Abstract
Immunoassays such as enzyme-linked-immunosorbent assays (ELISAs) and Western blotting have been the common choice for protein validation studies for the past several decades. Technical advancements and modifications are continuously being developed to enhance the detection sensitivity of these procedures. Among them, streptavidin containing Poly-horseradish peroxidase (PolyHRP) based detection strategies have been shown to improve signals in ELISA. The use of commercially available streptavidin and antibodies conjugated with many HRPs (PolyHRPs) to potentially enhance the detection sensitivity in Western blotting has not been previously investigated in a comprehensive manner. The use of PolyHRP-secondary antibody instead of HRP-secondary antibody increased the Western blotting sensitivity by up to 85% depending on the primary antibody used. The use of a biotinylated secondary antibody and commercially available streptavidin conjugated with HRP or PolyHRP all resulted in increased sensitivity with respect to antigen detection. Utilizing a biotinylated secondary antibody and streptavidin conjugated PolyHRP resulted in as much as a 110-fold increase in Western blotting sensitivity over traditional Western blotting methods. Quantification of troponin I in rat heart lysates showed that the traditional Western blotting method only detected troponin I in ≥ 2μg of lysate while streptavidin conjugated PolyHRP20 detected troponin I in ≥ 50ng of lysate. A modified blocking procedure is also described that eliminated the interference caused by the endogenous biotinylated proteins. These results suggest that streptavidin conjugated PolyHRP and PolyHRP secondary antibodies are likely to be commonly utilized for Western blots in the future.
Keywords: SDS PAGE, Western blotting, Horseradish peroxidase, PolyHRP, chemiluminescence, Streptavidin-Biotin
1. Introduction
Enzyme-linked-immunosorbent assays (ELISAs) and Western blotting are important in clinical diagnosis and experimental protein analysis. These indirect analytical strategies primarily rely on the binding of an antigen by a specific antibody (primary antibody), followed by the detection of this primary antibody by an enzyme or fluorescently labelled secondary antibody, allowing the semi-quantitative detection of a protein of interest [1–4]. The enzyme-linked secondary antibody is used to amplify and detect the signals from the primary antibody bound to its target antigen [1–4]. Horseradish peroxidase (HRP) and alkaline phosphatase (AP) linked antibodies used for secondary detection have been employed for more than 20 years in both Western blots and enzyme-linked immunosorbent assays (ELISAs) [1–4].
The signal intensity detected for each antigen is influenced by several factors, including antigen abundance, the type of colorimetric and chemiluminescent reagents used, and the quality of the antibody and dilution used [3–5]. Interestingly, there are significant differences in signal intensities generated through the HRP and AP methods [4]. Initially, the HRP colorimetric detection system had lower sensitivity when compared to colorimetric AP detection systems [6]. Several modifications of HRP-based detection strategies have been developed to improve the detection sensitivity using chemiluminescent systems. Over the last decade, more researchers started using HRP chemiluminescent detection systems due to the higher sensitivity compared to colorimetric AP detection systems, as well as being more convenient and cost-effective than AP-based detection methods [4]. One early development to increase immunoblotting sensitivity was the incorporation of biotin on the secondary antibody, and the use of HRP labelled avidin for detection [7]. However, the increase in sensitivity was relatively small (2-fold increase).
Recent developments for increasing the sensitivity of ELISAs include the addition of numerous HRPs (PolyHRPs), up to >80 HRPs on a single protein such as streptavidin (Strep-PolyHRP) [8, 9]. Strep-PolyHRP allows the incorporation of streptavidin-biotin binding that maximizes both capture and detection sensitivity of low abundant or problematic proteins or antigens in immune assays [9, 10]. A recent comparison of three different Strep-PolyHRP’s in ELISA using nanobodies showed a significantly improved sensitivity of detection of the target protein (>140-fold improvement) [11]. Multiple HRP molecules can also be added to the conventional secondary antibodies. The commonly used HRP labeled secondary antibodies seemed to be less effective than the PolyHRP-based detections in ELISA and Western blotting [8, 11, 12]. However, these previously published Western blotting procedures were only qualitative [8, 11, 12].
There are several engineered Strep-PolyHRPs that are commercially available. However, data regarding their suitability and effectiveness in Western blotting-based detection are sparse. To investigate if antibodies or streptavidin conjugates containing PolyHRP would significantly enhance Western blotting sensitivity, we designed our study to explore these engineered detection systems regarding their effectiveness and sensitivity in Western blotting. The commercially engineered conjugates (PolyHRP, Strep-HRP, Strep-PolyHRP20, Strep-PolyHRP80) and conventional HRPs were analyzed and compared via conventional and a modified Western blotting procedure as depicted in Fig. 1. The results of our study suggest that these modified streptavidin-conjugated polymer-HRPs have several-fold increases in signal intensities of proteins and can be used to detect antigens present at very low concentrations. Overall, the use of PolyHRP and streptavidin-conjugated PolyHRP has many advantages over traditional HRP-based secondary antibodies.
