Abstract
Objective
Infants with higher adiposity at birth may be at greater risk of developing obesity later in life. IGF-1 is important for intrauterine growth and may be a useful early life marker of adiposity, and thus later obesity risk. The aim of this study was to determine the relationship between cord blood IGF-1, neonatal anthropometrics and markers of neonatal adiposity.
Design, Patients and Measurements
A cross-sectional study design was utilized to study a multiethnic cohort of full-term neonates born to healthy mothers with normal glucose tolerance at a large university hospital. Neonatal cord blood was collected after birth and assayed for IGF-1, leptin and C-peptide. Neonatal body composition was measured between 24 and 72 h of life using the method of air displacement plethysmography.
Results
Cord blood IGF-1 was positively and significantly associated with markers of neonatal adiposity in models adjusted for maternal age at delivery, race, maternal prepregnancy BMI, gestational age at delivery and neonatal sex: birthweight (r = 0·62, P < 0·001), leptin (r = 0·33, P = 0·018), fat mass (r = 0·52, P < 0·001) and percent body fat (r = 0·51, P < 0·001). Cord blood IGF-1 was not associated with cord blood C-peptide.
Conclusions
Cord blood IGF-1 is strongly associated with all measures of neonatal adiposity suggesting that IGF-1 may be an important contributor to in utero neonatal fat accumulation.
Background
Insulin-like growth factor 1 (IGF-1) is an important regulator of foetal growth, previously illustrated by both murine and human studies. Studies of IGF1 gene knockout mice have demonstrated reductions in birthweight of 10–20% and 40% in heterozygous and homozygous knockouts, respectively.1 In humans, even partial IGF1 gene deletions have been associated with severe intrauterine growth retardation and postnatal growth failure.2–4
The relationship of IGF-1 to infant body composition is less well studied. Cord blood IGF-1 has previously been shown to be associated with birthweight in a number of studies of full-term healthy infants.5–9 Birthweight is not a reliable surrogate for body composition, as it is comprised of both adipose tissue and lean tissue. There have been few studies evaluating the association between IGF-1 and body composition, and each study employed different methods of estimating body composition.
Understanding the predictors of neonatal body composition is pertinent for the study of childhood obesity and the related risk of obesity in adulthood.10–12 Obese children have a higher likelihood of developing a myriad of comorbid metabolic disorders such as Type 2 diabetes mellitus, fatty liver disease, hyperlipidaemia and hypertension at earlier ages; therefore, prevention of childhood obesity is imperative. Infants born to mothers with gestational diabetes have been shown to have higher fat mass at birth compared to infants born to mothers with normal glucose tolerance, independent of birthweight.13,14 When evaluating maternal glucose on a continuum, rising glucose levels are associated with incremental increases in adiposity even at glucose levels below the diagnostic threshold for gestational diabetes.15 Foetal hyperinsulinism, characterized by elevated cord blood C-peptide levels, in response to maternal–foetal transmission of hyperglycaemia is an important mediator of adipose tissue accumulation in these infants.16 Even in infants without known exposure to gestational diabetes, it is prudent to evaluate for elevations in C-peptide when attempting to understand foetal fat accumulation.
Less is understood about obesity predictors in healthy infants of mothers with normal glucose tolerance. Leptin, a peptide hormone produced by both the placenta and adipocytes, is a marker that may signify excess fat accumulation. It is not well determined whether leptin plays a causal role in in utero fat deposition or primarily reflects production by foetal adipocytes. In addition, cord blood leptin may be derived from both foetal adipocytes and placental transfer of maternal leptin. Maternal leptin has not always correlated with neonatal adiposity or cord blood leptin, yet cord blood leptin has consistently correlated with foetal macrosomia and adiposity, suggesting that cord blood leptin may be a true reflection of adiposity at birth.5,17–21
Identification of early life markers, such as IGF-1, that may predict adiposity and obesity risk in healthy infants can ultimately inform earlier obesity prevention efforts and alter one’s metabolic trajectory. A better understanding of whether IGF-1 preferentially impacts the intrauterine growth of adipose versus lean tissue compartments is needed. In this study, the primary aim was to examine the relationship between IGF-1 and neonatal body composition using the validated, nonuser dependent method of air displacement plethysmography in a multiethnic cohort of full-term, healthy infants born to healthy mothers of varying prepregnancy BMI groups who had normal glucose tolerance. We hypothesized that infants with higher cord blood IGF-1 levels would have greater adiposity, measured by percent body fat and absolute fat mass. The secondary aim was to evaluate for associations between cord blood IGF-1 and additional measures of foetal growth and adiposity including birthweight, cord blood leptin and cord blood C-peptide levels.
