Abstract
Abstract
Xanthomonas translucens is a group of gram‐negative bacteria that can cause important diseases in cereal crops and forage grasses. Different pathovars have been defined according to their host ranges, and molecular and biochemical characteristics. Pathovars have been placed into two major groups: translucens and graminis. The translucens group contains the pathovars causing bacterial leaf streak (BLS) on cereal crops such as wheat, barley, triticale, rye, and oat. In recent years, BLS has re‐emerged as a major problem for many wheat‐ and barley‐producing areas worldwide. The biology of the pathogens and the host–pathogen interactions in cereal BLS diseases were poorly understood. However, recent genome sequence data have provided an insight into the bacterial phylogeny and identification and pathogenicity/virulence. Furthermore, identification of sources of resistance to BLS and mapping of the resistance genes have been initiated.
Taxonomy
Kingdom Bacteria; Phylum Proteobacteria; Class Gammaproteobacteria; Order Xanthomonadales; Family Xanthomonadacea e; Genus Xanthomonas; Species X. translucens; translucens group pathovars: undulosa, translucens, cerealis, hordei, and secalis; graminis group pathovars: arrhenatheri, graminis, poae, phlei; newly established pathovar: pistaciae.
Host range
X. translucens mainly infects plant species in the Poaceae with the translucens group on cereal crop species and the graminis group on forage grass species. However, some strains have been isolated from, and are able to infect, ornamental asparagus and pistachio trees. Most pathovars have a narrow host range, while a few can infect a broad range of hosts.
Genome
The complete genome sequence is available for two X. translucens pv. undulosa strains and one pv. translucens strain. A draft genome sequence is also available for at least one strain from each pathovar. The X. translucens pv. undulosa strain Xt4699 was the first to have its complete genome sequenced, which consists of 4,561,137 bp with total GC content approximately at 68% and 3,528 predicted genes.
Virulence mechanisms
Like most xanthomonads, X. translucens utilizes a type III secretion system (T3SS) to deliver a suite of T3SS effectors (T3Es) inside plant cells. Transcription activator‐like effectors, a special group of T3Es, have been identified in most of the X. translucens genomes, some of which have been implicated in virulence. Genetic factors determining host range virulence have also been identified.
Keywords: bacterial diseases, host resistance, pathogen virulence, wheat, Xanthomonas
This pathogen profile summarizes the current knowledge on Xanthomonas translucens that causes bacterial leaf streak on cereals.

1. INTRODUCTION
According to Bamberg (1936), the occurrence of leaf streak‐like disease on wheat and barley had been observed and recorded as early as 1893. However, the formal description of the disease and the causal bacterium was not made until 1917, when Jones and coworkers published the identification of bacterial blight on barley (Jones et al., 1917). The causal bacterium was named Bacterium translucens because of the translucent lesions on diseased leaves. Shortly after this the disease on wheat was described, but was named as black chaff, which referred to the disease symptoms on the spikes (Smith et al., 1919). Since then, leaf streak disease has been reported on other small grains and some grasses (Reddy et al., 1924; Hagborg, 1942; Wallin and Reddy, 1945; Fang et al., 1950; Cunfer and Scolari, 1982). On forage grasses, a group of genetically related bacteria cause bacterial wilt (Egli et al., 1975; Egli and Schmidt, 1982).
The classification and nomenclature of bacteria causing bacterial leaf streak (BLS) diseases has been very confusing and has undergone many changes, largely due to their morphological and biochemical similarity and overlapping host range. For a long time, these bacteria were classified as different pathovars (pv.) under the species of Xanthomonas campestris (Dye and Lelliott, 1974). Vauterin et al. (1992, 1995) proposed the re‐establishment of the species Xanthomonas translucens including strains that cause leaf streak on small grains and some grasses (the “translucens” group) and strains that cause bacterial wilt on forage grasses (the “graminis” group). This classification and nomenclature system has been supported by recent molecular and whole genome sequence data (Peng et al., 2016; Langlois et al., 2017; Hersemann et al., 2017). Genomic sequence data also suggest that the pathovars cerealis could be genetically separated from other translucens and graminis group pathovars.
Wheat and barley BLS diseases have been found in almost all wheat‐ or barley‐growing areas worldwide (Bamberg, 1936; Duveiller, 1990) and they can cause substantial yield losses and poor grain quality (Waldron, 1929; Shane et al., 1987; Duveiller and Maraite, 1993). Although BLS epidemics have been sporadic and usually occur in warm and humid subtropics regions, in the last decade the incidence of BLS has dramatically increased in the Midwestern United States, where the majority of the hard red spring wheat and durum wheat are produced (Adhikari et al., 2011). Most of the cultivars in this region, as well as other places, are highly susceptible and no chemical methods are available for BLS control in the field (McMullen and Adhikari, 2011). Furthermore, breeding for resistant wheat and barley cultivars is difficult due to the lack of sources of resistance and our knowledge regarding host–pathogen interactions.
Although research has been done on some aspects of the disease system, in particular the recent genome sequencing projects, there has been no review for this group of bacterial pathogens as well as the diseases they cause except a handbook published in 1997 by the International Maize and Wheat Improvement Center (CIMMYT) (Duveiller et al., 1997). In this work, we provide a comprehensive review mainly of the “translucens” group of X. translucens pathogens that cause BLS on small grains. This review summarizes the current knowledge regarding the diseases, virulence, and genomics of the bacterial pathogens, and the genetics of host resistance. We also provide some thoughts on the future direction of the research aiming to solve this disease problem.
2. DISEASE SYMPTOMS, DISTRIBUTION, AND IMPORTANCE
The symptoms of BLS disease are mainly observed on leaves and spikes. On the leaf, initially water‐soaked streaks develop that subsequently become translucent necrotic lesions (Figure 1a,b). Under warm and humid conditions, bacterial ooze (yellow exudates) can be seen on the leaf surface (Figure 1a). Under high disease pressure, the whole leaf area may be severely affected by the pathogen (Figure 1c). The disease, black chaff, refers to the dark‐purple streaks on the glumes (Figure 1d). The diagnosis of BLS is sometime difficult in the field mainly because the symptoms resemble those caused by fungal pathogens or genetic or environmental factors (Duveiller et al., 1997; McMullen and Adhikari, 2011). For example, wheat cultivars that possess stem rust resistance gene Sr2 develop a melanic reaction that mimics the black chaff symptoms caused by the X. translucens pathogens (Duveiller et al., 1993).
Figure 1.

Symptoms and signs associated with wheat bacterial leaf streak. (a) Wheat leaves at the early stage of disease development. Water‐soaking streaks are present with bacterial ooze. (b) Wheat leaf with longitudinal necrotic lesions caused by the bacterium. (c) Completely dead flag leaves caused by the bacterium. (d) Black chaff symptoms on the spike. (Photographs (a) and (d) were kindly provided by Justin Stanton, University of Minnesota, and Dr Erick DeWolf, Kansas State University, respectively)
BLS disease has been reported from many geographical regions worldwide where wheat is grown. According to Duveiller et al. (1997), the occurrence of BLS has been reported from the countries in North America (United States, Canada, Mexico), South America (Argentina, Bolivia, Brazil, Paraguay, Peru, Uruguay), Asia (China, Iran, India, Pakistan, Syria, Turkey, Kazakhstan, Yemen, Israel, Russia, Malaysia, Japan), Africa (Kenya, Ethiopia, South Africa, Tanzania, Tunisia, Libya, Madagascar, Morocco, Zambia), most parts of Europe (France, Romania, Russia, Turkey, Ukraine), and Australia. Recently, the global distribution of BLS has been updated on the web page of the European Plant Protection Organization (EPPO) Global Database (https://gd.eppo.int/taxon/XANTTR/distribution, accessed 20 June 2019). It appears that BLS disease has not been reported from western Europe, which is probably due to unfavourable environmental conditions and extensive quarantine efforts (Paul and Smith, 1989; Duveiller et al., 1997). In the United States, BLS was initially reported in barley and wheat fields in the Midwest (Jones et al., 1917; Smith et al., 1919). Since then, BLS has been found in many places in the United States with outbreaks and epidemics mostly having occurred in the southeastern regions (Milus and Mirlohi, 1994; Tubajika et al., 1999). In recent years, the incidence of BLS has increased in the Upper Midwest region of the United States, including North Dakota, Minnesota, and South Dakota (Adhikari et al., 2012a; Kandel et al., 2012; Curland et al., 2018).
