Abstract
Cavernous angioma (CA) is a vascular pathology caused by loss of function in one of the 3 CA genes (CCM1, CCM2, and CCM3) that result in rho kinase (ROCK) activation. We investigated a novel ROCK2 selective inhibitor for the ability to reduce brain lesion formation, growth and maturation. We used genetic methods to explore the use of a ROCK2-selective kinase inhibitor to reduce growth and hemorrhage of CAs. The role of ROCK2 in CA was investigated by crossing Rock1 or Rock2 hemizygous mice with Ccm1 or Ccm3 hemizygous mice, and we found reduced lesions in the Rock2 hemizygous mice. A ROCK2-selective inhibitor, BA-1049 was used to investigate efficacy in reducing CA lesions after oral administration to Ccm1+/− and Ccm3+/− mice that were bred into a mutator background. After assessing the dose range effective to target brain endothelial cells in an ischemic brain model, Ccm1+/− and Ccm3+/− transgenic mice were treated for three (Ccm3+/−) or four months (Ccm1+/−), concurrently, randomized to receive one of three doses of BA-1049 in drinking water, or placebo. Lesion volumes were assessed by micro-computed tomography. BA-1049 reduced activation of ROCK2 in Ccm3+/−Trp53−/− lesions. Ccm1+/−Msh2−/− (n=68) and Ccm3+/−Trp53−/− (n=71) mice treated with BA-1049 or placebo showed a significant dose-dependent reduction in lesion volume after treatment with BA-1049, and a reduction in hemorrhage (iron deposition) near lesions at all doses. These translational studies show that BA-1049 is a promising therapeutic agent for treatment of CA, a disease with no current treatment except surgical removal of the brain lesions.
Keywords: Vascular permeability, Rho kinase, cerebral cavernous malformation, hemangioma, therapeutics
Introduction
Cavernous angiomas (CA), also called cerebral cavernous malformations (CCMs), are common cerebrovascular anomalies affecting 0.2 to 0.4% of the population [1]. The lesions are multilobed clusters of grossly dilated and thin-walled capillaries in the brain and spinal cord [2]. The disease concept has been described as “hemorrhagic proliferative dysangiogenesis” [3]. CA lesions range in size from a few millimeters in diameter to several centimeters and are susceptible to chronic leakage or overt hemorrhagic events [4, 5]. Patients have a wide range of symptoms depending on the location of the lesions including nonspecific headaches, seizures, and focal neurological deficits including ataxia, diplopia, gait imbalance and vertigo, dysarthria, among other deficits [6].
The familial form of CA is transmitted as an autosomal dominant disorder and the disease predisposes patients to develop many lesions, with increasing numbers with age [4]. Sporadic form of the disease, accounting for up to 80% of cases, involves a single lesion developing spontaneously. A first symptomatic hemorrhage of a CA is associated with a 6% per year re-bleed rate, and 42% rate of recurrent bleed or focal neurologic deficit within 5 years [1]. Lesions in the brainstem confer greater risk of significant intracranial hemorrhage [1]. The clinical course of CA lesions that have hemorrhaged is quite serious yet surgical removal can lead to poor outcomes [7]. While the location and number of lesions in a given individual determines the severity of disease, the existence of even a single lesion may predispose a patient to seizures, stroke, neurological deficits, and death.
CA lesions form upon sporadic or germline loss-of-function mutations in one of three genes: CCM1 (KRIT1), CCM2 (malcavernin, MGC4607), or CCM3 (PDCD10) [8–10]. The same mutations in CCM1, CCM2, or CCM3 genes have been shown to underlie the formation of both spontaneous and familial CA [11]. Knudson’s ‘two-hit’ hypothesis explains the molecular mechanism for pathogenesis of both sporadic and familial lesions; but the second hit may be chemical or environmental, rather than genetic, such as exposure to cytokines in response to stress or inflammation [11–13].
Abnormal activation of the RhoA/Rho kinase (ROCK) signaling pathway is a key event in many diseases with endothelial dysfunction [14]. CA lesion pathogenesis has been directly linked to ROCK activation in the capillary endothelial cells (ECs) upon CCM1, CCM2, or CCM3 loss-of-function CA [15–17]. The CCM proteins are thought to form a complex at EC junctions, and loss of any one of the CA proteins results in increased actin stress fibers, phosphorylated myosin light chain (pMLC), and vascular permeability [18]. Surgically excised human CA lesions also show ROCK activation [16, 19]. Isoforms of ROCK, ROCK1 and ROCK2, have overlapping functions. ROCK2, the isoform most highly expressed in the central nervous system (CNS), is the key kinase that regulates EC barrier function [20] and vascular remodeling [21, 22]. Importantly, ROCK2 is thought to be a better drug target because of hypotensive and other side effects of non-specific ROCK inhibitors [23, 24]. The hypothesis of this study is that ROCK2 inhibition will inhibit lesion burden and hemorrhage in both mild and aggressive models of CCM disease.
CA can be clearly diagnosed by imaging, but there are no pharmacological treatment options. The standard of care is watchful waiting, until neurosurgical removal is deemed unavoidable. There are no drug treatments to prevent leakiness or hemorrhage and the behavior of individual lesions is variable and unpredictable. After diagnosis, patients are unable to prepare for or prevent re-occurrence of symptoms.
Here we investigate the potential of a ROCK2-selective ROCK inhibitor, BA-1049 [25] to prevent CA disease progression in two different transgenic animal models. BA-1049 was designed via a rational method based on structural comparison to non-specific inhibitors of ROCK, and IC50 assays show 20–80-fold greater potency for ROCK2 vs ROCK1. BA-1049 is metabolized to an active metabolite that also shows selectivity for ROCK2. Neither BA-1049 nor the metabolite inhibits PKA or PKC at physiological ATP concentrations, which is important for safety considerations [23].
Before initiating the study, we chose to study two of the three CA lines. We have studied Ccm1 transgenic mice because CCM1 is the most prevalent genotype [13] and is prevalent in American Hispanic populations, arising as a founder effect [8, 26]. We also studied transgenic mice with Ccm3 mutations because CCM3 lesions are particularly aggressive [19]. Since CA mutations are lethal in the homozygous state, we bred hemizygous Ccm mice with strains defective in DNA repair to enhance the rate of lesion formation [19, 27]. The results show that BA-1049 is a promising drug candidate to reduce lesion growth and hemorrhage in both CCM1 and CCM3 models of CA.
Materials and methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. An expanded Methods section is available in the supplementary file.
Study design
The study was designed to investigate the role of ROCK2 in development of CA and the functional impact of ROCK2 inhibition in vitro and in transgenic mice with CA Ccm1 or Ccm3 mutations. All experiments were randomized and rigorously blinded by dosing of animals coded in the Marchuk lab prior to sending to the Awad lab for analysis of brain CA lesions, masked for treatment rendered.
Experimental animals
To examine dose-response we used C57BL/6 mice at 8 to 12 weeks of age, the same line used to create the transgenic mice. A 60 minute transient ischemia was induced by middle cerebral artery occlusion (MCAO). Upon awakening, mice were scored for behavior on a 5-point scale, as described [28] and animals with circling behavior (scores of 2 or 3) were included in the study. Mice were euthanized 4 hours after reperfusion, brain tissue dissected into ischemic and contralateral hemispheres, prepared for Western blot and levels of phosphorylated Cofilin (pCofilin) determined.
Ccm3+/−Rock1+/− and Ccm3+/−Rock2+/− mice were generated by crossing mice hemizygous for deletion of the Ccm3 gene [19] with mice hemizygous for deletion of either Rock1 or Rock2 [29]. Ccm3+/− mice that were either wild-type or hemizygous for deletion of Rock1 or Rock2 were aged to 20 weeks and sacrificed by inhalation of carbon dioxide and decapitation. Brains were surgically extracted and placed into a 10% neutral buffered formalin fixative solution.
