Abstract
Purpose:
Design of intravaginal rings (IVRs) for delivery of antiretrovirals is often guided by in vitro release under sink conditions, based on the assumption that in vivo release will follow a similar release profile.
Methods:
We conducted a dose-ranging study in the female reproductive tract of pigtail macaques using matrix IVRs containing IQP-0528, a poorly soluble but highly potent antiretroviral drug with an IC90 of 146 ng/mL. These IVRs consisted of drug-loaded segments, 15.6% IQP-0528 in Tecoflex 85A, comprising either all, half, or a quarter of the entire ring.
Results:
In vitro release under sink conditions demonstrates loading-proportional release, with a cumulative 30-day release of 48.5 ± 2.2 mg for our 100% loaded ring, 24.8 ± .36 mg from our 50% loaded ring, and 13.99 ± 1.58 mg from our 25% loaded ring. In vivo, while drug concentration in vaginal fluid is well in excess of IQP-0528’s EC90, we find no statistical difference between the different ring loadings in either swab drug levels or drug released from our rings.
Conclusions:
We show that in vitro release may not accurately reflect in vivo release, particularly for poorly soluble drugs. All tested loadings of our IVRs are capable of delivering IQP-0528 well in excess of the IC90.
Keywords: IQP-0528, intravaginal rings, macaque, pharmacokinetics
INTRODUCTION
Intravaginal rings delivering antiretrovirals for pre-exposure prophylaxis remain a potent tool in the fight against HIV transmission, as demonstrated by two recent dapivirine intravaginal ring (IVR) Phase III clinical trials (Aspire and The Ring Study), which demonstrated a 56% reduction in risk among women over the age of 21 years and a 61% reduction in risk among women aged 25 years and older[1]. The design of intravaginal rings often requires extensive trial and error, guided by measuring the in vitro release kinetics [2–4]. Yet a tenuous and unknown connection can exist between in vitro and in vivo release rates. In this paper, we demonstrate that in vitro release rates from intravaginal rings under sink conditions may bear no resemblance to in vivo release, particularly for poorly soluble drugs. We have also presented a new design for a vaginal topical dose-ranging pharmacokinetic study of a series of matrix intravaginal rings containing highly potent anti-retroviral, IQP-0528. Rather than varying the drug loading within the entire ring, we have used drug-loaded segments of varying length, thus removing the potential for variability due to differences in concentration gradient between rings of different loading during release.
Like dapivirine, IQP-0528 (1-(3-Cyclopropyl)methyl-6-(3,5-dimethylbenzoyl)-5-isopropyl-2,4(1H,3H)-pyrimidinedione) is a highly potent nonnucleoside reverse transcriptase inhibitor (NNRTI) (Figure 1). Another similarity is that both IQP-0528 and dapivirine can move freely in and out of cells without being trapped in non-target cells, unlike tenofovir (TFV)[5]. Unlike dapivirine, IQP-0528 has a dual mechanism of action, and can prevent HIV-1 entry (EC50 of 12 nM) as well as reverse transcription[6]. A vaginal IQP-0528 IVR could be more effective at stopping HIV-1 without combining two drugs, such as was done in MTN-013 with dapivirine and the entry inhibitor maraviroc[7]. IQP-0528 uniquely demonstrates nanomolar activity against both HIV-1 and HIV-2, with an HIV-1 EC50 of 0.2 nM[8, 9] and a HIV-2 EC50 of 100 nM[6]. While IQP-0528 is considered generally stable, it has poor aqueous solubility, with a calculated LogP of 4.1[10].
Figure 1.
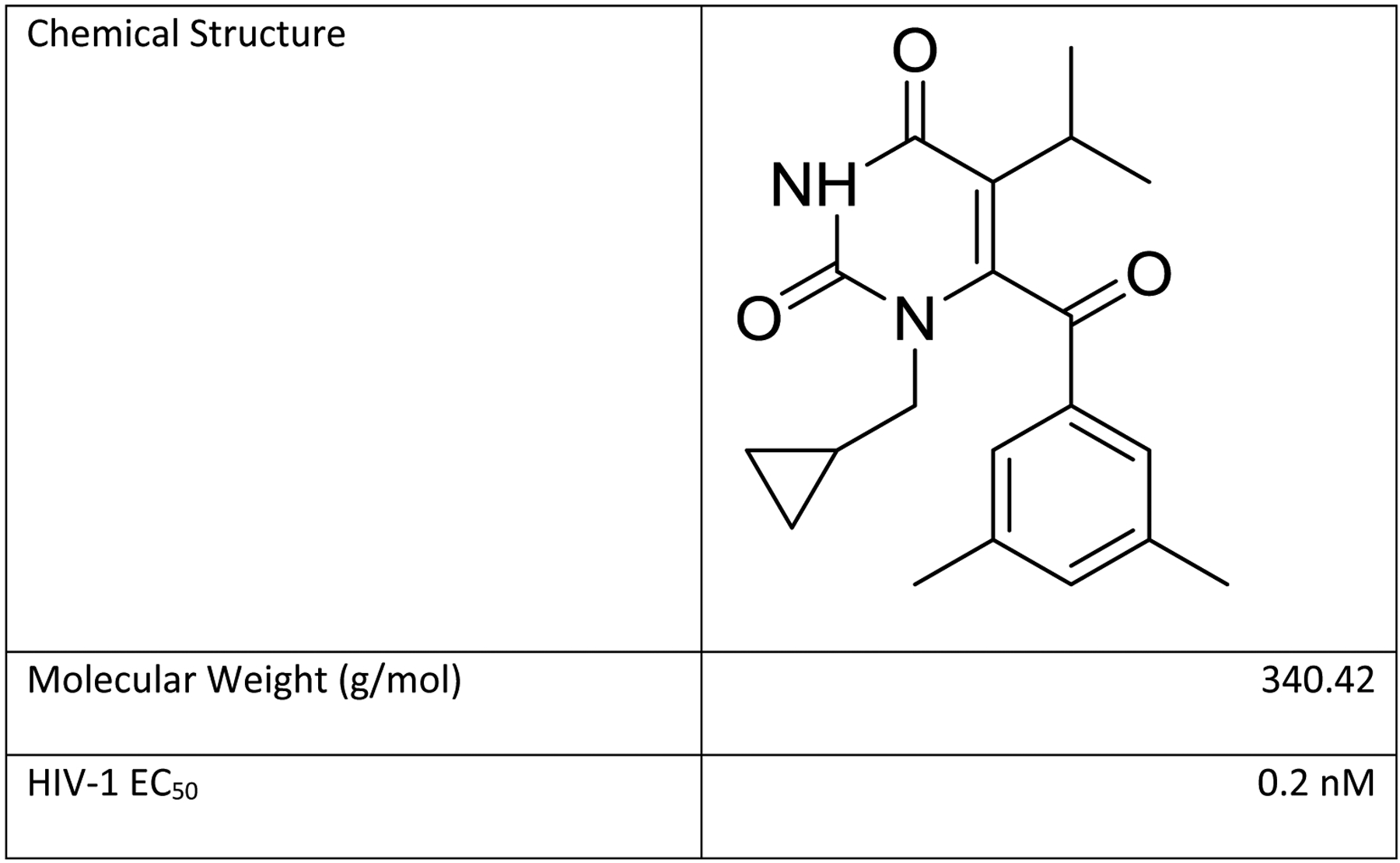
Chemical structure and properties of IQP-0528. HIV-1 EC50 from [8, 9].
IQP-0528 has been formulated in a number of ways for topical vaginal drug delivery, including vaginal films, IVRs, and gel formulations. IQP-0528 has been formulated as a 0.1% w/w vaginal film with complete release in vitro after 30 minutes[11]. Srinivasan et al[12] evaluated vaginal films loaded with 1.5 w/w% IQP-0528 in a pigtail macaque model and measured vaginal fluid concentrations one to five logs higher than the in vitro IC90. A 3.0% HEC gel containing 0.25% IQP-0528 was found to be safe and effective against HIV-1 in vitro and ex vivo[10], and a combination 2.5% TFV/1% IQP-0528 dual compartment gel was demonstrated to be effective against HIV-1 in both colorectal and ectocervical tissue, with an EC50 somewhere between that of IQP-0528 and TFV[13]. Pereira et al[14] conducted a pharmacokinetic and pharmacodynamics evaluation of a 1% IQP-0528 vaginal gel in a rhesus macaque model and found that they were able to achieve drug levels 5 to 7 logs higher than the in vitro EC50. Concerns about adherence with coitally dependent or daily dosage forms such as gels and films have led to development of sustained release formulations of IQP-0528.
Rastogi, Teller, Mesquita, Herold, and Kiser demonstrated the first use of an osmotic pump for sustained topical vaginal delivery, achieving greater than EC50 levels of IQP-0528 in sheep studies with their osmotic pump tablet[15]. Teller et al[16] demonstrated in vitro delivery of IQP-0528 over 30 days using a new flux controlled pump incorporated into a ring capable of delivering drug independent of interaction of drug with the ring elastomer. Simultaneous in vitro delivery of the HIV-1 maturation inhibitor SAMT-10 with IQP-0528 using a polyurethane IVR was also demonstrated[17]. Safety of matrix IQP-0528 IVRs was also evaluated in a pigtailed macaque model[8]. They were able to demonstrate use of the IVR to achieve micro-molar IQP-0528 concentrations in the pigtail macaque vaginal tract over 28 days, far in excess of the EC50 [8, 9]. In a follow-up to these studies, we have conducted a dose ranging topical vaginal pharmacokinetic study in pigtailed macaques using matrix rings containing IQP-0528.
MATERIALS AND METHODS
Materials.
