Abstract
We used functional magnetic resonance to investigate the effects of exposure to violence on early adolescent brain function in an inhibitory control task. We investigated the association among scores on self-reported exposure to violence, performance and brain activation. Thirty-seven early adolescents (ages 10–14) from a Latin-American urban region participated in the study. Results showed that recent and chronic exposure to violence was associated with less activation of a network of frontal regions, including the anterior cingulate gyrus and the superior frontal cortex; recent exposure to violence was also associated with less activation of the superior parietal lobe. Results also showed that less activation correlated with more prominent deterioration in the performance in the inhibitory control task (increased latency with time). The findings suggest that early adolescence exposure to violence is associated with differences in activation of a neural network commonly associated with executive function and control. The results underscore the urgency of addressing exposure to violence in adolescence, a period of high susceptibility to the environment, and are discussed in the light of the evidence of the effects of violence on adolescent brain function. Executive function training may be a candidate for targeted cognitive interventions aimed at mitigating these effects.
Keywords: violence, inhibitory Control, adolescence, frontoparietal network, anterior cingulate cortex
Early adolescence (9 to 14 years) is a period of heightened susceptibility to social context (Schriber and Guyer, 2016; Dahl and Suleiman, 2017). It is a critical juncture for negative behavior patterns, which in their turn are associated with increased risks for poor mental health outcomes (Dahl & Suleiman, 2017). The increase in risk-taking in adolescence is underpinned by brain maturation processes that make adolescents more prone to risk-taking, especially in the company of peers (Spear, 2013; Spielberg et al., 2014; Steinberg, 2008). Risk-taking behavior changes in adulthood as brain circuitry associated with self-regulation and executive functions develops (Steinberg, 2008; Spear, 2013). But negative, stressful environments can mar adolescent development and alter neurodevelopmental patterns.
Executive functions are higher-level processes involved in controlling and coordinating thoughts and action. They include inhibiting impulsive and automatic responses, shifting attention and updating the information in working memory (Friedman and Miyake, 2017). More specifically, inhibitory control refers to the ability to produce adaptive responses and suppress prepotent behavior that is presently not required to carry out goal-directed actions (Friedman and Miyake, 2017; Stocco et al., 2012; Fuster, 2000). To the point, inhibitory control involves the ability to control one’s attention, behavior, thoughts and/or emotions. It is paramount for resisting impulses, habits and actions in order to select a more appropriate behavior consistent with the completion of goals (Boecker et al., 2013; Diamond, 2013; Hughes, 2013), such as ordinary decisions of not eating a cake to keep with one's dietary plan (Aron, 2011) and more resilient behaviors as the ability to focus and study despite the lack of a safe place at home (Zhang et al., 2012).
Executive functions are underpinned by a distributed, anterior-posterior brain network that includes lateral pre-frontal cortex, anterior cingulate cortex, medial pre-frontal regions, posterior parietal cortex and the basal ganglia (D’Esposito et al., 1995; Fuster, 2000; Luna et al., 2010; Stocco et al., 2012; Hsu et al., 2014; Friedman and Miyake, 2017). The activation of the frontoparietal executive network has been involved in switching attention, response inhibition and interference (Sylvester et al., 2003; Simmonds et al., 2008). It is also involved in when more efficient distribution of limited brain resources is required: Increasing task difficulty has been shown to modulate activation of the frontoparietal systems across different domains, including executive function and language comprehension tasks (D’Esposito et al., 1995; Keller et al., 2001, 2003; Jaeggi et al., 2003; Just et al., 2008; Buchweitz et al., 2014).
Stress has been shown to affect cognition and the brain throughout the lifespan (Pollak, 2005; Taylor et al., 2006; Lupien et al., 2009; Rahdar and Galván, 2014; Hanson et al., 2015; Birn et al., 2017; Gupta et al., 2017). In adolescents, stress is associated with increased physiological response and risk for mental health problems (Lupien et al., 2009); the adolescent brain is more sensitive to stress-induced differences in activation and dysfunctions (Eiland and Romeo, 2013; Rahdar and Galván, 2014).
Negative, stressful experiences have been associated with differences in executive-function related performance and brain function, including working memory (Richmond et al., 1967; Spielberg et al., 2015; Noble et al., 2015), sustained attention (Lim et al., 2016) and response inhibition (Carrion et al., 2008; Mueller et al., 2012; Elton et al., 2014; Rahdar and Galván, 2014; Lim et al., 2015; Jankowski et al., 2017). High levels of stress culminate in declined intellectual ability and deteriorated academic performance (Pechtel and Pizzagalli, 2011). The effects of early-life stress on cognition and development have been extensively investigated (Shonkoff et al., 2012; Pollak, 2015); but less is known about the effects of stress on adolescent cognition and development (Romeo and McEven, 2006), especially in more vulnerable, low and middle income countries (Pellizzoni et al., 2019; Willoughby et al., 2019). The combination of stress-related susceptibilities with growing up in more stressful, violent environments may create the ‘perfect storm’ for adolescent brain development in more violent regions of the world.
Violence affects adolescents worldwide (Bustreo and Chestnov, 2013). In Latin America, violence affects youths disproportionately relative to adults (Cerqueira, 2016). Yet the neurobiological effects of violence and stress are poorly understood in Latin American adolescent populations. Studies of the effects of violence, poverty and other negative environmental factors on brain function are especially scarce in lower and middle income countries (see e.g. Buchweitz, et al., 2019b; Wijeakumar et al., 2019). The goal of the present study was to investigate the association among Latin-American early adolescents’ exposure to violence and brain activation and performance in an inhibitory control task.
Methods
We sent invitations to participate in the study through municipal schools to approximately 500 families; approximately 300 families attended meetings at the schools, during which we explained the project. A total 142 parents or guardians consented participation of their children and gave a signed informed consent in a sealed, anonymous envelope provided with the informed consent form. The study was approved by the ethics committee of the Pontifical Catholic University of Rio Grande do Sul.
Participants
The study involved evaluations at the participant’s school and evaluations and brain scanning sessions at the Brain Institute. Of the 142 participants whose guardians consented participation, 90 were excluded due to (i) IQ score below 75 (7 participants), (ii) voluntary withdrawal from the investigation, by the guardian or participant, during the evaluations at school (43 participants); (iii) illiteracy/inability to fill out the questionnaires and tests (10 participants); and (iv) frequent absence from school (at least two additional attempts at data collection were made if the participant missed school on data collection days) (5 participants). There were another 25 voluntary withdrawals from the study due to unavailability of a guardian to accompany the minor. Therefore, 52 participants were scanned, initially. Participation in the study was voluntary and at no cost to participants. We provided free transportation to the Brain Institute.
The present paper reports on 37 right-handed early adolescents (boys: n = 25; girls: n = 12; average age = 11.43 year; s.d. = 1.06 years; age range 9 to 14 years). Of the 52 participants scanned, nine were excluded due to excessive head motion (see below). A 10th participant was excluded due to focal demyelination on the left hemisphere temporal lobe. (A neuroradiological reading of the structural scans was carried out to ensure there were no lesions, malformations or other abnormalities in the brain). Additionally, five participants were excluded due to absence of socioeconomic scores, which were used as a covariable in the brain imaging analyses. The socioeconomic interview was carried with the accompanying guardian or parent while the participant was in the MRI scanner. Accompanying guardians were, at times, more distant relatives or friends of the family who were unable to provide the information for the SES questionnaire. In these cases, we subsequently attempted to reach the parents or guardians by phone. We were unable to reach parents or guardians of five participants.
