Abstract
BACKGROUND:
We conducted a secondary analysis to 1) compare changes in mood disorders and quality of life (QOL) among four groups of patients with heart failure (HF) in a home-based exercise program who had varying degrees of change in their exercise capacity; and 2) determine whether there was an association between exercise capacity, mood disorders, and QOL.
METHODS:
Seventy-one patients were divided into 4 groups based on changes in exercise capacity from baseline to 6 months: Group 1showed improvements >10% (n = 19); Group 2 showed improvements ≤10% (n = 16); Group 3 showed reductions ≤10% (n = 9); and Group 4 showed reductions >10% (n = 27).
RESULTS:
Over time, patients in all four groups demonstrated significantly lower levels of depression and hostility (P<.001) and higher levels of physical and overall QOL (P=.046). Group differences over time were noted in anxiety (P =.009), depression (P =.015), physical QOL (P <.001) and overall QOL (P =.002). Greater improvement in exercise capacity was strongly associated with lower depression scores (r=−.49, P=.01).
CONCLUSIONS:
An improvement in exercise capacity with exercise training was associated with a decrease in depression and anxiety and an increase in QOL in patients with HF.
Heart Failure (HF) is a debilitating chronic condition that affects an estimated 5.7 million Americans and the projections show that the prevalence will increase 46% from 2012 to 2030, resulting in >8 million Americans ≥ 18 years of age with HF.1 Most individuals with HF experience deficits in physical functioning, fatigue, and report decreased quality of life (QOL).2-4 A common finding in patients with HF is exercise intolerance, which causes progressive functional deterioration.5 In addition to physical impairments, patients with HF also exhibit psychological challenges, specifically anxiety and depression, and it is associated with increased morbidity and mortality.6 Recent HF guidelines advise regular exercise for patients with HF to improve their functional capacity and decrease their symptoms.2,7 Research supports the importance of promoting exercise training to reduce mortality, hospitalizations, and risk for other chronic diseases in patients with HF.8-10 Controlled clinical trials have also shown that exercise training programs improve aerobic capacity, delay onset of anaerobic metabolism, and improve autonomic balance.11-13 Furthermore, improvements in exercise capacity in this patient population led to increased metabolism, strength, and vitality14-17and significant reductions in depressive symptoms.18 The HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise TraiNing) trial also showed that greater physiological and clinical benefits appear likely in patients with HF who adhere to a higher volume of exercise.15
Although prior exercise research indicate that regular exercise represents an effective therapy in the management of patients with HF due to left ventricular systolic dysfunction, the benefits of exercise training on mood disorders and QOL remain inconsistent. Likewise, the relationship between exercise capacity and mood states and QOL vary across studies. The heterogeneity of HF populations, differences in exercise prescriptions (e.g., varying intensity and progression), and use of a variety of measures of exercise capacity, depressive symptoms and QOL likely explains the differences seen across studies. A number of investigators have found an improved QOL along with increased exercise capacity and cardiorespiratory fitness following participation in exercise training.19-24 One study showed improvements in both QOL and exercise capacity, but no significant correlations were found between the two,25 while yet another study demonstrated only a weak relationship between improvement in exercise capacity and improvement in QOL.26 Some investigators have reported improvements in exercise capacity without improvements in QOL20,21,27 or improvements in QOL with little or no change in exercise capacity,28,29 which strongly suggests that changes in exercise capacity and QOL are not related and changes in one may occur without associated changes in the other. In a randomized clinical trial conducted by our research team to examine the effects of a six-month, home-based exercise training program, we found no significant differences between the intervention and control groups in exercise capacity or in QOL measures upon follow-up.30 Finally, in the recently reported HF-ACTION study, 23,31 medically stable outpatients with HF and a reduced left ventricular ejection fraction were randomized to an aerobic exercise training group or control group and followed for a mean of 2.5 years with scheduled measurements of health status. Compared to baseline, the exercise group had significant improvements in health status at 3 months and these were sustained for the duration of the study. However, the health status questionnaire used did not measure mood disorders or QOL.31
Thus, there remain gaps in knowledge about the influence of exercise training on mood disorders and QOL in HF. Likewise, the association between physiologic and emotional factors is highly inconclusive based on past clinical trials of exercise. Therefore, we conducted a secondary analysis to 1) compare changes in mood disorders, specifically anxiety, depression, hostility, and QOL among four groups of patients who had all participated in a home-based exercise program and who had varying degrees of change in their exercise capacity; and 2) determine whether there was an association between exercise capacity, mood disorders, and QOL. Specifically, we wanted to determine if positive changes in mood states and QOL were dependent on the degree to which patients experienced significant improvements in their exercise capacity related to exercise training.
