Abstract
The development of the marmoset as a translational model for healthspan and lifespan studies relies on the characterization of health parameters in young and geriatric marmosets. This cross-sectional study examined health phenotypes in marmosets for five domains of interest for human health and aging: mobility, cognition, metabolism, homeostasis, and immune function. Geriatric marmosets were found to have significant executive function impairment when compared to young animals. While geriatric animals did not show gross abnormalities in mobility and measures of locomotion, their types of movement were altered from young animals. Geriatric marmosets had alterations in cardiac function, with significantly increased mean arterial pressures; metabolism, with significantly lower VO2; and suppressed immune function. Further, this study sought to characterize and describe histopathology for both young and geriatric healthy marmosets. Overall this study provides a characterization of health parameters for young and geriatric marmosets which will greatly enhance future aging and interventional testing in marmosets.
Keywords: Healthspan, longevity, biomarkers of aging, animal models, nonhuman primates
Graphical Abstract
Introduction
Research on aging is moving toward a stronger emphasis on health span versus life span – understanding those genetic and environmentally driven processes that lead to longer span of healthy life (Kaeberlein, 2016; Kennedy et al., 2014). This shift in emphasis has led to broad discussions in the aging research community of the definition of health. While an obvious simple Western definition of health might be “free of disease”, older individuals consistently point to abilities needed for independent living as the most important features of health – intact cognitive abilities, absence of chronic pain, and maintenance of physical function needed for routine activities, for example (Caffrey, Harris-Kojetin, Rome, & Sengupta, 2014; Leveille, Fried, & Guralnik, 2002; Stevens, Mack, Paulozzi, & Ballesteros, 2008). The manner in which these phenotypes relate to specific disease processes can be extremely complex, such that specific disease state may not be the best predictor of “health”. Of special interest to geriatricians is the set of phenotypes that not only predict independence but also predict an individual’s vulnerability to poor outcomes from late life challenges such as surgery, infection or psychosocial stress (Bandeen-Roche et al., 2006; Fried et al., 2001; Kennedy et al., 2014).
When the endpoints or phenotypes being measured to define health span become global, the application of animal models becomes more challenging. Death as an endpoint or phenotype has been powerfully used in various animal models exploring the factors controlling life span. However, translation and application of definitions of “health” between animals and humans is decidedly more complex. The most effective animal model for integrating complex phenotypes and translating findings from non-vertebrates and rodents to humans is a nonhuman primate. No other type of animal provides the combination of physiological, immunological, cognitive and psychosocial traits that are needed to reach a fully integrated understanding of health span that can be translated to humans.
Aging studies of nonhuman primates are hindered by the long life span of this taxonomic group. Therefore, there has been considerable interest in the last decade in understanding aging in the smallest, and therefore shortest lived primates (Austad, 1997; Tardif et al., 2008; Tardif, Mansfield, Ratnam, Ross, & Ziegler, 2011). Common marmosets (Callithrix jacchus) are small monkeys (300-400 grams) that begin puberty before one year of age and reach sexual maturity at 1.7 years(Tardif et al., 2008). The average lifespan for a captive marmoset is approximately 9-10 years and the maximum lifespan is around 21 years (Ross, Davis, Dobek, & Tardif, 2012; Tardif et al., 2011). Marmosets can be considered aged at around 8-10 years of age, based upon changes in behavior, appearance and pathology (e.g. amyloid deposition). Contrast this with the lifespan of a macaque or baboon, in which the average life span often exceeds 15-20 years and old age is not reached until 25-30 years (Hoffman, Higham, Mas-Rivera, Ayala, & Maestripieri, 2010; Maestripieri & Hoffman, 2011). In addition, breeding marmosets are routinely maintained in “nuclear” family groups, with an identifiable dam and sire and up to four litters of their twin offspring – this social arrangement closely mirrors that seen in the wild. This social structure offers an advantage in the use of this species in genetics studies as the dam and sire of each individual produced in the National Primate Research Center populations is known, making pedigree assessment and use easily manageable. Also, small body size combined with a normally small social network means that complex activities can be effectively measured within the animals’ normal caging/social environment. Because of their small size and associated shorter life span, marmosets have great potential as a species in which to better understand primate aging in a controlled environment. An initial characterization of aging in marmosets found that aged animals had decreased fat mass and decreased serum albumin values when compared to young healthy adults (Ross et al., 2012). There is also evidence that aged marmosets are more likely to suffer from cardiac and chronic kidney disease than are young marmosets (Ross et al., 2017; Ross et al., 2012; Tardif et al., 2011). While this previous work evaluated a few phenotypes of interest in health span assessment a broader assessment of healthy aged and young comparison animals is necessary to define aging in the marmosets.
This paper describes a cross-sectional study that compares, in young and old marmosets, a comprehensive array of phenotypes designed to capture health span. The phenotypes fall into five broad categories or domains. Two of these domains include those traits that most often underlie assessments of wellness in aged human populations: mobility and cognition. Three additional domains include those physiological systems that underlie the most common age-related or chronic diseases in humans: metabolic, homeostatic, and immune function. In this study we specifically evaluate daily activity levels using an actimeter and observation of daily behavior to assess mobility. To evaluate cognition, we evaluated the animals’ performance on the detoured reach task which assesses executive function. Metabolic function was appraised by assessing body composition using quantitative magnetic resonance (QMR), response to an oral glucose challenge, and measuring basal metabolic rate via indirect calorimetry. Homeostatic function was determined by measuring blood pressure, and daily circadian activity patterns. Immune function and inflammatory environment were assessed by comparing circulating cytokine concentrations and T cell functionality. Finally, we compared histopathological findings from eight young and ten geriatric marmosets. These data are particularly valuable, as most previous examinations of age-related pathology in this species have used spontaneous deaths, therefore necessarily using a population that is moribund regardless of age. These samples, though limited in number, offer the first opportunity to examine overall pathology in healthy young and healthy geriatric individuals.
Methods
Subjects:
Marmosets were housed singly or as male-female pairs with visual, auditory and olfactory contact with other marmosets at the Southwest National Primate Research Center. Husbandry and care of the animals followed that described in (Layne & Power, 2003). All procedures were reviewed and approved by the SNPRC Institutional Care and Use Committee and followed the guidelines put forth by the American Society of Primatologists. Geriatric animals (n= 15 males, n= 14 females) ranged in age from 13.5 y- 19.8 y and weighed an average of 360 ± 29.1 g. Young animals (n=5 males, n=5 females) ranged in age from 2.6 y – 4y and weighed an average of 384.5 ± 34.7g.
Mobility
Behavioral/Locomotion Observations:
In order to evaluate daily behavioral activity, all subjects were observed weekly for a ten-minute assessment of general activity and locomotion. Instantaneous sampling on twenty second intervals was used to assess the subject’s location within the cage, whether the animal was moving, resting or eating. If the animal was paired whether they were near, far or in contact with their mate was also recorded on twenty second intervals. All occurrences sampling was used to record the number of times the subject leapt (all four limbs leaving the substrate as the animal jumped to a new location), self-groomed, scent marked or genital displayed; and for paired subjects, instances of grooming and mating were also recorded. The amount of time spent in a hanging or stretched position was also recorded for all occurrences (Ross et al., 2012). Animals were observed for at least 15 weeks, with a maximum collection of 29 weeks. From each observation the percentage of time noted for each of the instantaneous state behaviors, and the frequency of the all occurrence behaviors were calculated and averaged for young and geriatric individuals. Repeated measures multivariate ANOVA was used to compare behaviors in young and old marmosets.
Daily Activity (Actimeter):
Daily activity patterns were assessed for individuals randomly selected from the larger cohort, geriatric (n=6, 3 female and 3 male, average age 15.2 years) and young (n=7, 4 female and 3 male, average age 2.3 years). Mini actiwatches (CamNtech) were placed in a marmoset pouch (Lomar) on a modified ferret harness (Petco). Subjects were gradually habituated to the ferret harness backpacks over the course of three weeks, increasing time in the harness incrementally throughout training until 24 hours in the harness had been achieved. For these trials animals were separated from each other within the cage and placed in harnesses with the actiwatch in the pouch secured across the back of the animal. Animals remained in the harness for 48 hours of data collection during which normal husbandry and feeding continued. At the end of the trial the animals were captured in transfer boxes and the harnesses were removed. The miniwatches are data loggers that batch data in 15 second epochs. The activity counts from the first 15 minutes and last 15 minutes of the collection were removed from analysis as these represented handling and cage manipulation. Average counts per hour were calculated for the twelve hours of light (day) and twelve hours of dark (night) and statistically compared for old and young animals using a t-test.
Cognition
Detoured Reach:
In order to evaluate cognitive executive function in geriatric and young marmosets the detoured reach task (Dias, Robbins, & Roberts, 1996) was evaluated for n= 10 geriatric (5 male, 5 female, average age 15.4 years) and n=7 young subjects (4 male, 3 female, average age 2.6 years). One male geriatric subject and one male young subject were dropped from further testing due to failure to habituate to the test cage and the testing device. In order to decrease handling or capture stress during cognitive testing, and to test the animals in cages that had the same configuration each animal was trained to transfer from their home cage to a test cage to perform the detoured reach object retrieval task. The test cage was surrounded by an opaque barrier to prevent visual contact with other marmosets during the testing. Animals participated in one session each day and returned to their home cage immediately following each session.
The testing device was a clear acrylic five sided cube (2”x2”x2”) presented on an opaque platform attached to the side of the testing cage. A visual screen was used to obstruct the subject’s view of the apparatus while the presenter manipulated the cube. Each trial began with the removal of the visual screen and lasted for either thirty seconds or until the subject successfully retrieved the reward. The subject was presented with ten trials in each session of two training sessions during which the clear five sided cube was altered to be opaque (covered in white paper) and the opening faced the subjects throughout the entirety of each session. This training allowed the animals to visually assess the opening of the box and the placement of the marshmallow reward.
The testing sessions consisted of twenty trials in which the five sided clear cube was presented, with the location of the opening varied from front, left and right between trials. During initial testing each testing session consisted of randomized presentations with each side (left, right, front) equally presented in the trials. Success was defined as the subject retrieving the reward. An unsuccessful attempt was specifically defined for this report as an animal reaching forward and making contact with the solid face of the box. Criterion was defined as a subject successfully retrieving 18/20 (≥90%) rewards in ≤6 seconds.