Although BLS is considered as a potential threat for wheat production worldwide, there are no recent reports on yield losses caused by BLS. Furthermore, the importance of BLS varies in different wheat‐growing regions and depends mainly on the level of resistance/susceptibility of wheat cultivars grown and the prevalent environmental conditions. Yield losses due to BLS are generally reported to be 10% or less, but severe infection can cause up to 40% yield losses on highly susceptible cultivars (Waldron, 1929; Forster and Schaad, 1988). Studies have indicated that yield loss due to BLS is generally negatively correlated with BLS severity on flag leaves, and up to 20% yield reduction is possible if 50% leaf area of the flag leaves is infected (Shane et al., 1987; Duveiller and Maraite, 1993). Yield losses are usually due to the reduction in grain weight and the number of kernels per spikes; however, severe infection can cause sterile spikes leading to a complete yield loss (Forster and Schaad, 1988; Tubajika et al., 1998). In addition, BLS infection can alter the protein content of the grains, resulting in quality reduction (Shane et al., 1987).
3. DISEASE CYCLE, EPIDEMIOLOGY, AND MANAGEMENT
Many aspects of the BLS aetiology have not been experimentally tested, but a general disease cycle has been proposed (Duveiller et al., 1997, Figure 2). Seed is thought to be an important source of primary inoculum for BLS (Boosalis, 1952; Tsilosani et al., 1977; Timmer et al., 1987; Forster and Schaad, 1988; Milus and Mirlohi, 1995; Rashid et al., 2013). The survival rate of bacteria on seeds and the possibility of transmission to seedlings were shown to be largely dependent on the storage conditions, the length of storage, and the level of susceptibility of genotypes (Boosalis, 1952; Forster and Schaad, 1990; Milus and Mirlohi, 1995). It has been reported that if the number of bacteria is less than 1,000 cfu per gram in the seed lots, no BLS symptoms could be visible in the emerged plants (Klykov, 1945; Duveiller et al., 1997). Studies have shown that weeds and grasses may serve as overwintering hosts or green bridges for the bacterium to spread from one season to other (Wallin, 1946; Fang et al., 1950; Boosalis, 1952; Thompson et al., 1989). The bacteria are also able to survive in soil and crop debris for a short period of time (Milus and Mirlohi, 1995; Duveiller et al., 1997; Stromberg et al., 2000).
Figure 2.
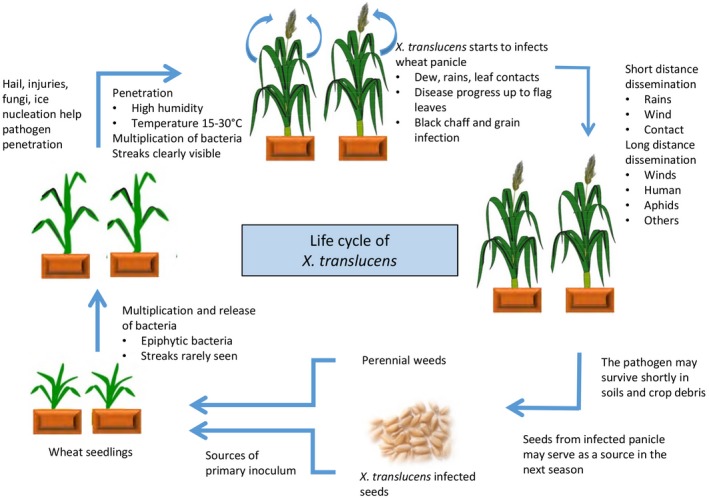
Life cycle of Xanthomonas translucens pathogens. The life cycle picture was redrawn based on the descriptions in Duveiller et al. (1997)
Although X. translucens pathogens enter plant tissues through natural openings, some of them have been reported to possess ice nucleation ability that can cause frost damage leading to the formation of wounding sites for direct entry (Kim et al., 1987; Azad and Schaad, 1988; Gurion‐Sherman and Lindow, 1993). Warm and humid conditions are thought to be important for BLS development because the disease has been found more in wet seasons or in sprinkler‐irrigated fields with warm temperature; however, the exact conditions conducive to BLS development are not well known. BLS epidemics have been reported to be sporadic and vary from year to year (Bamberg, 1936; Duveiller et al., 1991; Tubajika et al., 1998). Artificial introduction of disease in field plots is challenging which makes it difficult to determine the relationship between environmental conditions and BLS epidemics. Duveiller and Maraite (1995) showed that temperature is more important than other factors to initiate epidemics. Other factors implicated in BLS epidemics include damage from wind, hail and frost, dew period, and host genotypes (Duveiller et al., 1997).
Several cultural practices may help reduce BLS incidence; however, limited information is available on their efficacy (Duveiller et al., 1997; Adhikari et al., 2011). Crop rotation is not considered as a major control strategy because the bacteria cannot survive in debris for a long time (Milus and Mirlohi, 1995; Duveiller et al., 1997). Seed is considered an important source of primary inoculum, thus use of clean seed might be a way of reducing BLS incidence. Different methods have been developed to detect the bacterium in seeds, including dilution plating with the use of selective media, seedling infection assays, serodiagnostic assays, PCR amplification, and loop‐mediated isothermal amplification (Forster and Schaad, 1985; Bragard and Verhoyen, 1993; Maes et al., 1996; Langlois et al., 2017). Seed disinfection methods have also been developed to eliminate the bacterium by using chemical or physical methods (Atanasoff and Johnson, 1920; Forster et al., 1990). However, the effectiveness of seed treatment is still questionable because of contradictory results obtained from different studies (Braun, 1920; Duveiller et al., 1997). Using clean seeds or applying seed treatments may reduce disease incidence, but does not stop the spread of BLS inoculum between fields (Duveiller et al., 1997). Very limited studies have been conducted to test chemicals for controlling cereal BLS in the field. Silva et al. (2010) tested silicon compounds on BLS disease development but did not obtain conclusive results. Some copper‐based bactericides or antibiotics have been recommended for controlling bacterial diseases caused by other Xanthomonas spp. (McManus et al., 2002; Lamichhane et al., 2018), but their effects on cereal Xanthomonas spp. have not been tested.
4. CLASSIFICATION, NOMENCLATURE, AND IDENTIFICATION OF X. TRANSLUCENS
The early classification and nomenclature for this group of bacteria were mainly based on pathogenicity tests and host range. The early taxonomy for X. translucens had been very confusing because strains varied greatly in host range and levels of host specificity. Jones et al. (1917) first reported BLS disease on barley and named the pathogen B. translucens. Later, BLS was reported on wheat by Smith et al. (1919) where the pathogen was named B. translucens var. undulosum because it morphologically resembled the barley pathogen and was able to infect barley through artificial inoculation. Dowson (1939) created the genus Xanthomonas to include the species X. translucens that caused cereal BLS diseases. Based on the natural host as well as the ability to infect hosts using artificial inoculation, Hagborg (1942) classified X. translucens into five formae speciales (f. spp.): f. sp. hordei (barley), f. sp. undulosa (wheat, barley, and rye), f. sp. secalis (rye), f. sp. hordei‐avenae (barley and oat), and f. sp. cerealis (wheat, barley, rye, and oat). Fang et al. (1950) argued that f. sp. cerealis and hordei‐avenae should be combined with f. sp. undulosa and hordei, respectively, and given the name of f. sp. cerealis for strains that naturally occur on smooth bromegrass and quack grass but can infect wheat, barley, rye, and oat using artificial inoculations. Classification by Fang et al. (1950) also included f. sp. phleipratensis, which was originally identified by Wallin and Reddy (1945) from timothy grass. Because xanthomonad pathogens cannot be easily differentiated by morphology and bacteriological tests, later taxonomic efforts placed the entire X. translucens f. sp. into X. campestris as different pathovars, which included X. campestris pvs. cerealis, hordei, secalis, translucens, and undulosa (Dye and Lelliott, 1974; Young et al., 1978; Dye et al., 1980).