METHODS
Study Design
A complete description of the study design and methods of the parent study describing the effects of the six-month, home-based exercise program has been published elsewhere.30 In brief, we randomized patients with HF to an exercise group and a control group. All patients underwent cardiopulmonary exercise testing using a bicycle at baseline and 6 months to establish maximum VO2. Patients assigned to the exercise group were asked to perform a graduated, exercise protocol consisting of low-level aerobic exercise and resistive training five days per week. Aerobic training was initially 10 minutes at 40% maximal heart rate and progressively increased up to 45 minutes at 60% maximal heart rate for the remainder of the program. Participants in the control group maintained their usual level of daily activities, with no systematic exercise component. Participants were visited monthly by a nurse in order to review the exercise protocol and to collect daily exercise diaries and pedometer readings. The research protocol was reviewed and approved by the appropriate Institutional Review Board. Written informed consent was obtained from all participants. Data used for this secondary analysis were de-identified.
Study Participants
Thorough descriptions of the recruiting and screening processes, as well as the methods, have been previously published.30 Briefly, the study was limited to patients with chronic HF (defined as New York Heart Association [NYHA] Class II through IV and left ventricular systolic dysfunction with a LVEF ≤ 40%), aged 18 to 80 years, who read and spoke English. Exclusion criteria for participation in the study included myocardial infarction or recurrent angina within the previous three months, orthopedic impediments to exercise, severe obstructive pulmonary disease, stenotic valvular disease, history of uncontrolled ventricular tachyarrhythmias, or absence of an implantable cardioverter-defibrillator despite a history of sudden cardiac death. All participants were stable on standard medical therapy (e.g., angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, beta-blockers, diuretics, aldosterone blockers, and digoxin as necessary) at the time of baseline testing.
Seventy-one of the original 87 participants in the exercise group who had complete data at baseline and six months related to the current analyses were included in the secondary analysis. Four comparative groups were established based on documented changes in exercise capacity (peak VO2) from baseline to six months: group 1: participants with improvements greater that 10% (n = 19); group 2: participants with improvements less than 10% (n = 16); group 3: participants with reductions less than 10% (n = 9); and group 4: participants with reductions greater than 10% (n = 27). The selected cut-off points for the four groups were based on findings from a study that showed that improvements of ≥ 10% in exercise capacity were associated with lower mortality and hospital readmission for HF during a 5-year follow-up.32
Participants were asked to complete the Multiple Affect Adjective Checklist (MAACL) and the Minnesota Living with Heart Failure Questionnaire (MLHFQ) to assess mood disorders (i.e. anxiety, depression, and hostility) and QOL at baseline and 6 months. The MAACL is composed of 132 alphabetically arranged adjectives. Scores for anxiety range from 0 to 21 (norm 7 or below), depression from 0 to 40 (norm 11), and hostility from 0 to 28 (norm 7). Higher scores reflect higher levels of dysphoria. The reliability and validity of the MAACL has been demonstrated in HF patients.33 The MLHFQ is disease-specific, 21-item tool designed to measure the extent to which participants experienced various symptoms and prevented them from living as they wanted in the previous month. The items can be combined to form an overall QOL score as well as physical health (eight items) and emotional health (five items) scores. Response options are presented as six-point ordinal scales ranging from 0 (no) to 5 (very much), with a total maximum score of 105 (40 for physical and 25 for emotional health); a lower score indicates better QOL.34
Demographic information (i.e. age, race, marital status, education, current employment status and annual income) were obtained through self-reports. Information pertaining to medical history and current clinical status (e.g. etiology of HF, NYHA class, ejection fraction) were obtained from participants’ medical records.
Statistical Analysis
Descriptive baseline characteristics of groups were tabulated as means and standard deviations or as percentages. Differences in sociodemographics and clinical characteristics were calculated for the four groups of participants using Chi-square or Kruskal–Wallis test (non-parametric tests for unequal group sizes), depending on the level of measurement. Regression analyses were computed to describe changes in mood disorders and QOL between baseline and 6 months and to determine whether group differences were significant over time. For statistically significant analysis of covariance (P<.05), pairwise comparisons between the 2 groups of participants who showed improvements in exercise capacity and the 2 groups of participants who showed reductions in exercise capacity were made using the Bonferroni correction for multiple testing. Univariate analysis was conducted using Pearson’s product moment correlation coefficients. All analyses were performed using SPSS for Windows (version, 13.0, SPSS, Inc., Chicago, Ill).35
RESULTS
The demographic and clinical characteristics of participants who showed improvements in exercise capacity (Group 1 [n = 19] and 2 [n = 16]) and participants who showed reductions in exercise capacity (Group 3 [n = 9] and Group 4 [n = 27]) from baseline to 6 months are illustrated in Tables 1A and 1B. There were no significant differences in age, gender, race, marital status, employment status, and education among participants in the 4 groups. Additionally, there were no significant differences among the groups with regard to ejection fraction, peak VO2, six-minute walk test, NYHA class, HF etiology, medical history (i.e. comorbidities) and medication use. However, the four groups were significantly different by ANOVA on the number of hospitalizations during the year of study with Group 1 experiencing a mean of .56 hospitalizations, Group 2 a mean of .45, Group 3 a mean of 1.09 and Group 4 a mean of 1.7 (P=0.02).
