Abstract
Introduction
Major advances in the field of pediatric oncology have resulted from rigorous, prospective clinical oncology research trials. Optimizing access for all children and adolescents to clinical research trials is an important goal. Barriers to clinical trial enrollment are numerous, involving the health care system, research infrastructure, access to care, providers, and participants. The perspectives of pediatric oncologists may provide insight into the barriers of clinical trial enrollment for this unique population.
Methods and Materials
We conducted qualitative structured interviews over 2 months of pediatric oncologists in a community-based clinical network as part of a quality improvement project aimed at increasing enrollment rates at St. Jude Affiliate Clinics. We assessed barriers and facilitators to clinical trial opportunities for racial and ethnic minority pediatric participants. In the same fiscal year of the interviews, we tracked clinical trial enrollment by race and ethnicity of the participant over 12 months.
Results
The major barriers to clinical trial enrollment for pediatric cancer minority participants included language discordance, travel difficulties, and complex trial designs. In contrast, the major facilitators included building trust with participants and their parents, and education on the merits of clinical research studies. We did not observe any disparities in clinical trial enrollment among the racial and ethnic minority participants of the clinical trials conducted across our network of pediatric oncology clinics.
Conclusions
Identifying barriers and facilitators may improve clinical trial enrollment for under-represented participant groups.
Keywords: Clinical trials, enrollment, disparity
INTRODUCTION
Improved survival rates for childhood cancer patients can be directly attributed to enrollment in clinical research trials. 1-3 Indeed, overall survival of childhood acute lymphoblastic leukemia has dramatically improved over decades of successive clinical research trials. 4 Moreover, enrollment in clinical trials can positively affect participant care because of close monitoring of medical care and access to expert review. 3 The proportion of participants who enroll in pediatric oncology clinical trials outnumbers that of adult oncology clinical trials and suggests that clinical trials provide improved outcomes for children with cancer including access to innovative therapies and increased clinical surveillance. 5,6 Adult clinical oncology trials have documented a disparity of racial and ethnic minorities enrolled, as compared with that of the general population. This disparity limits generalization of the research findings and limits access to novel therapies for racial and ethnic minority participants. 7,8.9 A similar disparity of enrollment, albeit to a lesser degree, exists among racial minority participants in pediatric clinical trials. 10,11 Barriers to clinical trial enrollment can be attributed to multiple components, including the health care system, research infrastructure, individual participants and family members, and doctor–patient relationships. 12-14 The impact of each component may vary depending on the specific clinical trial, the individual patient population or the practice setting. Understanding these barriers may direct interventions to improve the gap for enrollment of minority participants in pediatric clinical trials.
To understand the barriers occurring at the doctor–patient relationship level, investigators have used qualitative research methods to ascertain the perspectives of various stakeholders in several National Cancer Institute (NCI)–designated cancer centers, including referring clinicians. 9 These qualitative interviews showed that referring clinicians cite similar barriers, such as unmet transportation needs, language discordance, and distrust of research. In addition, the referring clinicians relayed that more training with recruitment and a greater awareness of clinical trial availability may facilitate increased clinical trial enrollment for adult participants with cancer. 9
The Affiliate Program at St. Jude Children’s Research Hospital (St. Jude) is a network of eight collaborating institutions working to increase access to pediatric oncology clinical trials. Over the past several years, the affiliates contribute approximately 35% of enrollments on St. Jude-initiated clinical trials, however the range has varied. This work was the initiation of a quality improvement project which is ongoing. The problem statement was, in one fiscal year the affiliates in aggregate contributed 32% of primary therapeutic enrollments, however there was a wide range in accrual rates among the eight affiliates. Our aim statement was to achieve a doubling of clinical trial enrollments contributed by the lowest contributing affiliate and overall accrual of all affiliates at 40% clinical trial enrollment rate by the next fiscal year. The long-term goal was to guide future interventions to further increase access to clinical trials for all patients. We report here the results of the qualitative interviews focused on barriers and facilitators. In addition, we examined whether clinical trial enrollment varied according to the racial and ethnic demographics of the communities served by the St. Jude Affiliate Program in the same fiscal year as the structured interviews.
