Abstract
Background and Aim. Upper gastrointestinal bleeding is a threat to patients with gastric varices (GVs). Previous studies have concluded that both transjugular intrahepatic portosystemic shunt (TIPS) and balloon-occluded retrograde transvenous obliteration (BRTO) are effective treatments for patients with GV. We aimed to compare the efficiency and outcomes of these two procedures in GV patients through meta-analysis.
Methods
The PubMed, Cochrane Library, EMBASE, and Web of Science databases were searched using the keywords: GV, bleeding, TIPS, and BRTO to identify relevant randomized controlled trials and cohort studies. The overall survival (OS) rate, imminent haemostasis rate, rebleeding rate, technical success rate, procedure complication rate (hepatic encephalopathy and aggravated ascites), and Child-Pugh score were evaluated. Randomized clinical trials and cohort studies comparing TIPS and BRTO for GV due to portal hypertension were included in our meta-analysis. Two independent reviewers performed data extraction and assessed the study quality. A meta-analysis was performed to calculate risk ratios (RRs), mean differences (MDs), and 95% CIs using random effects models.
Results
A total of nine studies fulfilled the inclusion criteria. There was a significant difference between TIPS and BRTO in the OS rate (RR, 0.81 (95% CI, 0.66 to 0.98); P=0.03) and rebleeding rate (RR, 2.61 (95% CI, 1.75 to 3.90); P=0.03) and rebleeding rate (RR, 2.61 (95% CI, 1.75 to 3.90); P=0.03) and rebleeding rate (RR, 2.61 (95% CI, 1.75 to 3.90); P=0.03) and rebleeding rate (RR, 2.61 (95% CI, 1.75 to 3.90); P=0.03) and rebleeding rate (RR, 2.61 (95% CI, 1.75 to 3.90); P=0.03) and rebleeding rate (RR, 2.61 (95% CI, 1.75 to 3.90); P=0.03) and rebleeding rate (RR, 2.61 (95% CI, 1.75 to 3.90);
Conclusions
In this meta-analysis, BRTO brought more benefits to patients, with a higher OS rate and lower rebleeding rate. BRTO is a feasible method for GVB.
1. Introduction
Gastric varices (GVs) are a common complication associated with portal hypertension [1]. Previous studies [2–6] have found that gastric varices bleeding (GVB) is lower in prevalence compared with oesophageal varices bleeding (EVB) but is associated with considerably higher mortality (45–55%) and rebleeding (35–90%) rates.
The current treatment approaches for GVB caused by portal hypertension include endoscopic therapy, radiological intervention methods, and surgical management. Endoscopic therapies such as endoscopic variceal ligation (EVL) and endoscopic variceal obturation (EVO) are considered effective methods for treating GVB [7]. However, EVL and EVO are limited in value for GV bleeding. Current guidelines [8, 9] suggest that EVL should be performed on GVs smaller than 2 cm in diameter because ligation can be performed with standard rubber bands, whereas larger-diameter GVs require the use of larger detachable snares, which can increase the risk of rebleeding [10]. EVO is considered a more effective method of haemostasis [11, 12], but the complications of EVO and thromboembolic phenomena (splenic, renal, pulmonary, cerebral, spinal, and coronary) may be serious and even life-threatening.
Radiological intervention methods also have demonstrated efficiency and safety in managing GVB [13–16]. Transjugular intrahepatic portosystemic shunt (TIPS), a minimally-invasive interventional surgery, can decompress the portal circulation and is regarded as the primary approach for GVB management in the West (United States and Europe) [17]. TIPS reduces GVB and treats complications with liver cirrhosis, such as refractory ascites and hepatic encephalopathy. Balloon-occluded retrograde transvenous obliteration (BRTO) is also a suitable method for GVB because it scleroses the GV, and it is widely used in the East (Japan and Korea) [18]. However, the portal pressure may not decrease due to a lack of shunts, which can cause complications such as ascites and worsening of oesophageal varix pressure. With the development of radiation intervention techniques, TIPS and BRTO have been increasingly widely used in both Western and Eastern countries. The current studies [19–22] on TIPS and BRTO have shown that both methods can be beneficial for reducing the rebleeding rate and improving the overall survival rate. However, whether TIPS or BRTO is more beneficial for GVB patients, especially regarding the overall survival rate, is still unknown. We searched databases and found a meta-analysis [23] including five studies to compare the effects of TIPS and BRTO. Three of the studies were abstracts without the full article [24–26], which may have caused incomplete data bias. Therefore, we performed a systematic review and meta-analysis to evaluate the feasibility and safety of TIPS and BRTO.
2. Methods
This meta-analysis was performed according to the Cochrane Handbook for Systematic Reviews of Interventions and is presented based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
2.1. Search Trials
We searched the PubMed, Cochrane Library, EMBASE, and Web of Science databases from their inception dates to October 10, 2019, using the keywords gastric varices, bleeding, transjugular intrahepatic portosystemic shunt (TIPS), and balloon-occluded retrograde transvenous obliteration (BRTO), to identify relevant published randomized controlled trials and cohort studies. There were no language restrictions. We excluded trials and cohort studies that included only TIPS or BRTO without comparison.
