 (57.8%) | 0.9 | [0.5, 1.4] |
| None [ref] | 31 (46.3%) | 13 311 (42.4%) | ||
| Pregnancy intentionf | ||||
| Wanted to be pregnant then | 25 (44.6%) | 15 178 (58.2%) | 0.6 | [0.3, 1.0] |
| Other intention [ref] | 31 (55.4%) | 10 922 (41.8%) | ||
| Pre-pregnancy obesity (BMI kg/m2) | ||||
| ≥30 | 21 (31.3%) | 6244 (20.6%) | 1.8 | [1.1, 2.9] |
| <30 [ref] | 46 (68.7%) | 24 002 (79.4%) | ||
| Any early pregnancy folic acid useg | ||||
| Yes | 33 (49.3%) | 16 536 (52.4%) | 0.9 | [0.6, 1.4] |
| No [ref] | 34 (50.7%) | 15 047 (47.6%) | ||
| Any illicit drug useh | ||||
| Yes | 16 (23.9%) | 1964 (6.2%) | 4.7 | [2.7, 8.3] |
| No/Not Known [ref] | 51 (76.1%) | 29 620 (93.8%) | ||
| Any smokingb | ||||
| Yes | 40 (60.6%) | 6339 (20.2%) | 6.1 | [3.7, 10.0] |
| No [ref] | 26 (39.4%) | 24 995 (79.8%) | ||
| Any alcohol useb | ||||
| Yes | 28 (42.4%) | 11 931 (38.3%) | 1.2 | [0.7, 1.9] |
| No [ref] | 38 (57.6%) | 19 257 (61.7%) | ||
| Other Psychiatric Medication Use | ||||
| Anxiolytic medicationsb,i | ||||
| Yes | 7 (10.4%) | 289 (0.9%) | 12.6 | [5.7, 27.9] |
| No [ref] | 60 (89.6%) | 31 286 (99.1%) | ||
| Anticonvulsant medicationsb,i | ||||
| Yes | 25 (37.3%) | 215 (0.7%) | 86.8 | [52.0, 145.0] |
| No [ref] | 42 (62.7%) | 31 366 (99.3%) | ||
| Antidepressant medicationsb,i | ||||
| Yes | 44 (66.7%) | 1846 (5.9%) | 32.2 | [19.3, 53.8] |
| No [ref] | 22 (33.3%) | 29 707 (94.1%) |
Mothers of case infants reported use ot the following atypical antipsychotic medications: aripiprazole, asenapine, olanzapine, quetiapine, risperidone, and ziprasidone;
From the month before conception to pregnancy end;
Column counts and percentages may not equal the expected N due to missing data on maternal factors;
Bolded results in the table reflect elevated associations with cOR ≥2.0;
Includes previous live births and stillbirths;
Other intention includes women who wanted to wait until later to become pregnant, those who did not want to be pregnant at all, and those who did not care about becoming pregnant.
From the month before conception through the first month of pregnancy;
From three months before conception to pregnancy end;
Medications included in the anxiolytic, anticonvulsant, and antidepressant medication categories can be found in the National Institute of Mental Health mental health medication list;
Abbreviations: 95% CI, 95% confidence interval; cOR, crude (unadjusted) odds ratio; Ref, reference category.
3.3. Early Pregnancy Atypical Antipsychotic Exposure and Risk for Selected Birth Defects
After applying exclusion criteria, there were 36 case and 12 control mothers exposed to any atypical antipsychotic in the month before conception through the third month of pregnancy. The birth defect categories with ≥3 exposed case mothers are delineated in Table 3. We observed elevated associations (cOR ≥2.0) between maternal atypical antipsychotic use during early pregnancy and conotruncal defects (6 exposed cases; cOR: 2.3, 95% CI: 0.9–6.1), particularly with Tetralogy of Fallot (3 exposed cases; cOR: 2.5, 95% CI: 0.7–8.8). Associations between early pregnancy atypical antipsychotic use and cleft palate (4 exposed cases; cOR: 2.5, 95% CI: 0.8–7.6), anorectal atresia/stenosis (3 exposed cases; cOR: 2.8, 95% CI: 0.8–9.9), and gastroschisis (3 exposed cases; cOR: 2.1, 95% CI: 0.6–7.3) were also elevated.
