Abstract
Survival of mesenchymal stem cells (MSCs) against oxidative stress and inflammation is vital for effective stem cell therapy. The reactive oxygen species (ROS) result in apoptosis and release of inflammatory mediators. Adipose-derived stem cells (ASCs) have shown promise for stem cell therapy owing to their anti-inflammatory and anti-oxidant activity. Previously, we showed the benefits of vitamin E against hydrogen peroxide (H2O2)-induced oxidative stress in rat bone marrow-derived MSCs. In this study, we aim to evaluate the effect of vitamin E treatment on porcine adipose-derived mesenchymal stem cells (pASCs) against H2O2-induced oxidative stress. The oxidative stress was induced by treating pASCs with 500 μM H2O2 with or without vitamin E. Viability of pASCs is enhanced after vitamin E treatment. In addition, reduced cellular toxicity, total NO level, PGE2 production and caspase-3 activity were observed after vitamin E treatment. Gene expression analysis of vitamin E-treated pASCs showed down-regulated expression for the genes associated with oxidative stress and apoptosis, viz., NOS2, Casp3, p53, BAX, MDM2, NF𝜅B, HIF1α and VEGF-A genes. On the other hand, expression of anti-apoptotic and survival genes was up-regulated, viz., BCL2, BCL2L1 and MCL1. Furthermore, phosphorylation of Akt was attenuated following vitamin E treatment. The findings of this study may help in developing effective stem cell therapy for the diseases characterized by the oxidative stress and inflammation.
Keywords: Mesenchymal stem cells, Alpha-Tocopherol, Hydrogen peroxide, Porcine, Oxidative stress
Introduction
Mesenchymal stem cells (MSCs) were discovered from the bone marrow stroma as multipotent adult stem cells having the capacity of self-renewal and multi-lineage differentiation (Friedenstein et al. 1968; Tsuji et al. 2014). Subsequently, MSCs were isolated from other tissues such as adipose tissue (Ullah et al. 2015). The advantage of using adipose as a source of MSCs is that up to 300-fold greater cell number can be obtained by using just 100 g of adipose tissue as compared with 100 ml of bone marrow (Oedayrajsingh-Varma et al. 2006; Pittenger et al. 1999). Adipose-derived mesenchymal stem cells (ASCs) possess the ability to differentiate into various mesodermal lineages (Minteer et al. 2013). Furthermore, ASCs have a high proliferative capacity, secrete various proteins and have immunomodulatory effects (Li et al. 2015b). Therefore, ASCs have gained considerable attention in the field of regenerative medicine to repair various damaged tissues (Chen et al. 2014, 2015; Pers and Jorgensen 2016; Safford and Rice 2005).
Reactive oxygen species (ROS) such as hydroxyl radical, superoxide anions, hydrogen peroxide and peroxide are produced at the basal level as a result of normal cellular metabolism (Li et al. 2015a). The ROS-reducing mechanisms protect cells against the cellular damage mediated by ROS. However, excessive production of ROS can cause cellular damage (Yagi et al. 2013). For instance, H2O2 at concentrations exceeding the normal physiological state (> 100 nm) is known to induce oxidative stress that causes damage to the biomolecules. The H2O2-induced oxidative stress can in turn lead to inflammation via NFκB pathway (Sies 2017). Therefore, the pathogenesis of various diseases is a hallmark of oxidative stress that is primarily caused by disturbed redox state brought about by ROS species (Atashi et al. 2015; Djordjevic et al. 2008). ROS can have a deleterious effect on implanted mesenchymal stem cells (MSCs) by causing damage to all biomolecules (Lu and Zhao 2012). The sustained proliferation, differentiation and survival of MSCs is only possible at low levels of ROS as high levels can lead to cellular senescence, cell death, mitochondrial dysfunction and tissue inflammation (Denu and Hematti 2016).
Vitamin E is referred to as a “chain-breaking anti-oxidant” due to its radical scavenging activity that results in stabilization of the cell membrane against ROS-induced damage (Niki and Traber 2012; Upadhyay and Misra 2009). Furthermore, vitamin E has been reported to protect oxidant damage in various cell types such as neurons, fibroblasts, osteoblasts, chondrocytes, skeletal muscle cells and dental pulp cells (Bhatti et al. 2013; Calabrese et al. 2001; Kaneai et al. 2012; Nizar et al. 2011; Nunes et al. 2005; Upadhyay and Misra 2009). In addition to this, vitamin E plays an important role as an anti-oxidant against various diseases (Rizvi et al. 2014). Vitamin E has also been reported for its anti-inflammatory effects in early stage of surgically induced OA in dogs (Rhouma et al. 2013). Recently, we showed that vitamin E attenuated the harmful effects of hydrogen peroxide (H2O2)-induced oxidative stress in rat bone marrow MSCs (Bhatti et al. 2016).
Here, we examine the role played by vitamin E in porcine ASCs (pASCs) against H2O2-induced oxidative stress in vitro. The pASCs were treated with H2O2 with or without vitamin E. We observed that vitamin E treatment enhanced the survival of cultured pASCs by causing a reduction in oxidative stress, apoptosis, and inflammation.
Materials and methods
Isolation and culture of pASCs
The subcutaneous adipose tissue was isolated from 3- to 4-month-old healthy pigs according to a previously described method with slight modifications (Chen et al. 2016b). All pig tissues were taken from healthy pigs that were freshly euthanized for other experiments according to the approved protocol and the ethical guidelines of the Institutional Animal Care and Use Committee (IACUC) at the University of Tennessee Health Science Center (UTHSC).
The adipose tissue was washed three to four times with 1× phosphate-buffered saline (PBS) (Gibco, Grand Island, NY), minced in small pieces and incubated in 200 μg/ml collagenase II (Sigma-Aldrich, St. Louis, MO) prepared in serum-free Dulbecco’s modified Eagle’s medium-high glucose (DMEM-HG) (Gibco, Grand Island, NY) for 60 min at 37 °C and 5% CO2 with gentle agitation at 250 rpm. Later, collagenase was inactivated with 10% FBS and digested solution was filtered through a 100 μm mesh cell strainer to remove debris. The digestion solution was centrifuged at 1000×g for 10 min. The pellet was treated with 160 mM NH4Cl (Sigma-Aldrich, St. Louis, MO) for 10 min to lyse red blood cells. The resulting suspension was washed twice with 1× PBS by centrifugation at 1000×g for 10 min. Finally, the pellet was re-suspended in DMEM-HG containing 10% FBS and 1% solution of penicillin-streptomycin. Cells were seeded in 100 mm petri plates until 80–90% confluence. Medium was changed every 2–3 days. All the experiments were performed for pASCs at the second passage (P2).