Subjects and methods
Subjects
This study utilized a cohort of healthy maternal–neonatal pairs recruited between 2011 and 2013 at a large university hospital. Women carrying a singleton pregnancy with normal glucose tolerance evidenced by normal results on a 2-hour 75 g oral glucose tolerance test performed between 24 and 28 weeks gestation were eligible for the study.22 Women were excluded if they had a history of >3 term pregnancies or smoked during pregnancy as these factors are well known to be associated with excess or restricted growth, respectively.23 Newborns were excluded if they required intensive care and were too ill to undergo body composition measurements within the first 24–72 h of life. Newborns were also excluded if they had congenital anomalies as some of these are independently associated with abnormal foetal growth. Cord blood was collected after birth by labour and delivery staff, processed within 30 min and stored at −70 °C until laboratory assays were performed. Each mother provided written informed consent for herself and her neonate at the time of study enrolment. A total of 112 neonates were included in this analysis, of whom 103 had body composition data available. This study was approved by the Northwestern University Institutional Review Board.
Laboratory measurements
Samples for IGF-1 assays were batched and measured singly by liquid chromatography–mass spectrometry (Quest Diagnostics, San Juan Capistrano, CA, USA). The inter- and intra-assay coefficients of variation were 2·8–5·2% and 1·8–4·4%, respectively. Samples for C-peptide and leptin assays were batched and measured in duplicate with a radioimmunoassay kit (Millipore Corp, Billerica, MA, USA). The inter- and intra-assay coefficients of variation were 2·9–4·4% and 2·1–3·4%, respectively, for C-peptide. The inter- and intra-assay coefficients were 3·7–5·9% and 3·0–4·0%, respectively, for leptin.
Neonatal anthropometrics
Infant length was obtained with the use of a hard-surface measuring board. Measurements were recorded to the nearest 0·1 cm, performed in duplicate, and the results averaged. Weight and body composition were obtained by one of two trained clinicians utilizing an air displacement plethysmography system (PeaPod, Cosmed, Rome, Italy), a modality that is noninvasive, nonuser dependent, and has been validated in comparison with deuterium dilution in full-term infants between ages 0·4–21·7 weeks and of weight 2–8 kg.24,25 To measure weight, the infant was undressed and placed on the calibrated PeaPod scale and weight was recorded to the nearest 0·0001 kg. Next, the infant was placed inside the PeaPod volume chamber for 2 min to determine body volume. Density was calculated after which age- and sex-specific fat-free mass density values were used to determine absolute fat-free mass and fat mass. Per cent body fat is subsequently calculated from these values.25 In accordance with prior studies, body composition measurements were obtained after 24 h of life but prior to hospital discharge. Waiting until at least 24 h of life mitigates the effects of any possible fluid shifts or weight loss that are common in the immediate postbirth period.26
Statistical analysis
The associations between IGF-1 and percent body fat, birthweight, leptin, C-peptide, fat mass and fat-free mass were assessed using generalized linear models. The models were adjusted for neonatal sex, race, maternal prepregnancy BMI, gestational age at delivery and maternal age at delivery, which are all factors known to affect body composition and/or IGF-1 levels. A two-sided alpha at 5% significance level was considered significant. All statistical analyses were performed using SAS Version 9·4 (Cary, NC, USA).