During the 1970s and 1980s, bacterial wilt was reported on many forage grasses and the causal bacteria were shown to be closely related to X. campestris identified from cereals (Egli et al., 1975; Wilkins and Exley, 1977; Roberts et al., 1981; Egli and Schmidt, 1982; Channon and Hisset, 1984). Those bacteria were named as different pathovars under X. campestris, including pv. graminis, pv. arrhenatheri, pv. phlei, and pv. poae (Egli and Schmidt, 1982; Van den Mooter et al., 1987). Van den Mooter et al. (1987) also recognized that pv. phlei is the synonym of pv. phleipratensis, which was previously identified by Wallin and Reddy (1945) and Fang et al. (1950).
Since the late 1980s, modern DNA, protein, and other biochemical methods have been used in the taxonomy and identification of xanthomonads isolated from cereals and grasses (van den Mooter 1987; Azad and Schaad, 1988; Stead, 1989; Kersters et al., 1989; Vauterin et al., 1992; Rademaker et al., 2006). The main finding was that xanthomonads from cereals and grasses are phylogenetically related, while they can be easily separated by using high‐resolution fingerprinting techniques. Thus, the idea emerged to separate them into two main groups, the “translucens” and “graminis” groups, to describe xanthomonads from cereals and grasses, respectively (Vauterin et al., 1992). Therefore, the subsequent reclassification of xanthomonads made by Vauterin et al. (1995) re‐established the species name X. translucens to encompass all X. campestris pathovars infecting cereals (translucens group: pv. undulosa, pv. translucens, pv. cerealis, pv. hordei, pv. secalis) and grasses (graminis group: pv. graminis, pv. arrhenatheri, pv. phlei, pv. poae).
Beside cereals and forage grasses, X. translucens was also found to infect ornamental asparagus and pistachio trees, which are in the genetically distant plant families Liliaceae and Anacardiaceae, respectively (Rademaker et al., 2006; Marefat et al., 2006a, 2006b). Very surprisingly, the bacterial pathogens on asparagus were identified as pv. undulosa and were able to infect wheat by cross‐inoculation and vice versa (Rademaker et al., 2006). The bacterium on pistachio trees was designated a new pathovar, X. translucens pv. pistaciae, because it could not be classified into any of pathovars in X. translucens even though they are closely related (Giblot‐Ducray et al., 2009). The X. translucens pv. pistaciae has been added to the International Congress of Plant Pathology (ICPP) list of plant pathogenic bacteria along with other X. translucens pathovars (Bull et al., 2010, 2012).
Further studies have been conducted to examine the genetic similarity and diversity of the pathovars in translucens group by using DNA markers, biochemical profiling, and pathogenicity tests. The studies conducted by Bragard et al. (1995, 1997) suggested that X. translucens pv. translucens is a synonym of X. translucens pv. hordei, and the translucens group contains three true biological entities, cerealis, translucens and undulosa, with pv. translucens pathogenic on barley, pv. undulosa pathogenic to both barley and wheat, and pv. cerealis pathogenic to barley, wheat, oat, and bromegrass. Not many X. translucens pv. secalis strains were available for analyses, but they were shown to be clustered with pv. undulosa (Bragard et al., 1997; Giblot‐Ducray et al., 2009; Curland et al., 2018). Using a few strains isolated from wheat and barley, we showed that wheat strains (undulosa) were capable of causing lesions and water‐soaking symptoms on wheat, barley, and triticale (×Triticosecale), while barley strains (translucens) were only able to cause lesions and water‐soaking symptom on barley (Sapkota et al., 2018; Figure 3). However, Curland et al. (2018) reported that most X. translucens pv. undulosa and pv. translucens strains collected from the Upper Midwestern United States cause disease symptoms on both wheat and barley by infiltration, even though their virulence was generally correlated with their original host.
Figure 3.
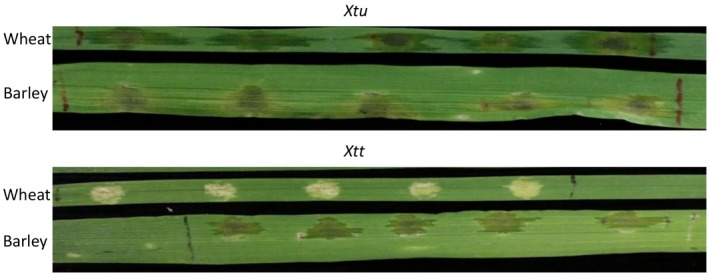
Reaction of wheat or barley to Xanthomonas translucens pv. undulosa (Xtu) and X. translucens pv. translucens (Xtt) strains. The strains were collected from North Dakota, USA
In recent years, X. translucens taxonomy has been performed using multilocus sequence analysis with data from the housekeeping gene and/or genome sequences (Wichmann et al., 2013; Gardiner et al., 2014; Peng et al., 2016; Langosis et al. 2017; Curland et al., 2018). The analyses with the DNA sequence data basically agreed with the previous classifications for X. translucens at both species and pathovar levels determined by DNA–DNA hybridization and DNA marker analysis and pathogenicity tests (Bragard et al., 1997). Evidence has also suggested that cerealis strains could be genetically separated from other translucens and graminis pathovars (Langlois et al., 2017). However, more cerealis strains need to be analysed in order to draw a solid conclusion.
5. VIRULENCE and PATHOGENICITY OF X. TRANSLUCENS
Similar to other xanthomonads, X. translucens bacteria are gram‐negative, rod‐shaped, non‐spore forming, 0.5–0.8 × 1.0–2.5 µm in size, contain a single polar flagellum, and form pale yellow colonies on nutrient agar medium (Figure 4a,b). Using a transmission electron microscope and the diseased wheat leaf samples collected at the fifth day after spray inoculation with a X. translucens pv. undulosa strain, we observed that bacterial cells were mainly distributed in the mesophyll tissue (Figure 4b). Thus, the bacterial pathogen probably enters plant tissue through stomata and mainly colonizes mesophyll tissues. However, using the same spray inoculation method, we observed little or no disease on barley leaves for X. translucens pv. translucens, suggesting that it may have a different tissue specificity (Liu et al. unpublished data). Leaf clipping and the dip inoculation method have been successfully used in the study by Pesce et al. (2017) to induce disease on barley. This suggests that X. translucens pv. translucens probably resides in the vascular tissue. Tissue specificity differences have been reported for other Xanthomonas species (Bogdanove et al., 2011). For example, X. oryzae pv. oryzae is a vascular bacterial pathogen causing rice leaf blight, while X. oryzae pv. oryzicola, the cause of bacterial leaf streak, is a nonvascular bacterial pathogen (Bogdanove et al., 2011).
Figure 4.
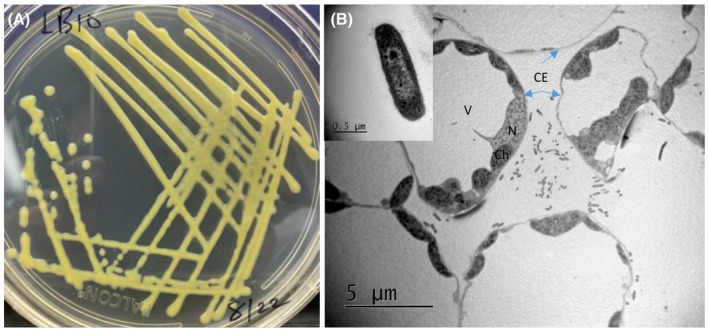
The bacterial pathogen in culture and in planta. (a) Bacterial culture on King's medium B. (b) Scanning electron microscopic photogram of leaf section infected with a Xanthomonas translucens pv. undulosa strain. The inserted box indicates a single bacterial cell. The parts of the plant cells are indicated: CE, cell envelope (blue arrows); Ch, chloroplast; N, nucleus; V, vacuole
Research in the last few decades has substantially advanced our understanding of the molecular mechanisms of pathogenicity and virulence for X. translucens. As mentioned above, X. translucens strains differ in their levels of host specificity, with some capable of infecting multiple host species (broad host range) but others only infecting one host species (narrow host range). The broad host range bacteria may harbour factors controlling virulence rather than narrow‐host range bacteria carrying avirulence factors (Mellano and Cooksey, 1988). Mutants with a narrow host range have been obtained from a wide host range in X. translucens by using Tn5 insertion mutagenesis, and host‐specific virulence (Hsv) genes have been identified that can restore the ability of the mutants to infect specific hosts (Mellano and Cooksey, 1988; Waney et al., 1991). However, the sequences for these Hsv genes have not been determined.