Materials and procedures
Evaluations included IQ tests and questionnaires about exposure to violence. IQ was evaluated using the Wechsler Abbreviated Scale of Intelligence™ (mean = 95.27; s.d. = 10.69; range 75–114). Exposure to violence was investigated using the Juvenile Victimization Questionnaire (JVQ) second revision (JVQ-R2) (Finkelhor et al., 2005). We also investigated socioeconomic status using a standardized questionnaire for socioeconomic classification in Brazil (ABEP, 2016), which provides a score based on schooling and possession of consumer goods. The scores allow for categorization of SES from A (highest) to D (lowest) and subcategories in between. The average SES score corresponded to level C1 SES in Brazil (the range of scores was from D lower SES to B1 higher SES).
Exposure to violence: the JVQ-R2
The JVQ-R2 is an instrument for evaluating self-reported interpersonal victimization in children and adolescents (Finkelhor et al., 2005). The JVQ-R2 gathers information on 34 items of specific forms of victimization, distributed into five modules: 9 items for Conventional Crime, 4 items for Maltreatment, 6 items for Peer and Sibling Victimization, 7 items for Sexual Victimization and 8 items for Witnessing and Indirect Victimization. It also allows for evaluation of more recent victimization (last year) and more chronic exposure to violence (lifetime). Assuming the Item-level Scores proposed by the manual1 for each item the reported presence of victimization is scored as 1, the absence is scored as zero; the sum of these scores makes up the total score. The JVQ-R2 can also be used to assess individual module scores. Each module can be scored to produce a LY or LT rate for, for example, Conventional Crime, or other type of victimization. Thus, a ‘yes’ or 1 for the Conventional Crime module indicates that at least one report of exposure to Conventional Crime occurred, whereas a ‘no’ or zero indicates no report of exposure to the type of victimization in that module.
The JVQ-R2 full interview was translated and adapted to Portuguese with the permission of its authors. In the present study, the JVQ-R2 was filled out in two separate occasions. The first occasion was at the schools. Participants filled out the reduced format of the questionnaire in groups of 10 to 20 individuals (i.e., the module that assesses the dichotomous presence/absence of types and instances of victimization only). The questionnaires were later scored and evaluated. Subsequently, a trained member of the clinical research team administered the full format of the questionnaire in an individual interview, in the second stage of the study. The full format of the JVQ-R2 gathers additional information about each of the types and instances of victimization reported in the reduced format.
Child Behavior Checklist for ages 6 to 18
Behavioral and mental health problems were assessed using the Child Behavior Checklist (CBL) for ages 6–18 (CBCL/6-18) and adapted for the Brazilian context (Bordin et al., 1995). The CBCL/6-18 is a psychological assessment questionnaire (Achenbach and Rescorla, 2001) used for screening of child and adolescent mental health. It is filled out by parents or guardians, and it has been adapted for the Brazilian context (Bordin et al., 1995). The questionnaire consists of 138 items: 20 assess social competence and 118 assess behavior problems. The checklist includes 11 subscales that evaluate symptoms of internalizing problems (withdrawn, somatic complaints and anxiety/depressed behaviors) and externalizing problems (delinquent and aggressive behaviors), as well as total problem scores (include externalizing, internalizing, social, school, thought and attention problems) (Achenbach, 2004).
We analyzed CBCL/6-18 scores for the three broad-spectrum scales: internalizing and externalizing problems and total problem scores. The data were analyzed using the Assessment Data Manager (ADM) software (ASEBA, Burlington, Vermont) to assess raw scores and generate clinical, non-clinical and borderline clinical profiles (Achenbach and Rescorla, 2001). The CBCL/6-18 scales in the present study showed good internal consistency reliability (Cronbach’s alpha estimated in 0.801). The internal consistency (Cronbach’s alpha) was also calculated for each broad-spectrum scale: internalizing problems (α = 0.760), externalizing problems (α = 0.527) and total problems (α = 0.729).
Functional magnetic resonance design: Change task
Functional magnetic resonance (fMRI) studies have applied different tasks to investigate executive functions, such as Stop tasks (McNab et al., 2008; Mueller et al., 2010; Levy and Wagner, 2011; Cai et al., 2014; Elton et al., 2014; Meyer and Bucci, 2016) and Go/No Go tasks (Menon et al., 2001; Mazzola-Pomietto et al., 2009; Gilman et al., 2018). In the present study, we investigated brain function associated with a variant of the Go/No Go and Stop tasks, called the Change task. Participants must inhibit the more frequent, prepotent left-hand button press and make a right-hand button press (Logan and Burkell, 1986; Boecker et al., 2011; Thomas et al., 2011; Boecker et al., 2013).
The task includes Go trials and Change trials. Go trials consist of the visual presentation of either an X or an O, to which participants have to respond by pressing a button with their left middle finger for X and index finger for O. Go trials made up 66% of the trials. The less-preponderant Change trials consist of the presentation of a blue square, to which participants have to press a button with their right index finger; Change trials made up 33% of the experiment. Go trials are more frequent, thus the task tests the ability of the participant to inhibit the prepotent response (Go—left middle and index fingers) and change it to a different, less frequent response (Change—right index finger).
The task lasted 8 min and 3 s and included 167 trials, 112 Go and 55 Change. Each trial began with a 500 ms fixation cross at the center of the screen, which was followed by the stimuli (presented for 1000 ms). The order of the presentation of the stimuli was randomized once, and each participant was presented with the same order. Stimulus presentation was offset by jittered intervals, which ranged from 0.75 to 2 s (in 0.25 s intervals) and were randomly inserted after each trial. A 6 s dummy scan was inserted at the beginning of the task to ensure T1 magnetization reached an equilibrium state. An additional 10 s rest was inserted at the end of the task. Response times and accuracy were recorded and computed for all trials using an MRI-safe buttonbox; stimulus was presented using E-Prime (Psychology Software Tools). Prior to the scanning session participants were given an out-of-scanner practice in an MRI simulator (Psychology Software Tools, Pittsburgh, PA). The goal was to help participants become acclimated with the scanner environment and noise. We used a shorter version of the task for the practice session.
Data collection
fMRI parameters
Data were collected on a GE HDxT 3.0T MRI scanner with an eight-channel head coil. Three MRI sequences were acquired: a T1 structural scan (TR/TE = 6.16/2.18 ms, isotropic 1 mm3 voxels); two task-related functional FMRI EPI sequences (run 1 = 8 min; run 2 = 8 min 04 s). For the task EPI sequence we used the following parameters: TR = 2000 ms, TE = 30 ms, 29 interleaved slices, slice thickness = 3.6 mm; slice gap = 0.3 mm; matrix size = 64 × 64, FOV = 220 × 220 mm, voxel size = 3.75 × 3.75 × 3.90 mm. During the scan, real-time motion detection software was used to monitor participant cooperation. In case participants presented more than 0.9 mm of motion in more than 20 TRs before completing the run, we interrupted the experiment and ran the task again. We made one attempt to re-run the task if it was stopped due to excessive head motion.
fMRI analyses
Functional data were processed using AFNI’s (http://afni.nimh.nih.gov/) afni_proc.py program (Cox, 1996). Preprocessing included slice-time and motion correction, smoothing with a 6 mm FWHM Gaussian kernel, and a non-linear spatial normalization to 3.5 × 3.5 × 3.5 mm voxel template (HaskinsPedsNL template) (Molfese et al., 2015). Time points between volumes with motion >0.9 mm were censored from the data. Nine participants who finished the scanning session were excluded due to excessive motion. The criterion for exclusion due to head motion was excessive motion in 20% of the TRs. The average head motion for the participants included in the study was mean = 0.1262 (s.d. = 0.065).