The Ccm3+/− mouse model was generated in the sensitized background of complete deletion of Trp53 [19]. The Ccm1+/− model was generated in the sensitized background of complete deletion of Msh2 [27]. Multiple mouse models of CCM disease are available, each with its inherent strengths and weaknesses. Heterozygous Ccm1 mutant mice do not develop a measurable lesion burden while Ccm3 mice develop a low but measurable burden. By contrast, the CCM mouse models in sensitized genetic backgrounds (Trp53 or Msh2 null backgrounds) develop CCM throughout the brain, and significantly, the larger lesions then develop the bleeding characteristic of the human phenotype. Thus these sensitized models are more useful in pre-clinical drug studies where CCMs gradually develop into the mature, hemorrhagic, and clinically-relevant lesion. We chose different sensitizers for the Ccm1 and Ccm3 mice based on years of attempting to generate a robust CCM phenotype for each genotype while exhibiting a low tumor burden and a reasonable breeding capacity [19, 27, 30].
Groups of mice to be compared were raised and treated contemporaneously. The neonatal mice were genotyped and those with the correct genotype were randomly recruited into the study in one of the 3 dose groups or placebo. The dose groups were 1, 10 or 100 mg/kg/day. Based on lesion burden in prior studies with the same models, and its non-normal distribution, we predetermined that a sample size of 20 mice per group would have a greater than 80% power to detect a therapeutic effect, by a non-parametric test for scale. The study was randomized, placebo controlled, and blinded to both treatment and analysis. All mice were put on drug or placebo treatment at weaning. The Ccm3+/−Trp53−/− mice were sacrificed at 3 months, and the Ccm1+/−Msh2−/− at 4 months unless a decline in animal health required earlier euthanasia. Mice were sacrificed by inhalation of carbon dioxide and decapitation. Brains were surgically extracted and placed into a 10% neutral buffered formalin fixative solution. Plasma was collected from all dose groups, and aorta, carotid artery, the inferior vena cava were collected from all animals in the 100 mg/kg/day treatment group. In some mice of the Ccm3+/−Trp53−/− cohort, the spinal cords were also dissected at time of sacrifice. A quantitative assessment of lesion volume was carried out by micro-computed tomography (micro-CT) as previously described [31]. The CA lesions in the spinal cord were photographed in situ, and dissected out, placed into individual cryotubes, and flash frozen. The samples were prepared for Western blots as describe in the Detailed Methods (in supplementary file). The analytical methods to determine plasma and tissue concentrations of BA-1049 and M1 are described in the supplemental methods.
Pharmacokinetic studies in mice
To understand pharmacokinetics in mice in preparation for transgenic mouse studies we compared oral gavage (30 mg/kg) and intravenous (I.V.) delivery (5 mg/kg) of BA-1049 HCl. The in vivo portion of the study was carried out at Calvert Labs (Scott Township, PA) and bioanalytical liquid chromatography-mass spectrometry (LC-MS) analysis to detect BA-1049 and the active M1 metabolite in plasma and whole brain was carried out by MPI Research/Charles River (Mattawan, MI). Analysis of plasma and brain was performed at multiple time points after oral or I.V. dosing.
In the transgenic mice, plasma was collected at the time of euthanasia. Vascular tissue exposure and quantification of BA-1049 and M1 was undertaken on tissue isolated immediately after blood collection. Inferior vena cava and abdominal aorta were harvested from just superior to their distal, caudal bifurcations rostrally to just superior of the renal vein and artery. Common carotid arteries were dissected from the neck bilaterally.
Statistical analysis
Statistical analyses were performed using SAS9.4 (SAS Institute Inc., Cary, NC), R v3.4.4 (R Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism 7.00 (GraphPad Software Inc., La Jolla, CA). All probability (P) values were considered to be statistically significant at P<0.05. Data from cell culture studies are presented as mean ± S.E.M. unless otherwise noted.
The primary outcome analysis for the transgenic mice studies were pre-specified to measure lesion volume by mouse brain volume, and to use non-parametric statistical tests. Previous studies showed that lesions are not normally distributed [32, 33]. When most of data per group was non-zero, the 2-sided Conover 2-sample test [34] was used because the individual data points are weighted to improve sensitivity. If one of the groups contained more zero values than non-zero values, the Mann-Whitney U test was used to test for significant differences per group to avoid possible statistical errors due to over emphasis of weighted outlying data points. Similar non-parametric statistical analyses were conducted with the non-heme iron deposition data which were not normally distributed. Outliers were removed if they were beyond 2 standard deviations from the mean. The Pearson χ2 test was used to compare the proportions of mice with and without lesions.
The body weights of mice were normally distributed, and parametric tests were used to test for statistical significance. The F test was used to evaluate the variances between two unpaired groups. The differences between the two groups were compared using Student’s t-test with equal variances and Welch’s t test with unequal variances. The log-rank (Mantel-Cox) test was used to compare the survival of animals between treatment groups.
Results
Ccm3+/−Rock2+/− mice have reduced CA burden
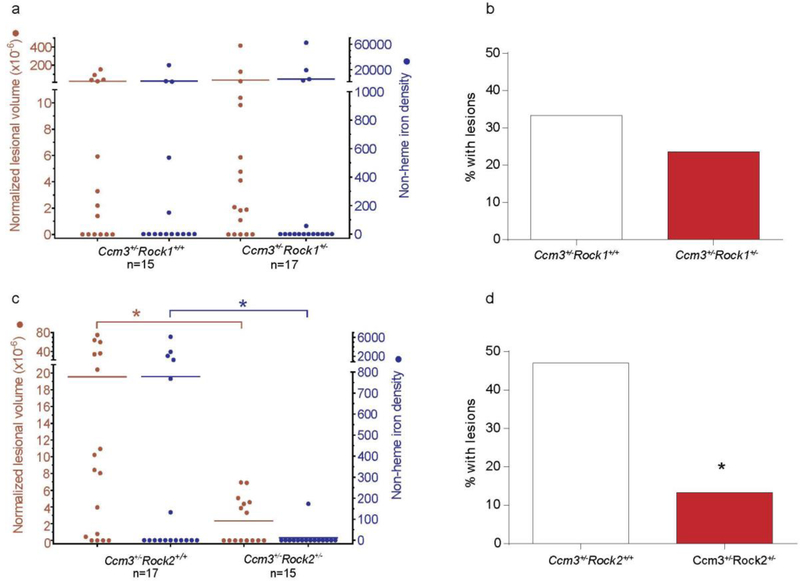
We used genetics to investigate the role of Rock1 versus Rock2 in formation and maturation of CAs. Mice hemizygous for deletion of the aggressive Ccm3 gene [19] were crossed with mice heterozygous for deletion of either Rock1 or Rock2 [35]. Homozygosity for either Rock1 or Rock2 loss is lethal in the embryonic stage but the Rock1 and Rock2 heterozygotes are viable with low rates of attrition (Table 1) and normal weight gain Table S1 (in supplementary file). Previous studies showed that they have approximately 50% less ROCK1 and ROCK2 transcript/protein, respectively [29]. At 20 weeks of age, the brains of the Ccm3+/− mice that were either wild-type or heterozygous for deletion of Rock1 or Rock2 were examined for presence of lesions by micro-CT, a non-destructive technique that allowed three-dimensional volumetric measurements on the transgenic mouse brains. The Rock1 heterozygotes had the same lesional CA volume per brain as the wild-type Rock1 mice and there was no difference in the leakage of non-heme iron from the CAs in these mice (Fig. 1a). Also, with the Ccm3+/−Rock1+/+ and the Ccm3+/−Rock1+/− mice, the number of mice with lesions was not significantly different (Fig. 1b). By contrast, the Rock2 heterozygotes had significantly fewer lesions than the wild-type Rock2 mice, and the leakage of non-heme iron in the brains of Ccm3+/−Rock2+/+ mice was significantly higher than in Ccm3+/−Rock2+/− mice (Fig. 1c). Also, there were significantly more mice with lesions in the wild-type Rock2 homozygotes compared to the hemizygotes (Fig. 1d). This experiment could not be performed with Ccm1+/− mice, as this less aggressive genotype develops no lesions in the absence of sensitization by concomitant Msh2 or Trp53 loss.