Characterization of pASCs
Flow cytometry was used to characterize the surface markers of pASCs (Chen et al. 2016b). Briefly, three groups of pASCs containing 1 × 105 cells/100 μl were obtained in 1× PBS after trypsinization and washing. Cells were incubated with CD29-Alexa Fluor® 647 (BD Biosciences, San Jose, CA), CD90-PE (BD Biosciences, San Jose, CA) and CD45-PerCP-Cy™ 5.5 (BD Biosciences, San Jose, CA) at recommended dilutions for 10 min at room temperature. Mouse IgG1-FITC (BD Biosciences, San Jose, CA) served as standard isotype control. Data were obtained using the LSR II Flow Cytometer (BD Biosciences, San Jose, CA) and analyzed by FlowJo software (Tree Star, Inc. Ashland, OR). Furthermore, pASCs were subjected to undergo adipogenesis, chondrogenesis and osteogenesis using the StemPro® Adipogenesis Differentiation Kit, StemPro® Chondrogenesis Differentiation Kit and StemPro® Osteogenesis Differentiation Kit (Gibco, Grand Island, NY), respectively, following the manufacturer’s instructions. Adipogenesis, chondrogenesis and osteogenesis were confirmed by Oil red O stain, alcian blue stain and alizarin red S stain, respectively.
Induction of oxidative stress and vitamin E treatment
The experiments were performed by dividing pASCs into three groups: (i) untreated pASCs (Control), (ii) H2O2-treated pASCs (H2O2), and (iii) H2O2 + vitamin E-treated pASCs (Vit.E + H2O2). The oxidative stress was induced with 500 μM H2O2 for 24 h in H2O2 and vitamin E-treated groups (Suppl. Fig. 1). In addition to H2O2, the vitamin E-treated group was supplemented with 500 μM α-tocopherol for 24 h (Sigma-Aldrich, St. Louis, MO) (Suppl. Fig. 2). All the experiments were done in triplicate and repeated thrice.
Viability of pASCs
The viability of pASCs observed by trypan blue exclusion assay (data not shown) was confirmed using Live and Dead Cell Assay Kit (Abcam, Cambridge, MA) according to the manufacturer’s instructions. Briefly, following treatment cells were washed twice with 1× PBS and labeled with the live/ dead stain at 5× concentration. At least 15 (n = 15) images per group were taken randomly using the Invitrogen™ EVOS™ FL Auto Imaging System (Invitrogen, Carlsbad, CA). Cells were counted manually at × 10 magnification.
Determination of cellular cytotoxicity
The viability of pASCs was measured by determining the lactate dehydrogenase (LDH) release in the media using the In Vitro Toxicology Assay Kit, Lactic Dehydrogenase based (Sigma-Aldrich, St. Louis, MO). Absorbance was read at 490 nm and background at 690 nm using SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale CA). The LDH release in each group was expressed as comparison with untreated pASCs lyzed with 1% Triton X-100 (Bio-Rad, Hercules, CA) for maximum LDH release.
Measurement of oxidative stress
The total NO assay in media was performed to determine the level of nitrates and nitrites in pASCs using the Nitrate/Nitrite Fluorometric Assay Kit according to the manufacturer’s instructions (Cayman Chemical Company, Ann Arbor, MI). Fluorescence was read at excitation and emission wavelengths of 375 and 417 nm, respectively, using SpectraMax M5 microplate reader. The total NO was determined by interpolation from the nitrate standard curve.
Prostaglandin E2 assay
The amount of prostaglandin E2 (PGE2) in media was examined using the Prostaglandin E2 Parameter Assay Kit (R&D Systems, Inc., Minneapolis, MN) according to the manufacturer’s instructions. Absorbance was read at 450 nm and wavelength correction at 690 nm using SpectraMax M5 microplate reader. The concentration of PGE2 was determined by interpolation from the PGE2 standard curve.
Detection of apoptosis
Apoptosis of pASCs was assessed using the EnzChek® Caspase-3 Assay Kit (Molecular Probes™, Eugene, OR) according to the manufacturer’s instructions. Fluorescence was read at excitation and emission wavelengths of 496 and 520 nm, respectively, using SpectraMax M5 microplate reader. The amount of fluorescence was used to determine the level of caspase-3 activity in pASCs.
Gene expression analysis of pASCs
Gene expression analysis was performed at the end of treatment for pASCs. The RNA was extracted using the GeneJET RNA Purification Kit (Thermo Fisher Scientific, Waltham, MA). The cDNA was prepared using 0.5 μg RNA by TaqMan® Reverse Transcription Reagents (Thermo Fisher Scientific, Waltham, MA). Semi-quantitative gene expression (qPCR) was done using TaqMan™ Gene Expression Assays (Thermo Fisher Scientific, Waltham, MA) for the following genes (symbol): nitric oxide synthase 2 (NOS2), tumor protein P53 (p53), BCL2-associated X protein (BAX), B cell leukemia/lymphoma 2 (BCL2), BCL2-like 1 (BCL2L1), myeloid cell leukemia 1 (MCL1), mouse double-minute 2 homolog (MDM2), nuclear factor kappa B (NFκB), hypoxia inducible factor 1 alpha subunit (HIF1α), caspase-3 (Casp3), vascular endothelial growth factor A (VEGF-A) and transforming growth factor beta (TGF-β). Beta-Actin (β-actin) served as an internal control. qPCR was performed on the LightCycler®480 (Roche, Basel, Switzerland). Data were analyzed using the LightCycler® 480 software (Roche, Basel, Switzerland). The gene expression value of control pASCs was arbitrarily set to 1.