TABLE 3.
Associations between any atypical antipsychotic medication use from one month before conception through the third month of pregnancy and specific selected birth defects,a National Birth Defects Prevention Study (NBDPS), 1997–2011
| Defect | Atypical Antipsychotic Medication Exposedb,c N | Atypical Antipsychotic Medication Unexposedb,d N | cOR (95% CI)e |
|---|---|---|---|
| Controls | 12 | 11,458 | |
| Any heart defect | 18 | 11,803 | 1.5 (0.7, 3.0) |
| Conotruncal defects | 6 | 2,495 | 2.3 (0.9, 6.1) |
| Tetralogy of Fallot | 3 | 1,154 | 2.5 (0.7, 8.8) |
| LVOTO | 4 | 2,169 | 1.8 (0.6, 5.5) |
| RVOTO | 3 | 2,037 | 1.4 (0.4, 5.0) |
| Septal defects | 4 | 4,529 | 0.8 (0.3, 2.6) |
| Atrial septal defect (secundum or NOS) | 4 | 2,914 | 1.3 (0.4, 4.1) |
| Any orofacial cleftf | 7 | 4,574 | 1.4 (0.6, 3.7) |
| Cleft palatef | 4 | 1,539 | 2.5 (0.8, 7.6) |
| Cleft lip +/− cleft palatef | 3 | 3,035 | 0.9 (0.3, 3.3) |
| Anorectal atresia/stenosis | 3 | 1,028 | 2.8 (0.8, 9.9) |
| Hypospadias, 2/3rd degreef | 3 | 2,510 | 0.8 (0.2, 2.9) |
| Craniosynostosis | 3 | 1,567 | 1.8 (0.5, 6.5) |
| Gastroschisis | 3 | 1,398 | 2.1 (0.6, 7.3) |
This table includes NBDPS birth defects with ≥3 exposed cases. Cases were included in the higher order defect category (e.g., “any heart defect”) even if the more detailed birth defect category was excluded (e.g., hypoplastic left heart syndrome, coarctation of the aorta). The total sample of cases presented in the Method section does not sum to Table 3 individual defects reported as cases may have ≥1 eligible defect;
Atypical antipsychotic medication components with case or control mother exposure included aripiprazole, asenapine, olanzapine, quetiapine, risperidone, and ziprasidone;
Mothers exposed to atypical antipsychotics from the month before conception through the third month of pregnancy;
Mothers unexposed to atypical antipsychotics from three months before conception through the end of pregnancy;
Bolded results in the table reflect elevated associations with cOR ≥2.0;
As any orofacial clefts, cleft palate, and cleft lip +/− cleft palate were only ascertained by a subset of the study sites in certain years, and hypospadias only affects male infants, controls for these analyses were similarly restricted. For orofacial clefts, cleft palate, and cleft lip +/− cleft palate, there were 11 324 unexposed controls and 12 controls who were exposed to atypical antipsychotic medication in early pregnancy. For hypospadias, there were 5,837 unexposed controls and 9 controls who were exposed to atypical antipsychotic medication in early pregnancy;
Abbreviations: 95% CI, 95% confidence interval; cOR, crude (unadjusted) odds ratio; LVOTO, left ventricular outflow tract obstruction; RVOTO, right ventricular outflow tract obstruction; NOS, not otherwise specified.