Table 1.A.
Baseline Characteristics (Sociodemograhic)
| All Participants (N=71) |
Group 1a (n = 19) |
Group 2b (n = 16) |
Group 3c (n = 9) |
Group 4d (n = 27) |
P value | |
|---|---|---|---|---|---|---|
| Age, years (Mean±SD) | 55.56 ± 11.13 | 55.21 ± 10.75 | 55.50 ± 12.51 | 56.33 ± 11.41 | 55.59 ± 11.09 | .996 |
| Male, N (%) | 47 (66.2%) | 13 (68.4%) | 10 (62.5%) | 6 (66.7%) | 18 (66.7%) | .986 |
| Race, N (%) | .298 | |||||
| Hispanic | 10 (14.1%) | 5 (26.3%) | 2 (12.5%) | 1 (11.1%) | 2 (7.4%) | |
| White | 47 (66.2%) | 11 (57.9%) | 9 (56.3%) | 8 (88.9%) | 19 (70.4%) | |
| African America | 14 (19.7%) | 3 (15.8%) | 5 (31.3% | 0 (0%) | 6 (22.2%) | |
| Married, N (%) | 43 (60.6%) | 10 (52.6%) | 9 (56.3%) | 8 (88.9%) | 16 (59.3%) | .300 |
| Employed, N (%) | 20 (28.3%) | 7 (36.8%) | 3 (18.8%) | 2 (22.2%) | 8 (29.6%) | .661 |
| Education, N (%) | .086 | |||||
| < 12 | 26 (26.6%) | 2 (7.7%) | 7 (26.9%) | 3 (11.5%) | 14 (53.8%) | |
| 12-16 | 29 (40.8%) | 10 (34.5%) | 7 (24.1%) | 5 (17.2%) | 7 (24.1%) | |
| > 16 | 16 (22.1%) | 7 (43.8%) | 2 (12.5%) | 9 (12.7%) | 6 (37.5%) |
Group 1: participants with improvements in peak VO2 greater that 10%
Group 2: participants with improvements in peak VO2 less than 10%
Group 3: participants with reductions in peak VO2 less than 10%
Group 4: participants with reductions in peak VO2 greater that 10%
Table 1.B.
Baseline Characteristics (Clinical)
| All participants (N=71) |
Group 1a (n = 19) |
Group 2b (n = 16) |
Group 3c (n = 9) |
Group 4d (n = 27) |
P value | |
|---|---|---|---|---|---|---|
| Ejection fraction, % (Mean±SD) | 26.68 ± 6.61 | 24.86 ± 6.40 | 29.00 ± 8.22 | 28.33 ± 6.49 | 26.02 ± 5.47 | .144 |
| Peak VO2, mg/kg/min (Mean±SD) | 13.72 ± 3.38 | 12.01 ± 2.92 | 13.95 ± 3.09 | 14.50 ± 6.49 | 14.50 ± 3.50 | .144 |
| Six minute walk, feet (Mean±SD) | 1436.86 ± 209.47 | 1457.18 ± 176.63 | 1357.57 ± 167.52 | 1573.25 ± 331.52 | 1420.33 ± 189.50 | .799 |
| NYHA class, N (%) | .539 | |||||
| Class II | 56 (78.9%) | 13 (68.4%) | 14 (87.5%) | 8 (88.9%) | 21 (77.8%) | |
| Class III | 13 (18.3%) | 5 (26.3%) | 1 (6.3%) | 1 (11.1%) | 6 (22.2%) | |
| Class IV | 2 (2.8%) | 1 (5.3%) | 1 (6.3%) | 0 (0%) | 0 (0%) | |
| HF Etiology, Ischemic N (%) | 30 (42.3%) | 7 (36.8%) | 6 (37.5%) | 4 (44.4%) | 13 (48.1%) | .855 |