First level analysis included modeling regressors for each condition, Change and Go, convolved with the canonical hemodynamic response function as implemented in AFNI (Cox, 1996). To correct for multiple comparisons, the 3dClustSim program using the autocorrelation function blurring estimates and performing 10 000 Monte Carlo simulations was used to calculate the cluster threshold for a corrected p-score of α < 0.05. Results showed that the threshold of p < 0.005 combined with a minimum cluster size of 71 voxels (3038.8 μl) corresponded to a corrected score of α < 0.05.
Correlations: fMRI, exposure to violence and behavior
We carried out correlations among the JVQ-R2 scores and the images collapsed across Change and Go trials. We collapsed across conditions to investigate the brain activation associated with the entire task. The correlation was calculated using the 3dRegAna function from the AFNI package (Cox, 1996). We used the 3dClustSim program autocorrelation function blurring estimates and performed 10 000 Monte Carlo simulations to calculate the p-value and cluster size combination that equate to a corrected p-score of α < 0.05 (Cox et al., 2017). The calculation showed that a threshold of p < 0.005 combined with a minimum cluster size of 71 voxels (3044.1 μl) corresponded to a corrected score of α < 0.05. All results thus represent α < 0.05 corrected for multiple comparisons. The correlation was carried out with the Lifetime and the Last Year scores for JVQ-R2 to investigate the effects of longer (chronic) exposure and more recent exposure to violence, respectively. We included the age, IQ and SES of participants as covariables in the fMRI analyses to control for effects associated with these variables.
Betas
We extracted the average betas for functional regions of interest for individual participants for the regions that negatively correlated with the JVQ-R2 scores (see Figure 1 and Table 1). These betas were subsequently correlated with the slope of the response times (an index of latency in response, see below).
Fig. 1.
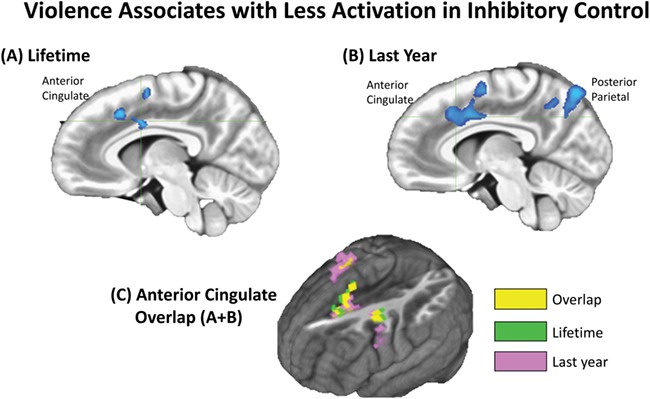
Negative correlation among JVQ-R2 Lifetime and Last Year scores with activation for all conditions (Go and Change) in the Change task. Clusters significant at p < 0.05 corrected for multiple comparisons (equivalent to a 71-voxel threshold and p < 0.005). (A) Sagittal slice showing anterior and middle cingulate cluster of negative correlation with Lifetime scores (crosshair at x = −6, y = 3, z = 35). (B) Sagittal slice showing anterior cingulate and posterior parietal clusters of negative correlation with Last Year scores (crosshair at x = −6, y = 19, z = 35). (C) Overlap among anterior cingulate negative correlation with Lifetime and Last Year exposure to violence: yellow-colored voxels represent the overlapping areas. AFNI (Cox, 1996).
Table 1.
Brain regions and number of voxels significantly correlated with JVQ-R2 scores. Number of voxels negatively correlated with the JVQ-R2 scores (p < 0.005; cluster threshold 71 voxels, which corresponds to a cluster corrected for multiple comparisons). There were no positive correlations. Brain regions from the Haskins pediatric atlas (Molfese et al., 2015); the atlas region number is reported in parentheses. The x, y, z coordinate indicates the peak region in the correlation
| Region (Haskins ped. atlas number) | Voxels | Peak | ||
|---|---|---|---|---|
| x | y | z | ||
| JVQ-R2 Lifetime | ||||
| Right-hemisphere | ||||
| Anterior cingulate (caudal) (75) | 3 | 3 | 6 | 39 |
| Precentral (96) | 2 | 47 | 2 | 38 |
| Superior frontal (66) | 30 | 9 | −1 | 55 |
| Left-hemisphere | ||||
| Anterior cingulate (caudal) (41) | 11 | −8 | 2 | 32 |
| Middle frontal (42) | 45 | −28 | −5 | 37 |
| Pars opercularis (56) | 62 | −45 | 4 | 12 |
| Precentral (62) | 33 | −45 | 3 | 34 |
| Superior frontal (66) | 52 | −23 | −8 | 55 |
| Insula (73) | 9 | −35 | 5 | 4 |
| JVQ-R2 Last Year | ||||
| Right-hemisphere | x | y | z | |
| Anterior cingulate (caudal) (75) | 4 | 4 | 2 | 40 |
| Middle frontal (76) | 13 | 29 | −3 | 40 |
| Postcentral (94) | 1 | 35 | −16 | 40 |
| Posterior cingulate (95) | 1 | 10 | −54 | 14 |
| Precentral (96) | 64 | 34 | −12 | 40 |
| Superior frontal (100) | 54 | 15 | 1 | 62 |
| Left-hemisphere | ||||
| Anterior cingulate (caudal) (41) | 40 | −7 | 7 | 36 |
| Middle frontal (caudal) (42) | 45 | −3 | 8 | 36 |
| Cuneus (43) | 9 | −14 | −76 | 30 |
| Precentral (62) | 25 | −52 | 4 | 29 |
| Precuneus (63) | 61 | −10 | −65 | 34 |
| Superior frontal (66) | 71 | −4 | 5 | 45 |
| Superior parietal (67) | 11 | −15 | −65 | 51 |
Behavioral data analyses
Task response and response times were recorded using E-Prime (Psychology Software Tools, Pittsburgh, PA) for all trials. Behavioral data were analyzed using Spearman’s rho correlation for significant association between response times and accuracy on the task and the JVQ scores. To investigate if participants’ performances changed with time, we correlated the accuracy and response time of each trial, for each participant, with the order of the presentation of the trials. The correlation was intended to represent an index of changes in the latency of response time. The correlation was calculated as follows: the response time of each Go and Change trial was correlated with the trial number in the experiment (e.g. the first trial is 1, the second, 2, and so on). The slope of the correlation between response time and the presentation was used as an index of the latency: a negative slope (r value) would represent a decrease in response time as the experiment progressed; a positive slope, an increase in response time as the experiment progressed. To investigate if there was an association among increase in latency or accuracy with exposure to violence, we correlated (Pearson’s correlation) the slope (r value) for each participant with their JVQ scores.