Virulence differences were also observed among strains within a pathovar (Cunfer and Scolari, 1982; Milus and Chalkly, 1994; Adhikari et al., 2011; Sapkota et al., 2018). Using pathogenicity tests on several wheat genotypes, Adhikari et al. (2011) found that X. translucens pv. undulosa strains collected from North Dakota are relatively diverse in virulence and there is a significant interaction of wheat–X. translucens pv. undulosa. Previous studies coupled with our recent studies showed that several triticale lines harbour dominant resistance genes to some X. translucens pv. undulosa strains, suggesting the presence of a gene‐for‐gene interaction in triticale (Johnson et al., 1987; Wen et al., 2018). However, it remains unknown if the wheat–X. translucens pathosystem involves a gene‐for‐gene interaction.
Many gram‐negative bacterial pathogens rely on the type III secretion system (T3SS) and T3SS‐delivered effectors (T3Es) for their pathogenicity and virulence. Phylogenetic analysis showed xanthomonads can be classified into two clades with the majority of species in clade 2, and they usually contain a highly conserved hrp gene cluster that encodes the T3SS (Bȕttner and Bonas, 2010; Bogdanove et al., 2011; Pesce et al., 2017). X. translucens phylogenetically belongs to clade 1, which has four other species: X. albilineans, X. hyacinthi, X. theicola, and X. sacchari (Young et al., 2008; Gardiner et al., 2014). Among the clade 1 species, X. albilineans and X. sacchari have no hrp cluster, but all the sequenced X. translucens strains have it (Pesce et al., 2017). The genetic structure and organization of the hrp locus in X. translucens genomes is highly conserved among sequenced strains and it is similar to that in clade 1 Xanthomonas species and β‐protebacteria (Wichmann et al., 2013; Pesce et al., 2017). Functional analyses have shown that X. translucens T3SS is essential for pathogenicity, hypersensitive response induction and effector delivery (Waney et al., 1991; Gardiner et al., 2014; Peng et al, 2016; Pesce et al., 2017). However, the hrp system in X. graminis strain Xtg29 has been shown not to be required for pathogenicity but to modulate virulence (Wichmann et al., 2013). This indicates that strains of graminis and translucens may have different biology and virulence mechanisms (Hersemann et al., 2017).
Like other Xanthamonas spp., genome sequence data indicate that X. translucens strains harbour a suite of T3Es (Whichmann et al., 2013; Gardiner et al., 2014; Pesce et al., 2015; Peng et al., 2016; Charkhabi et al., 2017; Hersemann et al., 2017). Sequence alignment and comparison showed that a core set of T3Es identified from other Xanthomonas spp. was also present in the sequenced X. translucens genomes. However, variations in the presence/absence and copy number of individual T3Es were detected among different pathovars and among different strains within a pathovar, and also frame‐shift and loss‐of‐function mutations of certain T3Es occur among different strains (Wichmann et al., 2013; Peng et al., 2016; Charkhabi et al., 2017). The unique repertoire of T3Es in different strains and pathovars may reflect the adaptation of strains to various hosts or virulence to specific genotypes of a host (Jacques et al., 2016). However, the function of the majority of T3Es in X. translucens in virulence or colonization have not been tested and determined.
Transcription activator‐like effectors (TALEs) are a special type of T3Es that are only present in Xanthomonas and some strains of Ralstonia solanacearum (Bogdanove et al., 2010). TALE genes have been identified from three X. translucens strains with a complete genome sequence as well as a few strains with a draft genome sequence (Pesce et al., 2015; Jaenicke et al., 2016; Peng et al., 2016; Charkhabi et al., 2017). X. translucens TALEs that have been well characterized were from two X. translucens pv. undulosa strains, Xt4699 and ICMP11055, which have eight and seven genes, respectively. Based on the sequences of repeat variable di‐residue (RVD) amino acids, it was found that four TALEs are common between the two strains and the rest are either partially conserved or have no obvious similarity (Charkhabi et al., 2017). Sequence comparison revealed that the two strains had several unique RVDs, including KG, QD, Y*, YK and YD, which have not been identified from other Xanthomas spp. (Peng et al., 2016; Charkhabi et al., 2017). It was also found that some TALEs that are widely distributed in other xanthomonads are less frequently present in X. translucens (Charkhabi et al., 2017).
TALEs function to induce host gene expression after binding to the promoter region of a gene. Using microarray analysis and TALE gene mutants, Peng et al. (2016) provided evidence that TALEs in XT4699 induce high expression of specific wheat genes. For example, the Tal6 was shown to particularly up‐regulate two wheat genes, Ta.7291.1.S1_S1_at (a succinate dehydrogenase subunit) and Ta.14164.1S1_x_at (bHLH family transcription factor). Very recently, it was found that Tal8 is associated with enhanced virulence in XT4699 by modulating ABA biosynthesis and production (Peng et al., 2019). Two TALE genes in ICMP11055 were also shown by site‐directed mutagensis to contribute to bacterial virulence based on lesion length (Charkhabi et al., 2017). More work is needed to investigate the roles of TALEs in virulence and their underlying molecular mechanisms.
6. GENOMICS of X. TRANSLUCENS
As mentioned above, genome sequencing and comparative genomics is a powerful tool to reveal candidate genes’ underlying virulence/pathogenicity as well as bacterial classification of the bacterial pathogens, and whole‐genome sequencing has been performed for X. translucens. The first genome sequence of X. translucens was obtained from X. translucens pv. graminis strain Xtg29 (Wichmann et al., 2013). So far, a total of 51 X. translucens genome assemblies have been deposited in the NCBI Genome Resources (https://www.ncbi.nlm.nih.gov/genome/genomes/14066, accessed 20 June 2019), which covers all pathovars in both translucens and graminis groups. Among them, three were sequenced with long‐reads sequencing techniques and had a complete circular genome sequence, including two X. translucens pv. undulosa strains (Xt4699 from the United States, ICMP11055 from Iran) and one X. translucens pv. translucens strain (DSM 18974 from the United States) (Jaenicke et al., 2016; Peng et al., 2016; Charkhabi et al., 2017). The main features for the three complete genomes are listed in Table 1. The remaining genome sequences were fragmented containing variable numbers of scaffolds or contigs (see the above web link) and the genome size of these strains ranged from 4.1 to 4.8 Mb. Size difference was observed among different pathovars and also among different strains within a pathovar (Charkhabi et al., 2017; Hersemann et al., 2017). The annotated protein‐encoding genes in published X. translucens genomes ranged from 3,160 to 4,413, with pv. graminis relatively having fewer. The available genome sequence data and comparative genomics will greatly facilitate our progress in the understanding host, cultivar, and tissue‐specificity in X. translucens species.
Table 1.
Summary of Xanthomonas translucens pv. translucens or pv. undulosa strains that have fully sequenced genomes
| X. translucens strain | |||
|---|---|---|---|
| ICMP11055 | XT4699 | DSM 18974T | |
| Pathovar name | undulosa | undulosa | translucens |
| Host of origin | Wheat | Wheat | Barley |
| Place of collection | Kerman, Iran | Kansas, United States | Minnesota, United States |
| Genome size (bp) | 4,761,583 | 4,561,137 | 4,715,357 |
| GC content (%) | 67.8 | 68.1 | 67.7 |
| Protein coding genes | 3,953 | 3,528 | 3,736 |
| Ribosomal RNA operons | 2 | 2 | 2 |
| Transfer RNAs | 54 | 54 | 54 |
| CRISPR array | 1 | Not detected | 1 |
| TALE genes | 7 | 8 | 8 |
| Non‐TALE T3E genes | 32 | 32 | – |
| Insertion sequence elements (complete/partial) | 83/58 | 74/56 | – |
| Reference | Charkhabi et al., 2017 | Peng et al., 2016 | Jaenicke et al., 2016 |
TALE, transcription activator‐like effector; T3E, type III secretion effector; CRISPR, clustered regularly interspaced short palindromic repeats; RNA, ribonucleic acid.
7. GENETICS AND MAPPING OF HOST RESISTANCE
Because no chemical control is available, host resistance appears to be the only way to control wheat BLS. Several sources of BLS resistance have been identified in diverse wheat or barley germplasm and related species (Hagborg, 1974; Akhtar and Aslam, 1986; Duveiller et al., 1993; Alizadeh et al., 1994; El Attari et al., 1996; Milus et al., 1996; Tillman et al., 1996; Adhikari et al., 2011; Kandel et al., 2012; Sapkota et al., 2018). These studies revealed a low percentage of resistant lines and no immune or highly resistant materials were found in the wheat germplasm. Nevertheless, several triticale lines were reported to possess high levels of BLS resistance (Cunfer and Scolari, 1982; Johnson et al., 1987; Sapkota et al., 2018).
