Abstract
The first example of metal-free cyanomethylenation from alkyl nitriles of sp3 C-H bonds to afford quaternary carbon centers is described. This oxidative protocol is operationally simple and features good functional group compatibility. This method provides a novel approach to highly functionalized fluorene and oxindole derivatives, which are commonly used in material and pharmaceutical areas. Control experiments provide evidence for a radical reaction process.
Graphical Abstract
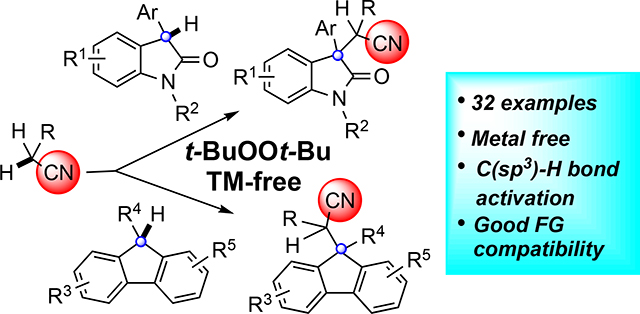
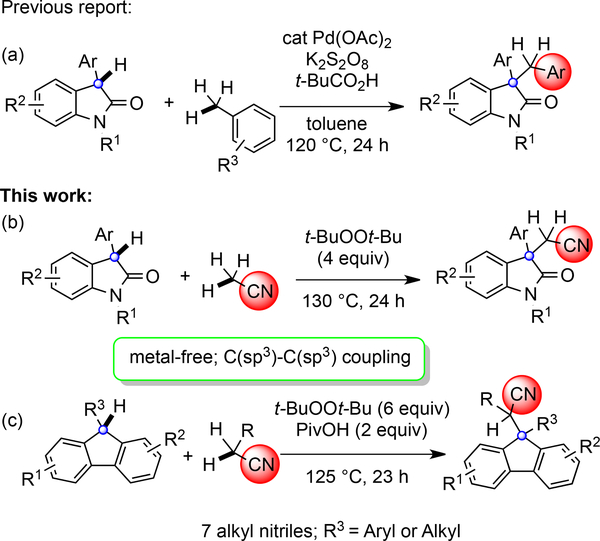
Nitriles are versatile functional groups in organic synthesis. In addition to being a very useful functional group in biologically active compounds, they can be readily converted into amines, carboxylic acids, ketones and even heterocycles.1 Compounds containing nitrile groups are frequently employed as building blocks in drug discovery programs. 2 Introduction of a nitrile group by activation of the α-hydrogen of simple aliphatic nitriles is one of the most efficient and environmentally benign entries to this class of structure. For example, generation and reaction of stable α-nitrile anions is well-explored with a range of electrophiles.3 The nitrile group also stabilizes radicals arising from α-hydrogen atom abstraction, and these radicals permit bond disconnections4 complementary to those available from the α-nitrile anions. Amongst these reactions, there are relatively few involving C-H activation of a second component, that is oxidative fragment coupling. Building off of our prior efforts in dual C-H activation of two components (Scheme 1a),5 the use of acetonitrile was investigated with oxindoles and fluorenes. To the best of our knowledge, there are no examples of cross-coupling between sp3 C-H bonds and alkyl nitriles under metal-free conditions. Herein, we communicate our efforts culminating in facile, metal-free oxidative cyanomethylenation of oxindoles and fluorenes with alkyl nitriles through C(sp3)-H oxidative radical functionalization using t-BuOOt-Bu as the oxidant (Scheme 1b–c).
Scheme 1.
Dual C-H Activation of Oxindoles and Fluorenes with Toluenes or Alkylnitriles to Generate Quaternary Centers
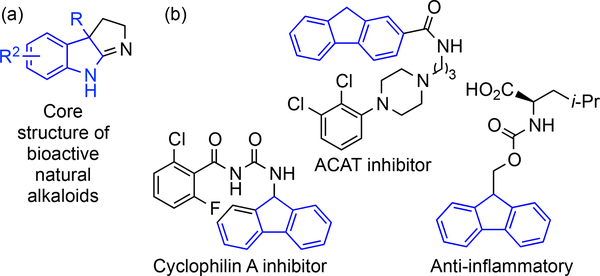
As an important structural unit, 2-oxindoles with a quaternary carbon center at the C3 position are a class of heterocycles existing in many natural products, pharmaceuticals and drug candidates (Figure 1a).6 Their importance has prompted considerable interest in developing new construction methods.
Figure 1.
Structures of Bioactive Compounds with a) Oxindole or b) Fluorene Moieties
Similarly, fluorenes have attracted much attention for a variety of applications involving advanced materials, including those used in semiconductors,7 optoelectronics,8 and solar cells. 9 In addition, fluorene derivatives have also been playing an increasing role in pharmaceuticals and biochemistry10 Pharmaceutical compounds also can incorporate fluorene moieties (Figure 1b).11 As a consequence, the development of practical synthetic methods for the construction of functionalized fluorenes is in demand.
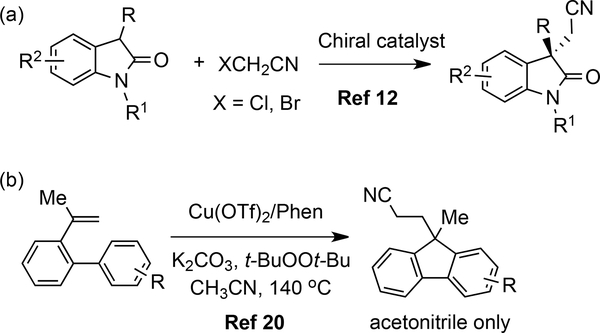
For example, the asymmetric cyanomethylenation of 3-substituted oxindoles using prefunctionalized cyanomethyl halides have been reported by different groups (Scheme 2a).12 Recently, many studies focused on the atom-transfer radical addition reactions of nitriles with olefins.13 Among them, the Zhu Group has made significant contributions to this research field.14 In 2016, Ge group reported the first example of the palladium-catalyzed cross-coupling of sp3 C-H bonds with acetonitrile. 15 Thereafter, Wu16 and Shen17 independently reported the direct oxidative cyanomethylenation reactions by adding acetonitrile to 1,3-dicarbonyls and tetrahydroisoquinolines respectively, however other alkyl nitrile coupling partners were unsuccessful.