IQP-0528 was provided by ImQuest Biosciences (Frederick, MD). Medical grade Tecoflex with a hardness of 85A was purchased from Lubrizol (Wickliffe, OH). Solutol HS-15 surfactant was purchased from Sigma-Aldrich (St. Louis, MO).
Methods.
Micronized IQP-0528 and pellets of medical grade Tecoflex 85A were mixed using a roll mixer. The drug/polymer mixture was then manually fed into a MiniLab benchtop twin-screw extruder (Thermo Haake, Waltham, MA) set to 140°C and 50-rpm screw speed through a 4.3-mm-diameter aluminum die. Drug loaded polymer strands were then pelletized using a micropelletizer (Randcastle Extrusion Systems Inc., Cedar Grove, New Jersey)[8].
IQP-0528 content of the drug/polymer pellets was confirmed through extraction as described previously [8]. Briefly, 50 mg of pellets were dissolved in a mixture of 50:50 dicholoromethane/ diethylacetamide in a 50 mL flask. 1 mL of this solution was transferred to a 10 mL flask, and the polymer precipitated with acetonitrile. After filtration, this solution was filtered into 1.5 mL HPLC vials. Spiked blanks with 8–10 mg drug and 40 mg polymer were created at the same time. Extraction revealed a drug loading of 17.0 ± 0.8 wt%; as this was higher than our target dose of 15%, additional blank polymer pellets were added during injection molding to achieve our target. Drug content of the injected molded ring segments was confirmed through extraction to be 15.6 ± 0.6 wt%.
This dose-ranging study was designed to change dose by varying the length of the ring segment, rather than changing the drug loading. For matrix-controlled systems the drug release is directly proportional to the surface area of the drug-loaded segment of the ring, varying length of drug-loaded segment allows for precise control of the relative drug release. That is, release (Q) will proceed according to the equation
Where ϕ is the fraction of the ring comprised of the drug loaded segment, and R is the release from a ring containing only a drug loaded segment. In comparison, changing of drug loading does not necessarily result in proportional release; a ring loaded with half as much drug as another is not guaranteed to release half as quickly because of changing concentration gradients within the ring. Thus, by changing only the length of the drug-loaded segment from a full-length to a half-length to a quarter-length segment, we are able to precisely control the amount of surface area available for diffusion and therefore precisely vary in vitro release.
Placebo and drug-loaded rings of Tecoflex EG85A were made through injection molding on a BabyPlast 610P (Molteno, Italy) and then dried overnight at 65.6 °C. The machine was cleaned and purged five times, then rings of pure drug-loaded Tecoflex EG85A were made using the same mold. Dimensions for our macaque-sized rings were an outer diameter of 25 mm and a segment diameter of 5 mm, consistent with previous work which demonstrated that IVRs of this size were well-tolerated and retained in the pigtail macaque reproductive tract[8, 18]. Macaque-sized rings containing three different drug-loaded lengths were created: full, half, and quarter (Figure 2). In order to create the half and quarter rings, a guide was created with the appropriate demarcations, and the rings were cut using a razor blade. Drug loaded segments were butt-welded using a thermoplastic welding blade (Fennder Drives, Manheim, PA) to the blank Tecoflex EG85A ring segments (Figure 2). Drug loaded segments were opaque and white, reflecting the micronized drug within (Figure 2).
Figure 2.

Macaque sized rings containing IQP-0528 were made using injection molding. Drug loaded segment is opaque white. Full rings (left) were made through injection molding. Half (middle) and quarter rings (right) were made by butt-welding a drug loaded segment to an appropriate unloaded segment, as illustrated above.
Drug content analysis for in vitro studies and post-in vivo extraction.
Drug content was quantified on an Agilent 1200 series HPLC attached to a diode array detector (DAD). An Agilent 4.6 × 150 mm, 5 μm C-18 column equipped with an Agilent 4.6 × 12.5 mm, 5 μm C-18 guard was used. An isocratic 12 minute method was used with a mobile phase consisting of 35:65 v/v deionized water/acetonitrile at a flow rate of 1.5 mL/min at 25°C. IQP-0528 was detected at 267 nm, with an average retention time of 4.5 minutes.
In vitro release was conducted under sink conditions in 100 mL of 5 w/v% solutol in pH 4.2 acetate buffer at 37°C, similar to the in vitro release medium chosen by Johnson et al[8]. Acetate buffer was chosen for release media to reflect the approximate pH of the female reproductive tract, and 5 w/v% solutol was added as solubilizer to achieve sink conditions during release. Release media was refreshed daily. Samples were collected at days 1, 3, 5, 7, 10, 15, 20, 25, and 30, and concentration of drug was measured using an Agilent 1200 series HPLC connected to a DAD.
IVRs of each type (100, 50, and 25% active portion) were tested in vivo in macaques for 28 days (Figure 3; see next section for details). Post-use rings were assayed for determination of final ring drug levels. The quantitative levels of IQP-0528 remaining within the IVRs were determined using a previously determined polyurethane (PU) dissolution method similar to that used to analyze the pellets (above).[8] The drug-containing portion of the IVR was excised from the ring and dissolved in a 10 mL volumetric flask of a 50:50 mixture of dimethylacetamide and dichloromethane. Solutions were left to shake overnight on a Glas-Col large capacity mixer (Terre Haute, Indiana) at a motor speed of 30. Once in solution, 1 mL of the solution was transferred to a 5 mL volumetric flask of methanol for precipitation. The sealed volumetric was slightly agitate to thoroughly precipitate the PU, which was then filtered out using a 0.22 μm polytetrafluoroethylene (PTFE) filter tip directly into a labeled HPLC vial and crimped for analysis. All IVRs under quantitative investigation were processed alongside control IVRs and spiked blank solutions to give additional confidence to the validity of the results.
Figure 3.
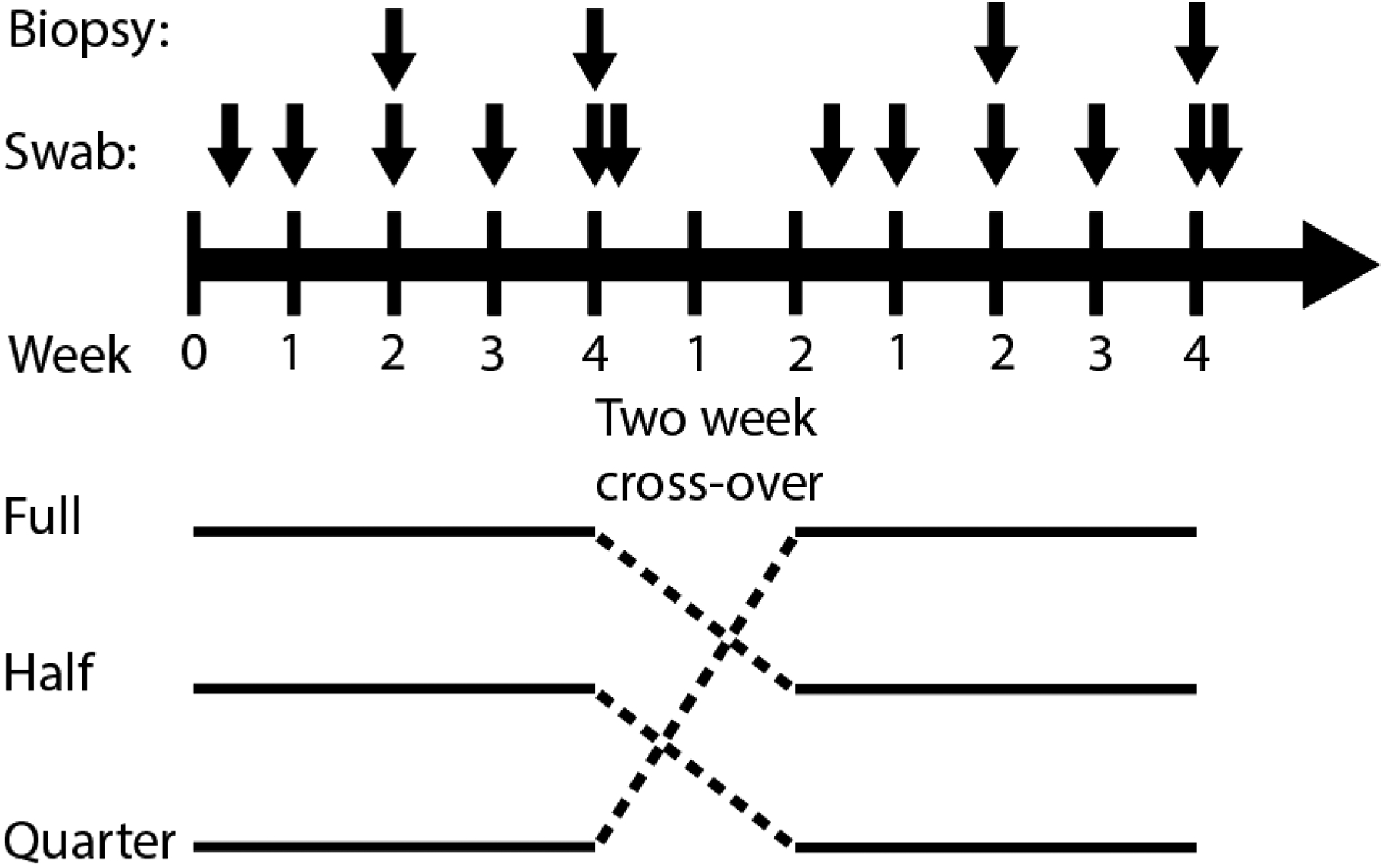
Schematic of in vivo macaque experiments. Six total pigtail macaques were used for each of the two rounds of studies. During each round, two macaques received a full, half, or quarter ring. There was a two week crossover between studies. The ring was inserted at day 0 and removed at day 28. Vaginal fluid sampling occurred at days 3, 7, 14, 21, 28, and 30. Vaginal biopsies occurred at days 14 and 28.