Analysis of total Akt and phospho-Akt by enzyme-linked immunosorbent (ELISA) assay
The phosphorylation of Akt is a vital indicator of cell survival. The expression of total Akt and phospho-Akt in pASCs was determined using Akt (pS473) + total Akt enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s instructions (Abcam, Cambridge, MA). Absorbance was read at 450 nm and wavelength correction at 690 nm using SpectraMax M5 microplate reader.
Statistical analysis
Statistical analysis was performed using GraphPad Prism v.5.00 for Windows (GraphPad Software, USA, http://www.graphpad.com). Data were expressed as mean ± SD, where n = 5 for all the experiments. Normality was determined by the Shapiro–Wilk test. One-way ANOVA followed by Bonferroni’s multiple-comparison post-test was performed to compare group mean differences. Student’s t test was done for unpaired comparison. Statistical significance was considered at P ≤ 0.05.
Results
Characterization of pASCs
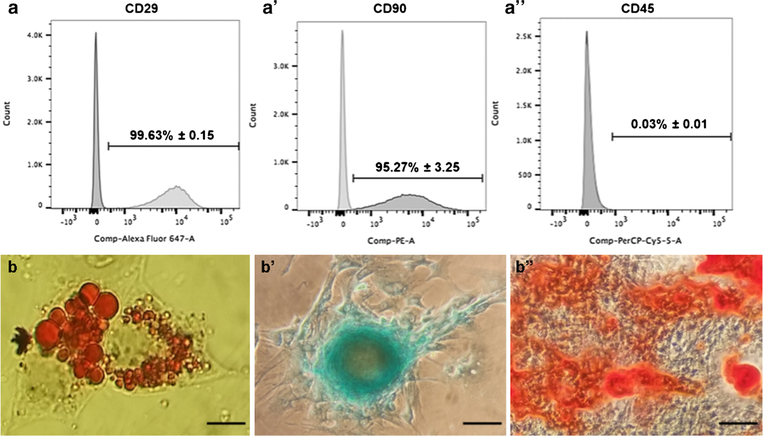
The phenotype of pASCs at P2 showed that the cultured cells have high expression of CD29 (99.63% ± 0.15) and CD90 (95.27% ± 3.25) compared with negligible expression of CD45 (0.03% ± 0.01) (Fig. 1a). Thus, there was high expression of positive mesenchymal stem cell surface makers and negative expression for the hematopoietic stem cell surface marker. Moreover, pASCs were able to undergo adipogenesis, chondrogenesis and osteogenesis that were examined by oil red, alcian blue and alizarin red S staining (Fig. 1b). These results confirmed the mesenchymal phenotype of pASCs that were used in subsequent experiments.
Fig. 1.
Characterization of pASCs. a Flow cytometry data for positive MSC surface markers (CD29 and CD90), and negative hematopoietic marker (CD45) is a negative marker for pASCs. Data presents percentage of cells positive for each marker. Data is represented as mean ± SD. b Left to right, adipogenesis (oil red O stain), chondrogenesis (alcian blue stain), and osteogenesis (alizarin red S stain). Magnification, × 80; scale bar = 10 μm
Determination of viability
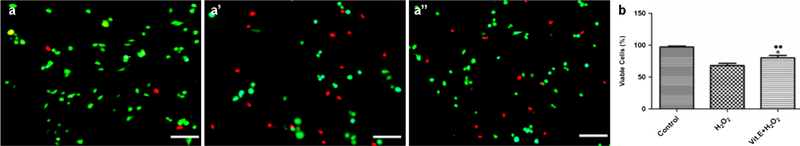
The live/dead cell assay showed that the viability (%) of untreated pASCs was significantly higher as compared with H2O2-induced pASCs (97.11% ± 1.64 vs. 67.99% ± 3.50; P = 0.0002) (Fig. 2). Vitamin E treatment of pASCs attenuated the effects of H2O2 by the increase in viability as compared with H2O2-induced pASCs (79.92% ± 3.92 vs. 67.99% ± 3.50; P = 0.0172). Furthermore, significant difference was found between untreated and vitamin E-treated pASCs (97.11% ± 1.64 vs. 79.92% ± 3.92; P = 0.0022). Nevertheless, viability of pASCs was enhanced after vitamin E treatment under H2O2-induced oxidative stress.
Fig. 2.
Live/dead assay for pASCs. a Representative images of different groups. Live cells are stained green, and dead cells are stained in red. Scale bar = 200 μm. b Graphical representation of live/dead cell assay. Data is represented as mean ± SD. ●●P <0.01 vs. Control, *P <0.05 vs. H2O2
Assessment of cytotoxicity
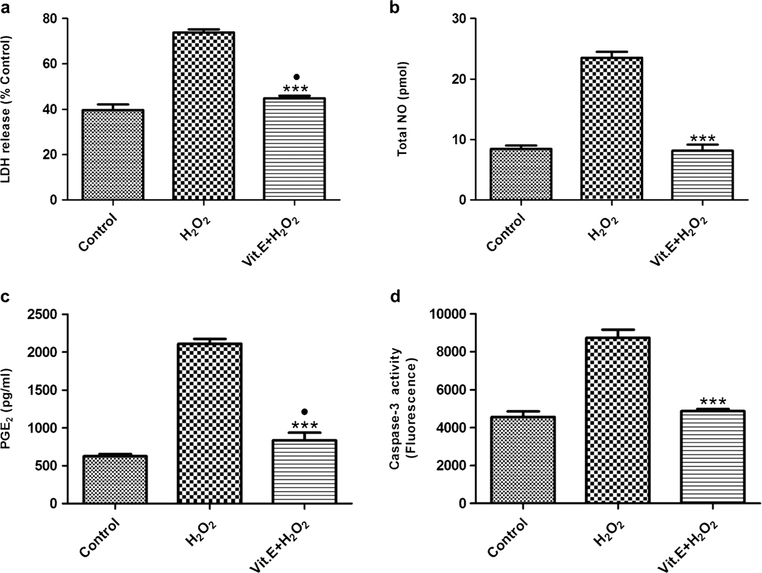
The treatment of pASCs with H2O2 increased the LDH release as compared with untreated pASCs (73.80 %Control ± 1.34 vs. 39.72 %Control ± 2.46; P < 0.0001) (Fig. 3a). Vitamin E treatment significantly reduced the LDH release as compared with H2O2-induced pASCs (73.80 %Control ± 1.34 vs. 44.89 %Control ± 1.04; P < 0.0001). In addition, a slight difference was found between control and vitamin E-treated pASCs (39.72 %Control ± 2.46 vs. 44.89 %Control ± 1.04; P = 0.0287). Thus, vitamin E treatment reduced cytotoxicity associated with H2O2-induced oxidative stress in pASCs.