Scheme 2.
Approaches to Cyanoalkyl Oxindoles and Fluorenes
In 2015, the Liu group developed a simple and efficient synthesis of 9-arylfluorenes via metal-free reductive coupling of arylboronic acids and N-tosylhydrazones.18 Also, extensive attention has been paid to generating fluorenes via transition-metal catalyzed cyclizations or direct dehydrogenative aryl-aryl coupling via C-H bond activation.19 In 2016, the Ji group developed a copper-mediated radical alkylarylation of unactivated alkenes with acetonitrile leading to methylene disubstituted fluorenes, which are not easily accessed by conventional methods (Scheme 2b).20 Nonetheless, this transformation still suffered from some drawbacks, such as the use of transition metal and ligand, narrow substrate scope (acetonitrile only), and only access to 9-alkyl substituted fluorenes, thus limiting its further applications.
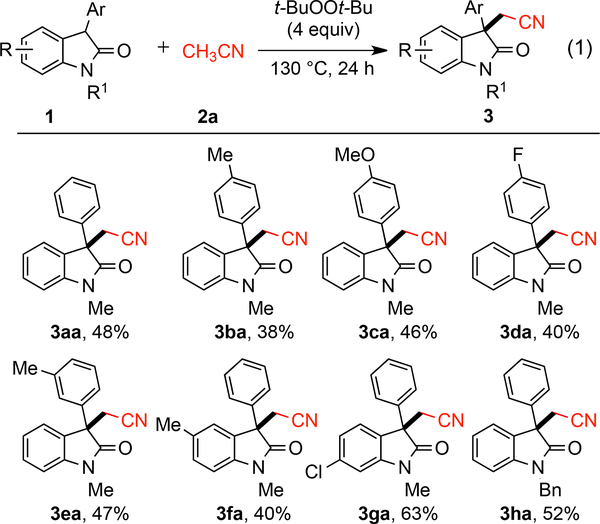
With a clear need for alternative strategies to construct highly functionalized oxindoles, we initiated our investigations by screening various metal source (Cu, Fe, Pd, Co, Mn and Sc) and reaction conditions (see the Supporting Information) for the t-BuOOt-Bu-mediated coupling of 3-substituted oxindoles with acetonitrile (eq 1). Notably, the protocols employed by Zhu and Li14a, 21 involving metal catalysts to generate acetonitrile radicals for additions to alkenes were not effective in these couplings. After extensive investigation of the reaction conditions (see the Supporting Information), control reactions revealed that the metal catalyst was unnecessary leading to a very straightforward oxidative method for introducing the cyanomethylene functionality to an oxindole. The optimum reaction conditions entailed heating a solution of 1a in acetonitrile (0.1 M) in the presence of t-BuOOt-Bu (4 equiv) at 130 °C for 24 h which provided 3aa in 48% yield.
Subsequently, a range of 3-monosubstituted oxindoles were explored for the cyanomethylenation at the C3-position with acetonitrile (Scheme 3). Electron-neutral (3aa), electron-donating (3ba, 3ca, 3ea, 3fa), and electron-withdrawing (3da) substituents on the phenyl ring were all well tolerated under the optimal reaction conditions. Substituents at the different positions did not affect the yields significantly. The 6-chloro-oxindole also gave the corresponding product 3ga in 63% yield. The N-benzyl substituted oxindole also exhibited good reactivity providing 3ha in 52% yield.
Scheme 3.
The Reaction of 3-Monosubstituted Oxindoles with Acetonitrilea
aConditions: 1 (0.15 mmol), 2a (1.5 mL, 0.1 M), t-BuOOt-Bu (4 equiv), 130 °C, under Ar, 24 h.
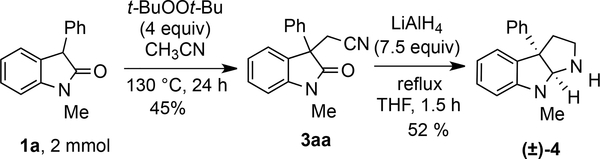
The cyanomethylenated products derived from 3-substituted oxindoles are versatile intermediates in organic synthesis and can be readily converted into other important building blocks including phenyl substituted pyrroloindolines.22 To show the utility of this method in producing useful precursors, a further transformation was carried out on product 3aa (Scheme 4). First, the 2 mmol scale synthesis of product 3aa proceeded successfully, delivering 3aa in 45% yield. Reductive cyclization of oxindole 3aa using LiAlH4 provided pyrroloindoline 4 in 52% yield. Overall, this route provides comparable or better efficiencies relative to other routes for generating target 4 with aryl substitution at the angular carbon.23
Scheme 4.