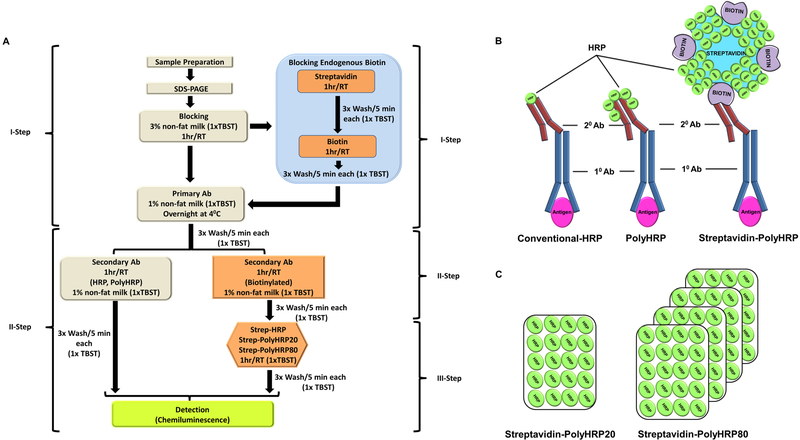
Figure 1.
Schematic representation of the modified Western blotting method. (A) Modified 3-step Western blotting method including the blocking of endogenous biotin method. (B) Diagram showing how antigens are detected using the conventional-HRP, PolyHRP, Streptavidin-conjugated PolyHRP (C) Schematic representation of Streptavidin-conjugated PolyHRP20 and Streptavidin-conjugated PolyHRP80. Ab, antibody; TBST, tris buffered saline containing tween 20; RT, room temperature.
2. Materials and method
2.1. Chemicals and reagents
Ponceau S was obtained from Alfa Aesar (MA, USA, catalog #J60744) and Tween-20 was obtained from Amresco (OH, USA, catalog # M147). Purified human troponin I was obtained as previously described [13, 14]. Primary antibodies for HSP40 (Santa Cruz Biotechnology, TX, USA, catalog #sc-398766), 20S proteasome β1 (Santa Cruz Biotechnology TX, USA, catalog# sc-67345), PSMA6 (Abcam, MA, USA, catalog# 109377), PSMA3 (Epitomics, CA, USA, catalog #3766–1), troponin I (Abcam, MA, USA catalog# 10225) as well as secondary antibodies, conventional antibodies labelled with HRP (Goat anti-mouse HRP, Sigma-Aldrich, MO, USA, catalog # A9044, Goat anti-rabbit HRP, Sigma-Aldrich, MO, USA, catalog # A0545 ), Biotinylated HRPs (Goat anti-mouse IgG, Kirkegaard and Perry Laboratories (KPL), MO, USA, catalog #16–18-06, Goat anti-rabbit, KPL, MO, USA, catalog #16–15-06), Goat anti-rabbit PolyHRP (PolyHRP, Thermo Scientific, MA, USA, 32260), Streptavidin-HRP (Thermo Scientific, USA, catalog# N100), Streptavidin-PolyHRP20 (Fitzgerald, MA,USA, catalog #65R-S107) and Streptavidin-PolyHRP80 (Fitzgerald, MA, USA, catalog # 65R-S105) were purchased commercially. The blocking reagents used for the modified Westerns, streptavidin (catalog #21122) and biotin (catalog # 230090010), were obtained from Thermo Scientific (MA, USA) and Arcos Organics (MA, USA) respectively. Pre-casted 4–20% gradient gels (catalog # 5671095), nitrocellulose membrane (catalog # 10026936), laemmli buffer (catalog #161–0737), and non-fat dry milk (catalog #170–6404) were obtained from Bio-Rad (CA, USA) while other laboratory reagents were purchased from Thermo Scientific, MA, USA.
2.2. Sample preparation
Heart tissue was obtained from healthy six week-old rats (Pel-Freez Biologicals, AK, USA). Heart tissue was kept at −80οC until needed, and then quickly chopped into small pieces at 4οC and total protein isolated through glass-based hand-held homogenization in ice-cold buffer, (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 5 mM MgCl2, pH 7.5). Samples were further cleared by centrifugation at 15000 x g for 15 minutes at 4οC and the supernatant (cytosolic fraction) removed. The supernatant concentrations were measured using a NanoDrop 2000c spectrophotometer (Thermo Scientific, MA, USA).
2.3. Western-immunoblotting
i). Conventional 2-step immunoblotting
Total protein heart samples were heated for 5 minutes at 95οC with laemmli buffer containing 5% (v/v) β-mercaptoethanol (Sigma-Aldrich, MO, USA, catalog # M3148) and loaded on 4–20% polyacrylamide gels in three different concentrations (500ng, 2μg, and 10μg). Resolved proteins were transferred to a nitrocellulose membrane and blocked with 3% non-fat milk in tris buffer saline (TBS) containing 0.05% Tween-20® (TBST) for 1 hour at room temperature (RT). Blots were further incubated with the primary antibodies at 4οC overnight in 1% non-fat milk in 1x TBST. After primary antibody incubation, blots were washed three times in 1x TBST for 5 minutes each and hybridized with either conventional HRP (1:15000) or PolyHRP (1:10000) at RT for 1 hour in 1% non-fat milk in 1x TBST. Blots were subsequently washed three times with 1x TBST for 5 minutes each and developed using enhanced chemiluminescence using a commercial chemiluminescent reagent (Clarity, Bio-Rad, catalog # 170–5061).