Using partial and high levels of BLS resistance in small grain crops, the heritability and genetics of BLS resistance were investigated (Duveiller et al., 1993; El Attari et al., 1996; Tillman and Harrison, 1996; Adhikari et al., 2012b; Kandel et al., 2015; Wen et al., 2018). The heritability of BLS resistance in wheat was reported to vary from low to high depending on the resistant lines used (El Attari et al., 1996; Tillman and Harrison, 1996). Classic genetic analysis showed that BLS resistance could be quantitative or qualitative. Duveiller et al. (1993) reported a total of five genes (Bls1, Bls2, Bls3, Bls4, and Bls5) conferring BLS resistance in three resistant wheat cultivars, with Bls1 present in all three partially resistant wheat cultivars and having the largest effect. Quantitative inheritance was suggested by disease level distribution based on the reaction of 19 wheat cultivars to 81 X. translucens pv. undulosa strains (Milus and Chalkey, 1994). Using three F2 populations derived from Terral 101 (resistant), Coker 9877 (moderately resistant), Pioneer 2548 (susceptible), and Coker 9766 (susceptible), Tillman and Harrison (1996) also detected the presence of multiple genes controlling BLS resistance in these wheat cultivars. However, resistance to BLS in a few triticale lines appears to be qualitative and dominant (Johnson et al., 1987; Wen et al., 2018).
To facilitate the use and transfer of host resistance in breeding programmes, quantitative trait locus (QTL) mapping or genome‐wide association studies (GWAS) have been conducted in recent years to identify genomic regions associated with BLS resistance and linked DNA markers. Using a doubled haploid population and a restriction fragment length polymorphism (RFLP) map, El Attari et al. (1998) identified three genomic regions associated with BLS resistance, two on chromosome 3H and one on 7H in barley line Morex. Adhikari et al. (2012b) conducted the first GWAS of BLS resistance using a panel of 566 spring wheat landraces and diversity array technology (DArT) markers, which led to the identification of five genomic regions on chromosomes 1A, 4A, 4B, 6B, and 7D associated with BLS resistance. Gurung et al. (2014) used the same wheat panel and phenotypic data, but with single nucleotide polymorphism (SNP) markers and identified four QTLs, two of which were on similar genomic regions as reported by Adhikari et al. (2012b). Kandel et al. (2015) identified two simple sequence repeat (SSR) markers on chromosomes 2A (Xwmc522) and 6B (Xbarc134) associated with resistance using the identity‐by‐descent mapping method. A total of four QTLs distributed on 2B, 6D, 7A, and 7B were identified by Ayana (2017) using a recombinant inbred lines (RILs) population from the cross of a partially resistant (SD52) and susceptible wheat lines (SD1001) and a genetic map consisting of 1,211 SNP markers. Wen et al. (2018) developed two triticale populations derived from a highly resistant genotype (Siskiyou) and two highly susceptible genotypes (UC38 and Villax St. Jose), and mapped a major resistance gene, Xct1, on chromosome 5R.
8. FUTURE RESEARCH DIRECTIONS
BLS is a common disease of wheat and barley that has the ability to cause a significant reduction in yield and quality. In the last decade, BLS has become an increasingly important problem in all major wheat‐ and barley‐growing areas around the world. Because there is a lack of effective management tools and the understanding of the disease system is limited, the impact of BLS disease on the world’s wheat and barley production will continue to rise. It is imperative to have a global research initiative aiming to solve or mitigate the BLS disease problem. We believe that future research should focus on, but not be limited to, the following areas.
First, an immediate need would be to identify chemicals that can be used at the field scale for disease control while waiting for resistant cultivars to be developed. There are a number of copper‐based commercial bactericides that have been recommended for other foliar diseases caused by xanthomonads. These products can be evaluated directly in the field for their efficacy and profitability. At the same time, new types of chemicals and novel delivery systems should be sought for the development of more efficient and profitable management methods.
Second, it is common knowledge that disease control using genetic resistance is the most desirable method as it is sustainable and environmentally friendly. Therefore, breeding programmes should place more emphasis on using current resistant materials and continue to search for novel sources of resistance. Research is needed to investigate the genetics of resistance in the identified wheat cultivars and breeding lines, and to identify DNA markers linked to the resistance genes or QTLs, which help quickly incorporate partial resistance into local cultivars. Although it could take a long time to move the resistance genes from triticale to wheat cultivars, it is worth doing because these genes confer high levels of resistance to BLS.
Third, a standardized, quick protocol is needed to evaluate pathogen virulence and host resistance. Previous studies have used different inoculation methods, for example direct spraying, injection, and infiltration, with a corresponding rating scale that makes the results incomparable. These inoculations and scoring methods should be further investigated in order to establish a good evaluation protocol in both the greenhouse and the field. This is in particularly important for breeding programmes.
Fourth, an effective management strategy relies on a good understanding of the biology of the disease cycles. There are many areas that need to be addressed, including (a) survival of the pathogens, (b) sources of inoculum and their importance under field conditions, (c) environmental factors favourable for disease development under field conditions, and (d) efficient and accurate identification and detection of the pathogens. Some of the research work mentioned above has been done for the winter wheat regions, but the results need to be confirmed and compared for spring wheat regions.
Lastly, a better understanding of host–pathogen interactions in X. translucens–cereals is needed for the development of wheat or barley cultivars with durable resistance. Genome sequences and comparative genomics can serve as a powerful tool to identify bacterial genes underlying bacterial virulence/avirulence and host range determinants. The host susceptibility genes that either directly or indirectly interact with these bacterial virulence genes can be subsequently identified, and then can be mutated through gene‐editing technologies (Li et al., 2012).
ACKNOWLEDGEMENTS
We would like to thank Dr James Buck for the critical review of the manuscript. This material is based on work supported, in part, by the National Institute of Food and Agriculture, United States Department of Agriculture (USDA), under Hatch project number ND02234.