Fig. 3.
Biochemical analysis of pASCs. a LDH release in media compared to %Control that represents untreated pASCs lyzed by 1% Triton-X 100. b Total NO in media concentration as determined by the nitrate standard curve. c PGE2 in media as determined by PGE2 standard curve. d Caspase-3 enzyme activity of pASCs determined after cell lysis. All biochemical assays were performed at the end of the treatment period. Data is represented as mean ± SD. ●P <0.05 vs. Control, ***P <0.001 vs. H2O2
Evaluation of oxidative stress due to total NO
There was a significant increase in the level of total NO after H2O2 induction as compared with an untreated control (23.52 pmol ± 0.95 vs. 8.43 pmol ± 0.57; P < 0.0001) (Fig. 3b). The anti-oxidant activity of vitamin E was evident in vitamin E-treated pASCs as compared with the H2O2-treated group (23.52 pmol ± 0.95 vs. 8.14 pmol ± 0.99; P < 0.0001). No significant difference was found between untreated and vitamin E-treated pASCs.
Assessment of inflammation via production of prostaglandin E2
The level of PGE2 was significantly higher in H2O2-treated group as compared with untreated pASCs (2109.16 pg/ml ± 64.97 vs. 624.58 pg/ml ± 27.88; P < 0.0001) (Fig. 3c). Vitamin E treatment significantly reduced the level of PGE2 as compared with the H2O2-treated group (833.50 pg/ml ± 102.43 vs. 2109.16 pg/ml ± 64.97; P < 0.0001). A very small significant difference was found between the control and vitamin E-treated pASCs (624.58 pg/ml ± 27.88 vs. 833.50 pg/ml ± 102.43; P = 0.0271). We assumed that H2O2 treatment resulted in a high level of PGE2 that in turn impeded proliferation of pASCs. On the other hand, vitamin E attenuated the effects of H2O2 induction.
Determination of apoptosis through caspase-3 enzyme activity
The caspase-3 activity almost increases 2-fold after H2O2 treatment as compared with the untreated pASCs (8725.62 fluorescence ± 439.72 vs. 4560.69 fluorescence ± 292.68; P = 0.0002) (Fig. 3d). However, vitamin E treatment resulted in the reduction of caspase-3 activity as compared with the H2O2-treated group (4867.29 fluorescence ± 117.78 vs. 8725.62 fluorescence ± 439.72; P = 0.0001). No significant difference was found between untreated and vitamin E-treated pASCs.
Gene expression analysis of pASCs
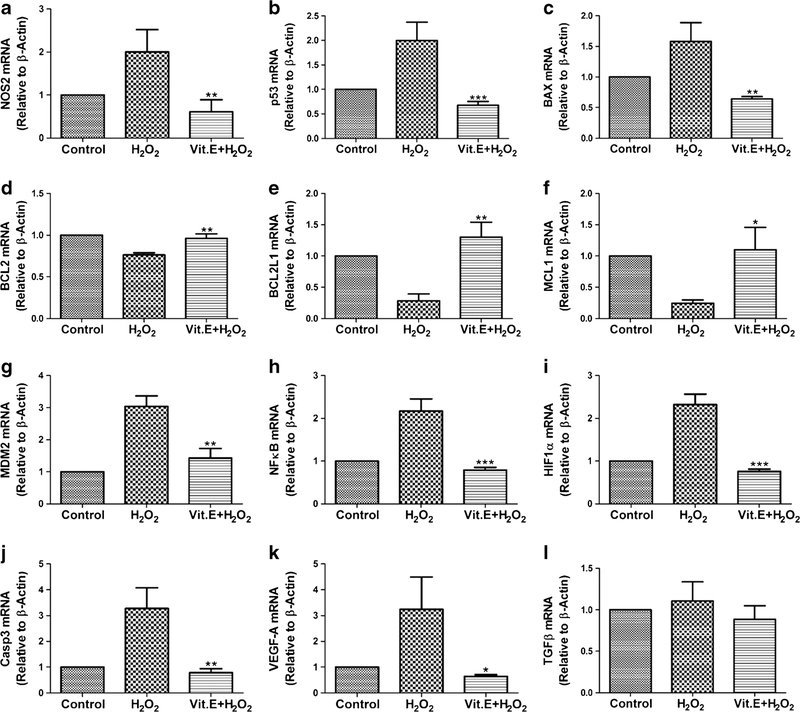
We studied gene expression levels of various genes (Fig. 4). The expression level of NOS2 gene, a marker of oxidative stress, was down-regulated following vitamin E treatment as compared with the H2O2-induced pASCs (0.60 ± 0.28-fold vs. 2.00 ± 0.51-fold; P = 0.0144). Similarly, the expression level of all the apoptotic genes decreased after vitamin E treatment as compared with the pASCs treated with H2O2 alone: Casp3 (0.78 ± 0.15-fold vs. 3.27 ± 0.80-fold; P = 0.0063), p53 (0.67 ± 0.07-fold vs. 1.99 ± 0.37-fold; P = 0.0039) and BAX (0.64 ± 0.03-fold vs. 1.58 ± 0.30-fold; P = 0.0062). Concomitantly, the expression level of anti-apoptotic genes was up-regulated after vitamin E treatment as compared with the H2O2-induced pASCs: BCL2 (0.96 ± 0.05-fold vs. 0.76 ± 0.02-fold; P = 0.0041), BCL2L1 (1.30 ± 0.23-fold vs. 0.28 ± 0.11-fold; P = 0.0024) and MCL1 (1.09 ± 0.35-fold vs. 0.24 ± 0.05-fold; P = 0.0148). We also studied the expression of MDM2 gene that degrades the p53 protein. We found that the expression level of MDM2 gene was up-regulated in H2O2-induced pASCs as compared with vitamin E-pretreated pASCs (3.03 ± 0.32-fold vs. 1.42 ± 0.29-fold; P = 0.0032). Further, no change was observed in the expression level of MDM2 gene between untreated and vitamin E-treated pASCs.