ii). Modified 3-step immunoblotting
To compare the detection sensitivities between conventional HRP antibodies and Streptavidin-conjugated PolyHRPs, a modified 3-step protocol was used that was based on streptavidin-biotin affinity (Fig. 1A). In this method, after primary antibody hybridization, blots were washed and incubated with the corresponding biotinylated secondary antibodies (1:10000) for 1 hour at RT with gentle shaking in 1% non-fat milk in 1x TBST. As an additional step, blots were further washed for three times with 1x TBST for 5 minutes each and incubated with either Streptavidin-HRP or Streptavidin-conjugated PolyHRP antibodies (Strep-HRP20 and Strep-HRP80) at 1:40000 dilutions in 1x TBST for 1 hour at RT. Blots were further washed three times in 1x TBST and developed with the same enhanced chemiluminescence method used for the conventional method described above.
iii). Modified blocking
Streptavidin is known for its strong binding to biotin, and one streptavidin can interact with four biotin molecules. Moreover, endogenous biotin that is an integral part of some proteins in different lysates may interfere in the specificity of Streptavidin-conjugated-HRP antibodies (Strep-HRP, Strep-PolyHRP20, and Strep-PolyHRP80) [15]. As such, we explored the incorporation of additional blocking steps for some experiments in which after conventional blocking with 3% non-fat milk, endogenous biotins were blocked with an excess of streptavidin (0.05mg/ml) for 1 hour at RT followed by three washes in 1x TBST for 5 minutes (Fig.1A). Since streptavidin contains high-affinity binding sites for biotin, these sites were further blocked by adding exogenous biotin (0.25mg/ml) dissolved in 1x TBST at RT for 1 hour and washed with 1x TBST for 5 minutes 3 times each. These blots were then further processed through the similar steps as mentioned for conventional Western blotting (Fig.1A).
To further validate the modified blocking procedure separate experiments were carried out where 10 mM of exogenous biotin was added to heart lysates and Western blots carried out as described above.
iv). Pre-hybridization method
We also utilized a previously described method to avoid endogenous biotin-induced interference in Western blots [16]. In this method, pre-hybridization complexes of Streptavidin-PolyHRP20 (0.01 mg/ml) and corresponding biotinylated-IgG antibody (0.05 mg/ml) were prepared through mixing and incubated for 1 hr at RT. The free biotin sites on the Streptavidin-PolyHRP20-biotinylated-IgG complex was then blocked with free biotin (0.06mg/ml) for 1hr at RT. After conventional blocking, the pre-hybridization complex was diluted with 1x TBST and incubated with blots for 1hr at RT followed by 3x washing for 5 minutes each, and the signal developed using chemiluminescence.
v). Statistical analysis
Band signal intensities were recorded by Image Lab® Software (Bio-Rad) and normalized with intensities obtained from loading control i.e., commercial Ponceau S. One-way ANOVA was done using a Sigma Plot® software (Systat Software Inc). All the experiments were carried out at least three times.
3. Results
To evaluate the effectiveness of Strep-PolyHRP conjugates as the detection system in the Western blotting, several commercially available conventional HRPs, PolyHRP, Streptavidin-HRP conjugates (Strep-HRP, Strep-PolyHRP20, Strep-PolyHRP80) were investigated. Primary antibodies previously used and validated in our laboratory: HSP40, 20S proteasome β1, PSMA6 and PSMA3 were utilized on heart lysates. Additionally, signal intensities were compared with conventional Western blotting methods. To eliminate the interference caused by endogenous biotin, a published blocking method was evaluated, and a modified blocking method was incorporated into the conventional Western blotting protocol for some proteins.
3.1. Heat shock protein-40 (HSP40)
We first compared Strep-HRP, Strep-PolyHRP20, Strep-PolyHRP80 conjugates using an antibody directed against the heat shock protein-40, HSP40. To test the detection limits of engineered streptavidin-conjugated HRPs and PolyHRP on protein concentration, three different concentrations of total protein samples were used (500ng, 2μg, and 10μg). The blots were stained with Ponceau S and the images used for normalization of the band intensities detected for the 2μg and 10μg of heart lysate. Ponceau S was unable to detect 500ng of total protein. As such, due to limited detection capabilities of Ponceau S, the relative sensitivities were not normalized for the 500ng group.
Our results suggest that all three engineered secondary antibodies (Strep-PolyHRP, Strep-PolyHRP20, and Strep-PolyHRP80) were able to detect HSP40 in heart lysates for all the concentrations used (Fig. 2 A-D). Strep-PolyHRP80 has up to a 22% enhanced signal over the Strep-PolyHRP20 for 10μg of total protein (Fig 2 A, D), which was statistically significant. Interestingly, Strep-PolyHRP20 and Strep-PolyHRP80 showed a 5–6-fold increase in sensitivity compared to Strep-HRP for lanes containing 500ng and 2μg of heart lysates. As the amount of target protein increased (such as when 10μg of heart lysate was used) the increase in sensitivity was only about 1.7–2-fold. In contrast, conventional HRP was not able to detect HSP40 in the lanes containing 500ng of heart lysates. While the conventional Western blots were able to detect HSP40 in lanes containing 2μg of heart lysate, the Strep-PolyHRP20 and Strep-PolyHRP80 showed >110-fold increases in signal intensities compared to the conventional Western blots. These increases in signal intensities are an underestimation since for HSP40 to be detected using the conventional Western blotting at the same imaging time as the Strep-PolyHRP’s the band intensities of the Strep-PolyHRP’s are all completely saturated (Fig. 2 E-G). Hence, the bands in Fig. 2 E-G, for the Strep-HRP and Strep-PolyHRP’s are not within a linear relationship with HSP40 protein concentration. As such, the increase in sensitivity is likely to be a significantly >110-fold increase. The lanes containing 10μg of heart lysate showed about 15–20-fold detection sensitivity increases for Strep-HRP and Strep-PolyHRP’s compared to conventional Western blots. As noted above, the values for the Strep-HRP and Strep-PolyHRP’s are also an underestimation since these bands were also saturated when bands detected using conventional Western blots are imaged and visible at the same time. Fig. 2H shows a graph of the correlation between the amount of heart lysate versus the band intensity for HSP40. Overall, our data suggest that there was an overall increase in detection signals for both Strep-PolyHRPs (Strep-PolyHRP20 and Strep-PolyHRP80) compared to Strep-HRP alone (Fig. 2 A-D), where Strep-PolyHRP80 produced highest detection sensitivity over Strep-PolyHRP20 conjugates.