After the initial five testing sessions there was an evident decline in engagement and refusal to participate observed in both the young and geriatric subjects. In order to get the animals to reengage with the testing apparatus subjects were given two reengagement training sessions. The first reengagement session was designed to promote the subjects’ engagement with the platform by removing the acrylic cube and simply presenting the subjects with the marshmallow reward on the platform immediately in front of the animal. The second reengagement session was designed to promote the subjects’ engagement with the acrylic cube. Subjects were presented with twenty trials during this session with the rewards displayed outside of the cube directly in front of the opening. For both reengagement sessions each trial lasted a maximum of 30 seconds. All subjects reengaged with the task and testing was begun in a modified manner. The testing format was altered to more closely mimic the design by (Dias et al., 1996) in which the location of the reward varied from placement at the center of the cube and the edge of the opening. The first three trials of every session began with the cube facing forward and then randomized presentation (left, right and center) for a total of twenty trials each session. Old and young subjects were compared for the following variables: number of sessions to reach criterion, number of unsuccessful reaches when the box was turned, and number of successful reaches following an unsuccessful reach when the box was turned, and number of persistent reaches in which an animal succeeded to one side and in the following trial continued to reach to the side to which it had previously succeeded.
Metabolic Function
Body composition:
Marmoset lean and fat mass was assessed for all subjects via quantitative magnetic resonance (QMR) imaging using an Echo MRI unit (Tardif et al., 2009). Unsedated animals (n=29 geriatric, n= 10 young) were placed in a plastic tube which was then inserted into the magnetic chamber with scans taking less than 2 minutes on average for each animal. Animals were weighed by placing a scale within the cage and rewarding the animals for maintaining position on the scale. Body composition for young and old animals were compared using repeated measures multivariate ANOVA.
Oral Glucose Tolerance Test:
Geriatric (n=10, 5 male and 5 female, average age 15.46 years) and young (n=10, 5 male and 5 female, average age 2.5 years) were fasted overnight (approximately 4 pm to 9 am) prior to an oral glucose tolerance test (Tardif et al., 2009), and placed in a restraint device used for blood collection to which they had previously been habituated. An EDTA coated needle and syringe were used to collect 0.5 ml of blood from the femoral vein for the baseline bleed. The animals were then dosed orally with a 40% dextrose solution receiving a calculated glucose dose equal to 0.5% of their current body weight. Subjects remained in the restraint for a 15 and 30-minute post dose blood sample drawn from the tail vein via an EDTA coated butterfly needle. Subjects were removed from the restraint device following the 30-minute sample and placed in a transport box until the 60-minute sample, and this was repeated for the 120-minute sample. Blood was analyzed using a glucometer for the 0, 15, 30, 60 and 120 minute time sampling. Following the 120-minute bleed the animals were returned to their home cage and fed.
Indirect calorimetry:
In order to evaluate the subjects’ metabolic function geriatric (n=10, 5 male and 5 female, average age 15.46 years) and young (n=10, 5 male and 5 female, average age 2.5 years) were placed in a transport box within a respirometry Fox Box chamber. A ten-minute baseline recording was done prior to placing the marmoset in the chamber, this baseline recording allowed the equipment to stabilize to room CO2 volumes. The animals were placed inside the respirometry unit and data was recorded every 5 minutes for one hour and 45 minutes. After the removal of the animal from the chamber a further 25 minutes of post-baseline data was collected. Oxygen and CO2 concentration, as well as flow rate and temperature were recorded throughout and used to calculate VO2 for each individual (Power, Tardif, Power, & Layne, 2003). VO2 for young and old individuals were compared using ANCOVA, with body mass as the covariate. One individual had a recorded VO2 of 566 ml O2/hour and as a statistical outlier was removed from further analysis.
Homeostasis
Blood Pressure:
All of the subjects were evaluated twice during the duration of this study for blood pressure using a high definition oscillometric device (VET HDO monitor MD PRO Marmoset; S+B medVET GmbH, Babenhausen, Germany) following (Mietsch et al., 2016). Marmosets were held in a gloved hand in a simulated quadrupedal position which allowed access to the right leg for each measurement. Three to five measurements were recorded for each animal. Measurements with normalized pressure waves and output curves were used to assess a daily average diastolic and systolic pressure which was then compared between young and old marmosets with a t-test.
Kidney function was assessed through clinical biomarkers as well as ex vivo tissue analyses. These extensive studies are reported in (H. J. Lee et al., in press).
Circadian patterns:
The time an individual settled at rest for the evening and arose in the morning was determined using the activity counts from the actimeter measurements described above. The time of rest at night was determined as the time nearest to lights out (7 pm) that had zero counts recorded for at least twenty15 second epochs (ie: at least 5 minutes of no activity). Time to arouse in the morning was determined as the epoch nearest to lights on (7 am) for which there were activity counts higher than 50 in a single 15 second epoch. Arousal and time to rest were compared between young and geriatric animals with a t-test.
Immune Function:
Immune function and inflammatory environment were assessed for all of the subjects by evaluating circulating cytokine concentrations measured using the Luminex system as validated for marmosets and other nonhuman primates (Giavedoni, 2005; Höglind et al., 2017). The Luminex system is a bead based bench top flow cytometer that can simultaneously quantify up to 100 molecules at a time from 0.5 ml femoral blood collected into EDTA coated tubes. The assay included evaluation of the following 11 analytes: interferon gamma (IFN-γ), interleukin-1 beta (IL-1β), IL-1 receptor antagonist (IL-1RA), monocyte chemoattractant protein 1 (MCP-1, CCL2), macrophage migration inhibitory factor (MIF), monokine induced by gamma interferon (MIG, CXCL9), macrophage inflammatory protein 1-alpha (MIP-1α, CCL3), MIP-1b (CCL4), regulated on activation, normal T cell expressed and secreted (RANTES, CCL5), tumor necrosis factor-alpha (TNF-α), and soluble intercellular adhesion molecule 1 (sICAM-1). Cytokine concentrations for young and geriatric animals were evaluated with multivariate ANOVA.
Lymphocyte phenotyping:
Phenotypic characterization of marmoset PBMCs was performed for geriatric (n=10, 5 male and 5 female, average age 15.46 years) and young (n=8, 4 male and 4 female, average age 2.5 years) by multicolor flow cytometry using direct immunofluorescence. Aliquots of 100 ml of EDTA whole blood were directly incubated with antibodies for 20 minutes at room temperature; red blood cells were lysed with ACK, and cells were then washed twice with PBS and fixed with 1.6% methanol-free formaldehyde before analysis in a CyAn LDP flow cytometer (Beckman-Coulter). The antibodies used for this analysis were conjugated to fluorescein isothiocyanate (FITC), Phycoerythrin (PE), Peridinin-chlorophyll-cyanin 5.5 (PerCP-Cy5.5), Phycoerythrin-cyanin 5.1 (PC5), Phycoerythrin-cyanin 7 (PC7), Pacific Blue, BD Horizon V500, Allophycocyanin (APC) or Alexa Fluor 700. Antibodies included in this study were: CD3 (clone SP34.2), CD4 (clone L200) and HLA-DR (clone G46.6/L243) from BD-Biosciences; CD14 (clone 322A-1 (My4), CD159a (NKG2A; clone Z199), CD20 (clone H299(B1)), CD335 (NKp46; clone BAB281) and CD337 (NKp30; clone Z25) from Beckman-Coulter; CD16 (clone 3G8), CD8 (clone HIT8a), CD86 (clone IT2.2) from Biolegend; and CD159c (NKG2C;clone 134522) from R&D Systems.
For analyses, lymphocytes were gated based on their characteristic forward and side scatter pattern, followed by T-cell selection using a second gate on the CD3-positive population. Thus, CD8 T cells were defined as CD8+/CD3+ and CD4 T cells as CD4+/CD3+. Natural Killer cells (NK) were defined as CD3−/CD20−/CD14− and analyzed by the expression of NK cell markers CD16+, CD8, NKG2A, NKG2C, NKp30 and NKp46. B cells were defined as CD20+/CD3−/CD14−. Values were compared between young and geriatric marmosets using multivariate ANOVA.
Necropsy and Histopathology
Following the cross-sectional assessment of the above measurements a few animals (geriatric n=10, 5 male and 5 female, average age 15.46 years) and young (n=8, 4 male and 4 female, average age 2.5 years) were selected randomly from the subject pool for tissue collection and further evaluation. Animals were euthanized and tissues were flash frozen and stored at −80°C until further assessment. The Southwest National Primate Research Center pathology department performed a post-mortem necropsy immediately after euthanasia and histological assessment of sample tissues. Samples of heart (left ventricle), lung (right apical lobe), kidney, urinary bladder, salivary gland, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, pancreas, liver, gallbladder, adipose tissue (omentum), skeletal muscle (Sartorius muscle), pituitary gland, thyroid glands, parathyroid glands, adrenal glands, testes, and axillary, mesenteric and hilar lymph nodes were fixed in 10% neutral buffered formalin, processed conventionally, paraffin embedded, cut at 5 microns, stained with hematoxylin and eosin, and reviewed by light microscopy by a board certified anatomic pathologist. Aorta, skin, gingiva, and bone were collected and examined via light microscopy on selected cases. Brain and female reproductive organs were not examined histologically. Kidney histopathology is detailed in a topic-dedicated manuscript (H. J. Lee et al., in press).
Statistical Analyses:
All analyses were evaluated at an α=0.05 and Bonferroni corrections were used.