METHODS
The St. Jude Affiliate Program allows more children to access the novel cancer treatment strategies afforded by a large academic research hospital. Geographically, the eight affiliate clinics are located throughout the southeastern and the midwestern United States and together contribute one-third of the patients enrolled in St. Jude–initiated pediatric oncology clinical trials. All eight clinics serve primarily rural and suburban communities with broad patient demographics (Table 1). The affiliate clinics are in Baton Rouge, Louisiana; Charlotte, North Carolina; Huntsville, Alabama; Johnson City, Tennessee; Peoria, Illinois; Shreveport, Louisiana; Springfield, Missouri and Tulsa, Oklahoma. Three clinics are associated with medical schools (Johnson City, Peoria and Shreveport). The staff and support services are similar. All the clinics have access to language translation services either in-person or videoconferencing. Patients are initially evaluated in the affiliate clinic. During the period of this project, participants in St. Jude-led clinical trials enrolled at St. Jude and then returned to their affiliate clinic to receive ongoing care. Three clinics are member institutions of the Children’s Oncology Group (COG). The combined number of new oncology patients from all eight affiliate clinics is approximately 350 patients per year.
TABLE 1.
Population and demographics of affiliate program site communities
| Affiliate Sites | ||||||||
|---|---|---|---|---|---|---|---|---|
| Population/Demographic | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Metro population (× 106) | 0.45 | 0.82 | 2.4 | 0.44 | 0.38 | 1 | 0.2 | 0.44 |
| White (%) | 40 | 41 | 45 | 65 | 63 | 62 | 90 | 90 |
| African-American (%) | 54 | 50 | 35 | 30 | 27 | 15 | 6 | 4 |
| Asian (%) | 1 | 4 | 5 | 2 | 5 | 2 | 0.3 | 1.5 |
| Native American (%) | 1 | 1 | 0.5 | 0.5 | 0.2 | 5 | 0.2 | 0.5 |
| Hispanic (%) | 4 | 4 | 13 | 2 | 5 | 14 | 2 | 4 |
NOTE. Data were obtained from the 2010 US Census.
This study was approved by the St. Jude Institutional Review Board. To address the aim statement, we started with a cause/effect approach. We used a structured interview survey of affiliate pediatric oncologists to identify possible causes that lead to the effect of low enrollment in pediatric oncology clinical trials. A previously reported standardized, open-ended interview method was used to create the cause/effect diagram. The causes were analyzed in a priority matrix for ease of use and impact of potential interventions. The interview guide was developed by the Consortium for Enhancing Minority Participation in Clinical Trials. 9 This standardized interview guide identified the themes related to the process of clinical trial recruitment and retention in NCI-designated cancer centers of racial and ethnic minority participants. It was used to identify potential causes of low clinical trial enrollment in our network. Specifically, the survey “Needs Assessment for Referring Clinicians” was used for this study. 9 In addition, a demographic questionnaire provided years in practice as a pediatric oncologist and age in years.
Qualitative one-on-one interviews were conducted with pediatric hematology-oncology medical directors (one clinic had 2 co-medical directors) at the start of the fiscal year. The interviews were completed in person or via webinar. The interviews ranged from 45 to 60 minutes and were conducted by one interviewer who received training from the Nursing Research Division at St. Jude. Transcribed results were coded independently by three coders by using grounded theory. 15 The coders reviewed each transcript individually and then the 3 coders meet-person to review each transcript together. The few disagreements were reviewed in detail until consensus was reached. The codes were grouped into categories inductively derived from the transcripts. The categories were grouped into themes of barriers or facilitators for clinical trial enrollment. The frequency of each code was calculated, and only codes mentioned in at least 50% of the interviews were included in the reported categories.
The first intervention tested was selected because of the ease of use. The intervention was to provide translated informed consents to all limited English proficiency participants and constituted the first plan-do-study-act (PDSA) cycle.
The numbers of new oncology patients accepted by each clinic were tracked during the same fiscal year as the interviews. Clinical trial enrollment in primary interventional therapeutic trials (both St. Jude and COG) was tracked for the same year. Race and ethnicity were self-identified at patient registration using the NCI categories for ethnicity (i.e., Hispanic or Latino and Not Hispanic nor Latino) and race (i.e., American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or other Pacific Islander, and White). The number of participants who refused to identify their ethnicity or race were designated as “refused to identify.” The number of primary clinical trial enrollments were calculated for each race and ethnicity category, or refused to identify for each specific affiliate clinic.
