Abstract
Statement of the Problem:
Recently, new compound of 3, 5-dimethyl-1-thiocarboxamide pyrazole has been composed with excellent antibacterial property. Biocompatibility and its effects on mechanical properties of dental composites should be considered before clinical use.
Purpose:
The purpose of this study was to evaluate the biocompatibility of 3, 5-dimethyl-1-thiocarboxamide pyrazole as a new antibacterial compound and its effect on the mechanical properties of dental composites.
Materials and Method:
In this experimental study, a new antibacterial compound was synthesis by reaction between Thiosemicarbazide and 2, 4-Pentandione and tested on thirty male albino Wistar rats weighting 200-250gr. Rats were randomly divided into 3 groups of 10, each rat received 3 implants of 3,5-dimethyl-1-thiocarboxamide pyrazole, penicillin v and empty polyethylene tube. A pathologist, who was unaware of types of tested materials and timing, performed the examination of specimens. The depth of cure and flexural strength of resin composite was measured using Iso4049 standard technique. Compressive strength was determined according to Iso9917 standard.
Results:
This compound was biocompatible and there was no significant difference in flexural strength and compressive strength of the composites containing 1% of this compound with the control group (p> 0.05).
Conclusion:
The 3, 5-dimethyl-1-thiocarboxamide pyrazole with a concentration of 1% in flowable composites can be very effective in preventing secondary caries.
Keywords: Pyrazole , Antibacterials agents , Streptococcus mutans, Dental Materials, Dental Caries, Biocompatibility
Introduction
Todays, dental composites have attracted many patients and dentists regarding their beauty and the convenience of their application [ 1 ]. Despite of the great progresses achived in these dental materials, one of the biggest problems that attracted the attention of many researchers is the weakness of these compounds against bacteria. This problem reduces their useful life and ultimately leads to substitution. Bacteria are a complex poly-microbial population that tends toward composites’ surface, as the bacteria's tendency for these compounds is more than tooth enamel [ 1 - 6 ]. The accumulation of these bacteria on the composite surface leads to secondary caries. In recent years, many studies have focused on creating antibacterial properties in composites [ 1 - 2 ]. One way to achieve this goal was to use the combination of 3,5-dimethyl-1-thiocarboxamide pyrazole [ 7 ].
Using this compound, a very good antibacterial property was obtained in flowable composites [ 7 ]. It should be noted that considering only antibacterial properties in composites are not efficient in dental restoration and should be biocompatible with suitable mechanical properties. In this study, tissue compatibility of 3, 5-dimethyl-1-thiocarboxamide pyrazole was studied. In order to evaluate the biological response due to the new dental material, in vivo methods such as implantation of these substances in subcutaneous tissue of laboratory animals were studied first [ 8 - 9 ].
Therefore, in this study, the tissue biocompatibility assessment of the composition of 3,5-dimethyl-1-thiocarboxamide pyrazole was investigated in rat subcutaneous tissue. Any restorative material should have good mechanical properties of tooth as well as good biocompatibility in order to be able to use it in tooth restoration. The mechanical properties of composites containing the aforementioned compound were also investigated.
Materials and Method
Synthesis of 3, 5-dimethyl-1-thiocarboxamide pyrazole
3, 5-dimethyl-1-thiocarboxamide pyrazole was synthesized by reaction between thiosemicarbazide and 2, 4-Pentandione with a molar ratio of one to one. 5 gr of thiosemicarbazide was dissolved in 250 ml cold water and 1ml of concentrated hydrochloric acid. Prepared liquid was filtered using filter paper and 5ml of 2, 4-Pentandione was added and stirred for 30 minutes. Passing one hour of stirring, a white precipitate, 3, 5-dimethyl-1-thiocarboxamide pyrazole was formed (See Figure 1) and purified using ethanol [ 10 ].
Figure1.