Scale-Up and Synthetic Transformation of the Cyanomethylenated Product 3aa
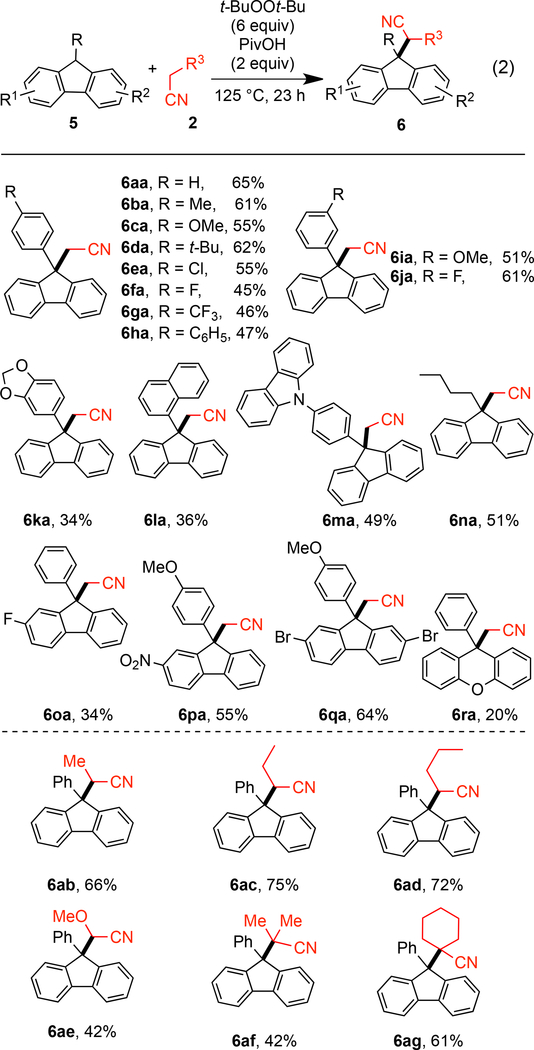
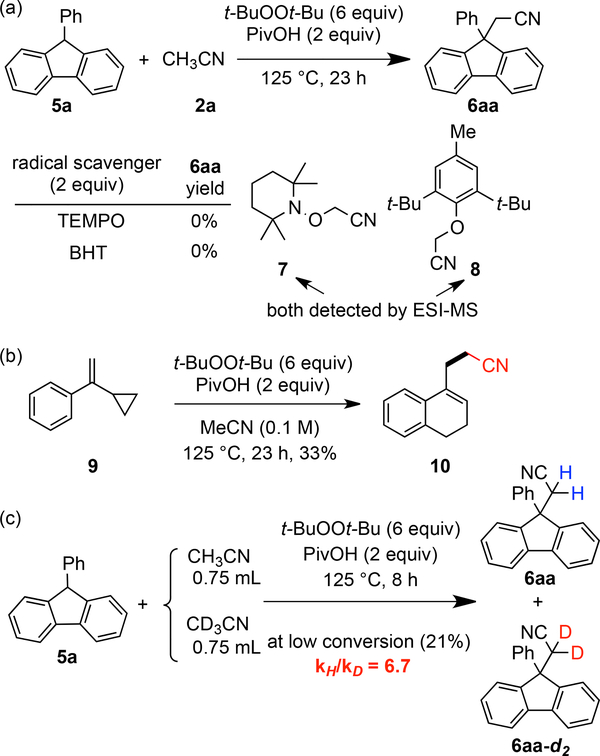
Application of the above conditions to the coupling of 9-phenyl-9H-fluorene 5a24 and acetonitrile 2a provided product 6aa (eq 2) in 49% yield (see SI). Further experimentation (see SI) ultimately revealed that carboxylic acid additives enhanced the outcome. The optimum conditions were t-BuOOt-Bu (6 equiv) with PivOH (2 equiv) at 125 °C for 23 h which provided 6aa in 65% yield (Scheme 5).
Scheme 5.
The Reaction of Various 9-Substituted Fluorenes with Alkyl Nitrilesa
aConditions: 5 (0.15 mmol), 2 (1.5 mL, 0.1 M), t-BuOOt-Bu (6 equiv) PivOH (2 equiv), 125 °C, under Ar, 23 h.
With these conditions in hand, the scope of the reaction with respect to the fluorene component and alkyl nitrile was evaluated (Scheme 5). First, different para-substituted aryl groups at C9 on the fluorene were explored. With either electron-donating or electron-neutral substituents, the products were formed in good yields (6aa-6da). Those bearing electron-withdrawing chloro, fluoro, trifluoromethyl or phenyl group gave the corresponding products in a slightly lower yields (6ea-6ha). C9-Aryl groups with either methoxy or fluoro groups at the meta-position reacted smoothly with 2a to give 6ia and 6ja in 51% and 61% yield, respectively. A range of bulkier aryl groups could be tolerated at C9 of the fluorene, including acetal derived, naphthyl, para-carbazolylphenyl affording the corresponding products in 34–49% yields (6ka-6ma). Of particular note, 9-butylfluorene can also react with acetonitrile to afford 6na in 51% yield.
Notably, functional groups such as methoxy, halogen, and nitro can be employed in different positions on the fluorene component (6oa-6qa). 9-Phenyl-9H-xanthene 5r also successfully reacted with acetonitrile, albeit with 20% yield (6ra). Next, other alkyl nitriles, such as propionitrile, n-butyronitrile, n-valeronitrile, and 2-methoxyacetonitrile, were discovered to be effective in this reaction, affording the corresponding fluorenes in 42–75% yield (6ab-6ae). The steric hindrance of these compounds is manifest as judged by the proton and carbon NMR spectra where the phenylfluorene is desymmetrizated from hindered rotation. Tertiary nitriles, such as isobutyronitrile 2f and cyclohexanecarbonitrile 2g smoothly underwent oxidative C-H activation at the α-position to give 6af and 6ag in 42% and 61% yields, respectively. Notably, these adducts arise from the approach of two hindered tertiary centers and give rise to compounds with two adjacent quaternary centers. However, some other nitriles were unreactive including: cyanocyclopropane, 2-methoxypropionitrile, bromoacetonitrile, and ethyl cyanoacetate.