Results
Maternal and neonatal characteristics are highlighted in Table 1. In our convenience sample, the majority of mothers had a normal prepregnancy BMI (4% underweight, 62·5% normal weight, 12·5% overweight and 21% obese). All mothers underwent a 2-hour oral glucose tolerance test between 24 and 28 weeks gestation and were found to have evidence of normal glucose tolerance per the International Association of Diabetes and Pregnancy Study Groups (IADPSG) guidelines, allowing us to remove the potential confounder of foetal hyperinsulinism and subsequent insulin mediated fat deposition.16,22 The majority of infants had a birthweight that was appropriate for gestational age (85% AGA, 15% LGA). None of the infants in our cohort were SGA, using the definition for SGA of birthweight <10%ile for gestational age.27 Mean cord blood IGF-1 level was not different between males and females (59·4 ng/ml vs 57·2 ng/ml, P = 0·63).
Table 1.
Demographics and sample characteristics
| Demographics | |
|---|---|
| Maternal race | 63% White 37% Non-White |
| Maternal characteristics (n = 112) | Mean ± SD |
| Age at delivery | 32 ± 3·9 years |
| Prepregnancy BMI | 25·0 ± 6·1 kg/m2 |
| OGTT fasting glucose | 76 ± 5·1 mg/dl 4·2 ± 0·3 mmol/l) |
| OGTT 1-hour glucose | 116·1 ± 26·1 mg/dl (6·4 ± 1·4 mmol/l) |
| OGTT 2-hour glucose | 100 ± 19 mg/dl (5·6 ± 1·1 mmol/l) |
| Neonatal characteristics (n = 112, unless noted) | Mean ± SD |
| Sex | 54% Male 46% Female |
| Gestational age | 39·6 ± 1 week |
| Birthweight | 3510 ± 511 g |
| SGA (n = 0) | 0% |
| AGA (n = 95) | 85% |
| LGA (n = 17) | 15% (4334 ± 340 g) |
| Length | 51·1 ± 2·2 cm |
| Fat-free mass (n = 103) | 2954 ± 371 g |
| Fat mass (n = 103) | 376·9 ± 170 g |
| Body fat (%) (n = 103) | 11 ± 3·8% |
| Cord blood IGF-1 (Somatomedin C) | 58·4 ± 24 ng/ml (7·6 ± 3·1 nmol/l) |
| Cord blood leptin (n = 110) | 10·7 ± 8·0 ng/ml (10·7 ± 8·7 mcg/l) |
| Cord blood C-peptide (n = 106) | 0·62 ± 0·24 ng/ml (0·21 ± 0·1 nmol/l) |
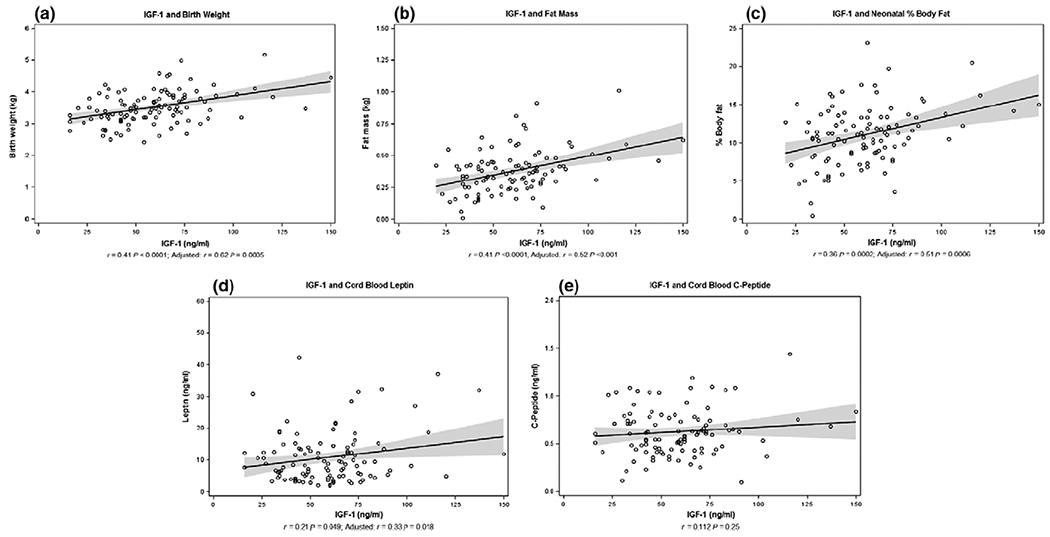
As shown in Fig. 1a–d, cord blood IGF-1 levels were positively correlated with birthweight (r = 0·62, P < 0·001), fat mass (r = 0·52, P < 0·001), per cent body fat (r = 0·51, P < 0·001) and leptin (r = 0·33, P = 0·018). Confidence intervals are depicted as the shaded grey area on each plot and values noted in Table 2. All analyses were adjusted for potential confounders that can impact growth including neonatal sex, race, gestational age, maternal prepregnancy BMI and maternal age at delivery. Cord blood C-peptide was not