Synthesis of 3, 5-dimethyl-1-thiocarboxamide pyrazole
Biocompatibility
In the present animal study, thirty male albino Wistar rats weighting 200-250 gr were used. The experiment was performed in accordance to “Principles of laboratory animal care” formulated by National Society for Medical Research and “Guide for the care and use of laboratory animals” prepared by Institute of Laboratory Animal Resources, National Research Council and published by the National Academy Press, revised 1996. Veterinarian performed rats’ anesthesia via the peritoneal method using 47.5 mg kg-1 of Ketamine hydrochloride and 0.01 mg kg-1 of Rompun 2%. For each animal, the hair was cut from the back of the animal to the right and left (two anterior regions and one posterior region) with 10% scarlet brown iodine. All regions were disinfected using green iodine and in each region a small incision with a length of approximately 12 mm was made by a razor scalpel number 20. Subsequently, using a blunt dissecting backside, a 20-mm bundle of subcutaneous tissue was cut for implanting tubes.
Rats were randomly divided into 3 groups of 10, each rat received 3 implants of 3,5-dimethyl-1-thiocarboxamide pyrazole, penicillin v, and empty polyethylene tube. Then, it was sutured plainly and individually with nylon yarn. The application of penicillin-v was because of its current use as a routine antibiotic. One week, one month and two months after the tubes were implanted [ 11 ]; the rats were sacrificed by injecting too much ketamine HCl. The hair extensions of the tubes were re-shaved, the skin and connective tissue surrounding the implant were brought out the block section and kept in the formalin 10% for at least 48 hours. After fixing, parallel sections with longitudinal axis and tissue were prepared for staining with Hematoxylin Eosin. A pathologist, without knowing the type of tested material and timing, performed the analysis of samples. To evaluate the histological response, studies of Stanford [ 11 ] and Yaltirik et al. [ 12 ], Ozbas et al. [ 13 ] and Derakhshan et al. [ 14 ] were used.
Histological criteria
The tissue reaction at both ends of the tubes was studied according to the following histological criteria: The size of the subcutaneous tissue around the tube was measured at 100 magnifications and in micrometers. The score were defined as (0) for no capsules, (1) when the thickness of the capsule was less than 150 μm, (2) when the thickness of the capsule was greater than 150 μm. The severity of inflammation was defined by observing the number of inflammatory cells in or around the subcutaneous tissue capsule with a magnification of 40 fold and scored as (0) for no inflammation, (1) for less than 25 cells, (2) between 25 and 50 cells, (3) between 50 and 75 cells, (4) for more than 75 cells.
For the extent of inflammation , the amount of expansion of inflammatory cells at both ends of the tube was observed at 40x magnification and scored as (1) when Inflammatory cells only appeared in the surface layer of the capsule,(2) when inflammatory cells were limited to fibrous capsules, (3) when inflammatory cells were seen around the capsule. For detecting necrosis, the disappearance of cells or the observation of the ghost of cells without a nucleus was observed with 40x magnification and scored as (0) for absence of necrosis, and (1) for presence of necrosis.
For assessing the inflammation type, different types of inflammation were defined according to the related inflammatory cells as acute inflammation (p (polymorphonuclear cells) and chronic inflammation (macrophages, plasma cells and lymphocytes) with the score of (0) for no inflammation, (1) for chronic inflammation, and (2) for acute inflammation. According to the available data presented in the literatures, 10 samples were utilized for each period [ 13 - 16 ].
Data analysis
By describing the findings, non-parametric methods such as Kruskal-Wallis were used based on the rank of the variables. Mann-whitney U test was applied for comparing the two methods and the significant correlation was modified with Bonferroni method.
Preparation of composites containing 3, 5-dimethyl-1- thiocarboxamide pyrazole
Six groups of the specimen were prepared by mixing of 3, 5-dimethyl-1-thiocarboxamide pyrazole with resin composite (Tetric flow, Ivoclar Vivadent, USA) in 1, 2, 3, 4 and 5 wt% and 0 wt% as the control group. Mixing was performed in a dark room for 15 min by spatula [ 17 ].