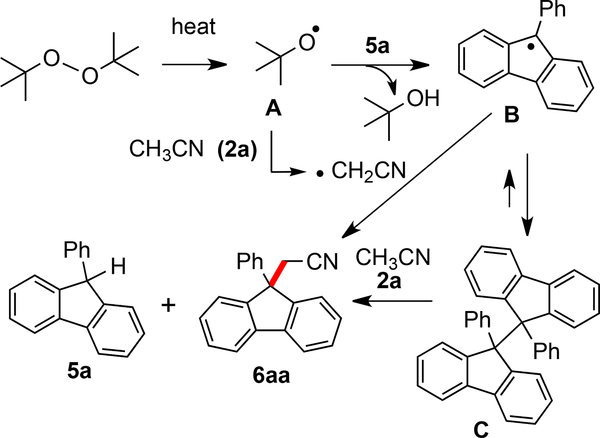
Some control experiments were carried out to gain a better understanding of the mechanism (Scheme 6). The cyanomethylenation reaction was completely inhibited when 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) or 2,6-di-tert-butyl-4-hydroxytoluene (BHT) was added into the reaction system (Scheme 6a). Moreover, the corresponding adducts 7 and 8 were detected in the reaction mixture by ESI-MS (see the Supporting Information). In addition, we found the compound 10 was isolated in 33% yield from (1-cyclopropylvinyl)benzene 9 (Scheme 6b). This adduct arises from sequential ring opening of a cyclopropylmethyl radical intermediate and cyclization,13c and this intermediate presumably arises from addition of a cyanomethylenyl radical to the alkene. Together, the above experiments suggest that the current reaction is triggered by a free-radical process. Moreover, all the experiments point to formation and reaction of a cyanomethylenyl radical. Next, an intermolecular kinetic isotopic effect (KIE) experiment was performed in a mixture of acetonitrile (0.75 mL) and acetonitrile-d3 (0.75 mL). As a result, a kH/kD = 6.7 was obtained (Scheme 6c), indicating that the acetonitrile C-H bond cleavage is involved in a product-determining step.
Scheme 6.
Control Experiments
The lack of adducts from either the fluorene or oxindole with any of the radical traps described above (Scheme 6a–b), implies that these stabilized radicals are less reactive than the cyanomethyl radical. It is likely that the resting state of the fluorenyl or oxindole radicals are the dimers as we5,25 and others26 have observed previously under oxidative conditions. Integrating the formation of dimer with reports of related systems,13f,16,27 we propose the mechanism outlined in Scheme 7. First, t-BuOOt-Bu decomposes to give the tert-butoxyl radical (A) at high temperature. The oxindole27 or fluorene undergoes facile hydrogen atom abstraction due to the weak C-H bonds (71 and 72 kcal/mol, respectively)5b forming tert-butanol and the corresponding radical B which is in equilibrium with its dimer C. Substrates lacking the 9-phenyl groups (e.g. fluorene) were not reactive, presumably due to the greater barrier to formation of the corresponding radical C, consistent with this hypothesis. In addition, the dimers of 1a (C’)5b and 5a (C)28 both gave rise to the product under the reaction conditions (see SI). At this stage the excess t-BuOOt-Bu may cause the alkyl nitrile (CH bond dissociation energy = 96 kcal/mol)29 to undergo hydrogen atom abstraction to generate the radical. Subsequent recombination with the oxindole or fluorene radical or dimer would generate the product (e.g. 6aa in Scheme 7). Alternately, the dimer (C) may react directly with the nitrile to generate one equivalent of product (6aa) and one equivalent of starting material (5a). Regardless, very hindered forms of the radical B are not expected to be able to react, which was supported by the lack of reactivity with 9(2’-methylphenyl)fluorene.
Scheme 7.
Proposed Mechanism
In summary, we have developed a novel and efficient metal-free method to activate the C(sp3)-H bond of alkyl nitriles for the synthesis of highly functionalized fluorene and oxindole derivatives. Based on the control experiments, the transformation is proposed to proceed via radical process. None of the compounds described herein have been reported previously illustrating the absence of methods to generate such hindered nitrile-derived structures. In particular, there are few examples in the literature of any nitrile derived fluorenes.18–20 Thus, this method contributes to new chemical space as well as providing a means to generate highly hindered quaternary centers, including compounds with adjacent quaternary/tertiary or quaternary/quaternary centers.