2.2. Inclusion Criteria
Trials were selected based on the following inclusion criteria: (1) RCTs and cohort studies comparing TIPS and BRTO; (2) patients with a clear diagnosis of GVs due to portal hypertension; and (3) studies providing original data on patients' baseline characteristics and postprocedure outcomes (postprocedure overall survival rate at five years, immediate haemostasis rate, rebleeding rate, technical success rate, procedure complication rate, and Child-Pugh score). The exclusion criteria were as follows: (1) randomized trials or cohort studies only about TIPS or BRTO without comparison; (2) case reports, reviews, meta-analyses, and studies or trials without specific dates on patient enrolment.
2.3. Risk-of-Bias Assessments
The methodological quality of the included RCTs was assessed independently by two researchers (Z. W. W. and P. F. C.) based on the Cochrane risk-of-bias criteria. Cohort studies were evaluated using the Newcastle–Ottawa Scale (NOS). In brief, a maximum of 9 points was assigned to each study: 4 for selection, 2 for comparability, and 3 for outcomes. Studies with a final score ≥6 were regarded as high quality. The rankings for each study are shown in Table 1.
Table 1.
Risk-of-bias assessment of the included studies.
| No. | Study ID | Newcastle–Ottawa quality assessment scale for cohort studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome | Total score | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |||
| 1 | Gimm et al. [27] | ★ | ★ | ★ | ● | ★ | ● | ★ | ★ | ★ | 7 |
| 2 | Kim et al. [28] | ★ | ★ | ★ | ● | ★ | ★ | ★ | ★ | ★ | 8 |
| 3 | Lee et al. [29] | ★ | ★ | ★ | ● | ★ | ★ | ★ | ★ | ★ | 8 |
| 4 | Sabri et al. [30] | ★ | ★ | ★ | ● | ★ | ★ | ★ | ★ | ● | 7 |
| 5 | Lee et al. [24] | ★ | ★ | ★ | ● | ★ | ● | ★ | ★ | ● | 6 |
| 6 | Sauk et al. [26] | ★ | ★ | ★ | ● | ★ | ● | ★ | ★ | ● | 6 |
| 7 | Lee et al. [25] | ★ | ★ | ★ | ● | ★ | ● | ★ | ★ | ● | 6 |
| 8 | Ninoi et al. [31] | ★ | ★ | ★ | ● | ★ | ★ | ★ | ★ | ★ | 8 |
1: representativeness of the exposed cohort; 2: selection of the nonexposed cohort; 3: ascertainment of exposure; 4: demonstration that the outcome of interest was not present at the start of the study; 5: study controls for the most important factor; 6: study controls for the second-most important factor; 7: assessment of outcome; 8: follow-up long enough for outcomes to occur; 9: adequacy of follow-up of cohorts. ★ was awarded if the respective information was available. ● was awarded if the respective information was unavailable.
2.4. Data Extraction
Two researchers (Z. W. W. and P. F. C.) independently extracted the following information for each study: lead author, publication year, country of origin, patient characteristics, overall survival rate, immediate haemostasis rate, rebleeding rate, technical success rate, procedure complication rate (hepatic encephalopathy and aggravated ascites), and Child-Pugh score. We extracted only the information and data of interest reported in the original articles. If a meta-analysis noted that unpublished data were provided by the primary authors, we extracted those missing data the from forest plots of the meta-analysis and reviewed the original articles to confirm whether the trials met our inclusion criteria. When those data were our outcomes of interest, we pooled them with the data from the primary trials.
The postprocedure overall survival rate was the primary outcome of interest because it represented the most direct benefit to patients. The secondary outcomes of interest were the number of patients with rebleeding after procedure, immediate haemostasis, technical success, and procedure complications as well as the Child-Pugh score. If a trial or study only reported the baseline Child-Pugh score or Child-Pugh grade without the score (Child-Pugh A, B, C), we did not consider those to be valid data.
2.5. Statistical Analysis
We performed a meta-analysis to calculate risk ratios (RRs), mean differences (MDs), and 95% confidence intervals (CIs) using the Mantel–Haenszel statistical method. If zero events were reported for one group in a comparison, a value of 0.5 was added to both groups for each such study. Based on the recommendation of the Cochrane Handbook, trials or studies with zero events in both the intervention and the control groups were not included in the meta-analysis when RRs were calculated.
A random effects model was used to pool the data, and statistical heterogeneity between summary data was evaluated using the χ2 test and I2 statistic. Sensitivity analysis was performed by excluding low-quality studies or trials with characteristics different from the others. If possible, potential publication bias was assessed by a visual inspection of the funnel plots of the primary outcome.
All meta-analyses were performed using RevMan version 5.3 (Cochrane Collaboration). All tests were 2-tailed, and P < 0.05 was considered statistically significant.
