Abstract
OBJECTIVE:
To compare the incidence of hypertensive disorders of pregnancy among women living with human immunodeficiency virus (HIV) on combination antiretroviral therapy (ART) to women without HIV, and to evaluate the association of hypertensive disorders of pregnancy with ART regimens or timing of ART initiation.
METHODS:
We conducted a retrospective cohort study among two overlapping pregnancy cohorts using preexisting databases at a single tertiary care hospital: all pregnant women who delivered during years 2016–2018 (cohort 1) and all women living with HIV who delivered during years 2011–2018 (cohort 2). The primary outcome for both cohorts was any hypertensive disorder of pregnancy; gestational hypertension and preeclampsia were also examined separately. The primary exposure variables were HIV status for cohort 1 and ART regimen (integrase strand transfer inhibitor–containing, protease inhibitor–containing, or non-nucleoside reverse transcriptase inhibitor–containing) for cohort 2. For estimation of risk ratios (RRs), we used a modified Poisson regression with robust error variances. Multivariate models among the women living with HIV in cohort 2 were tested for a statistical interaction between ART regimen and timing of initiation.
RESULTS:
In cohort 1, among 80 women living with HIV compared with 3,464 women without HIV, there was no difference in the risk of hypertensive disorders of pregnancy (29% in women living with HIV vs 30% in women without HIV, adjusted RR 0.9, 95% CI 0.6–1.3). In cohort 2, among 265 women living with HIV, integrase strand transfer inhibitor–containing regimens were associated with an increased risk for any hypertensive disorder of pregnancy (25% among integrase strand transfer inhibitor vs 10% among protease inhibitor, adjusted RR 2.8, 95% CI 1.5–5.1) and gestational hypertension (20% among integrase strand transfer inhibitor vs 8% among protease inhibitor, adjusted RR 2.8, 95% CI 1.3–5.9) compared with protease inhibitor–containing regimens. Timing of ART initiation was not associated with hypertensive disorders of pregnancy, nor did it significantly alter the associations between ART regimen and hypertensive disorders of pregnancy outcomes.
CONCLUSION:
Overall the risk of hypertensive disorders of pregnancy was similar among women living with HIV on ART and women without HIV. With greater integrase strand transfer inhibitor use, the greater frequency of hypertensive disorders of pregnancy with these regimens compared with protease inhibitor–containing regimens warrants future evaluation using cohorts with greater sample size.
Since the introduction of combination antiretroviral therapy (ART) in the early 1990s, the incidence of perinatal transmission of human immunodeficiency virus (HIV) has decreased by more than 90% and risk of perinatal transmission with ART use is less than 1%.1 The known benefits of ART during pregnancy far outweigh short-term risks. However, gaps remain in our understanding of the effects of maternal ART use on other maternal and perinatal outcomes and how specific ART regimens may influence these outcomes.
Hypertensive disorders of pregnancy encompass a spectrum of diseases including gestational hypertension, preeclampsia (including preeclampsia superimposed on chronic hypertension), eclampsia, and hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome. Hypertensive disorders of pregnancy are common complications of pregnancy with a reported population prevalence ranging from 5 to 8%.2 Hypertensive disorders of pregnancy are a leading cause of maternal morbidity and mortality,3 and their presence increases risk of placental abruption, preterm delivery, and infant death.
The mechanism of hypertensive disorders of pregnancy is likely multifactorial, but there is increasing evidence that immunologic mechanisms may contribute to its development. Human immunodeficiency virus causes a myriad of alterations to the immune system. Pregnant women living with HIV may have a dampened immunologic response reducing the risk for hypertensive disorders of pregnancy development, as suggested by some reports of decreased hypertensive disorders of pregnancy among women living with HIV.4–6 Alternatively, women treated with ART may have increased risk of hypertensive disorders of pregnancy compared with untreated women living with HIV or women without HIV.4,5,7–10 One potential mechanism for a possible increased risk of hypertensive disorders of pregnancy with ART is the immune reconstitution inflammatory syndrome,11 a hyperinflammatory state associated with ART initiation. This theory suggests timing of ART initiation or the regimen-dependent efficiency of viral decay may influence hypertensive disorders of pregnancy development. Integrase strand transfer inhibitors are the newest class of antiretrovirals and are reportedly associated with faster immune reconstitution and viral suppression.12,13 Few studies evaluate the association of contemporary ART regimen use with hypertensive disorders of pregnancy.
The objectives of our study were to examine: 1) whether pregnant women living with HIV taking contemporary ART regimens are at increased risk of developing hypertensive disorders of pregnancy compared with women without HIV and 2) whether, among women living with HIV, ART regimen or timing of ART initiation are associated with development of hypertensive disorders of pregnancy.
METHODS
We conducted a retrospective cohort study using preexisting data from two cohorts of women who delivered at Grady Memorial Hospital, a large tertiary care hospital. The institutional review board at Emory University and the Grady Research Oversight Committee provided ethical approval for this study.
For the comparison of pregnancies among women living with HIV to those among women without HIV, we included all pregnant women who delivered at Grady Memorial Hospital under the care of Emory physicians from July 1, 2016–June 30, 2018. Deliveries were identified using the Emory Medical Care Foundation database and cross-referenced with an ongoing record of all deliveries on the labor and delivery unit. For the analysis, we excluded pregnancies delivered before 20 weeks of gestation, multifetal pregnancies, and pregnancies among women living with HIV not treated with contemporary ART regimen (Fig. 1). We defined ART as a backbone of two nucleoside reverse transcriptase inhibitors plus at least one non-nucleoside reverse transcriptase inhibitor, protease inhibitor, or integrase strand transfer inhibitor. For the small percentage (3%) of women having multiple delivery dates within the 2-year period, we selected the last pregnancy for analysis.
Fig. 1.