correlated with fat mass when adjusting for the same confounders (r = 0·50, P = 0·24) (Fig. 1e). The correlation of IGF-1 and fat-free mass approached significance (r = 0·64, P = 0·05). Cord blood IGF-1 was not related to cord blood C-peptide (r = 0·112, P = 0·25). In the linear regression model, parameter estimates showed that for every 10 ng/ml increase in cord blood IGF-1, mean birthweight increased by 60 g (95% CI 28–94), mean fat mass increased by 25 g (95% CI 12·2–38·6) and mean percent body fat increased by 0·5 (95% CI 0·2–0·8) (Table 2). In our adjusted model, IGF-1 explains 26% of the variance in percent body fat, a measure of neonatal adiposity defined as (fat mass)/(fat mass + fat-free mass).
Fig. 1.
Association of cord blood IGF-1 with primary outcomes. A significant positive correlation was found between IGF-1 and birthweight (a), fat mass (b), percent body fat (c) and cord blood leptin (d). No correlation was identified between IGF-1 and cord blood C-peptide (e). The plots in Figure 1 represent the unadjusted models. Significance was maintained when linear regression models for (a–d) were adjusted for neonatal sex, race, maternal prepregnancy BMI, gestational age at delivery and maternal age at delivery. No adjustments were made for the model in (e). The solid line represents the linear regression line, and the shaded areas indicate the 95% confidence interval.
Table 2.
Relationship of IGF-1 to primary outcome variables
| Outcome | r | P | Parameter estimate (95% CI) |
|---|---|---|---|
| Birthweight (g) | 0·62 | 0·0005 | 6 (2·8-9·4) |
| Fat mass (g) | 0·52 | 0·0003 | 2·5 (1·22-3·86) |
| Fat-free mass (g) | 0·64 | 0·05 | 0·003 (0·005 × 10−2–0·005) |
| Body fat (%) | 0·51 | 0·005 | 0·05 (0·02-0·08) |
| Leptin (ng/ml) | 0·33 | 0·018 | 0·08 (0·02-0·15) |
All analyses were adjusted for: neonatal sex, race, maternal prepregnancy BMI, gestational age at delivery and maternal age at delivery. Parameter estimates are per 1 ng/ml increase in cord blood IGF-1.
Discussion
The results of our study demonstrate that IGF-1 is significantly associated with various measures of neonatal adiposity including birthweight, percent body fat, fat mass and cord blood leptin in a group of healthy neonates. The finding of increased IGF-1 levels in infants with higher birthweights confirmed the results of several prior studies.5–9 While birthweight is positively correlated with body fat, it is comprised of both lean and adipose tissue so it is not the most accurate reflection of the amount of adipose tissue present.