Results
Mobility:
Behavior:
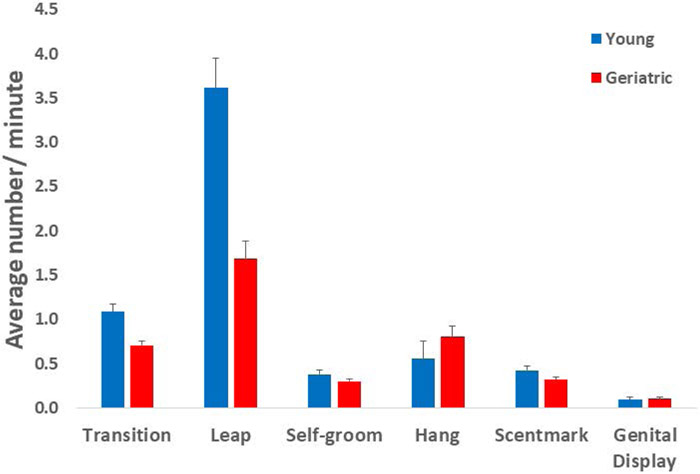
A repeated measures comparison of averaged behaviors for two months across the observation time to a maximum of eight months revealed no significant changes in behavior associated with time, therefore, all behaviors were averaged for each individual across all their observation times. There were no significant differences between young and geriatric marmosets for time spent moving, at rest or eating. The geriatric animals made significantly fewer transitions between quadrants of the cage than did young animals (F(1, 37)=14.126, p = 0.001), as well as performing fewer leaps (F(1,37) = 24.48, p = 0.0001) (Fig 1).
Figure 1-.
Marmoset activity behaviors recorded during ten-minute behavioral observations. The average number per minute (± SE) of transitions between quadrants of the cage, leaping, grooming, hanging (stretch), scent mark and genital displays. Geriatric marmosets made significantly fewer transitions between quadrants of the cage than did young animals (F(1, 37)=14.126, p = 0.001), as well as performing fewer leaps (F(1,37) = 24.48, p = 0.0001).
Daily activity:
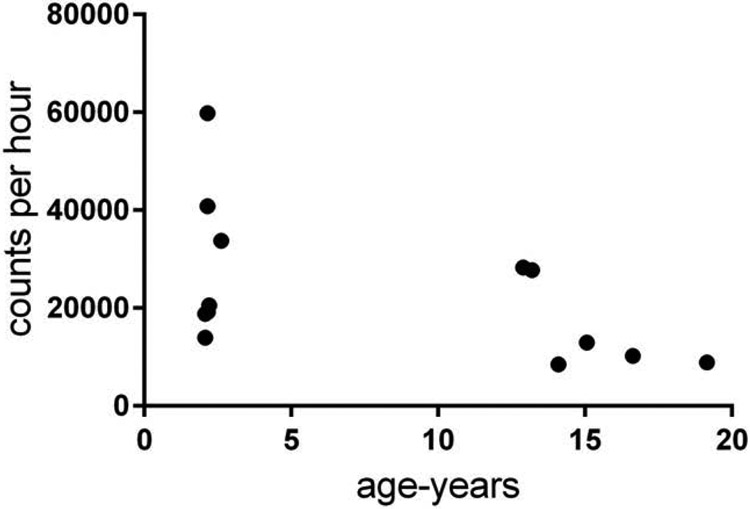
Activity, measured as daily activity counts per hour from an actimeter are presented in Fig 2. While, the average activity counts of the older animals was lower than the young, there was considerable variation in the young animals and the difference in central tendency was not statistically significant at p< 0.05 (t = 1.774, p=0.10).
Figure 2 –
Daily activity counts as recorded by an actimeter worn by the animals for 48 hours, there were no significant differences between geriatric and young animals (t = 1.774, p=0.10).
Cognition:
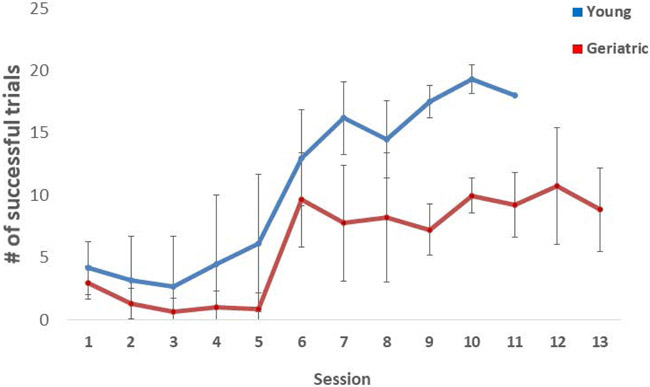
Fig 3 illustrates the performance of young and geriatric marmosets on the detoured reach task, as number of correct reaches within the allotted time per session. Young individuals were significantly more successful at the task as defined by number of sessions to reach criterion (t-test, p=0.002). By session 10, all young subjects had reached the criterion of 90% success, while no geriatric animal had reached criterion. Geriatric subjects were extended out to 13 sessions, but by session 13, only 1/9 geriatric subjects had reached criterion. Unsuccessful attempts, the number of times the animals bonked into the solid surface of the box when the box was turned, did not differ significantly between the young and geriatric subjects (t-test, p=0.06). However, importantly the number of successful grabs following a failed attempt differed between young (42.7±8.7) and geriatric subjects (25.9±10.4) (t-test, p=0.005). Geriatric animals had a significantly higher persistence to the side to which they had successfully retrieved a reward in the previous trial (young = 2.5±1.6, geriatric 6.5±5.2, t-test, p=0.05).
Figure 3 –
Cognitive function assessed via the detoured reach cognitive function task. The average number of successful trials (± SE) during each session for geriatric and young animals. Session 6 was the first test session following the two reengagement sessions and the altering of the presentation order for the test trials. Young animals were significantly more successful at the task (F(7,91)=2.495, p=0.022).
Metabolic function:
Body Composition:
There were no significant differences between geriatric and young animal body composition measures (results not shown).
Oral glucose challenge test:
Young and geriatric marmosets did not differ in individual time point values or area under the curve in an oral glucose tolerance test (results not shown).
Indirect calorimetry:
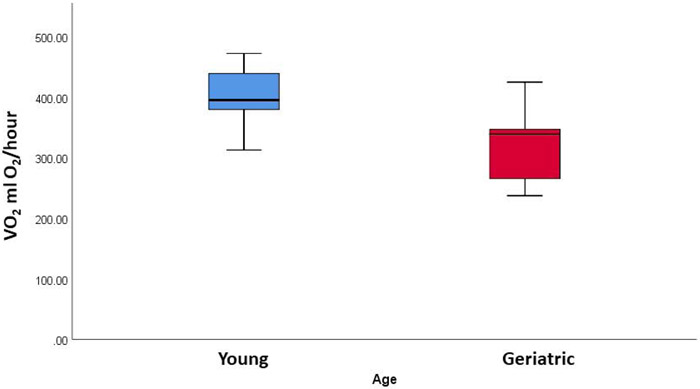
VO2 max was compared between the young and old marmosets with body mass as a covariate. One old animal was a found to be a statistical outlier with values over 550 ml O2/hour (more than 1.5IQ above the next value) and was eliminated from the analysis. The geriatric marmosets had a significantly reduced VO2 (326 ml O2/hour ± 63) compared to the young marmosets (400 ml O2/hour ± 47) (F(1,15) = 4.844, p = 0.044) (Fig 4).
Figure 4 –
Average VO2 (ml O2/hour) for young and geriatric marmosets as measured by indirect calorimetry. Geriatric animals had significantly reduced VO2 (F(1,15) = 4.844, p = 0.044).
Homeostasis:
Blood pressure:
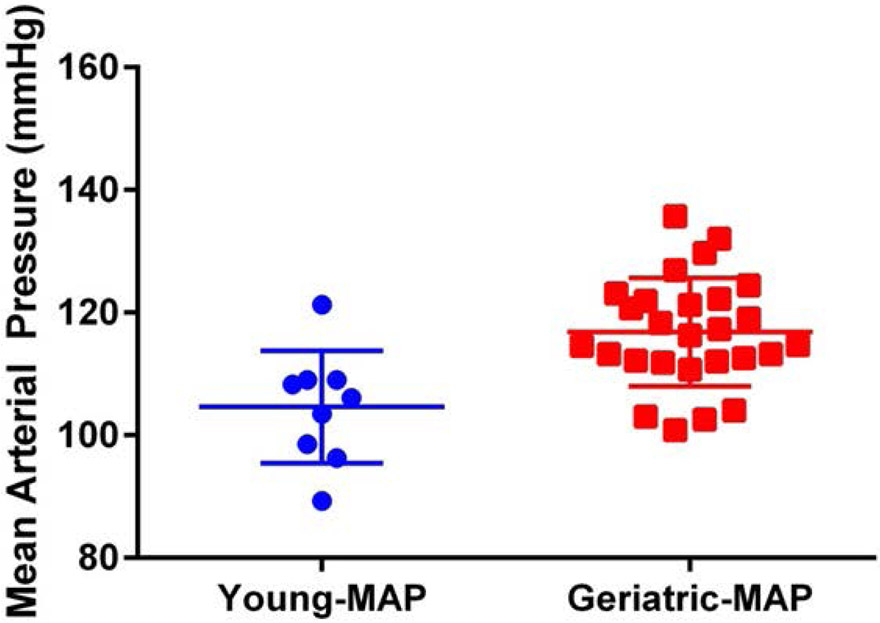
Young and geriatric marmosets differed significantly in diastolic and mean arterial pressure, with older animals having higher diastolic and mean arterial pressure (t= 3.562, df = 34, p = 0.0011) – see Fig 5.
Figure 5 –
Mean arterial pressure was significantly higher for geriatric marmosets than for young marmosets (t= 3.562, df = 34, p = 0.0011).
Circadian patterns:
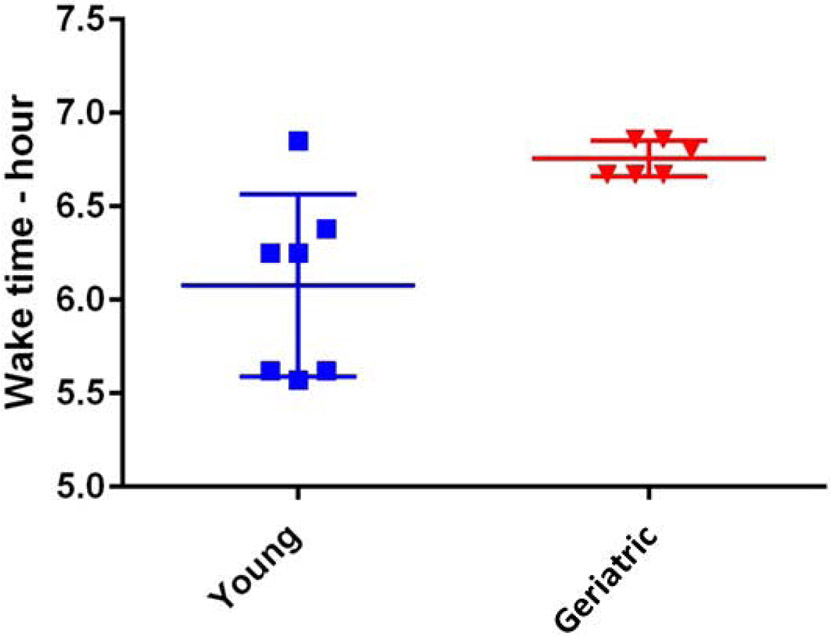
Young and geriatric marmosets did not differ in time to retire, as evidenced by actimeter measures. They did, however, differ in time to become active, with older marmosets becoming active significantly later than young individuals (t=3.333, df = 11, p=0.0067) – see Fig 6.