Table 1.
Total mice enrolled to study ROCK1 versus ROCK2
| Treatment | Completed Study | Attrition | Total |
|---|---|---|---|
| Genotypes | |||
| Ccm3+/−Rock1+/+ | 18 | 2 | 20 |
| Ccm3+/−Rock1+/− | 22 | 1 | 23 |
| Total mice | 40 | 3 | 43 |
| Genotypes | |||
| Ccm3+/−Rock2+/+ | 23 | 0 | 23 |
| Ccm3+/−Rock2+/− | 23 | 1 | 24 |
| Total mice | 46 | 1 | 47 |
Fig. 1.
The effect of Rock haploinsufficiency on cavernous angioma lesion burden and hemorrhage in Ccm3+/− models. Rock1 haploinsufficiency did not affect (a) lesion burden (left axis, red) and non-heme iron deposition (right axis, blue) per mouse, and (b) the percentage of mice with mature, multi-cavernous Stage 2 lesions. In contrast, Rock2 haploinsufficiency significantly decreased (c) lesion burden (P=0.011) and non-heme iron deposition (P=0.049) per mouse, and (d) the percentage of mice with Stage 2 lesions (P=0.040). The Mann Whitney U test (a, c) and χ2 test (b, d) were used to assess for significant differences. *P<0.05.
Dose-response studies with BA-1049
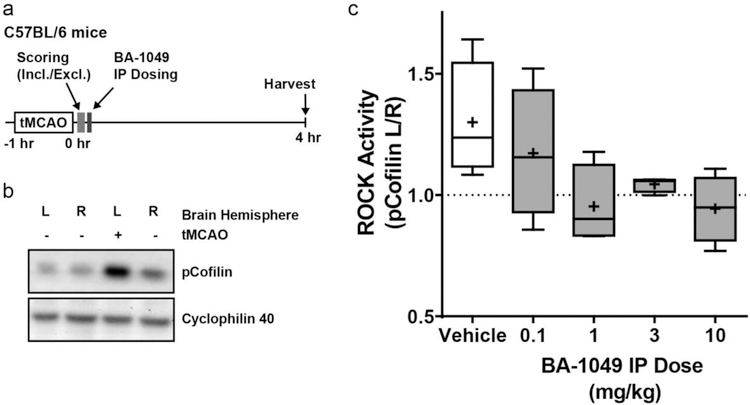
Cofilin is an actin-binding protein that is specifically phosphorylated by ROCK2, and not ROCK1 [36]. To examine the dose-response of BA-1049 to inhibit ROCK2 in brain endothelial cells, we used pCofilin as a biomarker of ROCK activation after transient ischemia because of the well-characterized activation of ROCK in in brain after ischemia [37] (Fig. 2a). In pilot experiments, we observed a dramatic increase in pCofilin on the ischemic side of the brain after a transient MCAO (Fig. 2b). To determine dose-response of BA-1049, we measured pCofilin in brain homogenates harvested 4 hours after a single intraperitoneal dose of BA-1049. Increasing concentrations of BA-1049 demonstrated a trend to dose-dependently decrease the ratio of pCofilin on the left (ischemic) versus right side of the brain (Fig. 2c). Results suggested that 1 mg/kg BA-1049 was the minimum effective dose, with effectiveness up to and including 10 mg/kg giving the most consistent response. Therefore, we chose to use 1, 10 and 100 mg/kg/day in drinking water for the transgenic mouse experiments.
Fig. 2.
Dose-response for BA-1049 to reduces ROCK hyperactivation in mice. (a) Experimental design of 60 minute transient middle cerebral artery occlusion (tMCAO) in C57BL/6 mice used to examine dose-response after a single application of BA-1049. (b) Increase in phosphorylated Cofilin (pCofilin) is detected in the left (L) ischemic side in brain after tMCAO compared to the right (R) contralateral side. (c) Dose-response determined as ratio of pCofilin expression on the ischemic left side of brain compared to right brain (pCofilin L/R) after MCAO lesion and treated with vehicle, 0.1, 1, 3 or 10 mg/kg BA-1049 HCl. There was a trend towards an effect of BA-1049 on ROCK activation (χ2(4) = 8.757, P=0.0675, n=4 animals per group).
Dose-dependent reduction in lesion formation in Ccm1 and Ccm3 mutant mice
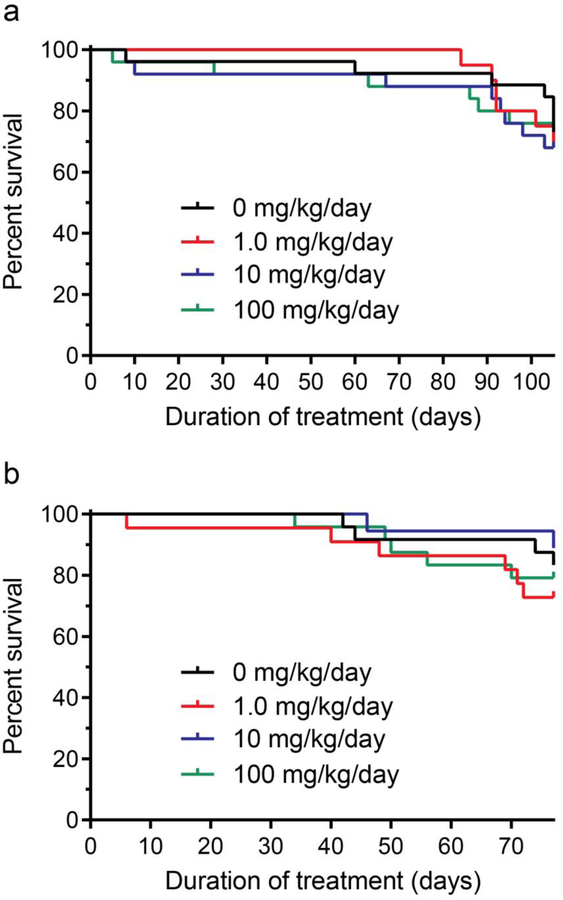
Dose-dependent effects of BA-1049 on CA lesion development were studied in 96 mice with a Ccm1+/−Msh2−/− genotype and 87 mice with Ccm3+/−Trp53−/− genotype. Mice bred into the mutator backgrounds have normal weight gain Table S2 (in supplementary file) but show high rates of attrition (Table 2) due to brain hemorrhage or other causes Table S3 and S4 (in supplementary file). The survival curves for the three doses of 1, 10 or 100 mg/kg/day provided in the drinking water in Ccm1+/−Msh2−/− mice (Fig. 3a) and Ccm3+/−Trp53−/− mice (Fig. 3b) showed no detrimental effect of any dose on survival. Some mice died or were euthanized before the end of the study, and attrition rates are shown in Tables S3 and S4 (in supplementary file). There was no noticeable change in neurological behavior or cognitive function in mice administered BA-1049. The Ccm1 genotype is less aggressive than Ccm3, and not all Ccm1+/−Msh2−/− mice develop lesions.
Table 2.