Supplementary Material
ACKNOWLEDGMENT
We are grateful to the NSF (CHE1827457) and the NIH (GM131902) for financial support of this research. Partial instrumentation support was provided by the NIH and NSF (1S10RR023444, 1S10RR022442, 3R01GM118510-03S, 3R01GM087605-06S1, CHE-0840438, CHE-0848460, 1S10OD011980, CHE-1827457) as well as the Vagelos Institute for Energy Science and Technology. G.H. and P.N. thank the Chinese Scholarship Council and the University of Guanajuato, respectively, for financial support. Dr. Charles W. Ross III (UPenn) is acknowledged for obtaining accurate mass data.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures, reaction condition screening, analytical data, and copies of spectra for all compounds (PDF)
REFERENCES
- (1) (a).Levay K; Hegedus L. Recent Achievements in the Hydrogenation of Nitriles Catalyzed by Transitional Metals Curr. Org. Chem 2019, 23, 1881–1900. [Google Scholar]; (b) Kouznetsov VV; Galvis CEP Strecker reaction and a-amino nitriles: Recent advances in their chemistry, synthesis, and biological properties Tetrahedron, 2018, 74, 773–810. [Google Scholar]; (c) Lindsay-Scott PJ; Gallagher PT Synthesis of heterocycles from arylacetonitriles: Powerful tools for medicinal chemists Tetrahedron Lett 2017, 58, 2629–2635. [Google Scholar]; (d) Opatz T The chemistryof deprotonated α-aminonitriles Synthesis, 2009, (12), 1941–1959.; (e) Friedrich K; Wallenfels K The Chemistry of the Cyano Group; WileyInterscience: New York, 1970; pp 341−421. [Google Scholar]
- (2) (a).Fleming FF; Yao LH; Ravikumar PC; Funk L; Shook BC Nitrile-Containing Pharmaceuticals: Efficacious Roles of the Nitrile Pharmacophore. J. Med. Chem. 2010, 53, 7902. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Caggiano TJ; Brazzale A; Ho DM; Kraml CM; Trybulski E; Christopher CC; Chippari S; Marcucci LB; Eckert A; Keith JC; Kenney T; Harnish DC Estrogen Receptor Dependent Inhibitors of NF-κB Transcriptional Activation-1 Synthesis and Biological Evaluation of Substituted 2-Cyanopropanoic Acid Derivatives: Pathway Selective Inhibitors of NF-κB, a Potential Treatment for Rheumatoid Arthritis. J. Med. Chem. 2007, 50, 5245. [DOI] [PubMed] [Google Scholar]
- (3).López R; Palomo C Cyanoalkylation: Alkylnitriles in Catalytic C-C Bond-Forming Reactions Angew.Chem.Int.Ed. 2015, 54, 13170. [DOI] [PubMed] [Google Scholar]
- (4).For recent review, see: Chu X-Q; Ge D; Shen Z-L; Loh T-P Recent Advances in Radical-Initiated C(sp3 )−H Bond Oxidative Functionalization of Alkyl Nitriles. ACS Catal 2018, 8, 258. [Google Scholar]
- (5) (a).Curto JM; Kozlowski MC Chemoselective Activation of sp3 vs sp2 C−H Bonds with Pd(II). J. Am. Chem. Soc. 2015, 137, 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hong G; Nahide PD; Neelam UK; Amadeo P; Vijeta A; Curto JM; Hendrick CE; VanGelder KF; Kozlowski MC Palladium-Catalyzed Chemoselective Activation of sp3 vs sp2 C–H Bonds: Oxidative Coupling To Form Quaternary Centers ACS Catal. 2019, 9, 3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6) (a).Dalpozzo R; Bartoli G; Bencivenni G Recent Advances in Organocatalytic Methods for the Synthesis of Disubstituted 2- and 3-Indolinones. Chem. Soc. Rev. 2012, 41, 7247. [DOI] [PubMed] [Google Scholar]; (b) Badillo JJ; Hanhan NV; Franz AK Enantioselective Synthesis of Substituted Oxindoles and Spirooxindoles with Applications in Drug Discovery. Curr. Opin. Drug Discovery Dev. 2010, 13, 758. [PubMed] [Google Scholar]; (c) Jensen BS BMS-204352: A Potassium Channel Opener Developed for the Treatment of Stroke. CNS Drug Rev. 2002, 8, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Chen J-Y; Yu X-Y; Xiao W-J Difunctionalization of Acrylamides through C–H Oxidative Radical Coupling: New Approaches to Oxindoles. Synthesis 2015, 47, 604. [Google Scholar]
- (7) (a).Ie Y; Umemoto Y; Nitani M; Aso Y Perfluoroalkyl-Annelated Conjugated Systems toward n-type Organic Semiconductors. Pure Appl. Chem. 2008, 80, 589. [Google Scholar]; (b) Anthony JE Functionalized Acenes and Heteroacenes for Organic Electronics. Chem. Rev. 2006, 106, 5028. [DOI] [PubMed] [Google Scholar]
- (8) (a).Bernius MT; Inbasekaran M; O’Brien J; Wu W Progress with Light-Emitting Polymers. Adv. Mater. 2000, 12, 1737. [Google Scholar]; (b) Scherf U; List EJW Semiconducting Polyfluorenes—Towards Reliable Structure–Property Relationships. Adv. Mater. 2002, 14, 477. [Google Scholar]; (c) Belfield KD; Yao S; Bondar MV Two-photon Absorbing Photonic Materials: From Fundamentals to Applications. Adv. Polym. Sci. 2008, 213, 97. [Google Scholar]; (d) Bendikov M; Wudl F; Perepichka DF Tetrathiafulvalenes, Oligoacenenes, and Their Buckminsterfullerene Derivatives: The Brick and Mortar of Organic Electronics. Chem. Rev. 2004, 104, 4891. [DOI] [PubMed] [Google Scholar]