To make spiked blanks, approximately 50 mg of PU EG85A was weighed out and placed into a 10 mL volumetric flask of 1:1 dimethylacetamide and dichloromethane. Additionally, a 1.2 mg/mL spike solution was prepared by weighing out 30 mg of drug into a 25 mL volumetric of methanol. One mL of this spike solution was used to spike the prepared solution, and was left to shake overnight alongside the previously prepared sample and control IVRs. Spiked blank solutions were processed and analyzed alongside the previously prepared sample and control IVRs.
Macaque Studies
The pharmacokinetic studies were conducted in pigtailed macaques (Macaca nemestrina) housed under biosafety level 2 containment conditions at the Centers for Disease Control and Prevention (CDC). The study was conducted under protocols approved by the CDC Institutional Animal Care and Use Committee (IACUC) with strict adherence to the standard principles described in the Guide for the Care and Use of Laboratory Animals [19]. Briefly, six sexually mature female pigtailed macaques were enrolled in a single cross-over PK (full, half, and quarter IQP-0528 IVRs) with a two week washout in between the cross-overs (Figure 3). Two macaques each received the full, half, and quarter IQP-0528 IVRs in each of the two rounds for a total of 4 animals with each dosing. Prior to all collection procedures, macaques were anesthetized using a 10 mg/kg Ketamine mixture injected intramuscularly which could be supplemented with Telazol, 2–6 mg/kg, as determined by the Animal Resources Branch (ARB) standard operating procedure or ARB staff including the attending Doctor of Veterinary Medicine (DVM).
The rings were inserted in the upper half of the posterior vagina, close to the ectocervix, on day 0 and were left in place for 28 days. Vaginal secretions were obtained proximal (ectocervix) and distal (introitus) to the cervix on days 0, 3, 7, 14, 21, 28 and two days post IVR removal (day 30) with 3.5 × 4 mm Ultracell surgical sponges in a multiswab device [20]. Vaginal biopsies (proximal, medial and distal to the cervix, 2 per site) were collected with Miltex Townsend #30–1445 biopsy forceps on days 14 and 28. The average weight of the vaginal biopsies was 12.68 mg. The menstrual cycles of the pigtailed macaques were monitored with weekly plasma progesterone measurements by enzyme immunoassay.
Drug concentration measurement in plasma, vaginal fluid and tissue
IQP-0528 was quantitated by liquid chromatography-tandem mass spectrometry (LC-MS/MS) in proximal and distal vaginal fluid and plasma obtained on days 0, 3, 7, 14, 21, 28, and 30 and in proximal, medial, and distal vaginal biopsies on days 14 and 28 and rectal tissue (day 28) as previously described [8, 21, 22]. The amount of vaginal fluid absorbed by the Ultracell sponge was measured by mass by weighing each swab before and after placement in the macaque vaginal cavity. The lower limit of quantification (LLOQ) was 0.5 ng/sample for vaginal fluid and the values are corrected for the volume collected on the sponge. The LLOQ for tissue and plasma was 0.5 ng/sample and 5 ng/mL respectively. The vaginal fluid and tissue densities were assumed to be 1.0 g/mL to convert weight/weight concentrations of IQP-0528 (nanograms IQP-0528 per gram of vaginal fluid or tissue) to molarity (μM).
Statistical methods
The Wilcoxon rank sum test was used to evaluate differences in vaginal fluid and tissue drug levels between the three ring loadings. All tests were performed using JMP (SAS Institute, Cary, North Carolina) with a significance level of 0.05.
Results
In this study, we have evaluated a range of loadings of our IQP-0528 matrix IVR, both in vitro and in vivo. Our in vitro results (Figure 4) clearly demonstrate release kinetics are directly proportional to length of drug-loaded segment, with the cumulative release at day 30 from the full ring of 48.5 ± 2.2 mg; double that of the half ring: 24.8 ± 0.36 mg. 30-day cumulative release from the quarter-segment ring: 14.0 ± 1.58 mg is similarly roughly a quarter that of the full sized ring, and half that of the half-segment ring (Figure 4). Because of the design of this study, percentage cumulative release is quite similar for all rings: 24.3 ± 0.01 % for the full ring, 22.8 ± 0.004 % for the half ring, and 23.2 ± 0.03 % for the quarter ring. This surface-area proportional release is to be expected given diffusion controlled drug release from a matrix ring.
Figure 4.
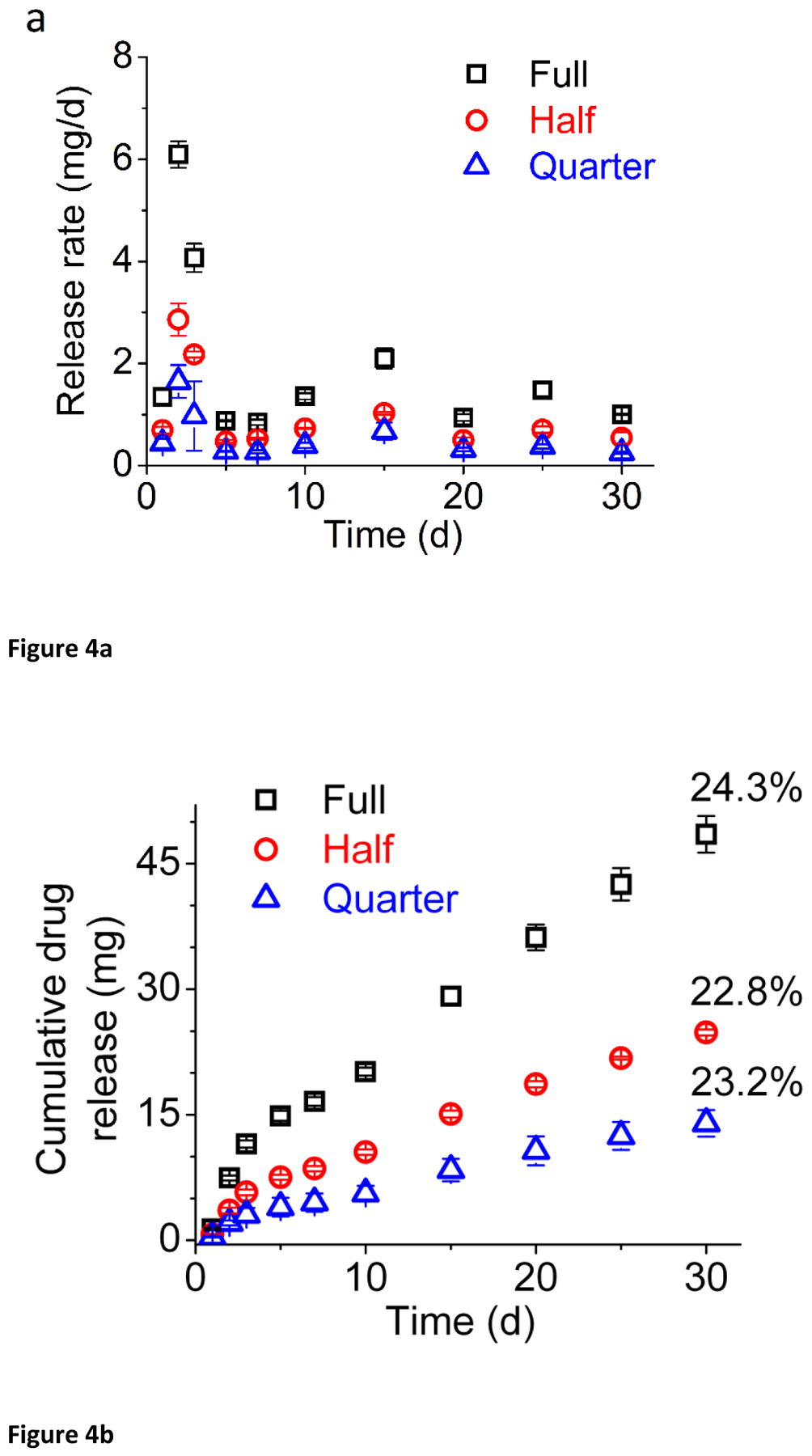
Daily (a) and cumulative (b) release of IQP-0528 for full (black), half (red), and quarter (blue) sized rings.
Drug extracted from post-trial rings used in vivo revealed an average of 0.03 ± 0.01 mg/day released from the full-segment rings, with a very similar .03 ± 0.02 mg/day released from the half-segment rings (Figure 5). The quarter-segment rings were found to have released .02 ± 0.02 mg/day. While the quarter segment rings had a lower average release, this difference is not statistically significant (single factor ANOVA, α = .05).
Figure 5.