Measurement of the depth of cure
The depth of cure of composite’s resin was measured using Iso4049 standard technique. The dental composite resin was placed inside a cylindrical mold (with 10 mm depth and 4 mm diameter) and cured by using a light cure instrument (LED, DEML, SDS Kerr, USA, with an intensity of circa 800 mW cm-2) from above for 40 seconds. Upon completion of the exposure, the cured composite was removed from the mold and uncured material was separated from the end of composite with the spatula, and then by using the digital caliper, the height of the cured resin was measured and finally, this value was divided into two and was reported as the depth of the cure [ 17 ]. The test was repeated three times and the mean of the obtained values was reported.
Flexural strength measurement
Flexural strength is one of the most important mechanical tests to determine the efficiency of dental resins. In accordance with Iso4049 standard, the resin was poured in a rectangular cube mold with a dimension of 25x2x25mm. The filled mold was closed by glass slides at the ends and was cured using a light cure instrument for 40 seconds through the both ends. The cured specimens were removed from the mold and put in deionized water at 37°C for 1 day. Later, both surfaces of the samples are polished in a wet environment. For each formulation, 10 samples were tested. The three-point bending test was performed using M350-10CT Testometric device at the rate of 0.5 mm / min. Finally, bending strength (Fs) was calculated using the following relation.
In the above relation, p, l, b and d present resistance at break point, distance between the two locations of the sample on the device (20 mm), sample width and sample thickness, respectively.
Compressive strength measurement
According to Iso9917 standard for compressive strength tests, the sample was located in a cylindrical metal frame with a diameter of 4 mm and a height of 6 mm. Prepared sample was cured for 40 seconds through both ends and put in 37°C water for 24 hours. The finalized samples were subjected to pressure using M350-10CT Testometric device at the rate of 1mm/min. Ten samples of each formulation were tested [ 15 ].
Data analysis
The resulted mechanical test data were analyzed by one-way ANOVA, and the Tukey post hoc HSD multiple comparison test. The level of significance was considered as p= 0.05.
Results
Evaluation of histopathologic observations in considered groups
Case 1: Empty polyethylene tube group
The capsule thickness was increased during the time, so that during 7 days, growth in thickness of the capsule was less than 30 days. Moreover, it was observed that the thickness growth of capsule was more significant after 60 days in comparison with 7days (p< 0.05).
Severity of inflammation, extent of inflammation, and presence of chronic inflammation were significantly higher in the 7-day interval than 30-day and 60-day periods (p< 0.05). However, no significant difference was observed between 30 and 60 days (p> 0.05).
Acute necrosis and inflammation were not observed in any of the periods.
Case 2: 3, 5-dimethyl-1-thiocarboxamide pyrazole group
In this case, thickness of the capsule increased over the time, although this difference was not significant (p> 0.05).The extent of inflammation decreased by 7, 30 and 60 days, but there was no apparent difference between the groups. The severity of inflammation decreased by 7, 30, and 60 days, and a significant decrease was seen for the time duration of 60 days in comparison with the 7 days (p< 0.05).Acute necrosis and inflammation were not observed in any group.
Case 3: Penicillin V group
The thickness of the capsule in the 30 and 60 days’ periods was severely higher than the 7-days period. However, no clear difference was seen (p> 0.05). The severity of inflammation and the extent of inflammation after 60 days was less than 30 days and 7 days, but this difference was not statistically significant (p> 0.05). The same as other groups, acute necrosis, and inflammation were not recognized in any of the samples.
Histological evaluation and comparison between the groups studied at different time periods
After seven days
There was no apparent difference in the amount of capsule thickness, intensity of inflammation and extent of inflammation between different groups during this time period (p> 0.05).
After thirty days
During this period, there was no significant variation in the thickness of the capsule, severity of inflammation, extent of inflammation and type of inflammation between the three different groups (p> 0.05).