Total mice enrolled to study effect of BA-1049 on CCM1 and CCM3 disease
| Treatment | Completed Treatment | Attrition | Total |
|---|---|---|---|
| Ccm1+/−Msh2−/− | |||
| Placebo | 19 | 7 | 26 |
| 1mg/kg/day | 14 | 6 | 20 |
| 10 mg/kg/day | 17 | 8 | 25 |
| 100 mg/kg/day | 18 | 7 | 25 |
| Ccm3+/−Trp53−/− | |||
| placebo | 20 | 4 | 24 |
| 1 mg/kg/day | 16 | 6 | 22 |
| 10 mg/kg/day | 16 | 2 | 18 |
| 100 mg/kg/day | 19 | 5 | 24 |
Fig. 3.
Attrition in cavernous angioma models treated with BA-1049. (a) Ccm1+/−Msh2−/− models were treated with 0 (n=26), 1.0 (n=20), 10 (n=25) or 100 (n=25) mg/kg/day BA-1049 from weaning to the earliest age for the end of treatment (105 days of age). (b) Ccm3+/−Trp53−/− models were treated with 0 (n=24), 1.0 (n=22), 10 (n=18) or 100 (n=24) mg/kg/day BA-1049 from weaning to the earliest age for the end of treatment (77 days of age). Kaplan-Meier plots show no significant effect of treatment on survival compared with placebos. The log-rank (Mantel-Cox) test was used to assess for significant differences.
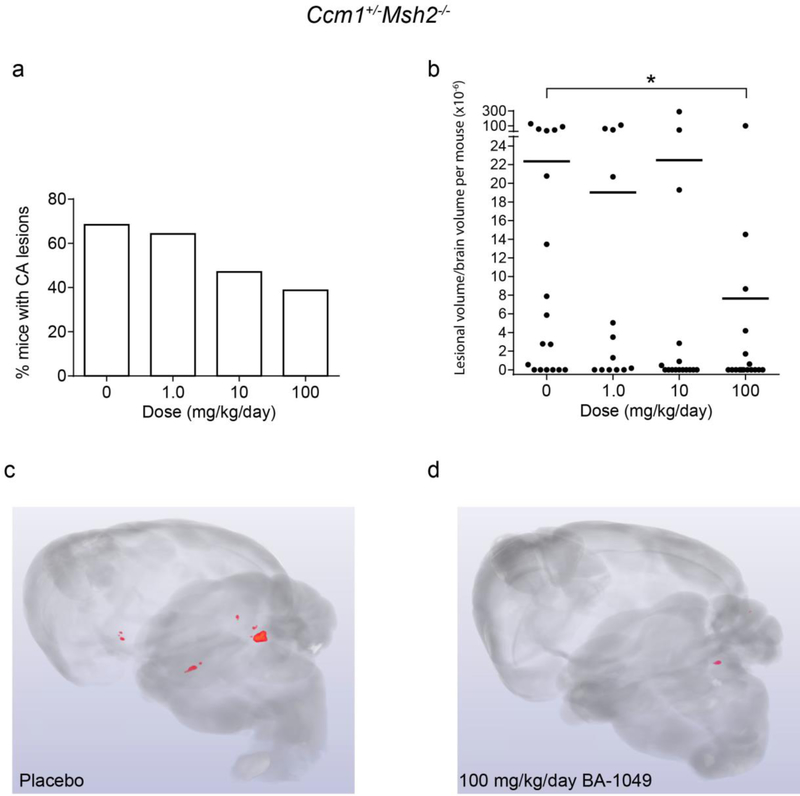
The number of Ccm1+/−Msh2−/− mice harboring CA lesions decreased with increasing doses of BA-1049 (Fig. 4a). The lesion volume per brain volume determined by micro-CT was significantly different from placebo for the high dose group in the Ccm1 mice (Fig. 4b–d). The effect was most marked on the large, multi-cavernous lesions in Fig. S2 (in supplementary file) called Stage 2 lesions [32].
Fig. 4.
The effect of BA-1049 therapy on cavernous angioma (CA) lesion burden in Ccm1+/−Msh2−/− models. (a) There was a trend (P=0.063) for fewer mice harboring CA lesions that were treated 100 mg/kg/day BA-1049 compared with placebos. (b) Treatment with 100 mg/kg/day BA-1049 significantly decreased lesion burden compared with placebos (P=0.022). Representative micro-computed tomography show (c) more prevalent CA lesions (red areas) in placebos than in (d) mice treated with 100 mg/kg/day BA-1049. No significant effect was found with treatment at lower doses of BA-1049. The χ2 (a) test and Mann Whitney U test (b) were used to assess for significance. *P<0.05. There were 18, 13, 16 and 17 animals per group treated with 0, 1.0, 10 and 100 mg/kg/day BA-1049 respectively.
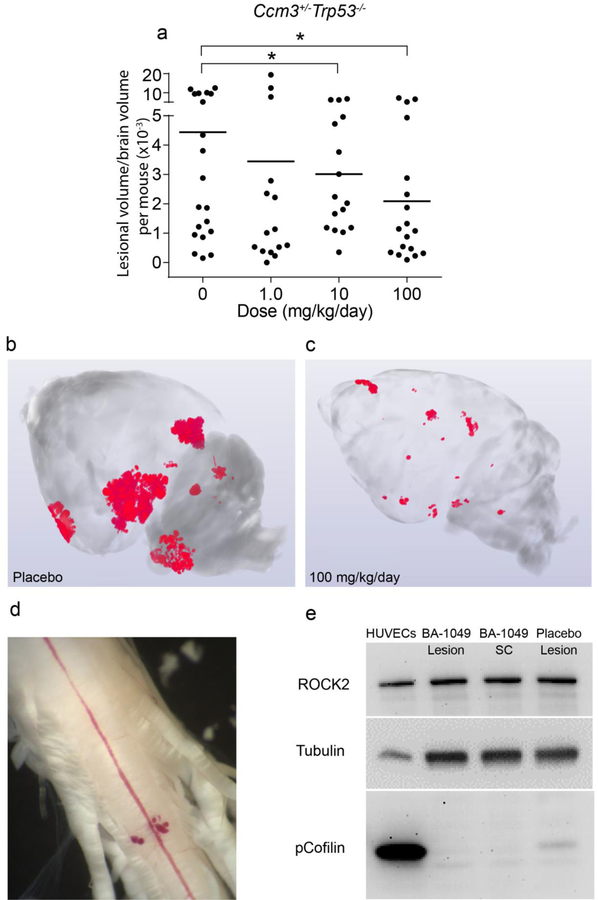
The Ccm3 genotype is a more aggressive genotype and most brains develop CA lesions and lesions are also detected in the spinal cord. Brains of the Ccm3+/−Trp53−/− mice were analyzed by micro-CT for lesion volume. The mice treated with 10 mg/kg/day and 100 mg/kg/day of BA-1049 showed significantly reduced lesion volume (Fig. 5a–c). The larger, multicavernous stage 2 lesions were those with reduced volume (Fig. S2 in supplementary file). We dissected the spinal cord of some of the treated and placebo mice (Fig. 5d) to take lesions for biochemical analysis of ROCK activation by Western blot, using pCofilin as a biomarker for in vivo ROCK activation. In the Ccm3+/−Trp53−/− mice treated with 100 mg/kg/day BA-1049, reduced pCofilin signal was detected, as compared to placebo (Fig. 5e).
Fig. 5.