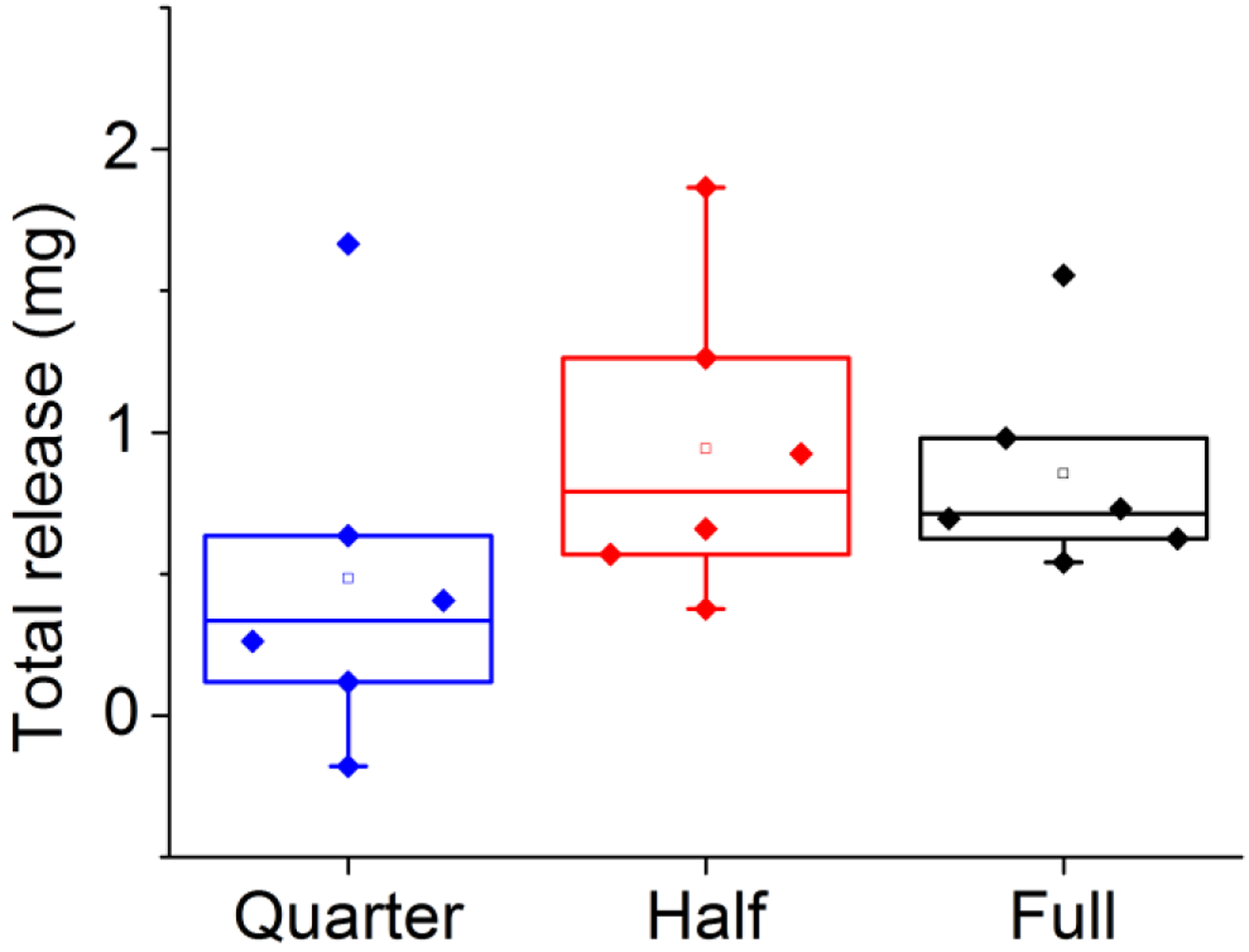
Total in vivo release for all three loadings: full (black), half (red), and quarter (blue) rings.
As is typical with these experiments, drug levels are highly variable: for days 3 to 28, with the ring present, the drug levels in the proximal vagina ranged from 6 × 102 to 3.2 × 105 ng/mL for the full ring (median 1.9 × 105 ng/mL); to 3 × 102 to 7.4 × 105 ng/mL for the half ring (median 3.8 × 104 ng/mL); and 1.4 × 102 to 1.0 × 105 ng/mL for the quarter ring (median 5× 103 ng/mL). Similarly, drug levels in the distal vagina ranged from 2 × 102 to 1.5 × 105 ng/mL for the full ring (median 1.6 × 103 ng/mL); to 2.5 to 3.0 × 105 ng/mL for the half ring (median 1.9 × 103 ng/mL); and 1.1 × 102 to 1.9 × 105 ng/mL for the quarter ring (median 1.2 × 103 ng/mL).
Statistical Analysis
Area under the first moment curves were calculated using the trapezoidal rule to create a summary statistic for release over the entire time period for each ring. The Wilcoxon rank sum test was used to compare the proximal area under the first moment curves (AUMCs) for vaginal fluid drug levels for full, half, and quarter rings (Figure 6), and found no difference (P = 0.05). Similarly, the Wilcoxon rank sum test (P = 0.05) showed no statistically significant difference between distal AUMCs for full, half, and quarter rings (Figure 7). Because no differences were found using the Wilcoxon rank sum test, no additional pairwise testing was necessary.
Figure 6.
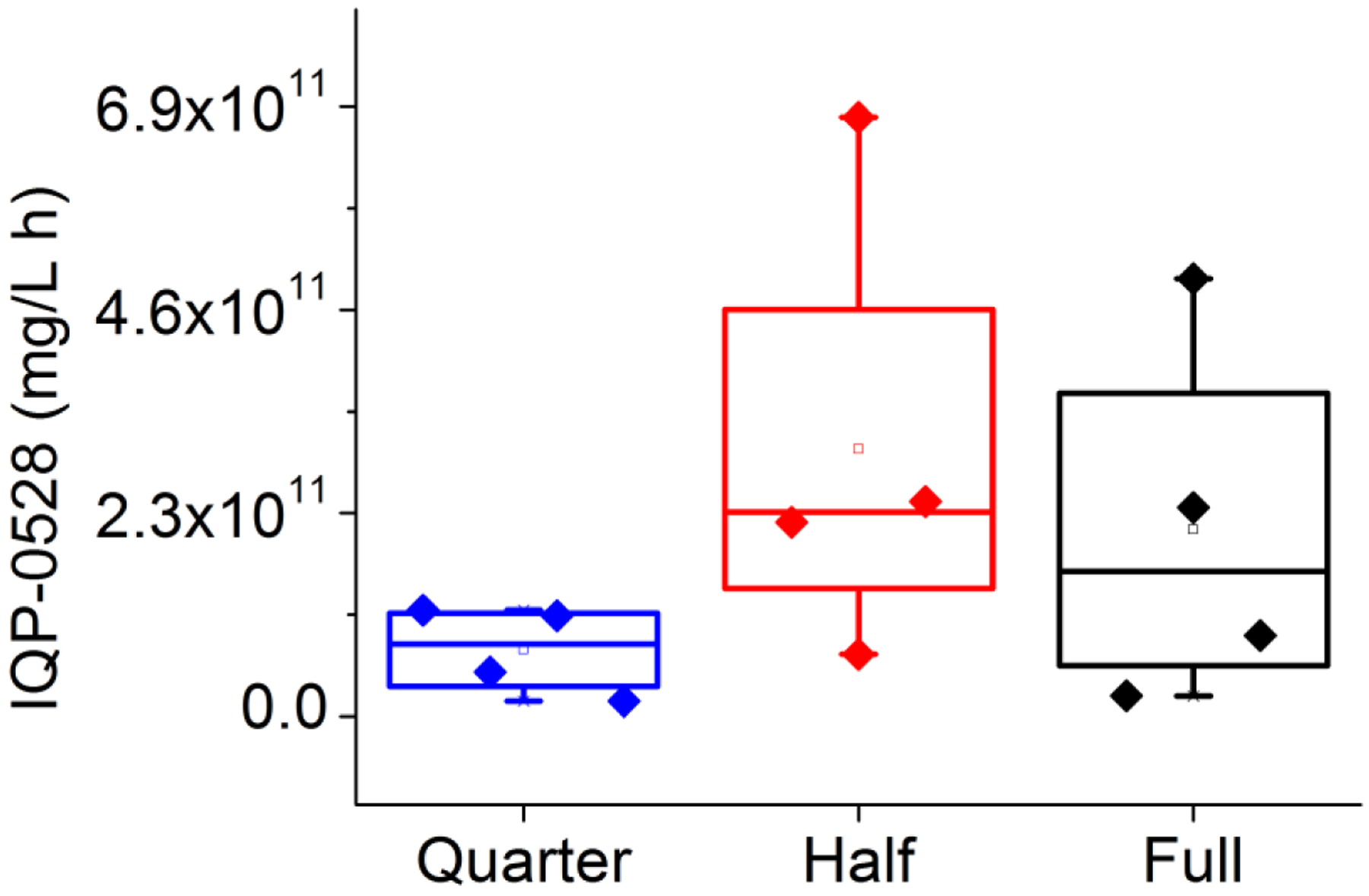
Comparison of the proximal vaginal fluid area under the first moment curve (AUMC). Full (blue), half (red), and quarter (black).
Figure 7.