- (9) (a).Li M; Liu Y; Ni W; Liu F; Feng H; Zhang Y; Liu T; Zhang H; Wan X; Kan B; Zhang Q; Russell TP; Chen Y A Simple Small Molecule as an Acceptor for Fullerene-Free Organic Solar Cells with Efficiency Near 8%. J. Mater. Chem. A 2016, 4, 10409. [Google Scholar]; (b) Kim Y; Song CE; Moon S-J; Lim E Effect of Dye End Groups in Non-Fullerene Fluorene- and Carbazole-Based Small Molecule Acceptors on Photovoltaic Performance. RSC Adv. 2015, 5, 62739. [Google Scholar]; (c) Kim Y; Song CE; Moon S-J; Lim E Rhodanine Dye-Based Small Molecule Acceptors for Organic Photovoltaic Cells. Chem. Commun. 2014, 50, 8235. [DOI] [PubMed] [Google Scholar]
- (10) (a).Kakadiya R; Dong H; Lee P-C; Kapuriya N; Zhang X; Chou T-C; Lee T-C; Kapuriya K; Shah A; Su T-L Potent Antitumor Bifunctional DNA Alkylating Agents, Synthesis and Biological Activities of 3a-Aza-Cyclopenta[A]Indenes. Bioorg. Med. Chem. 2009, 17, 5614. [DOI] [PubMed] [Google Scholar]; (b) Bradner WT Mitomycin C: A Clinical Update. Cancer Treat. Rev. 2001, 27, 35. [DOI] [PubMed] [Google Scholar]; (c) Tomasz M; Palom Y The Mitomycin Bioreductive Antitumor Agents: Cross-Linking and Alkylation of DNA as the Molecular Basis of Their Activity. Pharmacol. Ther. 1997, 76, 73. [DOI] [PubMed] [Google Scholar]
- (11)(a).Ni S; Yuan Y; Huang J; Mao X; Lv M; Zhu J; Shen X; Pei J; Lai L; Jiang H; Li J Discovering Potent Small Molecule Inhibitors of Cyclophilin A Using de Novo Drug Design Approach. J. Med. Chem. 2009, 52, 5295. [DOI] [PubMed] [Google Scholar]; (b) Burch RM; Weitzberg M; Blok N; Muhlhauser R; Martin D; Farmer SG; Bator JM; Connor JR; Green M; Ko C N-(fluorenyl-9-methoxycarbonyl) amino acids, a class of antiinflammatory agents with a different mechanism of action. Proc. Natl. Acad. Sci. U. S. A, 1991, 88, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Banala AK, Levy BA, Khatri SS, Furman CA, Roof RA, Mishra Y, Griffin SA, Sibley DR, Luedtke RR; Newman AH N-(3-Fluoro-4-(4-(2-methoxy or 2,3-dichlorophenyl)piperazine1-yl)butyl)arylcarboxamides as Selective Dopamine D3 Receptor Ligands: Critical Role of the Carboxamide Linker for D3 Receptor Selectivity. J. Med. Chem, 2011, 54, 3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12) (a).Lee TBK; Wong GSK Asymmetric Alkylation of Oxindoles: An Approach to the Total Synthesis of (−)-Physostigmine. J. Org. Chem. 1991, 56, 872. [Google Scholar]; (b) Deng F; Moteki SA; Maruoka K Catalytic Asymmetric Alkylation of 3-Aryl-Substituted Oxindoles to Give 3,3-Disubstituted Oxindoles under Phase-Transfer Conditions. Asian J. Org. Chem. 2014, 3, 395. [Google Scholar]; (c) Bleith T; Deng QH; Wadepohl H; Gade LH Radical Changes in Lewis Acid Catalysis: Matching Metal and Substrate. Angew. Chem. 2016, 128, 7983. [DOI] [PubMed] [Google Scholar]
- (13) (a).Xiao Y; Liu Z -Q. Free Radical Addition of Nitrile, Ketone, and Ester to Alkyne and the Selectivity Discussion. Org. Lett. 2019, 21, 8810. [DOI] [PubMed] [Google Scholar]; (b) Wang K; Chen X; Yuan M; Yao M; Zhu H; Xue Y; Luo Z; Zhang Y Silver-Mediated Cyanomethylation of Cinnamamides by Direct C(sp3)–H Functionalization of Acetonitrile. J. Org. Chem. 2018, 83, 1525. [DOI] [PubMed] [Google Scholar]; cb) Qiao K; Zhang D; Zhang K; Yuan X; Zheng MW; Guo TF; Fang Z; Wan L; Guo K Iron(II)-Catalyzed C-2 Cyanomethylation of Indoles and Pyrroles via Direct Oxidative Cross-Dehydrogenative Coupling with Acetonitrile Derivatives. Org. Chem. Front. 2018, 5, 1129. [Google Scholar]; (cd Wu XS; Riedel J; Dong VM Transforming Olefins into γ,δ-Unsaturated Nitriles through Copper Catalysis. Angew. Chem. Int. Ed. 2017, 56, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Zhu NB; Wang T; Ge L; Li YJ; Zhang XH; Bao LH γ-Amino Butyric Acid (GABA) Synthesis Enabled by Copper-Catalyzed Carboamination of Alkenes. Org. Lett. 2017, 19, 4718. [DOI] [PubMed] [Google Scholar]; (f) Hu M; Zou HX; Song RJ; Xiang JN; Li JH Copper-Catalyzed C−H Oxidative Radical Functionalization and Annulation of AnilineLinked 1,7-Enynes: Evidence for a 1,5-Hydride Shift Mechanism. Org. Lett. 2016, 18, 6460. [DOI] [PubMed] [Google Scholar]; (g) Lan XW; Wang NX; Bai CB; Lan CL; Zhang T; Chen SL; Xing YL Unactivated C(sp3)−H Bond Functionalization of Alkyl Nitriles with Vinylarenes and Mechanistic Studies. Org. Lett. 2016, 18, 5986. [DOI] [PubMed] [Google Scholar]