Patient characteristics were summarized and compared by whether the patient enrolled in a primary therapeutic St. Jude or COG trial. Categorical variables were summarized with counts and percentages. Categorical variables were compared using either Pearson’s chi-square test or Fisher’s exact test depending on counts. 16,17
RESULTS
Our study included nine interviews conducted over a 2-month period from all affiliate sites. All interviewees were board-certified pediatric hematologist-oncologists. The median age of the interviewees was 51 years. The years in practice ranged from 1 to 3 decades with a median of 15 years in practice as a pediatric hematologist-oncologist. Each interviewee stated that clinical trial enrollment is an important goal for the field of pediatric hematology-oncology.
Barriers
Based on the interviews, the three major barriers identified were transportation difficulties, complex trial designs, and language discordance (Table 2). Transportation factors included lack of or limited transportation to medical facilities and missing time away from work for parents. The interviewees stated that transportation to the clinics was more difficult for working parents. They relayed that many parents desired convenient medical care that was closer to their home community. Specific examples of travel concerns raised by the interviewees were as follows: (1) “We have many families in rural areas who are underserved, and the issue for them is resources. They do not have transportation and lack access to prompt medical care. This is a large barrier.” (2) “Travel is hard. More clinic visits makes it hard on families. It’s important to do as much locally as possible.”
TABLE 2.
Major themes of barriers and facilitators of clinical trial enrollment
| Barriers | Facilitators |
|---|---|
| Language discordance | Trust in provider |
| Transportation/travel difficulties | Acceptance of merits of scientific investigation |
| Complex trial designs |
Complex trial designs were also described as a barrier of clinical trial enrollment. Interviewees mentioned that multiple randomizations can be difficult for parents and participants to understand. They stated that when study participation timelines were unclear, the participants were less likely to consent for enrollment in a clinical trial. Specific examples of such concerns were as follows: (1) “Multiple arms of a trial, complex randomization, or if the trial has many ‘if/then’ scenarios, it is more difficult for participants to consent.” (2) “A study design that minimizes impact on the family with less time in the hospital and clinic is more desirable.”
Language discordance was also identified a barrier to enrollment. The interviewees cited the need for an interpreter to discuss the trial purpose and implications for some patients, and the inability to obtain a timely written translated informed consent as a barrier. One interviewee specifically stated, “Informed consent is a challenge, particularly with a language barrier. If there is a language difference, this is the main concern.” This identified barrier guided the first intervention which was to provide translated informed consents for all limited English proficiency participants.
Facilitators
Based on the interviews, the major facilitators of clinical trial enrollment were (1) participants and parents having general awareness and appreciation of scientific investigation; and (2) the importance of building a trusting relationship (Table 2). The interviewees relayed that education is a process and is best accomplished in stages over time. All interviewees stated that it was critically important to ensure that families recognize the benefits of clinical research. A specific example of this sentiment is as follows: (1) “It’s important to remind the patient of the benefits of clinical research.”
Nearly all the interviewees spoke of the importance of discussing clinical trial research with family members and family advocates. Having the support of family members or friends provides a foundation for parents and participants. These family advocates can help the family to ask appropriate questions and aid in the decision-making process. Interviewees said that involving key family members with the clinical team helped to build trust. One interviewee specifically stated that, “It is helpful to have advocates (other families, church members, bilingual friends) to help the family feel at peace with their decision.”
Outcome
Across the eight affiliates, there were a total of 353 patients in the same fiscal year of the interviews, of whom 128 (36%) enrolled in a therapeutic clinical trial (93% in a St. Jude trial; 7% in a COG trial). We examined the relationship between the race and ethnicity of participants and if the participant enrolled in a clinical trial. For those participants who stated their race, there were 7 Asian (2%), 44 Black (13%), 18 Mixed (5%), 7 Native American (2%), and 261 White (74%). For those participants who stated their ethnicity, there were 32 Hispanic (9%) and 307 Non-Hispanic (87%).