Results
fMRI results
Brain function and exposure to violence
Results show that more exposure to violence was associated with less activation of anterior and posterior clusters of brain regions that are part of the frontoparietal executive function network. The Lifetime score for violence was associated with less activation of an anterior cluster of brain regions that included the anterior cingulate gyrus, the middle and superior frontal gyri, the precentral gyrus and the insular cortex. More recent exposure to violence (Last Year score) was associated with less activation of a similar anterior network of regions and an additional posterior clusters, both of which included anterior cingulate, precentral, superior frontal gyri and posterior cingulate gyrus and posterior parietal lobe (Figure 1; Table 1). The anterior cluster of regions that negatively correlated with Lifetime and Last Year violence overlapped in pre-frontal regions, including the anterior cingulate cortex and precentral gyrus (Figure 1).
The results also show that an increase in latency in response time was associated with less activation of the anterior and posterior clusters of activation that negatively correlated with more exposure to violence. Beta values extracted from the brain regions of negative correlation with violence also showed a negative correlation with a more prominent increase in the response time over the experiment (an index of increase in latency of response, or faster deterioration in performance). Figure 2 shows the scatter plots for the beta values and the increase in response time (latency) [Spearman one-tailed correlations: RT LATENCY × L INSULA = −0.381 (p < 0.05); RT LATENCY × POSTERIOR = −0.286 (p = 0.046); RT LATENCY × ANTERIOR = −0.409 (p < 0.01)].
Fig. 2.
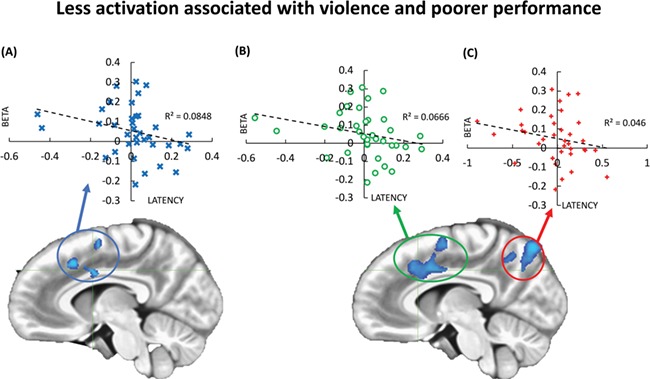
Association among increase in latency in response times with time and less activation for the clusters of negative correlation with exposure to violence. Participants who reported more exposure also showed more prominent deterioration in response times as the experiment progressed. (A) Dispersion plot for the betas for each participant’s activation for the anterior cingulate cluster (in association with JVQ-R2 Lifetime scores) and the slope of the latency in response times for each participant. (B) Dispersion plot for the betas for each participant’s activation for the anterior cingulate cluster (in association with JVQ-R2 Last Year scores) and the slope of the latency in response times for each participant. (C) Dispersion plot for the betas for each participant’s activation for the posterior parietal cluster (in association with JVQ-R2 Lifetime scores) and the slope of the latency in response times for each participant. Betas were extracted for the entire clusters. Latency represents the increase in latency during the experiment. AFNI (Cox, 1996).
Behavioral results
The mean accuracy (ACC) for the whole group was 0.83 (s.d. = 0.13) and mean response time (RT) was 636.7 (s.d. = 58.7). For the Change trials, mean ACC was 0.88 (s.d. = 0.09) and mean RT was 625.44 (s.d. = 64.32). For the Go trials, mean ACC was 0.80 (s.d. = 0.17) and mean RT was 642.49 (s.d. = 60.79). There were no significant correlations among RT and the JVQ scores (p = 0.341) or the CBCL (p = 0.376 for Internalizing Behaviors, p = 0.283 for Externalizing Behaviors and p = 0.477 for Total Problems Scale). There were no significant correlations among ACC and JVQ (p = 0.352) or CBLC (p = 0.308 for Internalizing Behaviors, p = 0.197 for Externalizing Behaviors and p = 0.356 for Total Problems Scale). The mean accuracy for all participants decreased over the experiment (p < 0.001), as the response time increased (p < 0.01). The correlations of JVQ scores and increase in RT or decrease in ACC were not significant (p = 0.121 for RT and p = 0.415 for ACC).
Exposure to violence: JVQ
The majority of preadolescents (36 participants, 85.7%) had experienced at least one form of victimization over the life span, and 31 (73.8%) reported being exposed to violence over the last year. The most common type of violence exposure was conventional crime (69%), followed by witnessing/indirect forms of violence (59.5%). Spearman’s rho correlation showed a significant negative correlation among the JVQ Modules 1 (Conventional Crime) and 2 (Maltreatment) with the CBCL Total Social Competence Score (p = 0.019 and p = 0.024, respectively). The Social Competence Score assesses social interaction patterns; for instance, if the respondent interacts well with other preadolescents and family members, how many close friends they have, how often they meet with the close friends and what is their level of independency for playing or working (Bordin et al., 2013). The correlation between low scores for Social Competence and high scores for exposure to violence suggest an association between the increased exposure to violence and diminished ability to socialize with peers and family. Table 2 shows descriptive statistics (mean ± s.d.) for each domain module of JVQ-R2 full interview, as well as for the total score. Studies have shown JVQ-R2 scores correlated with hair cortisol concentrations, thus suggesting self-reported scores provide a reliable index of more stressful experiences (Grassi-Oliveira et al., 2012; Buchweitz, et al., 2019b).
Table 2.
Descriptive data on types of exposure to violence (JVQ-R2)
| Participants, Exposure to Violence and Types of Exposure | |||||
|---|---|---|---|---|---|
| n | % | Min | Max | Mean (s.d.) | |
| Lifetime Conventional crime Maltreatment Peer and sibling victimization Sexual victimization | 36 29 13 14 04 | 85.7 69 31 33.3 9.5 | 1 1 1 1 1 | 20 7 2 4 2 | 5.25 (4.18) 2.55 (1.52) 1.23 (0.44) 1.71 (1.21) 1.25 (0.5) |
| Witnessing and other exposure | 25 | 59.5 | 1 | 5 | 2.64 (1.44) |
| Last Year | 31 | 73.8 | 1 | 18 | 3.35 (3.51) |
Internalizing and externalizing behaviors: CBCL
The analyses of CBCL/6-18 scores included 42 preadolescents (boys: n = 26; girls: n = 16). The results show that 64.3% (n = 27) of the sample scores for internalizing behaviors were at the clinical level (borderline level: 4.7%; non-clinical level: 31.0%), and 66.7% (n = 28) of the sample scores for externalizing behaviors were at the clinical level (borderline level: 9.5%; non-clinical level: 23.8%). The total problems scores were 61.9% (n = 26) at the clinical level (borderline level: 11.9%; non-clinical level: 26.2%). We investigated sex-specific effects and found no significant sex differences on CBCL/6-18 scores for the different symptoms: withdrawn (t = −0.726; P = 0.472), somatic complaints (t = −0.070; P = 0.944), anxiety/depression (t = −0.443; P = 0.660), rule-breaking behavior (t = 1.002; P = 0.322), aggressive behavior (t = −0.099; P = 0.922), internalizing problems (t = −0.435; P = 0.666), externalizing problems (t = 0.211; P = 0.834) and total problems (t = 0.372; P = 0.712).