| History of Hypertension, yes N (%) | 32 (45.1%) | 9 (47.4%) | 8 (50.0%) | 3 (33.3%) | 12 (44.4%) | .873 |
| History of Diabetes, yes N (%) | 22 (31.0%) | 6 (31.6%) | 5 (31.3%) | 4 (44.4%) | 7 (25.9%) | .780 |
| History of Dyslipidemia, yes N (%) | 34 (47.9%) | 9 (47.4%) | 7 (43.8%) | 4 (44.4%) | 14 (51.9%) | .955 |
| History of Depression, yes N (%) | 37 (52.1%) | 6 (16.2%) | 9 (24.3%) | 13 (35.1%) | 9 (24.3%) | .367 |
| Medications use, N (%) | ||||||
| ACE Inhibitors | 52 (73.2%) | 11 (57.9%) | 13 (81.3%) | 7 (77.8%) | 21 (77.8%) | .364 |
| Angiotensin Receptor Blockers | 13 (18.3%) | 5 (26.3%) | 3 (18.8) | 1 (11.1%) | 4 (14.8%) | .718 |
| Beta-Blockers | 50 (70.4%) | 15 (78.9%) | 9 (56.3%) | 7 (77.8%) | 19 (70.4%) | .486 |
| Diuretics | 61 (85.9%0 | 16 (84.2%) | 12 (75.0%) | 8 (88.9%) | 25 (92.6%) | .443 |
| Aldactone | 21 (29.6%) | 5 (26.3%) | 6 (37.5%) | 1 (11.1%) | 9 (33.3%) | .525 |
| Digoxin | 49 (69.0%) | 12 (63.2%) | 12 (75.0%) | 5 (55.6%) | 20 (64.1%) | .646 |
| Anti-depressants | 27 (38.0%) | 3 (11.1%) | 8 (29.6%) | 9 (33.3%) | 7 (25.9%) | .851 |
Group 1: participants with improvements in peak VO2 greater that 10%
Group 2: participants with improvements in peak VO2 less than 10%
Group 3: participants with reductions in peak VO2 less than 10%
Group 4: participants with reductions in peak VO2 greater that 10%
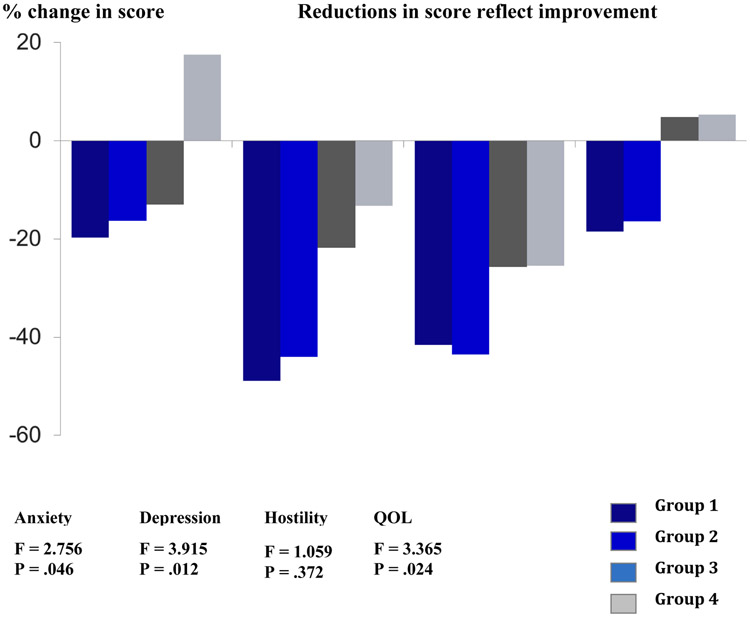
Table 2 summarizes the changes in mood disorders and QOL across the four groups from baseline to 6 months. Over time, all participants demonstrated significantly lower levels of depression and hostility (P < .001) and significantly higher levels of physical and overall QOL (P = .046). Group differences over time were noted in anxiety (P =.009), depression (P =.015), physical QOL (P <.001) and overall QOL (P =.002). Pairwise comparisons between the four groups on levels of anxiety and depression and physical and overall QOL were statistically significant between participants who demonstrated any improvement in exercise capacity (Group 1 and 2) when compared to participants who showed any reduction in exercise capacity (Group 3 and 4) (P<.05). Change scores for the four groups between baseline and 6 months are summarized in Figure 1. All scores showed decreasing effects in the psychological outcomes as changes in exercise capacity worsened.
Table 2.