4. Discussion
This is the largest analysis to date on the association between any atypical antipsychotic medication use and risk for specific birth defects, which used careful clinical review of the birth defect outcomes. The prevalence of atypical antipsychotic medication use during pregnancy was rare (0.2%) in our study population, but increased from the early (1997–2004) to the later (2005–2011) study years. Our findings are in line with other U.S.-based reports from administrative databases that suggest that atypical antipsychotic use is increasing among pregnant women, but our prevalence estimates were lower (Epstein et al., 2013; Park et al., 2017; Toh et al., 2013). However, Hanley and Mintzes (2014) observed a 0.2% prevalence of atypical antipsychotic prescriptions using claims data with a sample of privately-insured U.S. women from 2006–2011. Estimate variations may be due to methodological differences. Previous studies utilized administrative data that examined medications dispensed from pharmacies, while our study utilized retrospective case-control data with self-report of medications used. Differences in estimates may reflect differences between pregnant women who are Medicaid-insured (Epstein et al., 2013; Park et al., 2017) or those with private insurance (Hanley and Mintzes, 2014), and the NBDPS population, which included a population-based sample of pregnant women from sites across the United States. Triangulating evidence from all sources will be useful in determining accurate prevalence estimates of atypical antipsychotic use among U.S. pregnant women. In line with previous reports (e.g., Kallen et al., 2013; Park et al., 2017; Toh et al., 2013), we found a decreasing trend of atypical antipsychotic use across the months of pregnancy. We observed the most pronounced decrease during the 1st trimester, which corresponds with the timing of pregnancy recognition for most women (Branum and Ahrens, 2017). Although use of atypical antipsychotics declined in the 1st trimester of pregnancy, the highest prevalence of use occurred in early pregnancy, the period of fetal organogenesis.
In our analysis, control mothers exposed to atypical antipsychotics were more likely to report pre-pregnancy obesity, illicit drug use before and during pregnancy, smoking during pregnancy, alcohol use during pregnancy, and use of other psychiatric medications. These results were largely mirrored among case mothers, although findings for pre-pregnancy obesity and alcohol use did not meet our threshold as elevated associations. Other studies have reported higher pre-pregnancy weight, obesity, and/or diabetes among those using atypical antipsychotics (Habermann et al., 2013; Park et al., 2017; Petersen et al., 2016; Sadowski et al., 2013), which may be related to these medications’ known association with metabolic syndrome (Rummel-Kluge et al., 2010). Pregnant women who use atypical antipsychotics have reported using illicit drugs and smoking more commonly during pregnancy compared to women not using atypical antipsychotics (e.g., Bellet et al., 2015; McKenna et al., 2005). Moreover, use of anxiolytics, anticonvulsants, and antidepressants are commonly reported by women concurrently using atypical antipsychotics (Park et al., 2017; Petersen et al., 2016). The results for other factors examined in our analysis attempt to address conflicting evidence reported in other analyses. For example, it is unclear if pregnant women who take atypical antipsychotics are more likely to drink alcohol during pregnancy (Bellet et al., 2015; Park et al., 2017; Sadowski et al., 2013) or are less likely to plan their pregnancies (Habermann et al., 2013; McKenna et al., 2005). We observed an elevated association between atypical antipsychotic use and alcohol use among control mothers (but not case mothers), and we did not see an association with pregnancy planning. It is important to note that some of the factors examined in our analysis may be related to the underlying mental health disorder for which antipsychotics were prescribed (Boden et al., 2012); we were unable to address confounding by indication in our analysis.