Fig. 4.
Gene expression analysis of pASCs. a NOS2 (oxidative stress marker), b p53 (apoptotic marker), c BAX (apoptotic marker), d BCL2 (anti-apoptotic marker), e BCL2L1 (antiapoptotic marker), f MCL1 (anti-apoptotic marker), g MDM2 (Surival marker), h NFκB (apoptosis regulator), i HIF1α (oxidative stress marker), j Casp3 (apoptotic marker), k VEGF-A (angiogenic marker), and 1 TGFβ (Differentitaion marker for chondrogenesis). Data is represented as mean ± SD. The gene expression of control was arbitrarily set to 1. **P <0.01 and ***P <0.001 vs. H2O2
Next, we studied the expression level of NFκB, which is a transcription factor sensitive to oxidative stress and is an upstream protein complex controlling transcription of genes involved in inflammation. The expression level of NFκB was down-regulated by vitamin E treatment as compared with H2O2-induced pASCs (0.78 ± 0.06-fold vs. 2.17 ± 0.28-fold; P = 0.0011).
Although known to act under hypoxia, HIF1α can cause apoptosis under oxidative stress. We found that vitamin E treatment down-regulated the expression level of HIF1α gene as compared with the H2O2-induced pASCs (0.75 ± 0.05-fold vs. 2.32 ± 0.24-fold; P = 0.0004). This may suggest that vitamin E attenuated the apoptosis of pASCs mediated by HIF1α. One of the target genes of HIF1α is VEGF-A, which is also a marker of inflammation. We found that the expression level of VEGF-A gene was regulated by the expression of HIF1α gene. Similar to the results observed for HIF1α, it was found that vitamin E treatment down-regulated the expression level of VEGF-A gene as compared with the H2O2-induced pASCs (0.64 ± 0.06-fold vs. 3.23 ± 1.25-fold; P = 0.0233).
The expression of TGF-β gene is well known for its role in the chondrogenesis of stem cells (Yu et al. 2012). However, no significant difference was observed in the expression of the TGF-β gene either after H2O2 treatment with or without vitamin E treatment as compared to untreated pASCs.
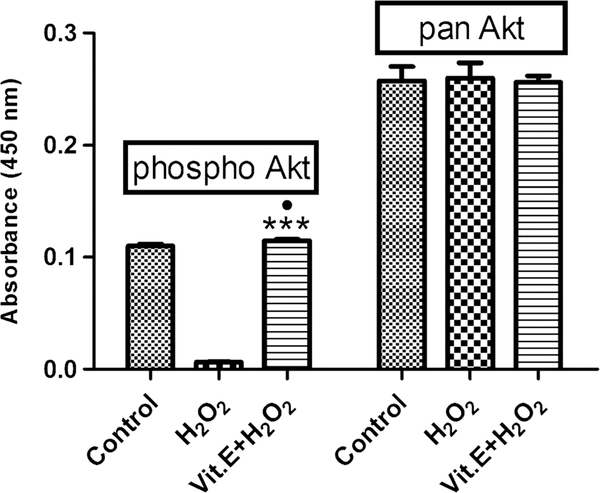
Activation of Akt by vitamin E
We observed significant reduction in the expression of phosphor-Akt after H2O2 treatment as compared with the untreated pASCs (0.0063 absorbance ± 0.0004 vs. 0.1101 absorbance ± 0.00169; P < 0.0001) (Fig. 5). Vitamin E treatment resulted in the up-regulated expression of phospho-Akt as compared with the H2O2-treated group (0.0063 absorbance ± 0.0004 vs. 0.1148 absorbance ± 0.00127; P < 0.0001). No significant difference was found between untreated and vitamin E-treated pASCs.
Fig. 5.
Akt ELISA of pASCs showing expression of phospho-Akt and total Akt. Significant difference can be observed for phospho-Akt after pASCs were induced with H2O2 with or without Vitamin E. The amount of total Akt under all conditions remains the same. Data is represented as mean ± SD. ●P <0.05 vs. Control, ***P <0.001 vs. H2O2
Discussion
Oxidative stress is a hall mark of various diseases and it can impede the progress of stem cell therapy by causing death of implanted cells (Wei et al. 2010; Ye et al. 2015). The key players involved are ROS generated at both physiological and pathological conditions (Simon et al. 2000). ASCs have been reported to better withstand ROS damage (Park et al. 2011). Nonetheless, the oxidative stress that the implanted cells have to resist is far more than a normal physiological state. The second challenge to the implanted cells is inflammation (Sokolove and Lepus 2013). ROS play a significant role in the progression of inflammatory diseases (Mittal et al. 2014). For instance, production of ROS during osteoarthritis (OA) trigger release of inflammatory mediators that in turn cause degradation of cartilage (Goldring and Otero 2011; Henrotin et al. 2003). Therefore, research has focused on using compounds that could target ROS as well as inflammatory mediators present in the osteoarthritic joint (Xue et al. 2017). A similar strategy may prove beneficial in the application of stem cell therapy against oxidative stress and inflammation in diseased tissues. Here, we selected vitamin E as it is known for its anti-oxidant and anti-inflammatory activity (Rhouma et al. 2013). Previously, we showed that vitamin E treatment of rat bone marrow-derived MSCs make them resistant against oxidative stress (Bhatti et al. 2017). In this study, we aimed to evaluate the findings of a previous study of ASCs due to their benefits over MSCs isolated from other sources. Moreover, the effect of vitamin E treatment is measured in terms of oxidative stress as well as inflammation. Briefly, we confirmed the hypothesis that treatment of pASCs with vitamin E may confer resistance against oxidative stress and inflammation.