Baseline and 6-month outcomes (N = 71)
| Group 1a (n = 19) | Group 2b (n = 16) | Group 3c (n = 9) | Group 4d (n = 27) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Baseline | 6-Month | Baseline | 6-Month | Baseline | 6-Month | Baseline | 6-Month | P (time) |
P (TxG) |
|
Mood Disorder (MAACL) |
||||||||||
| Anxiety | 7.6 ± 4.3 | 6.1 ± 4.4 | 8.6 ± 4.5 | 7.2 ± 4.4 | 9.2 ± 4.0 | 8.0 ± 3.6 | 7.4 ± 3.8 | 8.7 ± 4.0 | .087 | .009 |
| Depression | 13.3 ± 7.1 | 6.8 ± 3.8 | 14.3 ± 5.7 | 8.0 ± 3.2 | 15.1 ± 6.7 | 11.8 ± 5.8 | 14.4 ± 5.7 | 12.5 ± 4.9 | .000 | .015 |
| Hostility | 13.0 ± 6.9 | 7.6 ± 4.5 | 15.4 ± 5.2 | 8.7 ± 3.8 | 16.7 ± 6.2 | 12.4 ± 6.4 | 14.9 ± 7.4 | 11.1 ± 5.5 | .000 | .277 |
|
Quality of Life (MLHFQ) |
||||||||||
| Physical | 21.7 ± 9.5 | 16.9 ± 8.5 | 19.9 ± 10.7 | 17.4 ± 10.7 | 19.7 ± 8.8 | 19.9 ± 6.9 | 20.1 ± 9.4 | 22.0 ± 11.4 | .046 | .000 |
| Emotional | 10.8 ± 6.8 | 9.3 ± 6.3 | 11.8 ± 6.8 | 10.8 ± 8.4 | 13.1 ± 5.3 | 13.5 ± 4.5 | 12.1 ± 6.6 | 13.4 ± 8.1 | .807 | .325 |
| Overall | 48.0 ± 18.1 | 39.1 ± 20.4 | 43.9 ± 22.9 | 36.7 ± 21.7 | 45.5 ± 22.9 | 47.7 ± 20.9 | 46.9 ± 21.0 | 49.4 ± 25.0 | .046 | .002 |
Values are given as mean ± SD. T X G, time by group interaction
MAACL, Multiple Adjective Affect Check List; Scores for anxiety, depression, and hostility range from 0 to 21 (norm 7 or below), 0 to 40 (norm 11), and 0 to 28 (norm 7), respectively. Higher scores reflect higher levels of dysphoria.
MLHFQ, Minnesota Living with Heart Failure Questionnaire Higher scores indicate greater symptom interference and lower QOL
Group 1: participants with improvements in peak VO2 greater that 10%
Group 2: participants with improvements in peak VO2 less than 10%
Group 3: participants with reductions in peak VO2 less than 10%
Group 4: participants with reductions in peak VO2 greater that 10%
Figure 1.
Percent change from baseline to 6 months (N=71)
The correlation matrix for the key variables is presented in Table 3. Greater improvement in exercise capacity was strongly associated with lower depression but only weakly associated with lower anxiety and hostility and higher emotional QOL. An improvement in exercise capacity was not associated with changes in physical and overall QOL. Younger age and less education were associated with increased dysphorias and lower QOL. Higher levels of anxiety, depression, and hostility were associated with poorer QOL.
Table 3.
Correlational matrix of key variables (N=71)
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Group | 1.000 | ||||||||||
| 2. Age | −.016 | 1.000 | |||||||||
| 3. Education | .153 | −.077 | 1.000 | ||||||||
| 4. Ejection Fraction | −.022 | .003 | −.014 | 1.000 | |||||||
| 5. NYHA Class | .106 | −.225 | .015 | −.067 | 1.000 | ||||||
| 6. MAACL,a anxiety, 6m | −.281* | −.160 | −.254* | −.020 | −.104 | 1.000 | |||||
| 7. MAACL,a depression, 6m | −.491† | −.251* | −.400† | .036 | −.022 | .594† | 1.000 | ||||
| 8. MAACL,a hostility, 6m | −.293* | −.173 | −.331† | −.031 | −.151 | .670† | .544† | 1.000 | |||
| 9. MLHFQ,b physical, 6m | −.221 | −.063 | −.214 | .048 | −.073 | .262* | .388† | .310† | 1.000 | ||
| 10. MLHFQ,b emotional, 6m | −.238* | −.142 | −.255* | .133 | −.158 | .355† | .455† | .307† | .544† | 1.000 | |
| 11. MLHFQ,b overall, 6m | −.219 | −.209 | −.240* | .094 | −.193 | .254* | .440† | .368† | .880† | .650† | 1.000 |
Correlation is significant at the 0.01 level (2-tailed)
Correlation is significant at the 0.05 level (2-tailed).
Multiple Adjective Affect Check List; Higher scores reflect higher levels of dysphoria.