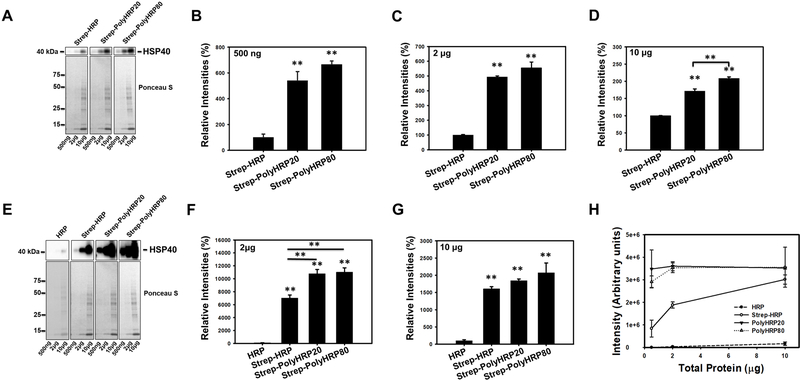
Figure 2.
Western Blots of rat heart lysates probed for heat shock protein 40 (HSP40) using Streptavidin-HRP (Strep-HRP) or Streptavidin-conjugated PolyHRPs (Strep-PolyHRP20, and Strep-PolyHRP80). (A) Figure shows representative unsaturated bands of HSP40 detected in 500ng, 2μg, and 10μg of heart lysates, and corresponding Ponceau S stained membranes. B-D shows the quantification of the HSP40 blots. Ponceau S was used for normalization of the Western blotting results. (B-D) Quantification of HSP40 using Strep-HRP, Strep-PolyHRP20, and Strep-PolyHRP80 on 500ng (B), 2μg (C), and 10μg (D) of heart lysate. (E) The upper figure shows representative blots of HSP40 detected in 500ng, 2μg, and 10μg of heart lysates. To detect the HSP40 using classical Western blotting methods together with the PolyHRP based methods the HSP40 detected using PolyHRPs had to be overexposed for the 2 and 10 μg of heart lysates. The lower figure shows Ponceau S staining which was used for normalization of the blots. Quantification of HSP40 using HRP, Strep-HRP, Strep-PolyHRP20, and Strep-PolyHRP80 on 2μg (F), and 10μg (G) of heart lysate with the bands using Strep-HRP, Strep-PolyHRP20, and Strep-PolyHRP80 all being saturated. (H) Graph showing the correlation between the amounts of heart lysate versus band intensity for HSP40. Values are means ± SE; (n = 3) per group. *P<0.05, **P<0.01.
3.2. 20S proteasome β1
We utilized an antibody to the 20S proteasome β1 subunit to further evaluate the effectiveness of the engineered Streptavidin conjugates (Strep-HRP, Strep-PolyHRP20, Strep-PolyHRP80) (Fig. 3A-D). Western blotting results showed enhancement of signals with all streptavidin conjugates (Strep-HRP, Strep-PolyHRP20, Strep-PolyHRP80) (Fig. 3A, B). As observed when using the β1 subunit antibody, the signal intensities were higher for both Strep-PolyHRP20 (4.8-fold), and Strep-PolyHRP80 (8.2-fold) compared to Strep-HRP (Fig. 3A, B). The Strep-PolyHRP80 exhibited 1.7-fold more intense detection signals compared to Strep-PolyHRP20 (Fig. 3 A, B), which was statistically significant. When the β1 subunit was detected using the conventional HRP Western blotting method, the β1 subunit bands were over-exposed (saturated) for all the other Strep-HRP and Strep-PolyHRP’s conjugates (Fig. 3C, D). Hence, an underestimate of the increased sensitivity of the Strep-HRP compared to the conventional HRP is that sensitivity increased 30-fold. At the time point where conventional HRP and Strep-HRP were compared, the Strep-PolyHRP20 and Strep-PolyHRP80 bands were even more over-exposed and not quantified (Fig. 3 C, D). Considering the Strep-PolyHRP80 was 8-fold greater than the Strep-HRP, and the Strep-HRP was 30-fold greater than the conventional HRP, the sensitivity using the Strep-PolyHRP80 could be as much as 240-fold greater than the conventional Western blot. To quantify the minimal total protein needed to be able to detect cardiac troponin I, a heart specific protein, Western blots (using anti-troponin I, 1:5000) were carried out using the conventional method as well as using Strep-HRP20. While troponin I was detectable in 50ng of total protein using the 3 step PolyHRP20 procedure, troponin I was only detectable in ≥2μg of total protein (Fig. 3E). Using purified human cardiac troponin I as a standard 14.2 ng of troponin I was detected in 10μg of total protein using the conventional Western blotting system. Using the 3 step PolyHRP20 13.8 ng of troponin I was detected in 500ng of total protein. The antibody used in these experiments recognized the same epitope in human and rat cardiac troponin I.