The effect of BA-1049 therapy on the Ccm3+/−Trp53−/− model. (a) BA-1049 therapy at 10 and 100 mg/kg/day significantly decreased cavernous angioma (CA) lesion burden (P=0.002, P=0.003 vs. placebo, respectively). There were 20, 15, 16 and 18 animals per group treated with 0, 1.0, 10 and 100 mg/kg/day BA-1049 respectively. Representative micro-computed tomography show (b) more prevalent CA lesions (red areas) in placebos than in (c) mice treated with 100 mg/kg/day BA-1049. (d) A spinal cord (SC) from a Ccm3+/−Trp53−/− mouse showing CA lesion on the ventral surface. (e) Western blot probed with antibodies to ROCK2, tubulin as control and phosphorylated Cofilin (pCofilin) as a biomarker of ROCK activation. Human umbilical vein ECs (HUVECs) treated with lipoprotein(a) to activate ROCK2 were used as a ROCK activation control. Lesion and SC samples from a mouse treated with 100 mg/kg/day BA-1049 is shown and compared with a mouse given placebo. The 2-sided Conover 2-sample test was used to assess for significant differences. *P<0.05.
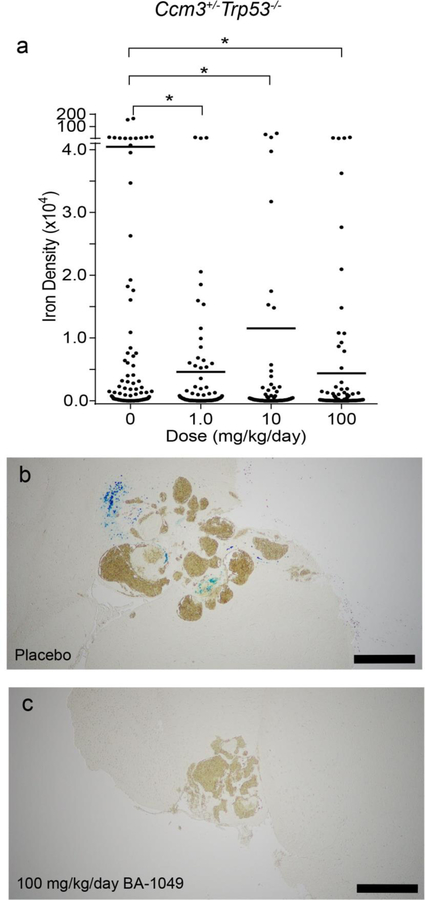
Reduction of non-heme iron deposition around CA lesions after treatment with BA-1049
After microCT the mouse brains were processed for Perls Prussian blue staining to detect non-heme iron, which is a hallmark of chronic hemorrhage in the lesions. The untreated control Ccm1+/−Msh2−/− mice did not have significant iron deposition, with only three of 19 mice showing detectable staining for non-heme iron and only one of 68 CA lesions with an integrated density of non-heme iron over 1 X 104, consistent with the lower penetrance and severity of the mouse model. By contrast, all 20 of the 20 Ccm3+/−Trp53−/− placebo-treated mice had detectable non-heme iron staining, with the blue staining was observed adjacent to lesions (Fig. 6a and b). Significantly, even the low dose mice treated with 1 mg/kg/day of BA-1049-treated showed decreased Perls staining adjacent to lesions (Fig. 6a). Quantitative analysis of the staining density (Fig. 6a) showed that the Ccm3+/−Trp53−/− mice have significant reduction in non-heme iron per lesional area in all dose groups.
Fig. 6.
The effect of BA-1049 therapy on hemorrhage in the Ccm3+/−Trp53−/− model. (a) BA-1049 therapy at 1.0, 10 and 100 mg/kg/day significantly decreased non-heme iron per Stage 2 lesional area compared with placebos (P=0.037, P=0.0015, P=0.00003 vs. placebo, respectively). There were 108, 85, 95 and 112 mature, multicavernous Stage 2 lesions per group in animals treated with 0, 1.0, 10 and 100 mg/kg/day BA-1049 respectively. Representative cavernous angioma lesions show (b) the presence of non-heme iron (blue Perls stain) in placebos and (c) non-heme iron lacking in mice treated with 100 mg/kg/day. The Mann Whitney U test was used to assess for significant differences. Bar, 500 µm. *P<0.05.
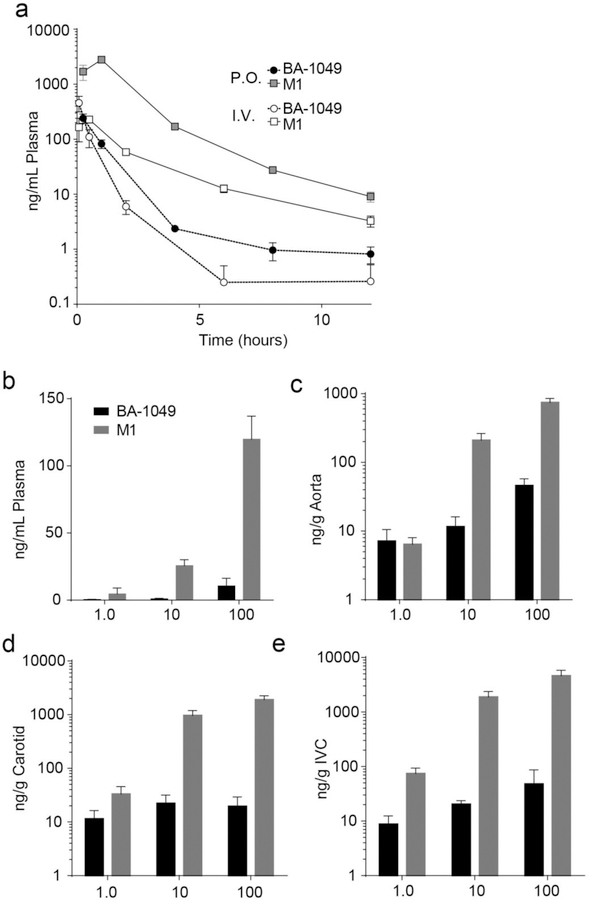
Analysis of drug plasma levels after oral delivery in drinking water
Pharmacokinetic studies in rats show that oral administration of BA-1049 generates an active metabolite, M1 [25]. Pharmacokinetic studies in mice show that the M1 metabolite is present in plasma after oral or IV administration of BA-1049 (Fig. 7a). To further understand the pharmacokinetics of BA-1049 and M1 in BA-1049-treated transgenic mice, we collected plasma and vascular tissues from some of the mice at the time of sacrifice (Fig. 7b–e). M1 was the dominant metabolite in mouse plasma with average levels of 4.6, 26 and 120 ng/mL in mice treated with 1, 10 and 100 mg/kg/day of BA-1049, respectively. Much greater of amounts of BA-1049 and M1 were observed in vascular tissue from the BA-1049-treated mice that included aorta (Fig. 7c), carotid artery (Fig. 7d), and inferior vena cava (Fig. 7e).
Fig. 7.
Pharmacokinetics and exposure of BA-1049 and M1 in Ccm mice. (a) Pharmacokinetic analysis of BA-1049 (circles) and metabolite M1 (squares) after oral (P.O.; 30 mg/kg; n = 4 at each time point) and intravenous (I.V.; 5 mg/kg; n = 4) delivery of BA-1049 in mice. (b) BA-1049 (gray) and M1 (black) content in plasma of Ccm1 and Ccm3 mice after 4 or 3 months of treatment (respectively) of 1.0 (n = 11), 10 (n = 14), or 100 (n = 38) mg/kg/day BA-1049 in drinking water. (c) BA-1049 (gray) and M1 (black) content in aorta of Ccm1 and Ccm3 mice after 4 or 3 months of treatment (respectively) of 1.0 (n = 9), 10 (n = 11), or 100 (n = 35) mg/kg/day BA-1049 in drinking water. (d) BA-1049 (gray) and M1 (black) content in carotid of Ccm1 and Ccm3 mice after 4 or 3 months of treatment (respectively) of 1.0 (n = 8), 10 (n = 10), or 100 (n = 25) mg/kg/day BA-1049 in drinking water. (e) BA-1049 (gray) and M1 (black) content in inferior vena cava (IVC) of Ccm1 and Ccm3 mice after 4 or 3 months of treatment (respectively) of 1.0 (n = 8), 10 (n = 20), or 100 (n = 23) mg/kg/day BA-1049 in drinking water.