Sapkota S, Mergoum M, Liu Z. The translucens group of Xanthomonas translucens: Complicated and important pathogens causing bacterial leaf streak on cereals. Molecular Plant Pathology. 2020;21:291–302. 10.1111/mpp.12909
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- Adhikari, T.B. , Gurung, S. , Hansen, J.M. and Bonman, J.M. (2012a) Pathogenic and genetic diversity of Xanthomonas translucens pv. undulosa in North Dakota. Phytopathology, 102, 390–402. [DOI] [PubMed] [Google Scholar]
- Adhikari, T.B. , Gurung, S. , Hansen, J.M. , Jackson, E.W. and Bonman, J.M. (2012b) Association mapping of quantitative trait loci in spring wheat landraces conferring resistance to bacterial leaf streak and spot blotch. The Plant Genome Journal, 5, 1–16. [Google Scholar]
- Adhikari, T.B. , Hansen, J.M. , Gurung, S. and Bonman, J.M. (2011) Identification of new sources of resistance in winter wheat to multiple strains of Xanthomonas translucens pv. undulosa . Plant Disease, 95, 582–588. [DOI] [PubMed] [Google Scholar]
- Akhtar, M.A. and Aslam, M. (1986) Xanthomonas campestris pv. undulosa on wheat. Rachis, 5, 34–37. [Google Scholar]
- Alizadeh, A. , Benetti, V. , Sarrafi, A. , Barrault, G. and Albertini, L. (1994) Genetic analysis for partial resistance to an Iranian strain of bacterial leaf streak (X. campestris pv. hordei) in barley. Plant Breeding, 113, 323–326. [Google Scholar]
- Atanasoff, D. and Johnson, A.G. (1920) Treatment of cereals seeds by dry heat. Journal of Agricultural Research, 18, 379–390. [Google Scholar]
- Ayana, G. (2017) Molecular Characterization of Spot Blotch and Bacterial Leaf Streak Resistance in Bread Wheat. Electronic Theses and Dissertations. 2260. Available at: https://openprairie.sdstate.edu/etd/2260 [Accessed 13 January 2020]. [Google Scholar]
- Azad, H. and Schaad, N.W. (1988) The relationship of Xanthomonas campestris pv. translucens to frost and the effect of frost on black chaff development in wheat. Phytopathology, 778, 95–100. [Google Scholar]
- Bamberg, R.H. (1936) Black chaff disease of wheat. Journal of Agricultural Research, 52, 397–417. [Google Scholar]
- Bogdanove, A.J. , Koebnik, R. , Lu, H. , Furutani, A. , Angiuoli, S.V. , Patil, P.B. et al (2011) Two new complete genome sequences offer insight into host and tissue specificity of plant pathogenic Xanthomonas spp. Journal of Bacteriology, 193, 5450–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanove, A.J. , Schornack, S. and Lahaye, T. (2010) TAL effectors: finding plant genes for disease and defense. Current Opinion in Plant Biology, 13, 394–401. [DOI] [PubMed] [Google Scholar]
- Boosalis, M.G. (1952) The epidemiology of Xanthomonas translucens (J.J. and R.) Dowson on cereals and grasses. Phytopathology, 42, 387–395. [Google Scholar]
- Bragard, C. and Verhoyen, M. (1993) Monoclonal antibodies specific for Xanthomonas campestris bacteria pathogenic on wheat and on other small grains, in comparison with polyclonal antisera. Phytopathology, 139, 217–228. [Google Scholar]
- Bragard, C. , Singer, E. , Alizadeh, A. , Vauterin, L. , Maraite, H. and Swings, J. (1997) Xanthomonas translucens from small grains: diversity and phytopathological relevance. Phytopathology, 87, 1111–1117. [DOI] [PubMed] [Google Scholar]
- Bragard, C. , Verdier, V. and Maraite, H. (1995) Genetic diversity among Xanthomonas campestris strains pathogenic for small grains. Applied and Environmental Microbiology, 61, 1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun, H. (1920) Presoak method seed treatment: A means of preventing seed injury due to chemical disinfectants and increasing germicidal efficiency. Journal of Agricultural Research, 19, 363–392. [Google Scholar]
- Bull, C.T. , De Boer, S.H. , Denny, T.P. , Firrao, G. , Fischer‐Le Saux, M. , Saddler, G.S. et al (2010) Comprehensive list of names of plant pathogenic bacteria, 1980–2007. Journal of Plant Pathology, 92, 551–592. [Google Scholar]
- Bull, C.T. , De Boer, S.H. , Denny, T.P. , Firrao, G. , Fischer‐Le Saux, M. , Saddler, G.S. et al (2012) List of new names of plant pathogenic bacteria (2008–2010). Journal of Plant Pathology, 94, 21–27. [Google Scholar]
- Büttner, D. and Bonas, U. (2010) Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiology Reviews, 34, 107–133. [DOI] [PubMed] [Google Scholar]
- Channon, A.G. and Hissett, R. (1984) The incidence of bacterial wilt caused by Xanthomonas campestris pv. graminis in pasture grasses in the West of Scotland. Plant Pathology, 33, 113–121. [Google Scholar]
- Charkhabi, N.F. , Booher, N.J. , Peng, Z. , Wang, L. , Rahimian, H. , ShamsBakhsh, M. et al (2017) Complete genome sequencing and targeted mutagenesis reveal virulence contributions of Tal2 and Tal4b of Xanthomonas translucens pv. undulosa ICMP11055 in bacterial leaf streak of wheat. Frontiers in Microbiology, 8, 1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunfer, B.M. and Scolari, B.L. (1982) Xanthomonas campestris pv. translucens on triticale and other small grains. Phytopathology, 72, 683–686. [Google Scholar]
- Curland, R.D. , Gao, L. , Bull, C.T. , Vinatzer, B. , Dill‐Macky, R. , Von Eck, L. et al (2018) Genetic diversity and virulence of wheat and barley strains of Xanthomonas translucens from the Upper Midwestern United States. Phytopathology, 108, 443–453. [DOI] [PubMed] [Google Scholar]
- Dowson, D.W. (1939) On the systematic position and generic names of the gram negative bacterial plant pathogens. Zentr. Bakter. Parasit. Infekt, 100, 177–193. [Google Scholar]
- Duveiller, E. (1990) Seed detection of Xanthomonas campestris pv. undulosa using a modification of Wilbrink’s agar medium. Parasitica, 40, 3–17. [Google Scholar]
- Duveiller, E. and Maraite, H. (1993) Study of yield loss due to Xanthomonas campestris pv. undulosa in wheat under high rainfall temperate conditions. Journal of Plant Diseases and Protection, 100, 453–459. [Google Scholar]
- Duveiller, E. and Maraite, H. (1995) Effect of temperature and air humidity on multiplication of Xanthomonas campestris pv. undulosa and symptom expression in susceptible and field tolerant wheat genotypes. Phytopathology, 143, 227–232. [Google Scholar]
- Duveiller, E. , Bragard, C. and Maraite, H. (1991) Bacterial diseases of wheat in the warmer areas‐reality or myth? In: Saunders D. (ed.), Wheat for the Nontraditional Warm Areas. Proceedings of the International Conference, Iguazu Falls, Brazil: CIMMYT, pp. 189–202. [Google Scholar]
- Duveiller, E. , Bragard, C. and Maraite, H. (1997) Bacterial leaf streak and black chaff caused by Xanthomonas translucens In: Duveiller E., Fucikovskil L. and Rudolph K. (Eds.) The Bacterial Disease of Wheat: Concept and Methods of Disease Management. Mexico, D.F: CIMMYT, pp. 25–32. [Google Scholar]
- Duveiller, E. , van Ginkel, M. and Thijssen, M. (1993) Genetic analysis of resistance to bacterial leaf streak caused by Xanthomonas campestris pv. undulosa in bread wheat. Euphytica, 66, 35–43. [Google Scholar]
- Dye, D.W. and Lelliott, R.A. (1974) Genus II. Xanthomonas In: Buchanan R.E. and Gibbons N.E. (Eds.) Bergey’s Manual of Determinative Bacteriology, 8th edition. Baltimore, MD: Williams and Wilkins, pp. 243–249. [Google Scholar]
- Dye, D.W. , Bradbury, J.F. , Goto, M. , Hayward, A.C. , Lelliot, R.A. and Schroth, M.N. (1980) International standards for naming pathovars of phytopathogenic bacteria and a list of pathovar names and pathotype strains. Annual Review of Plant Phytopathology, 59, 153–168. [Google Scholar]
- Egli, T. and Schmidt, D. (1982) Pathogenic variation among the causal agents of bacterial wilt of forage grasses. Journal of Phytopathology, 104, 138–150. [Google Scholar]
- Egli, T. , Goto, M. and Schmidt, D. (1975) Bacterial wilt, a new forage grass disease. Journal of Phytopathology, 82, 111–121. [Google Scholar]