Figure 6 –
Young animals displayed a wide range of times for arousal in the morning as compared to the later time and narrower time range of arousal for the geriatric animals (t=3.333, df = 11, p=0.0067).
Immune Function:
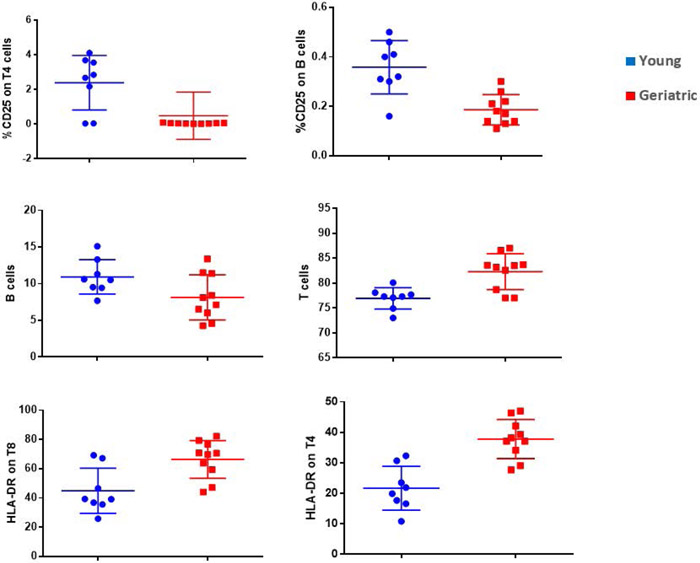
Only a few circulating cytokines were found to significantly differ between young and geriatric animals. The cytokines of particular interest IL8, IL13, TNFa, and C reactive protein did not differ significantly with age in the marmosets. MIP was found to be significantly higher in older animals (old = 22.875+2.86, young = 8.294+3.63 pg/mL) (F (1,38)=9.94, p=0.003). RANTES was found to be significantly higher in older animals (old = 0.777+0.07, young = 0.53+0.09 pg/mL) (F(1,38)=4.905, p=0.03). Finally, sICAM was also found to be significantly higher in older animals (old = 187.8+8.2, young = 154.6+10.4 pg/mL) (F(1,38)=6.32, p=0.016). Analysis of cellular function by flow cytometry revealed that older animals had significantly lower expression of proliferation markers as measured by percent CD25 in B cells (F(1,16)=18.19, p=0.001), and T4 cells (F(1,16)=7.58, p=0.014). Older animals also expressed heightened pro-inflammatory states as displayed by increased activation of HLA-DR in CD8 cells (F(1,16)=10.29, p=0.005) and CD4 cells (F(1,16)=25.27, p=0.000) Older animals also had a lower percentage of B cells (F(1,16)=4.51, p=0.05) and higher percentage of T-cells F(1,16)=13.8, p=0.002) (Fig 7.).
Figure 7 -.
Analysis of cellular function by flow cytometry revealed that older animals had significantly lower expression of proliferation markers as measured by CD25 in B cells (F(1,16)=18.19, p=0.001), and T4 cells (F(1,16)=7.58, p=0.014). Older animals also expressed heightened pro-inflammatory states as displayed by increased activation of HLA-DR in CD8 cells (F(1,16)=10.29, p=0.005) and CD4 cells (F(1,16)=25.27, p=0.000). Older animals also had a lower percentage of B cells (F(1,16)=4.51, p=0.05) and higher percentage of T-cells F(1,16)=13.8, p=0.002).
Necropsy and Histopathology:
All young adult and geriatric animals exhibited adequate weight, hydration status, skeletal muscle mass, and adipose stores. All animals were free of external and internal parasites on gross and microscopic examination. The thyroid gland of one young adult female and one young adult male marmoset exhibited symmetrical enlargement. There was mild diffuse gas distension of the small intestine in one male young marmoset. All other organs within this cohort were grossly unremarkable.
The geriatric cohort exhibited a wide range of gross findings in multiple organ systems. Two males and three females had atherosclerotic lesions of the aortic arch involving thoracic and abdominal portions of the descending aorta. Lesions were at different stages of severity ranging from early lesions characterized by irregular yellow thickened raised areas (fatty streaks) to advanced stages characterized by induration, irregular, white gritty areas and a significant loss of vascular elasticity. Four females had sessile white thickened areas along the dorsal surfaces of the caudal lung lobes that on histology corresponded to areas of fibrosis and osseous metaplasia. There was unilateral enlargement of right thyroid gland of two male marmosets. The liver of a female marmoset was firm and exhibited an enhanced reticular pattern; hepatic amyloidosis was identified histologically, and poorly formed stool was also noted in this marmoset. Gingivitis and periodontitis with loosening of the maxillary molars was observed in a female marmoset. This same individual also exhibited facial swelling and dermatitis on histopathology. There was radiographic evidence of degenerative joint disease involving the coxofemoral joint in a female marmoset resulting in reduced range of joint motion on physical exam. Two female marmosets had segmental areas of cortical bone thickening involving the ribs.
The morphologic diagnoses obtained from tissue evaluation of standardized collection and selected abnormal tissues are summarized in Table 1 and Table 2. The body systems with the most diagnoses were the endocrine (n = 66), digestive (n = 48), lymphatic (n = 24), cardiovascular (n = 15), reproductive (n = 5), respiratory (n = 5), and musculoskeletal (n = 3). The organs with the most diagnoses were the adrenal gland (n = 32), lymph node (n = 24), thyroid gland (n = 24), liver (n = 22), heart (n = 10), exocrine pancreas (n = 10), and gallbladder (n = 6). The most common diagnoses were adiposity in various tissues (n = 27), hepatic glycogenosis (n = 18), adrenal cortical lipid vacuolation (n = 11), extramedullary hematopoiesis (n = 11), cardiac fibrosis (n = 9), inflammation in various tissues (n = 9), and tattoo pigment within lymph nodes (n = 9). The kidney had the most morphologic diagnosis and is discussed in a separate dedicated manuscript (H. J. Lee et al., in press).
Table 1:
Histopathology results for young and geriatric marmosets. Lesions by system, organ and morphologic diagnosis.
| System | Organ | Diagnosis | Total (n=18) |
Young Total (n=8) |
Young Male (n=4) |
Young Female (n=4) |
Geriatric Total (n=10) |
Geriatric Male (n=5) |
Geriatric Female (n=5) |
|---|---|---|---|---|---|---|---|---|---|
| Endocrine | 66 | 18 | 10 | 8 | 48 | 24 | 24 | ||
| Adrenal gland | Total Adrenal | 32 | 7 | 6 | 1 | 25 | 14 | 11 | |
| Cortical lipid vacuolation | 11 | 4 | 4 | 0 | 7 | 4 | 3 | ||
| Adiposity | 6 | 0 | 0 | 0 | 6 | 1 | 5 | ||
| Extramedullary hematopoiesis | 10 | 3 | 2 | 1 | 7 | 4 | 3 | ||
| Cortical atrophy | 1 | 0 | 0 | 0 | 1 | 1 | 0 | ||
| Lymphocytic aggregates | 1 | 0 | 0 | 0 | 1 | 1 | 0 | ||
| Calcification | 3 | 0 | 0 | 0 | 3 | 3 | 0 | ||
| Thyroid gland | Total Thyroid | 24 | 10 | 4 | 6 | 14 | 7 | 7 | |
| Multinodular hyperplasia | 2 | 0 | 0 | 0 | 2 | 2 | 0 | ||
| C-cell hyperplasia | 3 | 0 | 0 | 0 | 3 | 1 | 2 | ||
| Follicular ectasia/distention | 4 | 1 | 1 | 0 | 3 | 1 | 2 | ||
| Lymphocytic aggregates | 4 | 4 | 1 | 3 | 0 | 0 | 0 | ||
| Thymic rest | 4 | 4 | 1 | 3 | 0 | 0 | 0 | ||
| Adiposity | 7 | 1 | 1 | 0 | 6 | 3 | 3 | ||
| Pancreas (Endocrine) | Islet Hyperplasia | 5 | 1 | 0 | 1 | 4 | 1 | 3 | |
| Pituitary gland | Cyst | 3 | 0 | 0 | 0 | 3 | 1 | 2 | |
| Parathyroid gland | Total Parathyroid | 2 | 0 | 0 | 0 | 2 | 1 | 1 | |
| Cyst | 1 | 0 | 0 | 0 | 1 | 0 | 1 | ||
| Hyperplasia | 1 | 0 | 0 | 0 | 1 | 1 | 0 | ||
| Urinary1 | 49 | 19 | 8 | 11 | 30 | 15 | 15 | ||
| Kidney | Total Kidney | 47 | 17 | 8 | 9 | 30 | 15 | 15 | |
| Mesangial expansion | 17 | 7 | 3 | 4 | 10 | 5 | 5 | ||
| Tubular ectasia and proteinosis | 15 | 5 | 2 | 3 | 10 | 5 | 5 | ||
| Interstitial nephritis | 14 | 5 | 3 | 2 | 9 | 4 | 5 | ||
| Extramedullary hematopoiesis | 1 | 0 | 0 | 0 | 1 | 1 | 0 | ||
| Urinary bladder | Inflammation | 2 | 2 | 0 | 2 | 0 | 0 | 0 | |
| Digestive | 48 | 16 | 8 | 8 | 32 | 13 | 19 | ||
| Liver | Total Liver | 21 | 8 | 4 | 4 | 13 | 7 | 6 | |
| Glycogenosis | 18 | 8 | 4 | 4 | 10 | 5 | 5 | ||
| Inflammation | 3 | 0 | 0 | 0 | 3 | 2 | 1 | ||
| Pancreas (Exocrine) | Total Pancreas (Exocrine) | 10 | 3 | 2 | 1 | 7 | 3 | 4 | |
| Adiposity | 4 | 0 | 0 | 0 | 4 | 2 | 2 | ||
| Inflammation | 4 | 3 | 2 | 1 | 1 | 0 | 1 | ||
| Atrophy | 1 | 0 | 0 | 0 | 1 | 0 | 1 | ||
| Exocrine hyperplasia | 1 | 0 | 0 | 0 | 1 | 1 | 0 | ||
| Gallbladder | Cholecystitis | 6 | 1 | 0 | 1 | 5 | 3 | 2 | |
| Duodenum | Total Duodenum | 5 | 2 | 0 | 2 | 3 | 0 | 3 | |
| Enteritis | 4 | 2 | 0 | 2 | 2 | 0 | 2 | ||
| Brunner's gland ectasia | 1 | 0 | 0 | 0 | 1 | 0 | 1 | ||
| Jejunum | Enteritis | 3 | 1 | 1 | 0 | 2 | 0 | 2 | |
| Cecum | Typhlitis | 2 | 0 | 0 | 0 | 2 | 0 | 2 | |
| Omentum | Ectopic spleen | 1 | 1 | 1 | 0 | 0 | 0 | 0 | |
| Lymphatic | Lymph Node | Total Lymph Node | 24 | 9 | 1 | 8 | 15 | 9 | 6 |
| Adiposity | 9 | 2 | 0 | 2 | 7 | 5 | 2 | ||
| Tattoo pigment | 9 | 1 | 0 | 1 | 8 | 4 | 4 | ||
| Hemorrhage | 3 | 3 | 1 | 2 | 0 | 0 | 0 | ||
| Histiocytosis | 3 | 3 | 0 | 3 | 0 | 0 | 0 | ||
| Cardiovascular | 15 | 1 | 0 | 1 | 14 | 6 | 8 | ||
| Heart | Total Heart | 10 | 1 | 0 | 1 | 9 | 4 | 5 | |