Our hypothesis that cord blood IGF-1 is related to adiposity is strongly supported by the significant associations we found between IGF-1 and percent body fat and IGF-1 and absolute fat mass, suggesting a possible preferential role of IGF-1 on adipose tissue development. Although the relationship of IGF-1 and fat-free mass approached significance, the correlation with fat mass was much stronger and implies a more robust role of IGF-1 and fat accumulation. Our findings are consistent with the few existing studies evaluating cord blood IGF-1 and neonatal body composition after birth in full-term newborns.28–30 However, the present study is the first to assess this relationship using the validated method of air displacement plethysmography to measure neonatal body composition. Javaid et al. found cord blood IGF-1 to be correlated with proportionate fat mass measured by DEXA, another noninvasive modality to study body composition. However, a significant proportion of mothers in that study smoked, which is known to impact foetal growth.28 Akcakus et al. demonstrated a relationship between cord blood IGF-1 and body fat using two different measures of adiposity – triceps skinfold thickness and fat mass measured by DEXA.29 A more recent study examining newborns in India found that cord blood IGF-1 was associated with adiposity measured by two sum of skinfolds; however, many mothers in this study were undernourished which independently affects foetal growth.30
The significant relationship between IGF-1 and cord blood leptin in the present study provides additional support for the association of IGF-1 with adiposity. If IGF-1 is associated with fat accumulation, and leptin is produced by adipocytes, then a correlation between IGF-1 and leptin is expected. Further study is necessary as causality cannot be determined and the physiologic mechanisms are not fully understood.
Prior studies have shown a relationship between foetal insulin resistance measured by cord blood C-peptide or HOMA-IR and neonatal body fat.15,31 Our results did not show this correlation, perhaps because the mothers in our study clearly had normal glucose tolerance with the majority also with a normal BMI. In mothers with normal glucose tolerance whose fetuses do not have hyperinsulinism, IGF-1 may be an independent factor related to neonatal fat accumulation. Additional study is necessary to evaluate for causal mechanisms behind this relationship. Furthermore, in utero growth and adiposity are likely to be affected by a multitude of factors in addition to IGF-1, including but not limited to genetics and the intrauterine milieu.
Although our study only demonstrated an association between IGF-1 and adipose tissue, it is physiologically conceivable that IGF-1 can have a direct effect on adipose tissue proliferation and differentiation. IGF-1 receptors are abundant on the surface of preadipocytes. In vitro studies on human preadipocytes demonstrated that activation of these receptors by IGF-1 promotes DNA synthesis and perhaps differentiation of preadipocytes.32 Murine studies demonstrated that preadipocytes differentiate into adipocytes in response to activation of IGF-1 and insulin receptors by IGF-1 and insulin, respectively, but that supraphysiologic doses of insulin are needed for differentiation as opposed to physiologic doses of IGF-1.33 These findings suggest that IGF-1 is a strong stimulator of adipocyte differentiation and may contribute to neonatal adipose tissue accumulation.
One strength of this study is the use of air displacement plethysmography to evaluate body composition. Various validated modalities exist to measure body composition; however, air displacement plethysmography is noninvasive, nonuser dependent, and is based on the principles of hydrostatic weighing which is the standard for body density measurement in humans. Fat mass and fat-free mass are calculated in this methodology using an assumed density of lean and adipose tissue; however, tissue composition may vary among groups of individuals. In addition, air displacement plethysmography only provides information on total body fat and cannot provide information on regional fat distribution.