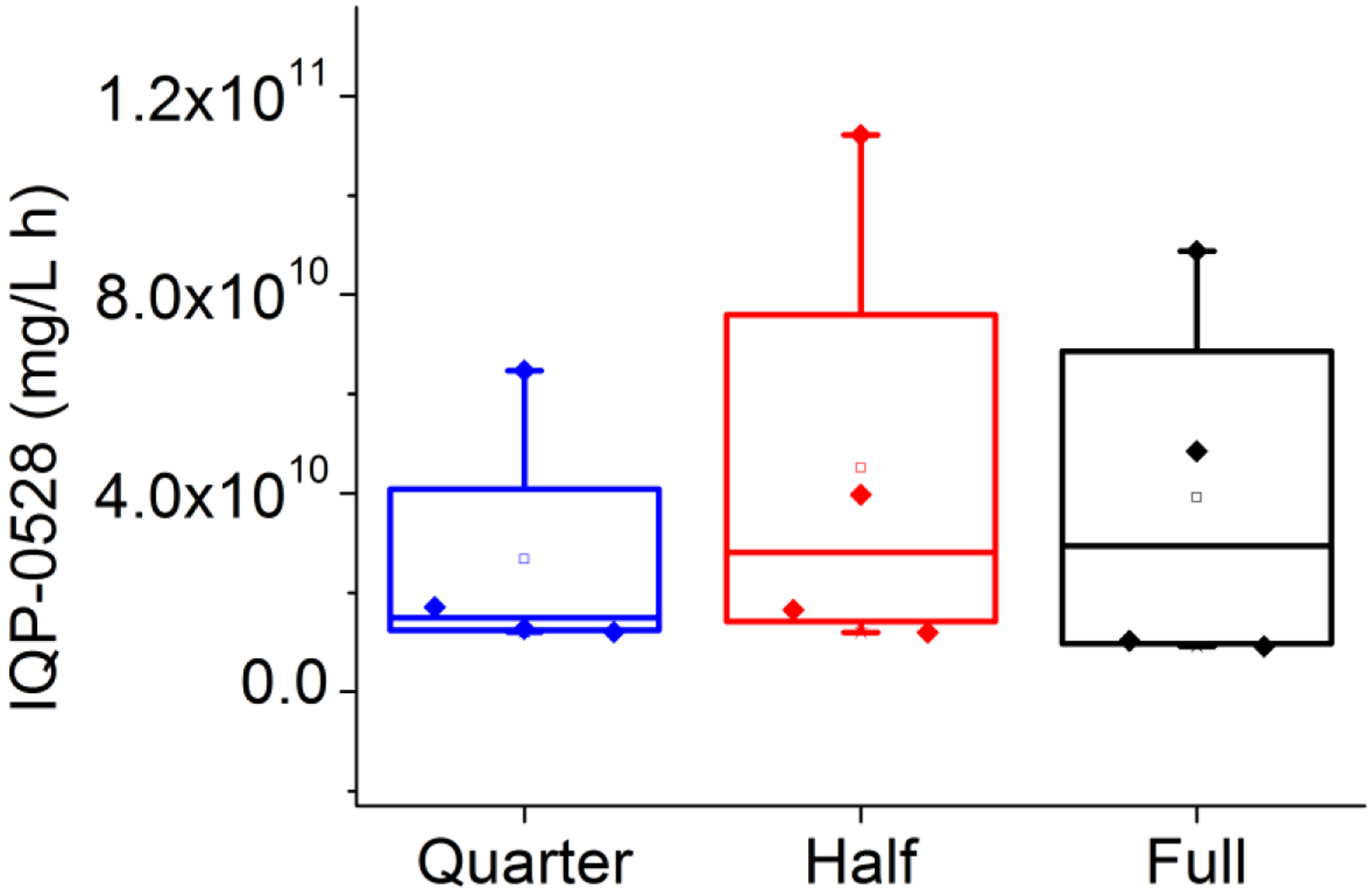
Comparison of the distal vaginal fluid area under the first moment curve (AUMC). Full (blue), half (red), and quarter (black).
A statistically significant drop in proximal vaginal fluid levels was observed 2 days after ring removal for full, half, and quarter rings (Wilcoxon rank sum test with P=0.05) when compared to day 28 levels (Figure 8, 10), with average proximal IQP-0528 levels dropping from an average of 9.4 × 104 ng/mL to an average 3 × 102 ng/mL for the full ring; and an average of 1.1 × 105 ng/mL to 4.5 × 102 ng/mL for the half ring (Figure 8, 10). While these average levels are above the in vitro IC90 (0.146 ug/mL)[12], several of the vaginal fluid levels lie below this concentration, indicating that any residual protection after ring removal has a short duration; shorter than the time for integration and dissemination of the virus.
Figure 8.
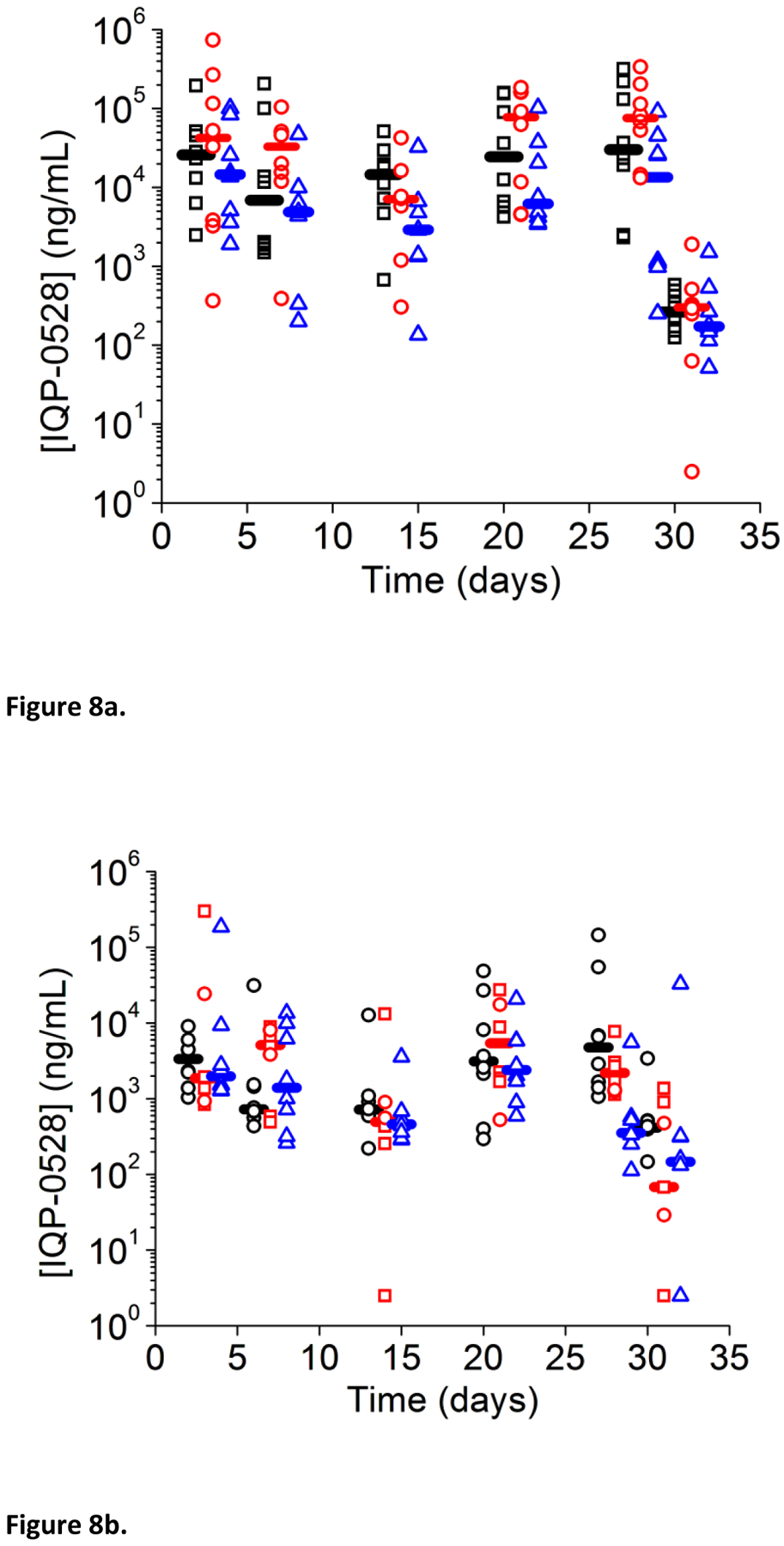
Vaginal fluid levels over time. Full (blue), half (red), and quarter (black). Horizontal bars indicate median release.
Figure 10.
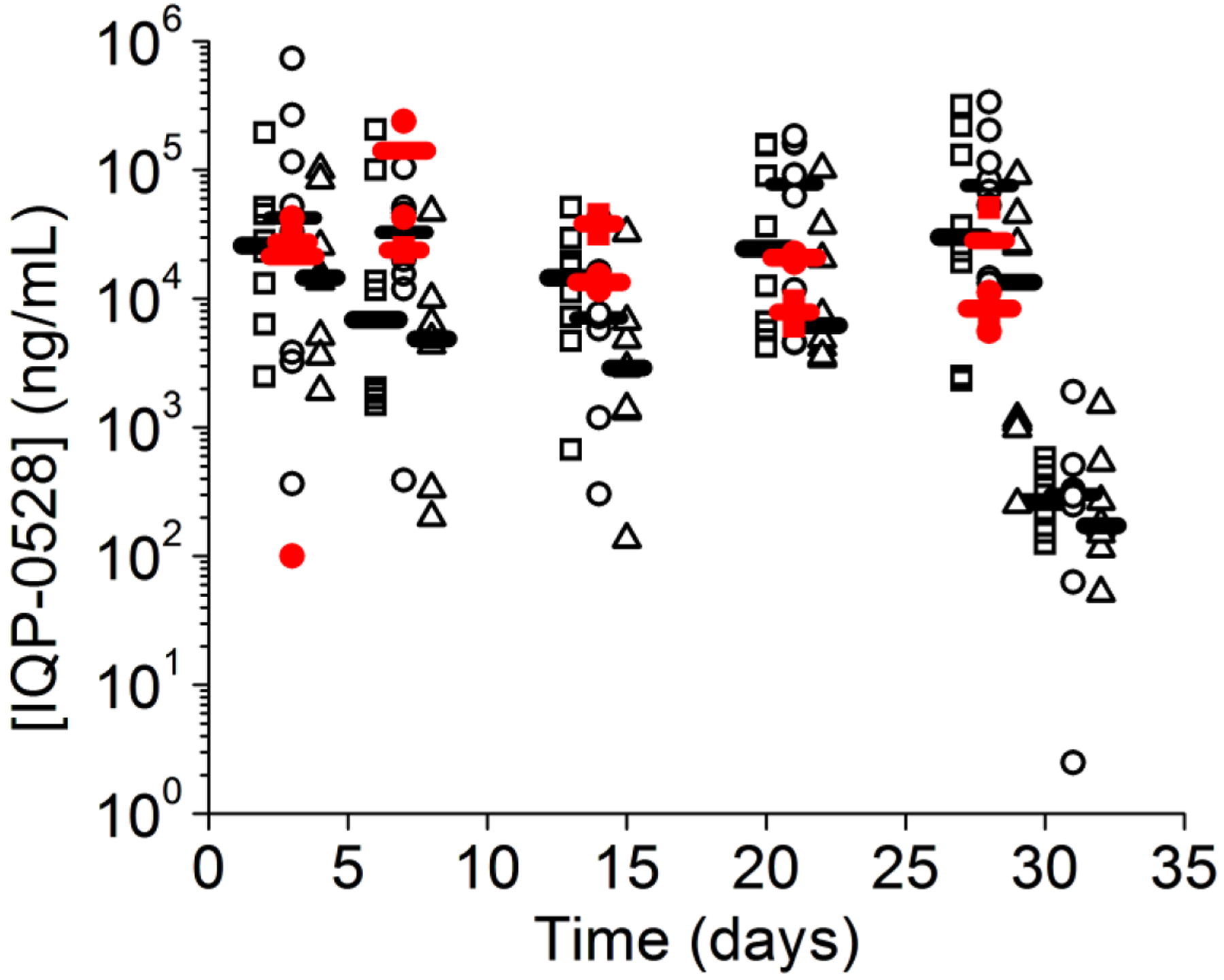
Comparison of proximal vaginal fluid levels (black) from our macaque study and those obtained by Johnson et al[8] (red).