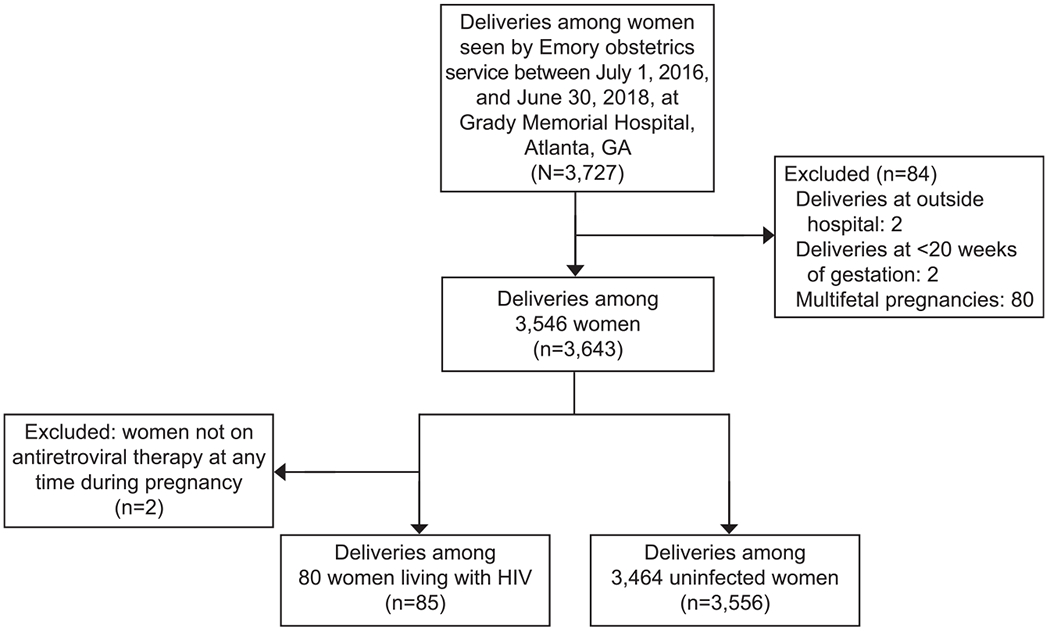
Pregnancies eligible and included in cohort 1: all pregnancies delivered to Emory obstetrics service at Grady Memorial Hospital from July 1, 2016 to June 30, 2018. HIV, human immunodeficiency virus.
Saums. Antiretrovirals and Hypertension in Pregnancy. Obstet Gynecol 2019.
We manually reviewed medical charts and directly entered variables of interest into a database using REDCap software on a secure server at Emory University.14 All researchers were trained in abstraction and used a codebook to ensure consistent methodology.
For the analysis of ART regimens on hypertensive disorders of pregnancy outcomes, the study population consisted of women living with HIV who delivered at Grady Memorial Hospital from January 1, 2011, through June 30, 2018. These data are maintained and updated regularly within an HIV perinatal database.15 In this database, sociodemographic and clinical data were collected from entry into antenatal care through the postpartum period via standardized extraction from the electronic medical record. The same women living with HIV in cohort 1 are included in cohort 2 unless they met exclusion criteria for cohort 2. Pregnancies without a known delivery date, delivered before 20 weeks of gestation, or that were multifetal were excluded (Fig. 2). Additionally, we excluded eight pregnancies in which ART regimen was unspecified. For women with multiple delivery dates during the study period (19%), only the last delivery was included to maintain consistency across both cohorts.
Fig. 2.
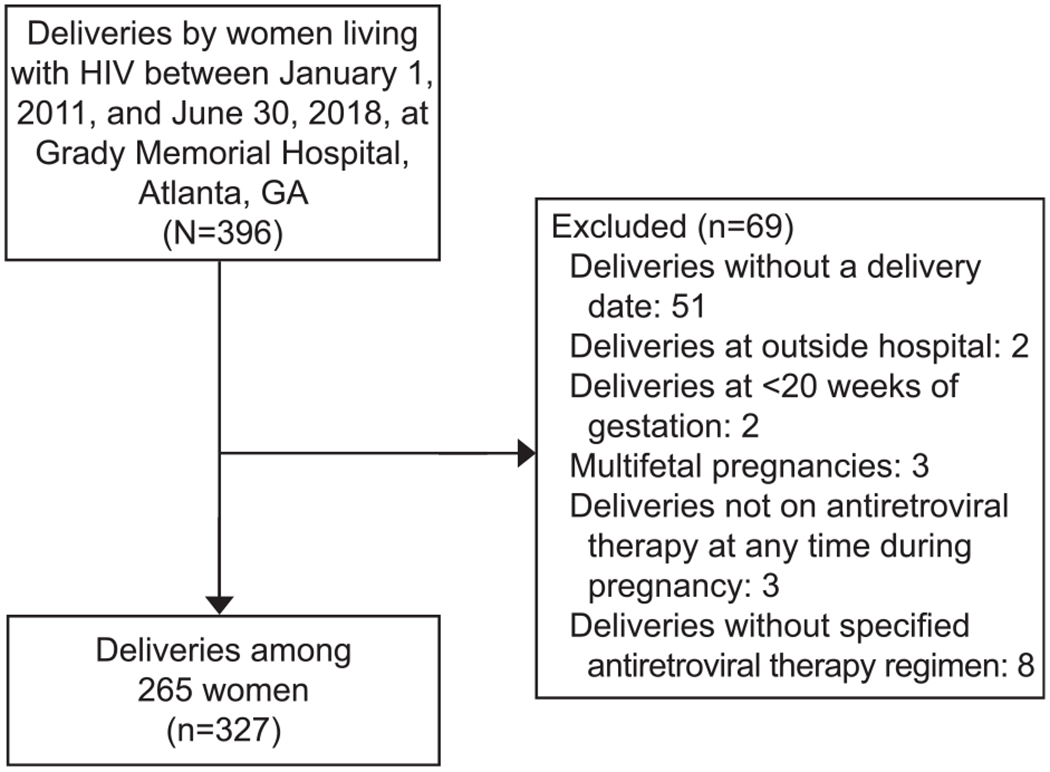
Pregnancies eligible and included in cohort 2: all pregnant women living with human immunodeficiency virus (HIV) who delivered at Grady Memorial Hospital from January 1, 2011–June 30, 2018.
Saums. Antiretrovirals and Hypertension in Pregnancy. Obstet Gynecol 2019.