Minnesota Living with Heart Failure Questionnaire; Higher scores indicate greater symptom interference and lower QOL
DISCUSSION
In this secondary analysis of data from a HF home exercise program, we examined the clinical outcomes of mood disorders and QOL in those participants who participated in exercise training (i.e., who were randomized to the experimental arm).30 In the parent trial, we had hypothesized that both mood disorders and QOL would improve as a consequence of exercise training. Similar to previous investigators,22 we found no difference in these outcomes between the experimental and control groups, although the trends were in the hypothesized direction.30 Therefore, we used a secondary analysis to compare participants in the exercise group who had positive changes in peak V02 from baseline with those participants who had negative changes in V02 from baseline in an attempt to understand the relationship between exercise and psychological outcomes. We found significant differences over time in anxiety, depression, physical QOL and overall QOL in the four patient groups. Greater improvement in exercise capacity was also strongly associated with lower depression scores. Thus it appears that positive psychological outcomes occurred with exercise training in those participants who experienced an increase in exercise capacity. These positive findings are validated by other studies that reported the impact of exercise training on psychological effects among participants with HF.18,36,37
Prior to our analysis, several competing theories could be offered to explain the lack of effect of exercise training on psychological outcomes. First, exercise may not be a powerful enough intervention to alter the emotional response of participants, many of whom have lived with a chronic, progressive syndrome for years. The development of complications (e.g., atrial arrhythmias) and the unplanned hospital admissions due to acute decompensation may affect the emotional status of these participants far more than participation in an exercise regimen. This interpretation is supported by data from the parent study. Hospitalizations occurred in 43% of participants in the control group and 40% of participants in the exercise group (P=0.75).38 Similarly, in the largest HF exercise trial conducted to date, the percent of participants who experienced a re-hospitalization over the period of follow-up (mean two and one-half years) were 63% in the exercise arm compared to 65% in the control arm. When examining the number of hospitalizations specifically for HF between the two groups, 30% in the exercise group had a total of 344 hospitalizations, while 34% in the control groups had a total of 393 hospitalizations (HR, 0.87 [95% CI, 0.75–1.00]; P = .06).38 Thus, participants in both the exercise and control groups experienced a significant number of hospitalizations, suggesting a relatively high vulnerability to acute decompensation, whether or not the participant exercised regularly.
A second theory is that participants with a certain clinical profile may adhere to the exercise prescription but may not achieve optimal benefit and therefore not experience positive psychological changes. Randomization procedures should insure that participants with a variety of clinical profiles (HF etiology, ejection fraction, peak VO2, medications, etc.) are equally represented across the treatment groups. But within the exercise arm, participants may have certain clinical characteristics that mute their response to exercise. This explanation is less convincing given our comparison of the four groups on a variety of sociodemographic and clinical variables. No significant differences were noted across groups. In particular, it can be noted that baseline peak VO2 values were not significantly different among the four groups.
A final explanation for the frequently conflicting findings related to exercise and emotional outcomes in HF is that participants’ adherence to an exercise regimen erodes with time. Adherence to exercise was assessed in the parent study by self-report activity logs and pedometer recordings because the exercise was a home-based walking program on city streets. Both techniques have limited accuracy since they rely on self-report or faithful use of the pedometer. However, investigators in the HF-ACTION trial gave subjects in the experimental arm either a bicycle or treadmill following the first 3 months of supervised exercise and used heart rate monitors as well as self-report to measure adherence.38 Adherence to the exercise prescription decreased from a median of 95 minutes per week during months 4 through 6 of follow-up to 74 minutes per week during months 10 through 12. In the third year of follow-up, participants in the exercise group exercised a median of 50 minutes per week (the training goal was 120 minutes). At all time points, only 30% of participants in HF-ACTION exercised at or above the target number of minutes per week.38 Clearly, some participants adhere to the prescribed exercise regimen throughout the study and these may be the ones who benefit psychologically from exercise.
In interpreting our results, it is clear that the resolution of mood disorders and the improvement in QOL in participants with HF was associated with the degree of improvement in exercise capacity. Participants in the two groups who experienced an improvement in peak VO2 may have been more adherent to the exercise protocol than participants who experienced a reduction in peak VO2. Given the limitations of self-report about adherence, this explanation remains speculative. It is also important to note that participants who had documented improvements in exercise capacity experienced significantly fewer hospitalizations than those who had reductions in exercise capacity. Whether fewer hospitalizations were related to higher levels of adherence to exercise (and therefore levels of improved exercise capacity) cannot be determined from our data.
Our findings are limited by being a secondary analysis conducted on subgroups of a parent study. The attention nurses provided to participants in the monthly home visits may have acted as an intervention that muted the power of the exercise intervention to alter the overall QOL of the participants. The age of our sample is considerably younger than the general population of HF participants. The subgroups were not randomized, but rather were identified based on the participants’ differing physiologic response to participation in an exercise training program. Thus, we cannot be sure that the differences in peak VO2 caused the differences noted in mood disorders and QOL. Likewise, our statistical analyses did not take into account the potential overlap between our predictor variables. However, our findings support the interpretation that improvements in physiological benefits may lead to improvements in mood disorders and QOL in patients with HF who participate in an exercise-training program.