Other common methodologies of body composition measurements such as BMI, DEXA and sum of skinfold thicknesses have their own strengths and limitations. BMI is not standardized across ethnicities and the inability to differentiate between the fat and lean mass contribution to body weight in a BMI calculation can lead to inaccurate conclusions regarding adiposity. DEXA is another noninvasive modality and has good correlation with air displacement plethysmography.34 However, there is radiation exposure and infant movement can impact the accuracy of measurements. Sum of skinfolds measured with calipers is an inexpensive modality. However, it is user dependent, requires the expertise of trained personnel and is an assessment only of subcutaneous fat and not abdominal adiposity. There is also great interobserver measurement variability and no existing standards on which skinfold sites to use, making comparability between studies difficult.35,36 A disadvantage of all modalities, including air displacement plethysmography, is the difficulty of following body composition longitudinally between infancy and early childhood due to age and weight requirements and need for full co-operation by the participant.
Another strength of this study is the use of a cohort of healthy, full-term infants born to healthy mothers with normal glucose tolerance, allowing for the elimination of potential common confounders of neonatal growth such as smoking, undernourishment and hypertension. Notably, the absence of any mothers with impaired glucose tolerance or gestational diabetes was a unique feature, allowing us to focus on adiposity contributors in healthy infants without hyperinsulinism.
The cross-sectional design of this study limits our ability to determine causality. While our results only reflect associations, those that we found are strong and confirm the findings of previous studies, reinforcing that a relationship between IGF-1 and adiposity may exist. Future research that follows IGF-1 levels and body composition over time would help us understand the long-term impact of these findings. Another potential limitation is the lack of normative IGF-1 data in neonates. We evaluated IGF-1 levels on a continuum; however, without normal ranges, we are unable to determine whether levels in higher fat infants were abnormal.
The generalizability of our study to all populations is limited as our enrolment only included women with normal glucose tolerance. The rationale for this enrollment criteria was to exclude mothers with hyperglycaemia, a known risk factor for foetal macrosomia.15 The use of a convenience sample yielded a cohort of mothers representative of the large, academic hospital where they gave birth as well as the general United States population. All infants in our cohort were either AGA or LGA; thus, we were unable to conclude if any associations exist between IGF-1 and adiposity in SGA infants. Growth and adiposity in SGA infants can be impacted by a multitude of confounders such as placental insufficiency, maternal hypertension, smoking and undernutrition. Given our findings, we can hypothesize that cord blood IGF-1 levels would be lower in SGA infants with lower body fat; however, consideration of potential confounders would be necessary.
In conclusion, this study is the first to demonstrate a strong association of IGF-1 with neonatal adiposity measured by a validated, noninvasive, nonuser dependent tool suggesting that IGF-1 may be a central player of foetal adipose tissue accumulation. While neonatal body composition is likely impacted by various hormonal, genetic and environmental factors, we propose that IGF-1 is an important, independent contributor. Further study is needed in larger, longitudinal cohorts of infants with validated measures of neonatal adiposity to assess reproducibility of these findings at birth and the impact on adiposity in childhood.
Acknowledgements
This work was supported by the National Institutes of Child Health and Human Development Grant K12 HD055884 and a Genentech Growth Disorders Grant G-29954. The authors would also like to acknowledge Dr. Boyd Metzger for his critical review and feedback during the preparation of this manuscript.
Footnotes
Disclosure statement
Nothing to declare.