Discussion
The present study shows that more exposure to violence was associated with less activation of anterior and posterior brain regions in an inhibitory control task; the activation of these brain networks was also associated with faster deterioration in performance in the task (more prominent increase in latency of response). Few studies have investigated inhibitory control in early adolescents exposed to violence or other forms of stress. To our knowledge, there are no brain imaging studies that investigated exposure to violence and executive functions in Latin-American children or adolescents. Our results corroborate the literature that shows the association among trauma, violence, institutionalization and other negative, stressful life events with alterations in brain function (Carrion et al., 2008). The direction of the differences in brain activation associated with stress varies (more or less activation), as do the directions of differences in brain connectivity and behavior patterns that emerge with increased exposure to violence (hypersensitivity to error, more risk-taking and reward-seeking behavior, among others). But one pattern is clear: negative, stressful experiences show associated effects on executive function performance and the underlying brain networks.
Chronic exposure to violence during childhood is associated with an increased risk for a broad range of developmental difficulties, including behavioral, emotional and learning problems (Moffitt and Tank, 2013; Tsavoussis et al., 2014; Bick and Nelson, 2016). Chronic exposure to violence is also associated with risk for psychosis, ADHD (Attention Deficit and Hyperactivity Disorder), depression, anxiety among other impairing conditions (Lupien et al., 2009; Banny et al., 2013; Fonzo et al., 2016). The deactivation of bilateral networks associated with inhibitory control and sustained attention suggests a deleterious effect of exposure to violence on brain function that, in its turn, is associated with an ability that is associated with quality of life.
Chronic exposure to violence (Lifetime score) was associated with less activation of an anterior cluster of brain regions, including the anterior cingulate cortex. More recent exposure (Last Year score) was associated with less activation of a similar, overlapping anterior network of regions, as shown in the overlapping clusters in Figure 1, and with less activation of the posterior parietal lobe. The posterior parietal cluster was identified only in association with more recent violence. The anterior and posterior regions identified in both correlations with violence scores are generally associated with cognitive control, behavioral flexibility, emotional regulation and working memory (Schneider, 2003; Chein and Schneider, 2005). The functional mapping of the anterior cingulate cortex is traditionally divided into rostral and caudal (dorsal) associations. The rostral anterior cingulate cortex is generally associated with affective processes; the caudal, in turn, is commonly associated with sensorimotor and higher order cognitive functions (Vogt et al., 1991; Bush et al., 2000). Stressful experiences and trauma have been associated with alterations in dorsal anterior cingulate cortex function, such as in cases of deprivation in institutionalized care (Mueller et al., 2010) and post-traumatic stress disorder in maltreated children (Carrion et al., 2008); moreover, significant morphological alterations in this portion of the anterior cingulate cortex have been identified in adults who experienced early-life stress (Cohen et al., 2006).
The effects on activation of anterior regions of the brain, including as the anterior cingulate cortex might imply deterioration in cognitive control. The common effects of recent and more cumulative violence of activation of the anterior cingulate cortex suggests that functions associated with this portion of the brain (and its parietal lobe connections, for example) may be especially sensitive to increased exposure to violence. This is a speculative statement at best, but the literature does show alterations associated with negative experiences in anterior cingulate function and anatomy, as indicated above. Effects on activation of the anterior cingulate cortex may compromise cognitive performance in other relevant domains. More activation of dorsal anterior cingulate cortex has been associated with good performance in early reading, for example (Shaywitz et al., 2002; Buchweitz, et al., 2019a) and with the ability to better benefit from reading interventions (compensated poor readers) (Shaywitz et al., 2003).
Policymaking aimed at mitigating the effects of violence in Latin-American youths may benefit from understanding the neurocognitive effects of exposure to violence and direct their focus to executive function training. Studies have shown benefits and transfer of executive functions training, such as in using computerized cognitive training (de Oliveira Rosa et al., 2019; Jaeggi et al., 2011; Salminen et al., 2012). Programs for building adolescent life skills include learning better goal-directed behavior and self-control (Lupien, 2017; Ward, 2017); programs that aim to address the effects of exposure to violence may thus benefit from evidence that executive function training help improve some of the abilities affected by violence and may represent a short-term alternative for mitigating its effects.
Participants were early adolescents whose ages ranged from 9 to 14 years. We underscore that age, intelligence and socioeconomic status were included as covariables in the investigation. These demographic and psychometric variables have been associated with differences in brain function and structure (Luna et al., 2010; Kim et al., 2013; Noble et al., 2015; Piccolo et al., 2016). The brain’s executive function network shows considerable overlap across ages (Luna et al., 2010), but studies show a developmental shift in anterior to more posterior activation with age (Velanova et al., 2008). Thus, we attempted to eliminate any associated development and SES effects. The literature shows different results for associations among accuracy and response time in the inhibitory control and clinical populations. Untreated bipolar adolescents showed significantly lower accuracy in the Change trials than the control group (Nelson et al., 2007), though most studies did not find differences in accuracy (Mueller et al., 2010; Kim et al., 2012; Bruce et al., 2013; Roberts and Husain, 2015; Jankowski et al., 2017). Studies have found that response time was slower in case of pre-SMA lesion patients (Roberts and Husain, 2015), but significant differences in response times were not identified (Kim et al., 2012; Jankowski et al., 2017; Nelson et al., 2017). In the present study, we used the calculation of a slope to evaluate faster deterioration in performance in the task. The results showed that the activation values for the brain regions whose activations negatively correlated with increased exposure to violence also correlated with faster deterioration in performance. A putative disengagement (i.e. less activation) of anterior and posterior brain regions that underpin executive functions also indicated that performance would deteriorate faster for these participants.
Of course, the present results are cross-sectional and associative. It remains to be understood if the alterations in brain function and deterioration in performance are associated with effects that are taking hold and will affect the development of executive functions into early adulthood. The literature suggests that alterations in brain function and behavior in adolescence may persist into adulthood (Romeo and McEven, 2006; Lupien et al., 2009; Burghy et al., 2012). If brain function associated with the ability to control inhibition is more permanently impaired by exposure to violence, there may be significant impacts in school performance and social-life behaviors (including impaired learning and studying abilities, impaired social abilities and poor impulse control). To be sure, executive functions are not crystallized and neither should we believe that the effects of violence identified in the present study are definitive. Rather, we hope the evidence suggests that these abilities can be included among targets for mitigation of the effects of exposure to violence during a crucial period of human development. Understanding the neural effects of exposure to violence may help operationalize multi-modal (brain and behavior), tangible measures to assess and develop targeted interventions for early adolescent violence.
Acknowledgments
The present study was funded by Inter-American Development Bank (IDB) consulting grant BRT-1322. We would like to thank the IDB and its Education Specialists Ryan Burgess, Aimee Verdisco and João Marcelo Borges for their support during different stages of the project. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior—Brasil (CAPES)—Finance Code 001 and by NIH R01DA044859. The authors have no competing interests to report.