While some studies have reported an increased risk of having a baby with any major structural birth defect or any congenital heart defect after pregnancy exposure to atypical antipsychotics (Coughlin et al., 2015; Terrana et al., 2015), there have been few reports on associations with specific birth defects. In our unadjusted but restricted analysis, we observed elevated associations (defined in our analysis as cOR ≥2.0) between early pregnancy atypical antipsychotic use and conotruncal heart defects (and specifically the conotruncal defect Tetralogy of Fallot), cleft palate, anorectal atresia/stenosis, and gastroschisis. Results from a global pharmacovigilance safety analysis suggested there may be an increased risk for cleft palate, esophageal disorders, and anorectal disorders after exposure to any antipsychotic during pregnancy (Montastruc et al., 2016). Other studies have noted increased risks for cardiac septal defects (Habermann et al., 2013) and hypospadias (Reis and Kallen, 2008; Kallen et al., 2013) after any antipsychotic exposure during pregnancy; we did not observe these associations in our analysis. Given limited literature on atypical antipsychotic use in early pregnancy and risk for specific birth defects, the exploratory findings from this analysis merit replication with another large sample of exposed pregnancies and specific birth defects. Researchers may also consider examining links between specific medications and individual birth defects; some medications in this chemically heterogeneous group may be associated with greater risk of adverse pregnancy outcomes (e.g., risperidone) (Huybrechts et al., 2016; Ennis and Damkier, 2015).
The NBDPS is among the largest studies worldwide that examines risk factors for specific birth defects. Clinical geneticists and pediatric cardiologists reviewed all cases to ensure eligibility and to classify birth defect cases using standard, detailed case definitions (Rasmussen et al., 2003; Reefhuis et al., 2015), which allowed us to accurately examine associations with specific defects. In the few instances when associations with specific defects have been examined previously, administrative data were used to capture birth defect outcomes, which is subject to misclassification bias overall (Grzeskowiak et al., 2012) and by specific birth defect (Cooper et al., 2008). We also focused on atypical antipsychotics specifically, whereas many previous studies have combined typical and atypical antipsychotics (e.g., Kulkarni et al., 2014; Montastruc et al., 2016; Petersen et al., 2016; Reis and Kallen, 2008). Notably, women who take typical antipsychotics during pregnancy are often prescribed these medications not for mental disorders but for antiemetic purposes (Goldstein et al., 2015; Reis and Kallen, 2008), with medications prescribed in lower doses and over shorter periods during pregnancy. These differences in the clinical use of the two types of antipsychotic medications could bias results towards the null if typical and atypical antipsychotics were collapsed. However, it is important to also note that the atypical antipsychotic class is a heterogeneous group of medications. Ideally, each medication and each birth defect pair should be examined; however, amassing a sufficient sample size to conduct this analysis proves to be a challenge in all available data sources.
There are also limitations of our study that should be considered. The NBDPS did not probe for specific mental disorders, which may have resulted in underreporting of atypical antipsychotic medication use and precluded our ability to examine the underlying indications for use. We also did not have information on the clinical course of the disorder for those who continued or discontinued treatment before and during pregnancy, on medication dosage, or on biomarkers for fetal drug exposure. NBDPS data collection ended with 2011 EDDs; new atypical antipsychotics (e.g., brexipiprazole, cariprazine) were not captured in this study. NBDPS does not include all birth defects; moreover, we excluded NBDPS-eligible birth defects with <3 exposed cases from our analysis in order to include only specific birth defects with sufficient data available to generate model estimates. In addition, study sites had varied ability to ascertain data on stillbirths and terminations over the study years (Reefhuis et al., 2015); it is unclear how these variations may have impacted our findings. While we restricted the birth defect analysis to account for variables that might be strong confounders for the association between atypical antipsychotic use and birth defects, the small number of exposed cases precluded our ability to adjust for additional confounders using multivariable models. Small exposed case counts also limited our ability to examine associations between individual atypical antipsychotics and specific birth defects. Although there were few women exposed overall to atypical antipsychotics, this analysis still makes an important contribution given the limited literature on associations with specific birth defects, and the growing use of these medications.