The primary exposure variables of interest were HIV status for cohort 1 and ART regimen and timing of ART initiation for cohort 2. Regimens of ART were categorized into integrase strand transfer inhibitor–containing, protease inhibitor–containing, and non-nucleoside reverse transcriptase inhibitor–containing. Because of faster viral suppression and immune reconstitution often observed with integrase strand transfer inhibitor–containing ART,12,13 women who were on regimens containing a combination of integrase strand transfer inhibitor, protease inhibitor, or non-nucleoside reverse transcriptase inhibitor were categorized using the following hierarchy: integrase strand transfer inhibitor>protease inhibitor>non-nucleoside reverse transcriptase inhibitor. Timing of ART initiation was categorized as before pregnancy or during pregnancy based on start date of latest ART regimen. Women who changed regimens during pregnancy were categorized by the regimen most proximate to delivery, unless taken for less than 28 days, in which her prior regimen was considered the exposure.10
The primary outcome was diagnosis of any new-onset hypertensive disorders of pregnancy, which included gestational hypertension and preeclampsia. Preeclampsia included diagnoses of preeclampsia, eclampsia, and HELLP syndrome. Additionally, gestational hypertension and preeclampsia were evaluated as separate outcomes. All diagnoses were based on the clinical diagnoses abstracted from the medical charts.
Covariates evaluated as potential confounders included limited risk factors for development or detection of hypertensive disorders of pregnancy: age, race, and tobacco or illicit drug use during pregnancy.16 HIV-related covariates, including viral load and CD4 count most proximate to delivery, are presented but not included in multivariate models owing to the effect of ART on these outcomes.
Descriptive statistics were calculated for variables of interest across levels of the main predictor variables (HIV status and ART regimen classification). P-values comparing categorical variables were derived using the χ2 test or Fisher exact test. Medians were compared using the Wilcoxon test across HIV status and the Kruskal Wallis test across the three categories of ART regimens. Statistical significance was assessed at the 0.05 level.
To estimate unadjusted and adjusted risk ratios (RRs) for outcomes, we used a modified Poisson approach that uses robust error variances to validly estimate 95% CIs.17 To attempt to control for potential confounding, we used a priori knowledge based on the literature of predictors for development or detection of the outcomes and of factors associated with the exposure (HIV status or ART regimen) in the source population along with causal (directed acyclic) diagram theory. For cohort 1, all initial models included HIV status, age, race, tobacco and illicit drug use during pregnancy. For cohort 2, all initial models included ART regimen classification, age, race, illicit drug use during pregnancy and timing of ART initiation. The model was also tested for a statistical interaction between ART regimen and timing of ART initiation.
We ran several analyses to test the sensitivity of the study findings to covariate adjustment, cohort selection, and statistical model. First, we ran regression models with a more inclusive list of risk factors for development or detection of hypertensive disorders of pregnancy: age, race, tobacco and illicit drug use plus parity, number of prenatal visits at Grady Memorial Hospital, preterm delivery, history of hypertensive disorders of pregnancy, and chronic diseases (chronic hypertension, diabetes, and obesity [defined as body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) 30 or higher or, if BMI was unknown, by chart-abstracted data on history of obesity before pregnancy]). We also evaluated a version of the latter models restricted to covariates with adjusted P<0.2 or that, when removed, changed the exposure-outcome RR by more than 5%. Given the difficulty of defining obesity in pregnancy and that women often entered prenatal care late in pregnancy, we also assessed these models with an alternate definition of BMI, based on trimester cutoffs for those without BMI in the first trimester: 35 or higher in the second trimester and 40 or higher in the third trimester. To assess the sensitivity of study findings to cohort selection, we reran the analyses among alternate cohort sets: 1) all deliveries (including multiple delivery dates on a given woman) during the study period using general estimating equations with an assumed exchangeable correlation between repeat pregnancies; 2) a cohort restricted to term (37 weeks of gestation or greater) deliveries; and 3) for cohort 1, we used propensity score matching to match women without HIV to women living with HIV 1:1 based on age, race, tobacco use, alcohol use, illicit drug use, history of hypertensive disorders of pregnancy, gestational age at entry, and gestational age at delivery. To verify consistency of results across modeling approaches, we reran models where convergence was possible using standard log-binomial models. Statistical analyses were performed using SAS University Edition 9.4.
RESULTS
A total of 80 women living with HIV and 3,464 women without HIV were eligible for inclusion in cohort 1 (Table 1). Women living with HIV in cohort 1 were older and more likely to be non-Hispanic black, to have used tobacco or alcohol during pregnancy, to have entered prenatal care earlier, and to have had more prenatal visits than women without HIV. The two groups were otherwise similar with regard to other characteristics (Table 1).
Table 1.
Cohort 1: Demographic and Clinical Characteristics
| Characteristic | HIV-Positive (n=80) | HIV-Negative (n=3,464) | P |
|---|---|---|---|
| Age at delivery (y) | .002 | ||
| 20 or younger | 4 (5) | 539 (16) | |
| 21–34 | 55 (69) | 2,409 (70) | |
| 35 or older | 21 (26) | 516 (15) | |
| Race | <.001 | ||
| Non-Hispanic black | 68 (86) | 2,321 (68) | |
| White, Hispanic, other | 11 (14) | 1,116 (33) | |
| Parity | .800 | ||
| 0 | 15 (19) | 801 (23) | |
| 1 | 6 (8) | 264 (8) | |
| 2 | 22 (28) | 838 (24) | |
| 3 or more | 36 (46) | 1,560 (45) | |
| History of HDP* | 1 (1) | 168 (5) | .183 |
| History of gestational diabetes | 2 (3) | 121 (4) | 1.000 |
| Chronic medical condition | |||
| Hypertension | 6 (8) | 317 (9) | .612 |
| Diabetes mellitus | 2 (3) | 90 (3) | 1.000 |
| Obesity† | 27 (34) | 1,173 (34) | .983 |
| Gestational age at entry | 16 (12–25) | 19 (12–31) | .046 |
| Substance use in pregnancy | |||
| Tobacco | 17 (21) | 375 (11) | .005 |
| Alcohol | 7 (9) | 91 (3) | .007 |
| Illicit drugs | 16 (20) | 461 (14) | .102 |
| No. of prenatal visits at GMH | 8 (5–11) | 6 (3–9) | <.001 |
| Gestational age at delivery (wk) | 38.7 (37.3–39.2) | 39.1 (38.0–40.0) | .003 |
| Preterm delivery‡ | 15 (19) | 487 (14) | .241 |
| Complications of pregnancy | |||
| HDP* | 23 (29) | 1,024 (30) | .875 |
| Gestational hypertension | 17 (23) | 611 (19) | .445 |
| Preeclampsia, eclampsia, HELLP | 6 (8) | 459 (13) | .132 |
HIV, human immunodeficiency virus; HDP, hypertensive disorders of pregnancy; GMH, Grady Memorial Hospital; HELLP, hemolysis, elevated liver enzymes, and low platelets.