Discussion
We report here studies in transgenic mice to investigate a novel ROCK2 selective inhibitor to stop progression of the disease phenotype in CA. Loss-of-function mutations in CCM1, CCM2, or CCM3 genes cause CAs in both hereditary and sporadic disease [11]. Solitary sporadic lesions can often be removed surgically, but there is no approved treatment to stop continued growth and development of multiple lesions in the hereditary form of the disease. ROCK is a kinase that critically regulates EC barrier function [38] and EC organization during angiogenesis [39]. ROCK has been identified as a potential target for treatment of CA [15, 40] and ROCK activation has been demonstrated in a number of surgically excised human CA lesion specimens from sporadic and all three familial genotypes [11, 16, 19]. The studies we report here demonstrate that ROCK2 is the important ROCK isoform for CA lesion development, and a ROCK2-selective inhibitor BA-1049 has potential to reduce disease progression.
Transgenic mice hemizygous for CCM1 or CCM3 and bred into a mutator background quite faithfully recapitulate the human disease. Both the Ccm1+/−Msh2−/− mice and Ccm3+/−Trp53−/− mice develop CA lesions, which are leaky clusters of EC lesions in the brain that increase in size and number over time [5, 27]. Other studies found that Fasudil, a non-specific ROCK inhibitor, decreased lesion burden and reduced permeability in Ccm1+/−Msh2−/− mice and Ccm2+/−Trp53−/− mice when provided in drinking water at 100 mg/kg/day [27]. Fasudil inhibits both isoforms of ROCK as well as protein kinase A and other kinases [41]. It is approved in Japan to treat vasospasm following subarachnoid hemorrhage, but it does not have sufficient safety profile for long-term use. Accumulating data show that drugs that specifically target the ROCK2 isoform are more promising for development of systemic therapies: ROCK1 and ROCK2 have distinct roles in vascular smooth muscle cell function [42] and targeting ROCK2 helps avoid unwanted cardiovascular side effects attributed to ROCK1 inhibition [23, 43, 44]. BA-1049 is an orally-available ROCK2-selective inhibitor [25] and here we report there was no dose-dependent attrition of the mice even after 4 months of 100 mg/kg/day oral delivery. We found that BA-1049 is effective in reducing lesion burden in both Ccm1+/−Msh2−/− mice and Ccm3+/−Trp53−/− mice, suggesting the potential for drug development for treatment of all forms of CA. Moreover, our studies with Ccm3+/−Rock1+/− and Ccm3+/−Rock2+/− mice showed that ROCK2 is the important isoform to target. While it would be interesting to investigate if any lesions reappeared at a later period after the 3- to 4- month treatment, this was beyond the scope of this study.
ROCK1 and ROCK2 are highly homologous kinases with overlapping functions. ROCK2 is the isoform highly expressed in the CNS, and both ROCK1 and ROCK2 are important in the regulation of actin cytoskeleton [36]. The Ccm3+/−Rock1+/− and Ccm3+/−Rock2+/− mice we used in our studies to investigate the ROCK isoform important in CA have reduced levels of ROCK1 and ROCK2, respectively [35]. Only the Ccm3+/−Rock2+/− mice showed reduced lesion volume compared to their Rock wild-type control. Although studies of silencing ROCK1 or ROCK2 in cells depleted of CCM1 point to a major role of ROCK1 in the regulation of actin cytoskeleton and adherens junctions in human umbilical vein ECs grown in culture and in zebrafish cardiac morphogenesis [45], this is the first study that demonstrates a critical role of the ROCK2 isoform in lesion growth and maturation in vivo. Oral in vivo administration of BA-1049 results in the production of an active metabolite [25] and was the predominant compound in plasma of mice fed BA-1049 in their drinking water (Fig. 7). While both BA-1049 and M1 are selective for ROCK2, M1 may inhibit some ROCK1 in vascular tissue where higher levels of both BA-1049 and M1 were found, compared to plasma.
In our transgenic mouse studies, it was not feasible to treat the animals by oral gavage because of the number of animals recruited into the study (n=96 Ccm1 and n=87 Ccm3) (Tables 1 and 2), the length of treatment over 3 or 4 months, and the initiation of treatment immediately after weaning. We treated the animals by addition BA-1049 to their drinking water, after confirming a minimum of 24 days stability of BA-1049 in aqueous solution, and we did not use solutions for more than a week. Mice are thought to drink approximately 5–8 times per day [46] and therefore the drug levels in plasma would increase and decrease according to time after drinking. With exposure in drinking water, the animals are not expected to show a large Cmax, compared to oral gavage, but to maintain a lower steady-state drug level. Plasma in the mice was collected at euthanasia, and showed very low levels of parent drug at all doses (below 20 ng/mL) with the M1 metabolite, which is an active metabolite [25], being the dominant form of the drug in plasma. The lowest dose of 1 mg/kg/day was effective for reduction in non-heme iron deposition around lesions, despite very low plasma levels, which is likely because ECs retained higher levels, as shown in Fig. 7. An examination of drug levels in blood vessels showed much higher levels of M1 in blood vessels than in plasma, and we speculate that ECs that make up the lesions do not rapidly export the drug, leading to sustained repression of ROCK2.
It is possible that the effectiveness of low doses of BA-1049 on reducing CA permeability reflect the difference of the importance of the two isoforms on different physiological aspects of CA lesions. In human disease, imaging shows that CA lesions have a defective cell permeability barrier [47] and Ccm3+/−Rock1+/− transgenic mice have substantial deposition of non-heme iron near lesions. Even the low dose of BA-1049 of 1 mg/kg/day dramatically reduced deposition of non-heme iron. Higher doses of BA-1049 effectively reduced lesion burden in both Ccm1 and Ccm3 transgenic mice. BA-1049 did not completely prevent formation and/or growth of all CA lesions despite the lowest doses being effective to reduce iron deposition. Atorvastatin, which acts on Rho through preventing isoprenylation, also reduces iron burden and growth of stage 2 lesions, but does not prevent lesion genesis [30]. While the safety of atorvastatin in humans with CA and recent symptomatic hemorrhage is currently being assessed in a Phase I-IIA proof of concept trial (clinicaltrials.gov: NCT02603328) [48], concern has been raised about brain hemorrhage with statins, and other pleiotropic effects beyond ROCK inhibition [49]. ROCK1 and ROCK2 have complex roles in normal angiogenesis and in the aberrant angiogenesis that causes lesion growth. In normal ECs, the ROCK1/2 inhibitor Y27632 reduces EC tube formation [15, 50], however in CCM-gene depleted ECs, Y-27632 has the opposite effect and rescues tube formation [15]. When ROCK is hyperactivated, Rho is also repressed by ROCK [51]. We speculate that this feedback loop may be defective in CAs, and therefore, perhaps a combination to repress both Rho with statins and ROCK with BA-1049 may more potently reduce de novo lesion formation. Many complex CNS disease respond better to combinations, and an important avenue for future research in CA will be to understand how multimodal therapies can not only reduce progression of disease, but prevent lesions from forming.
These studies demonstrate that BA-1049 has promise to reduce CA disease progression, both by reducing non-heme deposition near lesions as well as reducing lesion growth and maturation, while showing a promising safety profile after long-term (3–4 months) oral administration. BA-1049 treatment could provide alternatives to surgical removal of symptomatic CAs and will be developed in the future for the specific indication of reducing the burden of CA disease.