- (14) (a).Bunescu A; Wang Q; Zhu JP Copper-Mediated/Catalyzed Oxyalkylation of Alkenes with Alkylnitriles. Chem. Eur. J. 2014, 20, 14633. [DOI] [PubMed] [Google Scholar]; (b) Bunescu A; Wang Q; Zhu JP Copper-Catalyzed Cyanomethylation of Allylic Alcohols with Concomitant 1,2-Aryl Migration: Efficient Synthesis of Functionalized Ketones Containing an αQuaternary Center. Angew. Chem. Int. Ed. 2015, 54, 3132. [DOI] [PubMed] [Google Scholar]; (c) Sazepin CC; Wang Q; Sammis GM; Zhu JP Copper-Catalyzed Intermolecular Carboetherification of Unactivated Alkenes by Alkyl Nitriles and Alcohols. Angew. Chem. Int. Ed. 2015, 54, 5443. [DOI] [PubMed] [Google Scholar]; (d) Ha TM; Sazepin CC; Wang Q; Zhu JP Copper-Catalyzed Formal [2+2+1] Heteroannulation of Alkenes, Alkylnitriles, and Water: Method Development and Application to a Total Synthesis of (±)-Sacidumlignan D. Angew. Chem. Int. Ed. 2016, 55, 9249. [DOI] [PubMed] [Google Scholar]; (e) Bunescu A; Ha TM; Wang Q; Zhu JP Copper-Catalyzed Three-Component Carboazidation of Alkenes with Acetonitrile and Sodium Azide. Angew. Chem. 2017, 129, 10691. [DOI] [PubMed] [Google Scholar]; (f) Ha TM; Wang Q; Zhu JP Copper-Catalysed Cyanoalkylative Cycloetherification of Alkenes to 1,3-Dihydroisobenzofurans: Development and Application to the Synthesis of Citalopram. Chem. Commun. 2016, 52, 11100. [DOI] [PubMed] [Google Scholar]
- (15).Liu YB; Yang K; Ge HB Palladium-Catalyzed Ligand-Promoted Siteselective Cyanomethylation of Unactivated C(sp3)–H Bonds with Acetonitrile. Chem. Sci 2016, 7, 2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Wang CY; Li YB; Gong M; Wu Q; Zhang JY; Kim JK; Huang MM; Wu YJ Method for Direct Synthesis of α-Cyanomethyl-β-dicarbonyl Compounds with Acetonitrile and 1,3-Dicarbonyls. Org. Lett. 2016, 18, 4151. [DOI] [PubMed] [Google Scholar]
- (17).Zhang W; Yang S; Shen Z Copper-Catalyzed Cyanomethylation of Substituted Tetrahydroisoquinolines with Acetonitrile. Adv. Synth. Catal. 2016, 358, 2392. [Google Scholar]
- (18).Shen X; Gu NN; Liu P; Ma XW; Xie JW; Liu Y; He L; Dai B A Simple and Efficient Synthesis of 9-Arylfluorenes via Metal-Free Reductive Coupling of Arylboronic Acids and N-Tosylhydrazones in situ. RSC Adv 2015, 5, 63726. [Google Scholar]
- (19) (a).Hwang SJ; Kim HJ; Chang S Highly Efficient and Versatile Synthesis of Polyarylfluorenes via Pd-Catalyzed C−H Bond Activation. Org. Lett. 2009, 11, 4588. [DOI] [PubMed] [Google Scholar]; (b) Fuchibe K; Akiyama T Low-Valent Niobium-Mediated Double Activation of C−F/C−H Bonds: Fluorene Synthesis from o-Arylated α,α,α-Trifluorotoluene Derivatives. J. Am. Chem. Soc. 2006, 128, 1434. [DOI] [PubMed] [Google Scholar]; (c) Yeung CS; Dong VM Catalytic Dehydrogenative Cross-Coupling: Forming Carbon−Carbon Bonds by Oxidizing Two Carbon−Hydrogen Bonds. Chem. Rev. 2011, 111, 1215. [DOI] [PubMed] [Google Scholar]; (d) Itoh M; Hirano K; Satoh T; Shibata Y; Tanaka K; Miura M Rhodium- and Iridium-Catalyzed Dehydrogenative Cyclization through Double C–H Bond Cleavages to Produce Fluorene Derivatives. J. Org. Chem. 2013, 78, 1365. [DOI] [PubMed] [Google Scholar]
- (20).Chu XQ; Xing ZH; Meng H; Xu XP; Ji SJ Copper-Mediated Radical Alkylarylation of Unactivated Alkenes with Acetonitrile Leading to Fluorenes and Pyrroloindoles. Org. Chem. Front. 2016, 3, 165. [Google Scholar]
- (21) (a).Hu M; Li M; Tan FL; Song RJ; Xie YX; Li JH Oxidative Divergent Bicyclizations of 1,n-Enynes through α-C(sp3)–H Functionalization of Alkyl Nitriles. Adv. Synth. Catal. 2017, 359, 120. [Google Scholar]; (b) Liu YY; Yang XH; Song RJ; Luo SL; Li JH Oxidative 1,2-Carboamination of Alkenes with Alkyl Nitriles and Amines Toward G-Amino Alkyl Nitriles. Nat. Commun. 2017, 8, 14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22) (a).Zhou YJ; Duan KY; Zhu L; Liu ZF; Zhang CB; Yang LJ; Li MY; Zhang. H. B.; Yang, X. D. Synthesis and Cytotoxic Activity of Novel Hexahydropyrrolo[2,3-B] Indole Imidazolium Salts. Bioorg. Med. Chem. Lett. 2016, 26, 460. [DOI] [PubMed] [Google Scholar]; (b) Pinto A; Jia YX; Neuville L; Zhu JP Palladium-Catalyzed Enantioselective Domino Heck–Cyanation Sequence: Development and Application to the Total Synthesis of Esermethole and Physostigmine. Chem. Eur. J. 2007, 13, 961. [DOI] [PubMed] [Google Scholar]; (c) Becerril ER; Nathan PJ; Alvarez VMP; Rios MSM Synthesis and Biological Evaluation of (−)- and (+)-Debromoflustramine B and Its Analogues as Selective Butyrylcholinesterase Inhibitors. J. Med. Chem. 2008, 51, 5271. [DOI] [PubMed] [Google Scholar]