Apoptosis of ASCs have been reported on exposure to H2O2 (Cremers et al. 2014). The previous study reported that the toxic effects of H2O2 were observed at concentrations higher than 250 μM (Cremers et al. 2014). However, in our study, the toxic effects of H2O2 were observed at 500 μM (Suppl. Fig. 1). This difference may have arisen due to the difference in animal and tissue source of pASCs. Also, the effect of vitamin E was observed at a concentration of 500 μM. This dose is five times higher than that was used for rat MSCs in our previous study (Bhatti et al. 2016). The dose of vitamin E was optimized after testing different ranges of doses (50 μM to 2 mM) against H2O2-induced oxidative stress (Suppl. Fig. 2). Interestingly, at doses above 500 μM, an increase in cellular toxicity was observed by LDH assay.
LDH release increases during cell death due to compromised cell membrane (Chan et al. 2013). The cellular cytotoxicity revealed by the LDH release in our study was comparable with another study where bone marrow-derived MSCs were exposed to H2O2 at a concentration of 600 μM (Chen et al. 2016a). We assume that the reduction of LDH release after vitamin E treatment is associated with the reduction of oxidative stress as was observed by the decrease in the total NO level and expression level of NOS2 gene. A previous study also reported an increase in the NO production after the rat mesenteric venules were perfused with H2O2 (Zhou et al. 2013).
It has been reported previously that PGE2 decreased the proliferation of human tendon stem cells at a high concentration (> 1 ng/ml) while the proliferation increased at a concentration < 1 ng/ml (Zhang and Wang 2014). We observed > 1 ng/ml PGE2 level during H2O2-induced oxidative stress in pASCs. Vitamin E attenuated these effects by decreasing the level to PGE2 to < 1 ng/ml. These results seem to contradict the studies that report the significance of PGE2 at a concentration > 1 ng/ml for the survival, homing and immunosuppressive properties of various types of stem cells (Dhingra et al. 2013; Hoggatt et al. 2009; Yanez et al. 2010). However, at present, no such study has been conducted to examine the beneficial role of PGE2 during H2O2-induced oxidative stress. We suggest that the fate of cells may be determined by a high or low level of PGE2 production. Still, future work is required to confirm these assumptions. In addition to this, PGE2 is known to increase during inflammation (Ricciotti and FitzGerald 2011). The decrease in PGE2 production after vitamin E treatment suggests the anti-inflammatory role of vitamin E in H2O2-stimulated pASCs.
The activity of caspase-3 increased under H2O2-induced oxidative stress and results in apoptosis (Feng et al. 2007; Wang et al. 2013). It has been proposed that H2O2 may result in death of MSCs by a mitochondrial pathway that activates caspase-3 (Wei et al. 2010). We observed that vitamin E reduced the apoptosis of pASCs by affecting a cascade of genes involved in apoptosis of pASCs. Apoptosis under hypoxic conditions can be regulated by HIF1α through p53 and BAX (Piret et al. 2002). Interestingly, we observed that induction of pASCs with H2O2 increased the expression level of HIF1α, p53, BAX, as well as VEGF-A, which is a downstream target gene of HIF1α. Vitamin E caused down-regulation of all these genes and hence prevented H2O2-induced apoptosis of pASCs.
It is known that the oxidative stress induced by H2O2 results in the activation of NFκB that lies at the forefront of the inflammatory pathway in a plethora of diseases (Bowie and O’Neill 2000; Russo et al. 2015). Our results also showed a down-regulated expression of NF𝜅B gene after vitamin E treatment. Hence, vitamin E may be used to inhibit inflammation stimulated by the oxidative stress conditions.
The activation of MDM2 inactivates p53 and hence inhibits p53-dependent apoptosis (Gottlieb et al. 2002). Interestingly, the expression of MDM2 gene was up-regulated after H2O2 treatment while no difference was observed following vitamin E treatment as compared with untreated pASCs. We suggest that the expression of MDM2 was increased after stimulation with H2O2 as an intrinsic defense mechanism. Moreover, the expression of MDM2 is maintained by the levels of p53 through an autoregulatory feedback loop (Gottlieb et al. 2002). Since vitamin E down-regulated expression of p53 gene, we assume that its mechanism of action is independent of MDM2-mediated p53 down-regulation. However, further study is needed to elucidate this hypothesis.
TGF-β governs the chondrogenesis of stem cells (Yu et al. 2012). We suggest that pASCs maintain the basal level of TGF-β both under normal and oxidative conditions. No change in the expression of TGF-β gene in the presence of vitamin E may indicate that vitamin E does not interfere with the expression of TGF-β gene.
Akt mediates survival of cells under endoplasmic reticulum and oxidative stresses (Price et al. 2010; Wajid et al. 2013). We observed that vitamin E significantly enhanced the expression of phospho-Akt that may have resulted in enhanced survival of pASCs. However, the increased Akt activity did not show any effect on the expression level of MDM2 as has been suggested previously (Gottlieb et al. 2002). Therefore, we suggest that the increase in the activity of Akt after vitamin E treatment decreased the apoptosis of pASCs without the involvement of MDM2-p53 interaction.
Conclusions
Herein, we demonstrated that vitamin E protects pASCs against H2O2-induced oxidative stress in vitro. Vitamin E exerted its effects by enhancing the survival and viability of pASCs while reducing the factors that can cause inflammation and apoptosis of pASCs. Future in vitro studies on vitamin E-treated pASCs exploring the pathways mediated by Akt and NFκB may further help in understanding the mechanism of vitamin E action during H2O2-induced oxidative stress conditions. Furthermore, implantation of vitamin E-treated pASCs in an animal model of a disease characterized by oxidative stress and inflammation will be beneficial. The results of this investigation are promising to develop the stem cell implantation studies utilizing MSCs for various diseases involving oxidative stress and inflammation.
Supplementary Material
Acknowledgments
Funding sources: This work was supported by grants from the Arthritis Foundation (Discovery award, H.C) and Oxnard Medical Research Foundation (H.C). K.A.H was supported by grants from the Department of Veterans Affairs (VA Merit Review Award) and (Research Career Scientist Award). A.K.Y. was supported by grants from NIH/NIAMS (AR064723).