Supplementary Material
Acknowledgements
We thank Cenk Ayata (Massachusetts General Hospital), Mark Bear (Massachusetts Institute of Technology), Guy Rouleau (McGill), Dennis Choi (State University of New York), Rebecca Stockton (LABioMed) and Dongdong Zhang (University of Chicago) for helpful discussions throughout the study. We also thank Peter Pytel (University of Chicago) for help with autopsy of mice as well as Heidy Pardo and Erin Griffin (both of Duke University) for help with transgenic mice breeding.
Funding
Supported by a National Institute of Health Small Business Innovation Research (SBIR) 1R44NS095420–01 grant to LM, DM, and IA.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Competing interests
Dr. McKerracher is CEO of BioAxone, holds an ownership interest in the company and has a significant competing interest. Drs. Matthew Abbinanti, Ken Rosen and Joerg Ruschel were employees of BioAxone and have a modest conflict of interest. Drs. Doug Marchuk and Issam Awad were recipients of sponsored research through the SBIR grant to BioAxone. The other authors declare no conflict of interest.
Ethical Approval
All applicable international, national, and /or institutional guidelines for the care and use of animals were followed. All animal protocols were approved by the Tufts University Institutional Animal Care and Use Committee (IACUC) or the Duke University IACUC and mice were bred at Duke University in compliance with the NIH Guide for the Care and Use of Laboratory Animals.
References
- 1.Al-Shahi Salman R, Hall JM, Horne MA, Moultrie F, Josephson CB, Bhattacharya JJ et al. Untreated clinical course of cerebral cavernous malformations: a prospective, population-based cohort study. Lancet Neurol 2012;11(3):217–24. 10.1016/S1474-4422(12)70004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riant F, Bergametti F, Ayrignac X, Boulday G, Tournier-Lasserve E. Recent insights into cerebral cavernous malformations: the molecular genetics of CCM. FEBS J 2010;277(5):1070–5. 10.1111/j.1742-4658.2009.07535.x. [DOI] [PubMed] [Google Scholar]
- 3.Leblanc GG, Golanov E, Awad IA, Young WL, Biology of Vascular Malformations of the Brain NWC. Biology of vascular malformations of the brain. Stroke 2009;40(12):e694–702. 10.1161/STROKEAHA.109.563692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Holou WN, O’Lynnger TM, Pandey AS, Gemmete JJ, Thompson BG, Muraszko KM et al. Natural history and imaging prevalence of cavernous malformations in children and young adults. J Neurosurg Pediatr 2012;9(2):198–205. 10.3171/2011.11.PEDS11390. [DOI] [PubMed] [Google Scholar]
- 5.Plummer NW, Gallione CJ, Srinivasan S, Zawistowski JS, Louis DN, Marchuk DA. Loss of p53 sensitizes mice with a mutation in Ccm1 (KRIT1) to development of cerebral vascular malformations. Am J Pathol 2004;165(5):1509–18. 10.1016/S0002-9440(10)63409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horne MA, Flemming KD, Su IC, Stapf C, Jeon JP, Li D et al. Clinical course of untreated cerebral cavernous malformations: a meta-analysis of individual patient data. Lancet Neurol 2016;15(2):166–73. 10.1016/S1474-4422(15)00303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter PJ, Willinsky RA, Harper W, Wallace MC. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg 1997;87(2):190–7. 10.3171/jns.1997.87.2.0190. [DOI] [PubMed] [Google Scholar]
- 8.Sahoo T, Johnson EW, Thomas JW, Kuehl PM, Jones TL, Dokken CG et al. Mutations in the gene encoding KRIT1, a Krev-1/rap1a binding protein, cause cerebral cavernous malformations (CCM1). Hum Mol Genet 1999;8(12):2325–33. 10.1093/hmg/8.12.2325. [DOI] [PubMed] [Google Scholar]
- 9.Laaberge-le Couteulx S, Jung HH, Labauge P, Houtteville JP, Lescoat C, Cecillon M et al. Truncating mutations in CCM1, encoding KRIT1, cause hereditary cavernous angiomas. Nat Genet 1999;23(2):189–93. 10.1038/13815. [DOI] [PubMed] [Google Scholar]
- 10.Bergametti F, Denier C, Labauge P, Arnoult M, Boetto S, Clanet M et al. Mutations within the programmed cell death 10 gene cause cerebral cavernous malformations. Am J Hum Genet 2005;76(1):42–51. 10.1086/426952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonald DA, Shi C, Shenkar R, Gallione CJ, Akers AL, Li S et al. Lesions from patients with sporadic cerebral cavernous malformations harbor somatic mutations in the CCM genes: evidence for a common biochemical pathway for CCM pathogenesis. Hum Mol Genet 2014;23(16):4357–70. 10.1093/hmg/ddu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akers AL, Johnson E, Steinberg GK, Zabramski JM, Marchuk DA. Biallelic somatic and germline mutations in cerebral cavernous malformations (CCMs): evidence for a two-hit mechanism of CCM pathogenesis. Hum Mol Genet 2009;18(5):919–30. 10.1093/hmg/ddn430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gault J, Sain S, Hu LJ, Awad IA. Spectrum of genotype and clinical manifestations in cerebral cavernous malformations. Neurosurgery 2006;59(6):1278–84; discussion 84–5. 10.1227/01.NEU.0000249188.38409.03. [DOI] [PubMed] [Google Scholar]
- 14.Yao L, Romero MJ, Toque HA, Yang G, Caldwell RB, Caldwell RW. The role of RhoA/Rho kinase pathway in endothelial dysfunction. J Cardiovasc Dis Res 2010;1(4):165–70. 10.4103/0975-3583.74258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borikova AL, Dibble CF, Sciaky N, Welch CM, Abell AN, Bencharit S et al. Rho kinase inhibition rescues the endothelial cell cerebral cavernous malformation phenotype. J Biol Chem 2010;285(16):11760–4. 10.1074/jbc.C109.097220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stockton RA, Shenkar R, Awad IA, Ginsberg MH. Cerebral cavernous malformations proteins inhibit Rho kinase to stabilize vascular integrity. J Exp Med 2010;207(4):881–96. 10.1084/jem.20091258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitehead KJ, Chan AC, Navankasattusas S, Koh W, London NR, Ling J et al. The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nat Med 2009;15(2):177–84. 10.1038/nm.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer A, Zalvide J, Faurobert E, Albiges-Rizo C, Tournier-Lasserve E. Cerebral cavernous malformations: from CCM genes to endothelial cell homeostasis. Trends Mol Med 2013;19(5):302–8. 10.1016/j.molmed.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Shenkar R, Shi C, Rebeiz T, Stockton RA, McDonald DA, Mikati AG et al. Exceptional aggressiveness of cerebral cavernous malformation disease associated with PDCD10 mutations. Genet Med 2015;17(3):188–96. 10.1038/gim.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beckers CM, Knezevic N, Valent ET, Tauseef M, Krishnan R, Rajendran K et al. ROCK2 primes the endothelium for vascular hyperpermeability responses by raising baseline junctional tension. Vascul Pharmacol 2015;70:45–54. 10.1016/j.vph.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov 2005;4(5):387–98. 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- 22.Loirand G Rho Kinases in Health and Disease: From Basic Science to Translational Research. Pharmacol Rev 2015;67(4):1074–95. 10.1124/pr.115.010595. [DOI] [PubMed] [Google Scholar]
- 23.Green J, Cao J, Bandarage UK, Gao H, Court J, Marhefka C et al. Design, Synthesis, and Structure-Activity Relationships of Pyridine-Based Rho Kinase (ROCK) Inhibitors. J Med Chem 2015;58(12):5028–37. 10.1021/acs.jmedchem.5b00424. [DOI] [PubMed] [Google Scholar]
- 24.Xin YL, Yu JZ, Yang XW, Liu CY, Li YH, Feng L et al. FSD-C10: A more promising novel ROCK inhibitor than Fasudil for treatment of CNS autoimmunity. Biosci Rep 2015;35(5). 10.1042/BSR20150032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosen KM, Abbinanti MD, Ruschel J, McKerracher L, Bond L. Rho kinase inhibitor BA-1049 (R) and active metabolites thereof, United States Patent 10,106,525, October 23, 2018. In: USPTO Patent Full-Text and Image Database. 2018. patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=10106525.PN.&OS=PN/10106525&RS=PN/10106525. Accessed 29 Jul 2019.