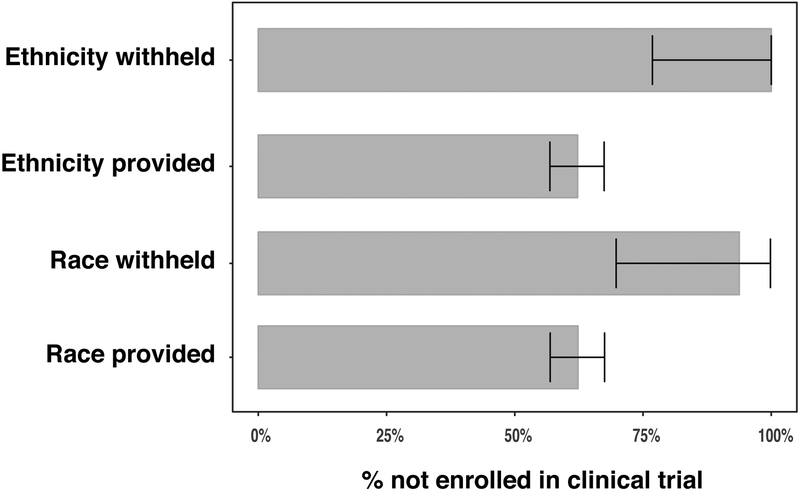
Participants who refused to state their race or ethnicity were less likely to enroll in a clinical trial (Figure 1). Specifically, of the 225 participants not on a clinical trial, 15 refused to state their race, whereas of the 128 participants on a clinical trial, only 1 refused to state their race (Fisher P-value=0.014). Similarly, of the 225 participants not on a clinical trial, 14 refused to state their ethnicity, whereas of the 128 participants on a clinical trial, none refused to state their ethnicity (Fisher P-value=0.003).
Figure 1.
Percentage of participants not enrolled in clinical trial according to whether the participant agreed to provide their ethnicity or race, with 95% binomial confidence intervals.
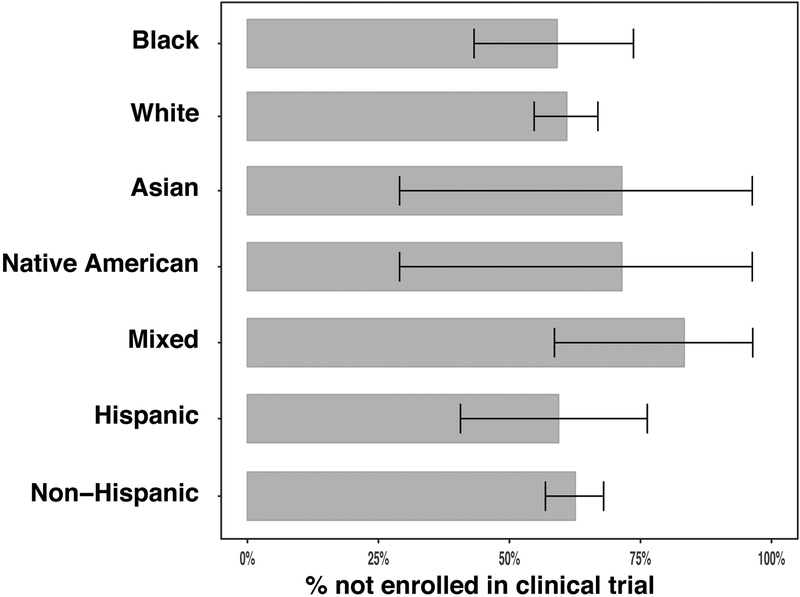
However, we observed no significant association between a specific race (Fisher P-value=0.37) or ethnicity (Fisher P-value=0.71) and enrollment in a clinical trial (Figure 2).
Figure 2.
Percentage of participants not enrolled in clinical trial according to self-identified race, with 95% binomial confidence intervals.
DISCUSSION
We describe the results of qualitative structured interviews of pediatric hematologist-oncologists in a community-based clinical network to gain insight into the barriers and facilitators of clinical trial enrollment as part of a quality improvement project. Every pediatric hematologist-oncologist we interviewed related that clinical trial participation is an important goal for current and future pediatric oncology patients; however, they identified several barriers. The physicians also purported approaches to foster clinical trial enrollment, which included access to translation services, minimizing travel, pragmatic clinical trial designs, education regarding clinical research, and building trust with participants and their families.
More than 50% of the interviewees recognized language discordance between participants and providers as a barrier to clinical trial enrollment. As shown by others, limited English proficiency can negatively affect patient care and communication. 18 The explanation and discussion of complicated clinical research trials are also likely to have a similar effect. Specifically, in a pediatric oncology population, Aristizabal et al. found that children of Spanish-speaking parents were less likely to be enrolled in a clinical trial. 10 Ensuring access to interpretation services for participants with limited English proficiency, together with providing translation of informed consent and clinical documents, is paramount for participants. The first intervention we tested was to provide translated informed consents for any participant with limited English proficiency. The minimal impact we observed from this intervention may be because of the limited number of Spanish-speaking participants in our population.