After sixty days
In this period, there was no clear difference in any cases of capsule thickness, intensity of inflammation, extent of inflammation and type of inflammation between 3 different groups (p> 0.05). Results are shown in Figure 2.
Figure2.
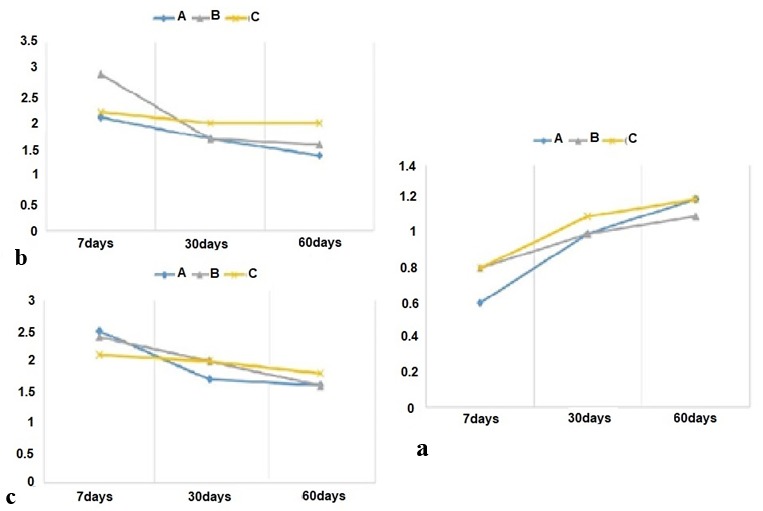
a: Thickness of subcutaneous tissue capsule in various time intervals, b: Severity of inflammation in different time intervals, c: Variation of extent of inflammation versus time. (A: Empty polyethylene tube, B: 3, 5-dimethyl-1-thiocarboxamide pyrazole, C: Penicillin V)
Depth of cure
It was seen that by increasing the percentage of composition of 3,5-dimethyl-1-thiocarboxamide pyrazole, the depth of cure decreases, so that there was no certain change between control group and 1% and 2% groups (p> 0.05). However, there was significant difference between control group and 3%, 4% and 5% groups, (p< 0.05). Results are depicted in Figure 3.
Figure3.

a: Depth of cure of flowable composites containing 3, 5-dimethyl-1-thiocarboxamide pyrazole, b: Flexural strength of flowable composites containing 3, 5-dimethyl-1-thiocarboxamide pyrazole, c: Compressive strength of flowable composites containing 3, 5-dimethyl-1-thiocarboxamide pyrazole
Flexural strength
By increasing the percentage of 3,5-dimethyl-1-thiocarboxamide pyrazole, the flexural strength increased so that there was no visible change between the control group and the 1% and 2% groups (p> 0.05). However, a significant difference with the rest of the groups was present (p< 0.05) (Figure 3).
Compressive strength
Increasing the composition of 3, 5-dimethyl-1-thiocarboxamide pyrazole, decreased the compressive strength, where no statistically significant difference between the control group and the 1% group was seen, p> 0.05, while the other groups had a significant variation in comparison with the control group (p< 0.05) (Figure 3).
Discussion
The success of antibacterial tests per se does not indicate the usefulness of these substances for clinical use. In addition to antibacterial characteristic, tissue biocompatibility and suitable mechanical properties are also important factors.
To use any substance on humans, tissue biocompatible tests should be done. There are currently four methods for measuring the biocompatibility of materials. These methods include cell evaluation in the culture medium, subcutaneous implantation, intrabony implantation, and ex vivo evaluation of periradicular tissue in human specimens [ 16 ].