Footnotes
1 http://www.unh.edu/ccrc/jvq/scoring.html
References
- ABEP (2016). Critério de classificação econômica Brasil. Associação Brasileira de Empresas de Pesquisa - ABEP. Retrieved January 1, 2017, from abep.org
- Achenbach T.M. (2004). Child behavior checklist In: Encyclopedia of Psychology, Vol. 2. (Vol. 7, VT: Burlington, pp. 69–70 10.1037/10517-028. [DOI] [Google Scholar]
- Achenbach T.M., Rescorla L.A. (2001). Manual for ASEBA In: University of Vermont, Research Center for Children, Youth, & Families, Burlington, VT. [Google Scholar]
- Aron A.R. (2011). From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biological Psychiatry, 69(12), e55–68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banny A.M., Cicchetti D., Rogosch F.A., Oshri A., Crick N.R. (2013). Vulnerability to depression: a moderated mediation model of the roles of child maltreatment, peer victimization, and serotonin transporter linked polymorphic region genetic variation among children from low socioeconomic status backgrounds. Development and Psychopathology, 25(3), 599–614. doi: 10.1017/S0954579413000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J., Nelson C.A. (2016). Early adverse experiences and the developing brain. Neuropsychopharmacology, 41(1), 177–96. doi: 10.1038/npp.2015.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn R.M., Roeber B.J., Pollak S.D. (2017). Early childhood stress exposure, reward pathways, and adult decision making. Proceedings of the National Academy of Sciences, 114(51), 13549–54. 10.1073/pnas.1708791114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boecker M., Drueke B., Vorhold V., Knops A., Philippen B., Gauggel S. (2011). When response inhibition is followed by response reengagement: an event-related fMRI study. Human Brain Mapping, 32(1), 94–106. doi: 10.1002/hbm.21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boecker M., Gauggel S., Drueke B. (2013). Stop or stop-change--does it make any difference for the inhibition process? International Journal of Psychophysiology, 87(3), 234–43. doi: 10.1016/j.ijpsycho.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Bordin I.A.S., Mari J.J., Caeiro M.F. (1995). Validação da versão Brasileira do “child behavior checklist” (CBCL) (invent rio de Comportamentos da Infância e Adolescência): dados preliminares. Revista ABP-APAL, 17(2), 55–66. [Google Scholar]
- Bruce J., Fisher P.A., Graham A.M., Moore W.E., Peake S.J., Mannering A.M. (2013). Patterns of brain activation in foster children and nonmaltreated children during an inhibitory control task. Development and Psychopathology, 25(4 Pt 1), 931–41. doi: 10.1017/S095457941300028X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchweitz A., Costa A.C., Toazza R. et al.. (2019a). Decoupling of the occipitotemporal cortex and the brain’s default-mode network in dyslexia and a role for the cingulate cortex in good readers: a brain imaging study of Brazilian children. Developmental Neuropsychology, 44(1), 146–157. 10.1080/87565641.2017.1292516 [DOI] [PubMed] [Google Scholar]
- Buchweitz A., de Azeredo L.A., Sanvicente-Vieira B. et al. (2019b). Violence and Latin-American preadolescents: a study of social brain function and cortisol levels. Developmental Science, e12799 10.1111/desc.12799 [DOI] [PubMed] [Google Scholar]
- Buchweitz A., Mason R.A., Meschyan G., Keller T.A., Just M.A. (2014). Modulation of cortical activity during comprehension of familiar and unfamiliar text topics in speed reading and speed listening. Brain and Language, 139, 49–57. 10.1016/j.bandl.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy C.A., Stodola D.E., Ruttle P.L. et al. (2012). Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience, 15(12), 1736–1741. 10.1038/nn.3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–22. 10.1016/S1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bustreo F., Chestnov O. (2013). Emerging issues in adolescent health and the positions and priorities of the World Health Organization. Journal of Adolescent Health, 52(2), S4 10.1016/j.jadohealth.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Carrion V.G., Garrett A., Menon V., Weems C.F., Reiss A.L. (2008). Posttraumatic stress symptoms and brain function during a response-inhibition task: an fMRI study in youth. Depression and Anxiety, 25(6), 514–26. 10.1002/da.20346. [DOI] [PubMed] [Google Scholar]
- Cerqueira D. (2016). Atlas da Violência, 2016. [Google Scholar]
- Chein J.M., Schneider W. (2005). Neuroimaging studies of practice-related change: fMRI and meta-analytic evidence of a domain-general control network for learning. Brain Research. Cognitive Brain Research, 25(3), 607–23. 10.1016/j.cogbrainres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Cohen R.A., Grieve S., Hoth K.F., et al. (2006). Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological Psychiatry, 59(10), 975–982. 10.1016/j.biopsych.2005.12.016 [DOI] [PubMed] [Google Scholar]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox R.W., Chen G., Glen D.R., Reynolds R.C., Taylor P.A. (2017). FMRI clustering in AFNI: false-positive rates redux. Brain Connectivity, 7(3), 152–71. 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M., Detre J.A., Alsop D.C., Shin R.K., Atlas S., Grossman M. (1995). The neural basis of the central executive system of working memory. Nature, 378(16), 279–81. 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- Dahl R., Suleiman A. (2017). Adolescent brain development: windows of opportunity In The adolescent brain: a second window of opportunity (pp. 21–28). Florence: UNICEF Office of Research - Innocenti; Retrieved fromhttps://www.unicef-irc.org/publications/pdf/adolescent_brain_a_second_window_of_opportunity_a_compendium.pdf [Google Scholar]
- Diamond A. (2013). Executive Functions. In (Vol. 64 pp. 135–168): Annual Review of Psychology. [DOI] [PMC free article] [PubMed]
- Eiland L., Romeo R.D. (2013, September 26). Stress and the Developing Adolescent Brain. Neuroscience. Pergamon; 10.1016/j.neuroscience.2012.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A., Tripathi S.P., Mletzko T. et al. (2014). Childhood maltreatment is associated with a sex-dependent functional reorganization of a brain inhibitory control network. Human Brain Mapping, 35(4), 1654–1667. 10.1002/hbm.22280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelhor D., Ormrod R.K., Turner H.A., Hamby S.L. (2005). Measuring poly-victimization using the juvenile victimization questionnaire. Child Abuse & Neglect, 29(11), 1297–312. doi: 10.1016/j.chiabu.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Fonzo G.A., Ramsawh H.J., Flagan T.M., Simmons A.N., Sullivan S.G., Allard C.B., Stein M.B. (2016). Early life stress and the anxious brain: evidence for a neural mechanism linking childhood emotional maltreatment to anxiety in adulthood. Psychological Medicine, 46(5), 1037–54. doi: 10.1017/S0033291715002603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman N.P., Miyake A. (2017). Unity and diversity of executive functions: individual differences as a window on cognitive structure. Cortex, 86, 186–204. 10.1016/j.cortex.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster J.M. (2000, July 10). Executive Frontal Functions. Experimental Brain Research. Springer-Verlag; 10.1007/s002210000401 [DOI] [PubMed] [Google Scholar]