Distant travel was identified as an additional barrier to clinical trial enrollment. In the St. Jude affiliate network, enrollment may require travel for participants. For working parents or parents of multiple children, leaving their home community can be a burden. Time away from work for parents, even with the benefit of the Family Medical Leave Act, is difficult. Time away from other siblings and community support may also be challenging. Nonparticipants in adult oncology trials cited travel as a primary reason to decline participation in clinical research trials because of either a lack of resources for travel or the extensive time away from work required. 19,20 Efforts to perform as much of the clinical trial procedures close to the home community was perceived as a positive impact for participants and family members. Digital health tools provide an opportunity to lessen the barrier of travel for participants, particularly for those participants living in rural areas with limited access to major cancer centers.
The barrier of travel may also be related to trial design. Pragmatic clinical trial designs that reduce the number and/or frequency of research-only tests, while preserving research integrity, is a laudable goal. Patient-engagement groups have advocated for a more patient-focused approach to clinical trial designs. 21.22 Complex randomizations or unclear endpoints may also be challenging for participants to comprehend and may contribute to non-accrual. Parent/patient advisory groups can provide input to guide the development of more practical clinical trial designs which may lead to increased accrual in clinical trials. Practicality in the informed consent document is likely to help participants in the decision process. Staged discussions of the clinical research aid a participant in understanding the research questions.
Trust in the provider was cited as a key element from the providers’ perspective of parent decisions to enroll children in clinical trials. This is not remarkable, as patient trust in health care providers is known to affect adherence to medical care. 8 Alternatively, distrust is a common reason for participants who refuse enrollment in clinical research trials. 7 Our finding that a significant number of participants refused to state either their race or ethnicity may be an indicator of such distrust. Indeed, a notable disparity in those participants who refused to identify both race or ethnicity and declining participation in clinical trials was evident. Specifically, the rate of clinical trial enrollment was significantly lower for the group of participants who refused to state their race and ethnicity than it was for the total group and those who identified their race and ethnicity groups.
Education of the positive aspects of clinical investigations is desirable. Public awareness of the advantages in which clinical trial research can improve survival and quality of life for children with cancer may guide participants. Education and awareness of clinical research was mentioned by the interviewees as a strategy to facilitate clinical trial enrollment. Here again, inclusion of patient/parent advisory groups in how best to deliver the education is important. The use of patient navigators has also been shown to be an effective way to educate potential participants. 7
The demographics of the affiliate areas varied regarding the race and ethnicity of the participants. We found no disparities in clinical trial enrollment by self-reported race or ethnicity of participants. However, this report reflects only 1 year of clinical trial enrollment data. For some race groups, the number of participants was small, which could account for the lack of disparity.
Prior studies in pediatric participants with cancer have demonstrated an under-enrollment of racial and ethnic minorities. Aristizabal et al. showed that participation in pediatric oncology clinical research trials was markedly lower among Hispanics compared to non-Hispanic whites. 10 Similarly, a 3-year study of clinical trial enrollment from the Children’s Oncology Group revealed subgroups of under-representation in trials among Hispanic and black children less than 10 years old. 11 Efforts to facilitate clinical trial enrollment have focused on the health care system, with interventions such as direct outreach to minority participants in community locations such as churches or social organizations. Interventions with social media campaigns have demonstrated increase in clinical trial accrual.7 The importance of appropriate communication among health care professionals and participants is a cited strategy to increase clinical trial enrollment and treatment goals. 23, 24
Although we did not observe under-enrollment by race or ethnicity at the St. Jude affiliate sites, a major limitation of our study is the limited sample size of participants with only one year of clinical trial enrollment data. We observed a slight increase in clinical trial enrollment from 32% to 36% in one year. Another shortcoming of this work is the limited Hispanic and limited English-proficiency patient population which may account for the small effect of the first intervention. Moreover, qualitative studies may be biased by the practice setting or regional differences. For example, if a practice setting has limited racial and ethnic diversity in the patient population, then a provider may have less experience or resources to provide culturally sensitive care.
Pediatric hematology-oncology physicians have unique insights regarding barriers and facilitators of clinical trial enrollment. Their input may guide future interventions to increase recruitment and retention in clinical research trials. We plan to test local enrollment at the affiliate sites and methods of communication to enhance a participant’s understanding of clinical trials.