One limitation of our study is due to number of animals used; each animal received a different ring after a two-week washout period. Despite this, each instance of an animal receiving a ring was treated as independent. The validity of this assumption was evaluated by comparing the vaginal fluid AUMC for each animal, each of which received two different rings (shown in Supplemental Figures 1 and 2). As before, this AUMC was highly variable and correlation between relative ring loading and AUMC was poor. While four out of the six animals displayed a decrease or increase in proximal vaginal fluid AUMC with correspondingly decreasing or increasing loading, the magnitude of AUMC change did not generally correspond to the change ring loading for the animal. Similarly, in the distal vagina, three out of the six macaques failed to show a decrease or increase in AUMC with a corresponding change in loading. Much as in the proximal vagina, the magnitude of AUMC change in the distal vagina did not correspond well to changes in ring drug loading.
Much like the concentrations observed in the vaginal fluid, vaginal tissue levels were highly variable. For the full ring at 14 days, vaginal tissue levels ranged from 1.3 × 102 – 2.6 × 103 (median 9.3 × 103) ng/g tissue in the proximal vagina to 54 – 2 × 103 (median 1.2 × 103) ng/g tissue in the medial vagina and 2.5 – 2 × 103 (median 6.6 × 102) ng/g tissue in the distal vagina (Figure 9). At 28 days, vaginal tissue levels ranged from 1.0 × 103 – 2.0 × 105 (median 8.2 × 103) ng/g tissue in the proximal vagina; 1.6 × 102 – 2.1 × 104 (median 7.8 × 103) ng/g tissue in the medial vagina; and 42 – 4.5 ×103 (median 1.7 × 102) ng/g tissue in the distal vagina.
Figure 9.
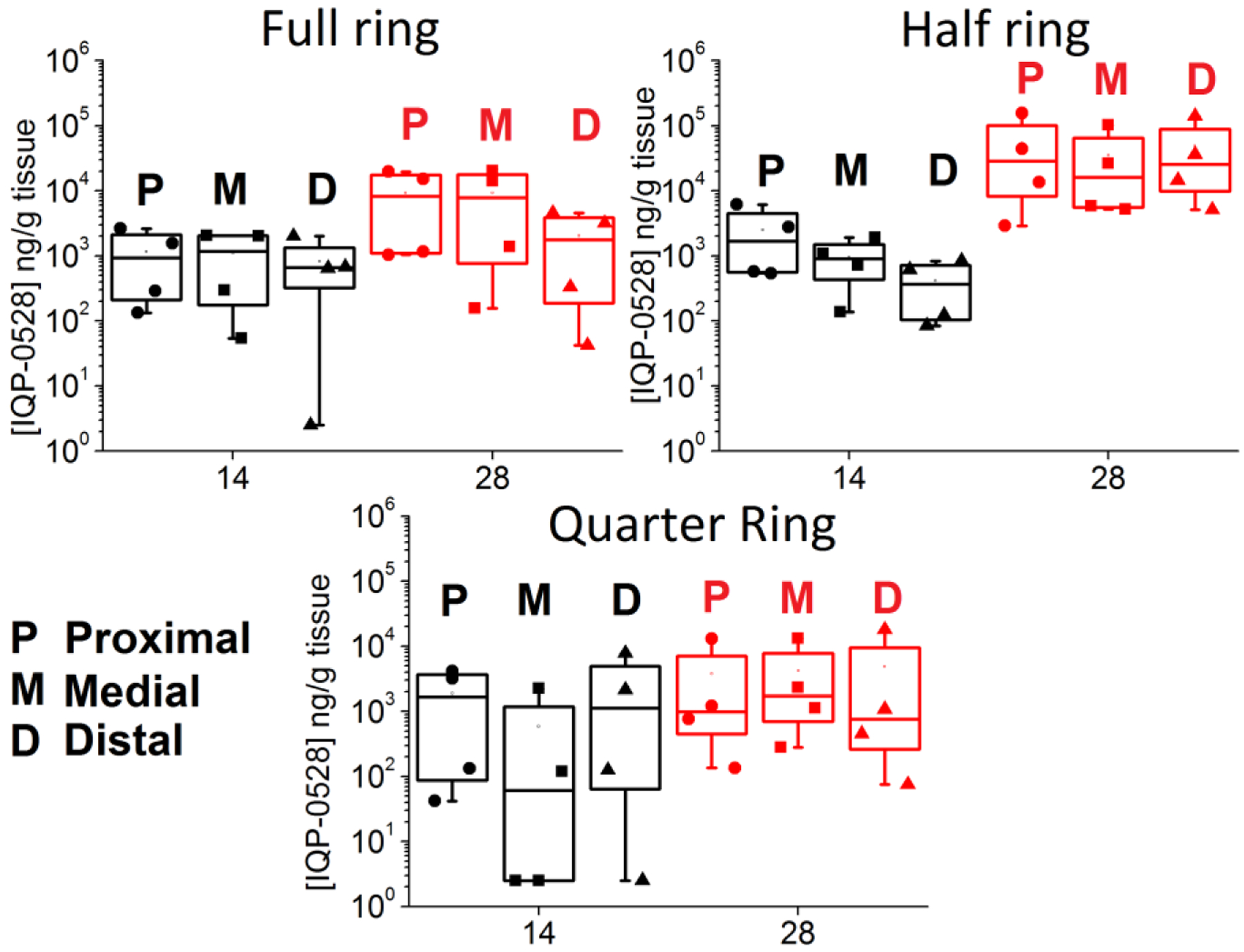
Vaginal tissue biopsy levels for the full, half, and quarter rings. Both 14 day (black) and 28 day (red) are compared.
Similarly, for the half ring, at 14 days, vaginal tissue levels ranged from 5.4 × 102 – 6.2×103 (median 1.7 × 103) ng/g tissue in the proximal vagina; 1.4 × 102 – 1.9 × 103 (median 9.0 × 102) ng/g tissue in the medial vagina; and 84 – 8.4 × 102 (median 3. 7 × 102) ng/g tissue in the distal vagina. After 28 days, tissue levels ranged from 2.9 × 103 – 1.6 × 105 (median 2.9 × 104) ng/g tissue in the proximal vagina; 5.2 × 103– 1.0 × 105 (median 1.6 × 104) ng/g tissue in the medial vagina; and 5.1 × 103 – 1.4 × 105 (median 2.5 × 104) ng/g tissue in the distal vagina.
For the quarter ring at 14 days, tissue levels ranged from 42 – 4.1 × 103 (median 1.7 × 103) ng/g tissue in the proximal vagina to 2.5 – 2.2 × 103 (median 61) ng/g tissue in the medial vagina, and 2.5 – 7.8 × 103 (median 1.1 × 103) ng/g tissue in the distal vagina. After 28 days, tissue levels ranged from 1.4 × 102 – 1.3 × 104 (median 9.9 × 102) ng/g tissue in the proximal vagina to 2.8 ×102 – 1.3 × 104 (median 1.7 × 103) ng/g tissue in the medial vagina to 75 – 1.8 × 104 (median 7.6 × 102) ng/g tissue in the distal vagina.
Vaginal tissue AUMC was calculated using the trapezoidal rule for ring type and location. The Wilcoxon rank sum test (P = 0.05) showed no statistically significant difference between AUMC based on location (proximal, medial, or distal vagina) for each of the full, half, or quarter rings, indicating that drug is distributed relatively evenly throughout the vaginal tissue for all rings. AUMC was also compared between ring types at each location (proximal, medial, and distal). This difference was not statistically significant based on the Wilcoxon rank sum test (P = 0.05).
Discussion
Despite our clearly dose-proportional in vitro release, the amount of drug released from these different rings in vivo is dependent on neither the total amount of drug in our IVR nor the surface area available for release. Our in vitro experiments are conducted in 100 mL of acetate buffer with 5 w/w% solutol as a solubilizer, constantly shaken, refreshed daily, and are thus under sink conditions; the concentration of drug outside the ring can be approximated as zero. (In practical terms, we conduct our in vitro release in enough media to exceed five times the solubility limit of the drug in the solution). Because the concentration at the boundary is zero, release from the in vitro rings is maximal under these conditions.