3. Results
3.1. Studies Retrieved and Characteristics
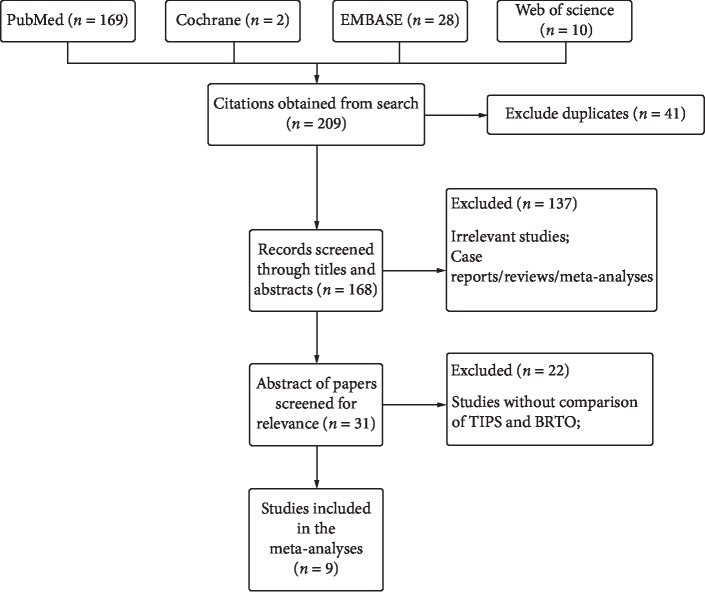
Based on the database search strategy, a total of 209 articles were identified. The titles and abstracts of these records were screened for inclusion. Forty-one articles were removed after finding duplicates. The full texts of thirty-one records were read, and nine [24–32] met the inclusion criteria (Figure 1): one RCT and eight cohort studies. The specific details of the papers that were included in our study are displayed in Table 2. The quality of the RCT was evaluated by the Cochrane ROB (good), and the quality of the cohort studies was evaluated by the Newcastle–Ottawa Scale with a final score ≥6 points, which means that all these studies had considerable methodological quality.
Figure 1.
Literature search and screening process.
Table 2.
Characteristics of the included RCT and cohort studies.
| Included studies | Country | Design | Sample size | Women, n (%) | Mean age | Follow-up | Traits of patients | Type of TIPS stents | Outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | TIPS | BRTO | TIPS | BRTO | ||||||||
| Gimm et al. [27] | Korea | Cohort | 176 | 19 | 157 | 37 (21) | 59.4 | 54.4 | 5 years | GV bleeding | Covered | IM, RB, C, OS |
| Kim et al. [28] | Korea | Cohort | 52 | 27 | 25 | 24 (46) | 58.0 | 59.0 | 1 year | GV bleeding (isolated GV) | Covered | TS, C, MELD score, IM, RB, OS |
| Lee et al. [29] | Korea | Cohort | 142 | 47 | 95 | 27 (19) | 55.6 | 59.4 | 3 years | GV bleeding | Covered | TS, Child-Pugh, C, RB, IM, OS |
| Sabri et al. [30] | USA | Cohort | 50 | 27 | 23 | 21 (42) | 55.0 | 52.0 | 1 year▲ | GV bleeding (isolated GV) | Covered | TS, C, OS, RB, H |
| Lee et al. [24] | Korea | Cohort | 135 | 49 | 86 | — | 58.5 | 58.5 | 2 years | GV bleeding | — | RB, OS, C, |
| Sauk et al. [26] | USA | Cohort | 52 | 27 | 25 | 24 (46) | 58.0 | 59.0 | — | GV bleeding (isolated GV) | — | C, RB, |
| Lee et al. [25] | Korea | Cohort | 100 | 32 | 68 | — | — | — | — | GV bleeding | — | TS, RB, C, OS |
| Ninoi et al. [31]◆ | Japan | Cohort | 76 | 27 | 58 | 34 (58) | 58.7 | 60.3 | 5 years∗ | GV bleeding | Bare | TS, OS, Child-Pugh, C, RB, H |
| Choi et al. [33] | Korea | RCT | 14 | 7 | 7 | 4 (29) | 54.0 | 60.3 | 1 year■ | GV bleeding | Bare/covered | OS, Child-Pugh, C, RB, IM |
OS: overall survival; PSG: portosystemic gradient; GV: gastric variceal; IM: immediate haemostasis; RB: rebleeding; C: complications; TS: technical success; H:haemostasis. ◆Ninoi et al. compared TIPS with transcatheter sclerotherapy (including BRTO). We extracted relevant data about BRTO from the article. ▲The mean duration of follow-up was 19.5 months for patients in the TIPS group (range, 1–52 mo.) and 18.2 months for patients in the BRTO group (range, 1–49 mo.). ∗The follow-up period after the procedures was 41.2 ± 32.4 months in the TIPS group and 26.9 ± 16.5 months in the transcatheter sclerotherapy group (including BRTO). ■Patients were followed up for 6 to 21 (mean, 14.4) months. _Not mentioned.
3.2. Meta-Analysis of the Overall Survival Rate
Five studies comparing the OS rates between TIPS and BRTO demonstrated that the OS rate in the BRTO group was higher than that in the TIPS group. There was a significant difference between the two groups (RR, 0.81 (95% CI, 0.66 to 0.98); P=0.03) according to a random effects model with no heterogeneity (P=0.14; Ι2 = 40%) (Figure 2).