Data are n (%) or median (interquartile range) unless otherwise specified.
P-values comparing categorical variables were derived using the χ2 test or Fisher exact test; medians were compared using the Wilcoxon test.
Data are missing for the following variables: race (n=28), parity (n=2), tobacco use (n=87), alcohol use (n=96), illicit drug use (n=80), gestational age at delivery (n=14), and preterm delivery (n=14).
Gestational hypertension, preeclampsia, eclampsia, HELLP syndrome.
Body mass index (BMI) 30.0 or greater for the 81% of patients with a prenatal BMI measurement (40% were measured in the first trimester, 40% in the second, and 20% in the third); for the 19% without a prenatal BMI measurement, chart-abstracted history of obesity was used.
Gestational age less than 37 weeks at delivery.
Hypertensive disorders of pregnancy occurred in about a quarter of pregnancies, both among women living with and without HIV, with the majority of cases gestational hypertension (Table 1). Preeclampsia did not differ by HIV status. Neither overall hypertensive disorders of pregnancy nor individual outcomes of gestational hypertension or preeclampsia differed by HIV status in unadjusted and adjusted analysis (Table 2). Findings remained consistent in sensitivity analyses and with propensity score matching.
Table 2.
Cohort 1: Unadjusted and Adjusted Risk Ratios for Hypertensive Disorders of Pregnancy Outcomes Comparing Pregnancies in Antiretroviral Therapy–Exposed Pregnant Women Living With Human Immunodeficiency Virus With Those in Pregnancies in Women Without Human Immunodeficiency Virus
| Outcome | RR (95% CI) | Adjusted RR (95% CI) |
|---|---|---|
| HDP* | 1.0 (0.7–1.4) | 0.9 (0.6–1.3) |
| Gestational hypertension | 1.2 (0.8–1.8) | 1.0 (0.7–1.6) |
| Preeclampsia | 0.6 (0.3–1.2) | 0.5 (0.3–1.2) |
RR, risk ratio; HDP, hypertensive disorders of pregnancy.
All models adjusted for age, race, and tobacco and illicit drug use.
One hundred nineteen (3%) observations had missing data for a covariate and are excluded from the analysis.
Gestational hypertension, preeclampsia, eclampsia or hemolysis, elevated liver enzymes, and low platelets syndrome.
A total of 265 women living with HIV were eligible for inclusion in cohort 2. The types of ART regimens used by pregnant women living with HIV at Grady Memorial Hospital changed over time, with a reduction in protease inhibitor–containing regimens and an increase in integrase strand transfer inhibitor–containing regimens (Fig. 3).
Fig. 3.
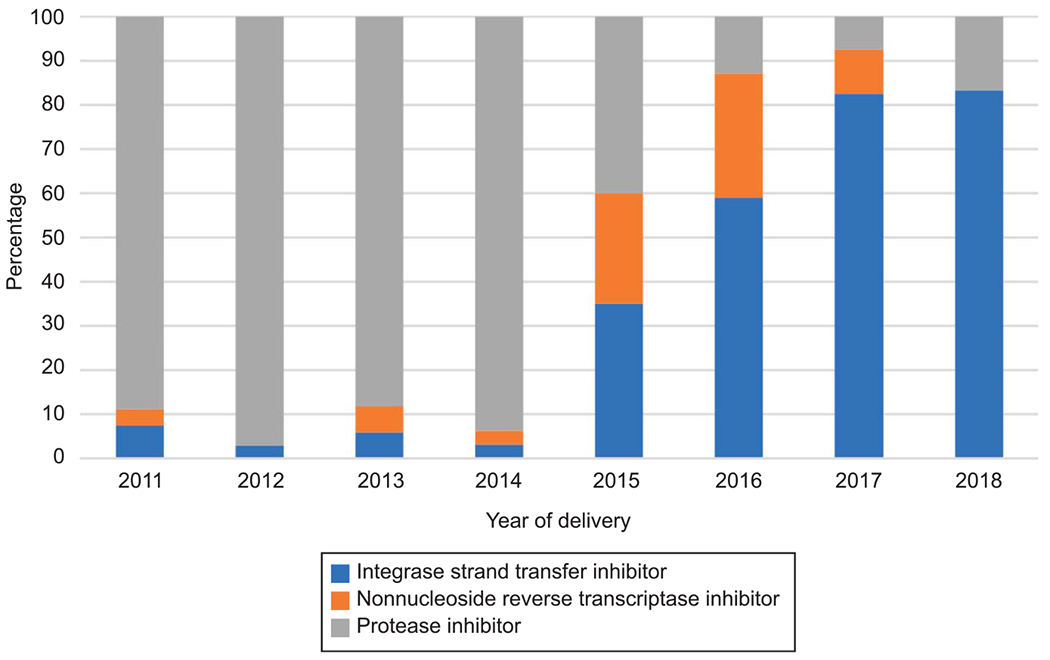
Distribution of antiretroviral therapy regimen from 2011 to 2018 among women living with human immunodeficiency virus who delivered at Grady Memorial Hospital.
Saums. Antiretrovirals and Hypertension in Pregnancy. Obstet Gynecol 2019.