| Fibrosis | 9 | 1 | 0 | 1 | 8 | 4 | 4 | ||
| Adiposity | 1 | 0 | 0 | 0 | 1 | 0 | 1 | ||
| Aorta | Atherosclerosis | 5 | 0 | 0 | 0 | 5 | 2 | 3 | |
| Reproductive 2 | Total Prostate Gland | 5 | 0 | 0 | N/A | 5 | 5 | N/A | |
| Prostate gland | Cystic atrophy | 5 | 0 | 0 | N/A | 5 | 5 | N/A | |
| Respiratory | Lung | Total Lung | 5 | 1 | 1 | 0 | 4 | 0 | 4 |
| Pleural Osseous Metaplasia | 4 | 0 | 0 | 0 | 4 | 0 | 4 | ||
| Alveolar septal calcification | 1 | 1 | 1 | 0 | 0 | 0 | 0 | ||
| Musculoskeletal | 3 | 0 | 0 | 0 | 3 | 0 | 3 | ||
| Rib | Focal osteosclerosis | 2 | 0 | 0 | 0 | 2 | 0 | 2 | |
| Hip | Osteoarthritis | 1 | 0 | 0 | 0 | 1 | 0 | 1 | |
| Multisystemic | 1 | 0 | 0 | 0 | 1 | 0 | 1 | ||
| Liver,adrenal, kidney | Systemic amyloidosis | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
Kidney histopathology is described in a topic dedicated manuscript (Lee et al in press)
Reproductive system results are from 4 young male and 5 geriatric male animals only.
Table 2:
The most common diagnoses in young and geriatric marmosets across organ systems.
| Young | Geriatric | |
|---|---|---|
| Diagnosis | Total (n=8) |
Total (n=10) |
| Adiposity | 3 | 10 |
| Tubular ectasia and proteinosis | 5 | 10 |
| Mesangial expansion | 7 | 10 |
| Glycogenosis | 8 | 10 |
| Interstitial nephritis | 5 | 9 |
| Fibrosis | 1 | 8 |
| Extramedullary hematopoiesis | 3 | 8 |
| Cortical lipid vacuolation | 4 | 7 |
| Atherosclerosis | 0 | 5 |
| Cystic atrophy | 0 | 5 |
| Cholecystitis | 1 | 5 |
| Cyst | 0 | 4 |
| Pleural Oseous Metaplasia/Calcification | 0 | 4 |
| Islet Hyperplasia | 1 | 4 |
| Enteritis | 3 | 4 |
| Inflammation | 5 | 4 |
Several lesions were encountered much more frequently in the geriatric cohort, including: adiposity in the lymph node, thyroid gland, exocrine pancreas, or heart; cardiac fibrosis; tattoo pigment within lymph nodes; renal tubular ectasia and proteinosis; extramedullary hematopoiesis of the adrenal gland; aortic atherosclerosis; cystic atrophy of the prostate gland (males); interstitial nephritis; cholecystitis; pituitary cysts; pleural osseous metaplasia/calcification (females); renal glomerular mesangial expansion; hyperplasia of the islets of Langerhans; and c-cell hyperplasia.
Thymic rests within the thyroid gland, lymph node hemorrhage, and lymph node histiocytosis were more often observed in young adult animals. No significant microscopic lesions were identified in the esophagus, salivary gland, stomach, ileum, colon and skeletal muscle. Neoplastic disease was not identified in either group of animals.
Discussion
In order to further the development of the marmoset as a model for aging and health span studies it is necessary to further refine the characterization of healthy aging marmosets. This cross sectional examination of healthy young and old marmosets evaluated phenotypic markers of particular interest for aging and health span studies focused upon five domains that are particularly important not only for characterizing the marmoset as a translational model for aging, but also to define health parameters and healthy aging. While many of the markers assessed in this study did not differ between old and young marmosets, there were many phenotypes that differed significantly and may be of particular interest for examining healthspan.
In humans neurological change associated with age results in disrupted executive functioning (Dahle, Jacobs, & Raz, 2009; Gunning-Dixon & Raz, 2000; Raz, Gunning-Dixon, Head, Dupuis, & Acker, 1998; Raz & Rodrigue, 2006). Executive functioning refers to a collection of cognitive processes such as goal directed attention, task switching, and response inhibition (Gunning-Dixon & Raz, 2000; Raz et al., 1998). Assessing declines in executive functioning in nonhuman primate models has been challenging due to the amount of time that is required to train animals to perform computerized performance tasks often used to assess human cognition (LaClair & Lacreuse, 2016; Munger, Takemoto, Raghanti, & Nakamura, 2017; Nakamura et al., 2018; Ridley, Cummings, Leow-Dyke, & Baker, 2006). The detoured reach task is an assessment of executive functioning as it assesses the individual’s ability to shift tasks when their path to the reward is blocked, as well as their ability to control the impulse to reach directly forward to the reward (Dias et al., 1996). All of the young animals in our assessment were able to reach criterion in this task, 90% success retrieval of the reward in less than six seconds, whereas only one geriatric animal was able to reach criterion even when given more testing sessions. There was some difficulty during the training and testing of this task due to marmosets disengaging from performance, probably due to increased difficulty in the task when compared to what had been previously described (Dias et al., 1996). In order to counteract their lack of engagement in the trials, all of the animals, young and old, participated in re-engagement sessions that are believed to have encouraged the animals to engage with the apparatus. The session presentations were altered to more closely mimic those of (Dias et al., 1996) which had been described in the development of the task. Following these changes the animals, young and old, remained engaged with the task during testing and the young animals went on to quickly reach criterion. The older animals, while engaged, were unable to reach criterion with the task. Although older animals maintained engagement with the task they were more likely to reach straight forward to “bonk” against the barrier, rather than shifting to a successful grab. The number of bonks did not significantly differ between older and younger animals suggesting that the geriatric animals did not simply lack motivation or the ability to reach, they instead lack the ability to shift to success. This was further supported with the finding that older animals were more likely to persist to the side that they were successful at in the previous task, and less likely to shift to success. All older animals were seen to be able to reach around to retrieve the reward, suggesting that the differences between animals was not due to motor ability restrictions but rather cognitive impulse control and ability to switch their task as the cube moved. Failure to reach criterion by the older animals was a failure to retrieve the number of rewards correctly rather than simply being a failure to retrieve the criterion number of rewards in the allotted time. It remains possible that the differences between the young and aged marmosets was an underlying difference in motivation to work hard and to continue to make attempts to achieve the goal, but we believe there is sufficient evidence to suggest that this assessment revealed significant impairment in cognitive executive functioning rather than simply impaired mobility in aged marmosets. This supports a previous report of aged marmosets displaying significant impairment in learning of a simple discrimination task as well as reduced ability to switch tasks during reversal learning (Munger et al., 2017).
Human aging is often characterized by increased prevalence of cardiovascular disease associated with hypertension and atherosclerosis (Bluher, 2008; Browner et al., 2004; Kloting & Bluher, 2005). Measures of blood pressure and hypertension are often a useful proxy to estimate the effects of age on cardiovascular health. Marmosets have previously been found to have significant age effects on mean arterial pressure and diastolic pressure, with no significant differences in systolic pressure (Mietsch et al., 2016; Mietsch & Einspanier, 2015). We have also found that mean arterial pressure and diastolic pressure were significantly associated with age, and no relationship with age was found for systolic pressure. The oldest marmoset measured in the previous report was 17 years old, and there were only three animals in that study within our geriatric range. Therefore, our findings strengthen the conclusion of a relationship between age and increasing MAP and diastolic pressure in the marmoset.