ACKNOWLEDGEMENTS
The authors thank Nisha Badders, Ph.D., ELS, for scientific editing of the manuscript, Dr. Mona Fouad for design guidance and Dr. Jeffrey Gossett for statistical support. The authors also thank affiliate site staff for their participation in the project and continuing determination to advance clinical research. This work was supported by the National Cancer Institute (St. Jude Cancer Center Support [CORE] under Grant [P30 CA21765] and the American Lebanese Syrian Associated Charities (ALSAC). The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
REFERENCES
- 1.Shochat SJ, Fremgen AM, Murphy SB, et al. : Childhood cancer: patterns of protocol participation in a national survey. CA Cancer J Clin 51:119–30, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Unger JM, Cook E, Tai E, et al. : The Role of Clinical Trial Participation in Cancer Research: Barriers, Evidence, and Strategies. Am Soc Clin Oncol Educ Book 35:185–98, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caldwell PH, Murphy SB, Butow PN, et al. : Clinical trials in children. Lancet 364:803–11, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Pui CH, Evans WE: Treatment of acute lymphoblastic leukemia. N Engl J Med 354:166–78, 2006 [DOI] [PubMed] [Google Scholar]
- 5.O’Leary M, Krailo M, Anderson JR, et al. : Progress in childhood cancer: 50 years of research collaboration, a report from the Children’s Oncology Group. Semin Oncol 35:484–93, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleyer A, Budd T, Montello M: Adolescents and young adults with cancer: the scope of the problem and criticality of clinical trials. Cancer 107:1645–55, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Hamel LM, Penner LA, Albrecht TL, et al. : Barriers to Clinical Trial Enrollment in Racial and Ethnic Minority Patients With Cancer. Cancer Control 23:327–337, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks SE, Muller CY, Robinson W, et al. : Increasing Minority Enrollment Onto Clinical Trials: Practical Strategies and Challenges Emerge From the NRG Oncology Accrual Workshop. J Oncol Pract 11:486–90, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durant RW, Wenzel JA, Scarinci IC, et al. : Perspectives on barriers and facilitators to minority recruitment for clinical trials among cancer center leaders, investigators, research staff, and referring clinicians: enhancing minority participation in clinical trials (EMPaCT). Cancer 120 Suppl 7:1097–105, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aristizabal P, Singer J, Cooper R, et al. : Participation in pediatric oncology research protocols: Racial/ethnic, language and age-based disparities. Pediatr Blood Cancer 62:1337–44, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lund MJ, Eliason MT, Haight AE, et al. : Racial/ethnic diversity in children’s oncology clinical trials: ten years later. Cancer 115:3808–16, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Hudson SV, Momperousse D, Leventhal H: Physician perspectives on cancer clinical trials and barriers to minority recruitment. Cancer Control 12 Suppl 2:93–6, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Eggly S, Barton E, Winckles A, et al. : A disparity of words: racial differences in oncologist-patient communication about clinical trials. Health Expect 18:1316–26, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barakat LP, Patterson CA, Mondestin V, et al. : Initial development of a questionnaire evaluating perceived benefits and barriers to pediatric clinical trials participation. Contemp Clin Trials 34:218–26, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Strauss AL, Corbin J: Basics of Qualitative Research: Grounded Theory Procedures and Techniques (ed 2) London, UK, Sage, 1998 [Google Scholar]
- 16.SAS Institute Inc., SAS 9.4 Help and Documentation, Cary, NC: SAS Institute Inc., 2002-2012 [Google Scholar]
- 17.R Core Team, R: A Language and Environment for Statistical Computing R Foundationfor Statistical Computing, Vienna, Austria: URL https://www.R-project.org/ [Google Scholar]
- 18.Kuo DZ, O’Connor KG, Flores G, et al. : Pediatricians’ use of language services for families with limited English proficiency. Pediatrics 119:e920–7, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Meropol NJ, Buzaglo JS, Millard J, et al. : Barriers to clinical trial participation as perceived by oncologists and patients. J Natl Compr Canc Netw 5:655–64, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Lara PN Jr., Higdon R, Lim N, et al. : Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol 19:1728–33, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Marsden J, Bradburn J: Patient and clinician collaboration in the design of a national randomized breast cancer trial. Health Expect 7:6–17, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DJ, Avulova S, Conwill R, et al. : Patient engagement in the design and execution of urologic oncology research. Urol Oncol 35:552–558, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Mannel RS, Walker JL, Gould N, et al. : Impact of individual physicians on enrollment of patients into clinical trials. Am J Clin Oncol 26:171–3, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Mack JW, Paulk ME, Viswanath K, et al. : Racial disparities in the outcomes of communication on medical care received near death. Arch Intern Med 170:1533–40, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]