Subcutaneous implantation method was utilized in the present study where the substance was placed in polyethylene and was implanted in subcutaneous connective tissue. This method is one of the most common methods for assessing the relative adaptability of dental materials, introduced by Torneck [ 18 ]. The effectiveness of this method was re-evaluated and confirmed by Olson et al. [ 19 ]. They showed that insertion of the investigated material in the final parts of the polyethylene tubes prevents the release of material into the connective tissue, so this method has more advantages than the direct method of injecting into the connective tissue. Makkes et al. [ 20 ], Safavi et al. [ 21 ], Kim et al. [ 22 ] and Bachmann et al. [ 23 ] used polyethylene tubes for implanting. These tubes form very little stimulation in the surrounding tissue, or do not produce any reaction in the surrounding tissue. These are easy to use and to implant, and it seems to have a stable chemical structure not affected by the substances or agents inside the tube [ 20 ]. Tubes should be selected with a small diameter to minimize the possibility of displacement and excessive movement of the material. A length of 7-5 mm of tube is good enough to compare the surface of the materials tested at the ends of the tubes, which are in direct contact with tissue [ 22 ]. In the present study, tubes with 5 mm length and 1.7 mm diameter were applied with two open ends.
Stanford [ 11 ] considered two periods of time to investigate the adaptive response but Olsson et al. [ 19 ] suggested three periods of time. Olsson’s study was designed in 3 periods of 7, 30 and 60 days.
There are several methods to compare the severity of the inflammatory response around the tubes containing the observing substance. According to Stanford method [ 11 ], the number of inflammatory cells is counted in different regions of the microscopic section [ 20 ]. Since the inflammatory response of the connective tissue has different aspects, focusing solely on the number of inflammatory cells does not reflect all aspects of the connective tissue response and does not appear to be comprehensive [ 24 ].
Therefore, method proposed by Yaltirik et al. [ 12 ], was used so that in addition to the number and extent of inflammatory cells, other aspects of inflammation, such as the formation of fibrous capsules and the presence of necrosis were included. Since in similar studies, the type of inflammatory cells was studied [ 13 - 14 , 24 - 25 ], this variable was also considered in the present study.
According to the results of this study, in the 30-day and 60-day periods, in most samples of each of the three tested materials, a fibrous capsule was formed around the tube, which suggested good tissue tolerance of these materials. Formation of fibrous capsule around the material can keep it from the surrounding tissue and prevents damage of the material in the tissue [ 11 , 26 - 27 ]. Formation of a fibrous capsule was reported in many similar studies [ 11 - 12 , 27 - 29 ].
According to the above studies, it was specified that the presence of 3, 5-dimethyl-1-thiocarboxamide pyrazole in flowable dental composites resulted in antimicrobial properties against Streptococcus mutans. It also had good biocompatibility, but the presence of this compound in flowable dental composites reduced mechanical properties of composites. This effect was not significant in composites containing 1% of the 3, 5-dimethyl-1-thiocarboxamide pyrazole.
Conclusion
According to the investigations, it was concluded that the compound 3, 5-dimethyl-1-thiocarboxamide pyrazole with concentration of 1% in flowable composites is very effective in preventing secondary caries without reducing the mechanical strength.
Acknowledgement
This work was supported by Kerman University of Medical Sciences. Also, authors would like to thank Hojatollah Khabazzadeh for his valuable contributions.
Conflict of Interest: The authors state that they have no conflict of interest.