References
- 1.Powell-Braxton L, Hollingshead P, Warburton C et al. (1993) IGF-I is required for normal embryonic growth in mice. Genes & development, 7, 2609–2617. [DOI] [PubMed] [Google Scholar]
- 2.Bonapace G, Concolino D, Formicola S et al. (2003) A novel mutation in a patient with insulin-like growth factor 1 (IGF1) deficiency. Journal of Medical Genetics, 40, 913–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woods KA, Camacho-Hubner C, Barter D et al. (1997) Insulin-like growth factor I gene deletion causing intrauterine growth retardation and severe short stature. Acta Paediatrica Supplementum, 423, 39–45. [DOI] [PubMed] [Google Scholar]
- 4.Walenkamp MJ, Karperien M, Pereira AM et al. (2005) Homozygous and heterozygous expression of a novel insulin-like growth factor-I mutation. The Journal of Clinical Endocrinology and Metabolism, 90, 2855–2864. [DOI] [PubMed] [Google Scholar]
- 5.Christou H, Connors JM, Ziotopoulou M et al. (2001) Cord blood leptin and insulin-like growth factor levels are independent predictors of fetal growth. The Journal of Clinical Endocrinology and Metabolism, 86, 935–938. [DOI] [PubMed] [Google Scholar]
- 6.Vatten LJ, Nilsen ST, Odegard RA et al. (2002) Insulin-like growth factor I and leptin in umbilical cord plasma and infant birth size at term. Pediatrics, 109, 1131–1135. [DOI] [PubMed] [Google Scholar]
- 7.Ong K, Kratzsch J, Kiess W et al. (2000) Size at birth and cord blood levels of insulin, insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-1 (IGFBP-1), IGFBP-3, and the soluble IGF-II/mannose-6-phosphate receptor in term human infants. The ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. The Journal of Clinical Endocrinology and Metabolism, 85, 4266–4269. [DOI] [PubMed] [Google Scholar]
- 8.Klauwer D, Blum WF, Hanitsch S et al. (1997) IGF-I, IGF-II, free IGF-I and IGFBP-1, -2 and -3 levels in venous cord blood: relationship to birthweight, length and gestational age in healthy newborns. Acta Paediatrica, 86, 826–833. [DOI] [PubMed] [Google Scholar]
- 9.Yang SW & Yu JS (2000) Relationship of insulin-like growth factor-I, insulin-like growth factor binding protein-3, insulin, growth hormone in cord blood and maternal factors with birth height and birthweight. Pediatrics International, 42, 31–36. [DOI] [PubMed] [Google Scholar]
- 10.Guo SS, Wu W, Chumlea WC et al. (2002) Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. The American Journal of Clinical Nutrition, 76, 653–658. [DOI] [PubMed] [Google Scholar]
- 11.Whitaker RC, Wright JA, Pepe MS et al. (1997) Predicting obesity in young adulthood from childhood and parental obesity. The New England Journal of Medicine, 337, 869–873. [DOI] [PubMed] [Google Scholar]
- 12.Freedman DS, Khan LK, Serdula MK et al. (2005) The relation of childhood BMI to adult adiposity: the Bogalusa Heart Study. Pediatrics, 115, 22–27. [DOI] [PubMed] [Google Scholar]
- 13.Catalano PM, Thomas A, Huston-Presley L et al. (2003) Increased fetal adiposity: a very sensitive marker of abnormal in utero development. American Journal of Obstetrics and Gynecology, 189, 1698–1704. [DOI] [PubMed] [Google Scholar]
- 14.Pettitt DJ, Nelson RG, Saad MF et al. (1993) Diabetes and obesity in the offspring of Pima Indian women with diabetes during pregnancy. Diabetes Care, 16, 310–314. [DOI] [PubMed] [Google Scholar]
- 15.HAPO Study Cooperative Research Group (2009) Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes 58, 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freinkel N (1980) Banting Lecture 1980. Of pregnancy and progeny. Diabetes, 29, 1023–1035. [DOI] [PubMed] [Google Scholar]
- 17.Varvarigou A, Mantzoros CS & Beratis NG (1999) Cord blood leptin concentrations in relation to intrauterine growth. Clinical Endocrinology, 50, 177–183. [DOI] [PubMed] [Google Scholar]
- 18.Harigaya A, Nagashima K, Nako Y et al. (1997) Relationship between concentration of serum leptin and fetal growth. The Journal of Clinical Endocrinology and Metabolism, 82, 3281–3284. [DOI] [PubMed] [Google Scholar]