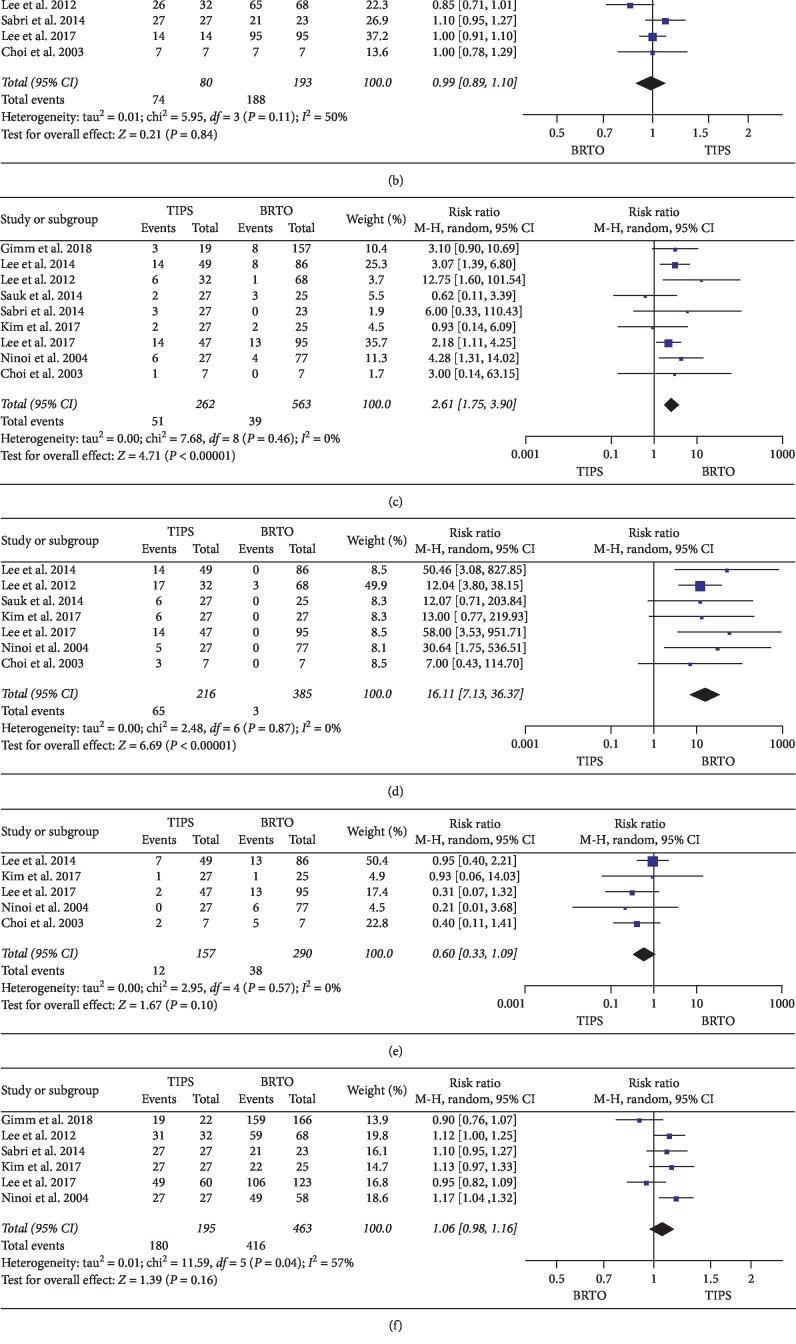
Figure 2.
Meta-analysis results: (a) overall survival rate, (b) haemostasis rate, (c) rebleeding rate, (d) hepatic encephalopathy rate, (e) aggravated ascites rate, (f) technical success rate, and (g) Child-Pugh change. There was a significant difference between TIPS and BRTO in the overall survival rate (RR, 0.81 (95% CI, 0.66 to 0.98); P=0.03) and rebleeding rate (RR, 2.61 (95% CI, 1.75 to 3.90); P < 0.00001). TIPS had a higher incidence rate of hepatic encephalopathy (RR, 16.11 (95% CI, 7.13 to 36.37); P < 0.00001). There was no significant difference between TIPS and BRTO in the immediate haemostasis rate (RR, 0.99 (95% CI, 0.89 to 1.10); P=0.84), technical success rate (RR, 1.06 (95% CI, 0.98 to 1.16); P=0.16), aggravated ascites rate (RR, 0.60 (95% CI, 0.33 to 1.09); P=0.10), or Child-Pugh change (MD, 0.22 (95% CI, −0.21 to 0.65); P=0.31).
3.3. Meta-Analysis of the Rebleeding Rate
All nine studies compared the postprocedure rebleeding rates between TIPS and BRTO. The rebleeding rates in the TIPS groups was higher than that in the BRTO group. There was a significant difference between the two groups (RR, 2.61 (95% CI, 1.75 to 3.90); P < 0.00001) according to a random effects model with no heterogeneity (P=0.46; Ι2 = 0%) (Figure 2).
3.4. Meta-Analysis of Postprocedure Complications
Four studies compared the postprocedure complication rate between TIPS and BRTO. The hepatic encephalopathy rate after the procedure in the TIPS group was significantly higher than that in the BRTO group (RR, 16.11 (95% CI, 7.13 to 36.37); P < 0.00001) under a random effects model, with no heterogeneity (P=0.87; Ι2 = 0%). The incidence rate of aggravated ascites in the BRTO group was higher than that in the TIPS group without a significant difference (RR, 0.60 (95% CI, 0.33 to 1.09); P=0.10) according to a random effects model with no heterogeneity (P=0.57; Ι2 = 0%) (Figure 2).