- Gilman J.M., Radoman M., Schuster R.M., Pachas G., Azzouz N., Fava M., Evins A.E. (2018). Anterior insula activation during inhibition to smoking cues is associated with ability to maintain tobacco abstinence. Addictive Behaviors Reports, 7, 40–6. doi: 10.1016/j.abrep.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi-Oliveira R., Pezzi J.C., Daruy-Filho L. et al. (2012). Hair cortisol and stressful life events retrospective assessment in crack cocaine users. The American Journal of Drug and Alcohol Abuse, 38(6), 535–538. 10.3109/00952990.2012.694538 [DOI] [PubMed] [Google Scholar]
- Gupta A., Mayer E.A., Acosta J.R. et al. (2017). Early adverse life events are associated with altered brain network architecture in a sex- dependent manner. Neurobiology of Stress, 7, 16–26. 10.1016/j.ynstr.2017.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Nacewicz B.M., Sutterer M.J., et al. (2015). Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biological Psychiatry, 77(4), 314–323. 10.1016/j.biopsych.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu N.S., Novick J.M., Jaeggi S.M. (2014). The development and malleability of executive control abilities. Frontiers in Behavioral Neuroscience, 8(June), 10.3389/fnbeh.2014.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J.A., Phillips G., Reed P. (2013). Brief exposure to a self-paced computer-based reading programme and how it impacts reading ability and behaviour problems. PLoS One, 8(11), e77867. doi: 10.1371/journal.pone.0077867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi S.M., Buschkuehl M., Jonides J., Shah P. (2011). Short- and long-term benefits of cognitive training. Proceedings of the National Academy of Sciences, 108(25), 10081–6. 10.1073/pnas.1103228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi S.M., Seewer R., Nirkko A.C. et al. (2003). Does excessive memory load attenuate activation in the prefrontal cortex? Load-dependent processing in single and dual tasks: functional magnetic resonance imaging study. NeuroImage, 19(2), 210–225. 10.1016/S1053-8119(03)00098-3 [DOI] [PubMed] [Google Scholar]
- Jankowski K.F., Bruce J., Beauchamp K.G. et al. (2017). Preliminary evidence of the impact of early childhood maltreatment and a preventive intervention on neural patterns of response inhibition in early adolescence. Developmental Science, 20(4), e12413 10.1111/desc.12413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just M.A., Keller T.A., Cynkar J. (2008). A decrease in brain activation associated with driving when listening to someone speak. Brain Research, 1205, 70–80. 10.1016/j.brainres.2007.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T.A., Carpenter P.A., Just M.A. (2001). The neural bases of sentence comprehension: a fMRI examination of syntactic and lexical processing. Cerebral Cortex, 11(3), 223–237. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11230094 [DOI] [PubMed] [Google Scholar]
- Keller T.A., Carpenter P.A., Just M.A. (2003). Brain imaging of tongue-twister sentence comprehension: twisting the tongue and the brain. Brain and Language, 84(2), 189–203. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12590911 [DOI] [PubMed] [Google Scholar]
- Kim P., Evans G.W., Angstadt M. et al. (2013). Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proceedings of the National Academy of Sciences of the United States of America, 110(46), 18442–18447. 10.1073/pnas.1308240110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P., Jenkins S.E., Connolly M.E., Deveney C.M., Fromm S.J., Brotman M.A., Leibenluft E. (2012). Neural correlates of cognitive flexibility in children at risk for bipolar disorder. Journal of Psychiatric Research, 46(1), 22–30. doi: 10.1016/j.jpsychires.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B.J., Wagner A.D. (2011). Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Annals of the New York Academy of Sciences, 1224, 40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L., Hart H., Mehta M.A., Simmons A., Mirza K., Rubia K. (2015). Neural correlates of error processing in young people with a history of severe childhood abuse: an fMRI study. American Journal of Psychiatry, 172(9), 892–900. 10.1176/appi.ajp.2015.14081042. [DOI] [PubMed] [Google Scholar]
- Lim M.M., Young L.J. (2006). Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Hormones and Behavior, 50(4), 506–17. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Logan G.D., Burkell J. (1986). Dependence and independence in responding to double stimulation: a comparison of stop, change, and dual-task paradigms. Journal of Experimental Psychology: Human Perception and Performance, 12(4), 549–63. 10.1037/0096-1523.12.4.549. [DOI] [Google Scholar]
- Luna B., Padmanabhan A., O’Hearn K. (2010). What has fMRI told us about the development of cognitive control through adolescence? Brain and Cognition, 72(1), 101–13. 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J. (2017). Helping teenagers develop resilience in the face of stress In: UNICEF , editor. The Adolescent Brain: a Second Window of Opportunity, UNICEF: Florence, Italy, pp. 57–64. [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews. Neuroscience, 10(6), 434–45. 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Mazzola-Pomietto P., Kaladjian A., Azorin J.M., Anton J.L., Jeanningros R. (2009). Bilateral decrease in ventrolateral prefrontal cortex activation during motor response inhibition in mania. Journal of Psychiatric Research, 43(4), 432–41. doi: 10.1016/j.jpsychires.2008.05.004. [DOI] [PubMed] [Google Scholar]
- McNab F., Leroux G., Strand F., Thorell L., Bergman S., Klingberg T. (2008). Common and unique components of inhibition and working memory: an fMRI, within-subjects investigation. Neuropsychologia, 46(11), 2668–82. doi: 10.1016/j.neuropsychologia.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Menon V., Adleman N.E., White C.D., Glover G.H., Reiss A.L. (2001). Error-related brain activation during a go/NoGo response inhibition task. Human Brain Mapping, 12(3), 131–43. doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H.C., Bucci D.J. (2016). Imbalanced activity in the orbitofrontal cortex and nucleus Accumbens impairs behavioral inhibition. Current Biology, 26(20), 2834–9. doi: 10.1016/j.cub.2016.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt T.E., Klaus-Grawe 2012 Think Tank (2013). Childhood exposure to violence and lifelong health: clinical intervention science and stress-biology research join forces. Development and Psychopathology, 25(4 Pt 2), 1619–34. 10.1017/S0954579413000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfese P., Glen D., Mesite L., Pugh K., Cox R. (2015). The Haskins pediatric brain atlas In: 21st annual meeting of the Organization for Human Brain Mapping (OHBM), Geneva. [Google Scholar]
- Mueller A., Candrian G., Kropotov J.D., Ponomarev V.A., Baschera G.M. (2010). Classification of ADHD patients on the basis of independent ERP components using a machine learning system. Nonlinear Biomed Phys, 4(Suppl 1, S1). doi: 10.1186/1753-4631-4-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S.C., Hardin M.G., Korelitz K. et al. (2012). Incentive effect on inhibitory control in adolescents with early-life stress: an antisaccade study. Child Abuse & Neglect, 36(3), 217–225. 10.1016/j.chiabu.2011.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.A. (2017). Hazards to early development: the biological embedding of early life adversity. Neuron, 96(2), 262–6. 10.1016/j.neuron.2017.09.027. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Vinton D.T., Berghorst L. et al. (2007). Brain systems underlying response flexibility in healthy and bipolar adolescents: an event-related fMRI study. Bipolar Disorders, 9(8), 810–819. 10.1111/j.1399-5618.2007.00419.x [DOI] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Brito N.H. et al. (2015). Family income, parental education and brain structure in children and adolescents. Nature Neuroscience, 18(5), 773–778. 10.1038/nn.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Rosa V., Rosa Franco A., Abrahão Salum Júnior G. et al. (2019). Effects of computerized cognitive training as add-on treatment to stimulants in ADHD: a pilot fMRI study. Brain Imaging and Behavior, 1–12. 10.1007/s11682-019-00137-0 [DOI] [PubMed] [Google Scholar]