Footnotes
Conflicts of interest The authors declare that they have no conflict of interest.
Compliance with ethical standards
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00441-018-2857-3) contains supplementary material, which is available to authorized users.
References
- Atashi F, Modarressi A, Pepper MS (2015) The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem Cells Dev 24:1150–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti FU, Mehmood A, Latief N, Zahra S, Cho H, Khan SN, Riazuddin S (2016) Vitamin E protects rat mesenchymal stem cells against hydrogen peroxide-induced oxidative stress in vitro and improves their therapeutic potential in surgically-induced rat model of osteoarthritis. Osteoarthr Cartil [DOI] [PubMed] [Google Scholar]
- Bhatti FU, Mehmood A, Latief N, Zahra S, Cho H, Khan SN, Riazuddin S (2017) Vitamin E protects rat mesenchymal stem cells against hydrogen peroxide-induced oxidative stress in vitro and improves their therapeutic potential in surgically-induced rat model of osteoarthritis. Osteoarthr Cartil 25:321–331 [DOI] [PubMed] [Google Scholar]
- Bhatti FU, Mehmood A, Wajid N, Rauf M, Khan SN, Riazuddin S (2013) Vitamin E protects chondrocytes against hydrogen peroxide-induced oxidative stress in vitro. Inflamm Res 62:781–789 [DOI] [PubMed] [Google Scholar]
- Bowie A, O’Neill LA (2000) Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol 59:13–23 [DOI] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Catalano C, Bates TE, Geraci D, Pennisi G, Giuffrida Stella AM (2001) Regulation of heat shock protein synthesis in human skin fibroblasts in response to oxidative stress: role of vitamin E. Int J Tissue React 23:127–135 [PubMed] [Google Scholar]
- Chan FK, Moriwaki K, De Rosa MJ (2013) Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol Biol 979: 65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Jin Y, Shi X, Qiu Y, Zhang Y, Cheng M, Wang X, Chen C, Wu Y, Jiang F, Li L, Zhou H, Fu Q, Liu X (2015) Adipose-derived stem cell-based treatment for acute liver failure. Stem Cell Res Ther 6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Qin F, Ge M, Shu Q, Xu J (2014) Application of adipose-derived stem cells in heart disease. J Cardiovasc Transl Res 7:651–663 [DOI] [PubMed] [Google Scholar]
- Chen M, Hou Y, Lin D (2016a) Polydatin protects bone marrow stem cells against oxidative injury: involvement of Nrf 2/ARE pathways. Stem Cells Int 2016:9394150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Liu HY, Chang YT, Cheng YH, Mersmann HJ, Kuo WH, Ding ST (2016b) Isolation and differentiation of adipose-derived stem cells from porcine subcutaneous adipose tissues J Vis Exp e53886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers NA, Lundvig DM, van Dalen SC, Schelbergen RF, van Lent PL, Szarek WA, Regan RF, Carels CE, Wagener FA (2014) Curcumin-induced heme oxygenase-1 expression prevents H2O2-induced cell death in wild type and heme oxygenase-2 knockout adipose-derived mesenchymal stem cells. Int J Mol Sci 15:17974–17999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denu RA, Hematti P (2016) Effects of oxidative stress on mesenchymal stem cell biology. Oxidative Med Cell Longev 2016:2989076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra S, Li P, Huang XP, Guo J, Wu J, Mihic A, Li SH, Zang WF, Shen D, Weisel RD, Singal PK, Li RK (2013) Preserving prostaglandin E2 level prevents rejection of implanted allogeneic mesenchymal stem cells and restores postinfarction ventricular function. Circulation 128:S69–S78 [DOI] [PubMed] [Google Scholar]
- Djordjevic VB, Zvezdanovic L, Cosic V (2008) Oxidative stress in human diseases. Srp Arh Celok Lek 136(Suppl 2):158–165 [DOI] [PubMed] [Google Scholar]
- Feng J, Yang S, Xu L, Tian H, Sun L, Tang X (2007) Role of caspase-3 inhibitor in induced anoikis of mesenchymal stem cells in vitro. J Huazhong Univ Sci Technolog Med Sci 27:183–185 [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP (1968) Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 6:230–247 [PubMed] [Google Scholar]
- Goldring MB, Otero M (2011) Inflammation in osteoarthritis. Curr Opin Rheumatol 23:471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb TM, Leal JF, Seger R, Taya Y, Oren M (2002) Cross-talk between Akt, p53 and MDM2: possible implications for the regulation of apoptosis. Oncogene 21:1299–1303 [DOI] [PubMed] [Google Scholar]
- Henrotin YE, Bruckner P, Pujol JP (2003) The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthr Cartil 11:747–755 [DOI] [PubMed] [Google Scholar]
- Hoggatt J, Singh P, Sampath J, Pelus LM (2009) Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood 113:5444–5455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneai N, Arai M, Takatsu H, Fukui K, Urano S (2012) Vitamin E inhibits oxidative stress-induced denaturation of nerve terminal proteins involved in neurotransmission. J Alzheimers Dis 28:183–189 [DOI] [PubMed] [Google Scholar]
- Li CJ, Sun LY, Pang CY (2015a) Synergistic protection of N-acetylcysteine and ascorbic acid 2-phosphate on human mesenchymal stem cells against mitoptosis, necroptosis and apoptosis. Sci Rep 5:9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Wu XY, Tong JB, Yang XX, Zhao JL, Zheng QF, Zhao GB, Ma ZJ (2015b) Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther 6:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu WY, Zhao MF (2012) Effect of oxidative stress on bone marrow mesenchymal stem cells. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 34:90–94 [PubMed] [Google Scholar]
- Minteer D, Marra KG, Rubin JP (2013) Adipose-derived mesenchymal stem cells: biology and potential applications. Adv Biochem Eng Biotechnol 129:59–71 [DOI] [PubMed] [Google Scholar]
- Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB (2014) Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20:1126–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki E, Traber MG (2012) A history of vitamin E. Ann Nutr Metab 61: 207–212 [DOI] [PubMed] [Google Scholar]