Of the 265 women living with HIV, 91 were on an integrase strand transfer inhibitor–containing regimen, 145 were on a protease inhibitor–containing regimen, and 29 were on a non-nucleoside reverse transcriptase inhibitor–containing regimen (Table 3). 19 women (7%) switched regimens during pregnancy. Those in the non-nucleoside reverse transcriptase inhibitor group were significantly older, less likely to initiate ART during pregnancy, and more likely to have CD4 greater than 500 cells/mm3 and viral load less than 200 copies/mL than women on the other regimens. Otherwise, women in all three regimen groups were similar (Table 3).
Table 3.
Cohort 2: Demographic and Clinical Characteristics
| ART Regimen |
|||||
|---|---|---|---|---|---|
| Characteristic | Total (n=265) | INSTI-Containing (n=91) | PI-Containing (n=145) | NNRTI-Containing (n=29) | P |
| Age at delivery (y) | |||||
| 20 or younger | 21 (8) | 8 (9) | 12 (8) | 1 (3) | .021 |
| 20–35 | 195 (74) | 69 (76) | 110 (76) | 16 (55) | |
| 35 or older | 49 (19) | 14 (15) | 23 (16) | 12 (41) | |
| Race | |||||
| Non-Hispanic black | 210 (84) | 74 (85) | 114 (84) | 22 (82) | .904 |
| White, Hispanic, other | 40 (16) | 13 (15) | 22 (16) | 5 (19) | |
| Parity | |||||
| 0 | 66 (25) | 24 (27) | 40 (28) | 2 (7) | .232 |
| 1 | 63 (24) | 18 (20) | 38 (26) | 7 (24) | |
| 2 | 54 (20) | 19 (21) | 26 (18) | 9 (31) | |
| 3 or more | 81 (31) | 29 (32) | 41 (28) | 11 (38) | |
| History of HDP* | 13 (5) | 5 (6) | 7 (5) | 1 (4) | 1.000 |
| History of gestational diabetes | 4 (2) | 2 (2) | 1 (1) | 1 (4) | .644 |
| Chronic medical condition | |||||
| Hypertension | 39 (15) | 17 (19) | 18 (12) | 4 (14) | .412 |
| Diabetes mellitus | 4 (2) | 1 (1) | 2 (1) | 1 (4) | .564 |
| Obesity† | 95 (37) | 35 (39) | 52 (38) | 8 (28) | .513 |
| Gestational age at entry | 16 (12–24) | 15 (11–24) | 17 (12–23) | 16 (10–29) | .621 |
| Substance use in pregnancy | |||||
| Tobacco | 54 (20) | 23 (25) | 25 (17) | 6 (21) | .329 |
| Alcohol | 16 (6) | 8 (9) | 7 (5) | 1 (4) | .380 |
| Illicit drugs | 39 (15) | 19 (21) | 18 (12) | 2 (7) | .092 |
| No. of prenatal visits at GMH | 9 (5–11) | 9 (6–11) | 9 (5–11) | 8 (5–10) | .829 |
| Initiated ART during pregnancy | 145 (55) | 57 (63) | 85 (60) | 3 (10) | <.001 |
| CD4‡ | 450 (274–608) | 423 (266–607) | 437 (264–604) | 530 (389–643) | .100 |
| Less than 200 | 34 (13) | 13 (14) | 21 (15) | 0 | .042 |
| 200–500 | 122 (46) | 47 (52) | 64 (44) | 11 (38) | |
| 500 or greater | 108 (41) | 30 (33) | 60 (41) | 18 (62) | |
| Viral load‡ | 0 (0–140) | 0 (0–60) | 0 (0–260) | 0 (0–0) | .047 |
| Less than 200 | 204 (77) | 70 (77) | 106 (73) | 28 (97) | .010 |
| 200–1,000 | 26 (10) | 5 (6) | 21 (15) | 0 | |
| 1,000 or greater | 35 (13) | 16 (18) | 18 (12) | 1 (4) | |
| Gestational age at delivery (wk) | 38.6 (37.3–39.3) | 38.6 (37.2–39.4) | 38.4 (37.4–39.3) | 39.0 (38.1–39.3) | .419 |
| Preterm delivery§ | 48 (18) | 18 (20) | 27 (19) | 3 (10) | .503 |
| Complications of pregnancy | |||||
| HDP | 44 (17) | 23 (25) | 14 (10) | 7 (24) | .004 |
| Gestational hypertension | 30 (13) | 15 (20) | 10 (8) | 5 (20) | .025 |
| Preeclampsia, eclampsia, HELLP | 15 (6) | 8 (9) | 5 (4) | 2 (7) | .214 |
| Timing of Initiation |
||||
|---|---|---|---|---|
| Characteristic | Before Pregnancy (n=117) | During Pregnancy (n=145) | P | |
| Age at delivery (y) | ||||
| 20 or younger | 6 (5) | 14 (10) | <.001 | |
| 20–35 | 76 (65) | 117 (81) | ||
| 35 or older | 35 (30) | 14 (10) | ||
| Race | ||||
| Non-Hispanic black | 92 (81) | 116 (87) | .269 | |
| White, Hispanic, other | 21 (19) | 18 (13) | ||
| Parity | ||||
| 0 | 26 (22) | 39 (27) | .612 | |
| 1 | 26 (22) | 37 (26) | ||
| 2 | 26 (22) | 27 (19) | ||
| 3 or more | 39 (33) | 41 (29) | ||
| History of HDP* | 3 (3) | 10 (7) | .108 | |
| History of gestational diabetes | 2 (2) | 2 (2) | 1.000 | |
| Chronic medical condition | ||||
| Hypertension | 18 (15) | 21 (15) | .838 | |
| Diabetes mellitus | 2 (2) | 2 (1) | 1.000 | |
| Obesity† | 43 (37) | 52 (37) | .998 | |
| Gestational age at entry | 13 (10–19) | 19 (13–27) | <.001 | |
| Substance use in pregnancy | ||||
| Tobacco | 22 (19) | 32 (22) | .516 | |
| Alcohol | 7 (6) | 9 (6) | .940 | |
| Illicit drugs | 10 (9) | 29 (20) | .010 | |
| No. of prenatal visits at GMH | 9 (7–11) | 8 (5–11) | .005 | |
| Initiated ART during pregnancy | 0 | 145 (100) | — | |
| CD4‡ | 473 (340–630) | 412 (263–607) | .120 | |
| Less than 200 | 11 (9) | 22 (15) | .191 | |
| 200–500 | 52 (44) | 69 (48) | ||
| 500 or greater | 54 (46) | 53 (37) | ||
| Viral load‡ | 0 (0–0) | 0 (0–400) | <.001 | |
| Less than 200 | 106 (91) | 97 (67) | <.001 | |
| 200–1,000 | 5 (4) | 20 (14) | ||
| 1,000 or greater | 6 (5) | 28 (19) | ||
| Gestational age at delivery (wk) | 38.6 (37.6–39.4) | 38.4 (37.3–39.3) | .723 | |
| Preterm delivery§ | 21 (18) | 26 (18) | .997 | |
| Complications of pregnancy | ||||
| HDP | 18 (15) | 26 (18) | .584 | |
| Gestational hypertension | 12 (12) | 18 (15) | .603 | |
| Preeclampsia, eclampsia, HELLP | 6 (5) | 9 (6) | .709 | |
ART, antiretroviral therapy; INSTI, integrase strand transfer inhibitor; PI, protease inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; HDP, hypertensive disorders of pregnancy; GMH, Grady Memorial Hospital; HELLP, hemolysis, elevated liver enzymes, and low platelets.