CONCLUSION
Patients with HF have mood disorders and reduced QOL that may be positively influenced by exercise training. The psychological benefits of exercise are well documented in healthy populations, but the results of exercise training in the HF population are more controversial with some trials showing negative or mixed results. Based on the findings of the current study, improvement in psychological outcomes is associated with positive changes in exercise capacity as a result of exercise training. It remains unclear if decreases in exercise capacity are related to non-adherence to an exercise protocol or to clinical deterioration despite adherence to exercise.
Acknowledgements:
This research was partially supported by a grant from the American Heart Association Western Division (NCR, 133-09, PI, K. Dracup)
Contributor Information
Lorraine S. Evangelista, Program in Nursing Science, University of California Irvine, Irvine, California.
Marysol Cacciata, Program in Nursing Science, University of California Irvine, Irvine, California.
Anna Stromberg, Department of Medical and Health Sciences and Department of Cardiology, Linkoping University, Linkoping, Sweden.
Kathleen Dracup, University of California San Francisco, San Francisco, California.
References
- (1).Mozaffarian D, Benjamin EJ, Go AS et al. Executive summary: Heart disease and stroke statistics -- 2016 Update: A report from the American Heart Association. Circulation 2016;133:447. [DOI] [PubMed] [Google Scholar]
- (2).Jessup M, Marwick T, Ponikowski P, Voors A, Yancy C. 2016 ESC and ACC/AHA/HFSA heart failure guideline update — what is new and why is it important? Nat Rev Cardiol 2016;13:623–628. [DOI] [PubMed] [Google Scholar]
- (3).Alpert CM, Smith MA, Hummel SL, Hummel EK. Symptom burden in heart failure: assessment, impact on outcomes, and management. Heart Fail Rev 2016;1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Bekelman DB, Rumsfeld J, Havranek EP et al. Symptom burden, depression, and spiritual well-being: a comparison of heart failure and advanced cancer patients. J Gen Intern Med 2009;24:592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Pina IL, Apstein CS, Balady GJ et al. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation 2003;107:1210–1225. [DOI] [PubMed] [Google Scholar]
- (6).Shen BJ, Eisenberg SA, Maeda U et al. Depression and anxiety predict decline in physical health functioning in patients with heart failure. Ann Behav Med 2011;41:373–382. [DOI] [PubMed] [Google Scholar]
- (7).Eckel RH, Jakicic JM, Ard JD et al. 2013 AHA/ACC Guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014;63:2960–2984. [DOI] [PubMed] [Google Scholar]
- (8).Davies EJ, Moxham T, Rees K et al. Exercise training for systolic heart failure: Cochrane systematic review and meta-analysis. Eur J Heart Fail 2010;12:706–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Chan E, Giallauria F, Vigoritto C, Smart N. Exercise training in heart failure patients with preserved ejection fraction: a systematic review and meta-analysis. Monaldi Arch Chest Dis 2016;86:759. [DOI] [PubMed] [Google Scholar]
- (10).Taylor RS, Sagar VA, Davies EJ et al. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev 2014. [DOI] [PMC free article] [PubMed]
- (11).Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation 1999;99:1173–1182. [DOI] [PubMed] [Google Scholar]
- (12).Coats AJ, Adamopoulos S, Radaelli A. Controlled trial of physical training in chronic heart failure: exercise performance, hemodynamics, ventilation and autonomic function. Circulation 1992;85:2119–2131. [DOI] [PubMed] [Google Scholar]
- (13).Smart N, Marwick T. Exercise training for patients with heart failure: A systematic review of factors that improve mortality and morbidity. Am J Med 2004;116:693–706. [DOI] [PubMed] [Google Scholar]
- (14).Adams V, Niebauer J. Reversing heart failure-associated pathophysiology with exercise: What actually improves and by how much? Heart Fail Clin 2015;11:17–28. [DOI] [PubMed] [Google Scholar]
- (15).Keteyian SJ, Pina IL, Hibner BA, Fleg JL. Clinical role of exercise training in the management of patients with chronic heart failure. J Cardiopulm Rehabil Prev 2010;30. [DOI] [PubMed] [Google Scholar]
- (16).Antunes-Correa L, Kanamura B, Melo R et al. Exercise training improves neurovascular control and functional capacity in heart failure patients regardless of age. Eur J Prev Cardiol 2012;19:822–829. [DOI] [PubMed] [Google Scholar]
- (17).Klecha A, Kawecka-Jaszcz K, Bacior B et al. Physical training in patients with chronic heart failure of ischemic origin: effect on exercise capacity and left ventricular remodeling. Eur J Cardiovasc Prev Rehabil 2007;14:85–91. [DOI] [PubMed] [Google Scholar]