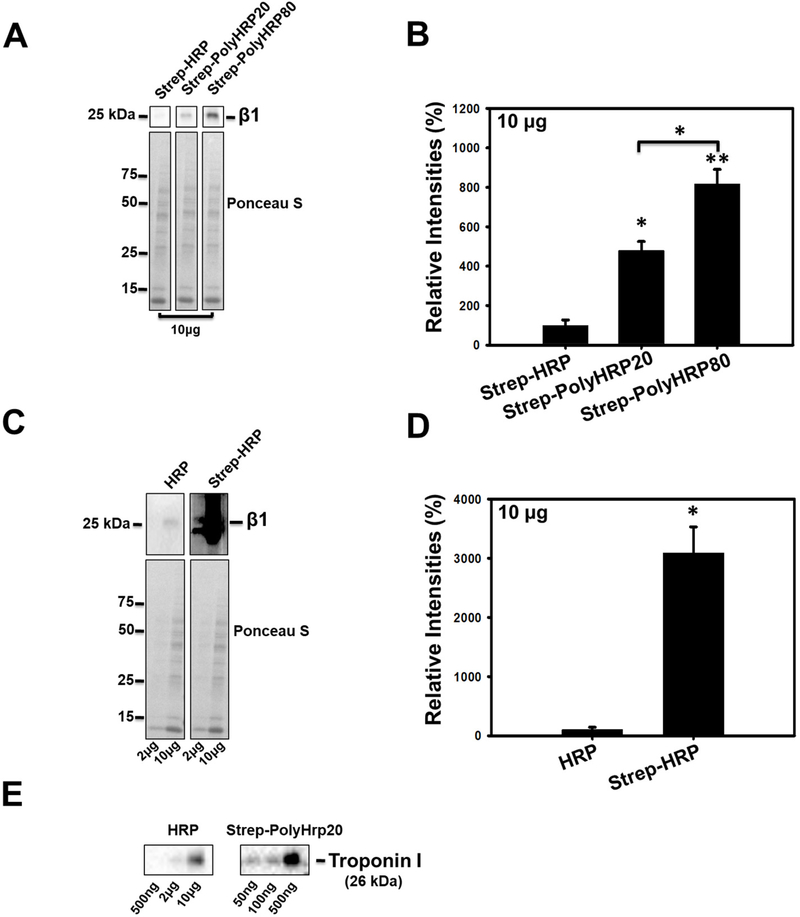
Figure 3.
Western Blots of rat heart lysates probed for proteasome β1 subunit using Streptavidin-HRP or Streptavidin-conjugated PolyHRPs. (A) Blots of β1 in 10μg of heart lysate using Strep-HRP, Strep-PolyHRP20, and Strep-PolyHRP80. The lower figure shows the Ponceau S stained membranes which were used for normalization. (B) Bar Charts showing the quantification of the proteasome β1 in 10μg of heart lysate using Strep-HRP, Strep-PolyHRP20, and Strep-PolyHRP80 (non-saturated). The conventional Western blotting method can detect the β1 subunit but when these blots are detected under the same conditions as the Strep-HRP blots, the β1 subunit bands in the Strep-HRP blots are saturated. (D) The bar chart shows the quantification of the β1 subunit in 10μg of heart lysate using conventional HRP and Strep-HRP (saturated). (E) Western blots of troponin I in heart lysates using the conventional Western blotting method and the three step PolyHRP20 method. Values are means ± SE; (n = 3) per group. *P<0.05, **P<0.01.
3.3. PSMA3 and PSMA6
To determine if the use of goat anti-rabbit PolyHRP instead of conventional goat anti-rabbit HRP (both used as secondary antibodies) significantly increased Western blotting sensitivity, we utilized two proteasome antibodies, anti-PSMA6 and anti-PSMA3. Comparisons with Strep-PolyHRP20 was also performed for the anti-PSMA6 antibody. Our data suggest that the PolyHRP significantly increased the PSMA6 band intensity by 1.85-fold compared to conventional HRP (Fig. 4A, B). The Strep-PolyHRP20 conjugate showed a 16-fold increase in sensitivity compared to the PolyHRP (Fig. 4C, D). To determine if the increased sensitivity of detection for the PolyHRP is common to all antibodies, anti-PSMA3 was utilized. There was a significant increase (0.89-fold) in detection sensitivity for 2 μg (Fig. 4E, F). Although a trend towards an increase in the sensitivity of detection using the PolyHRP was observed for 10μg, it was not statistically significant (Fig. 4E, G). These results suggest that the PolyHRP may be beneficial to increase the sensitivity of detection of low abundant, but not all target proteins.