Our findings support existing recommendations for the close monitoring of reproductive-aged women with mental health conditions that are treated by antipsychotic medications (ACOG, 2008; Zacher et al., 2013). In general, women who use atypical antipsychotics during pregnancy represent a subgroup of women at heightened baseline risk for having a baby with a major birth defect due to risks associated with the underlying indication (Cannon et al., 2002; Rusner et al., 2016), associated behaviors (e.g., smoking and substance use), and comorbidities (e.g., obesity, diabetes) that increase the risk of adverse pregnancy and infant outcomes, including major birth defects (Boden et al., 2012; Jablensky et al., 2005; Janssen et al., 2015; Parnell et al., 2017; US DHHS, 2014). Women with conditions treated by atypical antipsychotics generally access healthcare services before pregnancy (Toh et al., 2013), and health education could be provided to reduce pre-pregnancy obesity, smoking, and other correlates of atypical antipsychotic use to improve maternal and infant health in this population. Furthermore, we observed that some pregnant women discontinued the use of their medications around the time of pregnancy recognition (in the 1st trimester), although this also means that for many women, exposure during the period of fetal organogenesis would already have occurred. Ideally, healthcare providers would work with women taking atypical antipsychotics prior to pregnancy in order to determine a course of clinical action for before and during pregnancy that carefully weighs the potential for illness relapse and the consequent risks to the mother and baby related to that, against the potential risk of maintaining atypical antipsychotic treatment during pregnancy.
Acknowledgements.
Coding of drug information in the National Birth Defects Prevention Study used the Slone Drug Dictionary under license from the Slone Epidemiology Center of Boston University. Findings were presented at the 3rd Biennial Perinatal Mental Health Conference, Chicago, IL, November 7–10, 2017, the 2018 National Birth Defects Prevention Network Annual Meeting, Atlanta, GA, March 11–14, 2018, and the 31st Annual Meeting of the Society for Pediatric and Perinatal Epidemiologic Research, Baltimore, MD, June 18–19, 2018.
Funding body agreements and policies. This project was supported through Centers for Disease Control and Prevention (CDC) cooperative agreements under PA #96043, PA #02081, and FOA #DD09-001, and NOFO #DD18-001 to the Centers for Birth Defects Research and Prevention participating in the National Birth Defects Prevention Study (NBDPS) and/or the Birth Defects Study to Evaluate Pregnancy Exposures (BD-STEPS). William V. Bobo’s research has been supported by grants from the National Institute of Mental Health, Agency for Healthcare Research and Quality, and the Mayo Foundation for Medical Education and Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflicts of Interest. The authors have no conflicts of interest to report.
References
- American College of Obstetrics and Gynecology Practice Bulletin, 2008. Use of psychiatric medications during pregnancy and lactation. Obstet. Gynecol 111 (4) 1001–1020. [DOI] [PubMed] [Google Scholar]
- Bellet F, Beyens MN, Bernard N, et al. , 2015. Exposure to aripiprazole during embryogenesis: a prospective multicenter cohort study. Pharmacoepidemiol. Drug. Saf 24 (4) 368–380. [DOI] [PubMed] [Google Scholar]
- Boden R, Lundgren M, Brandt L, et al. , 2012. Risks of adverse pregnancy and birth outcomes in women treated or not treated with mood stabilisers for bipolar disorder: population based cohort study. BMJ. 345 e7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branum AM, Ahrens KA, 2017. Trends in timing of pregnancy awareness among US women. Matem. Child. Health. J 21 (4) 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M, Jones PB, Murray RM, 2002. Obstetric complications and schizophrenia: historical and meta-analytic review. Am. J. Psychiatry. 159 (7) 1080–1092. [DOI] [PubMed] [Google Scholar]
- Cooper WO, Hemandez-Diaz S, Gideon P, et al. , 2008. Positive predictive value of computerized records for major congenital malformations. Pharmacoepidemiol. Drug. Saf 17 (5)455–460. [DOI] [PubMed] [Google Scholar]