- Nizar AM, Nazrun AS, Norazlina M, Norliza M, Ima Nirwana S (2011) Low dose of tocotrienols protects osteoblasts against oxidative stress. Clin Ter 162:533–538 [PubMed] [Google Scholar]
- Nunes VA, Gozzo AJ, Cruz-Silva I, Juliano MA, Viel TA, Godinho RO, Meirelles FV, Sampaio MU, Sampaio CA, Araujo MS (2005) Vitamin E prevents cell death induced by mild oxidative stress in chicken skeletal muscle cells. Comp Biochem Physiol C Toxicol Pharmacol 141:225–240 [DOI] [PubMed] [Google Scholar]
- Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, Helder MN, Klein-Nulend J, Schouten TE, Ritt MJ, van Milligen FJ (2006) Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy 8:166–177 [DOI] [PubMed] [Google Scholar]
- Park SG, Kim JH, Xia Y, Sung JH (2011) Generation of reactive oxygen species in adipose-derived stem cells: friend or foe? Expert Opin Ther Targets 15:1297–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pers YM, Jorgensen C (2016) Adipose derived stem cells for regenerative therapy in osteoarticular diseases. Horm Mol Biol Clin Investig [DOI] [PubMed] [Google Scholar]
- Piret JP, Mottet D, Raes M, Michiels C (2002) Is HIF-1alpha a pro- or an anti-apoptotic protein? Biochem Pharmacol 64:889–892 [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 [DOI] [PubMed] [Google Scholar]
- Price J, Zaidi AK, Bohensky J, Srinivas V, Shapiro IM, Ali H (2010) Akt-1 mediates survival of chondrocytes from endoplasmic reticulum-induced stress. J Cell Physiol 222:502–508 [DOI] [PubMed] [Google Scholar]
- Rhouma M, de Oliveira El Warrak A, Troncy E, Beaudry F, Chorfi Y (2013) Anti-inflammatory response of dietary vitamin E and its effects on pain and joint structures during early stages of surgically induced osteoarthritis in dogs. Can J Vet Res 77:191–198 [PMC free article] [PubMed] [Google Scholar]
- Ricciotti E, FitzGerald GA (2011) Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31:986–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi S, Raza ST, Ahmed F, Ahmad A, Abbas S, Mahdi F (2014) The role of vitamin e in human health and some diseases. Sultan Qaboos Univ Med J 14:e157–e165 [PMC free article] [PubMed] [Google Scholar]
- Russo MA, Sansone L, Carnevale I, Limana F, Runci A, Polletta L, Perrone GA, De Santis E, Tafani M (2015) One special question to start with: can HIF/NFkB be a target in inflammation? Endocr Metab Immune Disord Drug Targets 15:171–185 [DOI] [PubMed] [Google Scholar]
- Safford KM, Rice HE (2005) Stem cell therapy for neurologic disorders: therapeutic potential of adipose-derived stem cells. Curr Drug Targets 6:57–62 [DOI] [PubMed] [Google Scholar]
- Sies H (2017) Hydrogen peroxide as a central redox signalling molecule in physiological oxidative stress: oxidative eustress. Redox Biol 11: 613–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon HU, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5:415–418 [DOI] [PubMed] [Google Scholar]
- Sokolove J, Lepus CM (2013) Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis 5:77–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji W, Rubin JP, Marra KG (2014) Adipose-derived stem cells: implications in tissue regeneration. World J Stem Cells 6:312–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah I, Subbarao RB, Rho GJ (2015) Human mesenchymal stem cells - current trends and future prospective. Biosci Rep 35: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J, Misra K (2009) Towards the interaction mechanism of to-copherols and tocotrienols (vitamin E) with selected metabolizing enzymes. Bioinformation 3:326–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajid N, Mehmood A, Bhatti FU, Khan SN, Riazuddin S (2013) Lovastatin protects chondrocytes derived from Wharton’s jelly of human cord against hydrogen-peroxide-induced in vitro injury. Cell Tissue Res 351:433–443 [DOI] [PubMed] [Google Scholar]
- Wang FW, Wang Z, Zhang YM, Du ZX, Zhang XL, Liu Q, Guo YJ, Li XG, Hao AJ (2013) Protective effect of melatonin on bone marrow mesenchymal stem cells against hydrogen peroxide-induced apoptosis in vitro. J Cell Biochem 114:2346–2355 [DOI] [PubMed] [Google Scholar]
- Wei H, Li Z, Hu S, Chen X, Cong X (2010) Apoptosis of mesenchymal stem cells induced by hydrogen peroxide concerns both endoplasmic reticulum stress and mitochondrial death pathway through regulation of caspases, p38 and JNK. J Cell Biochem 111:967–978 [DOI] [PubMed] [Google Scholar]
- Xue EX, Lin JP, Zhang Y, Sheng SR, Liu HX, Zhou YL, Xu H (2017) Pterostilbene inhibits inflammation and ROS production in chondrocytes by activating Nrf2 pathway. Oncotarget [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H, Tan J, Tuan RS (2013) Polyphenols suppress hydrogen peroxide-induced oxidative stress in human bone-marrow derived mesenchymal stem cells. J Cell Biochem 114:1163–1173 [DOI] [PubMed] [Google Scholar]
- Yanez R, Oviedo A, Aldea M, Bueren JA, Lamana ML (2010) Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Exp Cell Res 316:3109–3123 [DOI] [PubMed] [Google Scholar]
- Ye ZW, Zhang J, Townsend DM, Tew KD (2015) Oxidative stress, redox regulation and diseases of cellular differentiation. Biochim Biophys Acta 1850:1607–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D-A, Han J, Kim B-S (2012) Stimulation of Chondrogenic differentiation of Mesenchymal stem cells. Int J Stem Cells 5:16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang JH (2014) Prostaglandin E2 (PGE2) exerts biphasic effects on human tendon stem cells. PLoS One 9:e87706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Yuan D, Wang M, He P (2013) H2O2-induced endothelial NO production contributes to vascular cell apoptosis and increased permeability in rat venules. Am J Physiol Heart Circ Physiol 304:H82–H93 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.