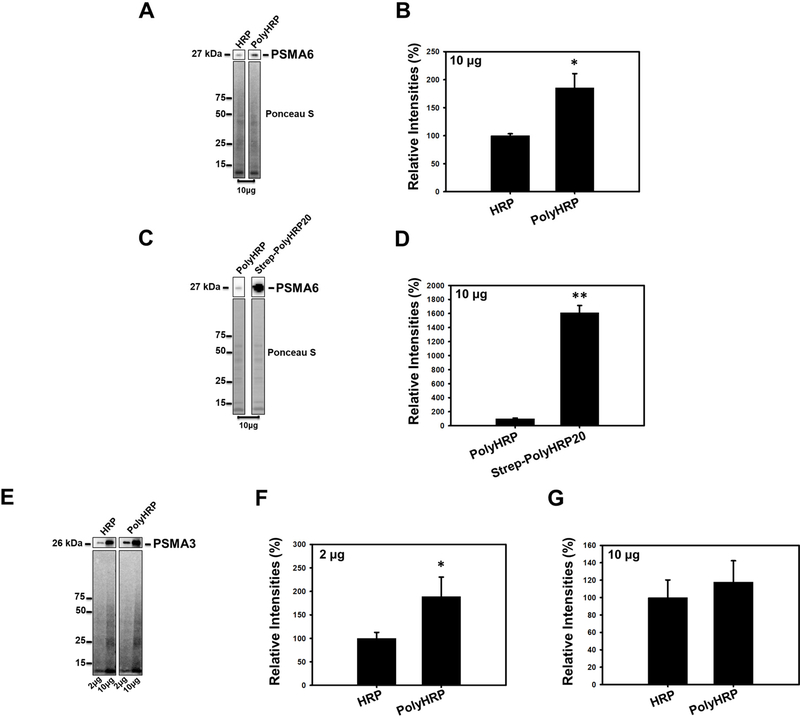
Figure 4.
Western Blots of rat heart lysates probed for proteasome PSMA6 and PSMA3 subunits using conventional HRP, PolyHRP and Streptavidin-conjugated PolyHRP20s. (A) Blot showing detection of PSMA6 in 10μg of heart lysate using conventional HRP and PolyHRP (goat anti rabbit HRP). (B) Quantification of PSMA6 on blots using HRP and PolyHRP. (C) Blot showing detection of PSMA6 in 10μg of heart lysate using PolyHRP and Strep-PolyHRP20. (D) Quantification of PSMA6 on blots using PolyHRP and Strep-PolyHRP2. (E) Blots of PSMA3 using conventional HRP and PolyHRP in 2μg of heart lysate. (F) Quantification of PSMA3 using conventional HRP and PolyHRP in 2μg and 10μg of heart lysate. (G) Quantification of PSMA3 using conventional HRP and PolyHRP in 10μg of heart lysate. Ponceau S staining of the blots was used for normalization of the results. Values are means ± SE; n = 3 per group. *P<0.05, **P<0.01.
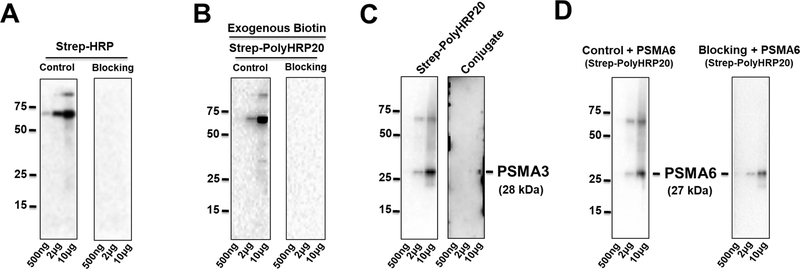
3.4. Blocking Endogenous and Exogenous biotin
Although Strep-HRP conjugates (Strep-HRP, Strep-PolyHRP20, and Strep-PolyHRP80) were shown to effectively enhance the secondary detection signals, endogenous biotins in protein lysate could interfere their detection specificity that may lead to producing ambiguous results [16]. Since the endogenous biotinylated proteins in different lysates give distinct patterns, such as a prominent band at 70 kDa in heart lysates but no bands below this molecular weight (Fig 5. A), it is possible to use the biotin-streptavidin system to detect proteins with molecular weights 55 kDa or less without pre-blocking or removing the endogenous biotin signal. However, since many proteins of interest are near the 70 kDa molecular weight, we evaluated a promising procedure for blocking the endogenous biotin signal in the lysate [16].
Figure 5.
Western Blots incorporating a blocking step for Strep-HRP and Strep-PolyHRP20 for 500ng, 2μg, and 10μg of heart lysate. (A) Modified Streptavidin-biotin blocking for Strep-HRP. (B) Effect of the modified blocking step on blocking endogenous biotin as well as 10 mM of exogenous biotin in heart lysates. (C) Blocking using a pre-hybridization method for Strep-PolyHRP20 for PSMA3; (D) Modified Streptavidin-biotin blocking step for PSMA6 with Strep-PolyHRP20. n = 3 per group. *P<0.05, **P<0.01.