The ability to use blood pressure measures as a noninvasive technique to assess health in the marmosets will greatly enhance the ability of researchers to assess homeostatic health in aging marmosets. There is variation in the susceptibility to develop atherosclerosis amongst different non-human primate species (Chamanza, 2011; Clarkson, Lehner, Bullock, Lofland, & Wagner, 1976; Malinow, 1965; Shelton, Clarkson, & Kaplan, 2012; Simmons, 2016). Spontaneous arteriosclerosis, atherosclerosis and arteriopathies in wild and laboratory bred common marmosets are rare and sporadically reported (Bleyer, Kunze, Gruber-Dujardin, & Mätz-Rensing, 2017; Chamanza, 2011; David, Dick Jr, & Hubbard, 2009; Dreizen, Levy, & Bernick, 1973; Shelton et al., 2012). Bleyer et. al. (2017) described an arteriopathy of pulmonary vasculature affecting 3/328 adult common marmosets (>30-months-old) characterized by hypertrophy and hyperplasia of the tunica intima and media respectively and mineralization of the tunica media (Bleyer et al., 2017). In our study, there were no pulmonary vascular abnormalities observed in the young adult or geriatric marmosets. Of greater interest, the aortas of multiple geriatric animals (2/5 males and 3/5 females) had gross and microscopic evidence of advanced stages (type V-VI) of atherosclerosis as defined by the American Heart Association Classification Criteria (Stary et al., 1995). The gross appearance and histopathology of atherosclerotic lesions were similar, although more severe, to those described in 2-4 year-old common marmosets fed high fat and glucose modified diets (Crook, Weisgraber, Boyles, & Mahley, 1990; Wachtman et al., 2011). Atherogenic diets have also resulted in similar vascular lesions in cotton top marmosets (Dreizen et al., 1973). In our study, atherosclerotic lesions or fatty streaks were not grossly evident in the young adult group; however, vascular structures within this cohort were not examined via light microscopy. Comorbidities of advanced stages of human atherosclerosis such as vascular rupture, hemorrhage, stenosis and mural thrombosis were not observed in the affected geriatric marmosets (Stary et al., 1995). As described by Simmons (2016), incidence of spontaneous atherosclerosis increases with age in rhesus macaques; this may also be the case for captive bred and laboratory maintained common marmosets (Simmons, 2016).
Cardiovascular fibrosis is a common background lesion of common marmosets (Chamanza, Parry, Rogerson, Nicol, & Bradley, 2006; David et al., 2009). Chamanza et. al. (2006) described a 2.5% incidence of cardiac fibrosis in a group of 284 captive bred 1-2.5-year-old common marmosets (Chamanza et al., 2006). David (David et al., 2009) identified 4/129 common marmosets with cardiac fibrosis, an incidence rate of ~3.1%. In our study, myocardial fibrosis was most frequently noted in the aged cohort with 8 of the 10 geriatric animals showing fibrosis. Stress-induced and chronic renal disease are considered as potential contributors to the genesis of myocardial fibrosis in non-human primates and humans, respectively (Chamanza et al., 2006; Lekawanvijit et al., 2012). In the geriatric animals assessed all 10 had signs of renal disease, and in the young animals only one animal had a cardiac symptom while 7 had kidney pathologies.
Kidney function was examined in this cross-sectional study and a detailed analysis is provided in (H. J. Lee et al., in press). In summary, biomarkers of impaired kidney function (albuminuria, etc.) were associated with age and were associated with marked kidney pathology in the geriatric group. Further, renal lesions were the most common lesion and of increased severity in the aged cohort. Histopathologic features were consistent with previously described renal pathologies in the common marmoset (Collins et al., 2014; Isobe et al., 2012; Tardif et al., 2011; Yamada, Sato, Kanno, Wako, & Tsuchitani, 2013)
While we found no significant differences between young and old marmosets in the percent of time they were active versus at rest during daily behavioral observations, we did find that the way they moved within the cage differed. Older marmosets were significantly less likely to transition between quadrants within the cage than were young animals, suggesting that while they are moving they are not covering the same distances within the cage. Older animals were also significantly less likely to leap as a means of movement within the cage. Leaping behavior was defined as jumps with all four feet leaving the substrate at the same time. Younger animals displayed twice as many leaps per minute as older animals, thus the older marmosets use a modified mode of locomotion from the younger animals. Further exploration of the basis of this difference – e.g. impaired joint mobility, impaired muscle function, pain from movement, impaired visual acuity – is merited to determine its specific worth as an aging assessment. Activity counts were not significantly lower in old than young animals but were on average lower; notably young animals displayed a great deal of variation in activity while aged animals did not, suggesting the possibility that an age difference might be observed in a situation that tested potential for maximum activity. Older animals were slower to arise in the morning than were the younger animals. Previous examinations of marmoset mobility in association with age did not find any differences in amount of movement or type of movement (Ross et al., 2012). However, one behavior that was previously reported as associated with risk of mortality that was not identified in the current study was the increased prevalence of stretching behavior (hang) in those with increased mortality in the following six months. It is possible that stretching is a useful biomarker for determining animals at risk of frailty or mortality, and it was not detected in this group of healthy aging marmosets.
The only measure of metabolic function that was found to significantly differ between the young and old marmosets was VO2 as assessed by indirect calorimetry. In humans VO2 maximizes early in adulthood (early 20’s) and then steadily declines with age (Matthews, Canepa, Strait, Lakatta, & Maurer, 2017). Individuals that maintain an active lifestyle with daily exercise lose VO2 at a slower rate than inactive individuals (Cartee, Hepple, Bamman, & Zierath, 2016). Further, VO2 serves as a biomarker for risk of mortality in aging humans, with individuals having lower values at higher risk of morbidity and mortality (Masakazu et al., 2017). VO2 is significantly linked to muscle mass, and individuals that maintain lean mass during aging, typically through exercise, maintain a higher VO2 and thus increased health span. The marmosets in this study did not have significantly different body composition and there was no difference in lean or fat mass with age. In previous reports marmosets did not display a significant relationship with age and body composition, but animals that were followed longitudinally displayed a loss of lean mass over three years (Ross et al., 2012). This suggests that the differences in VO2 with age in this study reflects aging effects on metabolic function that is independent of body composition.
Aging marmosets were found to have a number of significantly altered biomarkers associated with inflammation and altered immune functioning. The most commonly examined inflammatory biomarkers and cytokines associated with aging health in humans (IL6, CRP, TNFa) were not found to significantly differ in this marmoset population. However, other biomarkers (MIP, RANTES) as well as T cell proliferation were found to be significantly higher in older individuals indicating heightened immune and inflammatory response by these older marmosets. Increased inflammation and immune reactivity are hallmarks of cellular dysfunction with aging (Deleidi, Jäggle, & Rubino, 2015; Marcello et al., 2016).
Gross and histopathologic findings were consistent with previous surveys of background and pathologic lesions in captive and wild common marmosets (Bleyer et al., 2017; Chalmers, Murgatroyd, & Wadsworth, 1983; Chamanza, 2011; Chamanza et al., 2006; David et al., 2009; Foster, 2005; Kaspareit, Friderichs-Gromoll, Buse, & Habermann, 2006; Ludlage & Mansfield, 2003; Okazaki et al., 1996; Ross et al., 2012; Tardif et al., 2011; Tucker, 1984). Few reports have focused on further defining age-associated diseases in the common marmoset (Isobe et al., 2012; Ross et al., 2012; Tardif et al., 2011; Yamada et al., 2013). All of the geriatric animals were found to have multiple abnormal histopathologic findings across multiple organ systems, while only some of the younger animals had multiple histopathologies (Table 1 and Table 2). In this study, the majority of gross and histopathologic findings were not associated with debilitating or life threatening disease with the exception of the severe osteoarthritis affecting the coxofemoral joints in a 19.7-year-old geriatric female.
Miscellaneous lesions such as Brunner’s gland ectasia, adrenal cortical vacuolation and adiposity within organs are of relevance in toxicologic studies (Chamanza, 2011; Kaspareit et al., 2006; Tucker, 1984; Wallig, 2018) and are shown to occur as background lesions in young adult and aged common marmosets within our study group. Tissue adiposity is defined as the presence of mature adipose cells within the interstitium of non-atrophied organs (Mills, 2012). Overall, there was increased adiposity in multiple organs (lymph nodes, thyroid gland, exocrine pancreas, and heart) of aged common marmosets. Increased adiposity of the pancreas is associated with increased age, obesity and diabetes in humans (Mills, 2012). Increased adiposity of lymph nodes is also seen with obesity in humans (Mills, 2012). Age-related adipose redistribution in the heart, viscera, liver, muscle and bone marrow may result in significant morbidities including metabolic disease, impaired organ function and may predispose to bone fracture in human geriatric population (Kuk, Saunders, Davidson, & Ross, 2009). At the time of necropsy all animals exhibited adequate weight, therefore increased adiposity observed in multiple organs is potentially age related.
In this study, the most common background lesion of the lung was grossly evident as thick plaques along the caudo-dorsal lung lobes characterized histologically by pleural fibrosis, calcification and ossification. This was only observed in geriatric females, in contrast to small foci of alveolar septal calcification which was evident on histopathology in the one young adult male. Large retrospective studies of common marmoset lung pathology have reported that these lesions were noted in both sexes with no distinct gender predilection (Bleyer et al., 2017). Mechanisms of pulmonary calcification and ossification in humans include metastatic, dystrophic and idiopathic and are further characterized by anatomical distribution, histologic characteristics and comorbidities (Chan, Morales, Welsh, McDermott, & Schwarz, 2002). Generally, pulmonary calcification in the common marmoset is considered metastatic, secondary to dietary supplementation of calcium and vitamin D (Bleyer et al., 2017; Chamanza, 2011). Although the cause of pulmonary calcification in the aged females was not evident, the presence of pleural fibrosis suggests previous tissue injury therefore dystrophic calcification and ossification may be a plausible proposed mechanism.
All animals demonstrated varying amounts hepatocellular glycogen vacuolation without hepatomegaly. Glycogen accumulation can vary markedly among individual animals and it is largely dependent on diet (Foster, 2005). Some animals in the geriatric population had inflammatory infiltrates in the duodenum, jejunum or cecum of undetermined etiology. Similar findings of non-specific inflammation of the digestive system has been also described in other studies (David et al., 2009; Kaspareit et al., 2006). Although Kaspareit et. al. describes gallbladder inflammation occurring most frequently in juvenile and young adult marmosets, in our study cholecystitis was most frequently noted in the aged cohort.