The in vivo release from our matrix IVRs is diffusively controlled. The concentration of drug within the surrounding cervicovaginal fluid (CVF) is a balance between the release of drug from the ring versus clearance of drug from the CVF into the tissue. Drug flux is directly proportional to the difference between the surface concentration of the ring and the concentration in the surrounding medium. Because of the low aqueous solubility of IQP-0528, this difference is low. As the concentration of drug in the CVF increases, the concentration gradient becomes shallower, and drug release slows. Much less fluid volume is present in the female reproductive tract (FRT) as compared to our in vitro experiments: Mitchell et al report a mean volume of 0.51 mL of CVF in the human FRT[23], for example, and Owen and Katz report 0.5 – 0.75 g[24]. Our experiments are being conducted in the much smaller pigtailed macaque, and thus correspondingly less vaginal fluid is expected. Thus, drug release in vivo is decreased due to poor solubility and limited fluid volume combined with relatively slow clearance from the CVF. This decrease in drug release in vivo is obvious when comparing average daily release: in vitro, average daily release is 1.6 mg/day for the full ring and 0.49 mg/day for the quarter ring. In contrast, the in vivo release is more than an order of magnitude less: 0.02 – 0.03 mg/day, pointing to rapid saturation of the CVF with IQP-0528 and slow clearance of the drug from the CVF. Thus, in vivo release of IQP-0528 from the ring may be better modeled as partition-controlled: because in vivo diffusion of drug from the IVR is dominated by the solubility limit of drug in the surrounding vaginal fluid, release can be thought of as governed by this solubility limit[25].
Johnson et al tested two matrix IQP-0528 rings with a lower loading of 0.5 and 1 wt%[8]. Like the rings used in this study, the rings made by Johnson et al were IQP-0528 matrix rings made of medical grade Tecoflex EG-85A polyurethane[8], though the drug loading was different in comparison to the current study, since the drug was evenly dispersed throughout the entire ring. These rings were both evaluated in vitro and in vivo in pigtail macaques for safety. Interestingly, these rings also displayed dose-dependent release in vitro, though no statistically significant difference was observed in vivo proximal to the ring placement. Our more highly loaded rings (15.6%, 7.8%, and 3.9%), showed no dose dependence in vivo either proximal or distal to the ring placement. Comparison of data from the rings from the Johnson et al experiments reveals similar vaginal fluid levels as found in the current study (Figure 10). This indicates that even at low concentrations, IQP-0528 rapidly saturates the vaginal fluid proximal to the cervix. Thus, a relatively small loading of IQP-0528 is required vaginally to achieve levels far in excess of the IC90 of 146 ng/mL.
As mentioned in the introduction, dapivirine is another drug delivered with an IVR. Much like 0528, dapivirine is poorly soluble in water, with a solubility of 16 μM at pH 4.2[3]. 200 mg and 25 mg reservoir dapivirine rings were evaluated in vivo and found to achieve similar vaginal fluid concentrations; as expected, given that release rate from reservoir devices is governed largely by the rate controlling membrane [26, 27]. Nel et al evaluated a silicone elastomer matrix ring loaded with 25 mg of DPV and observed higher initial in vivo release and 42% release of loaded drug over 28 days of use[28]. The similarity between residual drug levels found in vivo and in vitro led to the conclusion that sink conditions were reached in vivo for dapivirine silicone matrix rings[28]. Interestingly, this contrasts with our results, which indicate that sink conditions were not reached for our IQP-0528 matrix rings in vivo. The fact that despite the low aqueous solubility of both drugs, in vivo release of dapivirine and IQP-0528 are very different, highlights the importance of dose-ranging our in vivo studies, as release is clearly dependent on more than simple aqueous solubility.
An advantage of delivery using an IVR is reduced systemic exposure to the drug, which can positively impact both safety profile and risk for resistance to the ARV[29]. Blood plasma samples were analyzed for drug content to determine systemic delivery. No drug was detected in any of the blood plasma samples, indicating that IQP-0528 is absorbed but remains locally in the vaginal fluid and surrounding tissues.
Conclusion
We have performed a dose-ranging study of an IQP-0528 ring in pigtailed macaque FRTs using macaque-sized matrix rings. By using full, half, and quarter segment macaque rings, the design of our study allowed us to measure in vivo and in vitro release from rings with an overall loading ranging from 15.6 to 3.9 wt% but with the same mechanism of release, varying only the surface area available for release. Despite the dose-proportional release found in vitro, no statistically significant difference in average daily release was found between the differently loaded IVRs in vivo for tissue samples or for vaginal fluid samples. This is likely due to a combination of low solubility of drug in vivo along with slow clearance from the cervicovaginal fluid leading to saturation of the CVF and shallow concentration gradients and therefore much lower release. Importantly, this indicates that in vitro release may be not be a good guide for in vivo release, particularly for poorly soluble drugs. Instead, these devices may be best modeled as partition controlled[25]. Similar results were also found for UC781-releasing vaginal rings by McConville et al[30]. Comparison of our release with the earlier 0.5% and 1 wt% loading rings considered by Johnson et al[8] likewise reveals release similar to our current, more highly-loaded rings, indicating that our conclusions can be extended to those loadings as well.
Additionally, despite a range of in vitro releases, all in vivo rings demonstrated the ability to achieve vaginal fluid levels well in excess of the IC90, indicating protective levels of IQP-0528 may be achieved even with very low loading. Thus, IQP-0528 may be appropriate for formulation in a combination IVR, since only a small amount of IQP-0528 is required to achieve generally protective levels in vivo. This also, however, indicates that achieving higher levels of vaginal IQP-0528 is not possible using devices based on passive diffusion.
Supplementary Material
Figure 11.
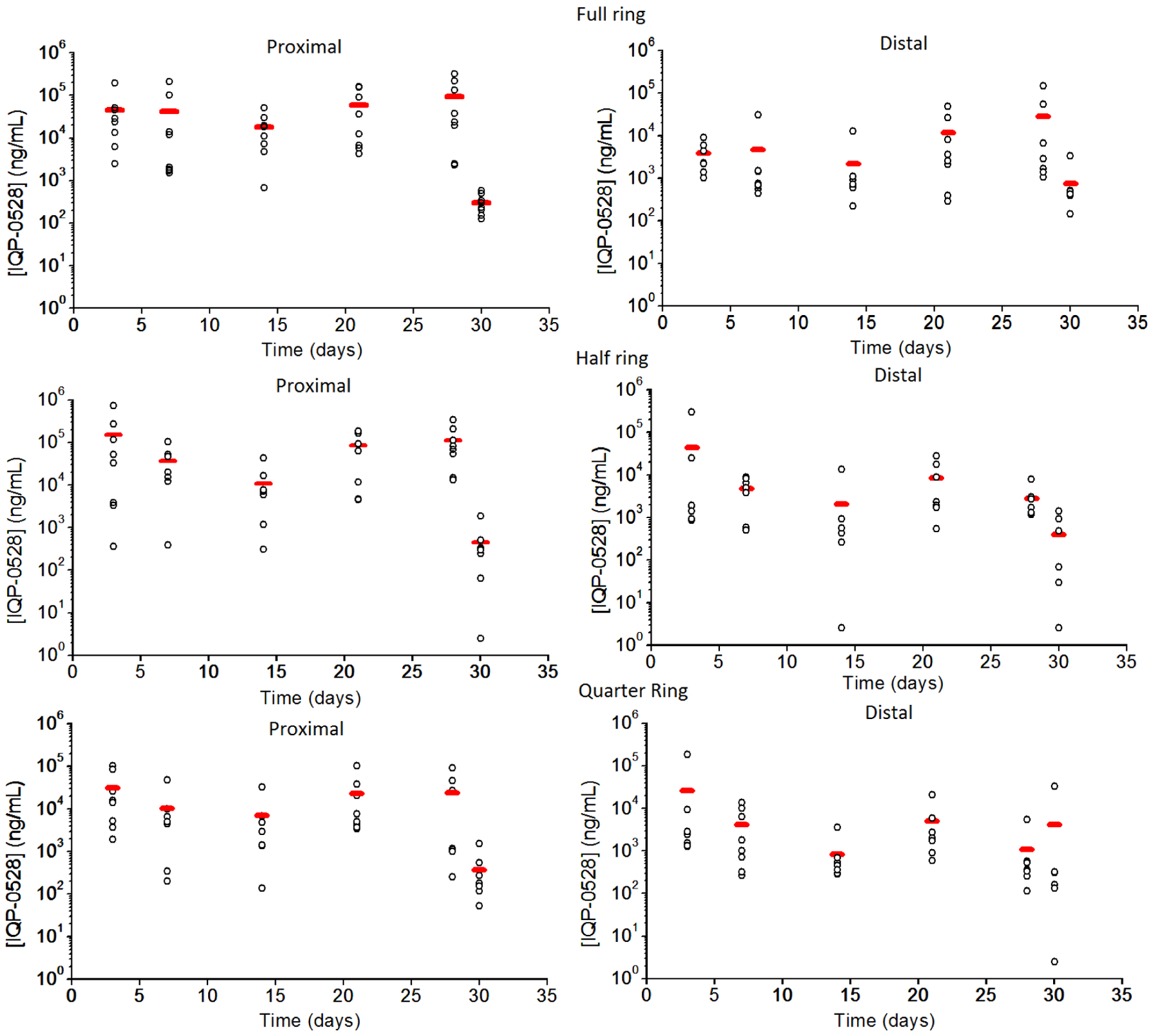
Overall summary of vaginal fluid levels per ring, both proximal and distal. Horizontal bar (red) indicates median.