- Correa A, Gilboa SM, Besser LM, et al. , 2008. Diabetes mellitus and birth defects. Am. J. Obstet. Gynecol 199 (3) 237.e1–237.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin CG, Blackwell KA, Bartley C, et al. , 2015. Obstetric and neonatal outcomes after antipsychotic medication exposure in pregnancy. Obstet. Gynecol 125 (5) 1224–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damkier P, Christensen LS, Broe A, 2018. Patterns and predictors for prescription of psychotropics and mood-stabilizing anti epileptics during pregnancy in Denmark 2000–2016. Brit J Clin Pharmaco. 84 (11) 2651–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demler LT, 2011. Labeling guidelines for antipsychotics during pregnancy. US. Pharmacist. 236 39–44. [Google Scholar]
- Ennis ZN, Damkier P, 2015. Pregnancy exposure to olanzapine, quetiapine, risperidone, aripiprazole and risk of congenital malformations: a systematic review. Basic. Clin. Pharmacol. Toxicol 116 315–320. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Bobo WV, Shelton RC, et al. , 2013. Increasing use of atypical antipsychotics and anticonvulsants during pregnancy. Pharmacoepidemiol. Drug. Saf 22 794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MD, Reilly K, Isenberg K, et al. , 2014. Antipsychotic patterns of use in patients with schizophrenia: polypharmacy versus monotherapy. BMC. Psychiatry. 14 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LH, Weber Schondorfer C, Berkovitch M, 2015. Nausea and vomiting during pregnancy, in: Schaefer C, Peters P, Miller RK (Eds.), Drugs during Pregnancy and Lactation. Elsevier, Boston, 75–91. [Google Scholar]
- Grzeskowiak LE, Gilbert AL, Morrison JL, 2012. Exposed or not exposed? Exploring exposure classification in studies using administrative data to investigate outcomes following medication use during pregnancy. Eur. J. Clin. Pharmacol 68 (5) 459–467. [DOI] [PubMed] [Google Scholar]
- Habermann F, Fritzsche J, Fuhlbruck F, et al. , 2013. Atypical antipsychotic drugs and pregnancy outcome: a prospective, cohort study. J. Clin. Psychopharmacol 33 (4) 453–462. [DOI] [PubMed] [Google Scholar]
- Hanley GE, Mintzes B, 2014. Patterns of psychotropic medicine use in pregnancy in the United States from 2006 to 2011 among women with private insurance. BMC. Pregnancy. Childbirth. 14 242–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huybrechts KF, Hemandez-Diaz S, Patorno E, et al. , 2016. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA. Pediatr 73 (9) 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablensky AV, Morgan V, Zubrick SR, et al. , 2005. Pregnancy, delivery, and neonatal complications in a population cohort of women with schizophrenia and major affective disorders. Am. J. Psychiatry. 162 (1) 79–91. [DOI] [PubMed] [Google Scholar]
- Janssen EM, McGinty EE, Azrin ST, et al. , 2015. Review of the evidence: prevalence of medical conditions in the United States population with serious mental illness. Gen. Hosp. Psychiatry. 37 (3) 199–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen B, Borg N, Reis M, 2013. The use of central nervous system active drugs during pregnancy. Pharmaceuticals. 6 (10) 1221–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury MJ, Moore CA, James LM, et al. 1992. The interaction between dsymorphology and epidemiology: methodologic issues of lumping and splitting. Teratology. 25 133–138. [DOI] [PubMed] [Google Scholar]
- Kulkarni J, Worsley R, Gilbert H, et al. , 2014. A prospective cohort study of antipsychotic medications in pregnancy: the first 147 pregnancies and 100 one year old babies. PLoS ONE. 9 (5) e94788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S, Pitschel-Walz G, Abraham D, et al. , 1999. Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo: a meta-analysis of randomized control trials. Schizophr. Res 35 (1) 51–68. [DOI] [PubMed] [Google Scholar]
- McKenna K, Koren G, Tetelbaum M, et al. , 2005. Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. Clin. Psychiatry. 66 (4) 444–449. [DOI] [PubMed] [Google Scholar]