Data are n (%) or median (interquartile range) unless otherwise specified.
P-values comparing categorical variables were derived using the χ2 test or Fisher exact test; medians were compared using the Kruskal Wallis test across ART regimens and the Wilcoxon test across timing of ART initiation.
Data are missing for the following variables: race (n=15), parity (1), history of gestational diabetes (81), obesity (9), gestational age at entry (6), initiated ART during pregnancy (3), and CD4 (1).
Gestational hypertension, preeclampsia, eclampsia, or HELLP syndrome.
Body mass index (BMI) 30.0 or greater for the 95% with a prenatal BMI measurement (43% were measured in the first trimester, 40% in the second, and 17% in the third); for 8 (2%) of the pregnancies, charts were abstracted for history of obesity as part of a 2016–2018 abstraction effort; for the remaining 9 (3%), obesity data were missing.
CD4 and viral load values were the closest values before delivery.
Gestational age less than 37 weeks at delivery.
Women on integrase strand transfer inhibitor–containing regimens had higher risk for hypertensive disorders of pregnancy and gestational hypertension compared with women on protease inhibitor–containing regimens in both unadjusted and adjusted analysis (hypertensive disorders of pregnancy 25% vs 10%, adjusted RR 2.8, 95% CI 1.5–5.1; gestational hypertension 20% vs 8%, adjusted RR 2.8, 95% CI 1.3–5.9) (Table 4). Although the point estimates comparing integrase strand transfer inhibitor with protease inhibitor regimens for preeclampsia were similar to those for gestational hypertension, the differences were not statistically significant for preeclampsia (9% vs 4%, adjusted RR 2.6, 95% CI 0.9–7.3). Comparing non-nucleoside reverse transcriptase inhibitor–containing to protease inhibitor–containing regimens, there was a significant difference in the risk of hypertensive disorders of pregnancy and gestational hypertension in adjusted analysis but no significant difference from protease inhibitor–containing regimens in unadjusted or adjusted analysis for preeclampsia (Table 4). These primary associations did not change in sensitivity analyses for different definitions and analytic methods.
Table 4.
Cohort 2: Unadjusted and Adjusted Risk Ratios Comparing Antiretroviral Therapy Regimens for Hypertensive Disorders of Pregnancy Outcomes Among Pregnant Women Living With Human Immunodeficiency Virus Who Delivered at Grady Memorial Hospital From 2011–2018
| INSTI-Containing vs PI-Containing Regimens |
NNRTI-Containing vs PI-Containing Regimens |
ART Initiation in Pregnancy vs Before Pregnancy [Adjusted RR (95% CI)] | ||||
|---|---|---|---|---|---|---|
| Outcome | RR (95% CI) | Adjusted RR (95% CI) | RR (95% CI) | Adjusted RR (95% CI) | P-Interaction | |
| HDP* | 2.6 (1.4–4.8) | 2.8 (1.5–5.1) | 2.5 (1.1–5.7) | 2.9 (1.2–6.9) | 1.5 (0.8–2.7) | .839 |
| Gestational hypertension | 2.6 (1.2–5.4) | 2.7 (1.3–5.9) | 2.5 (1.0–6.8) | 3.2 (1.1–9.0) | 1.5 (0.7–3.3) | .993 |
| Preeclampsia | 2.6 (0.9–7.6) | 2.6 (0.9–7.3) | 2.0 (0.4–9.8) | 2.1 (0.4–10.6) | 1.6 (0.5–4.9) | † |
INSTI, integrase strand transfer inhibitor; PI, protease inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; ART, antiretroviral therapy; RR, risk ratio; HDP, hypertensive disorders of pregnancy.
All multivariate models adjusted for age, race, illicit drug use, and initiation of ART or new ART regimen during pregnancy.
Eighteen women are excluded from this analysis for missing race (n=15) or timing of ART initiation (n=3) data.
Gestational hypertension, preeclampsia, eclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome.
Model with interaction term failed.
To assess the effect of timing of ART initiation on the study outcomes, three women with an unknown date of ART onset were excluded. Antiretroviral therapy was initiated before pregnancy for 117 (45%) and during pregnancy for 145 (55%) women. Of women initiating ART before pregnancy, 29.1% were on integrase strand transfer inhibitor–containing, 49% on protease inhibitor–containing, and 22% on non-nucleoside reverse transcriptase inhibitor–containing regimens; for women initiating ART during pregnancy, 39% were on integrase strand transfer inhibitor–containing, 59% on protease inhibitor–containing, and 2% on non-nucleoside reverse transcriptase inhibitor–containing regimens. Those who started ART during pregnancy were significantly younger, used illicit drugs during pregnancy, presented later in pregnancy, and were less likely to be virologically suppressed (Table 3). Otherwise, women who started ART before and during pregnancy were similar (Table 3).