- 19.Tsai PJ, Yu CH, Hsu SP et al. (2004) Cord plasma concentrations of adiponectin and leptin in healthy term neonates: positive correlation with birthweight and neonatal adiposity. Clinical Endocrinology, 61, 88–93. [DOI] [PubMed] [Google Scholar]
- 20.Clapp JF III & Kiess W (1998) Cord blood leptin reflects fetal fat mass. Journal of the Society for Gynecologic Investigation, 5, 300–303. [PubMed] [Google Scholar]
- 21.Hassink SG, de Lancey E, Sheslow DV et al. (1997) Placental leptin: an important new growth factor in intrauterine and neonatal development? Pediatrics, 100, E1. [DOI] [PubMed] [Google Scholar]
- 22.Metzger BE, Gabbe SG, Persson B et al. (2010) International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care, 33, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh CA, Mahony RT, Foley ME et al. (2007) Recurrence of fetal macrosomia in non-diabetic pregnancies. Journal of Obstetrics and Gynaecology, 27, 374–378. [DOI] [PubMed] [Google Scholar]
- 24.Ellis KJ, Yao M, Shypailo RJ et al. (2007) Body-composition assessment in infancy: air-displacement plethysmography compared with a reference 4-compartment model. The American Journal of Clinical Nutrition, 85, 90–95. [DOI] [PubMed] [Google Scholar]
- 25.Ma G, Yao M, Liu Y et al. (2004) Validation of a new pediatric air-displacement plethysmograph for assessing body composition in infants. The American journal of Clinical Nutrition, 79, 653–660. [DOI] [PubMed] [Google Scholar]
- 26.Hull HR, Thornton JC, Ji Y et al. (2011) Higher infant body fat with excessive gestational weight gain in overweight women. American Journal of Obstetrics and Gynecology 205, 211.e211–211.e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander GR, Himes JH, Kaufman RB et al. (1996) A United States national reference for fetal growth. Obstetrics and Gynecology, 87, 163–168. [DOI] [PubMed] [Google Scholar]
- 28.Javaid MK, Godfrey KM, Taylor P et al. (2004) Umbilical venous IGF-1 concentration, neonatal bone mass, and body composition. Journal of Bone and Mineral Research, 19, 56–63. [DOI] [PubMed] [Google Scholar]
- 29.Akcakus M, Koklu E, Kurtoglu S et al. (2006) The relationship among intrauterine growth, insulinlike growth factor I (IGF-I), IGF-binding protein-3, and bone mineral status in newborn infants. American Journal of Perinatology, 23, 473–480. [DOI] [PubMed] [Google Scholar]
- 30.Wiley AS, Lubree HG, Joshi SM et al. (2016) Cord IGF-1 concentrations in Indian newborns: associations with neonatal body composition and maternal determinants. Pediatric Obesity, 11, 151–157. [DOI] [PubMed] [Google Scholar]
- 31.Catalano PM, Presley L, Minium J et al. (2009) Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care, 32, 1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Back K, Brannmark C, Stralfors P et al. (2011) Differential effects of IGF-I, IGF-II and insulin in human preadipocytes and adipocytes–role of insulin and IGF-I receptors. Molecular and Cellular Endocrinology, 339, 130–135. [DOI] [PubMed] [Google Scholar]
- 33.Smith PJ, Wise LS, Berkowitz R et al. (1988) Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. Journal of Biological Chemistry, 263, 9402–9408. [PubMed] [Google Scholar]
- 34.Fields DA, Demerath EW, Pietrobelli A et al. (2012) Body composition at 6 months of life: comparison of air displacement plethysmography and dual-energy X-ray absorptiometry. Obesity (Silver Spring), 20, 2302–2306. [DOI] [PubMed] [Google Scholar]
- 35.Horan M, Gibney E, Molloy E et al. (2015) Methodologies to assess paediatric adiposity. Irish Journal of Medical Science, 184, 53–68. [DOI] [PubMed] [Google Scholar]
- 36.de Bruin NC, van Velthoven KA, Stijnen T et al. (1995) Body fat and fat-free mass in infants: new and classic anthropometric indexes and prediction equations compared with total-body electrical conductivity. The American Journal of Clinical Nutrition, 61, 1195–1205. [DOI] [PubMed] [Google Scholar]