Endocrine organs of young adult and aged common marmoset exhibited numerous background lesions. Age-associated endocrine organ functional and morphologic alterations have been extensively documented in human literature (Chahal & Drake, 2007; J. Lee et al., 2016). Altered histological patterns in endocrine organs do not necessarily correlate with hormonal alternations on hematological studies (J. Lee et al., 2016). In this study, animals were clinically healthy with no overt indication of endocrine disease; determination of subclinical endocrine disease requires organ specific hormone analysis which was not performed in this study. There was distinct variation in the islet cell size with an overall increased in islet size in 3 of the aged marmosets examined. Thyroid multinodular follicular hyperplasia seen in 2 aged marmosets resulted in marked asymmetric enlargement of the thyroid gland. One of the most common follicular changes among the aged common marmoset was variation of follicular size with some follicles exhibiting marked enlargement and lined by hypertrophied follicular epithelium. C-cells are concentrated in the middle third of the thyroid gland in marmosets, which is different than in most other species (Maile & Merker, 1996). Cell hyperplasia characterized by increased numbers and size of C-cells that extended beyond the middle third of the thyroid gland regionally disrupting the normal architecture was identified in 3 aged common marmosets; this finding is of undetermined clinical significance (Chamanza, 2011). Adrenal gland extramedullary hematopoiesis was common within both study groups and it is a well-known histologic finding of the common marmoset (Chamanza, 2011); this finding was more common in the geriatric group. Testicular tissue of males from the young adult and aged groups exhibited adequate spermatogenesis similar to previously described studies (Jackson & Edmunds, 1984). On gross evaluation prostate size was uniform. Benign prostatic hyperplasia is the most common age-related disease of the human male reproductive organs (Gunes, Hekim, Arslan, & Asci, 2016). Benign prostatic hyperplasia was not recognized in the adult or aged population of male common marmoset. Variation in the prostatic alveolar gland diameter was a feature noted throughout all prostatic glands of both young adult and aged marmosets. Prostatic glandular atrophy, characterized by decreased in nuclear to cytoplasmic ratio and thinning of prostatic epithelial lining with accumulations proteinaceous material within the lumens (corpora amylacea) similar to age-related pathology of prostatic glands of humans (Humphrey, 2003) was only observed in aged male common marmosets.
This examination of healthy young and old marmosets allowed us to evaluate biomarkers and phenotypes from five domains of health which have been determined to be significantly associated with health and aging in humans. Identification and characterization of these biomarkers, as well as characterizing typical histopathology of young and old healthy marmosets, will allow us to more broadly implement the marmoset as a translational model for aging studies.
Acknowledgements
The authors would like to thank Donna Layne-Colon, Jennifer Letts-Blake, and the marmoset husbandry staff at the Southwest National Primate Research Center for their dedicated care of the marmosets. This project was supported by funding from Southwest National Primate Research Center (5P51OD011133), Texas Biomedical Research Institute, and the San Antonio Claude D. Pepper Older Americans Independence Center (5P30AG044271).
References
- Austad SN (1997). Small nonhuman primates as potential models of human aging. Institute for Laboratory Animal Research Journal, 38(3), 142–147. doi: 10.1093/ilar.38.3.142 [DOI] [PubMed] [Google Scholar]
- Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Fried LP (2006). Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci, 61(3), 262–266. doi: 10.1093/gerona/61.3.262 [DOI] [PubMed] [Google Scholar]
- Bleyer M, Kunze M, Gruber-Dujardin E, & Mätz-Rensing K (2017). Spontaneous lung pathology in a captive common marmoset colony (Callithrix jacchus). Primate Biology, 4, 17–25. doi:doi: 10.5194/pb-4-17-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluher M (2008). Fat tissue and long life. Obes Facts, 1(4), 176–182. doi: 10.1159/000145930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browner WS, Kahn AJ, Ziv E, Reiner AP, Oshima J, Cawthon RM, Cummings SR (2004). The genetics of human longevity. Am J Med, 117(11), 851–860. doi: 10.1016/j.amjmed.2004.06.033 [DOI] [PubMed] [Google Scholar]
- Caffrey C, Harris-Kojetin L, Rome V, & Sengupta M (2014). Characteristics of residents living in residential care communities, by community bed size: United States, 2012. NCHS Data Brief(171), 1–8. [PubMed] [Google Scholar]
- Cartee GD, Hepple RT, Bamman MM, & Zierath JR (2016). Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metabolism, 23(6), 1034–1047. doi: 10.1016/j.cmet.2016.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal HS, & Drake WM (2007). The endocrine system and ageing. The Journal of Pathology, 211(2), 173–180. doi:doi: 10.1002/path.2110 [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Murgatroyd LB, & Wadsworth PF (1983). A survey of the pathology of marmosets (Callithrix jacchus) derived from a marmoset breeding unit. Laboratory Animals, 17(270–279). doi:https://doi.org/10.1258%2F002367783781062217 [DOI] [PubMed] [Google Scholar]
- Chamanza R (2011). Non-human primates: cynomolgus (Macaca fascicularis) and rhesus (Macaca mulatta) macaques and the common marmoset (Callithrix jacchus). Background Lesions in Laboratory Animals E-Book: A Color Atlas, 1. [Google Scholar]
- Chamanza R, Parry NMA, Rogerson P, Nicol JR, & Bradley AE (2006). Spontaneous Lesions of the Cardiovascular System in Purpose-Bred Laboratory Nonhuman Primates. Toxicologic Pathology, 34(4), 357–363. doi: 10.1080/01926230600809737 [DOI] [PubMed] [Google Scholar]
- Chan ED, Morales DV, Welsh CH, McDermott MT, & Schwarz MI (2002). Calcium Deposition with or without Bone Formation in the Lung. American Journal of Respiratory and Critical Care Medicine, 165(12), 1654–1669. doi: 10.1164/rccm.2108054 [DOI] [PubMed] [Google Scholar]
- Clarkson TB, Lehner ND, Bullock BC, Lofland HB, & Wagner WD (1976). Atherosclerosis in new world monkeys. Primates in medicine, 9, 90–144. [PubMed] [Google Scholar]
- Collins MG, Rogers NM, Jesudason S, Kireta S, Brealey J, & Coates PT (2014). Spontaneous glomerular mesangial lesions in common marmoset moneksy (Callitrhix jacchus): a benign non-progressive glomerulopathy. Journal of Medical Primatology, 43, 477–487. doi: 10.1111/jmp.12134 [DOI] [PubMed] [Google Scholar]
- Crook D, Weisgraber KH, Boyles JK, & Mahley RW (1990). Isolation and characterization of plasma lipoproteins of common marmoset monkey. Comparison of effects of control and atherogenic diets. Arteriosclerosis, Thrombosis, and Vascular Biology, 10(4), 633–647. doi: 10.1161/01.Atv.10.4.633 [DOI] [PubMed] [Google Scholar]
- Dahle CL, Jacobs BS, & Raz N (2009). Aging, vascular risk, and cognition: blood glucose, pulse pressure, and cognitive performance in healthy adults. Psychol Aging, 24(1), 154–162. doi: 10.1037/a0014283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David JM, Dick EJ Jr, & Hubbard GB (2009). Spontaneous pathology of the common marmoset (Callithrix jacchus) and tamarins (Saguinus oedipus, Saguinus mystax). Journal of Medical Primatology, 38(5), 347–359. doi: 10.1111/j.1600-0684.2009.00362.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleidi M, Jäggle M, & Rubino G (2015). Immune aging, dysmetabolism, and inflammation in neurological diseases. Frontiers in Neuroscience, 9(172). doi: 10.3389/fnins.2015.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, & Roberts AC (1996). Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci, 110(5), 872–886. doi: 10.1037//0735-7044.110.5.872 [DOI] [PubMed] [Google Scholar]
- Dreizen S, Levy BM, & Bernick S (1973). Diet-induced atherosclerosis in the marmoset. Proceedings of the Society for Experimental Biology and Medicine, 143(4), 1218–1223. doi:https://doi.org/10.3181%2F00379727-143-37505 [DOI] [PubMed] [Google Scholar]
- Foster JR (2005). Spontaneous and drug-induced hepatic pathology of the laboratory beagle dog, the cynomolgus macaque and the marmoset. Toxicologic Pathology, 33(1), 63–74. doi:https://doi.org/10.1080%2F01926230590890196 [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Cardiovascular Health Study Collaborative Research, G. (2001). Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci, 56(3), M146–156. doi: 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- Giavedoni LD (2005). Simultaneous detection of multiple cytokines and chemokines from nonhuman primates using luminex technology. J Immunol Methods, 301(1-2), 89–101. doi: 10.1016/j.jim.2005.03.015 [DOI] [PubMed] [Google Scholar]
- Gunes S, Hekim GNT, Arslan MA, & Asci R (2016). Effects of aging on the male reproductive system. Journal of assisted reproduction and genetics, 33(4), 441–454. doi:https://dx.doi.org/10.1007%2Fs10815-016-0663-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, & Raz N (2000). The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology, 14(2), 224–232. doi: 10.1037//0894-4105.14.2.224 [DOI] [PubMed] [Google Scholar]
- Hoffman CL, Higham JP, Mas-Rivera A, Ayala JE, & Maestripieri D (2010). Terminal investment and senescence in rhesus macaques (Macaca mulatta) on Cayo Santiago. Behav Ecol, 21(5), 972–978. doi: 10.1093/beheco/arq098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglind A, Areström I, Ehrnfelt C, Masjedi K, Zuber B, Giavedoni L, & Ahlborg N (2017). Systematic evaluation of monoclonal antibodies and immunoassays for the detection of Interferon-γ and Interleukin-2 in old and new world non-human primates. Journal of Immunological Methods, 441, 39–48. doi: 10.1016/j.jim.2016.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey PA (2003). Prostate pathology: American Society for Clinical Pathology; Chicago. [Google Scholar]