Timing of ART initiation was not significantly associated with any hypertensive disorder of pregnancy, gestational hypertension, or preeclampsia (Table 4). Furthermore, there was no statistical interaction between timing of initiation and ART regimen for hypertensive disorders of pregnancy or gestational hypertension.
DISCUSSION
Using data from a large, public hospital in the United States, we found women living with HIV using ART and women without HIV have similar risk for developing new-onset hypertensive disorders of pregnancy. However, our findings suggest that among pregnant women living with HIV, the use of integrase strand transfer inhibitor–containing regimens is increasing and may be associated with increased risk for hypertensive disorders of pregnancy, specifically gestational hypertension, compared with protease inhibitor–containing regimens. The timing of ART initiation was not associated with development of hypertensive disorders of pregnancy.
Evidence for an effect of ART on hypertensive disorders of pregnancy development is mixed. Several studies suggest women living with HIV taking ART may be at similar or increased risk of hypertensive disorders of pregnancy compared with women without HIV4,18,19 and at an increased risk compared with untreated women living with HIV.7,8 In agreement with previous studies, our results found similar risk for any hypertensive disorder of pregnancy among women living with HIV on ART compared with women without HIV, which is in contrast to other studies that found an increased risk. Inconsistencies in the literature between HIV status and hypertensive disorders of pregnancy risk may reflect clinical heterogeneity in the spectrum of hypertensive disorders of pregnancy and differences in the predominant classes of ART regimens across time and cohorts.
Among women living with HIV, our findings suggest that those on integrase strand transfer inhibitors have almost three times higher risk of hypertensive disorders of pregnancy than those on a protease inhibitor–containing regimen. Compared with other regimens, integrase strand transfer inhibitors lead to a more rapid viral decay12 and correspondingly increased CD4 counts and CD4 T-cell hyperactivation.13 Several studies implicate these immune-mediated mechanisms in development of hypertensive disorders of pregnancy.20,21 In contrast, protease inhibitors and non-nucleoside reverse transcriptase inhibitors may not fully restore the normal inflammatory state. Because we do not compare integrase strand transfer inhibitor use with use of no method, our findings of increased hypertensive disorders of pregnancy among integrase strand transfer inhibitors users compared with protease inhibitor users can either implicate elevated risk among integrase strand transfer inhibitor users or may alternatively suggest that protease inhibitors are associated with a low risk of hypertensive disorders of pregnancy compared with other regimens. Protease inhibitor regimens can also have more side effects, especially during pregnancy, resulting in less tolerability, lower adherence, and slower viral suppression.22 With so few women in our cohort on non-nucleoside reverse transcriptase inhibitor–containing regimens, we are hesitant to draw firm conclusions about the statistically significant increased risk for developing hypertensive disorders of pregnancy on this therapy.
In this study, timing of ART initiation was not significantly associated with hypertensive disorders of pregnancy outcomes, nor did it change the observed associations between ART classification and hypertensive disorders of pregnancy outcomes. Two other studies examined the effect of ART initiation in relation to pregnancy on preeclampsia risk. In a study of more than 1,500 pregnant women living with HIV in Latin American and Caribbean countries, there were twofold increased odds of preeclampsia in women taking ART at conception compared with those not taking ART at conception.7 Another study of 472 pregnant women living with HIV in Spain found preeclampsia to be associated with the use of ART before pregnancy compared with those not using ART before pregnancy or using ART suboptimally.8 These studies suggest that a mechanism for development of hypertensive disorders of pregnancy by ART may begin before conception; however, these studies did not directly compare initiation before with during pregnancy, nor did they include integrase strand transfer inhibitor–containing regimens. Although we did not find an interaction between timing of ART initiation and regimen, our analysis may be underpowered. Our cohort also showed many characteristic differences between those who initiated ART before pregnancy and those who initiated ART after, making meaningful comparison difficult. Larger studies are needed to further examine this factor.
Our study has some notable limitations. The absolute number of deliveries to women living with HIV in this report is relatively small and likely limits our power to detect important differences. Because women in our cohorts are able to support pregnancy, they may represent a healthier group of women living with HIV. However, this potentially healthier population of women living with HIV is nonetheless important, especially as contemporary ART regimens allow for the management of HIV as a chronic disease. Related, we do not have direct information on regimen compliance and instead used indirect measures of CD4 and viral load. Additionally, we attempted to account for the competing risk of those who delivered early without time to develop hypertensive disorders of pregnancy and those who delivered early for hypertensive disorders of pregnancy indication by limiting to term deliveries and found no change in outcome. However, this sensitivity analysis does not fully remove the influence of competing risk and is an important limitation in our cohort. A key covariate missing from our analysis is nadir CD4 count. People who start therapy when their CD4 count is high have generally less inflammation and immune activation than those who are severely immunocompromised at initiation. This is evident in lab testing and also in the rates of cardiovascular disease and various other inflammation-associated morbidities. It is possible that women enrolled in the earlier years of the cohort had lower nadir CD4 and were also more likely to be prescribed protease inhibitors, thus accounting for a difference in preeclampsia incidence.
Although chart abstraction was completed systematically, reliance on medical record documentation during patient care limits ability to examine all covariates of interest. The change in use of different ART regimen classes over the study period was consistent with shifts in treatment guidelines toward recommendation of integrase strand transfer inhibitor use in nonpregnant adults and in prenatal guidelines toward continuation of suppressive prepregnancy regimens. We also cannot exclude the possibility that, in conjunction with the steep increase in integrase strand transfer inhibitor use, recognition of hypertensive disorders of pregnancy may have changed over the 7 years of observation. These changes may influence the study findings; however, owing to the rare occurrence of study outcomes, we could not separate out effects of ART regimen and time.
In summary, among women living with HIV on modern ART regimens, we observed an increased risk of hypertensive disorders of pregnancy, specifically gestational hypertension, among those on integrase strand transfer inhibitor–containing regimens compared with protease inhibitor–containing regimens. Duplication of this finding in other cohorts would indicate a need for more careful screening for hypertensive disorders of pregnancy in women on integrase strand transfer inhibitors and may warrant implementation of risk-reducing strategies.