- El Attari, H. , Hayes, P.M. , Rebai, A. , Barrault, G. , Dechamp‐Guillaume, G. and Sarrafi, A. (1998) Potential of doubled‐haploid lines and localization of quantitative trait loci (QTL) for partial resistance to bacterial leaf streak (Xanthomonas campestris pv. hordei) in barley. Theoretical and Applied Genetics, 96, 95–100. [Google Scholar]
- El Attari, H. , Sarrafi, A. , Alizadeh, A. , Dechamp‐Guillaume, G. and Barrault, G. (1996) Genetic analysis of partial resistance to bacterial leaf streak (Xanthomonas campestris pv. cerealis) in wheat. Plant Pathology, 45, 736–741. [Google Scholar]
- Fang, C.T. , Allen, O.N. , Riker, A.J. and Dickson, J.G. (1950) The pathogenic, physiological and serological reactions of the form species of Xanthomonas translucens . Phytopathology, 40, 44–64. [Google Scholar]
- Forster, R.L. and Schaad, N.W. (1985) Evaluation of seed treatments for eradication of Xanthomonas campestris pv. translucens from wheat seed. Phytopathology, 75, 1385. [Google Scholar]
- Forster, R.L. and Schaad, N.W. (1988) Control of black chaff of wheat with seed treatment and a foundation seed health program. Plant Disease, 72, 935–938. [Google Scholar]
- Forster, E. , Rehms, L.D. , Sands, D.C. , Bjarko, M. and Lund, R.E. (1990) Eradication of Xanthomonas translucens from barley seed with dry heat treatments. Plant Disease, 74, 816–818. [Google Scholar]
- Forster, R.L. and Schaad, N.W. (1990) Longevity of Xanthomonas campestris pv. translucens in wheat seed under two storage conditions In: Klement A.Z. (ed.), Proceedings of the 7th International Conference of Plant Pathogenic Bacteria. Budapest, Hungary: Akadémiai Kiado; ´, pp. 329–331. [Google Scholar]
- Gardiner, D.M. , Upadhyaya, N.M. , Stiller, J. , Ellis, J.G. , Dodds, P.N. , Kazan, K. et al (2014) Genomic analysis of Xanthomonas translucens pathogenic on wheat and barley reveals cross‐kingdom gene transfer events and diverse protein delivery systems. PLoS ONE, 9, e84995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giblot‐Ducray, D. , Marefat, A. , Gillings, M.R. , Parkinson, N.M. , Bowman, J.P. , Ophel‐Keller, K. et al (2009) Proposal of Xanthomonas translucens pv. pistaciae pv. nov., pathogenic to pistachio (Pistacia vera). Systematic and Applied Microbiology, 32, 549–557. [DOI] [PubMed] [Google Scholar]
- Gurion‐Sherman, D. and Lindow, S.E. (1993) Bacterial ice nucleation: Significance and molecular basis. FASEB (Federation of American Societies for Experimental Biology) Journal, 7, 1338–1343. [DOI] [PubMed] [Google Scholar]
- Gurung, S. , Mamidi, S. , Bonman, J.M. , Xiong, M. , Brown‐Guedira, G. and Adhikari, T.B. (2014) Genome‐wide association study reveals novel quantitative trait loci associated with resistance to multiple leaf spot diseases of spring wheat. PLoS ONE, 9, e108179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagborg, W.A.F. (1942) Classification revision in Xanthomonas translucens . Canadian Journal of Research, 20, 312–326. [Google Scholar]
- Hagborg, W.A.F. (1974) Notes on bacterial diseases of cereals and some other crop plants. Canadian Plant Disease Survey, 54, 129–151. [Google Scholar]
- Hersemann, L. , Wibberg, D. , Blom, J. , Goesmann, A. , Widmer, F. , Vorholter, F.J. et al (2017) Comparative genomics of host adaptive traits in Xanthomonas translucens pv. graminis . BMC Genomics, 18, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques, M.A. , Arlat, M. , Boulanger, A. , Boureau, T. , Carrère, S. , Cesbron, S. et al (2016) Using ecology, physiology, and genomics to understand host specificity in Xanthomonas . Annual Review of Phytopathology, 54, 163–187. [DOI] [PubMed] [Google Scholar]
- Jaenicke, S. , Bunk, B. , Wibberg, D. , Spröer, C. , Hersemann, L. , Blom, J. et al (2016) Complete genome sequence of the barley pathogen Xanthomonas translucens pv. translucens DSM 1874T (ATCC 19319T). Genome Announcements, 4, e01334–e1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, J.W. , Cunfer, B.M. and Morey, D.D. (1987) Inheritance of resistance to Xanthomonas campestris pv. translucens in triticale. Euphytica, 36, 603–607. [Google Scholar]
- Jones, L.R. , Johnson, A.G. and Reddy, C.S. (1917) Bacterial blight of barley. Journal of Agricultural Research, 11, 625–643. [Google Scholar]
- Kandel, Y.R. , Glover, K.D. , Osborne, L.E. and Gonzalez‐Hernandez, J.L. (2015) Mapping quantitative resistance loci for bacterial leaf streak disease in hard red spring wheat using an identity by descent mapping approach. Euphytica, 201, 53–65. [Google Scholar]
- Kandel, Y.R. , Glover, K.D. , Tande, C.A. and Osborne, L.E. (2012) Evaluation of spring wheat germplasm for resistance to bacterial leaf streak caused by Xanthomonas campestris pv. translucens . Plant Disease, 96, 1743–1748. [DOI] [PubMed] [Google Scholar]
- Kersters, K. , Pot, B. , Hoste, B. , Gillis, M. and De Ley, J. (1989) Protein electrophoresis and DNA:DNA hybridizations of xanthomonads from grasses and cereals. EPPO Bulletin, 19, 51–55. [Google Scholar]
- Kim, H.K. , Orser, C. , Lindow, S.E. and Sands, D.C. (1987) Xanthomonas campestris pv. translucens strains active in ice nucleation. Plant Disease, 71, 994–997. [Google Scholar]
- Klykov, A.P. (1945) The viability of the causal agent of black bacteriosis in wheat seed. Mikrobiologiya (Moscow), 14, 413–414. [Google Scholar]
- Lamichhane, J.R. , Osdaghi, E. , Behlau, F. , Köhl, J. , Jones, J.B. and Aubertot, J.‐N. (2018) Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agronomy for Sustainable Development, 38, 28. [Google Scholar]
- Langlois, P.A. , Snelling, J. , Hamilton, J.P. , Bragard, C. , Koebnik, R. , Verdier, V. et al (2017) Characterization of the Xanthomonas translucens complex using draft genomes, comparative genomics, phylogenetic analysis and diagnostic LAMP assays. Phytopathology, 107, 519–527. [DOI] [PubMed] [Google Scholar]
- Li, T. , Liu, B. , Spalding, M.H. , Weeks, D.P. and Yang, B. (2012) High‐efficiency TALEN‐based gene editing produces disease‐resistant rice. Nature Biotechnology, 30, 390–392. [DOI] [PubMed] [Google Scholar]
- Maes, M. , Garbeva, P. and Kamoen, O. (1996) Recognition and detection in seed of the Xanthomonas pathogens that cause cereal leaf streak using rDNA spacer sequences and polymerase chain reaction. Phytopathology, 86, 63–69. [Google Scholar]
- Marefat, A. , Ophel‐Keller, K. , Scott, E. and Sedgley, M. (2006a) The use of ARMS PCR in detection and identification of xanthomonads associated with pistachio dieback in Australia. European Journal of Plant Pathology, 116, 57–68. [Google Scholar]
- Marefat, A. , Scott, E.S. , Ophel‐Keller, K. and Sedgley, M. (2006b) Genetic, phenotypic and pathogenic diversity among xanthomonads isolated from pistachio (Pistacia vera) in Australia. Plant Pathology, 55, 639–649. [Google Scholar]
- McManus, P.S. , Stockwell, V.O. , Sundin, G.W. and Jones, A.L. (2002) Antibiotic use in plant agriculture. Annual Review of Phytopathology, 40, 443–465. [DOI] [PubMed] [Google Scholar]
- McMullen, M. and Adhikari, T. (2011) Bacterial leaf streak and black chaff of wheat. Plant Disease Management. Fargo, ND, USA: North Dakota State University Extension Publication, PP1566. [Google Scholar]
- Mellano, V.J. and Cooksey, D.A. (1988) Development of host range mutants of Xanthomonas campestris pv. translucens . Applied and Environmental Microbiology, 54, 884–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milus, E.A. and Chalkley, D.B. (1994) Virulence of Xanthomonas campestris pv. translucens on selected wheat cultivars. Plant Disease, 78, 612–615. [Google Scholar]
- Milus, E.A. and Mirlohi, A.F. (1994) Use of disease reactions to identify resistance in wheat to bacterial streak. Plant Disease, 78, 157–161. [Google Scholar]
- Milus, E.A. and Mirlohi, A.F. (1995) Survival of Xanthomonas campestris pv. translucens between successive wheat crops in Arkansas. Plant Disease, 79, 263–265. [Google Scholar]
- Milus, E.A. , Duveiller, E. , Kirkpatrick, T.L. and Chalkey, D.B. (1996) Relationships between disease reactions under controlled conditions and severity of wheat bacterial streak in the field. Plant Disease, 80, 726–730. [Google Scholar]