3.5. Meta-Analysis of the Immediate Haemostasis Rate
Three studies compared the postprocedure immediate haemostasis rate between TIPS and BRTO. The postprocedure immediate haemostasis rate in the TIPS group was higher than that in the BRTO group. Nevertheless, there was no significant difference between the two groups (RR, 0.99 (95% CI, 0.89 to 1.10); P=0.84) according to a random effects model with no heterogeneity (P=0.11; Ι2 = 50%) (Figure 2).
3.6. Meta-Analysis of the Technical Success Rate
Five studies compared the technical success rate between TIPS and BRTO. The technical success rate in the TIPS group was higher than that in the BRTO group. Even so, there was no significant difference between the two groups (RR, 1.06 (95% CI, 0.98 to 1.16); P=0.16) according to a random effects model with heterogeneity (P=0.04; Ι2 = 57%) (Figure 2).
3.7. Meta-Analysis of Changes in Child-Pugh Score
Three studies compared the changes in the Child-Pugh score between TIPS and BRTO. The change in the Child-Pugh score in the BRTO group was higher than that in the TIPS group, but this difference did not reach statistical significance (MD, 0.22 (95% CI, −0.21 to 0.65); P=0.31) according to a random effects model with no heterogeneity (P=0.32; Ι2 = 13%) (Figure 2).
4. Discussion
The management of GVB is not incontrovertible and poses substantial clinical challenges. Given the limited efficacy of endoscopic treatment and candidacy restrictions of shunt surgery, endovascular treatments such as BRTO and TIPS have become valuable options for controlling GVB [34]. Therefore, the discussion of the choice of these two surgical methods is ongoing. The two treatments are completely different in their therapeutic concepts: TIPS aims to decrease the portal pressure to reduce gastric variceal bleeding [31], and BRTO scleroses the GV without decreasing portal pressure [33]. TIPS changes the haemodynamics of the portal vein: the blood flow from the portal vein into the inferior vena cava, which reduces the blood supply to the liver and increases the burden on the liver. On the contrary, some blood that has not been metabolized by the liver directly enters the systemic circulation and might lead to azotaemia, which in turn leads to hepatic encephalopathy and hepatic myelopathy. BRTO directly stops bleeding without reducing portal pressure, which may increase the risk of ascites and bacterial peritonitis. The long-term effects of BRTO on portal haemodynamics have been reported [35, 36]. Using BRTO for treating GV, as with the TIPS procedure, may preserve the function and synthetic capabilities of the liver. We pooled three studies to compare the changes in the Child-Pugh score after the procedure to evaluate liver function. The meta-analysis showed that there was no significant difference between the two groups in the Child-Pugh score change.
For patients, a long survival time is the most valuable benefit. Gimm et al. reported that the postprocedure OS after 5 years was longer after BRTO than after TIPS [27]. Ninoi et al. reported that among patients in Child-Pugh class A, survival (5 years) was significantly higher in the transcatheter sclerotherapy group (including BRTO) than in the TIPS group. In Child-Pugh classes B and C, no significant difference was seen between the TIPS and transcatheter sclerotherapy groups [31]. Lee et al. reported that the postprocedure OS after three years was longer after BRTO than after TIPS [29]. Choi et al. and Sabri et al. reported that the postprocedure OS after one year was higher in BRTO than in TIPS [30, 32]. Kim et al. reported that there was no statistically significant difference in the mean survival between the two groups (TIPS, 30 months; BRTO, 24 months; P=0.16), but the median survival of the patients who underwent TIPS was 16.6 months and that for patients who underwent BRTO was 26.6 months [28]. Therefore, we compared the OS rates between TIPS and BRTO. The combined data suggested that the BRTO group had a significantly higher OS rate than the TIPS group. Early TIPS treatment is still the first-line treatment for acute upper gastrointestinal bleeding. García-Pagán et al. [37] reported that the early use of TIPS was associated with a reduction in mortality. Qi et al. performed a meta-analysis and demonstrated that compared with medical/endoscopic therapy for acute variceal bleeding, TIPS with covered stents might improve overall survival [38]. Nevertheless, there were some limitations in that meta-analysis: (a) only 3 of 6 studies discussed TIPS for GV, and (b) the meta-analysis only compared medical/endoscopic therapy with TIPS for acute variceal bleeding, BRTO was not mentioned. In our study, we compared TIPS and BRTO for GV. The overall survival rate and rebleeding rate in the BRTO group were significantly higher than those in the TIPS group. This finding may provide new options for the future treatment of GV.