Acknowledgments
Dr. Haddad’s and Dr. Yee’s efforts are supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (LBH: NICHD-1K23HD078153; LMY: NICHD K12 HD050121-11). Dr. Sheth’s effort is supported by the National Institute of Allergy and Infectious Diseases (NIAID-1K23AI114407). This research was further supported by the 2018–2019 Marianne Ruby, MD Award from Emory University School of Medicine, Department of Gynecology and Obstetrics.
The authors thank Madeline Smith, MD, Tesia Kim, MD, Kamini Doraivelu, MPH, and Caitlin Szabo, MD, for their contributions to abstraction of medical records.
Financial Disclosure
Caroline C. King disclosed that Emory University School of Medicine provided her payment for contract services as epidemiologist performing statistical analysis on this paper. Anandi N. Sheth disclosed that their institution received a research grant to Emory University from Gilead Sciences, unrelated to the presented work. The other authors did not report any potential conflicts of interest.
Footnotes
Presented at the Annual Infectious Diseases Society for Obstetrics and Gynecology Meeting, August 8–10, 2019, Big Sky, Montana.
Each author has confirmed compliance with the journal’s requirements for authorship.
REFERENCES
- 1.Centers for Disease Control and Prevention. HIV among pregnant women, infants, and children. Available at: https://www.cdc.gov/hiv/group/gender/pregnantwomen/index.html. Updated March 21, 2018. Retrieved August 17, 2018.
- 2.Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res 2017;40:213–20. [DOI] [PubMed] [Google Scholar]
- 3.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014;2:e323–33. [DOI] [PubMed] [Google Scholar]
- 4.Wimalasundera RC, Larbalestier N, Smith JH, de Ruiter A, McG Thom SA, Hughes AD, et al. Pre-eclampsia, antiretroviral therapy, and immune reconstitution. Lancet 2002;360:1152–4. [DOI] [PubMed] [Google Scholar]
- 5.Stoner MC, Vwalika B, Smid MC, George S, Chi BH, Stringer EM, et al. A retrospective study of HIV, antiretroviral therapy, and pregnancy-associated hypertension among women in Lusaka, Zambia. Int J Gynaecol Obstet 2016;134:299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall D, Gebhardt S, Theron G, Grove D. Pre-eclampsia and gestational hypertension are less common in HIV infected women. Pregnancy Hypertens 2014;4:91–6. [DOI] [PubMed] [Google Scholar]
- 7.Machado ES, Krauss MR, Megazzini K, Coutinho CM, Kreitchmann R, Melo VH, et al. Hypertension, preeclampsia and eclampsia among HIV-infected pregnant women from Latin America and Caribbean countries. J Infect 2014;68: 572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suy A, Martínez E, Coll O, Lonca M, Palacio M, de Lazzari E, et al. Increased risk of pre-eclampsia and fetal death in HIV-infected pregnant women receiving highly active antiretroviral therapy. AIDS 2006;20:59–66. [DOI] [PubMed] [Google Scholar]
- 9.Sansone M, Sarno L, Saccone G, Berghella V, Maruotti GM, Migliucci A, et al. Risk of preeclampsia in human immunodeficiency virus-infected pregnant women. Obstet Gynecol 2016; 127:1027–32. [DOI] [PubMed] [Google Scholar]
- 10.Tooke LRL, Manila M, Harrison M. Antiretrovirals causing severe pre-eclampsia. Pregnancy Hypertens;6:266–8. [DOI] [PubMed] [Google Scholar]
- 11.Sebitloane HM, Moodley D. The impact of highly active antiretroviral therapy on obstetric conditions: a review. Eur J Obstet Gynecol Reprod Biol 2017;210:126–31. [DOI] [PubMed] [Google Scholar]
- 12.Kityo C, Szubert AJ, Siika A, Heyderman R, Bwakura-Dangarembizi M, Lugemwa A, et al. Raltegravir-intensified initial antiretroviral therapy in advanced HIV disease in Africa: a randomised controlled trial. PLoS Med 2018;15:e1002706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Negredo E, Massanella M, Puertas MC, Buzon MJ, Puig J, Perez-Alvarez N, et al. Early but limited effects of raltegravir intensification on CD4 T cell reconstitution in HIV-infected patients with an immunodiscordant response to antiretroviral therapy. J Antimicrob Chemother 2013;68:2358–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meade CM, Hussen SA, Momplaisir F, Badell M, Hackett S, Sheth AN. Long term engagement in HIV care among postpartum women with perinatal HIV infection in the United States. AIDS Care 2018;30:488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gestational hypertension and preeclampsia. ACOG Practice Bulletin No. 202. American College of Obstetricians and Gynecologists. Obstet Gynecol 2019;133:e1–25.30575675 [Google Scholar]
- 17.Zou G A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–6. [DOI] [PubMed] [Google Scholar]
- 18.Nourollahpour Shiadeh M, Riahi SM, Khani S, Alizadeh S, Hosseinzadeh R, Hasanpour AH, et al. Human immunodeficiency virus and risk of pre-eclampsia and eclampsia in pregnant women: a meta-analysis on cohort studies. Pregnancy Hypertens 2019;17:269–75. [DOI] [PubMed] [Google Scholar]
- 19.Premkumar ADA, Haddad LB, Yee LM. Combined antiretroviral therapy for HIV and the risk of hypertensive disorders of pregnancy: a systematic review. Pregnancy Hypertens 2019;17: 178–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomimatsu T, Mimura K, Endo M, Kumasawa K, Kimura T. Pathophysiology of preeclampsia: an angiogenic imbalance and long-lasting systemic vascular dysfunction. Hypertens Res 2017;40:305–10. [DOI] [PubMed] [Google Scholar]
- 21.Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 2012;125:911–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuk DM, Hughes CA, Foisy MM, Robinson JL, Singh AE, Houston S. Adverse effects of antiretrovirals in HIV-infected pregnant women. Ann Pharmacother 2009;43:1028–35. [DOI] [PubMed] [Google Scholar]