- Montastruc F, Salvo F, Arnaud M, et al. , 2016. Signal of gastrointestinal congenital malformations with antipsychotics after minimising competition bias: a disproportionality analysis using data from Vigibase. Drug. Saf 39 (7) 689–696. [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health, 2010. Mental health medications. NIH Publication 12-3929 1–26. [Google Scholar]
- National Institute of Mental Health, 2018. Mental health information. https://www.nimh.nih.gov/health/topics/index.shtml Accessed January 15, 2018
- Park Y, Huybrechts KF, Cohen JM, et al. , 2017. Antipsychotic medication use among publicly insured pregnant women in the United States. Psychiatr. Serv 68 (11) 1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell AS, Correa A, Reece EA, 2017. Pre-pregnancy obesity as a modifier of gestational diabetes and birth defects associations: a systematic review. Matern. Child. Health. J 21 (5) 1105–1120. [DOI] [PubMed] [Google Scholar]
- Petersen I, Sammon CJ, McCrea RL, et al. , 2016. Risks associated with antipsychotic treatment in pregnancy: Comparative cohort studies based on electronic health records. Schizophr. Res 176 349–356. [DOI] [PubMed] [Google Scholar]
- Poo SX, Agius M, 2015. Atypical antipsychotics for schizophrenia and/or bipolar disorder in pregnancy: current recommendations and updates in the NICE guidelines. Psychiatria. Danubina 27 (Suppl 1) S255–260. [PubMed] [Google Scholar]
- Rasmussen SA, Olney RS, Holmes LB, et al. , 2003. Guidelines for case classification for the National Birth Defects Prevention Study. Birth. Defects. Res. A. Clin. Mol. Teratol 67 (3) 193–201. [DOI] [PubMed] [Google Scholar]
- Reefhuis J, Gilboa SM, Anderka M, et al. , 2015. The National Birth Defects Prevention Study: a review of the methods. Birth. Defects. Res. A. Clin. Mol. Teratol 103 (8) 656–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis M, Kallen B, 2008. Maternal use of antipsychotics in early pregnancy and delivery outcome. J. Clin. Psychopharmacol 28 (3) 279–288. [DOI] [PubMed] [Google Scholar]
- Rummel-Kluge C, Komossa K, Schwarz S, et al. , 2010. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr. Res 123 (2-3) 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusner M, Berg M, Begley C, 2016. Bipolar disorder in pregnancy and childbirth: a systematic review of outcomes. BMC. Pregnancy. Childbirth. 1 331–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski A, Todorow M, Brojeni PY, et al. , 2013. Pregnancy outcomes following maternal exposure to second-generation antipsychotics given with other psychotropic drugs: a cohort study. BMJ. Open. 3 e003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesselman JJ, 1982. Case-control studies: design, conduct, and analysis. Oxford University Press, New York. [Google Scholar]
- Terrana N, Koren G, Pivovarov J, et al. , 2015. Pregnancy outcomes following in utero exposure to second-generation antipsychotics: a systematic review and meta-analysis. J. Clin. Psychopharmacol 35 (5) 559–565. [DOI] [PubMed] [Google Scholar]
- Toh S, Li Q, Cheetham TC, et al. , 2013. Prevalence and trends in the use of antipsychotic medications during pregnancy in the U.S., 2001–2007: a population-based study of 585,615 deliveries. Arch. Womens. Ment. Health. 16 (2) 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, 2014. The health consequences of smoking – 50 years of progress: A report of the Surgeon General, 2014. https://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html Accessed August 1, 2018.
- Wasserstein RL, Schirm AL, Lazar NA, 2019. Moving to a world beyond ‘p < 0.05’. The American Statistician. 73 (supl) 1–19. [Google Scholar]
- Werler MM, Ahrens KA, Bosco JL, et al. , 2011. Use of antiepileptic medications in pregnancy in relation to risks of birth defects. Ann. Epidemiol 21 (11) 842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacher J, Peterson J, Lempicki K, et al. , 2013. Comparing current practices of screening for pregnancy and contraceptive use in female veterans of child-bearing age prescribed psychotropic medications in a mental health versus a women’s health clinic. Mental. Health. Clinician. 3 (2) 71–77. [Google Scholar]