3.5. The Pre-hybridization method for blocking endogenous biotin
This method involves the pre-hybridization of Strep-PolyHRP20 (0.01 mg/ml) and the corresponding biotinylated-IgG antibody (0.05 mg/ml) to form complexes (12). After the complexes are formed, free biotin sites on the streptavidin-Poly HRP20 complex were blocked with free biotin and then the conjugates used to interact with blots that were already probed with either PSMA3 and PSMA6. We were unsuccessful in our attempts to use this method. One such attempt is shown in Fig. 5C. While the blots did not show the endogenous proteins containing biotin, they did not show the target protein band. Also concerning was that we observed several artifacts on the edges of the membrane (Fig. 5C). After a few attempts, we decided that this method would be difficult for most scientists doing Western blotting to be able to optimize.
3.6. Modified blocking for endogenous and exogenous biotin
Using protocols to block endogenous biotinylated proteins before immunohistochemistry with streptavidin reagents, we developed a modified blocking procedure to block endogenous biotin, eliminating the cross-reaction and interference by endogenous biotinylated proteins in the heart lysates (Fig.1 A and Fig. 5A). We incorporated an additional blocking step where endogenous biotins on proteins in the heart lysate were blocked by using exogenous streptavidin to bind the endogenous biotins and then using an excess of biotin to block the other empty sites on streptavidin bound to the endogenous biotin-containing proteins. The excess (unbound) biotin is then washed off and the blot continued by addition of the primary antibody (Fig. 1A).
In separate experiments, exogenous biotin (10 mM) was added to rat heart lysates and after running gels and transferring the lysates containing the exogenous biotin, the modified blocking procedure was carried out and the endogenous and exogenous biotin were found to be blocked by this method (Fig. 5B). Although this step results in additional time being required compared to the common Western blotting procedure, it eliminated the cross-reactivity of endogenous biotin with streptavidin conjugated secondary detections system (Fig. 5 A, B, D).
4. Discussion
Conventional HRPs are some of the most cost-effective entities for secondary detection in the Western blotting procedure. However, their detection sensitives are limited. Antigen concentration, low binding affinity, and low detection signals are the main factors that affect their efficiency in immune detection protocols [1–3]. To help with these issues, several modified HRPs are commercially available that claim superiority to each other. Among these modifications, incorporation of streptavidin, the addition of multiple HRPs, or conjugation of streptavidin with PolyHRPs are suggested to be very effective for secondary signal generation [8–10]. The modified conjugates were used in some immune detection methods such as ELISA [10, 15, 17], immunohistochemistry [10], nano-immune detections [18] and immunosensors [19, 20].
Our data suggest that multiple HRPs molecules on immunoglobins (IgG) (PolyHRP) were more efficient than conventional HRPs regarding the signal intensity of target detection, especially at lower total protein concentrations (such as 2μg). This enhancement may be due to the increased availability of signal generating HRP to a particular antigen, where multiple numbers of HRPs resulted in a collective enhancement of signals [8]. Since the cost of the PolyHRP is similar to the conventional HRP, it is worthwhile for investigators to try using the PolyHRP to determine if it is beneficial for their target protein.
Addition of a third step, instead of the classical two-step procedure shown in Fig. 1A, results in a Western blot that takes 75 mins longer than the traditional Western blot. The Western blotting sensitivity should be significantly increased for any increase in the procedure time required to be worthwhile. Our results strongly suggest that all Streptavidin-conjugates (Strep-HRP, Strep-PolyHRP20, Strep-PolyHRP80) enhance the Western blot detection sensitivities compared to both conventional HRPs and PolyHRP. These conjugates all worked at four-times higher dilution (1:40000) than conventional and PolyHRPs (1:10000) for all antigen tested in our study. Although these conjugates use an additional step in the Western blotting protocol, the improvement in detection sensitivities of >110-fold as well as the lower concentrations of primary antibody needed, suggests that it is well worth the extra 75 min protocol time.
Since streptavidin has a strong affinity towards biotin and can bind up to four different biotins molecules, it makes a great system for potentially amplifying Western blotting signals. Streptavidin can be easily attached to the antigen-primary antibody conjugates via a biotinylated secondary immunoglobin (IgG) and would provide a large number of HRPs for enhanced detection. Compared to conventional HRPs, these conjugates were extremely effective at detecting target proteins in very low amounts of total protein. Using Strep-HRP20, the same amount of troponin I was detected in 50ng of rat heart lysates as was detected in 2μg of rat heart lysate using the conventional HRP method. In other experiments, the Strep-PolyHRPs were able to routinely detect target proteins in 500ng of heart lysate, something that our conventional Western blotting technique was unable to do.
Although streptavidin-biotin binding strategies work well for Western blotting, this technique suffers from interference caused by endogenous biotins [16, 21, 22]. HRP bound Streptavidin-conjugates can bind to endogenous biotin molecules and may generate a non-specific signal and affect the interpretation of results [16, 21, 22]. Western blots can be carried out without endogenous blocking if controls are carried out to determine what endogenous bands are detected in the sample used. This Strep-PolyHRP method, without blocking of the endogenous biotin signal, would be extremely useful for detecting target proteins in purified or semi-purified samples (such as proteins being purified) as endogenous biotinylated proteins would be reduced or removed and very low amounts of the target protein can be detected by Western blotting.