- Isobe K, Adachi K, Hayashi S, Ito T, Miyoshi A, Kato A, & Suzuki M (2012). Spontaneous glomerular and tubulointerstitial lesions in common marmosets (Callithrix jacchus). Veterinary pathology, 49(5), 839–845. doi:https://doi.org/10.1177%2F0300985811427151 [DOI] [PubMed] [Google Scholar]
- Jackson M, & Edmunds J (1984). Morphological assessment of testicular maturity in marmosets (Callithrix jacchus). Laboratory Animals, 18(2), 173–178. doi:https://doi.org/10.1258%2F002367784780891262 [DOI] [PubMed] [Google Scholar]
- Kaeberlein M (2016). The Biology of Aging: Citizen Scientists and Their Pets as a Bridge Between Research on Model Organisms and Human Subjects. Vet Pathol, 53(2), 291–298. doi: 10.1177/0300985815591082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspareit J, Friderichs-Gromoll S, Buse E, & Habermann G (2006). Background pathology of the common marmoset (Callithrix jacchus) in toxicological studies. Experimental and Toxicologic Pathology, 57, 405–410. doi: 10.1016/j.etp.2006.02.013 [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Sierra F (2014). Geroscience: linking aging to chronic disease. Cell, 159(4), 709–713. doi: 10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloting N, & Bluher M (2005). Extended longevity and insulin signaling in adipose tissue. Exp Gerontol, 40(11), 878–883. doi: 10.1016/j.exger.2005.07.004 [DOI] [PubMed] [Google Scholar]
- Kuk JL, Saunders TJ, Davidson LE, & Ross R (2009). Age-related changes in total and regional fat distribution. Ageing research reviews, 8(4), 339–348. doi: 10.1016/j.arr.2009.06.001 [DOI] [PubMed] [Google Scholar]
- LaClair M, & Lacreuse A (2016). Reversal learning in gonadectomized marmosets with and without hormone replacement: are males more sensitive to punishment? Anim Cogn, 19(3), 619–630. doi: 10.1007/s10071-016-0966-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layne DG, & Power RA (2003). Husbandry, handling, and nutrition for marmosets. Comp Med, 53(4), 351–359. [PubMed] [Google Scholar]
- Lee HJ, Gonzalez O, Dick EJ Jr., Donati A, Feliers D, Choudhury GG, Kasinath BS (in press). Marmoset as a model to study kidney changes associated with aging Journal of Gerontology: Biological Sciences. doi: 10.1093/gerona/gly237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Yi S, Kang YE, Kim H-W, Joung KH, Sul HJ, Shong M (2016). Morphological and functional changes in the thyroid follicles of the aged murine and humans. Journal of pathology and translational medicine, 50(6), 426. doi:https://dx.doi.org/10.4132%2Fjptm.2016.07.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekawanvijit S, Kompa AR, Manabe M, Wang BH, Langham RG, Nishijima F, Krum H (2012). Chronic kidney disease-induced cardiac fibrosis is ameliorated by reducing circulating levels of a non-dialysable uremic toxin, indoxyl sulfate. PLoS One, 7(7), e41281. doi: 10.1371/journal.pone.0041281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveille SG, Fried L, & Guralnik JM (2002). Disabling symptoms: what do older women report? J Gen Intern Med, 17(10), 766–773. doi: 10.1046/j.1525-1497.2002.20229.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlage E, & Mansfield K (2003). Clinical care and diseases of the common marmoset (Callithrix jacchus). Comp Med, 53(4), 369–382. [PubMed] [Google Scholar]
- Maestripieri D, & Hoffman CL (2011). Chronic stress, allostatic load, and aging in nonhuman primates. Dev Psychopathol, 23(4), 1187–1195. doi: 10.1017/S0954579411000551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maile S, & Merker H-J (1996). C-cells of marmosets (Callithrix jacchus). Annals of Anatomy-Anatomischer Anzeiger, 178(2), 159–167. [DOI] [PubMed] [Google Scholar]
- Malinow MR (1965). Atherosclerosis in subhuman primates. Folia Primatologica, 3(4), 277–300. doi: 10.1159/000155039 [DOI] [PubMed] [Google Scholar]
- Marcello P, Victor A, Judith C, Daniela F, Tamas F, Delphine S, Andrea C (2016). Aging of the immune system: Focus on inflammation and vaccination. European Journal of Immunology, 46(10), 2286–2301. doi:doi: 10.1002/eji.201546178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masakazu S, R. SM, Amir E, Junichi I, Nicole E, Miroslava V, Stephan H (2017). Anorexia, functional capacity, and clinical outcome in patients with chronic heart failure: results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). ESC Heart Failure, 4(4), 448–457. doi:doi: 10.1002/ehf2.12209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews S, Canepa M, Strait J, Lakatta EG, & Maurer M (2017). Age Related Changes in Themyocardial Contraction Fraction and the Association with VO2 Max among Healthyparticipants of the BLSA. Journal of Cardiac Failure, 23(8), S45. doi: 10.1016/j.cardfail.2017.07.125 [DOI] [Google Scholar]
- Mietsch M, Baldauf K, Reitemeier S, Suchowski M, Schoon HA, & Einspanier A (2016). Blood pressure as prognostic marker for body condition, cardiovascular, and metabolic diseases in the common marmoset (Callithrix jacchus). J Med Primatol, 45(3), 126–138. doi: 10.1111/jmp.12215 [DOI] [PubMed] [Google Scholar]
- Mietsch M, & Einspanier A (2015). Non-invasive blood pressure measurement: values, problems and applicability in the common marmoset (Callithrix jacchus). Laboratory Animals, 49(3), 241–250. doi: 10.1177/0023677214565843 [DOI] [PubMed] [Google Scholar]
- Mills SE (2012). Histology for pathologists: Lippincott Williams & Wilkins. [Google Scholar]
- Munger EL, Takemoto A, Raghanti MA, & Nakamura K (2017). Visual discrimination and reversal learning in aged common marmosets (Callithrix jacchus). Neuroscience Research, 124, 57–62. doi: 10.1016/j.neures.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Koba R, Miwa M, Yamaguchi C, Suzuki H, & Takemoto A (2018). A Method to Train Marmosets in Visual Working Memory Task and Their Performance. Frontiers in Behavioral Neuroscience, 12. doi:ARTN 46 10.3389/fnbeh.2018.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki Y, Kurata Y, Makinodan F, Kidachi F, Yokoyama M, Wako Y, Tsuchitani M (1996). Spontaneous lesions detected in the common cotton-eared marmosets (Callithrix jacchus). Journal of veterinary medical science, 58(3), 181–190. doi: 10.1292/jvms.58.181 [DOI] [PubMed] [Google Scholar]
- Power ML, Tardif SD, Power RA, & Layne DG (2003). Resting energy metabolism of Goeldi's monkey (Callimico goeldii) is similar to that of Other callitrichids. American Journal of Primatology, 60(2), 57–67. doi:doi: 10.1002/ajp.10078 [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, & Acker JD (1998). Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology, 12(1), 95–114. doi: 10.1037//0894-4105.12.1.95 [DOI] [PubMed] [Google Scholar]
- Raz N, & Rodrigue KM (2006). Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev, 30(6), 730–748. doi: 10.1016/j.neubiorev.2006.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley RM, Cummings RM, Leow-Dyke A, & Baker HF (2006). Neglect of memory after dopaminergic lesions in monkeys. Behav Brain Res, 166(2), 253–262. doi: 10.1016/j.bbr.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Ross CN, Austad S, Brasky K, Brown CJ, Forney LJ, Gelfond JA, Tardif SD (2017). The development of a specific pathogen free (SPF) barrier colony of marmosets (Callithrix jacchus) for aging research. Aging (Albany NY), 9(12), 2544–2558. doi: 10.18632/aging.101340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CN, Davis K, Dobek G, & Tardif SD (2012). Aging Phenotypes of Common Marmosets (Callithrix jacchus). J Aging Res, 2012, 567143. doi: 10.1155/2012/567143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton KA, Clarkson TB, & Kaplan JR (2012). Nonhuman primate models of atherosclerosis In Nonhuman Primates in Biomedical Research (Second Edition) (pp. 385–411): Elsevier. [Google Scholar]
- Simmons H (2016). Age-associated pathology in Rhesus macaques (Macaca mulatta). Veterinary pathology, 53(2), 399–416. doi:https://doi.org/10.1177%2F0300985815620628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Wissler RW (1995). A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation, 92(5), 1355–1374. doi: 10.1161/01.ATV.15.9.1512 [DOI] [PubMed] [Google Scholar]
- Stevens JA, Mack KA, Paulozzi LJ, & Ballesteros MF (2008). Self-reported falls and fall-related injuries among persons aged>or=65 years--United States, 2006. J Safety Res, 39(3), 345–349. doi: 10.1016/j.jsr.2008.05.002 [DOI] [PubMed] [Google Scholar]
- Tardif SD, Arauajuo A, Arruda MF, French JA, Sousa MB, & Yamamoto K (2008). Reproduction and aging in marmosets and tamarins In Atsalis S & Margulis SW (Eds.), Primate Reproductive Aging: Interdisciplinary topics in gerontology and geriatrics (Vol. 36, pp. 29–48): Karger. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Mansfield KG, Ratnam R, Ross CN, & Ziegler TE (2011). The marmoset as a model of aging and age-related disease. Institute for Laboratory Animal Research Journal, 52(1), 54–65. doi: 10.1093/ilar.52.1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif SD, Power ML, Ross CN, Rutherford JN, Layne-Colon DG, & Paulik MA (2009). Characterization of obese phenotypes in a small nonhuman primate, the common marmoset (Callithrix jacchus). Obesity (Silver Spring), 17(8), 1499–1505. doi: 10.1038/oby.2009.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker MJ (1984). A survey of the pathology of marmosets (Callithrix jacchus) under experiment. Laboratory Animals, 18(4), 351–358. doi:https://doi.org/10.1258%2F002367784780865397 [DOI] [PubMed] [Google Scholar]
- Wachtman LM, Kramer JA, Miller AD, Hachey AM, Curran EH, & Mansfield KG (2011). Differential contribution of dietary fat and monosaccharide to metabolic syndrome in the common marmoset (Callithrix jacchus). Obesity, 19(6), 1145–1156. doi: 10.1038/oby.2010.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallig MA (2018). Endocrine System In Fundamentals of Toxicologic Pathology (Third Edition) (pp. 565–624): Elsevier. [Google Scholar]
- Yamada N, Sato J, Kanno T, Wako Y, & Tsuchitani M (2013). Morphological study of progressive glomerulonephropathy in common marmosets (Callithrix jacchus). Toxicologic Pathology, 41(8), 1106–1115. doi:https://doi.org/10.1177%2F0192623313478206 [DOI] [PubMed] [Google Scholar]