- 26.Gunel M, Awad IA, Finberg K, Anson JA, Steinberg GK, Batjer HH et al. A founder mutation as a cause of cerebral cavernous malformation in Hispanic Americans. N Engl J Med 1996;334(15):946–51. 10.1056/NEJM199604113341503. [DOI] [PubMed] [Google Scholar]
- 27.McDonald DA, Shenkar R, Shi C, Stockton RA, Akers AL, Kucherlapati MH et al. A novel mouse model of cerebral cavernous malformations based on the two-hit mutation hypothesis recapitulates the human disease. Hum Mol Genet 2011;20(2):211–22. 10.1093/hmg/ddq433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang SX, Lertvorachon J, Hou ST, Konishi Y, Webster J, Mealing G et al. Chlortetracycline and demeclocycline inhibit calpains and protect mouse neurons against glutamate toxicity and cerebral ischemia. J Biol Chem 2005;280(40):33811–8. 10.1074/jbc.M503113200. [DOI] [PubMed] [Google Scholar]
- 29.Zhu M, Liu PY, Kasahara DI, Williams AS, Verbout NG, Halayko AJ et al. Role of Rho kinase isoforms in murine allergic airway responses. Eur Respir J 2011;38(4):841–50. 10.1183/09031936.00125010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shenkar R, Peiper A, Pardo H, Moore T, Lightle R, Girard R et al. Rho Kinase Inhibition Blunts Lesion Development and Hemorrhage in Murine Models of Aggressive Pdcd10/Ccm3 Disease. Stroke 2019;50(3):738–44. 10.1161/STROKEAHA.118.024058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girard R, Zeineddine HA, Orsbon C, Tan H, Moore T, Hobson N et al. Micro-computed tomography in murine models of cerebral cavernous malformations as a paradigm for brain disease. J Neurosci Methods 2016;271:14–24. 10.1016/j.jneumeth.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shenkar R, Shi C, Austin C, Moore T, Lightle R, Cao Y et al. RhoA Kinase Inhibition With Fasudil Versus Simvastatin in Murine Models of Cerebral Cavernous Malformations. Stroke 2017;48(1):187–94. 10.1161/STROKEAHA.116.015013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi C, Shenkar R, Zeineddine HA, Girard R, Fam MD, Austin C et al. B-Cell Depletion Reduces the Maturation of Cerebral Cavernous Malformations in Murine Models. J Neuroimmune Pharmacol 2016;11(2):369–77. 10.1007/s11481-016-9670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conover WJ. Practical Nonparametric Statistics New York: John Wiley and Sons; 1999. [Google Scholar]
- 35.Zhu Y, Wu Q, Fass M, Xu JF, You C, Muller O et al. In vitro characterization of the angiogenic phenotype and genotype of the endothelia derived from sporadic cerebral cavernous malformations. Neurosurgery 2011;69(3):722–32. 10.1227/NEU.0b013e318219569f. [DOI] [PubMed] [Google Scholar]
- 36.Newell-Litwa KA, Badoual M, Asmussen H, Patel H, Whitmore L, Horwitz AR. ROCK1 and 2 differentially regulate actomyosin organization to drive cell and synaptic polarity. J Cell Biol 2015;210(2):225–42. 10.1083/jcb.201504046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H-H, Ayata C. Rho-Associated Kinases in Cerebrovascular Disease. In: Caplan LR, Leary MC, Thomas AJ, Zhang JH, Biller J, Lo EH et al. , editors. Primer on Cerebrovascular Diseases (Second Edition). Amsterdam: Elsevier; 2017. pp. 265–8. [Google Scholar]
- 38.Terry S, Nie M, Matter K, Balda MS. Rho signaling and tight junction functions. Physiology (Bethesda) 2010;25(1):16–26. 10.1152/physiol.00034.2009. [DOI] [PubMed] [Google Scholar]
- 39.Hoang MV, Whelan MC, Senger DR. Rho activity critically and selectively regulates endothelial cell organization during angiogenesis. Proc Natl Acad Sci U S A 2004;101(7):1874–9. 10.1073/pnas.0308525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDonald DA, Shi C, Shenkar R, Stockton RA, Liu F, Ginsberg MH et al. Fasudil decreases lesion burden in a murine model of cerebral cavernous malformation disease. Stroke 2012;43(2):571–4. 10.1161/STROKEAHA.111.625467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 2000;351(Pt 1):95–105. 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Zheng XR, Riddick N, Bryden M, Baur W, Zhang X et al. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ Res 2009;104(4):531–40. 10.1161/CIRCRESAHA.108.188524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hartmann S, Ridley AJ, Lutz S. The Function of Rho-Associated Kinases ROCK1 and ROCK2 in the Pathogenesis of Cardiovascular Disease. Front Pharmacol 2015;6:276 10.3389/fphar.2015.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Defert O, Boland S. Rho kinase inhibitors: a patent review (2014 – 2016). Expert Opin Ther Pat 2017;27(4):507–15. 10.1080/13543776.2017.1272579. [DOI] [PubMed] [Google Scholar]
- 45.Lisowska J, Rodel CJ, Manet S, Miroshnikova YA, Boyault C, Planus E et al. The CCM1-CCM2 complex controls complementary functions of ROCK1 and ROCK2 that are required for endothelial integrity. J Cell Sci 2018;131(15). 10.1242/jcs.216093. [DOI] [PubMed] [Google Scholar]
- 46.Kurokawa M, Akino K, Kanda K. A new apparatus for studying feeding and drinking in the mouse. Physiol Behav 2000;70(1–2):105–12. 10.1016/s0031-9384(00)00226-2. [DOI] [PubMed] [Google Scholar]
- 47.Mikati AG, Khanna O, Zhang L, Girard R, Shenkar R, Guo X et al. Vascular permeability in cerebral cavernous malformations. J Cereb Blood Flow Metab 2015;35(10):1632–9. 10.1038/jcbfm.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polster SP, Stadnik A, Akers AL, Cao Y, Christoforidis GA, Fam MD et al. Atorvastatin Treatment of Cavernous Angiomas with Symptomatic Hemorrhage Exploratory Proof of Concept (AT CASH EPOC) Trial. Neurosurgery In press. 10.1093/neuros/nyy539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eisa-Beygi S, Wen XY, Macdonald RL. A call for rigorous study of statins in resolution of cerebral cavernous malformation pathology. Stroke 2014;45(6):1859–61. 10.1161/STROKEAHA.114.005132. [DOI] [PubMed] [Google Scholar]
- 50.Uchida S, Watanabe G, Shimada Y, Maeda M, Kawabe A, Mori A et al. The suppression of small GTPase rho signal transduction pathway inhibits angiogenesis in vitro and in vivo. Biochem Biophys Res Commun 2000;269(2):633–40. 10.1006/bbrc.2000.2315. [DOI] [PubMed] [Google Scholar]
- 51.Mori K, Amano M, Takefuji M, Kato K, Morita Y, Nishioka T et al. Rho-kinase contributes to sustained RhoA activation through phosphorylation of p190A RhoGAP. J Biol Chem 2009;284(8):5067–76. 10.1074/jbc.M806853200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.