Acknowledgements
This work was supported by NIH U19AI03461. IQP-0528 was provided by ImQuest BioSciences. We acknowledge the following members of the CDC DHAP Laboratory Branch/Preclinical Evaluation Team for their contributions to our nonhuman primate research: David Garber, James Mitchell, Shanon Ellis, Frank Deyounks and Kristen Kelley. We thank Angela Fought for helpful discussion of the statistical analysis.
Abbreviations
- IVR
Intravaginal Ring
- ARV
Antiretroviral
- TFV
Tenofovir
- HEC
Hydroxyethyl cellulose
- NNRTI
nonnucleoside reverse transcriptase inhibitor
- IQP-0528
1-(3-Cyclopropyl)methyl-6-(3,5-dimethylbenzoyl)-5-isopropyl-2,4(1H,3H)-pyrimidinedione
- FRT
Female Reproductive Tract
- HPLC
High-performance Liquid Chromatography
- DAD
Diode Array Detector
- CDC
Centers for Disease Control and Prevention
- IACUC
Institutional Animal Care and Use Committee
- LLOQ
Lower Limit of Quantitation
- AUMC
Area Under the first Moment Curve
- PU
Polyurethane
Footnotes
Disclaimer
Publisher's Disclaimer: The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. , Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. N Engl J Med, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malcolm RK, Edwards KL, Kiser P, Romano J, and Smith TJ, Advances in microbicide vaginal rings. Antiviral Res, 2010. 88 Suppl 1: p. S30–9. [DOI] [PubMed] [Google Scholar]
- 3.Kiser PF, Johnson TJ, and Clark JT, State of the art in intravaginal ring technology for topical prophylaxis of HIV infection. AIDS Rev, 2012. 14(1): p. 62–77. [PubMed] [Google Scholar]
- 4.Chien YW, Mares SE, Berg J, Huber S, Lambert HJ, and King KF, Controlled drug release from polymeric delivery devices. III: In vitro-in vivo correlation for intravaginal release of ethynodiol diacetate from silicone devices in rabbits. J Pharm Sci, 1975. 64(11): p. 1776–81. [DOI] [PubMed] [Google Scholar]
- 5.Mesquita PM, Srinivasan P, Johnson TJ, Rastogi R, Evans-Strickfaden T, Kay MS, et al. , Novel preclinical models of topical PrEP pharmacodynamics provide rationale for combination of drugs with complementary properties. Retrovirology, 2013. 10: p. 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson Buckheit K, Yang L, and Buckheit RW Jr., Development of dual-acting pyrimidinediones as novel and highly potent topical anti-HIV microbicides. Antimicrob Agents Chemother, 2011. 55(11): p. 5243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen BA, Panther L, Marzinke MA, Hendrix CW, Hoesley CJ, van der Straten A, et al. , Phase 1 Safety, Pharmacokinetics, and Pharmacodynamics of Dapivirine and Maraviroc Vaginal Rings: A Double-Blind Randomized Trial. J Acquir Immune Defic Syndr, 2015. 70(3): p. 242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson TJ, Srinivasan P, Albright TH, Watson-Buckheit K, Rabe L, Martin A, et al. , Safe and sustained vaginal delivery of pyrimidinedione HIV-1 inhibitors from polyurethane intravaginal rings. Antimicrob Agents Chemother, 2012. 56(3): p. 1291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartman TL, Yang L, and Buckheit RW Jr., Antiviral interactions of combinations of highly potent 2,4(1H,3H)-pyrimidinedione congeners and other anti-HIV agents. Antiviral Res, 2011. 92(3): p. 505–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahalingam A, Simmons AP, Ugaonkar SR, Watson KM, Dezzutti CS, Rohan LC, et al. , Vaginal microbicide gel for delivery of IQP-0528, a pyrimidinedione analog with a dual mechanism of action against HIV-1. Antimicrob Agents Chemother, 2011. 55(4): p. 1650–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ham AS, Rohan LC, Boczar A, Yang L, W.B. K, and Buckheit RW Jr, Vaginal film drug delivery of the pyrimidinedione IQP-0528 for the prevention of HIV infection. Pharm Res, 2012. 29(7): p. 1897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srinivasan P, Zhang J, Martin A, Kelley K, McNicholl JM, Buckheit RW Jr., et al. , Safety and pharmacokinetics of quick dissolving polymeric vaginal films delivering the antiretroviral IQP-0528 for pre-exposure prophylaxis. Antimicrob Agents Chemother, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dezzutti CS, Shetler C, Mahalingam A, Ugaonkar SR, Gwozdz G, Buckheit KW, et al. , Safety and efficacy of tenofovir/IQP-0528 combination gels - a dual compartment microbicide for HIV-1 prevention. Antiviral Res, 2012. 96(2): p. 221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira LE, Mesquita PM, Ham A, Singletary T, Deyounks F, Martin A, et al. , Pharmacokinetic and Pharmacodynamic Evaluation following Vaginal Application of IQB3002, a Dual-Chamber Microbicide Gel Containing the Nonnucleoside Reverse Transcriptase Inhibitor IQP-0528 in Rhesus Macaques. Antimicrob Agents Chemother, 2015. 60(3): p. 1393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rastogi R, Teller RS, Mesquita PM, Herold BC, and Kiser PF, Osmotic pump tablets for delivery of antiretrovirals to the vaginal mucosa. Antiviral Res, 2013. 100(1): p. 255–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teller RS, Malaspina DC, Rastogi R, Clark JT, Szleifer I, and Kiser PF, Controlling the hydration rate of a hydrophilic matrix in the core of an intravaginal ring determines antiretroviral release. J Control Release, 2016. 224: p. 176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ugaonkar SR, Clark JT, English LB, Johnson TJ, Buckheit KW, Bahde RJ, et al. , An Intravaginal Ring for the Simultaneous Delivery of an HIV-1 Maturation Inhibitor and Reverse-Transcriptase Inhibitor for Prophylaxis of HIV Transmission. J Pharm Sci, 2015. 104(10): p. 3426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Promadej-Lanier N, Smith JM, Srinivasan P, McCoy CF, Butera S, Woolfson AD, et al. , Development and evaluation of a vaginal ring device for sustained delivery of HIV microbicides to non-human primates. J Med Primatol, 2009. 38(4): p. 263–71. [DOI] [PubMed] [Google Scholar]
- 19.NIH, Guide for the care and use of laboratory animals, 8th ed 2010, The National Academies Press: Washingotn DC. [Google Scholar]
- 20.Srinivasan P, Dinh C, Zhang J, Pau C-P, McNicholl JM, Lo Y, Herold BC, Teller R, Kiser P and Smith JM, Pharmacokinetic evaluation of tenofovir disoproxil fumarate released from an intravaginal ring in pigtailed macaques after 6 months of continuous use. Journal of Medical Primatology, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuklenyik Z, Martin A, Pau CP, Garcia-Lerma JG, Heneine W, Pirkle JL, et al. , Effect of mobile phase pH and organic content on LC-MS analysis of nucleoside and nucleotide HIV reverse transcriptase inhibitors. J Chromatogr Sci, 2009. 47(5): p. 365–72. [DOI] [PubMed] [Google Scholar]
- 22.Pereira LE, Mesquita PM, Ham A, Singletary T, Deyounks F, Martin A, et al. , Pharmacokinetic and pharmacodynamic evaluation following vaginal application of IQB3002, a dual chamber microbicide gel containing the NNRTI IQP-0528 in rhesus macaques. Antimicrob Agents Chemother, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell C, Paul K, Agnew K, Gaussman R, Coombs RW, and Hitti J, Estimating volume of cervicovaginal secretions in cervicovaginal lavage fluid collected for measurement of genital HIV-1 RNA levels in women. J Clin Microbiol, 2011. 49(2): p. 735–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owen DH and Katz DF, A vaginal fluid simulant. Contraception, 1999. 59(2): p. 91–5. [DOI] [PubMed] [Google Scholar]
- 25.Chien YW and Lambert HJ, Controlled Drug Release from Polymeric Delivery Devices II: Differentiation between Partition-Controlled and Matrix-Controlled Drug Release Mechanisms. Journal of Pharmaceutical Sciences, 1974. 63(4): p. 515–519. [DOI] [PubMed] [Google Scholar]
- 26.Malcolm KM,C; Woolfson D, Correlation between in vitro - in vivo release rates of the antiretroviral candidate, dapivirine, from silicone elastomer vaginal rings, in British Pharmaceutical Conference. 2008: Manchester, UK. [Google Scholar]
- 27.Romano J, Variano B, Coplan P, Van Roey J, Douville K, Rosenberg Z, et al. , Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res Hum Retroviruses, 2009. 25(5): p. 483–8. [DOI] [PubMed] [Google Scholar]
- 28.Nel A, Bekker LG, Bukusi E, Hellstrm E, Kotze P, Louw C, et al. , Safety, Acceptability and Adherence of Dapivirine Vaginal Ring in a Microbicide Clinical Trial Conducted in Multiple Countries in Sub-Saharan Africa. PLoS One, 2016. 11(3): p. e0147743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thurman AR, Clark MR, Hurlburt JA, and Doncel GF, Intravaginal rings as delivery systems for microbicides and multipurpose prevention technologies. Int J Womens Health, 2013. 5: p. 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McConville C, Smith JM, McCoy CF, Srinivasan P, Mitchell J, Holder A, et al. , Lack of in vitro-in vivo correlation for a UC781-releasing vaginal ring in macaques. Drug Delivery and Translational Research, 2015. 5(1): p. 27–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