The technical difficulty of TIPS lies in portal vein puncture: puncture may cause massive haemorrhage in the abdominal cavity. The appropriate positioning of the puncture point may lead to shunt dysfunction, which makes it difficult to reduce portal pressure [39, 40]. BRTO is also a relatively demanding interventional surgery that requires a well-knit and skilled basis in anatomy, radiology, and handling procedures. Wang et al. reported that BRTO was not more difficult to finish than TIPS [23]. In our meta-analysis, we reached a similar conclusion. The rebleeding rate of the TIPS group was significantly higher than that in the BRTO group. As previously shown, especially in shunts with bare metal stents, thrombosis could occur in the shunt, which leads to an increase in the rebleeding rate [41]. Qi et al. performed a meta-analysis and demonstrated that compared with bare stents, covered stents for TIPS may improve overall survival. Subgroup analysis for the effect of different brands of covered stents achieved no significant difference [42]. In our meta-analysis, there were only two early studies using bare stents [31, 32]. The original data of all the studies did not specifically record the postoperative changes in patients with bare or covered stents. We tried to exclude the early research that contained bare metal stents [31, 32] before analysing the data and reached the same conclusion on the rebleeding rate. Tripathi et al. and Chau et al. showed that GV can bleed at PSGs <12 mmHg and behave differently than EVs, which bleed at higher PSGs (average, 15.5 mmHg) [43, 44]. Thus, even though the portal shunt was established, PSGs might not decrease below 12 mmHg. Another meta-analysis suggested that TIPS in combination with variceal embolization is more effective for the prevention of variceal rebleeding [45]. In our included studies, a total of 23 patients underwent post-TIPS coil embolization. In the study by Sabri et al. [30], if any GV were visualized during the TIPS procedure, selective catheterization of the inflow portal venous branch was followed by embolization of the branch using metallic coils. In the study by Choi et al. [32] and Kim et al. [28], gastric varices were evaluated by CT after the TIPS procedure. If the GV persisted, additional embolization was performed. However, in the results analysis section, the above authors did not list the data on patients with TIPS combined with variceal embolization separately. We were unable to obtain raw data from these studies and to use these data for further analysis of whether a combination of TIPS with variceal embolization is superior to BRTO alone for GV. More RCTs are needed in the future. Except for the postprocedure complications (hepatic encephalopathy), the TIPS group and BRTO group did not have a significant difference in the immediate haemostasis rate, which meant that the two methods were both effective for GVB in emergency situations.
Our meta-analysis only contained one randomized controlled trial with a small sample size, which may have led to selection bias. Differences in the performance of different TIPS stents might have also affected the results. With the development of radiological intervention technology, more RCTs comparing TIPS and BRTO should be conducted.
5. Conclusions
In our meta-analysis, BRTO brought more benefits to patients than TIPS. BRTO is a suitable method for GVB with gastrorenal shunts.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Ma L., Tseng Y., Luo T., et al. Risk stratification for secondary prophylaxis of gastric varices due to portal hypertension. Digestive and Liver Disease. 2019;51(12):1678–1684. doi: 10.1016/j.dld.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 2.Goral V., Yılmaz N. Current approaches to the treatment of gastric varices: glue, coil application, TIPS, and BRTO. Medicina. 2019;55(7):p. 335. doi: 10.3390/medicina55070335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Pagán J. C., Barrufet M., Cardenas A., Escorsell À. Management of gastric varices. Clinical Gastroenterology and Hepatology. 2014;12(6):919–928.e1. doi: 10.1016/j.cgh.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim M., Mostafa I., Devière J. New developments in managing variceal bleeding. Gastroenterology. 2018;154(7):1964–1969. doi: 10.1053/j.gastro.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 5.Ryan B. M., Stockbrugger R. W., Ryan J. M. A pathophysiologic, gastroenterologic, and radiologic approach to the management of gastric varices. Gastroenterology. 2004;126(4):1175–1189. doi: 10.1053/j.gastro.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda T., Hirota S., Sugimura K. Long-term results of balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy. Journal of Vascular and Interventional Radiology. 2001;12(3):327–336. doi: 10.1016/s1051-0443(07)61912-5. [DOI] [PubMed] [Google Scholar]
- 7.Girotra M., Raghavapuram S., Abraham R. R., Pahwa M., Pahwa A. R, Rego R. F. Management of gastric variceal bleeding: role of endoscopy and endoscopic ultrasound. World Journal of Hepatology. 2014;6(3):p. 130. doi: 10.4254/wjh.v6.i3.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czaja A., Freese D. K. Diagnosis and treatment of autoimmune hepatitis. Hepatology. 2002;36(2):479–497. doi: 10.1053/jhep.2002.34944. [DOI] [PubMed] [Google Scholar]