However, because of the immense potential of this technique, proteins near or at the molecular weight of the endogenous biotinylated proteins may need to be detected. A published pre-hybridization method was initially utilized but was found to be difficult to optimize and obtain satisfactory results. Another blocking method was devised using protocols from immunohistochemistry, and another previously published method for Western blotting as the initial basis [21]. In this method, endogenous biotin was blocked by an excess of streptavidin, and the biotin sites on streptavidin blocked with exogenous biotin [21, 23]. Although the modified blocking step is time-consuming (adding 2.5 hours to the Western blotting procedure), it eliminated the signal generation by Streptavidin-conjugates that was due to the endogenous biotin or exogenous biotin added to the heart lysate. Collectively our study suggests that Streptavidin-conjugates are quite effective in increasing the Western blotting signal intensity.
5. Conclusion
All Streptavidin-PolyHRP conjugates (Strep-PolyHRP20 and Strep-PolyHRP80) resulted in > 2-log increases in antigen detection sensitivity for target proteins when compared to conventional methods utilizing secondary antibodies which contain only one or a few HRP reporter enzymes. Strep-HRP also significantly increased Western blotting sensitivity but this increase was significantly less than the increases observed with Strep-PolyHRP20, and Strep-PolyHRP80. Since the cost of the Strep-HRP is only about 10% cheaper than Strep-PolyHRP, it is better to use Strep-PolyHRP20 or Strep-PolyHRP80 to maximize the Western blotting signal intensity. The results also suggest that PolyHRP antibodies may be useful for some Western Blots. These results suggest that both Streptavidin-HRP conjugates and PolyHRP secondary antibodies are likely to become a commonly used reagent for Western blots.
6 References
- [1].Bass JJ, Wilkinson DJ, Rankin D, Phillips BE, Szewczyk NJ, Smith K, Atherton PJ, Scand J Med Sci Sports 2017, 27, 4–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Drijvers JM, Awan IM, Perugino CA, Rosenberg IM, Pillai S, in: Jalali M, Saldanha FYL, Jalali M (Eds.), Basic Science Methods for Clinical Researchers, Academic Press, Boston: 2017, pp. 119–133. [Google Scholar]
- [3].Mishra M, Tiwari S, Gomes AV, Expert Rev Proteomics 2017, 14, 1037–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gallagher S, Winston SE, Fuller SA, Hurrell JG, Curr Protoc Mol Biol 2008, Chapter 10, Unit 10 18. [DOI] [PubMed] [Google Scholar]
- [5].Ghosh R, Gilda JE, Gomes AV, Expert Rev Proteomics 2014, 11, 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kurien BT, Scofield RH, J Immunol Methods 2003, 274, 1–15. [DOI] [PubMed] [Google Scholar]
- [7].Liu P, Na N, Liu T, Huang L, He D, Hua W, Ouyang J, Proteomics 2011, 11, 3510–3517. [DOI] [PubMed] [Google Scholar]
- [8].Dhawan S, Peptides 2002, 23, 2091–2098. [DOI] [PubMed] [Google Scholar]
- [9].Vasilov RG, Tsitsikov EN, Immunol Lett 1990, 26, 283–284. [DOI] [PubMed] [Google Scholar]
- [10].Charbgoo F, Mirshahi M, Sarikhani S, Saifi Abolhassan M, Biotechnol Appl Biochem 2012, 59, 45–49. [DOI] [PubMed] [Google Scholar]
- [11].Li DY, Cui YL, Morisseau C, Gee SJ, Bever CS, Liu XJ, Wu J, Hammock BD, Ying YB, Anal Chem 2017, 89, 6249–6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fukuda T, Tani Y, Kobayashi T, Hirayama Y, Hino O, Anal Biochem 2000, 285, 274–276. [DOI] [PubMed] [Google Scholar]
- [13].Gilda JE, Xu Q, Martinez ME, Nguyen ST, Chase PB, Gomes AV, Arch Biochem Biophys 2016, 601, 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gomes AV, Liang JS, Potter JD, J Biol Chem 2005, 280, 30909–30915. [DOI] [PubMed] [Google Scholar]
- [15].Lakshmipriya T, Gopinath SC, Tang TH, PLoS One 2016, 11, e0151153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ahmed R, Spikings E, Zhou S, Thompsett A, Zhang T, J Immunol Methods 2014, 406, 143–147. [DOI] [PubMed] [Google Scholar]
- [17].Li D, Ying Y, Wu J, Niessner R, Knopp D, Comparison of monomeric and polymeric horseradish peroxidase as labels in competitive ELISA for small molecule detection, 2013. [Google Scholar]
- [18].Li D, Cui Y, Morisseau C, Gee SJ, Bever CS, Liu X, Wu J, Hammock BD, Ying Y, Anal Chem 2017, 89, 6248–6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lim GS, Seo SM, Paek SH, Kim SW, Jeon JW, Kim DH, Cho IH, Paek SH, Sci Rep 2015, 5, 14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ojeda I, Moreno-Guzman M, Gonzalez-Cortes A, Yanez-Sedeno P, Pingarron JM, Anal Bioanal Chem 2014, 406, 6363–6371. [DOI] [PubMed] [Google Scholar]
- [21].Vaitaitis GM, Sanderson RJ, Kimble EJ, Elkins ND, Flores SC, Biotechniques 1999, 26, 854–858. [DOI] [PubMed] [Google Scholar]
- [22].Banks RE, Craven RA, Harnden PA, Selby PJ, PROTEOMICS 2003, 3, 558–561. [DOI] [PubMed] [Google Scholar]
- [23].Wood GS, Warnke R, J Histochem Cytochem 1981, 29, 1196–1204. [DOI] [PubMed] [Google Scholar]