- (18).Blumenthal JA, Babyak MA, O’Connor C et al. Effects of exercise training on depressive symptoms in patients with chronic heart failure: The HF-ACTION randomized trial. JAMA 2012;308:465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Chrysohoou C, Tsitsinakis G, Vogiatzis I et al. High intensity, interval exercise improves quality of life of patients with chronic heart failure: a randomized controlled trial. QJM 2013;107:25. [DOI] [PubMed] [Google Scholar]
- (20).Jonsdottir S, Andersen K, Sigurosson A, Sigrosson S. The effect of physical training in chronic heart failure. Eur J Heart Fai 2006;8:97–101. [DOI] [PubMed] [Google Scholar]
- (21).Kitzman DW, Brubaker P, Morgan T et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: A randomized clinical trial. JAMA 2016;315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Flynn KE, Pina IL, Whellan DJ et al. Effects of exercise training on health status in patients with chronic heart failure: Findings from the HF-ACTION randomized controlled trial. JAMA 2009;301:1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Phil E, Cidar A, Stromberg A, Fridlund B, Martensson J. Exercise in elderly patients with chronic heart failure in primary care: effects on physical capacity and health-related quality of life. Eur J Cardiovasc Nurs 2011;10:150–158. [DOI] [PubMed] [Google Scholar]
- (24).Ostman C, Jewiss D, Smart NA. The effect of exercise training intensity on quality of life in heart failure patients: A aystematic review and meta-analysis. Cardiology 2017;136:79–89. [DOI] [PubMed] [Google Scholar]
- (25).Koukouvou G, Kouidi E, Iacovides A, Konstantinidou E, Kaprinis G, Deligiannis A. Quality of life, psychological and physiological changes following exercise training in patients with chronic heart failure. J Rehabil Med 2004;36:36–41. [DOI] [PubMed] [Google Scholar]
- (26).Quittan M, Sturm B, Wiesinger G, Pacher R, Fialka-Moser V. Quality of life in patients with chronic heart failure: a randomized controlled trial of changes induced by a regular exercise program. Scand J Rehabil Med 1999;39:223–228. [DOI] [PubMed] [Google Scholar]
- (27).Chien CL, Lee CM, Wu YW, Chen TA, Wu YT. Home-based exercise increases exercise capacity but not quality of life in people with chronic heart failure: a systematic review. Aust J Physiother 2008;54:87–93. [DOI] [PubMed] [Google Scholar]
- (28).Oka RK, DeMarco T, Haskell WL. Impact of a home-based walking and resistance training program on quality of life in patients with heart failure. Am J Cardiol 2000;85:365–369. [DOI] [PubMed] [Google Scholar]
- (29).Wielenga RP, Erdman RA, Huisveld IA, Bol E, Dunselman JM, Basilier MR. Effect of exercise training on the quality of life in patients with chronic heart failure. J Psychosom Res 1998;45:459–464. [DOI] [PubMed] [Google Scholar]
- (30).Dracup K, Evangelista L, Hamilton M et al. Effects of a home-based exercise program on clinical outcomes in heart failure. Am Heart J 2007;154:877–883. [DOI] [PubMed] [Google Scholar]
- (31).Flynn KE, Lin L, Moe GW et al. Relationships between changes in patient-reported health status and functional capacity in outpatients with heart failure. Am Heart J 2012;163:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Belardinelli R, Georgiou D, Cianci G, Purcaro A. 10-Year exercise training in chronic heart failure: A randomized controlled trial. J Am Coll Cardiol 2012;60:1521–1528. [DOI] [PubMed] [Google Scholar]
- (33).Dracup K, Walden J, Stevenson LW, Brecht L. Quality of life in patients with advanced heart failure. J Heart Lung Transp 1992;11:273–279. [PubMed] [Google Scholar]
- (34).Ni H, Toy W, Burgess D et al. Comparative responsiveness of Short-Form 12 and Minnesota Living with Heart Failure Questionnaire in patients with heart failure. J Card Fail 2000;6:83–91. [DOI] [PubMed] [Google Scholar]
- (35).SPSS User's Guide Version 13.0. 13th ed. Chicago, Illinois: SPSS Inc., 2006. [Google Scholar]
- (36).Nolte K, Herrmann-Lingen C, Wachter R et al. Effects of exercise training on different quality of life dimensions in heart failure with preserved ejection fraction: the Ex-DHF-P trial. Eur J Prev Cardiol 2015;22:582–593. [DOI] [PubMed] [Google Scholar]
- (37).Milani RV, Lavie CJ, Mehra MR, Ventura HO. Impact of exercise training and depression on survival in heart failure due to coronary heart disease. Am J Cardiol 2011;107:64–68. [DOI] [PubMed] [Google Scholar]
- (38).O'Connor CM, Whellan DJ, Lee KL et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]