- (23) (a).Wang X; Ma G; Peng Y; Pitsch CE; Moll BJ; Ly TD; Wang X; Gong H Ni-Catalyzed Reductive Coupling of Electron-Rich Aryl Iodides with Tertiary Alkyl Halides. J. Am. Chem. Soc. 2018, 140, 14490. [DOI] [PubMed] [Google Scholar]; (b) Tong S; Limouni A; Wang Q; Wang MX; Zhu JP Catalytic Enantioselective Double Carbopalladation/C-H Functionalization with Statistical Amplification of Product Enantiopurity: A Convertible Linker Approach. Angew. Chem. Int. Ed. 2017, 56, 14192. [DOI] [PubMed] [Google Scholar]; (c) Babu KN; Kariyandi NR; Saina SMK; Kinthada LK; Bisai A Lewis Acid-Catalyzed Malonate Addition onto 3-Hydroxy-2- oxindoles: Mechanistic Consideration and Synthetic Approaches to the Pyrroloindoline Alkaloids. J. Org. Chem. 2018, 83, 12664–12682. [DOI] [PubMed] [Google Scholar]; (d) Yen A; Lautens M Nickel-Catalyzed Intramolecular Arylcyanation for the Synthesis of 3,3-Disubstituted Oxindoles. Org. Lett. 2018, 20, 4323. [DOI] [PubMed] [Google Scholar]; (e) Zhu S; MacMillan DWC Enantioselective CopperCatalyzed Construction of Aryl Pyrroloindolines via an ArylationCyclization Cascade. J. Am. Chem. Soc. 2012, 134, 10815. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) He R; Ding C; Maruoka K Phosphonium Salts as Chiral Phase-Transfer Catalysts: Asymmetric Michael and Mannich Reactions of 3- Aryloxindoles. Angew. Chem., Int. Ed. 2009, 48, 4559–4561. [DOI] [PubMed] [Google Scholar]; (g) Ma S; Han X; Krishnan S; Virgil SC; Stoltz BM Catalytic Enantioselective Stereoablative Alkylation of 3‐Halooxindoles: Facile Access to Oxindoles with C3 All‐Carbon Quaternary Stereocenters. Angew. Chem., Int. Ed. 2009, 48, 8037–8041. [DOI] [PubMed] [Google Scholar]
- (24).Vougioukalakis GC; Roubelakis MM; Orfanopoulos M Radical Reactivity of Aza[60]fullerene: Preparation of Monoadducts and Limitations. J. Org. Chem. 2010, 75, 4124. [DOI] [PubMed] [Google Scholar]
- (25) (a).Kang H; Jemison AL; Nigro E; Kozlowski MC Oxidative Coupling of 3‐Oxindoles with Indoles and Arenes ChemSusChem 2019, 12, 3144–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) (c) Ohnishi R; Sugawara M; Akakabe M; Ezawa T; Koshino H; Sohtome Y; Sodeoka M Cross‐Coupling Reaction of Dimer‐Derived Persistent Tertiary‐Carbon‐Centered Radicals with Azo Compounds Asian. J. Org.Chem 2019, 8, 1017–1023. [Google Scholar]; (c) Tanaka T; Hashiguchi K; Tanaka T; Yazaki R; Ohshima T Chemoselective Catalytic Dehydrogenative Cross-Coupling of 2- Acylimidazoles: Mechanistic Investigations and Synthetic Scope. ACS Catal 2018, 8, 8430–8440. [Google Scholar]
- (26) (a).Kang H; Jemison AL; Nigro E; Kozlowski MC ChemSusChem 2019, 12, 3144–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kozlowski MC; DiVirgilio ES; Malolanarasimhan K; Mulrooney CA “Oxidation of Chiral ☒-Phenylacetate Derivatives: Formation of Dimers with Contiguous Quaternary Stereocenters versus Tertiary Alcohols” Tetrahedron: Asymmetry 2005, 16, 3599–3605. [Google Scholar]
- (27) (a).Chu XQ; Meng H; Zi Y; Xu XP; Ji SJ; Oxidative C(sp3)-H Functionalization of Acetonitrile and Alkanes with Allylic Alcohols Under Metal-Free Conditions. Org. Chem. Front. 2015, 2, 216. [Google Scholar]; (b) Huang HY; Cheng L; Liu JJ; Wang D; Liu L; Li CJ Transition-Metal-Free Alkynylation of 2-Oxindoles through Radical−Radical Coupling. J. Org. Chem. 2017, 82, 2656. [DOI] [PubMed] [Google Scholar]; (c) Kong DL; Cheng L; Yue T; Wu HR; Feng WC; Wang D; Liu L Cobalt-Catalyzed Peroxidation of 2-Oxindoles with Hydroperoxides. J. Org. Chem. 2016, 81, 5337. [DOI] [PubMed] [Google Scholar]; (d) Wei WT; Zhu WM; Ying WW; Wang YN; Bao WH; Gao LH; Luo YJ; Liang H Metal-Free Nitration of the C(sp3)–H Bonds of 2-Oxindoles through Radical Coupling Reaction at Room Temperature. Adv. Synth. Catal. 2017, 359, 3551. [Google Scholar]
- (28).Frenette M; Aliaga C; Font-Sanchis E; Scaiano JC Bond Dissociation Energies for Radical- Dimers Derived from Highly Stabilized Carbon-Centered Radicals Org. Lett. 2004, 6, 2579–2582. [DOI] [PubMed] [Google Scholar]
- (29) (a).Luo Y-R Handbook of Bond Dissociation Energy in Organic Compound, CRC Press, Boca Raton, 2002. [Google Scholar]; (b) Li Z-J; Xiao Y-X; Liu Z-Q A radical anti-Markovnikov addition of alkyl nitriles to simple alkenes via selective sp3 C–H bond functionalization. Chem. Commun. 2015, 51, 9969. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.