- Pechtel P., Pizzagalli D.A. (2011). Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology, 214(1), 55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellizzoni S., Apuzzo G.M., De Vita C., Agostini T., Passolunghi M.C. (2019). Evaluation and training of executive functions in genocide survivors. The case of Yazidi children. Developmental Science, e12798 10.1111/desc.12798. [DOI] [PubMed] [Google Scholar]
- Piccolo L.R., Merz E.C., He X., Sowell E.R., Noble K.G. (2016). Age-related differences in cortical thickness vary by socioeconomic status. PLoS One, 11(9), e0162511 10.1371/journal.pone.0162511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak S.D. (2005). Early adversity and mechanisms of plasticity: integrating affective neuroscience with developmental approaches to psychopathology. Development and Psychopathology, 17(03), 735–52. 10.1017/S0954579405050352. [DOI] [PubMed] [Google Scholar]
- Pollak S.D. (2015). Multilevel developmental approaches to understanding the effects of child maltreatment: recent advances and future challenges. Development and Psychopathology, 27(4pt2), 1387–97. 10.1017/S0954579415000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahdar A., Galván A. (2014). The cognitive and neurobiological effects of daily stress in adolescents. NeuroImage, 92, 267–73. 10.1016/j.neuroimage.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Richmond J.B., Covert C. (1967). Mental health and education conference. A report. Arch Gen Psychiatry, 17(5), 513–20. doi: 10.1001/archpsyc.1967.01730290001001. [DOI] [PubMed] [Google Scholar]
- Roberts R.E., Husain M. (2015). A dissociation between stopping and switching actions following a lesion of the pre-supplementary motor area. Cortex, 63, 184–95. doi: 10.1016/j.cortex.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo R.D., McEven B.S. (2006). Stress and the adolescent brain. Annals of the New York Academy of Sciences, 1094(1), 202–14 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- Salminen T., Strobach T., Schubert T. (2012). On the impacts of working memory training on executive functioning. Frontiers in Human Neuroscience, 6(166). 10.3389/fnhum.2012.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W. (2003). Controlled & automatic processing: behavior, theory, and biological mechanisms. Cognitive Science, 27(3), 525–59. 10.1016/S0364-0213(03)00011-9. [DOI] [Google Scholar]
- Schriber R.A., Guyer A.E. (2016). Adolescent neurobiological susceptibility to social context. Developmental Cognitive Neuroscience, 19, 1–18. 10.1016/J.DCN.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz B.A., Shaywitz S.E., Pugh K.R. et al. (2002). Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry, 52(2), 101–110. Retrieved fromhttp://www.ncbi.nlm.nih.gov/pubmed/12114001 [DOI] [PubMed] [Google Scholar]
- Shaywitz S.E., Shaywitz B.A., Fulbright R.K. et al. (2003). Neural systems for compensation and persistence: young adult outcome of childhood reading disability. Biological Psychiatry, 54(1), 25–33. 10.1016/S0006-3223(02)01836-X [DOI] [PubMed] [Google Scholar]
- Shonkoff J.P., Garner A.S., Siegel B.S. et al. (2012). The lifelong effects of early childhood adversity and toxic stress. Pediatrics, 129(1), e232–e246. 10.1542/peds.2011-2663 [DOI] [PubMed] [Google Scholar]
- Simmonds D.J., Pekar J.J., Mostofsky S.H. (2008). Meta-analysis of go/no-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia, 46(1), 224–32. 10.1016/J.NEUROPSYCHOLOGIA.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L.P. (2013). Adolescent neurodevelopment. Journal of Adolescent Health, 52(2), S7–S13. 10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg J.M., McGlinchey R.E., Milberg W.P., Salat D.H. (2015). Brain network disturbance related to posttraumatic stress and traumatic brain injury in veterans. Biological Psychiatry, 78(3), 210–6. doi: 10.1016/j.biopsych.2015.02.013. [DOI] [PubMed] [Google Scholar]
- Spielberg J.M., Olino T.M., Forbes E.E., Dahl R.E. (2014). Exciting fear in adolescence: does pubertal development alter threat processing? Developmental Cognitive Neuroscience, 8, 86–95. 10.1016/J.DCN.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. (2008). A social neuroscience perspective on adolescent risk-taking. Developmental Review, 28(1), 78–106. 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco A., Lebiere C., O’Reilly R.C., Anderson J.R. (2012). Distinct contributions of the caudate nucleus, rostral prefrontal cortex, and parietal cortex to the execution of instructed tasks. Cognitive, Affective, & Behavioral Neuroscience, 12(4), 611–28. 10.3758/s13415-012-0117-7. [DOI] [PubMed] [Google Scholar]
- Sylvester C.-Y.C., Wager T.D., Lacey S.C., et al. (2003). Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia, 41(3), 357–370. 10.1016/S0028-3932(02)00167-7 [DOI] [PubMed] [Google Scholar]
- Taylor S.E., Eisenberger N.I., Saxbe D., Lehman B.J., Lieberman M.D. (2006). Neural responses to emotional stimuli are associated with childhood family stress. Biological Psychiatry, 60(3), 296–301. 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Thomas L.A., Hall J.M., Skup M., Jenkins S.E., Pine D.S., Leibenluft E. (2011). A developmental neuroimaging investigation of the change paradigm. Developmental Science, 14(1), 148–61. doi: 10.1111/j.1467-7687.2010.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsavoussis A., Stawicki S.P., Stoicea N., Papadimos T.J. (2014). Child-witnessed domestic violence and its adverse effects on brain development: a call for societal self-examination and awareness. Frontiers in Public Health, 2, 178. doi: 10.3389/fpubh.2014.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. (2008). Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex, 18(11), 2505–22. 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt B.A., Finch D.M., Olson C.R. (1991). Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cerebral Cortex (New York, N.Y. : 1991), 2(6), 435–443. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1477524 [DOI] [PubMed] [Google Scholar]
- Ward E. (2017). Understanding adolescent neuroplasticity: a guide to developing resiliency programmes for adolescents In: U. O. of R.- Innocenti , editor. The Adolescent Brain: a Second Window of Opportunity, UNICEF: Florence, pp. 65–74. [Google Scholar]
- Wijeakumar S., Kumar A.M., Delgado Reyes L., Madhuri T., Spencer J.P. (2019). Early adversity in rural India impacts the brain networks underlying visual working memory. Developmental Science, e12822 10.1111/desc.12822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby M.T., Piper B., Oyanga A., Merseth King K. (2019). Measuring executive function skills in young children in Kenya: associations with school readiness. Developmental Science, e12818 10.1111/desc.12818. [DOI] [PubMed] [Google Scholar]
- Zhang K., Johnson B., Gay M., Horovitz S.G., Hallett M., Sebastianelli W., Slobounov S. (2012). Default mode network in concussed individuals in response to the YMCA physical stress test. Journal of Neurotrauma, 29(5), 756–65. doi: 10.1089/neu.2011.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]