References
- 1.Li F, Weir MD, Chen J, Xu HH. Comparison of quaternary ammonium-containing with nano-silver-containing adhesive in antibacterial properties and cytotoxicity. Dent Mater. 2013; 29: 450–461. doi: 10.1016/j.dental.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanner J, Vallittu PK, Söderling E. Effect of water storage of E-glass fiber-reinforced composite on adhesion of Streptococcus mutans. Biomaterials. 2001; 22: 1613–1618. doi: 10.1016/s0142-9612(00)00314-8. [DOI] [PubMed] [Google Scholar]
- 3.Tanner J, Carlén A, Söderling E, Vallittu PK. Adsorption of parotid saliva proteins and adhesion of Streptococcus mutans ATCC 21752 to dental fiber-reinforced composites. J Biomed Mater Res B Appl Biomater. 2003; 66: 391–398. doi: 10.1002/jbm.b.10012. [DOI] [PubMed] [Google Scholar]
- 4.Skjörland KK. Plaque accumulation on different dental filling materials. Scand J Dent Res. 1973; 81: 538–542. doi: 10.1111/j.1600-0722.1973.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 5.Skjorland KK. Bacterial accumulation on silicate and composite materials. J Biol Buccale. 1976; 4: 315–322. [PubMed] [Google Scholar]
- 6.Tanner J, Robinson C, Söderling E, Vallittu PK. Early plaque formation on fiber-reinforced composites in vivo. Clin Oral Invest. 2005; 9: 154–160. doi: 10.1007/s00784-005-0317-4. [DOI] [PubMed] [Google Scholar]
- 7.Eskandarizadeh A, Khabazzadeh A, Mohammadzadeh I, Khaleghi M, Askari A, Saidi A. creating excellent antibacterial properties in flowable dental composites with a new antibacterial compound. Annals Dent Spec. 2017; 5: 5–8. [Google Scholar]
- 8.Garcia Lda F, Marques AA, Roselino Lde M, Pires-de-Souza Fde C, Consani S. Biocompatibility evaluation of Epiphany/Resilon root canal filling system in subcutaneous tissue of rats. J Endod. 2010; 36: 110–114. doi: 10.1016/j.joen.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Beck F, Lettner S, Graf A, Bitriol B, Dumitrescu N, Bauer P, et al. Survival of direct resin restorations in posterior teeth within a 19-year period (1996-2015): A meta-analysis of prospective studies. Dent Mater. 2015; 31: 958–985. doi: 10.1016/j.dental.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Kovacs A, Nemcsok D, Pokol G, Meszaros Szecsenyi K, Leovac VM, Acimovic, ZK, et al. Structural, spectroscopic and computational studies of the HgL2Cl2 complex (L= 3,5-dimethyl-1-thiocarboxamide pyrazole) and the crystal structure of L. New Journal of Chemistry. 2005; 29: 833–840. [Google Scholar]
- 11.Stanford JW. Recommended standard practices for biological evaluation of dental materials. Fédération Dentaire International, Commission of Dental Materials, Instruments, Equipment and Therapeutics. Int Dent J . 1980; 30: 141–188. [PubMed] [Google Scholar]
- 12.Yaltirik M, Ozbas H, Bilgic B, Issever H. Reactions of connective tissue to mineral trioxide aggregate and amalgam. J Endod. 2004; 30: 95–99. doi: 10.1097/00004770-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Ozbas H, Yaltirik M, Bilgic B, Issever H. Reactions of connective tissue to compomers, composite and amalgam root-end filling materials. Int Endod J. 2003; 36: 281–287. doi: 10.1046/j.1365-2591.2003.00649.x. [DOI] [PubMed] [Google Scholar]
- 14.Derakhshan S, Adl A, Parirokh M, Mashadiabbas F, Haghdoost AA. Comparing subcutaneous tissue responses to freshly mixed and set root canal sealers. Iran Endod J. 2009; 4: 152–157. [PMC free article] [PubMed] [Google Scholar]