- Paul, V.H. and Smith, I.M. (1989) Bacterial pathogens of gramineae: Systematic review and assessment of quarantine status for the EPPO region. EPPO Bulletin, 19, 33–42. [Google Scholar]
- Peng, Z. , Hu, Y. , Xie, Z. , Potnis, N. , Akhunova, A. , Jones, J. et al (2016) Long read and single molecule DNA sequencing simplifies genome assembly and TAL effector gene analysis of Xanthomonas translucens . BMC Genomics, 17, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Z. , Hu, Y. , Zhang, J. , Huguet‐Tapia, J.C. , Block, A.K. , Park, S. et al (2019) Xanthomonas translucens commandeers the host rate‐limiting step in ABA biosynthesis for disease susceptibility. Proceedings of the National Academy of Sciences of the United States of America, 116, 20938–20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce, C. , Bolot, S. , Cunnac, S. , Portier, P. , Fischer‐Le Saux, M. , Jacques, M.A. et al (2015) High quality draft genome sequence of the Xanthomonas translucens pv. cerealis pathotype strain CFBP 2541. Genome Announcements, 3, e01574–e1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce, C. , Jacobs, J.M. , Berthelot, E. , Perret, M. , Vancheva, T. , Bragard, C. et al (2017) Comparative genomics identifies a novel conserved protein, HpaT, in proteobacterial type III secretion systems that do not possess the putative translocon protein HrpF. Frontiers in Microbiology, 8, 1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademaker, J.L.W. , Norman, D.J. , Forster, R.L. , Louws, F.J. , Schultz, M.H. and De Bruijn, F.J. (2006) Classification and identification of Xanthomonas translucens isolates, including those pathogenic to ornamental asparagus. Phytopathology, 96, 876–884. [DOI] [PubMed] [Google Scholar]
- Rashid, A. , Sajahan, M. , Inam‐Ul‐Haq, M. , Shahid, M. , Ehetisham‐ul‐Haq, M. , Waris, I.H. et al (2013) Distribution of black chaff disease of wheat caused by Xanthomonas campestris pv. translucens in different ecological zones of Pakistan and its management through plant extracts and bio‐products. European Journal of Experimental Biology, 3, 261–266. [Google Scholar]
- Reddy, C.S. , Godkin, J. and Johnson, A.G. (1924) Bacterial blight of rye. Journal of Agricultural Research, 28, 1039–1040. [Google Scholar]
- Roberts, D.L. , Vargas, J.M. , Baker, K.K. and Hooper, G.R. (1981) Association of a bacterium with a disease of toronto creeping bentgrass. Plant Disease, 65, 1014–1016. [Google Scholar]
- Sapkota, S. , Zhang, Q. , Chittem, K. , Mergoum, M. , Xu, S.S. and Liu, Z. (2018) Evaluation of triticale accessions for resistance to wheat bacterial leaf streak caused by Xanthomonas translucens pv. undulosa . Plant Pathology, 67, 595–602. [Google Scholar]
- Shane, W.W. , Baumer, J.S. and Teng, P.S. (1987) Crop losses caused by Xanthomonas streak on spring wheat and barley. Plant Disease, 71, 927–930. [Google Scholar]
- Silva, I.T. , Rodrigues, F.A. , Oliveira, J.R. , Pereira, S.C. , Andrade, C.C.L. , Silveira, P.R. et al (2010) Wheat resistance to bacterial leaf streak mediated by silicon. Journal of Phytopathology, 158, 253–262. [Google Scholar]
- Smith, E.F. , Jones, L.R. and Reddy, C.S. (1919) The black chaff of wheat. Science, 50, 48. [DOI] [PubMed] [Google Scholar]
- Stead, D.E. (1989) Grouping of Xanthomonas campestris pathovars of cereals and grasses by cellular fatty acid profiling. EPPO Bulletin, 19, 51–68. [Google Scholar]
- Stromberg, K.D. , Kinkel, L.L. and Leonard, K.J. (2000) Interactions between Xanthomonas translucens pv. translucens, the causal agent of bacterial leaf streak of wheat, and bacterial epiphytes in the wheat phyllosphere. Biological Control, 17, 61–72. [Google Scholar]
- Thompson, D.C. , Schaad, N.W. and Forster, R.L. (1989) New perennial hosts of epiphytic populations of Xanthomonas campestris pv. translucens . Phytopathology, 79, 1168. [Google Scholar]
- Tillman, B.L. and Harrison, S.A. (1996) Heritability of resistance to bacterial streak in winter wheat. Crop Science, 36, 412–418. [Google Scholar]
- Tillman, B.L. , Harrison, S.A. , Clark, C.A. , Milus, E.A. and Russin, J.S. (1996) Evaluation of bread wheat germplasm for resistance to bacterial streak. Crop Science, 36, 1063–1068. [Google Scholar]
- Timmer, L.W. , Marois, J.J. and Achor, D. (1987) Growth and survival of xanthomonads under conditions nonconductive to disease development. Phytopathology, 77, 1341–1345. [Google Scholar]
- Tsilosani, G.A. , Palavandishvili, I.V. , Kopaleishvili, A. and Tukhareli, A. (1977) Seeds as the source of bacterial disease of wheat caused by Xanthomonas translucens . Trudy Instituta Zashchity Rastenii Grus, USSR, 21, 127–130. [Google Scholar]
- Tubajika, K.M. , Russin, J.S. and Harrison, S.A. (1999) Analysis of bacterial leaf streak epidemics on winter wheat in Louisiana. Plant Disease, 83, 541–548. [DOI] [PubMed] [Google Scholar]
- Tubajika, K.M. , Tillman, B.L. , Russin, J.S. , Clark, C.A. and Harrison, S.A. (1998) Relationship between flag leaf symptoms caused by Xanthomonas translucens pv. translucens and subsequent seed transmission in wheat. Plant Disease, 82, 1341–1344. [Google Scholar]
- van den Mooter, M. , Steenackers, M. , Maertens, C. , Gossele, F. , De Vos, P. , Swings, J. et al (1987) Differentiation between Xanthomonas campestris pv. graminis ISPP L 1980, pv. phleipratensis ISPP List 1980 emend., pv. poae Egli an Schmidt 1982, and pv. arrhenatheris Egli and Schmidt 1982, by numerical analysis of phenotypic features and protein gel electrophoresis. Journal of Phytopathology, 118, 135–156. [Google Scholar]
- Vauterin, L. , Hoste, B. , Kersters, K. and Swings, J. (1995) Reclassification of Xanthomonas . International Journal of Systematic Bacteriology, 45, 472–489. [Google Scholar]
- Vauterin, L. , Yang, P. , Hoste, B. , Pot, B. , Swings, J. and Kersters, K. (1992) Taxonomy of xanthomonads from cereals and grasses based on SDS‐PAGE of proteins, fatty acid analysis and DNA hybridization. Journal of General Microbiology, 138, 1467–1477. [Google Scholar]
- Waldron, L.R. (1929) The relationship of black chaff disease of wheat to certain physical and pathological characters. Science, 70, 268. [DOI] [PubMed] [Google Scholar]
- Wallin, J.R. (1946) Parasitism of Xanthomonas translucens (J.J. and R.) Dowson on grasses and cereals. Iowa State College Journal of Science, 20, 171–193. [Google Scholar]
- Wallin, J.R. and Reddy, C.S. (1945) A bacterial streak disease of Phleum pratense L. Phytopathology, 35, 937–939. [Google Scholar]
- Waney, V.R. , Kingsley, M.T. and Gabriel, D.W. (1991) Xanthomonas campestris pv. translucens genes determining host‐specific virulence and general virulence on cereals identified by Tn5‐gusA insertion mutagenesis. Molecular Plant‐Microbe Interactions, 4, 623–627. [Google Scholar]
- Wen, A. , Jayawardana, M. , Fiedler, J. , Sapkota, S. , Shi, G. , Peng, Z. et al (2018) Genetic mapping of a major gene in triticale conferring resistance to bacterial leaf streak. Theoretical and Applied Genetics, 131, 649–658. [DOI] [PubMed] [Google Scholar]
- Wichmann, F. , Vorholter, F.J. , Hersemann, L. , Widmer, F. , Blom, J. , Niehaus, K. et al (2013) The noncanonical type III secretion system of Xanthomonas translucens pv. graminis is essential for forage grass infection. Molecular Plant Pathology, 14, 576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins, P.W. and Exley, J.K. (1977) Bacterial wilt of ryegrass in Britain. Plant Pathology, 26, 2. [Google Scholar]
- Young, J.M. , Dye, D.W. , Bradbury, J.F. , Panagopoilos, C.G. and Robbs, C.S. (1978) A proposed nomenclature and classification for plant pathogenic bacteria. New Zealand Journal of Agricultural Research, 21, 153–177. [Google Scholar]
- Young, J.M. , Park, D.C. , Shearman, H.M. and Fargier, E. (2008) A multilocus sequence analysis of the genus Xanthomonas . Systematic and Applied Microbiology, 5, 366–377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