- 9.Timmis A., Roobottom C. A. National Institute for Health and Care Excellence updates the stable chest pain guideline with radical changes to the diagnostic paradigm. Heart. 2017;103(13):982–986. doi: 10.1136/heartjnl-2015-308341. [DOI] [PubMed] [Google Scholar]
- 10.Sarin S. K., Kumar A. Endoscopic treatment of gastric varices. Clinics in Liver Disease. 2014;18(4):809–827. doi: 10.1016/j.cld.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Kahloon A., Chalasani N., DeWitt J., et al. Endoscopic therapy with 2-octyl-cyanoacrylate for the treatment of gastric varices. Digestive Diseases and Sciences. 2014;59(9):2178–2183. doi: 10.1007/s10620-014-3148-9. [DOI] [PubMed] [Google Scholar]
- 12.Bhat Y. M., Banerjee S., Barth B. A., et al. Tissue adhesives: cyanoacrylate glue and fibrin sealant. Gastrointestinal Endoscopy. 2013;78(2):209–215. doi: 10.1016/j.gie.2013.04.166. [DOI] [PubMed] [Google Scholar]
- 13.Lipnik A. J., Pandhi M. B., Khabbaz R. C., Gaba R. C. Endovascular treatment for variceal hemorrhage: TIPS, BRTO, and combined approaches. Seminars in Interventional Radiology. 2018;35(03):169–184. doi: 10.1055/s-0038-1660795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayakawa M., Ohnishi S., Suzuki H. Recent development of balloon-occluded retrograde transvenous obliteration. Journal of Gastroenterology and Hepatology. 2019;34(3):495–500. doi: 10.1111/jgh.14463. [DOI] [PubMed] [Google Scholar]
- 15.Strunk H., Marinova M. Transjugular intrahepatic portosystemic shunt (TIPS): pathophysiologic basics, actual indications and results with review of the literature. RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren. 2018;190(8):701–711. doi: 10.1055/a-0628-7347. [DOI] [PubMed] [Google Scholar]
- 16.Mantaka A., Tsetis D., Hatzidakis A., Kouroumalis E. A, Samonakis D. N. Successful management of refractory gastric variceal bleeding via a combined percutaneous approach in sinistral portal hypertension. Gastroenterol Hepatol Open Access. 2018;9(2):55–58. doi: 10.15406/ghoa.2018.09.00294. [DOI] [Google Scholar]
- 17.Saad W. E. A., Darcy M. Seminars in Interventional Radiology. 3. Vol. 28. Thieme Medical Publishers; 2011. Transjugular intrahepatic portosystemic shunt (TIPS) versus balloon-occluded retrograde transvenous obliteration (BRTO) for the management of gastric varices; pp. 339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim M. Y., Um S. H., Baik S. K., et al. Clinical features and outcomes of gastric variceal bleeding: retrospective Korean multicenter data. Clinical and Molecular Hepatology. 2013;19(1):p. 36. doi: 10.3350/cmh.2013.19.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arai H., Abe T., Takagi H., Mori M. Efficacy of balloon-occluded retrograde transvenous obliteration, percutaneous transhepatic obliteration and combined techniques for the management of gastric fundal varices. World Journal of Gastroenterology. 2006;12(24):3866–3873. doi: 10.3748/wjg.v12.i24.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arai H., Abe T., Takayama H., et al. Respiratory effects of balloon occluded retrograde transvenous obliteration of gastric varices: a prospective controlled study. Journal of Gastroenterology and Hepatology. 2011;26(9):1389–1394. doi: 10.1111/j.1440-1746.2011.06727.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang K., Sun X., Wang G., et al. Treatment outcomes of percutaneous transhepatic variceal embolization versus transjugular intrahepatic portosystemic shunt for gastric variceal bleeding. Medicine. 2019;98(18) doi: 10.1097/md.0000000000015464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orloff M. J., Hye R. J., Wheeler H. O., et al. Randomized trials of endoscopic therapy and transjugular intrahepatic portosystemic shunt versus portacaval shunt for emergency and elective treatment of bleeding gastric varices in cirrhosis. Surgery. 2015;157(6):1028–1045. doi: 10.1016/j.surg.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y.-B., Zhang J.-Y., Gong J.-P., Zhang F., Zhao Y. Balloon-occluded retrograde transvenous obliteration versus transjugular intrahepatic portosystemic shunt for treatment of gastric varices due to portal hypertension: a meta-analysis. Journal of Gastroenterology and Hepatology. 2016;31(4):727–733. doi: 10.1111/jgh.13248. [DOI] [PubMed] [Google Scholar]
- 24.Lee D. Y., Lee S. J., Kim M. D., et al. Transjugular intrahepatic portosystemic shunt (TIPS) versus balloon-occluded retrograde transvenous obliteration (BRTO) for the treatment of gastric varix. Cardio Vascular and Interventional Radiology. 2014;37:p. S350. [Google Scholar]
- 25.Lee J. Y., Lee Y. J., Min B. R., et al. Efficacy and safety of transjugular intrahepatic portosystemic shunt and balloon occluded retrograde transvenous obliteration for gastric variceal bleeding. Hepatology. 2012;56:p. 754A. [Google Scholar]
- 26.Sauk S., Niemeyer M., Kim S., Korenblat K. Outcomes from balloon-occluded retrograde transvenous obliteration (BRTO) versus transjugular intrahepatic portosystemic shunt (TIPS) in the management of isolated gastric varices: a retrospective study in single US medical center. Journal of Vascular and Interventional Radiology. 2014;25(3):p. S80. doi: 10.1016/j.jvir.2013.12.227. [DOI] [Google Scholar]