- 15.Yli-Urpo H, Lassila LV, Närhi T, Vallittu PK. Compressive strength and surface characterization of glass ionomer cements modified by particles of bioactive glass. Dent Mater. 2005; 21: 201–209. doi: 10.1016/j.dental.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Cox CF, Sübay RK, Suzuki S, Suzuki SH, Ostro E. Biocompatibility of various dental materials: pulp healing with a surface seal. Int J Periodontics Restorative Dent. 1996; 16: 240–251. [PubMed] [Google Scholar]
- 17.Tavassoli Hojati S, Alaghemand H, Hamze F, Ahmadian Babaki F, Rajab-Nia R, Rezvani MB, et al. Antibacterial, physical and mechanical properties of flowable resin composites containing zinc oxide nanoparticles. Dent Mater. 2013; 29: 495–505. doi: 10.1016/j.dental.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Torneck CD. Reaction of rat connective tissue to polyethylene tube implants. I. Oral Surg Oral Med Oral Pathol . 1966; 21: 379–387. doi: 10.1016/0030-4220(66)90077-6. [DOI] [PubMed] [Google Scholar]
- 19.Olsson B, Sliwkowski A, Langeland K. Subcutaneous implantation for the biological evaluation of endodontic materials. J Endod. 1981; 7: 355–367. doi: 10.1016/S0099-2399(81)80057-X. [DOI] [PubMed] [Google Scholar]
- 20.Makkes PC, van Velzen SK, Wesselink PR, de Greeve PC. Polyethylene tubes as a model for the root canal. Oral Surg Oral Med Oral Pathol. 1977; 44: 293–300. doi: 10.1016/0030-4220(77)90283-3. [DOI] [PubMed] [Google Scholar]
- 21.Safavi KE, Pascon EA, Langeland K. Evaluation of tissue reaction to endodontic materials. J Endod. 1983; 9: 421–429. doi: 10.1016/S0099-2399(83)80257-X. [DOI] [PubMed] [Google Scholar]
- 22.Kim JS, Baek SH, Bae KS. In vivo study on the biocompatibility of newly developed calcium phosphate-based root canal sealers. J Endod. 2004; 30: 708–711. doi: 10.1097/01.don.0000125318.90702.01. [DOI] [PubMed] [Google Scholar]
- 23.Bachmann MH, Mathiason-Dubard C, Learn GH, Rodrigo AG, Sodora DL, Mazzetti P, et al. Genetic diversity of feline immunodeficiency virus: dual infection, recombination, and distinct evolutionary rates among envelope sequence clades. J Virol. 1997; 71: 4241–4253. doi: 10.1128/jvi.71.6.4241-4253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pickett H. Shave and punch biopsy for skin lesions. Am Fam Physician. 2011; 84: 995–1002. [PubMed] [Google Scholar]
- 25.Sieuwerts AM, Klijn JG, Peters HA, Foekens JA. The MTT tetrazolium salt assay scrutinized: how to use this assay reliably to measure metabolic activity of cell cultures in vitro for the assessment of growth characteristics, IC50-values and cell survival. Eur J Clin Chem Clin Biochem. 1995; 33: 813–823. doi: 10.1515/cclm.1995.33.11.813. [DOI] [PubMed] [Google Scholar]
- 26.Khoramizadeh MR, Falak R. Basic practical methods in cell cultur J. Masters ed.; Animal Cell Culture: A Practical Approach. 3rd ed. Oxford University Press: New York; 2000. pp. 150–159. [Google Scholar]
- 27.Holland R, de Souza V, Nery MJ, Otoboni Filho JA, Bernabé PF, Dezan Júnior E. Reaction of rat connective tissue to implanted dentin tubes filled with mineral trioxide aggregate or calcium hydroxide. J Endod. 1999; 25: 161–166. doi: 10.1016/s0099-2399(99)80134-4. [DOI] [PubMed] [Google Scholar]
- 28.Balamurugan A, Balossier G, Laurent-Maquin D, Pina S, Rebelo AH, Faure J, et al. An in vitro biological and anti-bacterial study on a sol-gel derived silver-incorporated bioglass system. Dent Mater. 2008; 24: 1343–1351. doi: 10.1016/j.dental.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Van de Loosdrecht AA, Nennie E, Ossenkoppele GJ, Beelen RH, Langenhuijsen MM. Cell mediated cytotoxicity against U 937 cells by human monocytes and macrophages in a modified colorimetric MTT assay. A methodological study. J Immunol Methods . 1991; 141: 15–22. doi: 10.1016/0022-1759(91)90205-t. [DOI] [PubMed] [Google Scholar]