- 27.Gimm G., Chang Y., Kim H.-C., et al. Balloon-occluded retrograde transvenous obliteration versus transjugular intrahepatic portosystemic shunt for the management of gastric variceal bleeding. Gut and Liver. 2018;12(6):704–713. doi: 10.5009/gnl17515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim S. K., Lee K. A., Sauk S., Korenblat K. Comparison of transjugular intrahepatic portosystemic shunt with covered stent and balloon-occluded retrograde transvenous obliteration in managing isolated gastric varices. Korean Journal of Radiology. 2017;18(2):345–354. doi: 10.3348/kjr.2017.18.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S. J., Kim S. U., Kim M.-D., et al. Comparison of treatment outcomes between balloon-occluded retrograde transvenous obliteration and transjugular intrahepatic portosystemic shunt for gastric variceal bleeding hemostasis. Journal of Gastroenterology and Hepatology. 2017;32(8):1487–1494. doi: 10.1111/jgh.13729. [DOI] [PubMed] [Google Scholar]
- 30.Sabri S. S., Abi-Jaoudeh N., Swee W., et al. Short-term rebleeding rates for isolated gastric varices managed by transjugular intrahepatic portosystemic shunt versus balloon-occluded retrograde transvenous obliteration. Journal of Vascular and Interventional Radiology. 2014;25(3):355–361. doi: 10.1016/j.jvir.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Ninoi T., Nakamura K., Kaminou T., et al. TIPS versus transcatheter sclerotherapy for gastric varices. American Journal of Roentgenology. 2004;183(2):369–376. doi: 10.2214/ajr.183.2.1830369. [DOI] [PubMed] [Google Scholar]
- 32.Choi Y. H., Yoon C. J., Park J. H., Chung J. W., Kwon J. W., Choi G. M. Balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding: its feasibility compared with transjugular intrahepatic portosystemic shunt. Korean Journal of Radiology. 2003;4(2):109–116. doi: 10.3348/kjr.2003.4.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi Y. S., Lee J. H., Sinn D. H., et al. Effect of balloon-occluded retrograde transvenous obliteration on the natural history of coexisting esophageal varices. Journal of Clinical Gastroenterology. 2008;42(9):974–979. doi: 10.1097/mcg.0b013e318126c154. [DOI] [PubMed] [Google Scholar]
- 34.Saad W. E. Endovascular management of gastric varices. Clinics in Liver Disease. 2014;18(4):829–851. doi: 10.1016/j.cld.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Hong C. H., Kim H. J., Park J. H. Treatment of patients with gastric variceal hemorrhage: endoscopic N-butyl-2-cyanoacrylate injection versus balloon-occluded retrograde transvenous obliteration. Journal of Gastroenterology and Hepatology. 2009;24(3):372–378. doi: 10.1111/j.1440-1746.2008.05651.x. [DOI] [PubMed] [Google Scholar]
- 36.Inoue H., Emori K., Toyonaga A., et al. Long term results of balloon-occluded retrograde transvenous obliteration for portosystemic shunt encephalopathy in patients with liver cirrhosis and portal hypertension. The Kurume Medical Journal. 2014;61(1-2):1–8. doi: 10.2739/kurumemedj.ms63014. [DOI] [PubMed] [Google Scholar]
- 37.García-Pagán J. C., Caca K., Bureau C., et al. Early Use of TIPS in patients with cirrhosis and variceal bleeding. New England Journal of Medicine. 2010;362(25):2370–2379. doi: 10.1056/nejmoa0910102. [DOI] [PubMed] [Google Scholar]
- 38.Qi X., Jia J., Bai M., et al. Transjugular intrahepatic portosystemic shunt for acute variceal bleeding. Journal of Clinical Gastroenterology. 2015;49(6):495–505. doi: 10.1097/MCG.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 39.Gazzera C., Righi D., Doriguzzi Breatta A., et al. Emergency transjugular intrahepatic portosystemic shunt (TIPS): results, complications and predictors of mortality in the first month of follow-up. La Radiologia Medica. 2012;117(1):46–53. doi: 10.1007/s11547-011-0682-9. [DOI] [PubMed] [Google Scholar]
- 40.Henderson J. M., Boyer T. D., Kutner M. H., et al. Distal splenorenal shunt versus transjugular intrahepatic portal systematic shunt for variceal bleeding: a randomized trial. Gastroenterology. 2006;130(6):1643–1651. doi: 10.1053/j.gastro.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Bureau C., Garcia-Pagan J. C., Otal P., et al. Improved clinical outcome using polytetrafluoroethylene-coated stents for tips: Results of a randomized study. Gastroenterology. 2004;126(2):469–475. doi: 10.1053/j.gastro.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 42.Qi X., Tian Y., Zhang W., Yang Z., Guo X. Coveredversusbare stents for transjugular intrahepatic portosystemic shunt: an updated meta-analysis of randomized controlled trials. Therapeutic Advances in Gastroenterology. 2017;10(1):32–41. doi: 10.1177/1756283x16671286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tripathi D., Therapondos G., Jackson E., Redhead D. N., Hayes P. C. The role of the transjugular intrahepatic portosystemic stent shunt (TIPSS) in the management of bleeding gastric varices: clinical and haemodynamic correlations. Gut. 2002;51(2):270–274. doi: 10.1136/gut.51.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chau T. N., Patch D., Chan Y. W., Nagral A., Dick R., Burroughs A. K. “Salvage” transjugular intrahepatic portosystemic shunts: gastric fundal compared with esophageal variceal bleeding. Gastroenterology. 1998;114(5):981–987. doi: 10.1016/s0016-5085(98)00640-4. [DOI] [PubMed] [Google Scholar]
- 45.Qi X., Liu L., Bai M., et al. Transjugular intrahepatic portosystemic shunt in combination with or without variceal embolization for the prevention of variceal rebleeding: a meta-analysis. Journal of Gastroenterology and Hepatology. 2014;29(4):688–696. doi: 10.1111/jgh.12391. [DOI] [PubMed] [Google Scholar]